Polyphenols in the Central Nervous System: Cellular Effects and Liposomal Delivery Approaches
Abstract
1. Introduction
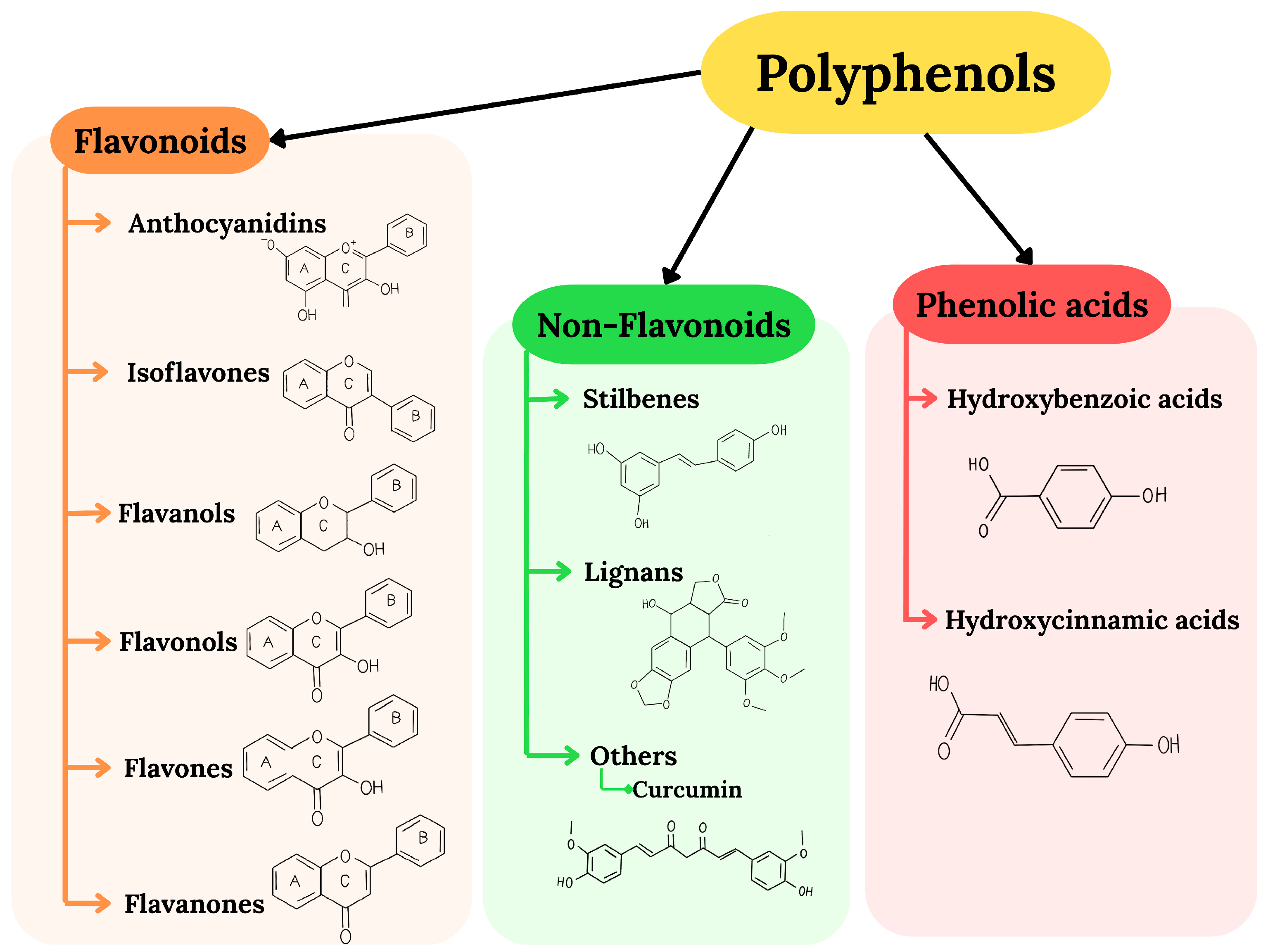
2. Polyphenols as Neuroprotective Agents: Antioxidant Properties, Bioavailability, and Mechanisms of Action
2.1. Bioavailability of Polyphenols and Their Biosafety
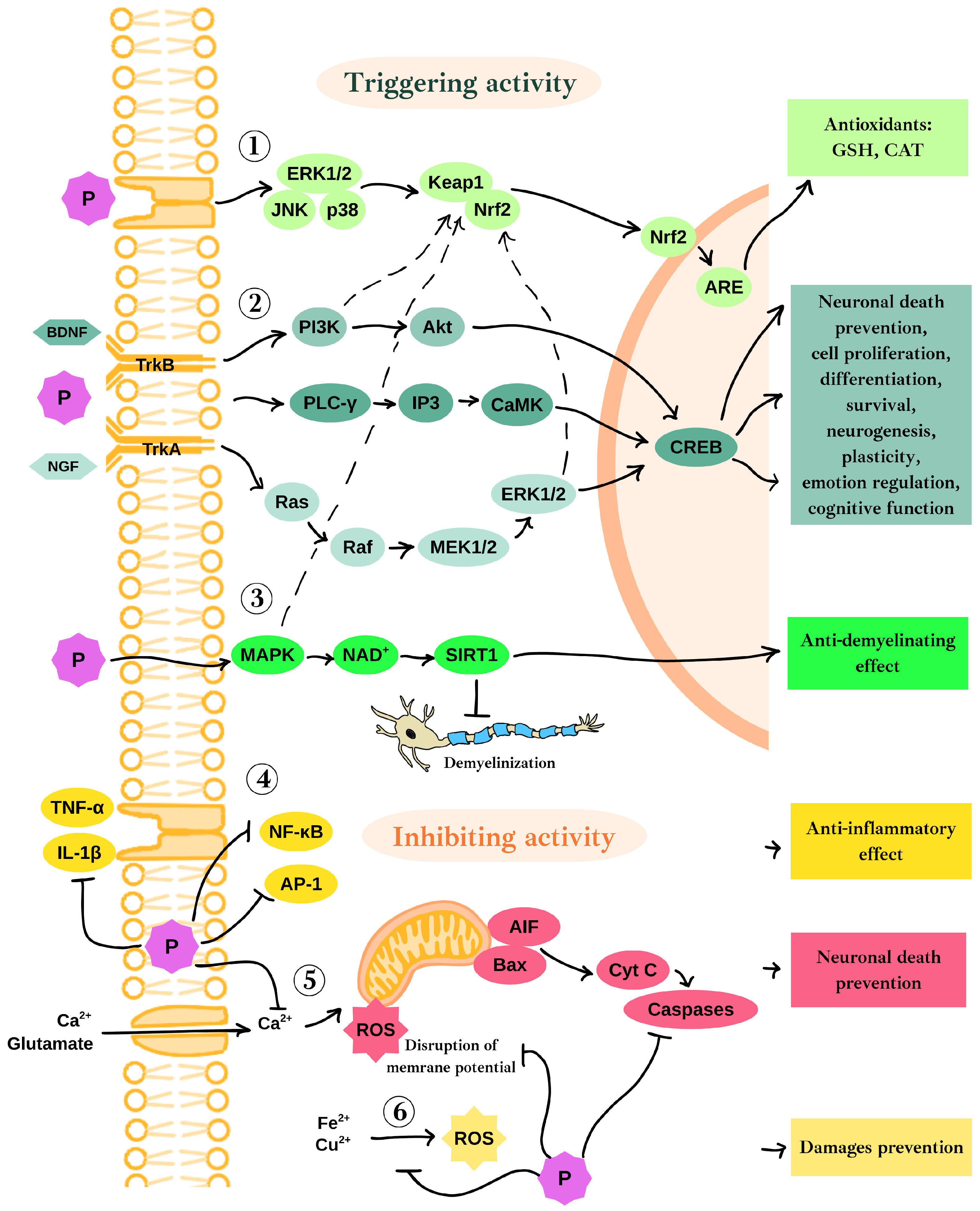
2.2. Neuroprotective Mechanisms of Polyphenols’ Action in the Context of Oxidative Stress and Neurodegeneration
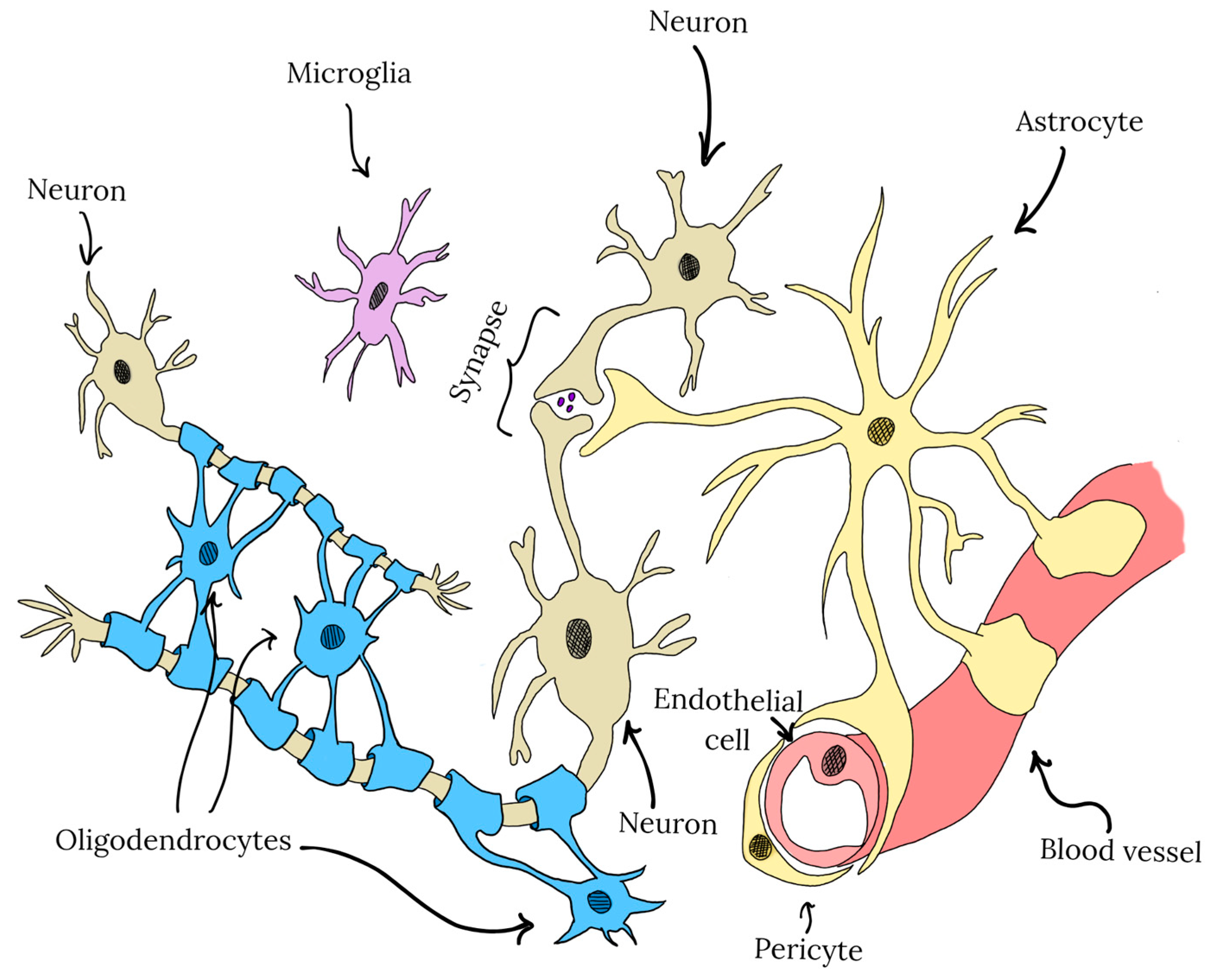
3. CNS Cell-Type Specific Effects of Polyphenols in the Central Nervous System
3.1. Neuroprotective Mechanisms of Selected Polyphenols in Neuronal Cells
3.1.1. Neuroprotective Effects of Epigallocatechin-3-Gallate in Neurons
3.1.2. Neuroprotective Effects of Berberine in Neurons
3.1.3. Neuroprotective Effects of Curcumin in Neurons
3.1.4. Neuroprotective Effects of Resveratrol in Neurons
3.1.5. Neuroprotective Effects of Quercetin in Neurons
3.2. Neuroprotective Mechanisms of Selected Polyphenols in Astrocytes
3.3. Neuroprotective Mechanisms of Selected Polyphenols in Microglial Cells
3.3.1. Influence of Curcumin on Microglia During the Course of Neurodegenerative Diseases
3.3.2. Influence of Luteolin on Microglia During the Course of Neurodegenerative Diseases
3.3.3. Influence of Resveratrol on Microglia During the Course of Neurodegenerative Diseases
3.3.4. Influence of Quercetin on Microglia During the Course of Neurodegenerative Diseases
3.4. Neuroprotective Mechanisms of Selected Polyphenols in OPCs and OLs
3.4.1. Influence of Quercetin on OPCs and OLs During the Course of Neurodegenerative Diseases
3.4.2. Influence of Resveratrol and Curcumin on OPCs and OLs During the Course of Neurodegenerative Diseases
3.4.3. Influence of Phloretin and Ellagic Acid on OPCs and OLs During the Course of Neurodegenerative Diseases
3.4.4. The Role of Other Polyphenols in OPCs and OLs Function During Neurodegenerative Diseases
4. Nanodelivery of Polyphenols: Liposomes as a Promising Platform
- Membrane composition adjustments, such as the incorporation of cholesterol, which stabilizes and stiffens the lipid bilayer [220].
- Surface functionalization, e.g., polyethylene glycol (PEG) coating, to prolong circulation time and reduce immune clearance (stealth effect) [214].
- Targeting strategies, involving the conjugation of antibodies or ligands to the liposomal surface, which can direct the nanocarrier to specific tissues or cell types [221].
Advances in Liposomal Nanocarriers for Brain-Targeted Polyphenol Therapy
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Van Schependom, J.; D’haeseleer, M. Advances in Neurodegenerative Diseases. J. Clin. Med. 2023, 12, 1709. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Bisignano, C.; Hsu, J.M.; Bryazka, D.; Cao, S.; Bhattacharjee, N.V.; Dalton, B.E.; Lindstedt, P.A.; Smith, A.E.; Ababneh, H.S.; et al. Burden of Disease Scenarios by State in the USA, 2022–2050: A Forecasting Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 404, 2341–2370. [Google Scholar] [CrossRef]
- Bomsdorf, E. Life Expectancy in Germany until 2050. Exp. Gerontol. 2004, 39, 159–163. [Google Scholar] [CrossRef]
- Forman, M.S.; Trojanowski, J.Q.; Lee, V.M.-Y. Neurodegenerative Diseases: A Decade of Discoveries Paves the Way for Therapeutic Breakthroughs. Nat. Med. 2004, 10, 1055–1063. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Lv, Q.; Long, J.; Gong, Z.; Nong, K.; Liang, X.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1–13. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, C.; Zhao, G. Ways to Enhance the Bioavailability of Polyphenols in the Brain: A Journey through the Blood-Brain Barrier. Food Rev. Int. 2022, 38, 812–828. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of Dietary Polyphenols: Factors Contributing to Their Clinical Application in CNS Diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Grabska-Kobylecka, I.; Kaczmarek-Bak, J.; Figlus, M.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Glabinski, A.; Nowak, D. The Presence of Caffeic Acid in Cerebrospinal Fluid: Evidence That Dietary Polyphenols Can Cross the Blood-Brain Barrier in Humans. Nutrients 2020, 12, 1531. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Resveratrol for Alzheimer Disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral Curcumin for Alzheimer’s Disease: Tolerability and Efficacy in a 24-Week Randomized, Double Blind, Placebo-Controlled Study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Chen, H.; Xu, X.; Li, Y.; Gui, Y. Neuroprotective Effect of Trans-Resveratrol in Mild to Moderate Alzheimer Disease: A Randomized, Double-Blind Trial. Neurol. Ther. 2021, 10, 905–917. [Google Scholar] [CrossRef]
- Micek, A.; Jurek, J.; Owczarek, M.; Guerrera, I.; Torrisi, S.A.; Castellano, S.; Grosso, G.; Alshatwi, A.A.; Godos, J. Polyphenol-Rich Beverages and Mental Health Outcomes. Antioxidants 2023, 12, 272. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Bouaziz, B.; Müller, P.; M Glenn, J.; Bott, N.T.; Müller, N.; Chtourou, H.; Driss, T.; et al. Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1598. [Google Scholar] [CrossRef]
- Hunt, T.; Pontifex, M.G.; Vauzour, D. (Poly)Phenols and Brain Health—Beyond Their Antioxidant Capacity. FEBS Lett. 2024, 598, 2949–2962. [Google Scholar] [CrossRef]
- Canevelli, M.; Adali, N.; Kelaiditi, E.; Cantet, C.; Ousset, P.-J.; Cesari, M. Effects of Gingko Biloba Supplementation in Alzheimer’s Disease Patients Receiving Cholinesterase Inhibitors: Data from the ICTUS Study. Phytomedicine 2014, 21, 888–892. [Google Scholar] [CrossRef]
- Szymkowiak, I.; Marcinkowska, J.; Kucinska, M.; Regulski, M.; Murias, M. Resveratrol Bioavailability After Oral Administration: A Meta-Analysis of Clinical Trial Data. Phytother. Res. 2025, 39, 453–464. [Google Scholar] [CrossRef]
- Williamson, G. Bioavailability of Food Polyphenols: Current State of Knowledge. Annu. Rev. Food Sci. Technol. 2025, 16, 315–332. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Zhou, Y.; Tan, Y.; Feng, W.; Peng, C. Interactions between Gut Microbiota and Polyphenols: A Mechanistic and Metabolomic Review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant Activity of a Flavonoid-Rich Extract of Hypericum perforatum L. In Vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Li, B.; Cheng, Z.; Sun, X.; Si, X.; Gong, E.; Wang, Y.; Tian, J.; Shu, C.; Ma, F.; Li, D.; et al. Lonicera caerulea L. Polyphenols Alleviate Oxidative Stress-Induced Intestinal Environment Imbalance and Lipopolysaccharide-Induced Liver Injury in HFD-Fed Rats by Regulating the Nrf2/HO-1/NQO1 and MAPK Pathways. Mol. Nutr. Food Res. 2020, 64, 1901315. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant Activity of Food Constituents: An Overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Mayer, J.M. Understanding Hydrogen Atom Transfer: From Bond Strengths to Marcus Theory. Acc. Chem. Res. 2011, 44, 36–46. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Dai, F.; Chen, W.-F.; Zhou, B. Antioxidant Synergism of Green Tea Polyphenols with α-Tocopherol and l-Ascorbic Acid in SDS Micelles. Biochimie 2008, 90, 1499–1505. [Google Scholar] [CrossRef]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and in Vivo Studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- de Groot, H.; Littauer, A. Hypoxia, Reactive Oxygen, and Cell Injury. Free Radic. Biol. Med. 1989, 6, 541–551. [Google Scholar] [CrossRef]
- Yusa, T.; Beckman, J.S.; Crapo, J.D.; Freeman, B.A. Hyperoxia Increases H2O2 Production by Brain in Vivo. J. Appl. Physiol. 1987, 63, 353–358. [Google Scholar] [CrossRef]
- Turrens, J.F.; Freeman, B.A.; Levitt, J.G.; Crapo, J.D. The Effect of Hyperoxia on Superoxide Production by Lung Submitochondrial Particles. Arch. Biochem. Biophys. 1982, 217, 401–410. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Biagi, M.; Bertelli, A.A.E. Wine, Alcohol and Pills: What Future for the French Paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Tenore, G.C.; Campiglia, P.; Giannetti, D.; Novellino, E. Simulated Gastrointestinal Digestion, Intestinal Permeation and Plasma Protein Interaction of White, Green, and Black Tea Polyphenols. Food Chem. 2015, 169, 320–326. [Google Scholar] [CrossRef]
- Jaramillo Flores, M.E. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients 2019, 11, 751. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic Activity, Biological Effect and Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A Natural Compound with Promising Pharmacological Activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Hollman, P.; de Vries, J.; van Leeuwen, S.; Mengelers, M.; Katan, M. Absorption of Dietary Quercetin Glycosides and Quercetin in Healthy Ileostomy Volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of Food Matrix on the Content and Bioavailability of Flavonoids. Trends Food Sci. Technol. 2021, 117, 15–33. [Google Scholar] [CrossRef]
- Lund, K.C.; Pantuso, T. Combination Effects of Quercetin, Resveratrol and Curcumin on In Vitro Intestinal Absorption. J. Restor. Med. 2014, 3, 112–120. [Google Scholar] [CrossRef]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the Bioavailability of Resveratrol by Combining It with Piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef]
- Bailey, H.H.; Johnson, J.J.; Lozar, T.; Scarlett, C.O.; Wollmer, B.W.; Kim, K.; Havinghurst, T.; Ahmad, N. A Randomized, Double-Blind, Dose-Ranging, Pilot Trial of Piperine with Resveratrol on the Effects on Serum Levels of Resveratrol. Eur. J. Cancer Prev. 2021, 30, 285–290. [Google Scholar] [CrossRef]
- Renaud, J.; Martinoli, M.-G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of Dietary Polyphenols on Metabolic Syndrome Features in Humans: A Systematic Review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Ijaz, M.; Buabeid, M.; Kharaba, Z.J.; Yaseen, H.S.; Murtaza, G. Therapeutic Effects and Safe Uses of Plant-Derived Polyphenolic Compounds in Cardiovascular Diseases: A Review. Drug Des. Dev. Ther. 2021, 15, 4713–4732. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Chen, J.; Guo, R.; Yan, H.; Tian, L.; You, Q.; Li, S.; Huang, R.; Wu, K. Naringin Inhibits ROS-activated MAPK Pathway in High Glucose-induced Injuries in H9c2 Cardiac Cells. Basic Clin. Pharmacol. Toxicol. 2014, 114, 293–304. [Google Scholar] [CrossRef]
- Biglu, M.-H.; Ghavami, M.; Biglu, S. Cardiovascular Diseases in the Mirror of Science. J. Cardiovasc. Thorac. Res. 2016, 8, 158–163. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Effects of Green Tea Supplementation on Elements, Total Antioxidants, Lipids, and Glucose Values in the Serum of Obese Patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef]
- Pasdar, Y.; Oubari, F.; Zarif, M.N.; Abbasi, M.; Pourmahmoudi, A.; Hosseinikia, M. Effects of Quercetin Supplementation on Hematological Parameters in Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Clin. Nutr. Res. 2020, 9, 11. [Google Scholar] [CrossRef]
- Abrahamse, E.; Minekus, M.; van Aken, G.A.; van de Heijning, B.; Knol, J.; Bartke, N.; Oozeer, R.; van der Beek, E.M.; Ludwig, T. Development of the Digestive System—Experimental Challenges and Approaches of Infant Lipid Digestion. Food Dig. 2012, 3, 63–77. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The Inhibitory Effect of Polyphenols on Human Gut Microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Kim, T.-E.; Shin, K.-H.; Park, J.-E.; Kim, M.-G.; Yun, Y.-M.; Choi, D.-H.; Kwon, K.J.; Lee, J. Effect of Green Tea Catechins on the Pharmacokinetics of Digoxin in Humans. Drug Des. Dev. Ther. 2018, 12, 2139–2147. [Google Scholar] [CrossRef]
- Lown, K.S.; Bailey, D.G.; Fontana, R.J.; Janardan, S.K.; Adair, C.H.; Fortlage, L.A.; Brown, M.B.; Guo, W.; Watkins, P.B. Grapefruit Juice Increases Felodipine Oral Availability in Humans by Decreasing Intestinal CYP3A Protein Expression. J. Clin. Investig. 1997, 99, 2545–2553. [Google Scholar] [CrossRef]
- Weber, K.; Setchell, K.; Stocco, D.; Lephart, E. Dietary Soy-Phytoestrogens Decrease Testosterone Levels and Prostate Weight without Altering LH, Prostate 5alpha-Reductase or Testicular Steroidogenic Acute Regulatory Peptide Levels in Adult Male Sprague-Dawley Rats. J. Endocrinol. 2001, 170, 591–599. [Google Scholar] [CrossRef]
- Hutchins, A.M.; McIver, I.E.; Johnston, C.S. Soy Isoflavone and Ascorbic Acid Supplementation Alone or in Combination Minimally Affect Plasma Lipid Peroxides in Healthy Postmenopausal Women. J. Am. Diet. Assoc. 2005, 105, 1134–1137. [Google Scholar] [CrossRef]
- Vanhees, K.; de Bock, L.; Godschalk, R.W.L.; van Schooten, F.J.; van Waalwijk van Doorn-Khosrovani, S.B. Prenatal Exposure to Flavonoids: Implication for Cancer Risk. Toxicol. Sci. 2011, 120, 59–67. [Google Scholar] [CrossRef]
- Cui, K.; Luo, X.; Xu, K.; Ven Murthy, M.R. Role of Oxidative Stress in Neurodegeneration: Recent Developments in Assay Methods for Oxidative Stress and Nutraceutical Antioxidants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 771–799. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Dineshkumar, K.; Karthik, S.; Sireesh, D.; Hopper, W.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene-Mediated Nrf2 Activation: Mechanistic Insights on Keap1:Nrf2 Interface. Bioorganic Med. Chem. 2016, 24, 3378–3386. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Liu, H.; Johnston, L.J.; Wang, F.; Ma, X. Triggers for the Nrf2/ARE Signaling Pathway and Its Nutritional Regulation: Potential Therapeutic Applications of Ulcerative Colitis. Int. J. Mol. Sci. 2021, 22, 11411. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Mini Review. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Trk Receptors: Roles in Neuronal Signal Transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef]
- Miningou, N.; Blackwell, K.T. The Road to ERK Activation: Do Neurons Take Alternate Routes? Cell. Signal. 2020, 68, 109541. [Google Scholar] [CrossRef]
- del Rosario Campos-Esparza, M.; Adriana Torres-Ramos, M. Neuroprotection by Natural Polyphenols: Molecular Mechanisms. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 269–277. [Google Scholar] [CrossRef]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular Characterization of Mitochondrial Apoptosis-Inducing Factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Ho, D.J.; Calingasan, N.Y.; Wille, E.; Dumont, M.; Beal, M.F. Resveratrol Protects against Peripheral Deficits in a Mouse Model of Huntington’s Disease. Exp. Neurol. 2010, 225, 74–84. [Google Scholar] [CrossRef]
- Fonseca-Kelly, Z.; Nassrallah, M.; Uribe, J.; Khan, R.S.; Dine, K.; Dutt, M.; Shindler, K.S. Resveratrol Neuroprotection in a Chronic Mouse Model of Multiple Sclerosis. Front. Neurol. 2012, 3, 84. [Google Scholar] [CrossRef]
- Schneider, S.A.; Hardy, J.; Bhatia, K.P. Syndromes of Neurodegeneration with Brain Iron Accumulation (NBIA): An Update on Clinical Presentations, Histological and Genetic Underpinnings, and Treatment Considerations. Mov. Disord. 2012, 27, 42–53. [Google Scholar] [CrossRef]
- Barres, B.A.; Barde, Y.-A. Neuronal and Glial Cell Biology. Curr. Opin. Neurobiol. 2000, 10, 642–648. [Google Scholar] [CrossRef]
- Blanchette, M.; Daneman, R. Formation and Maintenance of the BBB. Mech. Dev. 2015, 138, 8–16. [Google Scholar] [CrossRef]
- Baxter, P.S.; Hardingham, G.E. Adaptive Regulation of the Brain’s Antioxidant Defences by Neurons and Astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef]
- Gao, H.-M.; Hong, J.-S. Why Neurodegenerative Diseases Are Progressive: Uncontrolled Inflammation Drives Disease Progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef]
- Kooshki, L.; Zarneshan, S.N.; Fakhri, S.; Moradi, S.Z.; Echeverria, J. The Pivotal Role of JAK/STAT and IRS/PI3K Signaling Pathways in Neurodegenerative Diseases: Mechanistic Approaches to Polyphenols and Alkaloids. Phytomedicine 2023, 112, 154686. [Google Scholar] [CrossRef]
- Sarallah, R.; Jahani, S.; Soltani Khaboushan, A.; Moaveni, A.K.; Amiri, M.; Majidi Zolbin, M. The Role of CXCL12/CXCR4/CXCR7 Axis in Cognitive Impairment Associated with Neurodegenerative Diseases. Brain Behav. Immun. Health 2025, 43, 100932. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The Role of Zinc, Copper, Manganese and Iron in Neurodegenerative Diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Zhang, H.; Diao, X.; Zhao, S.; Zhou, W. Reduction in Autophagy by (-)-Epigallocatechin-3-Gallate (EGCG): A Potential Mechanism of Prevention of Mitochondrial Dysfunction After Subarachnoid Hemorrhage. Mol. Neurobiol. 2017, 54, 392–405. [Google Scholar] [CrossRef]
- Ding, M.; Ma, H.; Man, Y.; LV, H. Protective Effects of a Green Tea Polyphenol, Epigallocatechin-3-Gallate, against Sevoflurane-Induced Neuronal Apoptosis Involve Regulation of CREB/BDNF/TrkB and PI3K/Akt/MTOR Signalling Pathways in Neonatal Mice. Can. J. Physiol. Pharmacol. 2017, 95, 1396–1405. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, L.; Hu, X.; Cao, S.; Yang, J. Effects and Mechanism of Epigallocatechin-3-Gallate on Apoptosis and MTOR/AKT/GSK-3β Pathway in Substantia Nigra Neurons in Parkinson Rats. Neuroreport 2019, 30, 60–65. [Google Scholar] [CrossRef]
- Kalaiselvi, P.; Rajashree, K.; Bharathi Priya, L.; Padma, V.V. Cytoprotective Effect of Epigallocatechin-3-Gallate against Deoxynivalenol-Induced Toxicity through Anti-Oxidative and Anti-Inflammatory Mechanisms in HT-29 Cells. Food Chem. Toxicol. 2013, 56, 110–118. [Google Scholar] [CrossRef]
- Srividhya, R.; Jyothilakshmi, V.; Arulmathi, K.; Senthilkumaran, V.; Kalaiselvi, P. Attenuation of Senescence-induced Oxidative Exacerbations in Aged Rat Brain by (−)-epigallocatechin-3-gallate. Int. J. Dev. Neurosci. 2008, 26, 217–223. [Google Scholar] [CrossRef]
- Islam, M.R.; Rauf, A.; Akter, S.; Akter, H.; Al-Imran, M.I.K.; Islam, S.; Nessa, M.; Shompa, C.J.; Shuvo, M.N.R.; Khan, I.; et al. Epigallocatechin 3-Gallate-Induced Neuroprotection in Neurodegenerative Diseases: Molecular Mechanisms and Clinical Insights. Mol. Cell. Biochem. 2025, 480, 3363–3383. [Google Scholar] [CrossRef]
- Tripathi, S.; Mishra, R.; Shrivastava, R.; Srivastava, V.; Singh, G. Neuroprotection Induced by Epigallocatechin-3-Gallate. In Natural Molecules in Neuroprotection and Neurotoxicity; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1321–1339. [Google Scholar]
- Cheng, Z.; Kang, C.; Che, S.; Su, J.; Sun, Q.; Ge, T.; Guo, Y.; Lv, J.; Sun, Z.; Yang, W.; et al. Berberine: A Promising Treatment for Neurodegenerative Diseases. Front. Pharmacol. 2022, 13, 845591. [Google Scholar] [CrossRef]
- Hsu, Y.-Y.; Chen, C.-S.; Wu, S.-N.; Jong, Y.-J.; Lo, Y.-C. Berberine Activates Nrf2 Nuclear Translocation and Protects against Oxidative Damage via a Phosphatidylinositol 3-Kinase/Akt-Dependent Mechanism in NSC34 Motor Neuron-like Cells. Eur. J. Pharm. Sci. 2012, 46, 415–425. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Li, H.; Li, Z.; Chen, M.; Ma, S.; Shen, R.; Lou, X. Berberine Protects against Neomycin-Induced Ototoxicity by Reducing ROS Generation and Activating the PI3K/AKT Pathway. Neurosci. Lett. 2023, 817, 137518. [Google Scholar] [CrossRef]
- Hsu, Y.-Y.; Tseng, Y.-T.; Lo, Y.-C. Berberine, a Natural Antidiabetes Drug, Attenuates Glucose Neurotoxicity and Promotes Nrf2-Related Neurite Outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef]
- Tian, E.; Sharma, G.; Dai, C. Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants 2023, 12, 1883. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine Improves Cognitive Impairment by Promoting Autophagic Clearance and Inhibiting Production of β-Amyloid in APP/Tau/PS1 Mouse Model of Alzheimer’s Disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Bae, J.; Lee, D.; Kim, Y.K.; Gil, M.; Lee, J.-Y.; Lee, K.J. Berberine Protects 6-Hydroxydopamine-Induced Human Dopaminergic Neuronal Cell Death through the Induction of Heme Oxygenase-1. Mol. Cells 2013, 35, 151–157. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M.; et al. Berberine Protects against 6-OHDA-Induced Neurotoxicity in PC12 Cells and Zebrafish through Hormetic Mechanisms Involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 Pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Kysenius, K.; Brunello, C.A.; Huttunen, H.J. Mitochondria and NMDA Receptor-Dependent Toxicity of Berberine Sensitizes Neurons to Glutamate and Rotenone Injury. PLoS ONE 2014, 9, e107129. [Google Scholar] [CrossRef]
- Shin, K.S.; Choi, H.S.; Zhao, T.T.; Suh, K.H.; Kwon, I.H.; Choi, S.O.; Lee, M.K. Neurotoxic Effects of Berberine on Long-Term l-DOPA Administration in 6-Hydroxydopamine-Lesioned Rat Model of Parkinson’s Disease. Arch. Pharm. Res. 2013, 36, 759–767. [Google Scholar] [CrossRef]
- Kim, M.; Cho, K.-H.; Shin, M.-S.; Lee, J.-M.; Cho, H.-S.; Kim, C.-J.; Shin, D.-H.; Yang, H.J. Berberine Prevents Nigrostriatal Dopaminergic Neuronal Loss and Suppresses Hippocampal Apoptosis in Mice with Parkinson’s Disease. Int. J. Mol. Med. 2014, 33, 870–878. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the Active Substance of Turmeric: Its Effects on Health and Ways to Improve Its Bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Jo, W.H.; Hoang, N.H.M.; Kim, M.-S. Curcumin-Attenuated TREM-1/DAP12/NLRP3/Caspase-1/IL1B, TLR4/NF-ΚB Pathways, and Tau Hyperphosphorylation Induced by 1,2-Diacetyl Benzene: An in Vitro and in Silico Study. Neurotox. Res. 2022, 40, 1272–1291. [Google Scholar] [CrossRef]
- Garodia, P.; Hegde, M.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin, Inflammation, and Neurological Disorders: How Are They Linked? Integr. Med. Res. 2023, 12, 100968. [Google Scholar] [CrossRef]
- De Lorenzi, E.; Franceschini, D.; Contardi, C.; Di Martino, R.M.C.; Seghetti, F.; Serra, M.; Bisceglia, F.; Pagetta, A.; Zusso, M.; Belluti, F. Modulation of Amyloid β-Induced Microglia Activation and Neuronal Cell Death by Curcumin and Analogues. Int. J. Mol. Sci. 2022, 23, 4381. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, Z.; Wu, H.; Zhou, G.; He, D.; Chang, Y.; Li, Y.; Wang, G.; Xie, M. BDMC Protects AD in Vitro via AMPK and SIRT1. Transl. Neurosci. 2020, 11, 319–327. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Fang, C.-H.; Yang, C.-Y.; Liang, Y.-J.; Lin, F.-H. Investigating a Curcumin-Loaded PLGA-PEG-PLGA Thermo-Sensitive Hydrogel for the Prevention of Alzheimer’s Disease. Antioxidants 2022, 11, 727. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Wang, Y. Curcumin Alleviates Aβ42-Induced Neuronal Metabolic Dysfunction via the Thrb/SIRT3 Axis and Improves Cognition in APPTG Mice. Neurochem. Res. 2021, 46, 3166–3178. [Google Scholar] [CrossRef]
- Jaisin, Y.; Thampithak, A.; Meesarapee, B.; Ratanachamnong, P.; Suksamrarn, A.; Phivthong-ngam, L.; Phumala-Morales, N.; Chongthammakun, S.; Govitrapong, P.; Sanvarinda, Y. Curcumin I Protects the Dopaminergic Cell Line SH-SY5Y from 6-Hydroxydopamine-Induced Neurotoxicity through Attenuation of P53-Mediated Apoptosis. Neurosci. Lett. 2011, 489, 192–196. [Google Scholar] [CrossRef]
- Pandey, N.; Strider, J.; Nolan, W.C.; Yan, S.X.; Galvin, J.E. Curcumin Inhibits Aggregation of α-Synuclein. Acta Neuropathol. 2008, 115, 479–489. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Gao, Z.; Xie, K.; Zhang, Q.; Jiang, H.; Pang, Q. Effects of Curcumin on Learning and Memory Deficits, BDNF, and ERK Protein Expression in Rats Exposed to Chronic Unpredictable Stress. Behav. Brain Res. 2014, 271, 116–121. [Google Scholar] [CrossRef]
- Kemadjou Dibacto, R.E.; Akamba Ambamba, B.D.; Ella, F.A.; Biyegue Nyangono, C.F.; Kamga Nanhah, J.V.; Fonkoua, M.; Minka, R.S.; Ngondi, J.L. The Neuroprotective Effect of Xylopia Parviflora against Aluminum Chloride-Induced Neurotoxicity in Rats. Heliyon 2022, 8, e09896. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Kinsella, J.E. Inhibition of Human LDL Oxidation by Resveratrol. Lancet 1993, 341, 1103–1104. [Google Scholar] [CrossRef]
- Vikal, A.; Maurya, R.; Bhowmik, S.; Khare, S.; Raikwar, S.; Patel, P.; Das Kurmi, B. Resveratrol: A Comprehensive Review of Its Multifaceted Health Benefits, Mechanisms of Action, and Potential Therapeutic Applications in Chronic Disease. Pharmacol. Res.-Nat. Prod. 2024, 3, 100047. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, T.; Ma, Y.; Huang, S.; Lei, L.; Wen, A.; Ding, Y. Resveratrol: Multi-Targets Mechanism on Neurodegenerative Diseases Based on Network Pharmacology. Front. Pharmacol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, J.-S.; Zhou, H.; Wilson, B.; Hong, J.-S.; Gao, H.-M. Resveratrol Protects Dopamine Neurons Against Lipopolysaccharide-Induced Neurotoxicity through Its Anti-Inflammatory Actions. Mol. Pharmacol. 2010, 78, 466–477. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhu, J.X.; Xie, W.; Le, W.; Fan, Z.; Jankovic, J.; Pan, T. Resveratrol-Activated AMPK/SIRT1/Autophagy in Cellular Models of Parkinson’s Disease. Neurosignals 2011, 19, 163–174. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.-F.; Wu, Q.; Liu, J.; Shi, J.-S. Resveratrol Promotes Neurotrophic Factor Release from Astroglia. Exp. Biol. Med. 2012, 237, 943–948. [Google Scholar] [CrossRef]
- Danisman, B.; Kelek, S.E.; Aslan, M. Resveratrol in Neurodegeneration, in Neurodegenerative Diseases, and in the Redox Biology of the Mitochondria. Psychiatry Clin. Psychopharmacol. 2023, 33, 147–155. [Google Scholar] [CrossRef]
- Shojaei, S.; Panjehshahin, M.R.; Shafiee, S.M.; Khoshdel, Z.; Borji, M.; Ghasempour, G.; Owji, A.A. Differential Effects of Resveratrol on the Expression of Brain-Derived Neurotrophic Factor Transcripts and Protein in the Hippocampus of Rat Brain. Iran. J. Med. Sci. 2017, 42, 32–39. [Google Scholar]
- Huang, T.-C.; Lu, K.-T.; Wo, Y.-Y.P.; Wu, Y.-J.; Yang, Y.-L. Resveratrol Protects Rats from Aβ-Induced Neurotoxicity by the Reduction of INOS Expression and Lipid Peroxidation. PLoS ONE 2011, 6, e29102. [Google Scholar] [CrossRef]
- Dajas, F.; Abin-Carriquiry, J.A.; Arredondo, F.; Blasina, F.; Echeverry, C.; Martínez, M.; Rivera, F.; Vaamonde, L. Quercetin in Brain Diseases: Potential and Limits. Neurochem. Int. 2015, 89, 140–148. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Zhou, D.; Bai, X.; Zhou, W.; Huang, C.; Song, J.; Meng, F.; Wu, C.; Li, L.; et al. Quercetin Protects against the Aβ25–35-Induced Amnesic Injury: Involvement of Inactivation of RAGE-Mediated Pathway and Conservation of the NVU. Neuropharmacology 2013, 67, 419–431. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic Insights and Perspectives Involved in Neuroprotective Action of Quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Arredondo, F.; Echeverry, C.; Abin-Carriquiry, J.A.; Blasina, F.; Antúnez, K.; Jones, D.P.; Go, Y.-M.; Liang, Y.-L.; Dajas, F. After Cellular Internalization, Quercetin Causes Nrf2 Nuclear Translocation, Increases Glutathione Levels, and Prevents Neuronal Death against an Oxidative Insult. Free Radic. Biol. Med. 2010, 49, 738–747. [Google Scholar] [CrossRef]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant Properties of Quercetin. In Oxygen Transport to Tissue XXXII; Springer: Berlin/Heidelberg, Germany, 2011; pp. 283–289. [Google Scholar]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive Effects of Quercetin in the Central Nervous System: Focusing on the Mechanisms of Actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef]
- Song, K.-S.; Yang, E.-J.; Kim, G.-S.; Kim, J. Protective Effects of Onion-Derived Quercetin on Glutamate-Mediated Hippocampal Neuronal Cell Death. Pharmacogn. Mag. 2013, 9, 302. [Google Scholar] [CrossRef]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-Enhancing Effect of Quercetin in a Rat Model of Parkinson’s Disease Induced by 6-Hydroxydopamine. Evid.-Based Complement. Altern. Med. 2012, 2012, 823206. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Purushotham, S.S.; Buskila, Y. Astrocytic Modulation of Neuronal Signalling. Front. Netw. Physiol. 2023, 3, 1205544. [Google Scholar] [CrossRef]
- Albini, M.; Krawczun-Rygmaczewska, A.; Cesca, F. Astrocytes and Brain-Derived Neurotrophic Factor (BDNF). Neurosci. Res. 2023, 197, 42–51. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, Neurons, Synapses: A Tripartite View on Cortical Circuit Development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Arizono, M.; Inavalli, V.V.G.K.; Panatier, A.; Pfeiffer, T.; Angibaud, J.; Levet, F.; Ter Veer, M.J.T.; Stobart, J.; Bellocchio, L.; Mikoshiba, K.; et al. Structural Basis of Astrocytic Ca2+ Signals at Tripartite Synapses. Nat. Commun. 2020, 11, 1906. [Google Scholar] [CrossRef]
- Szpakowski, P.; Ksiazek-Winiarek, D.; Turniak-Kusy, M.; Pacan, I.; Glabinski, A. Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli. Biomedicines 2022, 10, 1769. [Google Scholar] [CrossRef]
- Szpakowski, P.; Ksiazek-Winiarek, D.; Czpakowska, J.; Kaluza, M.; Milewska-Jedrzejczak, M.; Glabinski, A. Astrocyte-Derived Exosomes Differentially Shape T Cells’ Immune Response in MS Patients. Int. J. Mol. Sci. 2023, 24, 7470. [Google Scholar] [CrossRef]
- Czpakowska, J.; Głąbiński, A.; Szpakowski, P. The Potential Role of Exosomes in Communication Between Astrocytes and Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 4676. [Google Scholar] [CrossRef]
- Nurkenov, T.; Tsoy, A.; Olzhayev, F.; Abzhanova, E.; Turgambayeva, A.; Zhussupova, A.; Avula, B.; Ross, S.; Aituarova, A.; Kassymova, D.; et al. Plant Extract of Limonium Gmelinii Attenuates Oxidative Responses in Neurons, Astrocytes, and Cerebral Endothelial Cells In Vitro and Improves Motor Functions of Rats after Middle Cerebral Artery Occlusion. Antioxidants 2021, 10, 1814. [Google Scholar] [CrossRef]
- Alami, M.; Boumezough, K.; Zerif, E.; Zoubdane, N.; Khalil, A.; Bunt, T.; Laurent, B.; Witkowski, J.; Ramassamy, C.; Boulbaroud, S.; et al. In Vitro Assessment of the Neuroprotective Effects of Pomegranate (Punica granatum L.) Polyphenols Against Tau Phosphorylation, Neuroinflammation, and Oxidative Stress. Nutrients 2024, 16, 3667. [Google Scholar] [CrossRef]
- Ajit, D.; Simonyi, A.; Li, R.; Chen, Z.; Hannink, M.; Fritsche, K.L.; Mossine, V.V.; Smith, R.E.; Dobbs, T.K.; Luo, R.; et al. Phytochemicals and Botanical Extracts Regulate NF-ΚB and Nrf2/ARE Reporter Activities in DI TNC1 Astrocytes. Neurochem. Int. 2016, 97, 49–56. [Google Scholar] [CrossRef]
- Bahia, P.K.; Rattray, M.; Williams, R.J. Dietary Flavonoid (−)Epicatechin Stimulates Phosphatidylinositol 3-kinase-dependent Anti-oxidant Response Element Activity and Up-regulates Glutathione in Cortical Astrocytes. J. Neurochem. 2008, 106, 2194–2204. [Google Scholar] [CrossRef]
- Song, S.-Y.; Jung, Y.Y.; Hwang, C.J.; Lee, H.P.; Sok, C.H.; Kim, J.H.; Lee, S.M.; Seo, H.O.; Hyun, B.K.; Choi, D.Y.; et al. Inhibitory Effect of Ent-Sauchinone on Amyloidogenesis via Inhibition of STAT3-Mediated NF-ΚB Activation in Cultured Astrocytes and Microglial BV-2 Cells. J. Neuroinflammation 2014, 11, 118. [Google Scholar] [CrossRef]
- Lee, T.-H.; Chen, J.-L.; Liu, P.-S.; Tsai, M.-M.; Wang, S.-J.; Hsieh, H.-L. Rottlerin, a Natural Polyphenol Compound, Inhibits Upregulation of Matrix Metalloproteinase-9 and Brain Astrocytic Migration by Reducing PKC-δ-Dependent ROS Signal. J. Neuroinflammation 2020, 17, 177. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Ksiazek-Winiarek, D.; Glabinski, A.; Szpakowski, P. Dietary Polyphenols Decrease Chemokine Release by Human Primary Astrocytes Responding to Pro-Inflammatory Cytokines. Pharmaceutics 2023, 15, 2294. [Google Scholar] [CrossRef]
- Nones, J.; Spohr, T.C.L.d.S.; Gomes, F.C.A. Effects of the Flavonoid Hesperidin in Cerebral Cortical Progenitors in Vitro: Indirect Action through Astrocytes. Int. J. Dev. Neurosci. 2012, 30, 303–313. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.-Y.; Liu, H.; Lu, Y.-F.; Wu, Q.; Liu, J.; Shi, J.-S. Resveratrol Produces Neurotrophic Effects on Cultured Dopaminergic Neurons through Prompting Astroglial BDNF and GDNF Release. Evid.-Based Complement. Altern. Med. 2012, 2012, 937605. [Google Scholar] [CrossRef]
- Chai, L.; Guo, H.; Li, H.; Wang, S.; Wang, Y.; Shi, F.; Hu, L.; Liu, Y.; Adah, D. Scutellarin and Caffeic Acid Ester Fraction, Active Components of Dengzhanxixin Injection, Upregulate Neurotrophins Synthesis and Release in Hypoxia/Reoxygenation Rat Astrocytes. J. Ethnopharmacol. 2013, 150, 100–107. [Google Scholar] [CrossRef]
- Xu, S.L.; Bi, C.W.C.; Choi, R.C.Y.; Zhu, K.Y.; Miernisha, A.; Dong, T.T.X.; Tsim, K.W.K. Flavonoids Induce the Synthesis and Secretion of Neurotrophic Factors in Cultured Rat Astrocytes: A Signaling Response Mediated by Estrogen Receptor. Evid.-Based Complement. Altern. Med. 2013, 2013, 127075. [Google Scholar] [CrossRef]
- Jin, X.; Liu, P.; Yang, F.; Zhang, Y.; Miao, D. Rosmarinic Acid Ameliorates Depressive-Like Behaviors in a Rat Model of CUS and Up-Regulates BDNF Levels in the Hippocampus and Hippocampal-Derived Astrocytes. Neurochem. Res. 2013, 38, 1828–1837. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia Emerge as Central Players in Brain Disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Timmerman, R.; Burm, S.M.; Bajramovic, J.J. An Overview of in Vitro Methods to Study Microglia. Front. Cell. Neurosci. 2018, 12, 242. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-Mediated Neuroinflammation in Neurodegenerative Diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Li, Z.-H.; Liu, L.; Tang, W.-X.; Wang, Y.; Dong, M.-R.; Xiao, C. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. Front. Pharmacol. 2016, 7, 261. [Google Scholar] [CrossRef]
- Yazdani, Y.; Zamani, A.R.N.; Majidi, Z.; Sharafkandi, N.; Alizadeh, S.; Mofrad, A.M.E.; Valizadeh, A.; Idari, G.; Radvar, A.D.; Safaie, N.; et al. Curcumin and Targeting of Molecular and Metabolic Pathways in Multiple Sclerosis. Cell Biochem. Funct. 2023, 41, 779–787. [Google Scholar] [CrossRef]
- Xu, L.; Hao, L.-P.; Yu, J.; Cheng, S.-Y.; Li, F.; Ding, S.-M.; Zhang, R. Curcumin Protects against Rotenone-Induced Parkinson’s Disease in Mice by Inhibiting Microglial NLRP3 Inflammasome Activation and Alleviating Mitochondrial Dysfunction. Heliyon 2023, 9, e16195. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective Effects of Flavone Luteolin in Neuroinflammation and Neurotrauma. BioFactors 2021, 47, 190–197. [Google Scholar] [CrossRef]
- Chen, H.-Q.; Jin, Z.-Y.; Wang, X.-J.; Xu, X.-M.; Deng, L.; Zhao, J.-W. Luteolin Protects Dopaminergic Neurons from Inflammation-Induced Injury through Inhibition of Microglial Activation. Neurosci. Lett. 2008, 448, 175–179. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Chen, J.; Xu, S.; Luo, Y. Luteolin Mitigates Dopaminergic Neuron Degeneration and Restrains Microglial M1 Polarization by Inhibiting Toll Like Receptor 4. J. Integr. Neurosci. 2024, 23, 185. [Google Scholar] [CrossRef]
- Kwon, Y. Luteolin as a Potential Preventive and Therapeutic Candidate for Alzheimer’s Disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Y. The Role of Natural Flavonoids on Neuroinflammation as a Therapeutic Target for Alzheimer’s Disease: A Narrative Review. Neural Regen. Res. 2023, 18, 2582–2591. [Google Scholar] [CrossRef]
- Darwish, S.F.; Elbadry, A.M.M.; Elbokhomy, A.S.; Salama, G.A.; Salama, R.M. The Dual Face of Microglia (M1/M2) as a Potential Target in the Protective Effect of Nutraceuticals against Neurodegenerative Diseases. Front. Aging 2023, 4, 1231706. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Shen, Y.; Xiao, H. Resveratrol Attenuates Rotenone-Induced Inflammation and Oxidative Stress via STAT1 and Nrf2/Keap1/SLC7A11 Pathway in a Microglia Cell Line. Pathol. Res. Pract. 2021, 225, 153576. [Google Scholar] [CrossRef]
- Schlotterose, L.; Pravdivtseva, M.S.; Ellermann, F.; Jansen, O.; Hövener, J.-B.; Sönnichsen, F.D.; Cossais, F.; Lucius, R.; Hattermann, K. Resveratrol Mitigates Metabolism in Human Microglia Cells. Antioxidants 2023, 12, 1248. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Protective Effects of Quercetin and Vitamin C against Oxidative Stress-Induced Neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.; Han, J. Quercetin Regulates the Polarization of Microglia through the NRF2/HO1 Pathway and Mitigates Alzheimer’s Disease. Actas Esp. Psiquiatr. 2024, 52, 786–799. [Google Scholar] [CrossRef]
- Gu, M.; Yin, Q.; Wu, G. Metagenomic Analysis of Facilitation Mechanism for Azo Dye Reactive Red 2 Degradation with the Dosage of Ferroferric Oxide. J. Water Process Eng. 2021, 41, 102010. [Google Scholar] [CrossRef]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef]
- Bankston, A.N.; Mandler, M.D.; Feng, Y. Oligodendroglia and Neurotrophic Factors in Neurodegeneration. Neurosci. Bull. 2013, 29, 216–228. [Google Scholar] [CrossRef]
- Fernandez-Castaneda, A.; Gaultier, A. Adult Oligodendrocyte Progenitor Cells—Multifaceted Regulators of the CNS in Health and Disease. Brain Behav. Immun. 2016, 57, 1–7. [Google Scholar] [CrossRef]
- Wilkins, A.; Majed, H.; Layfield, R.; Compston, A.; Chandran, S. Oligodendrocytes Promote Neuronal Survival and Axonal Length by Distinct Intracellular Mechanisms: A Novel Role for Oligodendrocyte-Derived Glial Cell Line-Derived Neurotrophic Factor. J. Neurosci. 2003, 23, 4967–4974. [Google Scholar] [CrossRef]
- Zeis, T.; Schaeren-Wiemers, N. Lame Ducks or Fierce Creatures?—The Role of Oligodendrocytes in Multiple Sclerosis. J. Mol. Neurosci. 2008, 35, 91–100. [Google Scholar] [CrossRef]
- Zha, Z.; Liu, S.; Liu, Y.; Li, C.; Wang, L. Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis. Antioxidants 2022, 11, 1495. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J. Oxidative Stress and Its Impact on Neurons and Glia in Multiple Sclerosis Lesions. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2016, 1862, 506–510. [Google Scholar] [CrossRef]
- Roth, A.D.; Núñez, M.T. Oligodendrocytes: Functioning in a Delicate Balance Between High Metabolic Requirements and Oxidative Damage. In Glial Cells in Health and Disease of the CNS; Springer: Berlin/Heidelberg, Germany, 2016; pp. 167–181. [Google Scholar]
- Steudler, J.; Ecott, T.; Ivan, D.C.; Bouillet, E.; Walthert, S.; Berve, K.; Dick, T.P.; Engelhardt, B.; Locatelli, G. Autoimmune Neuroinflammation Triggers Mitochondrial Oxidation in Oligodendrocytes. Glia 2022, 70, 2045–2061. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; D’Onofrio, G.; Fazel Nabavi, S.; Daglia, M.; Rastrelli, L.; Mohammad Nabavi, S. Neuroprotective Effects of Quercetin: From Chemistry to Medicine. CNS Neurol. Disord. Drug Targets 2016, 15, 964–975. [Google Scholar] [CrossRef]
- Fan, H.; Tang, H.-B.; Shan, L.-Q.; Liu, S.-C.; Huang, D.-G.; Chen, X.; Chen, Z.; Yang, M.; Yin, X.-H.; Yang, H.; et al. Quercetin Prevents Necroptosis of Oligodendrocytes by Inhibiting Macrophages/Microglia Polarization to M1 Phenotype after Spinal Cord Injury in Rats. J. Neuroinflammation 2019, 16, 206. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Yao, R.-Q.; Liu, X.; Huang, J.-J.; Qi, D.-S.; Yang, L.-H. Quercetin Protects Oligodendrocyte Precursor Cells from Oxygen/Glucose Deprivation Injury in Vitro via the Activation of the PI3K/Akt Signaling Pathway. Brain Res. Bull. 2011, 86, 277–284. [Google Scholar] [CrossRef]
- Wu, X.; Qu, X.; Zhang, Q.; Dong, F.; Yu, H.; Yan, C.; Qi, D.; Wang, M.; Liu, X.; Yao, R. Quercetin Promotes Proliferation and Differentiation of Oligodendrocyte Precursor Cells After Oxygen/Glucose Deprivation-Induced Injury. Cell. Mol. Neurobiol. 2014, 34, 463–471. [Google Scholar] [CrossRef]
- van Meeteren, M.E.; Hendriks, J.J.A.; Dijkstra, C.D.; van Tol, E.A.F. Dietary Compounds Prevent Oxidative Damage and Nitric Oxide Production by Cells Involved in Demyelinating Disease. Biochem. Pharmacol. 2004, 67, 967–975. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, Z.; Ni, X.; Liu, X.; Xu, D. Fracture and Damage Model of Composite Ceramics. Vibroengineering Procedia 2020, 35, 39–45. [Google Scholar] [CrossRef]
- Qu, X.; Qi, D.; Dong, F.; Wang, B.; Guo, R.; Luo, M.; Yao, R. Quercetin Improves Hypoxia-Ischemia Induced Cognitive Deficits via Promoting Remyelination in Neonatal Rat. Brain Res. 2014, 1553, 31–40. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Cai, Q.; Yao, Z. ID2: A Negative Transcription Factor Regulating Oligodendroglia Differentiation. J. Neurosci. Res. 2012, 90, 925–932. [Google Scholar] [CrossRef]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Mol. Neurobiol. 2017, 54, 3219–3229. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Bobermin, L.D.; Latini, A.; Wajner, M.; Souza, D.O.; Gonçalves, C.-A.; Gottfried, C. Resveratrol Protects C6 Astrocyte Cell Line against Hydrogen Peroxide-Induced Oxidative Stress through Heme Oxygenase 1. PLoS ONE 2013, 8, e64372. [Google Scholar] [CrossRef]
- Sakata, Y.; Zhuang, H.; Kwansa, H.; Koehler, R.C.; Doré, S. Resveratrol Protects against Experimental Stroke: Putative Neuroprotective Role of Heme Oxygenase 1. Exp. Neurol. 2010, 224, 325–329. [Google Scholar] [CrossRef]
- Son, Y.; Lee, J.H.; Chung, H.-T.; Pae, H.-O. Therapeutic Roles of Heme Oxygenase-1 in Metabolic Diseases: Curcumin and Resveratrol Analogues as Possible Inducers of Heme Oxygenase-1. Oxid. Med. Cell. Longev. 2013, 2013, 639541. [Google Scholar] [CrossRef]
- Rosa, P.M.; Martins, L.A.M.; Souza, D.O.; Quincozes-Santos, A. Glioprotective Effect of Resveratrol: An Emerging Therapeutic Role for Oligodendroglial Cells. Mol. Neurobiol. 2018, 55, 2967–2978. [Google Scholar] [CrossRef]
- Iles, K.E.; Liu, R.-M. Mechanisms of Glutamate Cysteine Ligase (GCL) Induction by 4-Hydroxynonenal. Free Radic. Biol. Med. 2005, 38, 547–556. [Google Scholar] [CrossRef]
- ELBini-Dhouib, I.; Manai, M.; Neili, N.; Marzouki, S.; Sahraoui, G.; Ben Achour, W.; Zouaghi, S.; BenAhmed, M.; Doghri, R.; Srairi-Abid, N. Dual Mechanism of Action of Curcumin in Experimental Models of Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 8658. [Google Scholar] [CrossRef]
- Motavaf, M.; Sadeghizadeh, M.; Babashah, S.; Zare, L.; Javan, M. Dendrosomal Nanocurcumin Promotes Remyelination through Induction of Oligodendrogenesis in Experimental Demyelination Animal Model. J. Tissue Eng. Regen. Med. 2020, 14, 1449–1464. [Google Scholar] [CrossRef]
- Bernardo, A.; Plumitallo, C.; De Nuccio, C.; Visentin, S.; Minghetti, L. Curcumin Promotes Oligodendrocyte Differentiation and Their Protection against TNF-α through the Activation of the Nuclear Receptor PPAR-γ. Sci. Rep. 2021, 11, 4952. [Google Scholar] [CrossRef]
- Dierckx, T.; Vanherle, S.; Haidar, M.; Grajchen, E.; Mingneau, F.; Gervois, P.; Wolfs, E.; Bylemans, D.; Voet, A.; Nguyen, T.; et al. Phloretin Enhances Remyelination by Stimulating Oligodendrocyte Precursor Cell Differentiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2120393119. [Google Scholar] [CrossRef]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.-J.; Gao, Y.; Bennett, M.V.L.; et al. Peroxisome Proliferator-Activated Receptor γ (PPARγ): A Master Gatekeeper in CNS Injury and Repair. Prog. Neurobiol. 2018, 163–164, 27–58. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Soliman, M.M.; Iftikhar, A.; Iftikhar, M.; Aboelenin, S.M.; Wang, C. Protective Effects of Ellagic Acid Against Alcoholic Liver Disease in Mice. Front. Nutr. 2021, 8, 744520. [Google Scholar] [CrossRef]
- Kiasalari, Z.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Azadi-Ahmadabadi, E.; Esmaeil-Jamaat, E.; Fahanik-Babaei, J.; Fakour, M.; Fereidouni, F.; Ghasemi-Tarie, R.; Jalalzade-Ogvar, S.; et al. Ellagic Acid Ameliorates Neuroinflammation and Demyelination in Experimental Autoimmune Encephalomyelitis: Involvement of NLRP3 and Pyroptosis. J. Chem. Neuroanat. 2021, 111, 101891. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Jamieson, S.; Pandey, A.K.; Bishayee, A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv. Nutr. 2021, 12, 1211–1238. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kulkarni, V.H.; Chakraborty, M.; Habbu, P.V.; Ray, A. Ellagic Acid Restored Lead-Induced Nephrotoxicity by Anti-Inflammatory, Anti-Apoptotic and Free Radical Scavenging Activities. Heliyon 2021, 7, e05921. [Google Scholar] [CrossRef]
- Xu, X.; Han, C.; Wang, P.; Zhou, F. Natural Products Targeting Cellular Processes Common in Parkinson’s Disease and Multiple Sclerosis. Front. Neurol. 2023, 14, 1149963. [Google Scholar] [CrossRef]
- Zhang, Y.; Taveggia, C.; Melendez-Vasquez, C.; Einheber, S.; Raine, C.S.; Salzer, J.L.; Brosnan, C.F.; John, G.R. Interleukin-11 Potentiates Oligodendrocyte Survival and Maturation, and Myelin Formation. J. Neurosci. 2006, 26, 12174–12185. [Google Scholar] [CrossRef]
- Wang, W.-W.; Lu, L.; Bao, T.-H.; Zhang, H.-M.; Yuan, J.; Miao, W.; Wang, S.-F.; Xiao, Z.-C. Scutellarin Alleviates Behavioral Deficits in a Mouse Model of Multiple Sclerosis, Possibly Through Protecting Neural Stem Cells. J. Mol. Neurosci. 2016, 58, 210–220. [Google Scholar] [CrossRef]
- Siddiqui, S.; Kamal, A.; Khan, F.; Jamali, K.S.; Saify, Z.S. Gallic and Vanillic Acid Suppress Inflammation and Promote Myelination in an in Vitro Mouse Model of Neurodegeneration. Mol. Biol. Rep. 2019, 46, 997–1011. [Google Scholar] [CrossRef]
- La Rosa, G.; Sozio, C.; Pipicelli, L.; Raia, M.; Palmiero, A.; Santillo, M.; Damiano, S. Antioxidant, Anti-Inflammatory and Pro-Differentiative Effects of Chlorogenic Acid on M03-13 Human Oligodendrocyte-like Cells. Int. J. Mol. Sci. 2023, 24, 16731. [Google Scholar] [CrossRef]
- Ebrahim-Tabar, F.; Nazari, A.; Pouramir, M.; Ashrafpour, M.; Pourabdolhossein, F. Arbutin Improves Functional Recovery and Attenuates Glial Activation in Lysolecethin-Induced Demyelination Model in Rat Optic Chiasm. Mol. Neurobiol. 2020, 57, 3228–3242. [Google Scholar] [CrossRef]
- Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent Advances in the Conjugation Approaches for Enhancing the Bioavailability of Polyphenols. Food Hydrocoll. 2024, 146, 109221. [Google Scholar] [CrossRef]
- Rudrapal, M.; Mishra, A.K.; Rani, L.; Sarwa, K.K.; Zothantluanga, J.H.; Khan, J.; Kamal, M.; Palai, S.; Bendale, A.R.; Talele, S.G.; et al. Nanodelivery of Dietary Polyphenols for Therapeutic Applications. Molecules 2022, 27, 8706. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, R.; Wang, Y.; Liu, M.; Hu, D.; Wang, Y.; Yang, L. Nanoparticle Delivery for Central Nervous System Diseases and Its Clinical Application. Nano Res. 2024, 17, 6305–6322. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Yeo, Y. Recent Advances in Stealth Coating of Nanoparticle Drug Delivery Systems. WIREs Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef]
- Squillaro, T.; Cimini, A.; Peluso, G.; Giordano, A.; Melone, M.A.B. Nano-Delivery Systems for Encapsulation of Dietary Polyphenols: An Experimental Approach for Neurodegenerative Diseases and Brain Tumors. Biochem. Pharmacol. 2018, 154, 303–317. [Google Scholar] [CrossRef]
- Zhang, T.; Peng, Q.; San, F.-Y.; Luo, J.-W.; Wang, M.-X.; Wu, W.-Q.; Gong, T.; Zhang, Z.-R. A High-Efficiency, Low-Toxicity, Phospholipids-Based Phase Separation Gel for Long-Term Delivery of Peptides. Biomaterials 2015, 45, 1–9. [Google Scholar] [CrossRef]
- Singh, A.; Fatima, Z.; Srivastava, D. A Comprehensive Review on Polyphenols Based Nanovesicular System for Topical Delivery. Curr. Drug Deliv. 2025, 22, 123–139. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Witika, B.A.; Poka, M.S.; Demana, P.H.; Matafwali, S.K.; Melamane, S.; Malungelo Khamanga, S.M.; Makoni, P.A. Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date. Pharmaceutics 2022, 14, 836. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Gatto, M.S.; Johnson, M.P.; Najahi-Missaoui, W. Targeted Liposomal Drug Delivery: Overview of the Current Applications and Challenges. Life 2024, 14, 672. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-Modified Lipid Nanocarriers for Crossing the Blood-Brain Barrier (BBB): A Current Overview of Active Targeting in Brain Diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, D.; Singh, J. GLUT-1: An Effective Target To Deliver Brain-Derived Neurotrophic Factor Gene Across the Blood Brain Barrier. ACS Chem. Neurosci. 2020, 11, 1620–1633. [Google Scholar] [CrossRef]
- Lakkadwala, S.; dos Santos Rodrigues, B.; Sun, C.; Singh, J. Dual Functionalized Liposomes for Efficient Co-Delivery of Anti-Cancer Chemotherapeutics for the Treatment of Glioblastoma. J. Control. Release 2019, 307, 247–260. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, B.; Lakkadwala, S.; Kanekiyo, T.; Singh, J. Development and Screening of Brain-Targeted Lipid-Based Nanoparticles with Enhanced Cell Penetration and Gene Delivery Properties. Int. J. Nanomed. 2019, 14, 6497–6517. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Zhang, Y.-Q.; Wang, Z.-Z.; Wu, K.; Lou, J.-N.; Qi, X.-R. Enhanced Brain Distribution and Pharmacodynamics of Rivastigmine by Liposomes Following Intranasal Administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Barro, L.; Tsai, S.-T.; Feng, T.-W.; Wu, X.-Y.; Chao, C.-W.; Yu, R.-S.; Chin, T.-Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef]
- Taylor, M.; Moore, S.; Mourtas, S.; Niarakis, A.; Re, F.; Zona, C.; La Ferla, B.; Nicotra, F.; Masserini, M.; Antimisiaris, S.G.; et al. Effect of Curcumin-Associated and Lipid Ligand-Functionalized Nanoliposomes on Aggregation of the Alzheimer’s Aβ Peptide. Nanomedicine 2011, 7, 541–550. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Fu, Q.; Ma, R.; Xiang, J. Protective Effect of Resveratrol Derived from Polygonum Cuspidatum and Its Liposomal Form on Nigral Cells in Parkinsonian Rats. J. Neurol. Sci. 2011, 304, 29–34. [Google Scholar] [CrossRef]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Daniello, V.; Di Gioia, S.; Conese, M.; Ingrosso, C.; Ciriaco, F.; Catucci, L. Polymer Encapsulated Liposomes for Oral Co-Delivery of Curcumin and Hydroxytyrosol. Int. J. Mol. Sci. 2023, 24, 790. [Google Scholar] [CrossRef]
- Blanco, I.M.R.; Barbosa, R.d.M.; Borges, J.M.P.; de Melo, S.A.B.V.; El-Bachá, R.d.S.; Viseras, C.; Severino, P.; Sanchez-Lopez, E.; Souto, E.B.; Cabral-Albuquerque, E. Conventional and PEGylated Liposomes as Vehicles of Copaifera Sabulicola. Pharmaceutics 2023, 15, 671. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Desii, A.; Lavarello, C.; Genchi, G.; Petretto, A.; Ciofani, G. Liposomes Loaded with Polyphenol-Rich Grape Pomace Extracts Protect from Neurodegeneration in a Rotenone-Based in Vitro Model of Parkinson’s Disease. Biomater. Sci. 2021, 9, 8171–8188. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-Targeted, Resveratrol-Loaded Liposomes for the Treatment of Glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Tasciotti, E.; Molinaro, R.; Taraballi, F.; Toledano Furman, N.; Sherman, M.; Parodi, A.; Salvatore, F.; Corbo, C. Effects of the Protein Corona on Liposome–Liposome and Liposome–Cell Interactions. Int. J. Nanomed. 2016, 11, 3049–3063. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E.; et al. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef]
- Pagano, R.E.; Huang, L.; Wey, C. Interaction of Phospholipid Vesicles with Cultured Mammalian Cells. Nature 1974, 252, 166–167. [Google Scholar] [CrossRef]
- Adams, D.H.; Joyce, G.; Richardson, V.J.; Ryman, B.E.; Wiśniewski, H.M. Liposome Toxicity in the Mouse Central Nervous System. J. Neurol. Sci. 1977, 31, 173–179. [Google Scholar] [CrossRef]
- Pondman, K.; Le Gac, S.; Kishore, U. Nanoparticle-Induced Immune Response: Health Risk versus Treatment Opportunity? Immunobiology 2023, 228, 152317. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hussain, S.; Garantziotis, S.; Demokritou, P.; Castranova, V.; Cassee, F.R. The Yin: An Adverse Health Perspective of Nanoceria: Uptake, Distribution, Accumulation, and Mechanisms of Its Toxicity. Environ. Sci. Nano 2014, 1, 406–428. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Priprem, A.; Watanatorn, J.; Sutthiparinyanont, S.; Phachonpai, W.; Muchimapura, S. Anxiety and Cognitive Effects of Quercetin Liposomes in Rats. Nanomedicine 2008, 4, 70–78. [Google Scholar] [CrossRef]
- Lv, J.-M.; Ismail, B.B.; Ye, X.-Q.; Zhang, X.-Y.; Gu, Y.; Chen, J.-C. Ultrasonic-Assisted Nanoencapsulation of Kiwi Leaves Proanthocyanidins in Liposome Delivery System for Enhanced Biostability and Bioavailability. Food Chem. 2023, 416, 135794. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Giunchedi, P. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics 2020, 12, 1246. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-Inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Andrade, S.; Loureiro, J.A.; Ramirez, S.; Catumbela, C.S.G.; Soto, C.; Morales, R.; Pereira, M.C. Multi-Dose Intravenous Administration of Neutral and Cationic Liposomes in Mice: An Extensive Toxicity Study. Pharmaceuticals 2022, 15, 761. [Google Scholar] [CrossRef]
- Qiang, F.; Shin, H.-J.; Lee, B.-J.; Han, H.-K. Enhanced Systemic Exposure of Fexofenadine via the Intranasal Administration of Chitosan-Coated Liposome. Int. J. Pharm. 2012, 430, 161–166. [Google Scholar] [CrossRef]
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics 2023, 15, 746. [Google Scholar] [CrossRef]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; De Bonis, P.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid Nanoparticles for Intranasal Administration: Application to Nose-to-Brain Delivery. Expert Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the Transferrin Receptor for Brain Drug Delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Huang, M.; Wang, X.-R.; Fu, J.; Han, M.; Shen, Y.-Q.; Xia, Z.; Gao, J.-Q. Transferrin-Modified Liposome Promotes α-Mangostin to Penetrate the Blood–Brain Barrier. Nanomedicine 2016, 12, 421–430. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Gomes, B.; Coelho, M.A.; do Carmo Pereira, M.; Rocha, S. Immunoliposomes Doubly Targeted to Transferrin Receptor and to α-Synuclein. Future Sci. OA 2015, 1, FSO71. [Google Scholar] [CrossRef]
- Ying, X.; Wen, H.; Lu, W.-L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.-J.; Yang, T.-Y.; et al. Dual-Targeting Daunorubicin Liposomes Improve the Therapeutic Efficacy of Brain Glioma in Animals. J. Control. Release 2010, 141, 183–192. [Google Scholar] [CrossRef]
| Polyphenol (Dosage) | Adverse Effect | Study Type | Reference |
|---|---|---|---|
| Epigallocatechin gallate (379 mg/day for 3 months) | Decreased blood iron levels | Randomized controlled trials, obese patients | [58] |
| Quercetin (500 mg/day for 12 weeks) | Decreased blood iron levels | Randomized, double-blind, placebo-controlled study | [59] |
| Raspberry and strawberry extracts (50 μg/mL GAE 1) | Significant reduction in lipase activity | In vitro | [60] |
| Quercetin (minimal inhibitory concentration = 20–50 µg/mL); Naringenin and hesperetin (minimal inhibitory concentration ≥ 250 µg/mL) | Strong negative impact on physiological intestinal microbiota | In vitro | [61] |
| Green tea catechins (630 mg) | Significantly reduced systemic digoxin levels | In vivo human study | [62] |
| Grapefruit juice (8 oz, 3× a day for 6 days) | Increased felodipine availability due to CYP3A4 inhibition | In vivo human study | [63] |
| Isoflavones (approximately 600 µg/g for 5 weeks) | 35-fold increase in plasma phytoestrogen levels; decreased body and prostate weight | In vivo rat model | [64] |
| Soy isoflavones (5 mg/kg) | Elevated blood pressure | Human case study | [65] |
| Genistein (270 mg/kg); quercetin (302 mg/kg) | Dose-dependent DNA double-strand break in hematopoietic cells | In vitro model | [66] |
| Liposome-Based Composition | Functionalization | Size (nm) | Load | Encapsulation Efficiency (%) | Effect | Model | Source |
|---|---|---|---|---|---|---|---|
| Phosphatidylserine | - | 132.86 ± 2.05 | EGCG | 70.4 | Increased anti-inflammatory and neuroprotective effects | In vitro/in vivo | [227] |
| Liposomes composed of L-α-phosphatidylcholine | - | 155.2 ± 1.23 | EGCG | 55.4 | Increased anti-inflammatory and neuroprotective effects | In vitro/in vivo | [227] |
| Liposomes composed of sphingomyelin | - | 97.6 ± 3.1 | Curcumin | 1.3 mol% (with respect to total phospholipid content) | Inhibited formation of Aβ1–42 fibrils | In vitro | [228] |
| Liposomes composed of lecithin | - | 146–585 | Resveratrol | 73.54% | Increased dopaminergic neuron protection | In vivo (rat PD model) | [229] |
| Liposomes composed of Lipoid S100 | PEG 1 | 100.6 ± 0.7 | Curcumin | 87 ± 3 | Increased bilayer stability; decreased polyphenol release | In vitro | [230] |
| Liposomes composed of phosphatidylcholine | PEG 1 | 107.4–116.1 | Copaifera sabulicola leaves extract | 81.89–86.30 | Reduced glioma cell viability by 93% | In vitro | [231] |
| Liposomes composed of brain lipids | Anti-TfR Ab 2 | 133.0 ± 27.0 | Polyphenol-rich grape pomace extracts | 20.15 μg/mL of polyphenols in 1 mg/mL of vehicles | Increased protection against neurodegeneration | In vivo (rat PD model) | [232] |
| Liposomes composed of egg phosphatidylcholine | Transferrin moieties | 211.2 ± 0.8 | Resveratrol | 70−75 | Increased cytotoxicity and apoptosis in glioblastoma | In vitro/in vivo | [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaluza, M.; Ksiazek-Winiarek, D.; Szpakowski, P.; Czpakowska, J.; Fijalkowska, J.; Glabinski, A. Polyphenols in the Central Nervous System: Cellular Effects and Liposomal Delivery Approaches. Int. J. Mol. Sci. 2025, 26, 6477. https://doi.org/10.3390/ijms26136477
Kaluza M, Ksiazek-Winiarek D, Szpakowski P, Czpakowska J, Fijalkowska J, Glabinski A. Polyphenols in the Central Nervous System: Cellular Effects and Liposomal Delivery Approaches. International Journal of Molecular Sciences. 2025; 26(13):6477. https://doi.org/10.3390/ijms26136477
Chicago/Turabian StyleKaluza, Mateusz, Dominika Ksiazek-Winiarek, Piotr Szpakowski, Joanna Czpakowska, Julia Fijalkowska, and Andrzej Glabinski. 2025. "Polyphenols in the Central Nervous System: Cellular Effects and Liposomal Delivery Approaches" International Journal of Molecular Sciences 26, no. 13: 6477. https://doi.org/10.3390/ijms26136477
APA StyleKaluza, M., Ksiazek-Winiarek, D., Szpakowski, P., Czpakowska, J., Fijalkowska, J., & Glabinski, A. (2025). Polyphenols in the Central Nervous System: Cellular Effects and Liposomal Delivery Approaches. International Journal of Molecular Sciences, 26(13), 6477. https://doi.org/10.3390/ijms26136477






