Metabolomic and Pharmacological Approaches for Exploring the Potential of Tanacetum parthenium L. Root Culture as a Source of Bioactive Phytochemicals
Abstract
1. Introduction
2. Results
2.1. Selection of Fractions
2.2. The Three Fractions Exhibited Pharmacological Activity
2.3. Phytochemical Characterization of the Three Fractions
2.3.1. Total Secondary Metabolite Contents Profile of Fractions
2.3.2. Differential Abundance of Compounds Between the Three Fractions from Untargeted Metabolomics
2.3.3. The Phytochemical and Pharmacological Activities and Compound Abundances of the Three Fractions Were Correlated
2.3.4. Putative Identification of Metabolites Correlated with Pharmacological Effects
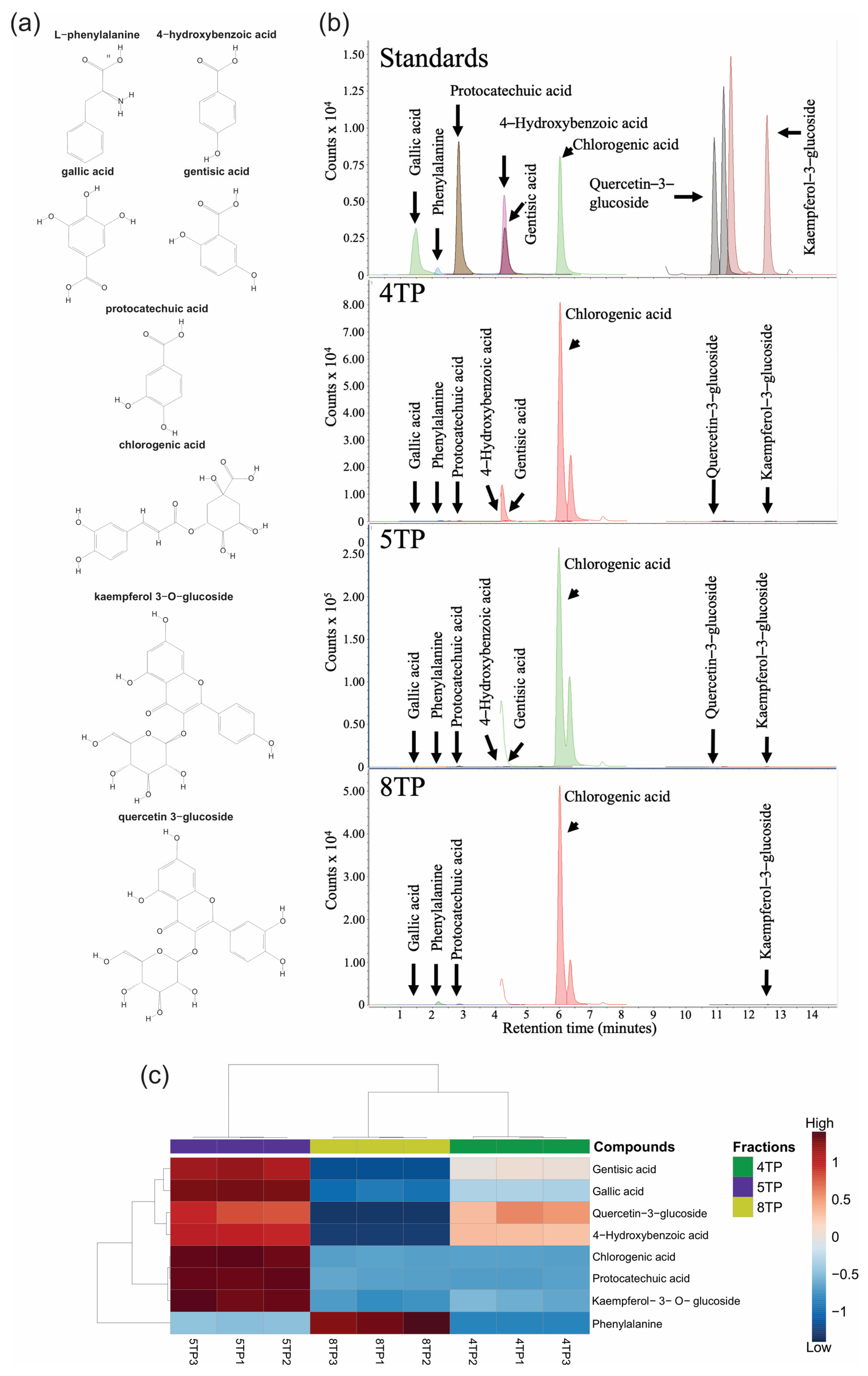
2.3.5. Targeted Metabolomics in Fractions
3. Discussion
3.1. Fractions Obtained from Tanacetum parthenium Root Culture Showed Increased Total Secondary Metabolite Contents Regarding Crude Extracts
3.2. Fractions Obtained from Tanacetum parthenium Root Culture Exhibited Enhanced Pharmacological Activity as Compared to Crude Extracts
3.3. Correlations Among Pharmacological Activities, Phytochemical Profiles, and Untargeted Metabolomics of Fractions Enabled the Identification of Metabolites
| Compound | Pharmacological Activity | Reference | |||
|---|---|---|---|---|---|
| Biological Effect | MIC | IC50 | % Inhibition | ||
| 4-hydroxybenzoic acid | Antibacterial against E. coli | 125 µg/mL | [59] | ||
| Antibacterial against S. aureus | 62.5 µg/mL | ||||
| α-Amylase inhibition | 3.552 mg/mL | [60] | |||
| Antioxidant | 321.72 µg/mL for DPPH | [59] | |||
| Cytotoxic | 20.8 µg/mL in MCF-7 | ||||
| Behenic acid | Cytotoxic | 7.52 µM in HepG2 | [61] | ||
| 11.86 µM in MCF-7 | |||||
| 12.28 µM in PC3 | |||||
| Antioxidant | ˃1 mg/mL for DPPH | [62] | |||
| Chlorogenic acid | Antibacterial against E. coli | 6 mg/mL | [63] | ||
| Antibacterial against S. aureus | 3 mg/mL | ||||
| α-Amylase inhibition | 9.1 µg/mL | [64] | |||
| Antioxidant | 51.23 µg/mL for DPPH | ||||
| Cytotoxic | 52% at 100 µM in 2OS | [65] | |||
| 27% at 100 µM in MG-63 | |||||
| Gallic acid | Antibacterial against E. coli | 1 µg/mL | [66] | ||
| Antibacterial against S. aureus | 1 µg/mL | ||||
| α-Amylase inhibition | 1.09 µg/mL | [67] | |||
| Antioxidant | 5.73 µM for DPPH | ||||
| Cytotoxic | 50 µM in SMMC-7721 | [68] | |||
| 80 µM in HL-60 | |||||
| 4 µM in k562 | |||||
| 40 µM in Wehi231 | |||||
| 80 µM in HeLa | |||||
| Gentisic acid | Antibacterial against E. coli | 4 mg/mL | [69] | ||
| Antibacterial against S. aureus | 4.15 mg/mL | ||||
| α-Amylase inhibition | 2.07 mg/mL | [70] | |||
| Antioxidant | 7.6 µM for DPPH | [71] | |||
| Cytotoxic | 14 mM in HepG2 | [69] | |||
| Isochlorogenic acid b | Antioxidant | EC50 9.4 µg/mL for DPPH | [72] | ||
| Kaempferol-3-O-glucoside | Antibacterial against E. coli | 1.25 µg/mL | [73] | ||
| Antibacterial against S. aureus | 0.625 µg/mL | ||||
| Antioxidant | 1.25 µg/mL for DPPH | ||||
| Lignoceric acid | Antibacterial against E. coli | ˃1 mg/mL | [62] | ||
| Antibacterial against S. aureus | ˃1 mg/mL | ||||
| Antioxidant | ˃1 mg/mL for DPPH | ||||
| Oleic acid | Antibacterial against E. coli | 0.512 mg/mL | |||
| Antibacterial against S. aureus | 0.256 mg/mL | ||||
| Antioxidant | 0.5 mg/mL for DPPH | ||||
| Protocatechuic acid | Antibacterial against E. coli | 2.5 mg/mL | [63] | ||
| Antibacterial against S. aureus | 0.45 mg/mL | [74] | |||
| α-Amylase inhibition | 1.12 µg/mL | [67] | |||
| Antioxidant | 8.29 µM for DPPH | ||||
| Cytotoxic | 55% at 8 µmol/L in MCF-7 | [75] | |||
| 60% at 8 µmol/L in A549 | |||||
| 45% at 8 µmol/L in HepG2 | |||||
| 42% at 8 µmol/L in HeLa | |||||
| 65% at 8 µmol/L in LNCaP | |||||
| Quercetin-3-glucoside | Antioxidant | 2.39 mM TEAC | [76] | ||
| Cytotoxic | 70.44% at 50 µg/mL in HeLa | [77] | |||
| Sodium diacetate | Antibacterial against E. coli | 0.31% (w/v) | [78] | ||
| Antibacterial against S. aureus | 0.31% (w/v) | ||||
| Variabilin | Cytotoxic | 87.74 µM in PC3 | [58] | ||
| 38.08 µM in MCF-7 | |||||
| ˃100 µM in HT-29 | |||||
3.4. Future Perspectives of the Findings
4. Materials and Methods
4.1. Root Biomass Production
4.2. Obtaining Fractions and Phytochemical Analysis
4.2.1. Methanolic Extraction from Root Biomass
4.2.2. Fractionation of Methanolic Extract
4.3. In Vitro Assays of Selected Fractions
4.3.1. Antioxidant Assay
4.3.2. Antibacterial Assay
4.3.3. Cytotoxicity Assay
4.3.4. α-Amylase Inhibitory Assay
4.4. Phytochemical Analysis of the Selected Fractions
Total Secondary Metabolite Contents
4.5. Metabolomics of Fractions
4.5.1. Untargeted Metabolomics
4.5.2. Targeted Metabolomics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4TP, 5TP or 8TP | Fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture |
| AJS | Agilent jet stream |
| ATCC | American Type Culture Collection |
| CFU | Colony-forming units |
| DMSO | Dimethyl sulfoxide |
| DNSA | Dinitrosalicylic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EC50 | Half maximal effective concentration |
| ESI | Electrospray ionization |
| FBS | Fetal bovine serum |
| IC50 | Half maximal inhibitory concentration |
| LC-MS | Liquid chromatography coupled with mass spectrometry |
| MeOH | Methanol |
| mg AE/gF | Milligrams of acarbose equivalents per gram of fraction |
| mg GAE/gF | Milligrams of gallic acid equivalents per gram of fraction |
| mg QE/gF | Milligrams of quercetin equivalents per gram of fraction |
| mg VBE/gF | Milligrams of verbascoside equivalents per gram of fraction |
| mg PTNE/gF | Milligrams of parthenolide equivalents per gram of fraction |
| MIC | Minimum inhibitory concentration |
| MS | Mass spectrometry |
| m/z | Mass charge signal |
| PBS | Phosphate buffer solution |
| PCA | Principal component analysis |
| PGR | Plant growth regulators |
| PLS-DA | Partial least squares discriminant analysis |
| PTN | Parthenolide |
| Q-TOF/MS | Quadrupole time-of-flight mass spectrometer |
| rt | Retention time in minutes |
| TFC | Total flavonoids content |
| TPAC | Total phenolic acid content |
| TPC | Total phenolic compound content |
| TSLC | Total sesquiterpene lactone content |
| UPLC | Ultrahigh resolution liquid chromatograph |
| VIP | Variable importance in projection |
| ZHI | Zone halo inhibition |
References
- Twaij, B.M.; Jazar, Z.H.; Hasan, M.N. Trends in the use of tissue culture, applications and future aspects. Int. J. Plant Biol. 2020, 11, 8385. [Google Scholar] [CrossRef]
- Abdulhafiz, F.; Mohammed, A.; Hanif, R.M.F.; Kari, Z.A.; Wei, L.S.; Goh, K.W. Plant cell culture technologies: A promising alternatives to produce high-value secondary metabolites. Arab. J. Chem. 2022, 15, 104161. [Google Scholar] [CrossRef]
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Supriyono; Suhara, C.; Kade, S.I.B.; Makarti, M.W.; Khurshid, W.A. Enhancing secondary metabolite production in plants: Exploring traditional and modern strategies. J. Agr. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Marchev, A.S.; Yordanova, Z.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef]
- Rohini, M.R.; Rajasekharan, P.E. Scale-up production of bioactive compounds using bioreactors. In Nutraceuticals Production from Plant Cell Factory; Belwal, T., Ed.; Springer: Singapore, 2022; pp. 69–81. [Google Scholar]
- Verdú-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. The advent of plant cells in bioreactors. Front. Plant Sci. 2023, 14, 1310405. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.L.; Laudy, A.E.; Majewska, E.; Tarnowska, K.; Małajowicz, J.; Ziarno, M. Antioxidant and antibacterial activity of extracts from selected plant material. Appl. Sci. 2022, 12, 9871. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural antioxidant compounds as potential pharmaceutical tools against neurodegenerative diseases. ACS omega 2022, 7, 25974–25990. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Bansal, Y.; Mujib, A.; Mamgain, J.; Syeed, R.; Mohsin, M.; Nafees, A.; Dewir, Y.H.; Mendler-Drienyovszki, N. Integrated GC-MS and UPLC-ESI-QTOF-MS based untargeted metabolomics analysis of in vitro raised tissues of Digitalis purpurea L. Front. Plant Sci. 2024, 15, 1433634. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants metabolome study: Emerging tools and techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Waris, M.; Kocak, E.; Gonulalan, E.M.; Demirezer, L.O.; Kır, S.; Nemutlu, E. Metabolomics analysis insight into medicinal plant science. TrAC Trends Anal. Chem. 2022, 157, 116795. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Othman, E.M.; Fahim, J.R.; Desoukey, S.Y.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Schirmeister, T.; Tawfike, A.; Abdelmohsen, U.R. Metabolomics analysis and biological investigation of three Malvaceae plants. Phytochem. Anal. 2020, 31, 204–214. [Google Scholar] [CrossRef]
- Shen, S.; Huang, J.; Li, T.; Wei, Y.; Xu, S.; Wang, Y.; Ning, J. Untargeted and targeted metabolomics reveals potential marker compounds of an tea during storage. LWT 2022, 154, 112791. [Google Scholar] [CrossRef]
- Maggi, F. Feverfew (Tanacetum parthenium L.) Sch. Bip.). In Nonvitamin and Nonmineral Nutritional Supplements; Mohammad, N.S., Sanchez, S.A., Eds.; Elsevier: Amsterdam, Netherlands, 2019; pp. 223–225. [Google Scholar]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef]
- Kashkooe, A.; Jalali, A.; Zarshenas, M.M.; Hamedi, A. Exploring the phytochemistry, signaling pathways, and mechanisms of action of Tanacetum parthenium (L.) Sch.Bip.: A comprehensive literature review. Biomedicines 2024, 12, 2297. [Google Scholar] [CrossRef]
- Binh, H.H.; Van Nghia, V. Chemical composition, antimicrobial, and anticancer activity of essential oil from Tanacetum parthenium (L.) Sch.Bip leaves cultivated in Dalat, Vietnam. Chem. Biodivers. 2025, e202403430. [Google Scholar] [CrossRef]
- Nithin, B.; Revathi, S.; Penabaka, V.; Chandra, P. Review on Tanacetum parthenium. Int. J. Indig. Herbs Drugs 2025, 10, 7–13. [Google Scholar] [CrossRef]
- Rabito, M.F.; Almeida, M.B.; Iglesias, A.H.; Paula, F.; Silva, B.P.; Cortez, D.A.; Nixdorf, S.L.; Ferreira, I.C.P. Development and validation of a method for simultaneous determination of bioactive compounds of Tanacetum parthenium (L.) Schultz-Bip. J. Braz. Chem. Soc. 2014, 10, 1824–1831. [Google Scholar] [CrossRef]
- Long, C.; Sauleau, P.; David, B.; Lavaud, C.; Cassabios, V.; Ausseil, F.; Massiot, G. Bioactive flavonoids of Tanacetum parthenium revisited. Phytochemistry 2003, 64, 567–569. [Google Scholar] [CrossRef]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020, 37, 441–565. [Google Scholar] [CrossRef]
- Izadi, Z.; Esna-Ashri, M.; Piri, K.; Davoodi, P. Chemical composition and antimicrobial activity of feverfew (Tanacetum parthenium) essential oil. Int. J. Agric. Biol. 2010, 12, 759–763. [Google Scholar]
- Izadi, Z.; Aghaalikhani, M.; Esna-Ashari, M.; Davoodi, P. Determining chemical composition and antimicrobial activity of feverfew (Tanacetum parthenium L.) essential oil on some microbial strains. Zahedan J. Res. Med. Sci. 2013, 15, 8–13. [Google Scholar]
- Shafaghat, A.; Ghorban-Dadras, O.; Mohammadhosseini, M.; Akhavan, M.; Shafaghatlonbar, M.; Panahi, A. A comparative study on chemical composition and antimicrobial activity of essential oils from Tanacetum parthenium (L.) Schultz. Bip. and Tanacetum punctatum (Desr.) Grierson. leaves from Iran. J. Essent. Oil-Bear. Plants 2017, 20, 1143–1150. [Google Scholar] [CrossRef]
- Ghavam, M. Tripleurospermum disciforme (C.A.Mey.) Sch.Bip., Tanacetum parthenium (L.) Sch.Bip, and Achillea biebersteinii Afan.: Efficiency, chemical profile, and biological properties of essential oil. Chem. Biol. Technol. Agric. 2021, 8, 45. [Google Scholar] [CrossRef]
- Banthorpe, D.V.; Brown, G.D. Growth and secondary metabolism in cell cultures of Tanacetum, Mentha and Anethum species in buffered media. Plant Sci. 1990, 67, 107–113. [Google Scholar] [CrossRef]
- Brown, G.D. Production of anti-malarial and anti-migraine drugs in tissue culture of Artemisia annua and Tanacetum parthenium. Acta Hortic. 1993, 330, 269–276. [Google Scholar] [CrossRef]
- Stojakowska, A.; Kisiel, W. Acetylenes in Agrobacterium rhizogenes transformed root cultures of Tanacetum parthenium. Pol. J. Chem. 1997, 71, 509–512. [Google Scholar]
- Sy, L.-K.; Brown, G.D. Coniferaldehyde derivatives from tissue culture of Artemisia annua and Tanacetum parthenium. Phytochemistry 1999, 50, 781–785. [Google Scholar] [CrossRef]
- Rateb, M.E.M.; El-Hawary, S.S.; El-Shamy, A.M.; Yousef, E.M.A. Production of parthenolide in organ and callus cultures of Tanacetum parthenium (L.). Afr. J. Biotechnol. 2007, 6, 1306–1316. [Google Scholar]
- Nieto-Trujillo, A.; Buendía-González, L.; García-Morales, C.; Román-Guerrero, A.; Cruz-Sosa, F.; Estrada-Zúñiga, M.E. Phenolic compounds and parthenolide production from in vitro cultures of Tanacetum parthenium. Rev. Mex. Ing. Quim. 2017, 16, 371–383. [Google Scholar]
- Pourianezhad, F.; Rahnama, H.; Mousavi, A.; Khosrowshahli, M.; Mafakheri, S. Effects of combined elicitors on parthenolide production and expression of parthenolide synthase (TpPTS) in Tanacetum parthenium hairy root culture. Plant Biotechnol. Rep. 2019, 13, 211–218. [Google Scholar] [CrossRef]
- Mahood, H.E.; Abbas, M.K.; Zahid, N.A. Micropropagation of feverfew (Tanacetum parthenium) and quantification of parthenolide content in its micropropagated and conventionally grown plants. Horticulturae 2022, 8, 50. [Google Scholar] [CrossRef]
- Saray, L.A.; Ahmadabadi, M.; Kamran, R.V. Efficient tissue culture and regeneration systems for the medicinal plant Tanacetum parthenium. Braz. Arch. Biol. Technol. 2022, 65, e22210264. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Rushing, J.W.; Rajapakse, N.C.; Thomas, R.L.; Riley, M.B. Phenolics and parthenolide levels in feverfew (Tanacetum parthenium) are inversely affected by environmental factors. J. Appl. Hort. 2008, 10, 36–39. [Google Scholar] [CrossRef]
- Majdi, M.; Charnikhova, T.; Bouwmeester, H. Genetical, developmental and spatial factors influencing parthenolide and its precursor costunolide in feverfew (Tanacetum parthenium L. Schulz Bip.). Ind. Crops Prod. 2013, 47, 270–276. [Google Scholar] [CrossRef]
- Hojati, M.; Modarres-Sanavy, S.A.M.; Tahmasebi, E.S.; Majdi, M.; Ghanati, F.; Farzadfar, S. Differential deployment of parthenolide and phenylpropanoids in feverfew plants subjected to divalent heavy metals and trans-cinnamic acid. Plant Soil 2016, 399, 41–59. [Google Scholar] [CrossRef]
- El-Shamy, A.M.; El-Hawary, S.S.; Rateb, M.E. Quantitative estimation of parthenolide in Tanacetum parthenium (L.) Schultz-Bip. cultivated in Egypt. J. AOAC Int. 2007, 90, 21–27. [Google Scholar] [CrossRef]
- Rezaei, F.; Jamei, R.; Heidari, R. Evaluation of the phytochemical and antioxidant potential of aerial parts of Iranian Tanacetum parthenium. Pharm. Sci. 2017, 23, 136–142. [Google Scholar] [CrossRef]
- Wu, C.; Chen, F.; Wang, X.; Kim, H.J.; He, G.Q.; Haley-Zitlin, V.; Huang, G. Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem. 2006, 96, 220–227. [Google Scholar] [CrossRef]
- Michalak, M.; Stryjecka, M.; Żarnowiec, P.; Zagórska-Dziok, M.; Kiełtyka-Dadasiewicz, A. Chemical composition of extracts from various parts of feverfew (Tanacetum parthenium L.) and their antioxidant, protective, and antimicrobial activities. Int. J. Mol. Sci. 2024, 25, 12179. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, H.-Y.; Quispe, Y.N.G.; Wang, Z.; Zuo, G.; Lim, S.S. Aldose reductase, protein glycation inhibitory and antioxidant of Peruvian medicinal plants: The case of Tanacetum parthenium L. and its constituents. Molecules 2019, 24, 2010. [Google Scholar] [CrossRef] [PubMed]
- Yur, S.; Tekin, M.; Göger, F.; Başer, K.H.C.; Özek, T.; Özek, G. Composition and potential of Tanacetum haussknechtii Bornm. Grierson as antioxidant and inhibitor of acetylcholinesterase, tyrosinase, and α-amylase enzymes. Int. J. Food Prop. 2017, 29, S2359–S2378. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Şenkardes, I.; Dogan, A.; Sinan, K.I.; Uysal, S.; Aumeeruddy-Elalfi, Z.; et al. Modern and traditional extraction techniques affect chemical composition and bioactivity of Tanacetum parthenium (L.) Sch. Bip. Ind. Crops Prod. 2020, 146, 112202. [Google Scholar] [CrossRef]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Tanacetum vulgare L. (Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef]
- Polatoğlu, K.; Demirci, F.; Demirci, B.; Gören, N.; Can, B.K.H. Antibacterial activity and the variation of Tanacetum parthenium (L.) Schultz Bip. essential oils from Turkey. J. Oleo Sci. 2010, 59, 177–184. [Google Scholar] [CrossRef]
- Ivănescu, B.; Tuchilus, C.; Corciova, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.M.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Yapici, I.; Altay, A.; Sarikaya, B.Ö.; Korkmaz, M.; Atila, A.; Gülçin, I.; Köksal, E. In vitro antioxidant and cytotoxic activities of extracts of endemic Tanacetum erzincanense together with phenolic content by LC-ESI-QTOF-MS. Chem. Biodivers. 2021, 18, e2000812. [Google Scholar] [CrossRef] [PubMed]
- Devrnja, N.; Anđelković, B.; Aranđelović, S.; Radulović, S.; Soković, M.; Krstić-Milošević, D.; Ristić, M.; Ćalić, D. Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. S. Afr. J. Bot. 2017, 111, 212–221. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Fu, M.; Yao, Q.; Zhuo, H.; Lu, Q.; Niu, X.; Zhang, P.; Pei, Y.; Zhang, K. Parthenolide induces apoptosis and lytic cytotoxicity in Epstein-Barr virus-positive Burkitt lymphoma. Mol. Med. Rep. 2012, 6, 477–482. [Google Scholar] [CrossRef]
- Kouhpaikar, H.; Sadeghian, M.H.; Rafatpanah, H.; Kazemi, M.; Iranshahi, M.; Delbari, Z.; Khodadadi, F.; Ayatollahi, H.; Rassouli, F.B. Synergy between parthenolide and arsenic trioxide in adult T-cell leukemia/lymphoma cell in vitro. Iran J. Basic Med. Sci. 2020, 23, 616–622. [Google Scholar]
- Chen, T.; Xiao, Z.; Liu, X.; Wang, T.; Wang, Y.; Ye, F.; Su, J.; Yao, X.; Xiong, L.; Yang, D.-H. Natural products for combating multidrug resistance in cancer. Pharmacol. Res. 2024, 202, 107099. [Google Scholar] [CrossRef]
- Lerata, M.S.; D’Souza, S.; Sibuyi, N.R.; Dube, A.; Meyer, M.; Samaai, T.; Beukes, D.R. Encapsulation of variabilin in stearic acid solid lipid nanoparticles enhances its anticancer activity in vitro. Molecules 2020, 25, 830. [Google Scholar] [CrossRef] [PubMed]
- Jansi, R.S.; Khusro, A.; Agastian, P.; Almutairi, M.H.; Almutairi, B.O. Bioassay-guided fractionation and biological activities of antimycin A and 4-hydroxybenzoic acid isolated from Nocardiopsis sp. strains LC-9. BioResources 2024, 19, 7673–7697. [Google Scholar] [CrossRef]
- Guan, L.; Long, H.; Ren, F.; Li, Y.; Zhang, H. A structure-activity relationship study of the inhibition of α-amylase by benzoic acid and its derivatives. Nutrients 2022, 14, 1931. [Google Scholar] [CrossRef]
- Samra, R.M.; Soliman, A.F.; Ashour, A.; Al-Karmalawy, A.A.; Hassan, M.A.; Zaghloul, A.M. Bioassay-guided isolation of a new cytotoxic ceramide from Cyperus rotundus L. S. Afr. J. Bot. 2021, 139, 210–216. [Google Scholar] [CrossRef]
- Kofi, A.; Stephen, G.; Francis, A. Antibacterial and radical scavenging activity of fatty acids from Paullinia pinnata L. Pharmacogn. Mag. 2009, 5, S1–S4. [Google Scholar]
- Chai, B.; Jiang, W.; Hu, M.; Wu, Y.; Si, H. In vitro synergistic interactions of protocatechuic acid and chlorogenic acid in combination with antibiotics against animal pathogens. Synergy 2019, 9, 100055. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic acid enhances doxorubicin-mediated cytotoxic effect in osteosarcoma cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pu, C.; Tang, W.; Wang, S.; Sun, Q. Gallic acid liposomes decorated with lactoferrin: Characterization, in vitro digestion and antibacterial activity. Food Chem. 2019, 293, 315–322. [Google Scholar] [CrossRef]
- Adeniyi, B.A.; Adetoye, A.; Ayeni, F.A. Antibacterial activities of lactic acid bacteria isolated from cow faeces against potential enteric pathogens. Afr. Health Sci. 2015, 15, 888–895. [Google Scholar] [CrossRef]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Cunha, L.B.; Lepore, E.D.; Medeiros, C.C.B.; Sorrechia, R.; Pietro, R.C.L.R.; Corrêa, M.A. Can gentisic acid serve as a high-performance antioxidant with lower toxicity for a promising new topical application? Life 2024, 14, 1022. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Al Kamaly, O.M.; Bousta, D. Insight into gentisic acid antidiabetic potential using in vitro and in silico approaches. Molecules 2021, 26, 1932. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, F.O.; Oyedeji, O.; Osundahunsi, M.T. Antimicrobial and antioxidant properties of kaemferol-3-O-glucoside and 1-(4-hydroxyphenyl)-3-phenylprpan-1-one isolated from the leaves of Annona muricate (Linn.). J. Pharm. Res. Int. 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Habibu, R.S.; Saidu, K.; Haliru, F.Z.; Ajiboye, H.O.; Aliyu, N.O.; Ibitoye, O.B.; Uwazie, J.N.; Muritala, H.F.; Bello, S.A.; et al. Involvement of oxidative stress in protocatechuic acid-mediated bacterial lethality. Microbiol. Open 2017, 6, e472. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Lin, C.C.; Wu, H.C.; Tsao, S.M.; Hsu, C.K. Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: Potential mechanism of action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, K.; Jung, S. Molecular dynamics simulations on the coplanarity of quercetin backbone for the antioxidant activity of quercetin-3-monoglycoside. Bull. Korean Chem. Soc. 2006, 27, 325–328. [Google Scholar] [CrossRef][Green Version]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.-W. Quercetin-3-glucoside extracted from apple pomace induces cell cycle arrest and apoptosis by increasing intracellular ROS levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef] [PubMed]
- Tangwatcharin, P.; Teemeesuk, W. Control of pathogenic bacteria in cooked duck blood curd using sodium diacetate and sodium chloride. Curr. Appl. Sci. Technol. 2019, 19, 306–312. [Google Scholar][Green Version]
- Muyumba, N.W.; Mutombo, S.C.; Sheridan, H.; Nachtergael, A.; Duez, P. Quality control of herbal drugs and preparations: The methods of analysis, their relevance and applications. Talanta Open 2021, 4, 100070. [Google Scholar] [CrossRef]
- Fasina, O.O.; Hallman, H.; Craig-Schmidt, M.; Clements, C. Predicting temperature-dependence viscosity of vegetable oils from fatty acid composition. J. Am. Oil Chem. Soc. 2006, 83, 899–903. [Google Scholar] [CrossRef]
- Zhou, L.G.; Wu, J.Y. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Nat. Prod. Rep. 2006, 23, 789–810. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Nieto-Trujillo, A.; Cruz-Sosa, F.; Luria-Pérez, R.; Gutiérrez-Rebolledo, G.A.; Román-Guerrero, A.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Estrada-Zúñiga, M.E. Arnica montana cell culture establishment, and assessment of its cytotoxic, antibacterial, α-amylase inhibitor, and antioxidant in vitro bioactivities. Plants 2021, 10, 2300. [Google Scholar] [CrossRef]
- Salapovic, H.; Geier, J.; Reznicek, G. Quantification of sesquiterpene lactones in Asteraceae plant extracts: Evaluation of their allergenic potential. Sci. Pharm. 2013, 81, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Morales-Merida, B.E.; Grimaldi-Olivas, J.C.; Cruz-Mendívil, A.; Villicaña, C.; Valdez-Torres, J.B.; Heredia, J.B.; León-Chan, R.G.; Lightbourn-Rojas, L.A.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; et al. Integrating proteomics and metabolomics approaches to elucidate the mechanism of responses to combined stress in the bell pepper (Capsicum annuum). Plants 2024, 13, 1861. [Google Scholar] [CrossRef] [PubMed]
- Monribot-Villanueva, J.L.; Altúzar-Molina, A.; Aluja, M.J.A.; Zamora-Briseño, J.; Elizalde-Contreras, J.M.; Bautista-Valle, M.V.; Arellano, J.; Sánchez-Martínez, D.E.; Rivera-Reséndiz, F.; Vázquez-Rosas-Landa, M.; et al. Integrating proteomics and metabolomics approaches to elucidate the ripening process in white Psidium guajava. Food Chem. 2022, 367, 130656. [Google Scholar] [CrossRef] [PubMed]
 4TP,
4TP,  5TP, and
5TP, and  8TP fractions obtained from the methanolic extract of the root biomass of a T. parthenium culture. (a) Antioxidant, inhibitory effect of (b) α-amylase, on (c) E. coli, (d) S. aureus, cytotoxic effect against the Ramos RA-1 lymphoma cell line (e) alone and (f) combined with 1 nM vincristine. The data are presented as the means ± SD. In (a,b), the letters indicate significant differences at the 5% level of probability; from (c) to (e), * indicates significant differences at the 5% level of significance compared to the negative control, and ** indicates the treatments showing the lowest values at the 5% level of significance compared to the negative control. The corresponding controls tested for those assays were as follows: (a) MeOH could not scavenge DPPH radical; (b) the positive control (acarbose) had an IC50 of 2.48 ± 0.31 μg/mL; (c,d) the negative control for both antibacterial assays was 3% DMSO, while the positive control for E. coli was 1 μg chloramphenicol/disk and the positive control for S. aureus was 1 μg vancomycin/disk; and (e) the negative control was the culture medium (which induced a cellular viability of 94.71% ± 1.5%), while 1 nM vincristine was used as a positive control (reducing cellular viability at 72.61% ± 1.45%).
8TP fractions obtained from the methanolic extract of the root biomass of a T. parthenium culture. (a) Antioxidant, inhibitory effect of (b) α-amylase, on (c) E. coli, (d) S. aureus, cytotoxic effect against the Ramos RA-1 lymphoma cell line (e) alone and (f) combined with 1 nM vincristine. The data are presented as the means ± SD. In (a,b), the letters indicate significant differences at the 5% level of probability; from (c) to (e), * indicates significant differences at the 5% level of significance compared to the negative control, and ** indicates the treatments showing the lowest values at the 5% level of significance compared to the negative control. The corresponding controls tested for those assays were as follows: (a) MeOH could not scavenge DPPH radical; (b) the positive control (acarbose) had an IC50 of 2.48 ± 0.31 μg/mL; (c,d) the negative control for both antibacterial assays was 3% DMSO, while the positive control for E. coli was 1 μg chloramphenicol/disk and the positive control for S. aureus was 1 μg vancomycin/disk; and (e) the negative control was the culture medium (which induced a cellular viability of 94.71% ± 1.5%), while 1 nM vincristine was used as a positive control (reducing cellular viability at 72.61% ± 1.45%).
 4TP,
4TP,  5TP, and
5TP, and  8TP fractions obtained from the methanolic extract of the root biomass of a T. parthenium culture. (a) Antioxidant, inhibitory effect of (b) α-amylase, on (c) E. coli, (d) S. aureus, cytotoxic effect against the Ramos RA-1 lymphoma cell line (e) alone and (f) combined with 1 nM vincristine. The data are presented as the means ± SD. In (a,b), the letters indicate significant differences at the 5% level of probability; from (c) to (e), * indicates significant differences at the 5% level of significance compared to the negative control, and ** indicates the treatments showing the lowest values at the 5% level of significance compared to the negative control. The corresponding controls tested for those assays were as follows: (a) MeOH could not scavenge DPPH radical; (b) the positive control (acarbose) had an IC50 of 2.48 ± 0.31 μg/mL; (c,d) the negative control for both antibacterial assays was 3% DMSO, while the positive control for E. coli was 1 μg chloramphenicol/disk and the positive control for S. aureus was 1 μg vancomycin/disk; and (e) the negative control was the culture medium (which induced a cellular viability of 94.71% ± 1.5%), while 1 nM vincristine was used as a positive control (reducing cellular viability at 72.61% ± 1.45%).
8TP fractions obtained from the methanolic extract of the root biomass of a T. parthenium culture. (a) Antioxidant, inhibitory effect of (b) α-amylase, on (c) E. coli, (d) S. aureus, cytotoxic effect against the Ramos RA-1 lymphoma cell line (e) alone and (f) combined with 1 nM vincristine. The data are presented as the means ± SD. In (a,b), the letters indicate significant differences at the 5% level of probability; from (c) to (e), * indicates significant differences at the 5% level of significance compared to the negative control, and ** indicates the treatments showing the lowest values at the 5% level of significance compared to the negative control. The corresponding controls tested for those assays were as follows: (a) MeOH could not scavenge DPPH radical; (b) the positive control (acarbose) had an IC50 of 2.48 ± 0.31 μg/mL; (c,d) the negative control for both antibacterial assays was 3% DMSO, while the positive control for E. coli was 1 μg chloramphenicol/disk and the positive control for S. aureus was 1 μg vancomycin/disk; and (e) the negative control was the culture medium (which induced a cellular viability of 94.71% ± 1.5%), while 1 nM vincristine was used as a positive control (reducing cellular viability at 72.61% ± 1.45%).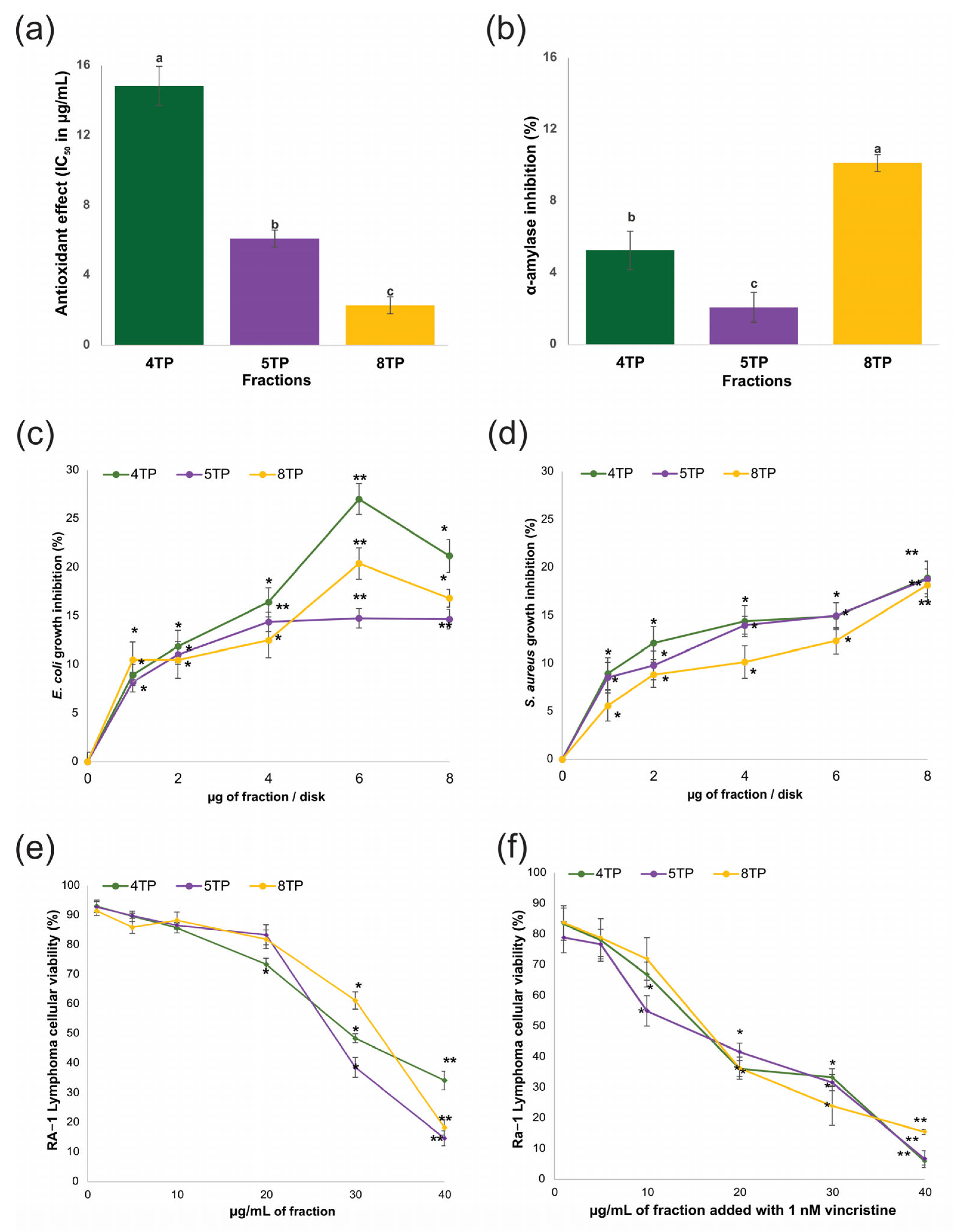
 4TP,
4TP,  5TP, and
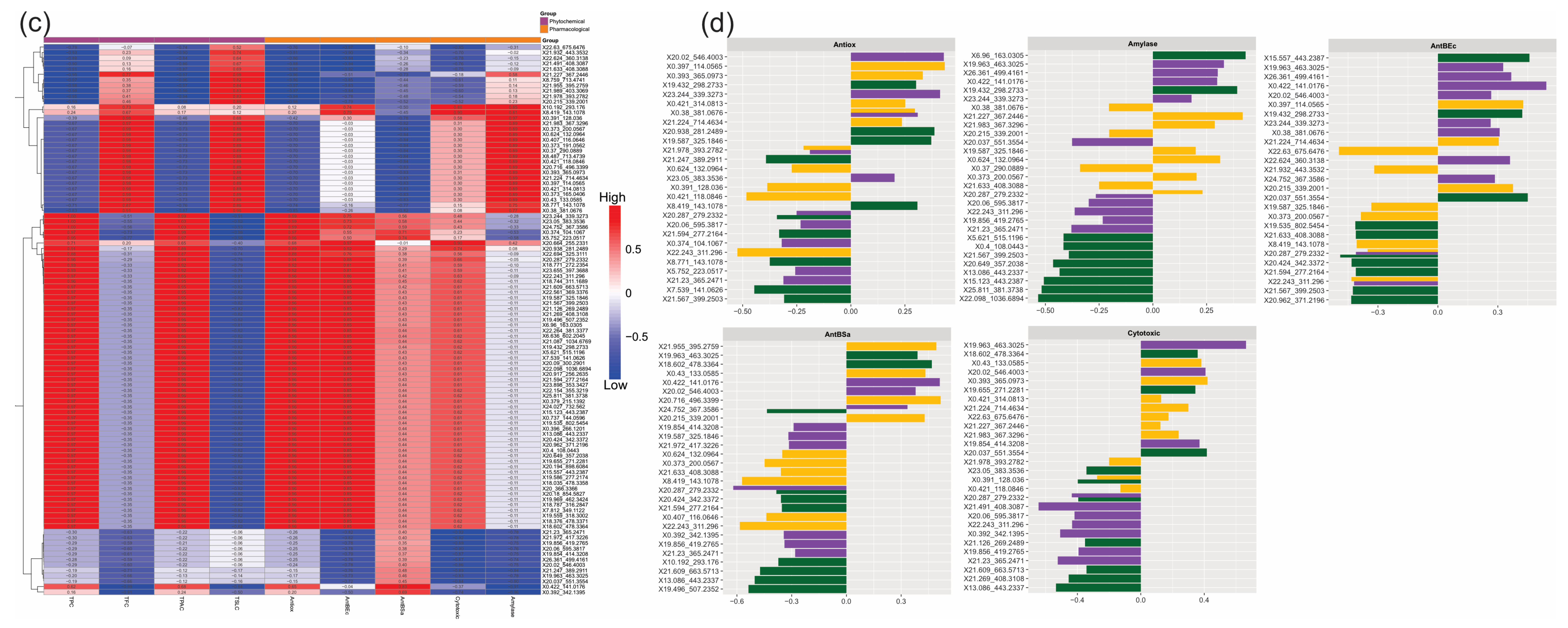
5TP, and  8TP fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture. (a) Total secondary metabolite contents: phenolic, phenolic acid, flavonoid, and sesquiterpene lactone (TPC, TPAC, TFC, and TSLC, respectively); LC-MS chromatogram obtained via electrospray ionization in (b) negative ionization mode and (c) positive ionization mode; (d) Venn diagram; (e) PCA; (f) PSL-DA; (g) VIP score of the top 20 compounds; and (h) heatmap of all identified compounds. In (a), data show the means ± SD; letters indicate significant differences at the 5% probability level, and the results for TPC, TPAC, TFC, and TSLC are expressed as milligrams of gallic acid, verbascoside, quercetin, or parthenolide equivalents per gram of fraction, respectively (mg GAE/gF, mg VBE/gF, mg QE/gF, or mg PTNE/gF). The Venn diagram in (d) was constructed online at http://www.interactivenn.net/, accessed on 20 August 2024. Data from (e) to (h) were analyzed for the rt_m/z signal and its abundance (peak intensity) obtained from LC-MS results.
8TP fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture. (a) Total secondary metabolite contents: phenolic, phenolic acid, flavonoid, and sesquiterpene lactone (TPC, TPAC, TFC, and TSLC, respectively); LC-MS chromatogram obtained via electrospray ionization in (b) negative ionization mode and (c) positive ionization mode; (d) Venn diagram; (e) PCA; (f) PSL-DA; (g) VIP score of the top 20 compounds; and (h) heatmap of all identified compounds. In (a), data show the means ± SD; letters indicate significant differences at the 5% probability level, and the results for TPC, TPAC, TFC, and TSLC are expressed as milligrams of gallic acid, verbascoside, quercetin, or parthenolide equivalents per gram of fraction, respectively (mg GAE/gF, mg VBE/gF, mg QE/gF, or mg PTNE/gF). The Venn diagram in (d) was constructed online at http://www.interactivenn.net/, accessed on 20 August 2024. Data from (e) to (h) were analyzed for the rt_m/z signal and its abundance (peak intensity) obtained from LC-MS results.
 4TP,
4TP,  5TP, and
5TP, and  8TP fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture. (a) Total secondary metabolite contents: phenolic, phenolic acid, flavonoid, and sesquiterpene lactone (TPC, TPAC, TFC, and TSLC, respectively); LC-MS chromatogram obtained via electrospray ionization in (b) negative ionization mode and (c) positive ionization mode; (d) Venn diagram; (e) PCA; (f) PSL-DA; (g) VIP score of the top 20 compounds; and (h) heatmap of all identified compounds. In (a), data show the means ± SD; letters indicate significant differences at the 5% probability level, and the results for TPC, TPAC, TFC, and TSLC are expressed as milligrams of gallic acid, verbascoside, quercetin, or parthenolide equivalents per gram of fraction, respectively (mg GAE/gF, mg VBE/gF, mg QE/gF, or mg PTNE/gF). The Venn diagram in (d) was constructed online at http://www.interactivenn.net/, accessed on 20 August 2024. Data from (e) to (h) were analyzed for the rt_m/z signal and its abundance (peak intensity) obtained from LC-MS results.
8TP fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture. (a) Total secondary metabolite contents: phenolic, phenolic acid, flavonoid, and sesquiterpene lactone (TPC, TPAC, TFC, and TSLC, respectively); LC-MS chromatogram obtained via electrospray ionization in (b) negative ionization mode and (c) positive ionization mode; (d) Venn diagram; (e) PCA; (f) PSL-DA; (g) VIP score of the top 20 compounds; and (h) heatmap of all identified compounds. In (a), data show the means ± SD; letters indicate significant differences at the 5% probability level, and the results for TPC, TPAC, TFC, and TSLC are expressed as milligrams of gallic acid, verbascoside, quercetin, or parthenolide equivalents per gram of fraction, respectively (mg GAE/gF, mg VBE/gF, mg QE/gF, or mg PTNE/gF). The Venn diagram in (d) was constructed online at http://www.interactivenn.net/, accessed on 20 August 2024. Data from (e) to (h) were analyzed for the rt_m/z signal and its abundance (peak intensity) obtained from LC-MS results.
 4TP,
4TP,  5TP, and
5TP, and  8TP). Values ranging from −1 to 1 represent the value of each correlation.
8TP). Values ranging from −1 to 1 represent the value of each correlation.
 4TP,
4TP,  5TP, and
5TP, and  8TP). Values ranging from −1 to 1 represent the value of each correlation.
8TP). Values ranging from −1 to 1 represent the value of each correlation.
 4TP,
4TP,  5TP, and
5TP, and  8TP fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture correlated with pharmacological effects, and those compounds were determined mainly by the VIP score. (a) Putatively identified compounds in the 4TP, 5TP, and 8TP fractions showing correlation values related to pharmacological effects; (b) putatively identified compounds showing the main values of the VIP score.
8TP fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture correlated with pharmacological effects, and those compounds were determined mainly by the VIP score. (a) Putatively identified compounds in the 4TP, 5TP, and 8TP fractions showing correlation values related to pharmacological effects; (b) putatively identified compounds showing the main values of the VIP score.
 4TP,
4TP,  5TP, and
5TP, and  8TP fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture correlated with pharmacological effects, and those compounds were determined mainly by the VIP score. (a) Putatively identified compounds in the 4TP, 5TP, and 8TP fractions showing correlation values related to pharmacological effects; (b) putatively identified compounds showing the main values of the VIP score.
8TP fractions obtained from the methanolic extract of the root biomass of the T. parthenium culture correlated with pharmacological effects, and those compounds were determined mainly by the VIP score. (a) Putatively identified compounds in the 4TP, 5TP, and 8TP fractions showing correlation values related to pharmacological effects; (b) putatively identified compounds showing the main values of the VIP score.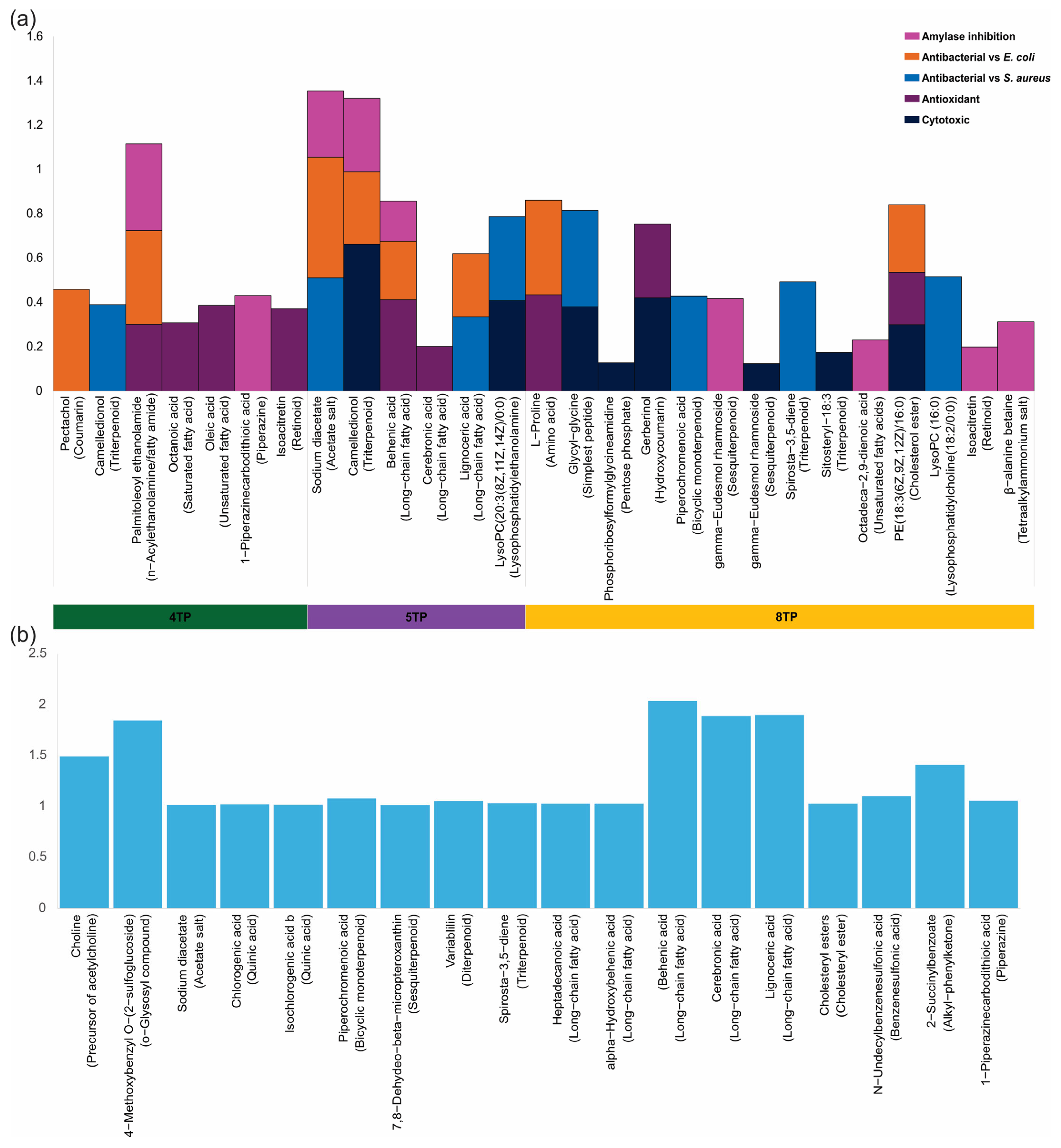
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto-Trujillo, A.; Luria-Pérez, R.; Cruz-Sosa, F.; Zepeda-Gómez, C.; González-Pedroza, M.G.; Burrola-Aguilar, C.; Sunny, A.; Correa-Basurto, J.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; et al. Metabolomic and Pharmacological Approaches for Exploring the Potential of Tanacetum parthenium L. Root Culture as a Source of Bioactive Phytochemicals. Int. J. Mol. Sci. 2025, 26, 7209. https://doi.org/10.3390/ijms26157209
Nieto-Trujillo A, Luria-Pérez R, Cruz-Sosa F, Zepeda-Gómez C, González-Pedroza MG, Burrola-Aguilar C, Sunny A, Correa-Basurto J, Guerrero-Analco JA, Monribot-Villanueva JL, et al. Metabolomic and Pharmacological Approaches for Exploring the Potential of Tanacetum parthenium L. Root Culture as a Source of Bioactive Phytochemicals. International Journal of Molecular Sciences. 2025; 26(15):7209. https://doi.org/10.3390/ijms26157209
Chicago/Turabian StyleNieto-Trujillo, Aurelio, Rosendo Luria-Pérez, Francisco Cruz-Sosa, Carmen Zepeda-Gómez, María G. González-Pedroza, Cristina Burrola-Aguilar, Armando Sunny, José Correa-Basurto, José A. Guerrero-Analco, Juan L. Monribot-Villanueva, and et al. 2025. "Metabolomic and Pharmacological Approaches for Exploring the Potential of Tanacetum parthenium L. Root Culture as a Source of Bioactive Phytochemicals" International Journal of Molecular Sciences 26, no. 15: 7209. https://doi.org/10.3390/ijms26157209
APA StyleNieto-Trujillo, A., Luria-Pérez, R., Cruz-Sosa, F., Zepeda-Gómez, C., González-Pedroza, M. G., Burrola-Aguilar, C., Sunny, A., Correa-Basurto, J., Guerrero-Analco, J. A., Monribot-Villanueva, J. L., & Estrada-Zúñiga, M. E. (2025). Metabolomic and Pharmacological Approaches for Exploring the Potential of Tanacetum parthenium L. Root Culture as a Source of Bioactive Phytochemicals. International Journal of Molecular Sciences, 26(15), 7209. https://doi.org/10.3390/ijms26157209










