1. Introduction
Phenanthrenes, a class of specialized secondary metabolites, are increasingly recognized for their diverse biological activities and structural uniqueness [
1,
2]. Among the plant families producing phenanthrenes, Juncaceae (the rush family) is one of the richest sources of these compounds [
3]. The wide distribution of phenanthrenes is concentrated mainly in the genus
Juncus within the family, with the structures ranging from simple monophenanthrenes, substituted with methyl, hydroxyl, and vinyl groups, to complex derivatives with additional functional groups. These compounds exhibit remarkable biological activities, including antioxidant, anti-inflammatory, antimicrobial, and cytotoxic effects, underscoring their potential as therapeutic agents [
4]. Besides their pharmacological effects, phenanthrenes are hypothesized to play ecological roles in plant defense and stress tolerance [
5]. However, to date, only a limited number of
Juncus species (
n = 13) have been investigated from phytochemical and pharmacological points of view. The most common phenanthrenes are juncunol, juncusol, effusol, juncuenin B, and dehydrojuncusol, of which juncusol can be considered a ubiquitous compound, as it has been isolated almost from all investigated species [
4]. Dimeric phenanthrenes are rare in this genus; to date, most have been isolated from
J. acutus, and seven of them have unusual hepta- and octacyclic structures [
6,
7,
8]. Many
Juncus species remain unexplored, leaving a wealth of potential bioactive compounds undiscovered.
Several phenanthrenes isolated from
Juncus species have been screened for their in vitro cytotoxic activity in various cancer cell lines. First, juncusol was investigated, which proved to be active against NCI 90 KB (human epidermoid nasopharynx carcinoma) cells with an ED
50 of 0.3 μg/mL [
9]. The effects of effusol, dehydroeffusol, juncusol, dehydrojuncusol, juncuenin B, dehydrojuncuenin B, and juncuenin D on cell survival in HT22 cells were tested in a mouse hippocampal neuroblastoma cell line at 10, 30, and 100 µM for 24 h using the MTT assay. Among them, effusol, juncusol, juncuenin B, dehydrojuncuenin B, and juncuenin D resulted in a decrease in MTT reduction (9.9, 25.4, 13.6, 8.4, and 23.7%, respectively) at 100 µM and destroyed neuronal integrity [
4,
10].
With almost 2 million new cases and 1 million deaths worldwide in 2020, colorectal cancer ranks as the third and second most frequent cause of cancer incidence and mortality, respectively [
11]. Doxorubicin is a commonly used chemotherapeutic drug in this type of human cancer as a result of its wide range of pharmacological activities, but at the same time, it causes a wide range of side effects (cardiotoxicity, neuropathy, hepatotoxicity, nephrotoxicity, alopecia, myelosuppression, neutropenia, anemia, and thrombocytopenia) [
12,
13]. In addition, common problems with monotherapy include drug resistance at the cellular and tumor levels; therefore, combination chemotherapy regimens containing two or more classical anticancer drugs have been applied for decades in clinical practice to treating a variety of cancers [
14].
Juncus tenuis, commonly known as path rush, is a 10–80 cm height, widespread species in various ecological habitats, including disturbed soils and wetlands. It occurs in North America and Western and Central Europe, as well as East Asia, Australia, and New Zealand [
15,
16]. Despite its broad distribution and ecological adaptability,
J. tenuis has been underexplored for its secondary metabolites. Previously, three phenanthrenes, namely effusol, juncusol, and 2,7-dihydroxy-1,8-dimethyl-5-vinyl-9,10-dihydrophenanthrene, were isolated from this plant and their antiproliferative activity tested against different tumor (A2780, A2780cis, KCR, MCF-7, HeLa, HTB-26, and T47D) and normal (MRC-5) cell lines. Juncusol and effusol possessed high activity in HeLa cells, with IC
50 values of 0.5 and 2.3 μM [
17].
This study presents the isolation and structural elucidation of phenanthrenes from J. tenuis using advanced chromatographic and spectroscopic techniques. We further evaluated their pharmacological properties, focusing on their antiproliferative activity against the COLO 205 (doxorubicin-sensitive) and COLO 320 (-resistant) human tumor cell lines. We also tested the synergistic effect of phenanthrenes with the therapeutically used anticancer agent doxorubicin. This work aims to expand our chemical and biological understanding of the phenanthrenes in J. tenuis. It contributes to the broader exploration of the Juncaceae family as a reservoir of bioactive natural products.
2. Results and Discussion
Air-dried aerial parts of
J. tenuis were ground into a powder and extracted using MeOH. After concentration, the extract was dissolved in 50% aqueous MeOH, and
n-hexane, CHCl
3, and EtOAc were used to perform solvent–solvent partitioning. Several chromatographic techniques [column chromatography (CC), vacuum liquid chromatography (VLC), Sephadex LH-20 gel chromatography, and high-performance liquid chromatography (HPLC)] were applied to isolating 24 compounds from the CHCl
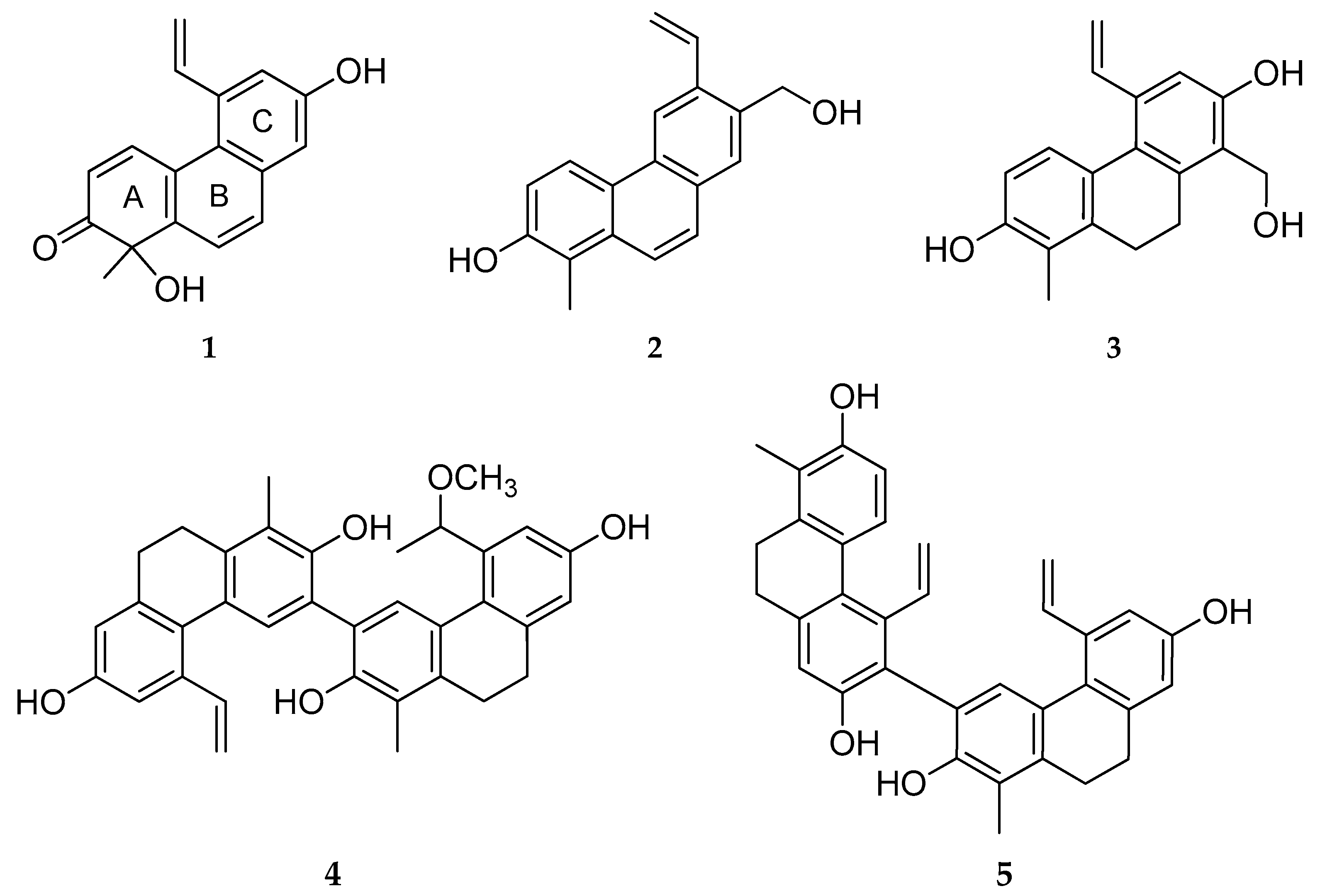
3 fraction, including 5 new (
1–
5) (
Figure 1) and 14 known (
6–
19) phenanthrenes and 5 other compounds.
The structures of the compounds were elucidated using a comprehensive spectroscopic analysis, including one- and two-dimensional NMR (1H–1H COSY, HSQC, HMBC, NOESY) and high-resolution electrospray ionization mass spectrometry (HRESIMS) measurements, complemented by comparisons with previously reported spectral data.
2.1. Structural Elucidation of the Isolated Compounds
2.1.1. Tenuin A (1)
Tenuin A (
1) was isolated as a yellow amorphous solid, exhibiting an optical rotation of [α]
D26 0 (
c 0.1, MeOH). HRESIMS revealed its molecular formula to be C
17H
14O
3, based on the observed [M–H]
− ion at
m/
z 265.0871 (calculated for C
17H
13O
3−, 265.0870) (
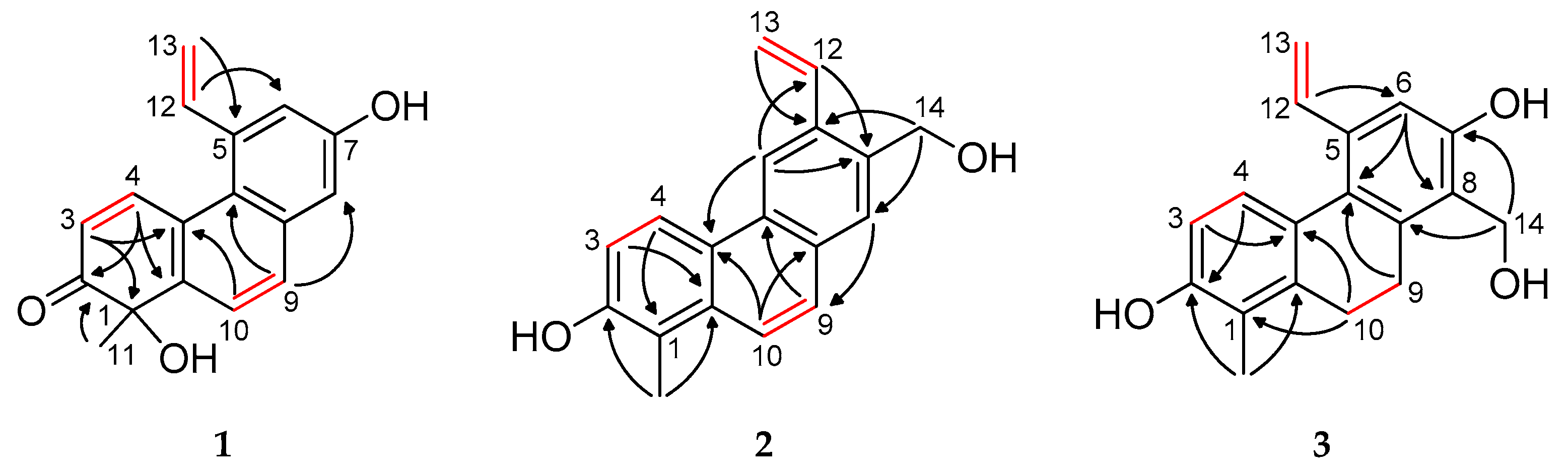
Figure S8). The
1H NMR spectrum (
Table 1,
Figure S1) showed resonances corresponding to two
ortho-coupled aromatic protons [
δH 6.13 d (1H, d,
J = 10.4 Hz, H-3), 8.73 d (1H, d,
J = 10.4 Hz, H-4)] and four aromatic protons as singlets [
δH 7.09 (1H, s, H-8), 7.18 (1H, s, H-6), and 2×7.79 (1H, s, H-9,10)]; one methyl singlet at
δH 1.55 (3H, s, Me-11); and a vinylic system at
δH 5.69 d (17.3 Hz), 5.44 d (10.8 Hz) (H-13), and 7.35 dd (17.3, 10.7) (H-12). In the JMOD spectrum, 17 carbon signals were detected (
Table 1,
Figure S2).
The resonance of a singlet aromatic proton at
δH 7.79 (2H) was observed, which correlated in the HSQC spectrum (
Figure S3) with carbon signals at
δC 131.6 and 124.7 and was attributed to protons H-9 and H-10 in a phenanthrene scaffold. The
1H–
1H COSY spectrum revealed three distinct spin systems: H-3/H-4 (
δH 6.13, d and 8.73, d), H-12/H-13a (
δH 7.35, dd and 5.69, d), and H-12/H-13b (
δH 7.35, dd and 5.44, d) (
Figure S4). A signal at
δC 206.9 in the
13C JMOD NMR spectrum indicated the presence of a carbonyl group, which was assigned to C-2 based on the HMBC correlations from both the methyl protons of CH
3-11 (
δH 1.55, s) and H-4 (
δH 8.73, d) to C-2 (δC 206.9) (
Table 1,
Figure 2 and
Figure S5). The
ortho-coupled doublets at
δH 6.13 and 8.73 were assigned to H-3 and H-4, respectively, supported by long-range correlations: H-3 with C-1 and C-4a and H-4 with C-1a. The methyl group at
δH 1.55 (s) was located at C-1, as confirmed by its HMBC cross-peaks with quaternary carbons at
δC 146.7 (C-1a), 78.3 (C-1), and 206.9 (C-2). The attachment of hydroxy groups to C-1 and C-7 was inferred from the chemical shifts in the quaternary carbons at
δC 78.3 (C-1) and
δC 156.2 (C-7), respectively. Furthermore, HMBC correlations from H-13a and H-13b to C-5 and from H-12 to C-6 indicated the presence of a vinyl group at position C-5.
The structure of compound
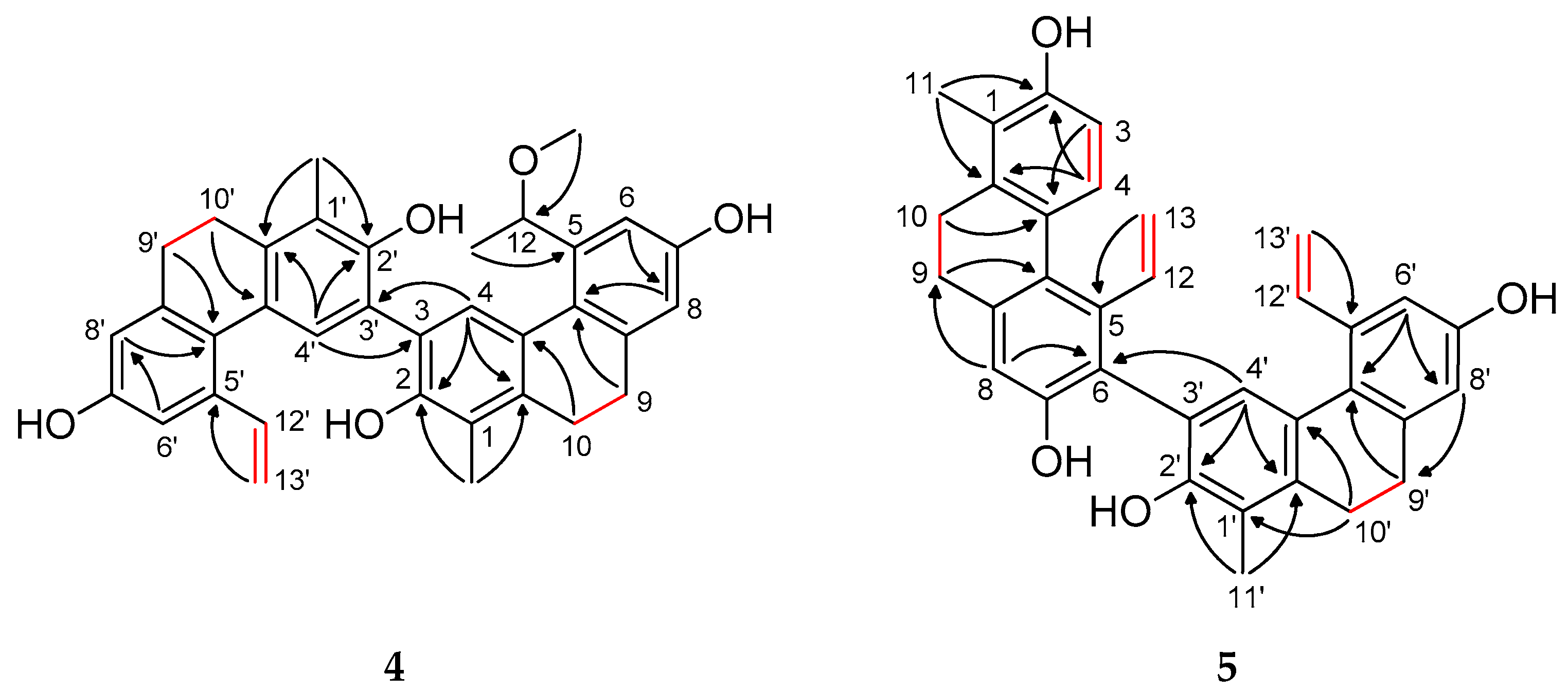
1 was corroborated further by NOESY data, which revealed key nuclear Overhauser enhancements between H-3/H-4, H-4/H-12, H-13a/H-6, and H-9/H-8 (
Figure 3 and
Figure S6). These findings conclusively established the planar structure of compound
1, herein named tenuin A.
Tenuin A (
1) is a chiral natural product; however, it exhibited zero specific rotation (
c 0.1, MeOH). Therefore, a chiral HPLC analysis of
1 was performed, revealing only one peak on a Lux Amylose-1 column. This result can be attributed to the low enantioselectivity of the biosynthetic step, which has also been observed for other natural phenanthrenes [
18].
Compound
1 is structurally similar to luzulin A, isolated previously from
Luzula luzuloides (Juncaceae) [
19]. Ring A is the same in the case of the two compounds, containing a chiral carbon atom (C-1) substituted with methyl and hydroxyl groups and a carbonyl group at C-2 (
Figure 1).
2.1.2. Tenuin B (2)
Compound
2 (tenuin B) was obtained as a light yellow amorphous solid. Its molecular formula was established as C
18H
16O
2 according to the molecular ion at
m/
z 263.1078 [M–H]
− (calcd for C
18H
15O
2−, 263.1067) in HRESIMS (
Figure S16). Based on the
1H NMR spectrum (
Table 1,
Figure S9), this compound is also a vinyl-substituted phenanthrene. In addition, the
1H NMR spectrum indicated one aromatic methyl group (
δH 2.55 s), four
ortho-coupled aromatic protons (
δH 7.22 d, 7.72 d, 7.90 d, 8.50 d), two
para-coupled aromatic protons (
δH 7.84 s, 8.75 s), and one methylene signal (
δH 4.86 s). The
13C JMOD NMR spectrum (
Table 1,
Figure S10) confirmed the presence of 18 carbon atoms (2 bearing oxygen atoms), 8 quaternary carbons, 7 methine, 2 methylene, and a methyl carbon. These NMR and MS data suggested a phenanthrene skeleton similar to dehydrojuncuenin A isolated from
J. sethcuensis and
J. inflexus [
20]. The difference between the two compounds is a hydroxymethyl group in
2 instead of the methyl group in dehydrojuncuenin A at C-7 (
Figures S11 and S12). The substitution of
2 was determined through an HMBC experiment (
Figure S13). The key correlations between H
3-11 (
δH 2.55, s)/C-1 (
δC 118.9), C-2 (
δC 154.7), C-1a (
δC 133.9), H-5 (
δH 8.75, s)/C-12 (
δC 136.1), H-13b (
δH 5.43, dd)/C-6 (
δC 136.7), H
2-14 (
δH 4.86, s)/C-6 (
δC 136.7), and C-8 (
δC 128.7) suggested the methyl group at C-1, the vinyl substituent at C-6, and the hydroxymethyl group at C-7 (
Figure 2).
The structure of compound
2 was further confirmed by the NOESY experiment (
Figure S14). Overhauser effects were detected between H
3-11/H-10, H-3/H-4, H-4/H-5, H-5/H-13a, H
2-14/H-8, and H-8/H-9, confirming the planar structure of
2, and it was named tenuin B (
Figure 3).
2.1.3. Tenuin C (3)
Tenuin C (
3) was determined to possess the molecular formula C
18H
18O
3, as established by its [M–H]
− peak at
m/
z 281.1184 in the HRESIMS (calculated for C
18H
17O
3−, 281.1183) (
Figure S24). The
1H and
13C JMOD NMR spectra (
Figures S17 and S18) revealed features consistent with a 9,10-dihydrophenanthrene skeleton, evidenced by the methylene signals at
δH 2.76 and 2.69 (each 2H, m) and the corresponding carbon signals at
δC 26.9 and 26.5. The substitution pattern included one methyl group, two hydroxyl groups, a hydroxymethyl, and a vinyl moiety (
Figures S19 and S20). Additionally, the
1H NMR spectrum displayed an isolated aromatic singlet at
δH 6.92 (1H) and two
ortho-coupled aromatic protons at
δH 6.63 and 7.13 (each 1H, d,
J = 8.4 Hz). The HMBC correlations of H
3-11 with C-1 and C-2, H-3 with C-4a, and H-4 with C-2 confirmed the assembly of ring A (
Figure 2 and
Figure S21). The methylene protons H
2-9 and H
2-10 exhibited correlations with C-8a, C-1, C-1a, and C-4a, thereby connecting rings A and C. The vinyl group and the hydroxymethyl substituent were positioned at C-5 and C-8, respectively, as indicated by the HMBC cross-peaks from H-12 to C-6 and from H
2-14 to both C-7 and C-8. Additional HMBC correlations from H-6 (
δH 6.92) to C-5a, C-7 (
δC 155.3), and C-8 (
δC 124.4) supported the placement of a hydroxyl group at C-7, leading to the full structural assignment of compound
3 as 2,7-dihydroxy-8-hydroxymethyl-1-methyl-5-vinyl-9,10-dihydrophenanthrene. The NOESY spectrum further supported this structure by showing key spatial correlations between H
2-9/H
2-14, H-4/H-12, and H
2-10/H
3-11 (
Figure 3 and
Figure S22). Structurally, tenuin C (
3) closely resembles 2,7-dihydroxy-1,8-dimethyl-5-vinyl-9,10-dihydrophenanthrene, previously reported from
Juncus effusus and
Juncus acutus [
6]. The only structural difference lies in the substitution at C-8; in tenuin C (
3), a hydroxymethyl group can be found instead of a methyl group at C-8.
2.1.4. Tenuin D (4)
The molecular formula for compound
4 (tenuin D) is C
35H
34O
5 based on the HRESIMS analysis (
m/
z 533.2330 [M–H]
−, calcd for C
35H
33O
5–, 533.2334) (
Figure S32). Analysis of the
1H NMR,
13C NMR JMOD, HSQC and
1H–
1H COSY spectra (
Figures S25–S28) of this compound revealed a heterodimeric phenanthrenoid structure comprising two dihydrophenanthrene units (
Table 2). Through an evaluation of the 2D NMR data, it was apparent that the building blocks of compound
4 are effususol A and effusol (
10) [
10]. The C-3–C-3′ connection of the two monomers was proved by observing the HMBC correlations of H-4 (
δH 7.04 s) with C-3′ (
δC 125.3) and H-4′ (
δH 7.41 s) with C-3 (
δC 125.2) (
Figure 2 and
Figure S29). The NOESY correlations between H-4/H-12, H-4′/H-12′, H-6/H-13a, and H-6′/H-13′a corroborated the elucidated structure (
Figure 3 and
Figure S30).
Compound 4 contains a stereogenic center at position C-12 within unit A. The specific optical rotation of the compound was measured as [α]D26 0 (c 0.1, MeOH), indicating the absence of optical activity. The chiral HPLC analysis of tenuin D (4) revealed two well-resolved peaks of an equal area, each displaying identical UV spectra. These results indicate that compound 4 is a racemic mixture.
2.1.5. Tenuin E (5)
The molecular formula for compound
5 (tenuin E) is C
34H
30O
4 based on the HRESIMS analysis (
m/
z 501.2066 [M–H]
−, calcd for C
34H
29O
4−, 501.2071) (
Figure S40). An analysis of the
1H NMR,
13C NMR JMOD, HSQC, and NOESY spectra (
Figures S33–S35 and S38) for this compound revealed a dihydrophenanthrenoid dimer comprising two effusol (
10) units (
Table 2). In compound
5, the methine groups at C-6 of unit A and C-3′ of unit B were replaced by quaternary carbons (
δC 123.8 and 122.4). This hypothesis was confirmed further through an analysis of the
1H−
1H COSY (
Figure S36) and HMBC data (
Figure 2 and
Figure S37). The HMBC long-range correlation observed from H-4′ (
δH 7.00 s) to C-6 (
δC 123.8) proved the C-6–C-3′connection of the two monomers (
Figure 2), establishing the structure of
5 as 1,1′-dimethyl-5,5′-divinyl-9,9′,10,10′-tetrahydro-[6,3′-biphenanthrene]-2,2′,7,7′-tetraol. The anisotropic effect of the aromatic ring of monomer B, which caused shielding of the H-12 and H-13 signals, can be observed (
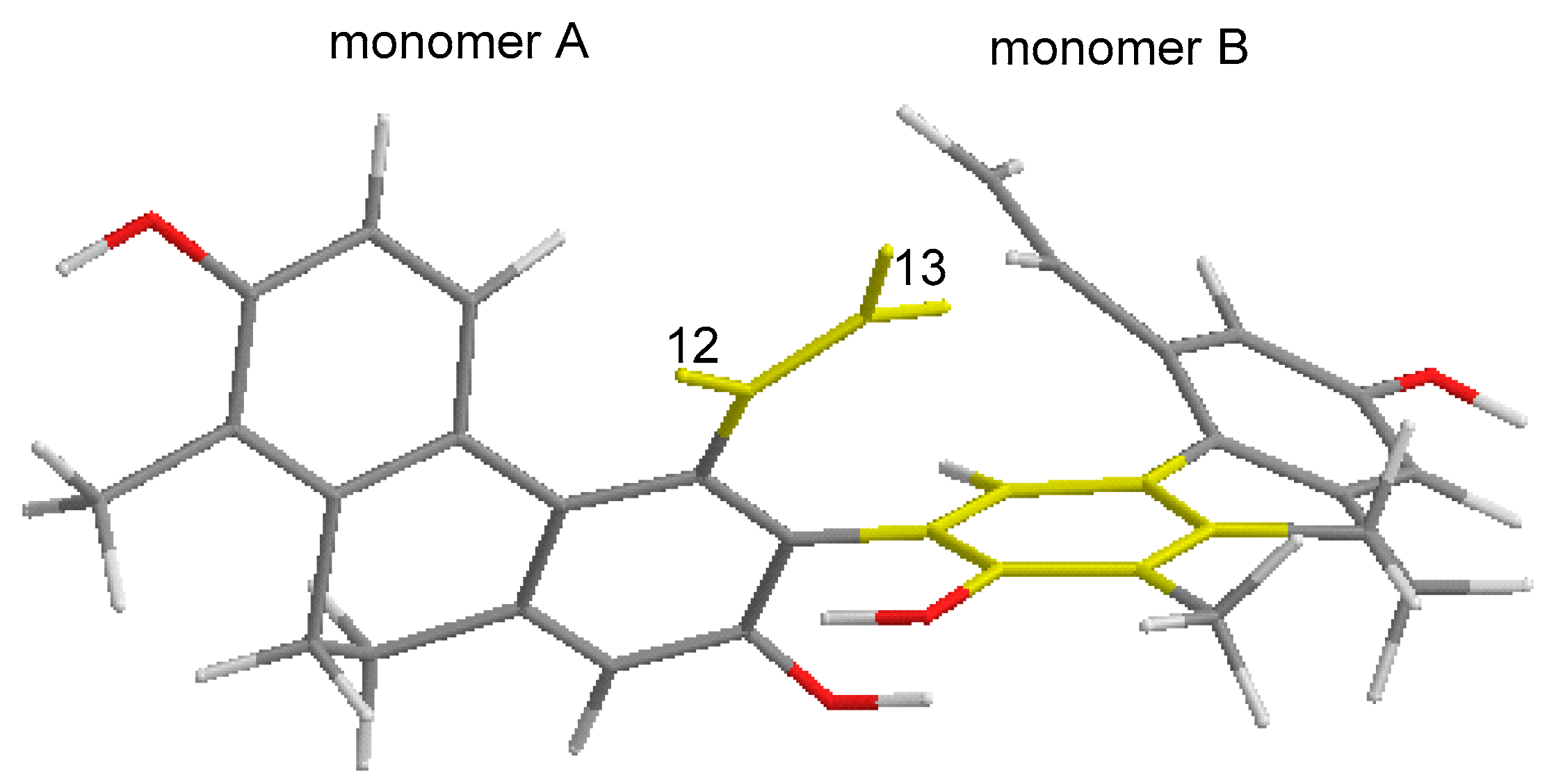
Figure 4).
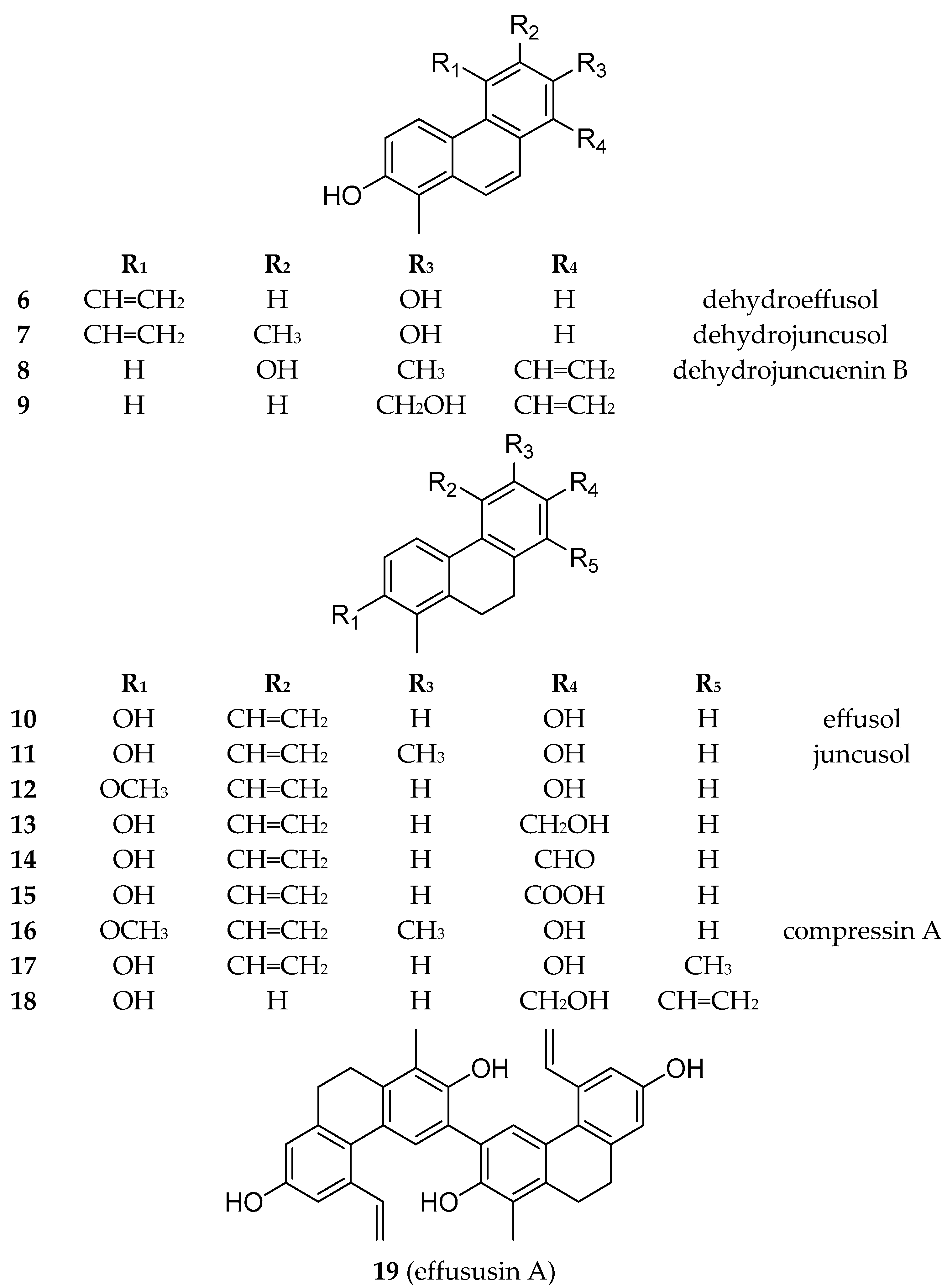
Besides the new compounds tenuins A–E (
1–
5), 14 known phenanthrenes (
6–
19) (
Figure 5), with 4 dehydrophenanthrenes among them, namely dehydroeffusol (
6) (
Figure S41) [
21], dehydrojuncusol (
7) (
Figure S42) [
22], dehydrojuncuenin B (
8) (
Figure S43) [
20], and its hydroxyderivative (
9) (
Figure S44) [
23]; 9 dihydrophenanthrenes (
10–
18) (
Figures S45–S53) [
24,
25,
26,
27,
28,
29]; 1 dimer (effususin A,
19) (
Figure S54) [
30]; luteolin; 5,7-dihydroxy-4-chromone;
p-hydroxybenzoic [
31] acid; vanillic [
32] acid; and
trans-p-coumaric acid were identified from
J. tenuis. Structural characterization was carried out using HRESIMS, together with one- and two-dimensional NMR experiments. The
1H and
13C chemical shifts were assigned and compared with data from the literature for confirmation. Except for effusol (
10), juncusol (
11), and 2,7-dihydroxy-1,8-dimethyl-5-vinyl-9,10-dihydrophenanthrene (
17), all of the compounds were isolated from this plant species for the first time.
Effusol (
10) and juncusol (
11) are the main phenanthrenes from
J. tenuis. Dehydro derivatives (
6 and
7) of these compounds were also identified from the plant. Similarly, compounds
9 and
18 are also dehydro–dihydro pairs. Most of the phenanthrenes are dihydro derivatives (
3–
5,
10–
19). Among the phenanthrenes, mono- (
1–
3,
6–
18) and diphenanthrenes (
4,
5,
19) also occur. All compounds are vinyl-substituted. The vinyl group is mainly joined at C-5; in the case of
2, it was connected at C-6, while in
8,
9, and
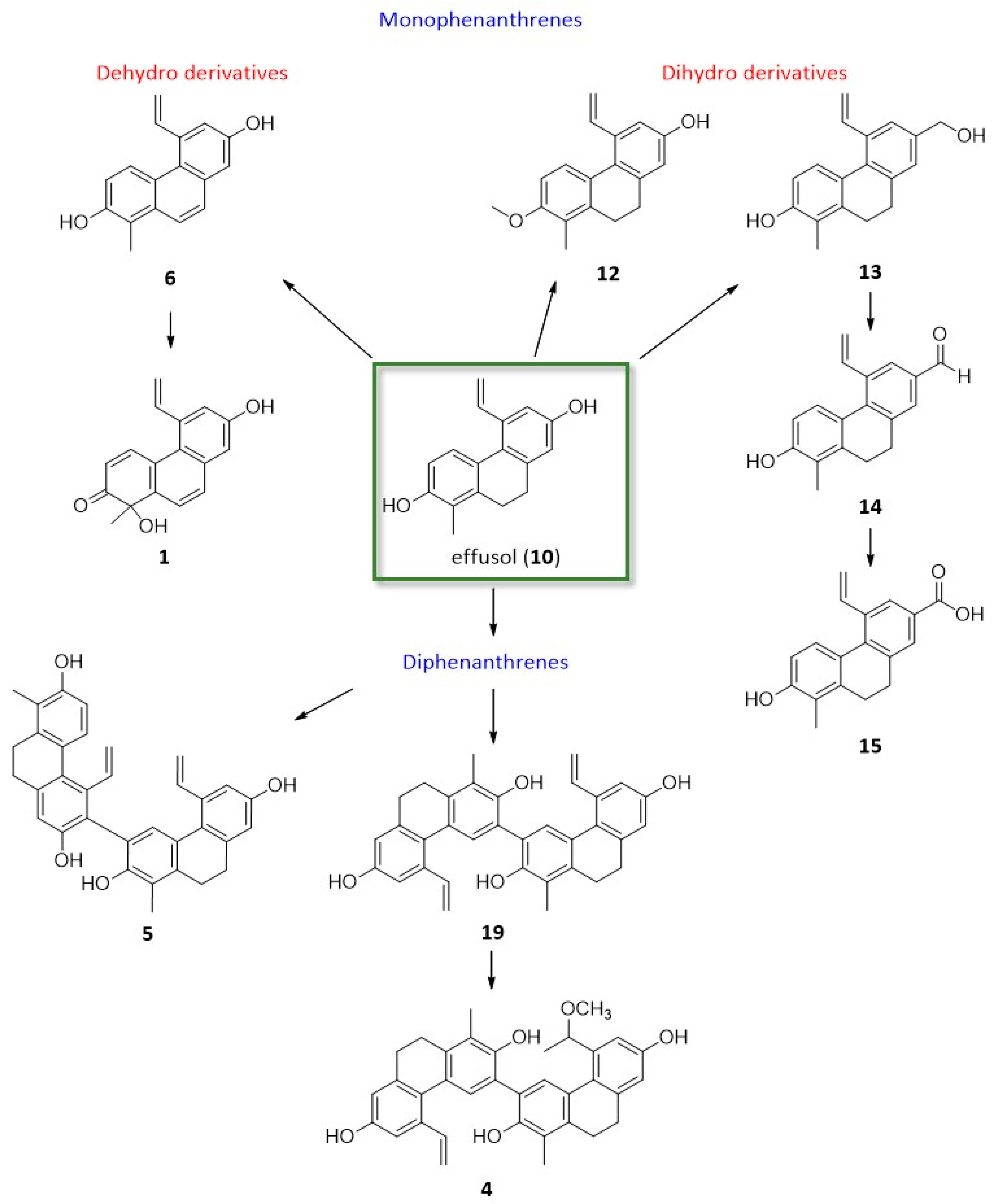
18, it was connected at C-8. Most probably, the 5-vinyl phenanthrenes could originate from effusol (
10) and juncusol (
11) (
Figure 6). In compound
4, one of the vinyl groups was modified into a methoxy ethylene group. Compounds
10 and
13–
15 differ only in the substituent at C-7: the hydroxyl group in
10, the hydroxymethyl group in
13, the formyl group in
14, and the carboxyl group in
15. Based on our results, it can be stated that the phenanthrene content of this plant sample was very different from that investigated previously by our group.
2.2. The Antiproliferative Activity of the Compounds
The antiproliferative effects of the isolated phenanthrenes (compounds
1–
19) were evaluated using human cancer cell lines, including doxorubicin-sensitive colonic adenocarcinoma COLO 205 and multidrug-resistant colonic adenocarcinoma COLO 320/MDR-LRP (which expresses P-glycoprotein [MDR1] and LRP), as well as non-cancerous human lung fibroblast CCD-19Lu cells. CCD-19Lu was chosen because it is a well-characterized, non-tumorigenic cell line commonly used to assess general cytotoxicity. Moreover, data from the literature support the use of lung fibroblast cell lines—including CCD-19Lu—as normal controls in studies involving colon cancer cells such as Colo 205 and Colo 320 [
33]. The thiazolyl blue tetrazolium bromide (MTT) assay was employed to determine the concentration of each compound required to inhibit 50% of the cell viability (IC
50), as summarized in
Table 3. Among the tested compounds, the dimeric phenanthrene tenuin D (compound
4) exhibited the most potent antiproliferative activity against the COLO 205 cell line, with an IC
50 value of 7.60 μM. The other two dimers (
5 and
19) were also proven to be active, especially against the COLO 205 cell line (IC
50 values of 11.6 μM for
5 and 10.9 μM for
19, respectively). Effusol (
10), the monomer of the dimers, was less active (IC
50 values of 52.7 μM in COLO 205 and 49.7 μM in the COLO 320 cell line). Saturation of the double bond between C-9 and C-10 led to decreased activity in the case of dehydroeffusol (
6) and effusol (
10), whereas no significant difference was observed for the other pairs,
7 and
11, and
9 and
18.
The isolated phenanthrenes were slightly selective or non-selective towards the tumor cells. Compound 4 was the most selective towards one of the tumor cell lines (COLO 205, SI = 0.49), while compressin A (16) showed selectivity towards the multi-drug resistant COLO 320 cells (SI = 1.87). Considering the tumor selectivity, the highest selectivity index (SI CCD-19Lu/COLO 320) was observed for tenuin B (2, SI = 2.51) and compound 18 (SI = 2.25).
All of the tested compounds were evaluated in four parallel measurements. The selectivity index (SI) was calculated as the ratio of the IC50 value in the non-tumorigenic cells to the IC50 value in the cancer cell lines. An SI value greater than 6 indicates strong selectivity toward cancer cells, values between 3 and 6 denote moderate selectivity, values between 1 and 3 indicate slight selectivity, and an SI below 1 suggests a lack of selectivity.
2.3. The Drug Combination Assay
Many types of cancer exhibit significant resistance to the currently available chemotherapeutic agents, highlighting the need for novel, effective, and well-tolerated therapeutic strategies. One promising approach involves the discovery of new bioactive natural products. To explore this, a chemosensitivity assay was conducted to examine the in vitro interactions between the isolated compounds and the antineoplastic agent doxorubicin, a substrate of P-glycoprotein (P-gp). A combination chemotherapy model was applied using human COLO 320 colon carcinoma cells. The combination index (CI), calculated according to the Chou–Talalay method, was used to evaluate drug–drug interactions, classifying them as synergistic (CI < 1), additive (CI = 1), or antagonistic (CI > 1) (
Table 4) [
34]. As shown in
Table 4, the majority of the tested compounds demonstrated synergistic interactions with doxorubicin (CI < 1) in the COLO 320 cell line. Tenuin B (compound
2) exhibited a particularly strong synergistic effect, with combination index (CI) values below 0.1. This compound showed weak antiproliferative activity (IC
50: 48.4 and 38.3 μM) (
Table 3). Moreover, strong synergisms were detected for compounds
6,
7,
13,
14, and
18.
In recent years, several phenanthrenes from the Juncaceae family have been tested for their in vitro cytotoxicity against various cancer cell lines using different test systems, exhibiting promising activities. Their in vitro effects may be mediated by several potential mechanisms, e.g., cell membrane rupture, impaired cell metabolism, DNA damage, or a combination of these [
36]. The anticancer activity of phenanthrenes is strongly influenced by their structural features, including the oxidation state, degree of saturation, substitution pattern, and dimerization. In this study, newly isolated phenanthrenes from
J. tenuis, particularly the diphenanthrenes tenuin D (
4), tenuin E (
5), and effususin A (
19), showed the most potent in vitro antiproliferative activity against the COLO 205 colorectal cancer cell line, with IC
50 values in the range of 7.6–11.6 μM. Their superior activity compared to that of their monomeric counterparts suggests that dimerization enhances their cytotoxic potential, potentially by increasing the molecular size, lipophilicity, or interaction with multiple cellular targets. Reports from the literature also support the enhanced bioactivity of phenanthrene dimers. For example, effususin B isolated from
J. effusus showed strong cytotoxic activity against three cancer cell lines (SMMC-7721, HepG-2, and MCF-7), with IC
50 values of 13.60, 12.93, and 12.49 µM, respectively [
30].
Among the monomers, e.g., juncunol had an IC
50 value of 18 µM in the HepG2 cells, and it induced an increase in the number of apoptotic cells in a concentration-dependent manner (IC
50 value ± 25%) accompanied by a decrease in Δψm [
37]. The compound induced cel cycle arrest in the G0/G1 phase, while showing no hemolytic properties. In silico studies indicate that that compound seems to bind between GC base pairs and thus may act as a DNA intercalator [
38].
The presence and position of vinyl groups can affect the activity of Juncaceae phenanthrenes. Most of the phenanthrenes in this study carried a vinyl substituent, often at C-5. However, tenuin B (2), with a vinyl group at C-6, demonstrated a unique profile, including very strong synergism with doxorubicin (CI = 0.021), despite its relatively weak antiproliferative activity. This suggests that the side-chain positioning may modulate interactions with efflux transporters or drug-metabolizing enzymes, potentially influencing chemosensitization.
Further studies incorporating molecular docking, metabolic profiling, and target identification are needed to deepen our understanding of how these structural motifs govern anticancer efficacy and selectivity.
3. Materials and Methods
3.1. The General Procedures
Optical rotations were measured using a JASCO P-2000 polarimeter (JASCO, Tokyo, Japan), and the UV spectra were obtained using a Shimadzu UV-1800 spectrophotometer. Vacuum liquid chromatography (VLC) was performed on silica gel (silica gel GF254, 15 µm, Merck, Darmstadt, Germany). Sephadex LH-20 (25–100 µm, Sigma-Aldrich, Budapest, Hungary) was employed for gel filtration. HPLC was performed on a Shimadzu HPLC system using reversed-phase columns (Phenomenex Luna Phenyl-Hexyl, 5 µm, 100 A, 250 × 10 mm; Phenomenex Kinetex Phenyl-Hexyl, 5 µm, 100 A, 150 × 4.6 mm). For the analysis of the compounds with chiral carbon atoms, a Lux Amylose-1 column (5 µm, 250 × 4.6 mm, Phenomenex, Torrance, CA, USA) was utilized with cyclohexane–isopropanol 82:18 as the mobile phase. All of the solvents used for CC were of at least analytical grade (VWR Ltd., Szeged, Hungary).
The NMR spectra were recorded in methanol-d4 using a Bruker Avance DRX 500 spectrometer (Bruker, Germany) operating at 500 MHz for 1H and 125 MHz for 13C. The residual solvent signals of CD3OD (δH 4.78 and 3.31; δC 49.2) were used as internal references. The chemical shift values (δ) are reported in parts per million (ppm), and coupling constants (J) are given in hertz (Hz). Two-dimensional NMR experiments were performed using standard Bruker software (TopSpin 3.6.2), employing gradient-enhanced techniques for the COSY, HSQC, and HMBC spectra. High-resolution mass spectrometry (HRMS) data were acquired using a Thermo Scientific Q-Exactive Plus Orbitrap mass spectrometer equipped with an electrospray ionization (ESI) source, operating in both positive and negative ionization modes. The data acquisition and processing were carried out using MassLynx software (Version 4.1).
3.2. The Plant Material
Whole plants of Juncus tenuis Willd. were collected during the flowering period in June 2020 from a sandy, dried lakebed near Barcs, Hungary (GPS coordinates: 45°58′30.817″ N, 17°32′17.764″ E). The plant material was botanically identified by Dragica Purger (Department of Pharmacognosy, University of Pécs, Hungary). A voucher specimen (No. 901) was deposited into the herbarium of the Department of Pharmacognosy, the University of Szeged, Szeged, Hungary.
3.3. Extraction and Isolation
The air-dried whole plant material of Juncus tenuis (3.38 kg) was ground and extracted at room temperature through percolation with methanol (30 L). The resulting crude methanolic extract (0.5 kg) was concentrated under reduced pressure and stored at −20 °C until further processing. Then, it was subjected to solvent–solvent partitioning using n-hexane (8 × 500 mL), chloroform (CHCl3, 10 × 500 mL), and ethyl acetate (EtOAc, 9 × 500 mL). The concentrated chloroform-soluble fraction (40 g) was separated further through vacuum liquid chromatography (VLC) on silica gel, employing a gradient elution system of cyclohexane–EtOAc–MeOH (ranging from 98:2:0 to 50:50:0; 1500 mL per eluent), followed by MeOH. A total of 11 major fractions (designated as fractions 1–11) were collected in 100 mL portions. The fractionation was monitored through thin-layer chromatography (TLC), and fractions with similar profiles were combined based on their chromatographic patterns. Subsequently, all major fractions were separated further through gel chromatography on a Sephadex LH-20 stationary phase using CH2Cl2–MeOH (1:1) as the eluent.
Fraction 11/1 was pure and yielded luteolin (26.6 mg). Fraction 1/3 was purified through RP-HPLC using a MeOH–H2O gradient solvent system (from 78:22 to 88:12 in 9 min; flow rate: 1 mL/min) as the mobile phase, yielding compounds 12 (tR = 5.50 min, 2.1 mg) and 16 (tR = 6.53 min, 1.4 mg). Purification of fraction 2/2 was performed through RP-HPLC under gradient conditions using MeOH–H2O (from 78:22 to 88:12 in 9 min; flow rate: 1 mL/min) as the mobile phase, yielding compound 14 (tR = 6.63 min, 1.0 mg). Fraction 4/2 was purified through RP-HPLC using MeOH–H2O (65:35) as the eluent at a flow rate of 1 mL/min, yielding compounds 10 (tR = 5.46 min, 574.9 mg), 11 (tR = 6.78 min, 374.7 mg), and 17 (tR = 8.12 min, 48.3 mg). Fraction 5/3 was separated through gel chromatography on a Sephadex LH-20 stationary phase using CH2Cl2–MeOH (1:1) as the eluent. Subfraction 5/3/2 was separated further through RP-HPLC under gradient conditions using MeOH–H2O (from 8:2 to 1:0 in 9 min; flow rate: 3 mL/min) as the mobile phase to yield compound 7 (tR = 8.32 min, 1.0 mg). Fraction 6/3 was purified through RP-HPLC using a gradient solvent system of MeOH–H2O (from 85:15 to 1:0 in 8 min; flow rate: 3 mL/min) as the mobile phase, yielding seven subfractions. Subfraction 6/3/2 was separated further through RP-HPLC using a gradient solvent system of MeOH–H2O (from 55:45 to 1:0 in 7 min; flow rate: 1 mL/min) as the mobile phase, yielding compound 1 (tR = 5.18 min, 2.4 mg). Purification of subfraction 6/3/4 was performed through RP-HPLC using MeOH–H2O (65:35) as the eluent at a flow rate of 1 mL/min, yielding compounds 13 (tR = 6.30 min, 20.4 mg) and 18 (tR = 6.79 min, 2.2 mg). Purification of fraction 7/2 was performed through RP-HPLC under gradient conditions using MeOH–H2O (from 7:3 to 1:0 in 11 min; flow rate: 3 mL/min) as the mobile phase, yielding six subfractions. Subfraction 7/2/1 was purified further through RP-HPLC using a gradient solvent system of MeOH–H2O (from 45:55 to 1:0 in 9 min; flow rate: 1 mL/min), yielding 5,7-dihydroxy-4-chromone (tR = 5.00 min, 1.5 mg,). Subfraction 7/2/2 was separated through RP-HPLC using a gradient solvent system of MeOH–H2O (from 62:38 to 77:23 in 9 min; flow rate: 1 mL/min) to yield compounds 2 (tR = 5.91 min, 1.5 mg) and 15 (tR = 7.78 min, 3.1 mg). Purification of subfraction 7/2/3 was performed through RP-HPLC using a gradient solvent system of MeOH–H2O (from 7:3 to 85:15 in 10 min; flow rate: 1 mL/min) as the mobile phase, yielding compound 5 (tR = 7.28 min, 1.9 mg). Subfraction 7/2/4 was separated through RP-HPLC under gradient conditions using MeOH–H2O (from 73:27 to 8:2 in 10 min; flow rate: 1 mL/min) as the mobile phase, yielding four subfractions. Subfraction 7/2/4/1 was pure and yielded compound 8 (tR = 5.96 min, 0.9 mg). Subfraction 7/2/4/3 was purified further through RP-HPLC using a gradient solvent system of MeOH–H2O (from 1:1 to 8:2 in 8 min; flow rate 1 mL/min) as the mobile phase, yielding compound 19 (tR = 6.30 min, 0.8 mg).
Fraction 8/3 was separated using gel chromatography with a Sephadex LH-20 stationary phase using CH2Cl2–MeOH (1:1) as the eluent. Subfraction 8/3/2 was purified further through RP-HPLC using a gradient solvent system of MeOH–H2O (from 85:15 to 1:0 in 9 min; flow rate: 3 mL/min) as the mobile phase, yielding six subfractions. Further purification of fraction 8/3/2/1 was performed through RP-HPLC under gradient conditions using MeOH–H2O (from 27:73 to 1:1 in 10 min; flow rate: 1 mL/min) as the mobile phase, yielding p-hydroxybenzoic acid (tR = 4.93 min, 1.6 mg), vanillic acid (tR = 5.46 min, 7.0 mg), and trans-p-coumaric acid (tR = 7.20 min, 3.2 mg). Subfraction 8/3/2/2 was separated further through RP-HPLC under gradient conditions using MeOH–H2O (from 25:75 to 52:48 in 8.5 min; flow rate: 1 mL/min) as the mobile phase to yield compound 3 (tR = 7.79 min, 1.2 mg). Subfraction 8/3/2/3 was purified further through RP-HPLC using a gradient solvent system of MeOH–H2O (from 35:65 to 85:15 in 8 min; flow rate: 1 mL/min) as the mobile phase, yielding compound 9 (tR = 6.65 min, 1.6 mg). Further purification of fraction 8/3/2/6 was performed through RP-HPLC using MeOH–H2O (73:27) as the eluent at a flow rate of 1 mL/min, yielding compound 4 (tR = 7.24 min, 1.5 mg). Purification of fraction 9/2 was performed through RP-HPLC using a gradient solvent system of MeOH–H2O (from 55:45 to 90:10 in 8 min; flow rate: 1 mL/min) as the mobile phase, yielding six subfractions. Subfraction 9/2/5 was purified further through RP-HPLC using a gradient solvent system of MeOH–H2O (from 63:37 to 8:2 in 7 min; flow rate: 1 mL/min) as the mobile phase, yielding compound 6 (tR = 5.76 min, 1.0 mg).
3.4. Physical Characteristics of New Compounds
Tenuin A (
1). Yellow amorphous solid; [α]
D26 0 (c 0.1, MeOH); UV (MeOH) λ
max (log ε) = 222 (4.09), 278 (3.85), 356 (3.12) nm (
Figure S7);
1H and
13C NMR data: see
Table 1; (–)-HRESIMS
m/
z 265.0871 [M–H]
− (calcd for C
17H
14O
3, 265.0870).
Tenuin B (
2). Light yellow amorphous solid; UV (MeOH) λ
max (log ε) = 215 (4.52), 269 (4.43) nm (
Figure S15);
1H and
13C NMR data: see
Table 1; (–)-HRESIMS
m/
z 263.1078 [M–H]
− (calcd for C
18H
16O
2, 263.1067).
Tenuin C (
3). Light brown amorphous solid; UV (MeOH) λ
max (log ε) = 210 (3.92), 266 (3.51) nm (
Figure S23);
1H and
13C NMR data: see
Table 1; (–)-HRESIMS
m/
z 281.1184 [M–H]
− (calcd for C
18H
18O
3, 281.1183).
Tenuin D (
4). Light yellow amorphous solid; [α]
D26 0 (c 0.1, MeOH); UV (MeOH) λ
max (log ε) = 212 (3.96), 244 (3.64), 272 (3.54) nm (
Figure S31);
1H and
13C NMR data: see
Table 2; (–)-HRESIMS
m/
z 533.2330 [M–H]
− (calcd for C
35H
34O
5, 533.2334).
Tenuin E (
5). Yellow amorphous solid; UV (MeOH) λ
max (log ε) = 215 (4.49), 283 (3.84) nm (
Figure S39);
1H and
13C NMR data: see
Table 2; (–)-HRESIMS
m/
z 501.2066 [M–H]
− (calcd for C
34H
30O
4, 501.2071).
3.5. The Antiproliferative Assays
3.5.1. Cell Lines
The human colon adenocarcinoma cells—COLO 205 (CCL-222, doxorubicin-sensitive) and COLO 320/MDR-LRP (ATCC-CCL-220.1, multidrug-resistant, expressing P-gp)—were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM of L-glutamine, 1 mM of sodium pyruvate, and 100 mM of HEPES. The non-cancerous human lung fibroblast cell line CCD-19Lu was maintained in Eagle’s Minimal Essential Medium (EMEM; containing 4.5 g/L of glucose) supplemented with a non-essential amino acid mixture, selected vitamins, and 10% heat-inactivated fetal bovine serum. All of the cell lines were detached using 0.25% trypsin and 0.02% EDTA for 5 min at 37 °C. The cell lines were obtained from LGC Promochem (Teddington, England).
3.5.2. The Antiproliferative Assay
Human colonic adenocarcinoma cell lines—doxorubicin-sensitive COLO 205 and multidrug-resistant COLO 320—as well as the non-cancerous human lung fibroblast cell line CCD-19Lu were used to evaluate the effects of the test compounds on cell proliferation. The assays were conducted in 96-well flat-bottomed microtiter plates. Stock solutions of the compounds were prepared in dimethyl sulfoxide (DMSO), with the final DMSO concentration kept below 1% in all samples. The compounds were diluted in 100 µL of the appropriate culture medium. Adherent cells were cultured in EMEM supplemented with 10% heat-inactivated fetal bovine serum and seeded into 96-well plates at a density of 6 × 103 cells per 100 µL per well. The cells were allowed to adhere for 24 h at 37 °C in a humidified incubator with 5% CO2. After incubation, the medium was removed and replaced with 100 µL of fresh medium. Serial dilutions of the compounds were prepared in separate plates and added to the cells, starting from a concentration of 100 µM with twofold serial dilutions (final concentration range: 100–0.19 µM). The cells were then incubated for 72 h at 37 °C. At the end of the incubation period, 20 µL of thiazolyl blue tetrazolium bromide (MTT) solution (5 mg/mL, Sigma) was added to each well. After 4 h of incubation at 37 °C, 100 µL of 10% sodium dodecyl sulfate (SDS) in 0.01 M HCl was added to solubilize the formazan crystals, and the plates were incubated overnight at 37 °C. Cell viability was assessed by measuring the optical density (OD) at 540/630 nm using a Multiscan EX ELISA reader (Thermo Labsystems, Cheshire, WA, USA).
The inhibitory concentration that reduced cell growth by 50% (IC
50) was determined from the dose–response curves. The percent inhibition was calculated using the formula
Dose–response curves were generated by plotting the percent inhibition against the logarithm of the compound concentrations and fitted using Prism5 software (Version 6, GraphPad Software Inc., San Diego, CA, USA). The IC
50 values were derived from four independent experiments conducted in parallel for each cell line and are reported as the mean values [
38].
All reagents and cell culture media used for cell maintenance were obtained from Merck (Darmstadt, Germany).
3.5.3. The Drug Combination Assay
The multidrug-resistant COLO 320 cell line was used to perform the drug combination assay. Doxorubicin (2 mg/mL, Teva Pharmaceuticals, Budapest, Hungary) was serially diluted horizontally, starting with 8.6 µM. The resistance modifier was subsequently diluted vertically, with the starting concentration determined based on the IC
50 value. Doxorubicin was diluted horizontally in 100 µL, while the resistance modifiers were diluted vertically in 50 µL in the microtiter plate. The compounds and doxorubicin were prepared as separate dilutions. The cells were seeded at a density of 6 × 10
3 cells per well into 100 µL of medium and incubated for 24 h at 37 °C in a 5% CO
2 atmosphere prior to treatment. After this period, the culture medium was removed, and 50 µL of fresh medium was added to each well, followed by the addition of 50 µL of the diluted compounds, resulting in a final volume of 200 µL per well. The plates were then incubated for 72 h at 37 °C under 5% CO
2. The cell viability was assessed at the end of incubation using the MTT assay, as described previously. Drug interactions were analyzed using CompuSyn software (Version 1.00) [
39].
The dose–response curves for individual agents and their combinations were fitted to a linear model based on the median-effect equation to calculate the median effective dose (IC
50) and the slope (m) [
35,
40]. The quality of the fit was evaluated using the linear correlation coefficient (r), and only data with r > 0.90 are reported. The extent of drug interaction was quantified using the combination index (CI), where CI ≈ 1 indicated an additive effect, CI < 1 signified synergy, and CI > 1 denoted antagonism.
4. Conclusions
In this study, 19 phenanthrenes—including tenuins A–E (1–5) as new natural products and 14 known compounds (6–19)—along with luteolin, 5,7-dihydroxy-4-chromone, p-hydroxybenzoic acid, vanillic acid, and trans-p-coumaric acid were characterized from the whole plant of J. tenuis. Comprehensive spectroscopic data elucidated the planar structures of these compounds. Except for effusol (10), juncusol (11), and 2,7-dihydroxy-1,8-dimethyl-5-vinyl-9,10-dihydrophenanthrene (17), all of the compounds were reported from this plant for the first time. The phenanthrene dimers 4, 5, and 19 exhibited in vitro antiproliferative activity against the COLO 205 tumor cell line, with tenuin D (4) identified as the most promising compound, with a strong effect (IC50 = 7.6 μM). In addition, tenuin B (2) exhibited very strong synergism with doxorubicin in the drug combination assay.
These findings expand the phytochemical and pharmacological knowledge of J. tenuis and support the Juncaceae family as a valuable source of bioactive phenanthrenes. Future research should focus on further mechanistic studies to understand the molecular basis of the synergistic effects observed with doxorubicin, particularly in multidrug-resistant cancer models. Additionally, structure–activity relationship (SAR) studies and semi-synthetic modifications of the newly identified phenanthrenes may help improve their tumor selectivity and pharmacokinetic profiles. In vivo evaluations and formulation approaches such as nanoencapsulation or targeted delivery systems could enhance their therapeutic potential while minimizing their toxicity. Continued exploration of other Juncus species and related genera may also uncover novel phenanthrene scaffolds with promising biological properties.