Fantastic Ferulic Acid Esterases and Their Functions
Abstract
1. Introduction
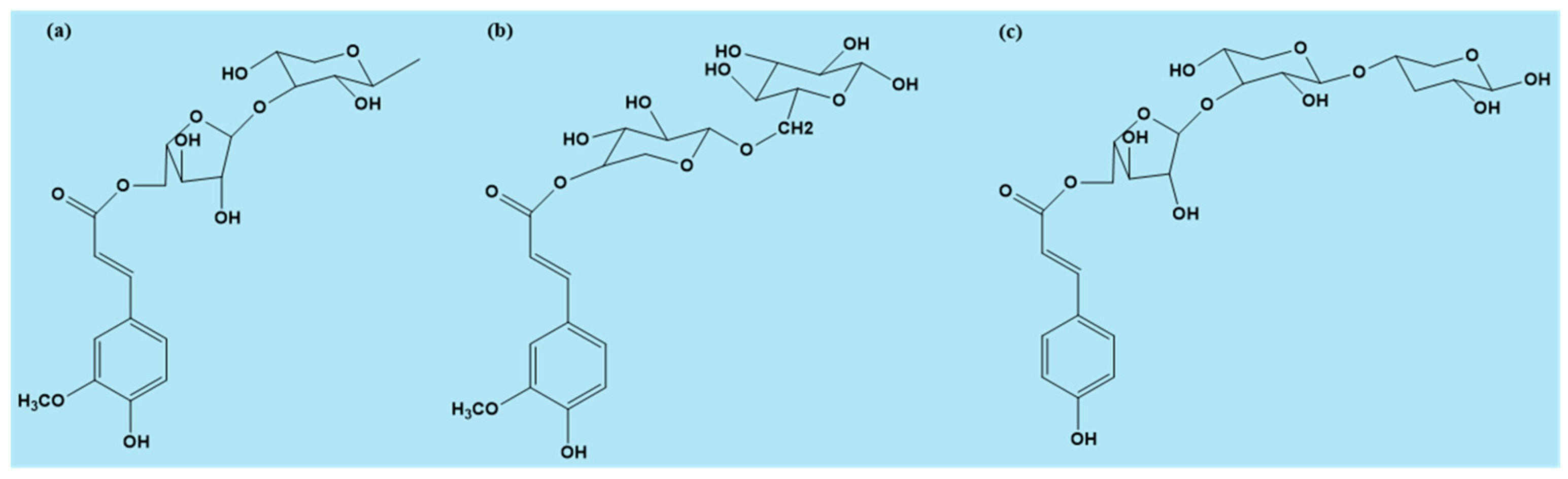
2. Origin and Occurrence of Different Ferulic Acid Forms
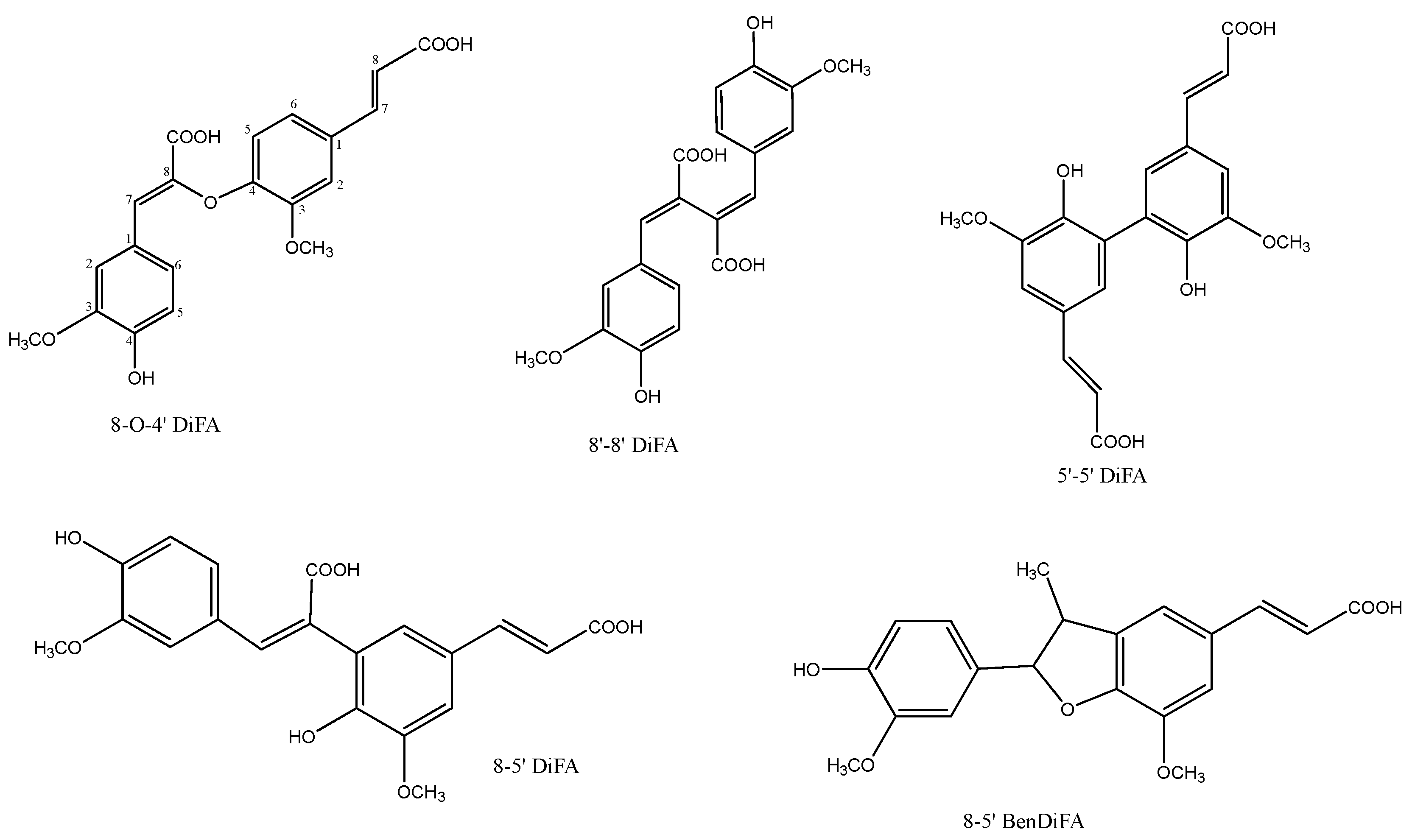
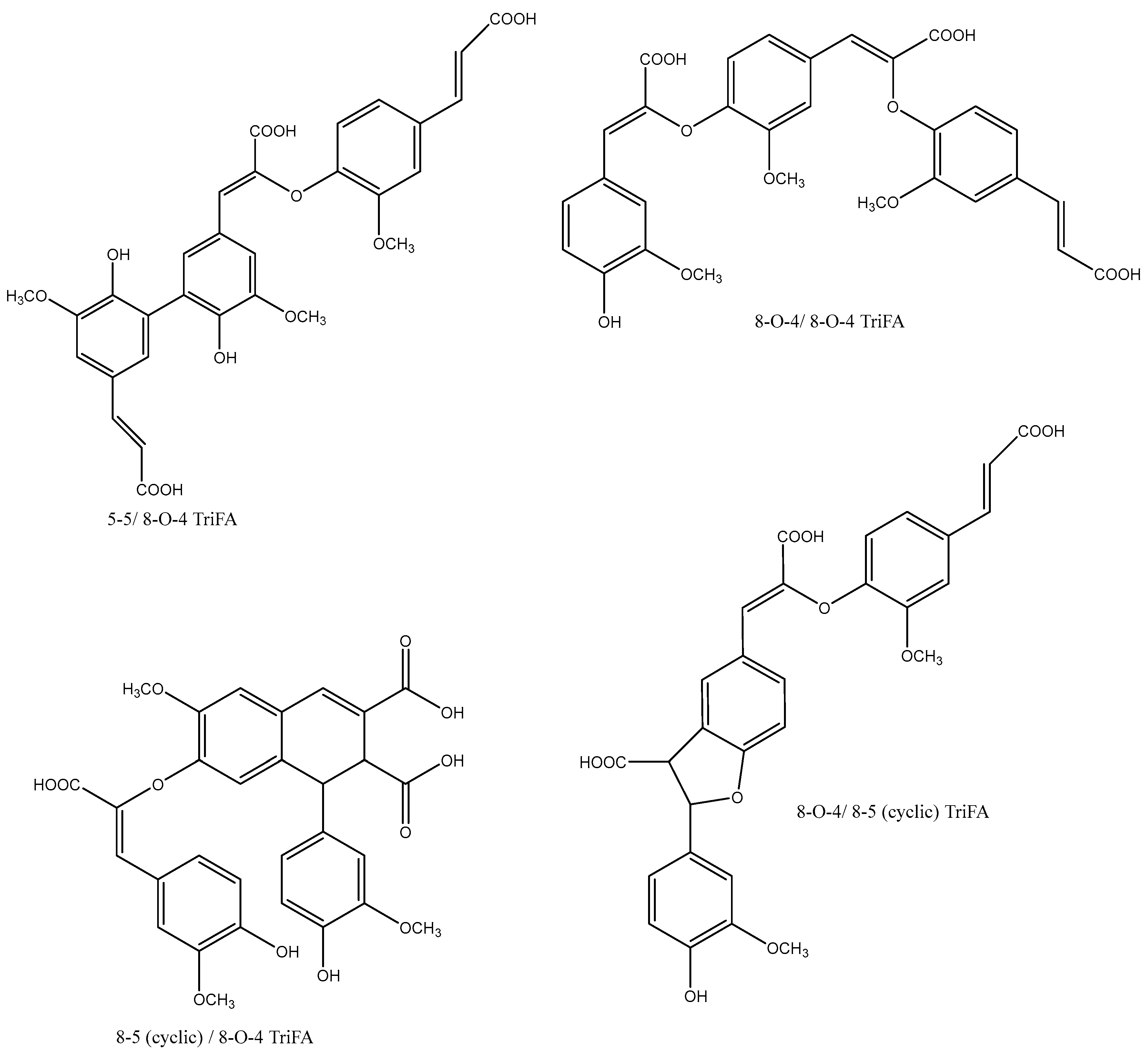
3. Ferulate Dimers and Cross-Links
- 8-O-4′-DiFA:(Z)-β-{4-[(E)-2-carboxyvinyl]-2-methoxyphenoxy}-4-hydroxy-3-methoxy-cinnamic acid;
- 8-5′ DiFA: (E,E)-4,4′-dihydroxy-3,5′-dimethoxy-β,3′-bicinnamic acid;
- 8-5′-BenDiFA: in the benzofuran form;
- 8-8′-DiFA: (E,E)-4,4′-dihydroxy-3,3′-dimethoxy-β,β′-bicinnamic acid;
- 5-5′ DiFA: (E,E)-4,4′-dihydroxy-5,5′dimethoxy-β,3′-bicinnamic acid.
4. Feruloyl (Ferulic Acid) Esterases and Their Classification
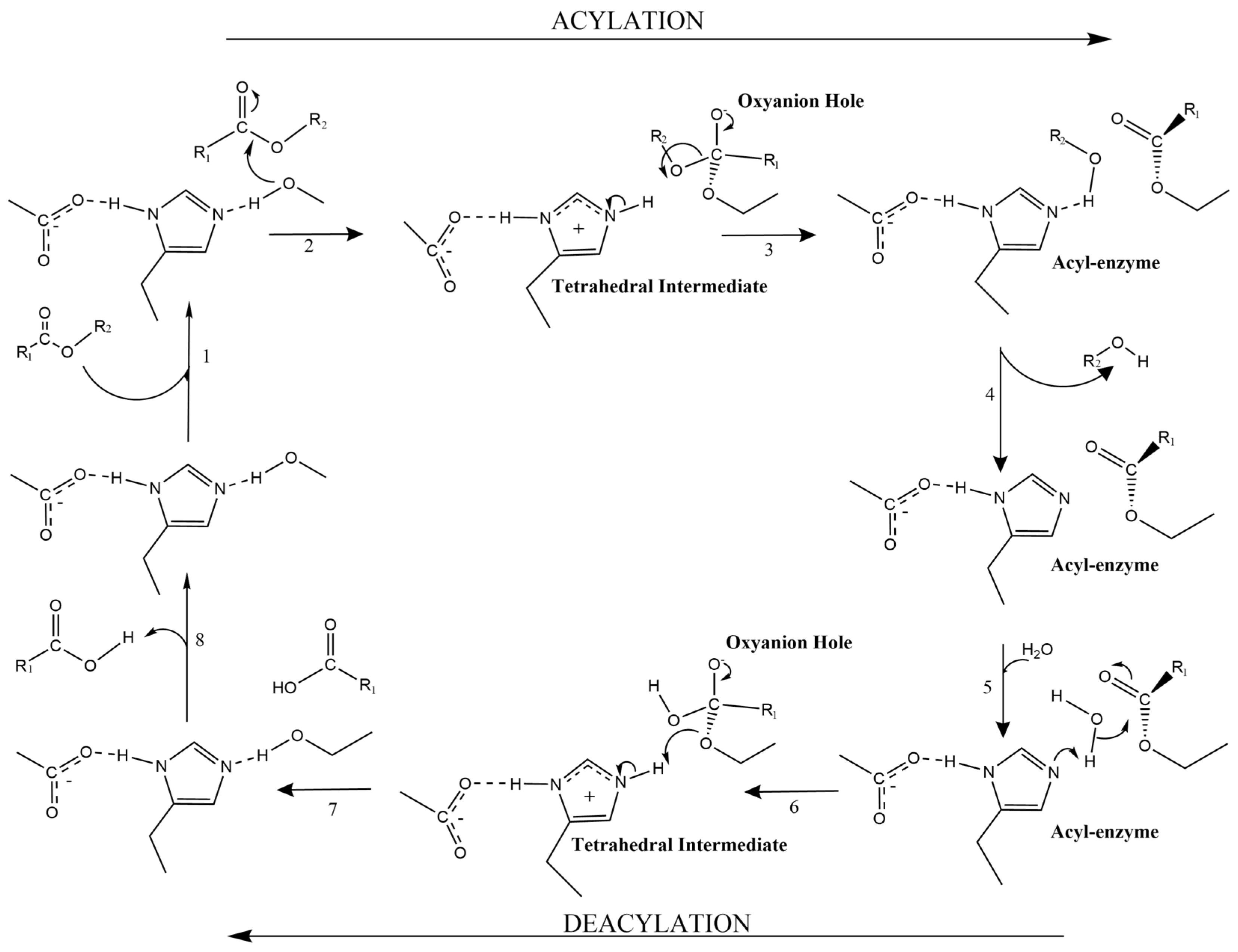
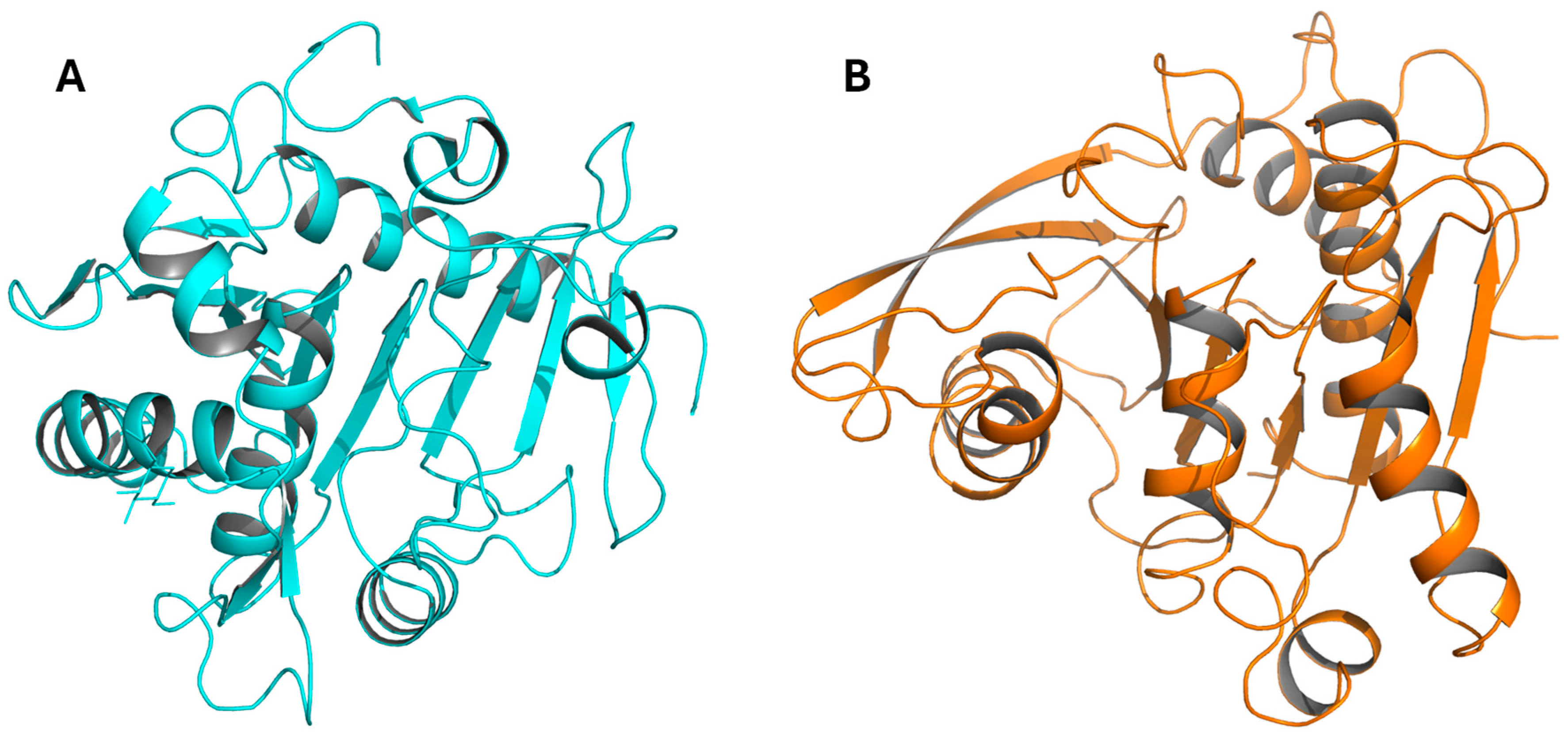
5. Catalytic Mechanism and Well-Characterized FAEs
6. FAEs in (Trans)Esterification
7. Synergistic Degradation and Bifunctional Esterases
8. Estimation of Enzymatic Activity and Detection of FA
9. Applications of FAEs and FA
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FA | Ferulic Acid |
| FAE | Ferulic Acid Esterase |
| CAZymes | Carbohydrate-Active Enzymes |
| GAX | Glucuronoarabinoxylan |
| AX | Arabinoxylan |
| DiFA | Di-Ferulic Acid |
| BenDiFA | Benzofuran Form of Di-Ferulic Acid |
| HRP | Horseradish Peroxidase |
| CBM | Carbohydrate-Binding Module |
| CE1 | Carbohydrate Esterase Family 1 |
| MFA | Methyl Ferulate |
| MCA | Methyl Caffeate |
| MSA | Methyl Sinapate |
| MpCA | Methyl p-Coumarate |
| GH | Glycoside Hydrolase Family |
| HPLC | High-Performance Liquid Chromatography |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| CZE | Capillary Zone Electrophoresis |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| TLC | Thin-Layer Chromatography |
| HPTLC | High-Performance Thin-Layer Chromatography |
| pNP | Para Nitrophenol |
| ROS | Reactive Oxygen Species |
References
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef]
- Terrett, O.M.; Dupree, P. Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr. Opin. Biotechnol. 2019, 56, 97–104. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Giannetti, I.; Dattilo, C.; Scaccini, C. Coffee drinking influences plasma antioxidant capacity in humans. J. Agric. Food Chem. 2002, 50, 6211–6216. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Hiroi, T. Linkage of phenolic acids to cell-wall polysaccharides of bamboo shoot. Carbohydr. Res. 1990, 206, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Locher, R.; Martin, H.V.; Grison, R.; Pilet, P.E. Cell wall-bound trans-and cis-ferulic acids in growing maize roots. Physiol. Plant. 1994, 90, 734–738. [Google Scholar] [CrossRef]
- Tang, Y.; Hao, J.; Fan, C.; Cao, X. Preparative separation of high-purity trans-and cis-ferulic acid from wheat bran by pH-zone-refining counter-current chromatography. J. Chromatogr. A 2021, 1636, 461772. [Google Scholar] [CrossRef]
- Rombouts, F.M.; Thibault, J.-F. Feruloylated pectic substances from sugar-beet pulp. Carbohydr. Res. 1986, 154, 177–187. [Google Scholar] [CrossRef]
- Fry, S.C. Feruloylated pectins from the primary cell wall: Their structure and possible functions. Planta 1983, 157, 111–123. [Google Scholar] [CrossRef]
- Colquhoun, I.J.; Ralet, M.-C.; Thibault, J.-F.; Faulds, C.B.; Williamson, G. Structure identification of feruloylated oligosaccharides from sugar-beet pulp by NMR spectroscopy. Carbohydr. Res. 1994, 263, 243–256. [Google Scholar] [CrossRef]
- Parr, A.J.; Waldron, K.W.; Ng, A.; Parker, M.L. The wall-bound phenolics of Chinese water chestnut (Eleocharis dulcis). J. Sci. Food Agric. 1996, 71, 501–507. [Google Scholar] [CrossRef]
- Massiot, P.; Rouau, X.; Thibault, J.-F. Characterisation of the extractable pectins and hemicelluloses of the cell wall of carrot. Carbohydr. Res. 1988, 172, 229–242. [Google Scholar] [CrossRef]
- Renard, C.; Champenois, Y.; Thibault, J.-F. Characterisation of the extractable pectins and hemicelluloses of the cell wall of glasswort, Salicornia ramosissima. Carbohydr. Polym. 1993, 22, 239–245. [Google Scholar] [CrossRef]
- Sanchez, M.; Pena, M.J.; Revilla, G.; Zarra, I. Changes in dehydrodiferulic acids and peroxidase activity against ferulic acid associated with cell walls during growth of Pinus pinaster hypocotyl. Plant Physiol. 1996, 111, 941–946. [Google Scholar] [CrossRef]
- Ward, G.; Hadar, Y.; Bilkis, I.; Konstantinovsky, L.; Dosoretz, C.G. Initial steps of ferulic acid polymerization by lignin peroxidase. J. Biol. Chem. 2001, 276, 18734–18741. [Google Scholar] [CrossRef]
- Nordkvist, E.; Salomonsson, A.C.; Åman, P. Distribution of insoluble bound phenolic acids in barley grain. J. Sci. Food Agric. 1984, 35, 657–661. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Ralph, J.; Grabber, J.H. Cell wall cross-linking by ferulates and diferulates in grasses. J. Sci. Food Agric. 1999, 79, 403–407. [Google Scholar] [CrossRef]
- Ford, C.W.; Hartley, R.D. GC/MS characterisation of cyclodimers from p-coumaric and ferulic acids by photodimerisation—A possible factor influencing cell wall biodegradability. J. Sci. Food Agric. 1989, 46, 301–310. [Google Scholar] [CrossRef]
- Ford, C.W.; Hartley, R.D. Cyclodimers of p-coumaric and ferulic acids in the cell walls of tropical grasses. J. Sci. Food Agric. 1990, 50, 29–43. [Google Scholar] [CrossRef]
- Hartley, R.D.; Whatley, F.R.; Harris, P.J. 4, 4′-Dihydroxytruxillic acid as a component of cell walls of Lolium multiflorum. Phytochemistry 1988, 27, 349–351. [Google Scholar] [CrossRef]
- Bartolome, B.; Faulds, C.B.; Kroon, P.A.; Waldron, K.; Gilbert, H.J.; Hazlewood, G.; Williamson, G. An Aspergillus niger esterase (ferulic acid esterase III) and a recombinant Pseudomonas fluorescens subsp. cellulosa esterase (Xy1D) release a 5-5′ferulic dehydrodimer (diferulic acid) from barley and wheat cell walls. Appl. Environ. Microbiol. 1997, 63, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Ralph, J.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J. Sci. Food Agric. 2001, 81, 653–660. [Google Scholar] [CrossRef]
- Funk, C.; Ralph, J.; Steinhart, H.; Bunzel, M. Isolation and structural characterisation of 8–O–4/8–O–4-and 8–8/8–O–4-coupled dehydrotriferulic acids from maize bran. Phytochemistry 2005, 66, 363–371. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Funk, C.; Steinhart, H. Structural elucidation of new ferulic acid-containing phenolic dimers and trimers isolated from maize bran. Tetrahedron Lett. 2005, 46, 5845–5850. [Google Scholar] [CrossRef]
- Rouau, X.; Cheynier, V.; Surget, A.; Gloux, D.; Barron, C.; Meudec, E.; Louis-Montero, J.; Criton, M. A dehydrotrimer of ferulic acid from maize bran. Phytochemistry 2003, 63, 899–903. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Funk, C.; Steinhart, H. Isolation and identification of a ferulic acid dehydrotrimer from saponified maize bran insoluble fiber. Eur. Food Res. Technol. 2003, 217, 128–133. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Brüning, P.; Steinhart, H. Structural identification of dehydrotriferulic and dehydrotetraferulic acids isolated from insoluble maize bran fiber. J. Agric. Food Chem. 2006, 54, 6409–6418. [Google Scholar] [CrossRef]
- Bunzel, M.; Heuermann, B.; Kim, H.; Ralph, J. Peroxidase-catalyzed oligomerization of ferulic acid esters. J. Agric. Food Chem. 2008, 56, 10368–10375. [Google Scholar] [CrossRef] [PubMed]
- Iiyama, K.; Lam, T.B.-T.; Stone, B.A. Covalent cross-links in the cell wall. Plant Physiol. 1994, 104, 315. [Google Scholar] [CrossRef] [PubMed]
- Oudgenoeg, G.; Dirksen, E.; Ingemann, S.; Hilhorst, R.; Gruppen, H.; Boeriu, C.G.; Piersma, S.R.; van Berkel, W.J.; Laane, C.; Voragen, A.G. Horseradish peroxidase-catalyzed oligomerization of ferulic acid on a template of a tyrosine-containing tripeptide. J. Biol. Chem. 2002, 277, 21332–21340. [Google Scholar] [CrossRef] [PubMed]
- Boeriu, C.G.; Oudgenoeg, G.; Spekking, W.T.J.; Berendsen, L.B.; Vancon, L.; Boumans, H.; Gruppen, H.; van Berkel, W.J.; Laane, C.; Voragen, A.G. Horseradish peroxidase-catalyzed cross-linking of feruloylated arabinoxylans with β-casein. J. Agric. Food Chem. 2004, 52, 6633–6639. [Google Scholar] [CrossRef]
- Ferreira, L.; Wood, T.M.; Williamson, G.; Faulds, C.; Hazlewood, G.P.; Black, G.W.; Gilbert, H.J. A modular esterase from Pseudomonas fluorescens subsp. cellulosa contains a non-catalytic cellulose-binding domain. Biochem. J. 1993, 294, 349–355. [Google Scholar] [CrossRef]
- Kroon, P.A.; Williamson, G.; Fish, N.M.; Archer, D.B.; Belshaw, N.J. A modular esterase from Penicillium funiculosum which releases ferulic acid from plant cell walls and binds crystalline cellulose contains a carbohydrate binding module. Eur. J. Biochem. 2000, 267, 6740–6752. [Google Scholar] [CrossRef]
- Holck, J.; Fredslund, F.; Møller, M.S.; Brask, J.; Krogh, K.B.; Lange, L.; Welner, D.H.; Svensson, B.; Meyer, A.S.; Wilkens, C. A carbohydrate-binding family 48 module enables feruloyl esterase action on polymeric arabinoxylan. J. Biol. Chem. 2019, 294, 17339–17353. [Google Scholar] [CrossRef]
- Mamiya, A.; Sakka, M.; Kosugi, A.; Katsuzaki, H.; Tanaka, A.; Kunitake, E.; Kimura, T.; Sakka, K. Significance of a family-6 carbohydrate-binding module in a modular feruloyl esterase for removing ferulic acid from insoluble wheat arabinoxylan. Enzym. Microb. Technol. 2020, 138, 109546. [Google Scholar] [CrossRef] [PubMed]
- Holck, J.; Djajadi, D.T.; Brask, J.; Pilgaard, B.; Krogh, K.B.; Meyer, A.S.; Lange, L.; Wilkens, C. Novel xylanolytic triple domain enzyme targeted at feruloylated arabinoxylan degradation. Enzym. Microb. Technol. 2019, 129, 109353. [Google Scholar] [CrossRef]
- Lin, S.; Hunt, C.J.; Holck, J.; Brask, J.; Krogh, K.B.; Meyer, A.S.; Wilkens, C.; Agger, J.W. Fungal feruloyl esterases can catalyze release of diferulic acids from complex arabinoxylan. Int. J. Biol. Macromol. 2023, 232, 123365. [Google Scholar] [CrossRef]
- Crepin, V.F.; Faulds, C.B.; Connerton, I. Functional classification of the microbial feruloyl esterases. Appl. Microbiol. Biotechnol. 2004, 63, 647–652. [Google Scholar] [CrossRef]
- Benoit, I.; Danchin, E.G.; Bleichrodt, R.-J.; de Vries, R.P. Biotechnological applications and potential of fungal feruloyl esterases based on prevalence, classification and biochemical diversity. Biotechnol. Lett. 2008, 30, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Udatha, G. Descriptor-based computational analysis reveals a novel classification scheme for feruloyl esterase enzyme families. In Proceedings of the International Conference on Bioinformatics, Kuala Lumpur, Malaysia, 30 November 2011–2 December 2011. [Google Scholar]
- Dilokpimol, A.; Mäkelä, M.R.; Aguilar-Pontes, M.V.; Benoit-Gelber, I.; Hildén, K.S.; de Vries, R.P. Diversity of fungal feruloyl esterases: Updated phylogenetic classification, properties, and industrial applications. Biotechnol. Biofuels 2016, 9, 231. [Google Scholar] [CrossRef]
- Lombard, V.; Henrissat, B.; Garron, M.-L. CAZac: An activity descriptor for carbohydrate-active enzymes. Nucleic Acids Res. 2025, 53, D625–D633. [Google Scholar] [CrossRef]
- Faber, K.; Faber, K. Biotransformations in Organic Chemistry: A Textbook; Springer: Heidelberg, Germany, 2004; Volume 6. [Google Scholar]
- Kasmaei, K.M.; Kalyani, D.C.; Reichenbach, T.; Jiménez-Quero, A.; Vilaplana, F.; Divne, C. Crystal structure of the feruloyl esterase from Lentilactobacillus buchneri reveals a novel homodimeric state. Front. Microbiol. 2022, 13, 1050160. [Google Scholar] [CrossRef]
- Faulds, C.B.; Molina, R.; Gonzalez, R.; Husband, F.; Juge, N.; Sanz-Aparicio, J.; Hermoso, J.A. Probing the determinants of substrate specificity of a feruloyl esterase, AnFaeA, from Aspergillus niger. FEBS J. 2005, 272, 4362–4371. [Google Scholar] [CrossRef]
- Dilokpimol, A.; Mäkelä, M.R.; Mansouri, S.; Belova, O.; Waterstraat, M.; Bunzel, M.; de Vries, R.P.; Hildén, K.S. Expanding the feruloyl esterase gene family of Aspergillus niger by characterization of a feruloyl esterase, FaeC. New Biotechnol. 2017, 37, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Faulds, C.B.; Williamson, G. Purification and characterization of a ferulic acid esterase (FAE-III) from Aspergillus niger: Specificity for the phenolic moiety and binding to microcrystalline cellulose. Microbiology 1994, 140, 779–787. [Google Scholar] [CrossRef]
- De Vries, R.P.; Michelsen, B.; Poulsen, C.; Kroon, P.; Van Den Heuvel, R.; Faulds, C.; Williamson, G.; Van Den Hombergh, J.; Visser, J. The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in degradation of complex cell wall polysaccharides. Appl. Environ. Microbiol. 1997, 63, 4638–4644. [Google Scholar] [CrossRef]
- de Vries, R.P.; vanKuyk, P.A.; Kester, H.C.; Visser, J. The Aspergillus niger faeB gene encodes a second feruloyl esterase involved in pectin and xylan degradation and is specifically induced in the presence of aromatic compounds. Biochem. J. 2002, 363, 377–386. [Google Scholar] [CrossRef]
- Hermoso, J.A.; Sanz-Aparicio, J.; Molina, R.; Juge, N.; González, R.; Faulds, C.B. The crystal structure of feruloyl esterase A from Aspergillus niger suggests evolutive functional convergence in feruloyl esterase family. J. Mol. Biol. 2004, 338, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Aliwan, F.O.; Kroon, P.A.; Faulds, C.B.; Pickersgill, R.; Williamson, G. Ferulic acid esterase-III from Aspergillus niger does not exhibit lipase activity. J. Sci. Food Agric. 1999, 79, 457–459. [Google Scholar] [CrossRef]
- Prates, J.A.; Tarbouriech, N.; Charnock, S.J.; Fontes, C.M.; Ferreira, L.S.M.; Davies, G.J. The structure of the feruloyl esterase module of xylanase 10B from Clostridium thermocellum provides insights into substrate recognition. Structure 2001, 9, 1183–1190. [Google Scholar] [CrossRef]
- Schubot, F.D.; Kataeva, I.A.; Blum, D.L.; Shah, A.K.; Ljungdahl, L.G.; Rose, J.P.; Wang, B.-C. Structural basis for the substrate specificity of the feruloyl esterase domain of the cellulosomal xylanase Z from Clostridium thermocellum. Biochemistry 2001, 40, 12524–12532. [Google Scholar] [CrossRef]
- Blum, D.L.; Kataeva, I.A.; Li, X.-L.; Ljungdahl, L.G. Feruloyl esterase activity of the Clostridium thermocellum cellulosome can be attributed to previously unknown domains of XynY and XynZ. J. Bacteriol. 2000, 182, 1346–1351. [Google Scholar] [CrossRef]
- Suzuki, K.; Hori, A.; Kawamoto, K.; Thangudu, R.R.; Ishida, T.; Igarashi, K.; Samejima, M.; Yamada, C.; Arakawa, T.; Wakagi, T. Crystal structure of a feruloyl esterase belonging to the tannase family: A disulfide bond near a catalytic triad. Proteins Struct. Funct. Bioinform. 2014, 82, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Topakas, E.; Vafiadi, C.; Stamatis, H.; Christakopoulos, P. Sporotrichum thermophile type C feruloyl esterase (StFaeC): Purification, characterization, and its use for phenolic acid (sugar) ester synthesis. Enzym. Microb. Technol. 2005, 36, 729–736. [Google Scholar] [CrossRef]
- Gao, L.; Wang, M.; Chen, S.; Zhang, D. Biochemical characterization of a novel feruloyl esterase from Penicillium piceum and its application in biomass bioconversion. J. Mol. Catal. B Enzym. 2016, 133, S388–S394. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhu, M.; Meng, L.; Wang, H.; Ng, T.B. An acidic feruloyl esterase from the mushroom Lactarius hatsudake: A potential animal feed supplement. Int. J. Biol. Macromol. 2016, 93, 290–295. [Google Scholar] [CrossRef]
- Lai, K.-K.; Stogios, P.J.; Vu, C.; Xu, X.; Cui, H.; Molloy, S.; Savchenko, A.; Yakunin, A.; Gonzalez, C.F. An inserted α/β subdomain shapes the catalytic pocket of Lactobacillus johnsonii cinnamoyl esterase. PLoS ONE 2011, 6, e23269. [Google Scholar] [CrossRef]
- Yang, W.; Sun, L.; Dong, P.; Chen, Y.; Zhang, H.; Huang, X.; Wu, L.; Chen, L.; Jing, D.; Wu, Y. Structure-guided rational design of the Geobacillus thermoglucosidasius feruloyl esterase GthFAE to improve its thermostability. Biochem. Biophys. Res. Commun. 2022, 600, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Hwang, J.; Do, H.; Le, L.T.H.L.; Lee, C.W.; Yoo, W.; Lee, M.J.; Shin, S.C.; Kim, K.K.; Kim, H.-W. Feruloyl Esterase (La Fae) from Lactobacillus acidophilus: Structural Insights and Functional Characterization for Application in Ferulic Acid Production. Int. J. Mol. Sci. 2023, 24, 11170. [Google Scholar] [CrossRef]
- Gruninger, R.J.; Cote, C.; McAllister, T.A.; Abbott, D.W. Contributions of a unique β-clamp to substrate recognition illuminates the molecular basis of exolysis in ferulic acid esterases. Biochem. J. 2016, 473, 839–849. [Google Scholar] [CrossRef]
- Topakas, E.; Stamatis, H.; Biely, P.; Kekos, D.; Macris, B.; Christakopoulos, P. Purification and characterization of a feruloyl esterase from Fusarium oxysporum catalyzing esterification of phenolic acids in ternary water–organic solvent mixtures. J. Biotechnol. 2003, 102, 33–44. [Google Scholar] [CrossRef]
- Romero-Borbón, E.; Grajales-Hernández, D.; Armendáriz-Ruiz, M.; Ramírez-Velasco, L.; Rodríguez-González, J.A.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Mateos-Díaz, J.C. Type C feruloyl esterase from Aspergillus ochraceus: A butanol specific biocatalyst for the synthesis of hydroxycinnamates in a ternary solvent system. Electron. J. Biotechnol. 2018, 35, 1–9. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Iancu, L.; Jütten, P.; Piechot, A.; Rova, U.; Christakopoulos, P. Screening of novel feruloyl esterases from Talaromyces wortmannii for the development of efficient and sustainable syntheses of feruloyl derivatives. Enzym. Microb. Technol. 2019, 120, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, X.; Gao, S.; Zhang, Y.; An, Y. Biochemical characterization of an engineered bifunctional xylanase/feruloyl esterase and its synergistic effects with cellulase on lignocellulose hydrolysis. Bioresour. Technol. 2022, 355, 127244. [Google Scholar] [CrossRef]
- Giuliani, S.; Piana, C.; Setti, L.; Hochkoeppler, A.; Pifferi, P.; Williamson, G.; Faulds, C. Synthesis of pentylferulate by a feruloyl esterase from Aspergillus niger using water-in-oil microemulsions. Biotechnol. Lett. 2001, 23, 325–330. [Google Scholar] [CrossRef]
- Couto, J.; St-Louis, R.; Karboune, S. Optimization of feruloyl esterase-catalyzed synthesis of feruloylated oligosaccharides by response surface methodology. J. Mol. Catal. B Enzym. 2011, 73, 53–62. [Google Scholar] [CrossRef]
- Zeng, Y.; Yin, X.; Wu, M.-C.; Yu, T.; Feng, F.; Zhu, T.-D.; Pang, Q.-F. Expression of a novel feruloyl esterase from Aspergillus oryzae in Pichia pastoris with esterification activity. J. Mol. Catal. B Enzym. 2014, 110, 140–146. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Xue, Y.; Luo, G.; Gan, L.; Liu, J.; Mao, L.; Long, M. High efficiency co-production of ferulic acid and xylooligosaccharides from wheat bran by recombinant xylanase and feruloyl esterase. Biochem. Eng. J. 2017, 120, 41–48. [Google Scholar] [CrossRef]
- Schmitz, E.; Leontakianakou, S.; Norlander, S.; Karlsson, E.N.; Adlercreutz, P. Lignocellulose degradation for the bioeconomy: The potential of enzyme synergies between xylanases, ferulic acid esterase and laccase for the production of arabinoxylo-oligosaccharides. Bioresour. Technol. 2022, 343, 126114. [Google Scholar] [CrossRef]
- Dilokpimol, A.; Verkerk, B.; Li, X.; Bellemare, A.; Lavallee, M.; Frommhagen, M.; Underlin, E.N.; Kabel, M.A.; Powlowski, J.; Tsang, A. Screening of novel fungal Carbohydrate Esterase family 1 enzymes identifies three novel dual feruloyl/acetyl xylan esterases. FEBS Lett. 2022, 596, 1932–1943. [Google Scholar] [CrossRef]
- Schmitz, E.; Leontakianakou, S.; Adlercreutz, P.; Nordberg Karlsson, E.; Linares-Pastén, J.A. Novel function of CtXyn5A from Acetivibrio thermocellus: Dual arabinoxylanase and feruloyl esterase activity in the same active site. ChemBioChem 2023, 24, e202200667. [Google Scholar] [CrossRef]
- Pussayanawin, V.; Wetzel, D.L. High-performance liquid chromatographi determination of ferulic acid in wheat milling fractions as a measure of bran contamination. J. Chromatogr. A 1987, 391, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Razboršek, M.I.; Ivanović, M.; Kolar, M. Validated stability-indicating GC-MS method for characterization of forced degradation products of trans-caffeic acid and trans-ferulic acid. Molecules 2021, 26, 2475. [Google Scholar] [CrossRef] [PubMed]
- Kvasnička, F.; Čopíková, J.; Ševčík, R.; Krátká, J.; Syntytsia, A.; Voldřich, M. Determination of phenolic acids by capillary zone electrophoresis and HPLC. Cent. Eur. J. Chem. 2008, 6, 410–418. [Google Scholar] [CrossRef]
- Jong, T.-T.; Lee, M.-R.; Chiang, Y.-C.; Chiang, S.-T. Using LC/MS/MS to determine matrine, oxymatrine, ferulic acid, mangiferin, and glycyrrhizin in the Chinese medicinal preparations Shiau-feng-saan and Dang-guei-nian-tong-tang. J. Pharm. Biomed. Anal. 2006, 40, 472–477. [Google Scholar] [CrossRef]
- Li, N.; Liu, C.; Mi, S.; Wang, N.; Zheng, X.; Li, Y.; Huang, X.; He, S.; Chen, H.; Xu, X. Simultaneous determination of oleanolic acid, p-coumaric acid, ferulic acid, kaemperol and quercetin in rat plasma by LC–MS-MS and application to a pharmacokinetic study of Oldenlandia diffusa extract in rats. J. Chromatogr. Sci. 2012, 50, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Waterstraat, M.; Hildebrand, A.; Rosler, M.; Bunzel, M. Development of a QuEChERS-based stable-isotope dilution LC-MS/MS method to quantitate ferulic acid and its main microbial and hepatic metabolites in milk. J. Agric. Food Chem. 2016, 64, 8667–8677. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K.; Singh, B. Thin-layer chromatography of gallic acid, methyl gallate, pyrogallol, phloroglucinol, catechol, resorcinol, hydroquinone, catechin, epicatechin, cinnamic acid, p-coumaric acid, ferulic acid and tannic acid. J. Chromatogr. A 1998, 822, 167–171. [Google Scholar] [CrossRef]
- Hingse, S.S.; Digole, S.B.; Annapure, U.S. Method development for simultaneous detection of ferulic acid and vanillin using high-performance thin layer chromatography. J. Anal. Sci. Technol. 2014, 5, 21. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kaneko, S.; Yoshida, M. Extracellular carbohydrate esterase from the basidiomycete Coprinopsis cinerea released ferulic and acetic acids from xylan. Biosci. Biotechnol. Biochem. 2010, 74, 1722–1724. [Google Scholar] [CrossRef][Green Version]
- Shi, Q.; Ma, J.; Abdel-Hamid, A.M.; Li, Y.; Zhong, P.; Wang, D.; Sun, Z.; Tu, T.; Zhu, W.; Cheng, Y. Mining of latent feruloyl esterase resources in rumen and insight into dual-functional feruloyl esterase-xylanase from Pecoramyces ruminantium F1. Bioresour. Technol. 2025, 418, 131854. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The antioxidant properties, metabolism, application and mechanism of ferulic acid in medicine, food, cosmetics, livestock and poultry. Antioxidants 2024, 13, 853. [Google Scholar] [CrossRef]
- Janus, E.; Pinheiro, L.R.; Nowak, A.; Kucharska, E.; Świątek, E.; Podolak, N.; Perużyńska, M.; Piotrowska, K.; Duchnik, W.; Kucharski, Ł. New ferulic acid and amino acid derivatives with increased cosmeceutical and pharmaceutical potential. Pharmaceutics 2022, 15, 117. [Google Scholar] [CrossRef]
- Neopane, D.; Ansari, V.A.; Singh, A. Ferulic acid: Signaling pathways in aging. Drug Res. 2023, 73, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-H.; Lin, J.-Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Zhao, D.-S.; Wang, J.; Zhou, H.; Wang, L.; Mao, J.-L.; He, J.-X. The treatment of cardiovascular diseases: A review of ferulic acid and its derivatives. Die Pharm. -Int. J. Pharm. Sci. 2021, 76, 55–60. [Google Scholar]
- Dwivedi, S.; Singh, D.; Deshmukh, P.T.; Soni, R.; Trivedi, R. Healing potential of ferulic acid on dermal wound in diabetic animals. Asian J. Mol. Model 2015, 1, 1–16. [Google Scholar]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef]
- Myrtollari, K.; Calderini, E.; Kracher, D.; Schöngaßner, T.; Galusic, S.; Slavica, A.; Taden, A.; Mokos, D.; Schrüfer, A.; Wirnsberger, G. Stability Increase of Phenolic Acid Decarboxylase by a Combination of Protein and Solvent Engineering Unlocks Applications at Elevated Temperatures. ACS Sustain. Chem. Eng. 2024, 12, 3575–3584. [Google Scholar] [CrossRef]
- Petermeier, P.; Bittner, J.P.; Jonsson, T.; de María, P.D.; Byström, E.; Kara, S. Integrated preservation of water activity as key to intensified chemoenzymatic synthesis of bio-based styrene derivatives. Commun. Chem. 2024, 7, 57. [Google Scholar] [CrossRef]
- Myrtollari, K.; Chánique, A.M.; Kracher, D.; Herrera, D.P.; Gutierrez-Benavente, J.; Schüller, A.; Kourist, R. Engineering Substrate Acceptance of Resveratrol O-Methyltransferase from Vitis vinifera for the Selective Synthesis of O-Methyl Protected Biobased Hydroxystyrenes. ChemCatChem 2025, 17, e202402027. [Google Scholar] [CrossRef]
- Kaur, B.; Chakraborty, D.; Kumar, B. Phenolic biotransformations during conversion of ferulic acid to vanillin by lactic acid bacteria. BioMed Res. Int. 2013, 2013, 590359. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, F.; Xu, L.; Khazi, M.I.; Ali, S.; Rahman, M.U.; Zhu, D. Extraction, purification, and applications of vanillin: A review of recent advances and challenges. Ind. Crops Prod. 2023, 204, 117372. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The applications of ferulic-acid-loaded fibrous films for fruit preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef] [PubMed]

| Classification System | Description | Main Features | Limitations |
|---|---|---|---|
| CAZy (CE1) | Groups FAEs into the CE1 based on sequence similarity and enzymatic catalytic mechanism. | Standardized database for carbohydrate-active enzymes. | Does not consider the functional diversity of FAEs. |
| Crepin (2004) [39] | Categorizes FAEs into types A, B, C, D, and putative E based on sequence similarity and substrate specificity. | Correlations based on sequence similarities and specificity on synthetic methyl esters. | Based on limited data; it can cluster unrelated enzymes within the same group. |
| Benoit (2008) [40] | Seven fungal FAE subfamilies based on phylogenetic analysis of fungal genomes. | Identifies evolutionary relationships within fungal species. | Limited to fungal genomic data. Excludes bacterial FAEs. |
| Udatha (2011) [41] | Twelve FAE families based on sequence and structure from fungi, bacteria, and plants. | Validation via computational and experimental methods. | Lacks functional correlation for some enzymes. |
| Dilokpimol (2016) [42] | Thirteen subfamilies of fungal FAEs based on phylogenetics and substrate specificity. | Divides fungal FAEs into subfamilies within CE1 based on biochemical data from new fungal genomes. | Error-prone in grouping bacterial and fungal FAEs together. |
| Enzyme | Organism | Oligomeric State | pH Opt. | Temp Opt. | Interesting Characteristics |
|---|---|---|---|---|---|
| AnFAEA | Aspergillus niger | Monomer | 6.0 * | 37 °C * | Tyr80 and Trp260 mutations to accommodate bigger substrates [46] (PDB 2BJH). |
| AoFAEB | Aspergillus oryzae | Dimer | 7.0 * | 37 °C * | C residues neighboring the catalytic S and H form a disulfide bond (motif found in tannases), which is crucial for activity [56] (PDB 3WMT). |
| XynZ | Clostridium thermocellum | Not mentioned | 6.0 | 60 °C | CBM containing FAEs that are active with ΔCBM truncation but inactive when unknown (non-catalytic domain) is omitted [55]. |
| StFaeC | Sporotrichum thermophile | Dimer | 6.0 | 55 °C | Type C FAE, active on all methyl hydroxycinnamic acids [57]. |
| PpFAE | Penicillium piceum | Not mentioned | 3.0 | 70 °C | FAE type C with broad pH activity range 2.0–8.0 [58]. |
| LhFAE | Lactarius hatsudake | Monomer | 4.0 | 30 °C | Metal ion-tolerant: retains 80% of activity in 5 mM metal ions; Mn2+ boosts activity to 114% [59]. |
| LbFAE | Lentilactobacillus buchneri | Dimer | 6.5 | 40 °C | Internally facing active site; inactive on dehydrodimers [45]. |
| LJ0536 | Lactobacillus johnsonii N6.2 | Dimer | - | - | Open canal catalytic site; solvent exposed [60]. |
| GthFAE | Geobacillus thermoglucosidasius | Dimer | 8.5 | 50 °C | Mutagenesis increased Tm, but activity decreased in variants [61]. |
| LaFae | Lactobacillus acidophilus | Dimer | 8.0 | 25–37 °C | Phe→Ala mutation near catalytic site increased activity; cap domain flexibility [62]. |
| AmCE1 | Anaeromyces mucronatus | Monomer | 7.2 * | 25 °C * | Structurally based loop domain “β-clamp” responsible for exolytic activity [63] (PDB 5CXU). |
| FAE-II | Fusarium oxysporum | Not mentioned | 7.0 | 45 °C | Synergistic interaction with xylanase; active in a ternary solvent system [64]. |
| AocFaeC | Aspergillus ochraceus | Monomer | 6.5 | 40 °C | Butanol-specific biocatalyst, with 5x higher butyl caffeate synthesis rate compared to type B FAE from A. niger [65]. |
| Fae125 | Talaromyces wortmannii | Not mentioned | 4.7/4.7 | 24.5 °C/38.9 °C | The optimal values of pH and temperature correspond to the synthesis of PFA and AFA, respectively [66] **. |
| XynII-Fae | Prevotella ruminicola 23 | Not mentioned | 7.0 | 40 °C | Bifunctional xylanase/feruloyl esterase, with each activity originating from a distinct domain [67]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leontakianakou, S.; Adlercreutz, P.; Nordberg Karlsson, E. Fantastic Ferulic Acid Esterases and Their Functions. Int. J. Mol. Sci. 2025, 26, 7474. https://doi.org/10.3390/ijms26157474
Leontakianakou S, Adlercreutz P, Nordberg Karlsson E. Fantastic Ferulic Acid Esterases and Their Functions. International Journal of Molecular Sciences. 2025; 26(15):7474. https://doi.org/10.3390/ijms26157474
Chicago/Turabian StyleLeontakianakou, Savvina, Patrick Adlercreutz, and Eva Nordberg Karlsson. 2025. "Fantastic Ferulic Acid Esterases and Their Functions" International Journal of Molecular Sciences 26, no. 15: 7474. https://doi.org/10.3390/ijms26157474
APA StyleLeontakianakou, S., Adlercreutz, P., & Nordberg Karlsson, E. (2025). Fantastic Ferulic Acid Esterases and Their Functions. International Journal of Molecular Sciences, 26(15), 7474. https://doi.org/10.3390/ijms26157474






