Daidzein and Genistein: Natural Phytoestrogens with Potential Applications in Hormone Replacement Therapy
Abstract
1. Introduction
1.1. Hormone Replacement Therapy (HRT) for Menopausal Symptoms and Related Symptoms
1.2. Natural Product and Phytoestrogen: The Alternative Hormone Replacement Therapy
2. Results
2.1. Daidzein and Genistein: Chemical Structure, Chemical Information
2.2. Source of Daidzein and Genistein
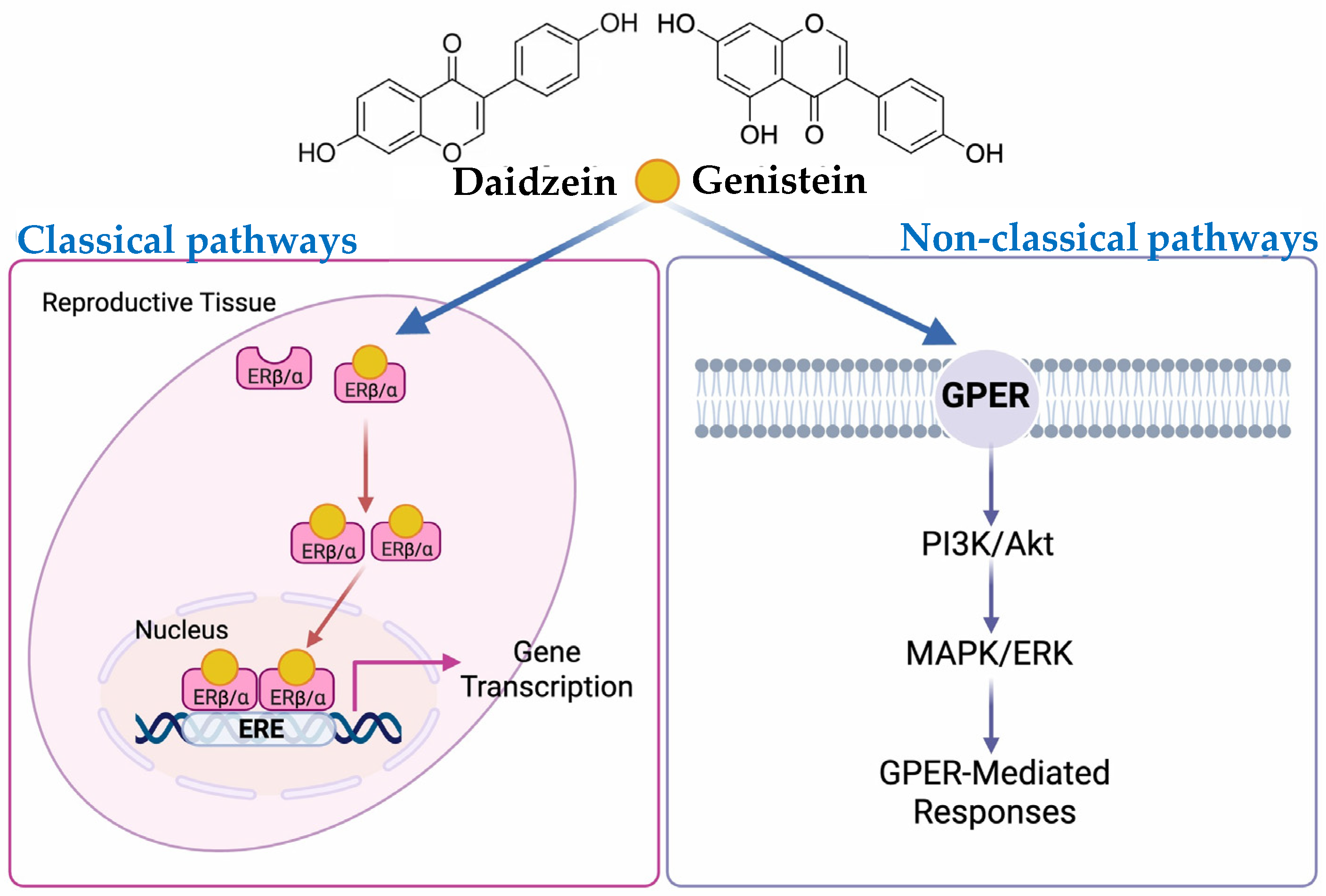
2.3. Bioactivities: Estrogenic Activity of Daidzein and Genistein
2.3.1. In Silico
2.3.2. In Vitro
2.3.3. In Vivo
2.3.4. Clinical Trials
2.4. Other Bioactivities of Daidzein and Genistein
2.5. Pharmacokinetic and Toxicity
3. Future Aspects
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 17β-HSD | 17β-Hydroxysteroid Dehydrogenase |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| ADME | Absorption, Distribution, Metabolism, and Excretion |
| AF-1/AF-2 | Activation Function-1/Activation Function-2 |
| AFP | Alpha-fetoprotein |
| Akt | Protein Kinase B |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AMPK | AMP-Activated Protein Kinase |
| Ang II | Angiotensin II |
| ANS | 8-anilino-1-naphthalenesulfonic acid |
| APP | Amyloid-β precursor protein |
| AREG | Amphiregulin |
| AST | Aspartate transaminase |
| AT1R | Angiotensin II Type 1 Receptor |
| BAD | Bcl-2-associated agonist of cell death |
| BDNF | Brain-Derived Neurotrophic Factor |
| CAT | Catalase |
| CCl4 | Carbon Tetrachloride |
| CD | Circular dichroism |
| CIRI | Cerebral ischemia/reperfusion injury |
| CNS | Central nervous system |
| CREB | cAMP Response Element-Binding Protein |
| CRP | c-reactive protein |
| CUMS | Chronic unpredictable mild stress |
| CXCL-12 | C-X-C Motif Chemokine Ligand 12 |
| CXCL1 | CXC Motif Chemokine Ligand 1 |
| CYP3A4 | Cytochrome P450 3A4 |
| DBD | DNA-Binding Domain |
| DENA | Diethylnitrosamine |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| DMS | Dimethylhydrazine dihydrochloride |
| DRIA | Daidzein-rich isoflavones aglycone |
| DSS | Dextran sodium sulfate |
| EGFR | Epidermal Growth Factor Receptor |
| EGR-1 | Early Growth Response Protein 1 |
| ER | Estrogen Receptor |
| ERK | Extracellular signal-regulated kinase |
| ERα | Estrogen Receptor Alpha |
| ERβ | Estrogen Receptor Beta |
| FAAH | Fatty acid amide hydrolase |
| FoxM1 | Forkhead Box M1 |
| GLUT1/GLUT4 | Glucose Transporter 1/4 |
| GnRH | Gonadotropin-Releasing Hormone |
| GPC3 | Glypican-3 |
| GPER | G-protein-coupled Estrogen Receptor |
| GPER | G protein-coupled estrogen receptor |
| GSH | Glutathione |
| GSK3 | Glycogen synthase kinase 3 |
| GSK3αβ | Glycogen Synthase Kinase 3 alpha/beta |
| GSSG | Glutathione disulfide |
| GST | Glutathione S-transferases |
| H2O2 | Hydrogen Peroxide |
| HCC | Hepatocellular carcinoma |
| HMGB1 | High mobility group box 1 |
| HRT | Hormone Replacement Therapy |
| I/R | Ischemia/reperfusion |
| IC50 | Half-maximal inhibitory concentration |
| IG | Intragastrically |
| IGD | Isoflavone Genistein and Daidzein combination |
| IL | Interleukin |
| IP | Intraperitoneally |
| LBD | Ligand-Binding Domain |
| LFPI | Lateral fluid percussion injury |
| LPO | lipid peroxidation |
| MAPK | Mitogen-Activated Protein Kinase |
| MAPK/ERK | Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase |
| MCAO | Middle cerebral artery occlusion |
| MKRN3 | Makorin Ring Finger Protein 3 |
| MMP-9 | Matrix Metalloproteinase 9 |
| MnSOD | Manganese Superoxide Dismutase |
| MPO | Myeloperoxidase |
| mTOR | Mechanistic Target of Rapamycin |
| NLRP3 | Nod-like receptor protein 3 |
| NO | Nitric oxide |
| NSCLC | Non-small cell lung cancer |
| OGD/R | Oxygen-glucose deprivation/reoxygenation |
| OPG | Osteoprotegerin |
| OVX | Ovariectomy or Oophorectomy |
| PDB | Protein Data Bank |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| PGC-1α | Peroxisome proliferator-activated receptor-gamma coactivator 1α |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol 3-Kinase |
| PKCγ | Protein Kinase C Gamma |
| PO | Per oral |
| PPAR-γ | Peroxisome proliferator-activated receptor-γ |
| PTZ | Pentylenetetrazole |
| RANKL | Receptor activator of nuclear factor κB ligand |
| RAS | Renin–Angiotensin System |
| RCT | Randomized Controlled Trial |
| ROS | Reactive Oxygen Species |
| Runx-2 | Runt-related transcription factor 2 |
| SC | Subcutaneously |
| SERM | Selective Estrogen Receptor Modulator |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| SRC3 | Steroid Receptor Coactivator 3 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SULTs | Sulfotransferases |
| T2DM | Type 2 diabetes mellitus |
| TACE | Tumor necrosis factor-α converting enzyme |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TBI | Traumatic brain injury |
| TEM | Transmission electron microscopy |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TOP | Topical administration |
| Trpv6 | Transient Receptor Potential Vanilloid 6 |
| TXA2 | Thromboxane A2 |
| UGTs | UDP-Glucuronosyltransferases |
| VEGF | Vascular endothelial growth factor |
| VMS | Vasomotor Symptoms |
| YES assay | Yeast Estrogen Screen Assay |
References
- Peacock, K.; Carlson, K.; Ketvertis, K.M.; Doerr, C. Menopause (Nursing). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kaunitz, A.M.; Manson, J.E. Management of menopausal symptoms. Obstet. Gynecol. 2015, 126, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Stuenkel, C.A.; Davis, S.R.; Gompel, A.; Lumsden, M.A.; Murad, M.H.; Pinkerton, J.V.; Santen, R.J. Treatment of symptoms of the menopause: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 3975–4011. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological management of osteoporosis in postmenopausal women: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef] [PubMed]
- Gregson, C.L.; Armstrong, D.J.; Bowden, J.; Cooper, C.; Edwards, J.; Gittoes, N.J.; Harvey, N.; Kanis, J.; Leyland, S.; Low, R. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2022, 17, 58. [Google Scholar] [CrossRef]
- Faubion, S.S.; Crandall, C.J.; Davis, L.; El Khoudary, S.R.; Hodis, H.N.; Lobo, R.A.; Maki, P.M.; Manson, J.E.; Pinkerton, J.V.; Santoro, N.F. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause 2022, 29, 767–794. [Google Scholar] [CrossRef]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Caughey, A.B.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Jaén, C.R. Hormone therapy for the primary prevention of chronic conditions in postmenopausal persons: US Preventive Services Task Force recommendation statement. Jama 2022, 328, 1740–1746. [Google Scholar]
- National Institute for health and care excellence (NICE). NICE Guideline [NG23]: Menopause: Identification and Management 2024; National Institute for Health and Care Excellence: London, UK, 2024. [Google Scholar]
- Mauvais-Jarvis, F.; Manson, J.E.; Stevenson, J.C.; Fonseca, V.A. Menopausal hormone therapy and type 2 diabetes prevention: Evidence, mechanisms, and clinical implications. Endocr. Rev. 2017, 38, 173–188. [Google Scholar] [CrossRef]
- Kim, Y.J.; Soto, M.; Branigan, G.L.; Rodgers, K.; Brinton, R.D. Association between menopausal hormone therapy and risk of neurodegenerative diseases: Implications for precision hormone therapy. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12174. [Google Scholar] [CrossRef]
- Vigneswaran, K.; Hamoda, H. Hormone replacement therapy–Current recommendations. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 8–21. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Walia, R.; Saxena, S.; Madaan, S.; Roy, S. Complementary and nature based treatment for menopausal women- a better approach. Nat. Volatiles Essent. Oils 2021, 8, 16324–16352. [Google Scholar]
- Patra, S.; Gorai, S.; Pal, S.; Ghosh, K.; Pradhan, S.; Chakrabarti, S. A review on phytoestrogens: Current status and future direction. Phytother. Res. 2023, 37, 3097–3120. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, X.; Li, X.; Wells, A.; Luo, Y.; Neapolitan, R. Conjugated equine estrogen and medroxyprogesterone acetate are associated with decreased risk of breast cancer relative to bioidentical hormone therapy and controls. PLoS ONE 2018, 13, e0197064. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.H.; Chowdhury, R.; Troup, J.; Voortman, T.; Kunutsor, S.; Kavousi, M.; Oliver-Williams, C.; Muka, T. Use of plant-based therapies and menopausal symptoms: A systematic review and meta-analysis. Jama 2016, 315, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Kuo, H.; Chang, S.; Wu, T.; Huang, K. The assessment of efficacy of Diascorea alata for menopausal symptom treatment in Taiwanese women. Climacteric 2011, 14, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D. Insight into the potential mechanisms of endocrine disruption by Dietary Phytoestrogens in the context of the etiopathogenesis of endometriosis. Int. J. Mol. Sci. 2023, 24, 12195. [Google Scholar] [CrossRef]
- Fan, Y.; Qian, H.; Wu, Z.; Li, Z.; Li, X.; Zhang, Y.; Xu, Q.; Lu, C.; Wang, X. Exploratory analysis of the associations between urinary phytoestrogens and thyroid hormones among adolescents and adults in the United States: National Health and Nutrition Examination Survey 2007–2010. Environ. Sci. Pollut. Res. 2022, 29, 2974–2984. [Google Scholar] [CrossRef]
- Russell, A.L.; Grimes, J.M.; Larco, D.O.; Cruthirds, D.F.; Westerfield, J.; Wooten, L.; Keil, M.; Weiser, M.J.; Landauer, M.R.; Handa, R.J. The interaction of dietary isoflavones and estradiol replacement on behavior and brain-derived neurotrophic factor in the ovariectomized rat. Neurosci. Lett. 2017, 640, 53–59. [Google Scholar] [CrossRef]
- Goyal, A.; Verma, A.; Agrawal, N. Dietary phytoestrogens: Neuroprotective role in Parkinson’s disease. Curr. Neurovascular Res. 2021, 18, 254–267. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Pisani, S.L.; Neese, S.L.; Doerge, D.R.; Helferich, W.G.; Schantz, S.L.; Korol, D.L. Acute genistein treatment mimics the effects of estradiol by enhancing place learning and impairing response learning in young adult female rats. Horm. Behav. 2012, 62, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Boué, S.M.; Tilghman, S.L.; Elliott, S.; Zimmerman, M.C.; Williams, K.; Payton-Stewart, F.; Miraflor, A.P.; Howell, M.H.; Shih, B.Y.; Carter-Wientjes, C.H. Identification of the potent phytoestrogen glycinol in elicited soybean (Glycine max). Endocrinology 2009, 150, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, H.; Grange, R.W. Genistein activates the 3′, 5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology 2005, 146, 1312–1320. [Google Scholar] [CrossRef]
- Siow, R.C.; Mann, G.E. Dietary isoflavones and vascular protection: Activation of cellular antioxidant defenses by SERMs or hormesis? Mol. Asp. Med. 2010, 31, 468–477. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef]
- Tjeerdsma, A.M.; van Hunsel, F.P.; van de Koppel, S.; Ekhart, C.; Vitalone, A.; Woerdenbag, H.J. Analysis of Safety Concerns on Herbal Products with Assumed Phytoestrogenic Activity. Pharmaceuticals 2023, 16, 1137. [Google Scholar] [CrossRef]
- Konar, N.; Poyrazoğlu, E.S.; Demir, K.; Artik, N. Determination of conjugated and free isoflavones in some legumes by LC–MS/MS. J. Food Compos. Anal. 2012, 25, 173–178. [Google Scholar] [CrossRef]
- Bensaada, S.; Raymond, I.; Pellegrin, I.; Viallard, J.-F.; Bennetau-Pelissero, C. Validation of ELISAs for isoflavones and enterolactone for phytoestrogen intake assessment in the French population. Nutrients 2023, 15, 967. [Google Scholar] [CrossRef]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef]
- Charoensup, W.; Intharuksa, A.; Yanaso, S.; Khamnuan, S.; Chansakaow, S.; Sirisa-ard, P.; Jantrawut, P.; Ditchaiwong, C.; Chaemcheun, K. Botanical Biometrics: Exploring Morphological, Palynological, and DNA Barcoding Variations in White Kwao Krua (Pueraria candollei Grah. ex Benth. and P. mirifica Airy Shaw & Suvat.). Horticulturae 2024, 10, 162. [Google Scholar] [CrossRef]
- Intharuksa, A.; Kitamura, M.; Peerakam, N.; Charoensup, W.; Ando, H.; Sasaki, Y.; Sirisa-Ard, P. Evaluation of white Kwao Krua (Pueraria candollei Grah. ex Benth.) products sold in Thailand by molecular, chemical, and microscopic analyses. J. Nat. Med. 2020, 74, 106–118. [Google Scholar] [CrossRef]
- Suntichaikamolkul, N.; Tantisuwanichkul, K.; Prombutara, P.; Kobtrakul, K.; Zumsteg, J.; Wannachart, S.; Schaller, H.; Yamazaki, M.; Saito, K.; De-Eknamkul, W. Transcriptome analysis of Pueraria candollei var. mirifica for gene discovery in the biosyntheses of isoflavones and miroestrol. BMC Plant Biol. 2019, 19, 581. [Google Scholar] [CrossRef]
- Pohjanvirta, R.; Nasri, A. The potent phytoestrogen 8-prenylnaringenin: A friend or a foe? Int. J. Mol. Sci. 2022, 23, 3168. [Google Scholar] [CrossRef]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic perspectives of 8-prenylnaringenin, a potent phytoestrogen from hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef]
- Zierau, O.; Gester, S.; Schwab, P.; Metz, P.; Kolba, S.; Wulf, M.; Vollmer, G. Estrogenic activity of the phytoestrogens naringenin, 6-(1,1-dimethylallyl) naringenin and 8-prenylnaringenin. Planta Medica 2002, 68, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; De Bruyne, T.; Apers, S.; Vanden Berghe, D.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent developments. Planta Medica 2003, 69, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahsan, F.; Ansari, J.A.; Mahmood, T.; Shamim, A.; Bano, S.; Tiwari, R.; Ansari, V.A.; Shafiurrahman; Kesari, M. A review on daidzein as food supplement: Exploring its phytopharmacological and preclinical status. eFood 2024, 5, e70008. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxidative Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant polyphenols: Structure, occurrence and bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar]
- Xu, Z.; Wu, Q.; Godber, J.S. Stabilities of daidzin, glycitin, genistin, and generation of derivatives during heating. J. Agric. Food Chem. 2002, 50, 7402–7406. [Google Scholar] [CrossRef] [PubMed]
- Coward, L.; Smith, M.; Kirk, M.; Barnes, S. Chemical modification of isoflavones in soyfoods during cooking and processing. Am. J. Clin. Nutr. 1998, 68, 1486S–1491S. [Google Scholar] [CrossRef] [PubMed]
- Miksicek, R.J. Estrogenic flavonoids: Structural requirements for biological activity. Proc. Soc. Exp. Biol. Med. 1995, 208, 44–50. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Martin, P.M.; Horwitz, K.B.; Ryan, D.S.; Mcguire, W.L. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology 1978, 103, 1860–1867. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Manas, E.S.; Xu, Z.B.; Unwalla, R.J.; Somers, W.S. Understanding the selectivity of genistein for human estrogen receptor-β using X-ray crystallography and computational methods. Structure 2004, 12, 2197–2207. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.-A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Rüfer, C.E.; Bub, A.; Möseneder, J.; Winterhalter, P.; Stürtz, M.; Kulling, S.E. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: A randomized, double-blind, crossover study. Am. J. Clin. Nutr. 2008, 87, 1314–1323. [Google Scholar] [CrossRef]
- Singla, N.; Gupta, G.; Kulshrestha, R.; Sharma, K.; Bhat, A.A.; Mishra, R.; Patel, N.; Thapa, R.; Ali, H.; Mishra, A. Daidzein in traditional Chinese medicine: A deep dive into its Ethnomedicinal and therapeutic applications. Pharmacol. Res.-Mod. Chin. Med. 2024, 12, 100460. [Google Scholar] [CrossRef]
- Hasanah, Y.; Nisa, T.C.; Armidin, H.; Hanum, H. Isoflavone content of soybean [Glycine max (L). Merr.] cultivars with different nitrogen sources and growing season under dry land conditions. J. Agric. Environ. Int. Dev. (JAEID) 2015, 109, 5–17. [Google Scholar]
- Qiu, M.; Tian, M.; Sun, Y.; Li, H.; Huang, W.; Ouyang, H.; Lin, S.; Zhang, C.; Wang, M.; Wang, Y. Decoding the biochemical dialogue: Metabolomic insights into soybean defense strategies against diverse pathogens. Sci. China Life Sci. 2024, 67, 2234–2250. [Google Scholar] [CrossRef]
- Modolo, L.V.; Cunha, F.Q.; Braga, M.R.; Salgado, I. Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol. 2002, 130, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic engineering of isoflavones: An updated overview. Front. Plant Sci. 2021, 12, 670103. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.J.; López-Lara, I.M.; Geiger, O.; Romero-Puertas, M.C.; Van Dillewijn, P. Rhizobial volatiles: Potential new players in the complex interkingdom signaling with legumes. Front. Plant Sci. 2021, 12, 698912. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; Jung, W.; Shi, J.; Croes, R.A.; Fader, G.M.; McGonigle, B.; Odell, J.T. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiol. 2000, 124, 781–794. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0; US Department of Agriculture: Annapolis, MD, USA, 2008; Volume 15.
- Yue, X.; Abdallah, A.M.; Xu, Z. Distribution of isoflavones and antioxidant activities of soybean cotyledon, coat and germ. J. Food Process. Preserv. 2010, 34, 795–806. [Google Scholar] [CrossRef]
- Shao, S.; Duncan, A.M.; Yang, R.; Marcone, M.F.; Rajcan, I.; Tsao, R. Tracking isoflavones: From soybean to soy flour, soy protein isolates to functional soy bread. J. Funct. Foods 2009, 1, 119–127. [Google Scholar] [CrossRef]
- Letizia, F.; Fusco, G.M.; Fratianni, A.; Gaeta, I.; Carillo, P.; Messia, M.C.; Iorizzo, M. Application of Lactiplantibacillus plantarum LP95 as a Functional Starter Culture in Fermented Tofu Production. Processes 2024, 12, 1093. [Google Scholar] [CrossRef]
- Kanno, R.; Koshizuka, T.; Miyazaki, N.; Kobayashi, T.; Ishioka, K.; Ozaki, C.; Chiba, H.; Suzutani, T. Protection of fatty liver by the intake of fermented soybean paste, Miso, and its pre-fermented mixture. Foods 2021, 10, 291. [Google Scholar] [CrossRef]
- Xiang, A.; Wang, J.; Xie, B.; Hu, K.; Chen, M.; Sun, Z. Determination of 14 isoflavone isomers in natto by UPLC-ESI-MS/MS and antioxidation and antiglycation profiles. Foods 2022, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ramasamy, K.; Majeed, A.B.A.; Mani, V. Enhancement of β-secretase inhibition and antioxidant activities of tempeh, a fermented soybean cake through enrichment of bioactive aglycones. Pharm. Biol. 2015, 53, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Saviranta, N.M.; Anttonen, M.J.; von Wright, A.; Karjalainen, R.O. Red clover (Trifolium pratense L.) isoflavones: Determination of concentrations by plant stage, flower colour, plant part and cultivar. J. Sci. Food Agric. 2008, 88, 125–132. [Google Scholar] [CrossRef]
- Wang, S.W.; Chen, Y.; Joseph, T.; Hu, M. Variable isoflavone content of red clover products affects intestinal disposition of biochanin A, formononetin, genistein, and daidzein. J. Altern. Complement. Med. 2008, 14, 287–297. [Google Scholar] [CrossRef]
- Andres, S.; Hansen, U.; Niemann, B.; Palavinskas, R.; Lampen, A. Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food Funct. 2015, 6, 2017–2025. [Google Scholar] [CrossRef]
- Butkutė, B.; Lemežienė, N.; Dabkevičienė, G.; Jakštas, V.; Vilčinskas, E.; Janulis, V. Source of variation of isoflavone concentrations in perennial clover species. Pharmacogn. Mag. 2014, 10, S181. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.; Runswick, S.; Atkinson, C.; Coward, W.; Bingham, S. Daidzein and genistein contents of vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000, 11, 326–331. [Google Scholar] [CrossRef]
- Lutz, M.; Martínez, A.; Martínez, E.A. Daidzein and Genistein contents in seeds of quinoa (Chenopodium quinoa Willd.) from local ecotypes grown in arid Chile. Ind. Crops Prod. 2013, 49, 117–121. [Google Scholar] [CrossRef]
- Liggins, J.; Mulligan, A.; Runswick, S.; Bingham, S. Daidzein and genistein content of cereals. Eur. J. Clin. Nutr. 2002, 56, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of cereals and cereal-based foods consumed in the UK. Nutr. Cancer 2009, 61, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Tesoriere, L.; Butera, D.; Fazzari, M.; Monastero, M.; Allegra, M.; Livrea, M.A. Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. J. Agric. Food Chem. 2007, 55, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Kaufman, P.B.; Warber, S.; Bolling, S.; Chang, S.C.; Duke, J.A. Quantification of major isoflavonoids and L-canavanine in several organs of kudzu vine (Pueraria montana) and in starch samples derived from kudzu roots. Plant Sci. 2003, 164, 883–888. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Intharuksa, A.; Sasaki, Y. A Promising View of Kudzu Plant, Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep: Flavonoid phytochemical compounds, taxonomic data, traditional uses and potential biological activities for future cosmetic application. Cosmetics 2020, 7, 12. [Google Scholar] [CrossRef]
- Jungsukcharoen, J.; Dhiani, B.A.; Cherdshewasart, W.; Vinayavekhin, N.; Sangvanich, P.; Boonchird, C. Pueraria mirifica leaves, an alternative potential isoflavonoid source. Biosci. Biotechnol. Biochem. 2014, 78, 917–926. [Google Scholar] [CrossRef]
- Peerakam, N.; Sirisa-Ard, P.; Huy, N.Q.; On, T.; Long, P.T.; Intharuksa, A. Isoflavonoids and phytoestrogens from Pueraria candollei var. mirifica related with appropriate ratios of ethanol extraction. Asian J. Chem. 2018, 30, 2086–2090. [Google Scholar] [CrossRef]
- Eumkeb, G.; Tanphonkrang, S.; Sirichaiwetchakoon, K.; Hengpratom, T.; Naknarong, W. The synergy effect of daidzein and genistein isolated from Butea superba Roxb. on the reproductive system of male mice. Nat. Prod. Res. 2017, 31, 672–675. [Google Scholar] [CrossRef]
- Faltas, C.L.; LeBron, K.A.; Holz, M.K. Unconventional Estrogen Signaling in Health and Disease. Endocrinology 2020, 161, bqaa030. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-b.; Bird, I.M.; Zheng, J.; Magness, R.R. Membrane Estrogen Receptor-Dependent Extracellular Signal-Regulated Kinase Pathway Mediates Acute Activation of Endothelial Nitric Oxide Synthase by Estrogen in Uterine Artery Endothelial Cells. Endocrinology 2004, 145, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Gronemeyer, H.; Gustafsson, J.-Å.; Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen–estrogen receptor signaling. Reprod. Med. Biol. 2017, 16, 4–20. [Google Scholar] [CrossRef]
- Cowley, S.M.; Parker, M.G. A comparison of transcriptional activation by ERα and ERβ. J. Steroid Biochem. Mol. Biol. 1999, 69, 165–175. [Google Scholar] [CrossRef]
- Pike, A.C.W.; Brzozowski, A.M.; Hubbard, R.E.; Bonn, T.; Thorsell, A.G.; Engström, O.; Ljunggren, J.; Gustafsson, J.Å.; Carlquist, M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999, 18, 4608–4618. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Pike, A.C.W.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engström, O.; Öhman, L.; Greene, G.L.; Gustafsson, J.-Å.; Carlquist, M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef]
- Satpathy, S.; Patra, A.; Hussain, M.D.; Kazi, M.; Aldughaim, M.S.; Ahirwar, B. A fraction of Pueraria tuberosa extract, rich in antioxidant compounds, alleviates ovariectomized-induced osteoporosis in rats and inhibits growth of breast and ovarian cancer cells. PLoS ONE 2021, 16, e0240068. [Google Scholar] [CrossRef]
- Dhananjaya, K.; Sibi, G.; Mallesha, H.; Ravikumar, K.R.; Awasthi, S. Insilico studies of daidzein and genistein with human estrogen receptor α. Asian Pac. J. Trop. Biomed. 2012, 2, S1747–S1753. [Google Scholar] [CrossRef]
- Ye, H.; Shaw, I.C. Dietary isoflavone-induced, estrogen receptor-β-mediated proliferation of Caco-2 cells is modulated by gallic acid. Food Chem. Toxicol. 2020, 145, 111743. [Google Scholar] [CrossRef]
- Kumar, V.; Chauhan, S.S. Daidzein Induces Intrinsic Pathway of Apoptosis along with ER α/β Ratio Alteration and ROS Production. Asian Pac. J. Cancer Prev. 2021, 22, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef] [PubMed]
- Zand, R.S.R.; Jenkins, D.J.A.; Diamandis, E.P. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res. Treat. 2000, 62, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective Estrogen Receptor Modulators and Phytoestrogens. Planta Medica 2008, 74, 1656–1665. [Google Scholar] [CrossRef]
- Marik, R.; Madhan, A.; Ravi, A.; Vered, S.; Christopher, B.U.; Khan, S. Potent genistein derivatives as inhibitors of estrogen receptor alpha-positive breast cancer. Cancer Biol. Ther. 2011, 11, 883–892. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, Z.; Brinton, R.D. A Select Combination of Clinically Relevant Phytoestrogens Enhances Estrogen Receptor β-Binding Selectivity and Neuroprotective Activities in Vitro and in Vivo. Endocrinology 2009, 150, 770–783. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, P.; Madak-Erdogan, Z.; Martin, T.; Jeyakumar, M.; Carlson, K.; Khan, I.; Smillie, T.J.; Chittiboyina, A.G.; Rotte, S.C.; et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013, 27, 4406–4418. [Google Scholar] [CrossRef]
- Uifălean, A.; Schneider, S.; Gierok, P.; Ionescu, C.; Iuga, C.A.; Lalk, M. The Impact of Soy Isoflavones on MCF-7 and MDA-MB-231 Breast Cancer Cells Using a Global Metabolomic Approach. Int. J. Mol. Sci. 2016, 17, 1443. [Google Scholar] [CrossRef]
- Mesía-Vela, S.; Kauffman, F.C. Inhibition of rat liver sulfotransferases SULT1A1 and SULT2A1 and glucuronosyltransferase by dietary flavonoids. Xenobiotica 2003, 33, 1211–1220. [Google Scholar] [CrossRef]
- Mohamed, M.-E.F.; Frye, R.F. Effects of Herbal Supplements on Drug Glucuronidation. Review of Clinical, Animal, and In Vitro Studies. Planta Medica 2011, 77, 311–321. [Google Scholar] [CrossRef]
- Ronis, M.J. Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug Metab. Rev. 2016, 48, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, A.; Stojan, J.; Krastanova, I.; Kristan, K.; Brunskole Švegelj, M.; Lamba, D.; Lanišnik Rižner, T. Structural basis for inhibition of 17β-hydroxysteroid dehydrogenases by phytoestrogens: The case of fungal 17β-HSDcl. J. Steroid Biochem. Mol. Biol. 2017, 171, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Esch, H.L.; Wagner, J.; Rohnstock, L.; Metzler, M. Estrogenic and genotoxic potential of equol and two hydroxylated metabolites of Daidzein in cultured human Ishikawa cells. Toxicol. Lett. 2005, 158, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Lewis, T.D.; Barbier, C.S.; Makowski, L.; Kaufman, D.G. Genistein effects on stromal cells determines epithelial proliferation in endometrial co-cultures. Exp. Mol. Pathol. 2011, 90, 257–263. [Google Scholar] [CrossRef]
- Bovee, T.F.H.; Helsdingen, R.J.R.; Rietjens, I.M.C.M.; Keijer, J.; Hoogenboom, R.L.A.P. Rapid yeast estrogen bioassays stably expressing human estrogen receptors α and β, and green fluorescent protein: A comparison of different compounds with both receptor types. J. Steroid Biochem. Mol. Biol. 2004, 91, 99–109. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-Induced Activation of Erk-1 and Erk-2 Requires the G Protein-Coupled Receptor Homolog, GPR30, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Olde, B.; Leeb-Lundberg, L.M.F. GPR30/GPER1: Searching for a role in estrogen physiology. Trends Endocrinol. Metab. 2009, 20, 409–416. [Google Scholar] [CrossRef]
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef]
- Xu, F.; Ma, J.; Wang, X.; Wang, X.; Fang, W.; Sun, J.; Li, Z.; Liu, J. The Role of G Protein-Coupled Estrogen Receptor (GPER) in Vascular Pathology and Physiology. Biomolecules 2023, 13, 1410. [Google Scholar] [CrossRef]
- Ariyani, W.; Miyazaki, W.; Amano, I.; Hanamura, K.; Shirao, T.; Koibuchi, N. Soy Isoflavones Accelerate Glial Cell Migration via GPER-Mediated Signal Transduction Pathway. Front. Endocrinol. 2020, 11, 554941. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol. Cell. Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef]
- Kajta, M.; Rzemieniec, J.; Litwa, E.; Lason, W.; Lenartowicz, M.; Krzeptowski, W.; Wojtowicz, A.K. The key involvement of estrogen receptor β and G-protein-coupled receptor 30 in the neuroprotective action of daidzein. Neuroscience 2013, 238, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Naciff, J.M.; Overmann, G.J.; Torontali, S.M.; Carr, G.J.; Tiesman, J.P.; Daston, G.P. Impact of the phytoestrogen content of laboratory animal feed on the gene expression profile of the reproductive system in the immature female rat. Environ. Health Perspect. 2004, 112, 1519–1526. [Google Scholar] [CrossRef]
- Xiong, J.; Tian, Y.; Ling, A.; Liu, Z.; Zhao, L.; Cheng, G. Genistein affects gonadotrophin-releasing hormone secretion in GT1-7 cells via modulating kisspeptin receptor and key regulators. Syst. Biol. Reprod. Med. 2022, 68, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Rios, E.; Castro, L.; Liu, J.; Yan, Y.; Dixon, D. Genistein: Dual Role in Women’s Health. Nutrients 2021, 13, 3048. [Google Scholar] [CrossRef]
- Azgomi, R.N.D.; Jazani, A.M.; Karimi, A.; Pourreza, S. Potential roles of genistein in polycystic ovary syndrome: A comprehensive systematic review. Eur. J. Pharmacol. 2022, 933, 175275. [Google Scholar] [CrossRef]
- Amanat, S.; Ashkar, F.; Eftekhari, M.H.; Tanideh, N.; Doaei, S.; Gholamalizadeh, M.; Koohpeyma, F.; Mokhtari, M. The effect of genistein on insulin resistance, inflammatory factors, lipid profile, and histopathologic indices in rats with polycystic ovary syndrome. Clin. Exp. Reprod. Med. 2021, 48, 236. [Google Scholar] [CrossRef]
- Picherit, C.; Coxam, V.; Bennetau-Pelissero, C.; Kati-Coulibaly, S.; Davicco, M.J.; Lebecque, P.; Barlet, J.P. Daidzein is more efficient than genistein in preventing ovariectomy-induced bone loss in rats. J. Nutr. 2000, 130, 1675–1681. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J. Bone Miner. Res. 2008, 23, 715–720. [Google Scholar] [CrossRef]
- Sun, J.; Sun, W.J.; Li, Z.Y.; Li, L.; Wang, Y.; Zhao, Y.; Wang, C.; Yu, L.R.; Li, L.Z.; Zhang, Y.L. Daidzein increases OPG/RANKL ratio and suppresses IL-6 in MG-63 osteoblast cells. Int. Immunopharmacol. 2016, 40, 32–40. [Google Scholar] [CrossRef]
- Jia, J.; He, R.; Yao, Z.; Su, J.; Deng, S.; Chen, K.; Yu, B. Daidzein alleviates osteoporosis by promoting osteogenesis and angiogenesis coupling. PeerJ 2023, 11, e16121. [Google Scholar] [CrossRef]
- Poschner, S.; Maier-Salamon, A.; Zehl, M.; Wackerlig, J.; Dobusch, D.; Pachmann, B.; Sterlini, K.L.; Jäger, W. The Impacts of Genistein and Daidzein on Estrogen Conjugations in Human Breast Cancer Cells: A Targeted Metabolomics Approach. Front. Pharmacol. 2017, 8, 290682. [Google Scholar] [CrossRef]
- Luo, M.; Yang, Z.Q.; Huang, J.C.; Wang, Y.S.; Guo, B.; Yue, Z.P. Genistein protects ovarian granulosa cells from oxidative stress via cAMP-PKA signaling. Cell Biol. Int. 2020, 44, 433–445. [Google Scholar] [CrossRef]
- Greathouse, K.L.; Bredfeldt, T.; Everitt, J.I.; Lin, K.; Berry, T.; Kannan, K.; Mittelstadt, M.L.; Ho, S.-m.; Walker, C.L. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol. Cancer Res. 2012, 10, 546–557. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, X.X.; Kong, F.X.; Chu, X.L. Effect of genistein on the gene and protein expressions of CXCL–12 and EGR–1 in the rat ovary. J. Anim. Physiol. Anim. Nutr. 2021, 105, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.; Gosselin, S.; Welsh, M.; Johnston, J.; Balistreri, W.; Kramer, L.; Dresser, B.; Tarr, M. Dietary estrogens—A probable cause of infertility and liver disease in captive cheetahs. Gastroenterology 1987, 93, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, A.; Sirotkin, A.; Taradajnik, T.; Chrenek, P. Effects of genistein and lavendustin on reproductive processes in domestic animals in vitro. J. Steroid Biochem. Mol. Biol. 1997, 63, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Bhupalam, V.; Awad, N.; Hetzel, J.D. The Therapeutic Role of Genistein in Perimenopausal and Postmenopausal Women. J. Clin. Aesthetic Dermatol. 2024, 17, 45–53. [Google Scholar]
- Voss, A.; Fortune, J. Estradiol-17β has a biphasic effect on oxytocin secretion by bovine granulosa cells. Biol. Reprod. 1993, 48, 1404–1409. [Google Scholar] [CrossRef]
- Mlynarczuk, J.; Wrobel, M.; Kotwica, J. The adverse effect of phytoestrogens on the synthesis and secretion of ovarian oxytocin in cattle. Reprod. Domest. Anim. 2011, 46, 21–28. [Google Scholar] [CrossRef]
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy Food Intake and Breast Cancer Survival. JAMA 2009, 302, 2437–2443. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Na Takuathung, M.; Teekachunhatean, S.; Chansakaow, S.; Klinjan, P.; Inpan, R.; Kongta, N.; Tipduangta, P.; Tipduangta, P.; Dukaew, N.; Sakuludomkan, C.; et al. The effects of SOY extract nutraceuticals on postmenopausal women’s health: A randomized, double-blind, placebo-controlled trial. J. Funct. Foods 2024, 113, 106055. [Google Scholar] [CrossRef]
- Sansai, K.; Takuathung, M.N.; Khatsri, R.; Teekachunhatean, S.; Hanprasertpong, N.; Koonrungsesomboon, N. Effects of isoflavone interventions on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2020, 31, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Inpan, R.; Na Takuathung, M.; Sakuludomkan, W.; Dukaew, N.; Teekachunhatean, S.; Koonrungsesomboon, N. Isoflavone intervention and its impact on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2024, 35, 413–430. [Google Scholar] [CrossRef]
- Inpan, R.; Dukaew, N.; Na Takuathung, M.; Teekachunhatean, S.; Koonrungsesomboon, N. Effects of isoflavone interventions on bone turnover markers and factors regulating bone metabolism in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Arch. Osteoporos. 2024, 20, 2. [Google Scholar] [CrossRef]
- Ganai, A.A.; Farooqi, H. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed. Pharmacother. 2015, 76, 30–38. [Google Scholar] [CrossRef]
- Garbiec, E.; Cielecka-Piontek, J.; Kowalówka, M.; Hołubiec, M.; Zalewski, P. Genistein—Opportunities Related to an Interesting Molecule of Natural Origin. Molecules 2022, 27, 815. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Ye, Y.; Xiao, L.; Rahman, K.; Xia, W.; Zhang, H. Daidzein: A review of pharmacological effects. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 117. [Google Scholar] [CrossRef]
- Ubaid, M.; Salauddin; Shadani, M.A.; Kawish, S.M.; Albratty, M.; Makeen, H.A.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Halawi, M.A.; et al. Daidzein from Dietary Supplement to a Drug Candidate: An Evaluation of Potential. ACS Omega 2023, 8, 32271–32293. [Google Scholar] [CrossRef]
- Rane, A.R.; Paithankar, H.; Hosur, R.V.; Choudhary, S. Modulation of α-synuclein fibrillation by plant metabolites, daidzein, fisetin and scopoletin under physiological conditions. Int. J. Biol. Macromol. 2021, 182, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Zada, W.; Murtaza, G.; Iqbal, G.; Abbas, G.; Khan, S.A.; Mannan, A. Antidepressant Potential of Daidzein through Modulation of Endocannabinoid System by Targeting Fatty Acid Amide Hydrolase. Sains Malays. 2022, 51, 3383–3399. [Google Scholar] [CrossRef]
- Abdulkadir, W.S.; Puana, F.; Taupik, M.; Tungadi, R.; Hutuba, A.H.; Djuwarno, E.N.; Ramahdani, F.N.; Hiola, F. In Silico Analysis of Isoflavone Compounds in Soybean (Glycine max L.) as Anti-Breast Cancer Agents Targeting Estrogen Receptor Alpha. Trop. J. Phytochem. Pharm. Sci. 2024, 3, 375–379. [Google Scholar] [CrossRef]
- Pulavarthy, V.; Mane, S.; Kotwal, N.; Pandey, V.; Srikanth, M.; Upadhyay, D.; Dm, C.; Rawat, S.; Evam, R.; Vigyan, V.; et al. Computational Analysis of Some Phytoconstituents for Breast Cancer as Potential Anticancer Drugs. Afr. J. Biol. Sci. 2024, 6. [Google Scholar] [CrossRef]
- Arzuk, E.; Armağan, G. Genistein and daidzein induce ferroptosis in MDA-MB-231 cells. J. Pharm. Pharmacol. 2024, 76, 1599–1608. [Google Scholar] [CrossRef]
- Montalesi, E.; Cipolletti, M.; Cracco, P.; Fiocchetti, M.; Marino, M. Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers 2020, 12, 167. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Li, Y.; Li, Y.; Feng, C.; Li, Z. Daidzein-rich isoflavones aglycone inhibits lung cancer growth through inhibition of NF-κB signaling pathway. Immunol. Lett. 2020, 222, 67–72. [Google Scholar] [CrossRef]
- Gundogdu, G.; Yavuz, D.; Meltem, C.; Mucahit, S.; Cicek, B. The cytotoxic and genotoxic effects of daidzein on MIA PaCa-2 human pancreatic carcinoma cells and HT-29 human colon cancer cells. Drug Chem. Toxicol. 2020, 43, 581–587. [Google Scholar] [CrossRef]
- Salama, A.A.A.; Allam, R.M. Promising targets of chrysin and daidzein in colorectal cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharmacol. 2021, 892, 173763. [Google Scholar] [CrossRef]
- Kitagawa, A.; Yamaguchi, M.; Fujiwara, A.; Shimizu, M.; Takahashi, A.; Sone, H.; Kamiyama, S. Genistein inhibits chondrogenic differentiation and mineralization of ATDC5 cells. Biochem. Biophys. Res. Commun. 2021, 566, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Harahap, I.A.; Olejnik, A.; Kowalska, K.; Suliburska, J. Effects of Daidzein, Tempeh, and a Probiotic Digested in an Artificial Gastrointestinal Tract on Calcium Deposition in Human Osteoblast-like Saos-2 Cells. Int. J. Mol. Sci. 2024, 25, 1008. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.J.; Nam, G.S.; Nam, K.S. Daidzein Inhibits Human Platelet Activation by Downregulating Thromboxane A(2) Production and Granule Release, Regardless of COX-1 Activity. Int. J. Mol. Sci. 2023, 24, 11985. [Google Scholar] [CrossRef] [PubMed]
- Nisha; Paramanik, V. Neuroprotective Roles of Daidzein Through Extracellular Signal-Regulated Kinases Dependent Pathway in Chronic Unpredictable Mild Stress Mouse Model. Mol. Neurobiol. 2025, 62, 4899–4921. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Y.; Kang, Q.; Wu, Y.; Liu, Y.; Su, Y.; Wang, X.; Xiu, M.; He, J. The protective effect and bioactive compounds of Astragalus membranaceus against neurodegenerative disorders via alleviating oxidative stress in Drosophila. FASEB J. 2024, 38, e23727. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, M.; Chen, M.; Lu, Y.; Shi, D.; Wang, J.; Liu, C. Neuroprotective Effect of Daidzein Extracted from Pueraria lobate Radix in a Stroke Model via the Akt/mTOR/BDNF Channel. Front. Pharmacol. 2021, 12, 772485. [Google Scholar] [CrossRef]
- Haider, T.; Khan, S.; Bibi, T.; Zahra, S.A.; Ali, H.; Din, F.u.; Shah, F.A.; Youn, I.; Seo, E.K. Daidzein ameliorates experimental traumatic brain injury-induced neurological symptoms by suppressing oxidative stress and apoptosis. J. Biochem. Mol. Toxicol. 2024, 38, e70019. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.; Ebaid, H.; Al-Tamimi, J.; Hassan, I.; Omara, E.A.; Elbaset, M.A.; Alhazza, I.M.; Siddique, J.A. Protective Effect of Daidzein against Diethylnitrosamine/Carbon Tetrachloride-Induced Hepatocellular Carcinoma in Male Rats. Biology 2023, 12, 1184. [Google Scholar] [CrossRef]
- Harahap, I.A.; Kuligowski, M.; Cieslak, A.; Kołodziejski, P.A.; Suliburska, J. Effect of Tempeh and Daidzein on Calcium Status, Calcium Transporters, and Bone Metabolism Biomarkers in Ovariectomized Rats. Nutrients 2024, 16, 651. [Google Scholar] [CrossRef]
- Liu, Z.-m.; Chen, B.; Li, S.; Li, G.; Zhang, D.; Ho, S.C.; Chen, Y.-m.; Ma, J.; Qi, H.; Ling, W.-h. Effect of whole soy and isoflavones daidzein on bone turnover and inflammatory markers: A 6-month double-blind, randomized controlled trial in Chinese postmenopausal women who are equol producers. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820920555. [Google Scholar] [CrossRef]
- Banu, Z.; Poduri, R.R.; Bhattamisra, S.K. Phytochemical profiling, in silico molecular docking and ADMET prediction of alkaloid rich fraction of Elaeocarpus angustifolius blume seeds against Alzheimer’s disease. Nat. Prod. Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Wang, M.; Li, M.; Liu, Y.; Chen, H.; Chen, J.; Tao, W.; Huang, L.; Zhao, S. Gen inhibiting the Wnt/Ca(2+) signaling pathway alleviates cerebral ischemia/reperfusion injury. Sci. Rep. 2025, 15, 4661. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wei, H.; Gu, T.; Wang, J.; Wu, Z.; Yang, Q. Genistein Attenuates Acute Cerebral Ischemic Damage by Inhibiting the NLRP3 Inflammasome in Reproductively Senescent Mice. Front. Aging Neurosci. 2020, 12, 153. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, C.; Choi, D.; Lee, Y.; Lee, S.-H. Effect of Soy Isoflavone on Prostate Cancer Cell Apoptosis Through Inhibition of STAT3, ERK, and AKT. Curr. Issues Mol. Biol. 2024, 46, 12512–12526. [Google Scholar] [CrossRef]
- Ono, M.; Mikako, T.; Asuka, N.; Takako, H.; Nakano, S. Genistein Suppresses v-Src-Driven Proliferative Activity by Arresting the Cell-Cycle at G2/M through Increasing p21 Level in Src-Activated Human Gallbladder Carcinoma cells. Nutr. Cancer 2021, 73, 1471–1479. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H. Genistein Increases Gene Expression by Demethylation of WNT5α Promoter in Colon Cancer Cell Line SW1116. Anticancer. Res. 2010, 30, 4537–4545. [Google Scholar] [PubMed]
- Wang, S.; Zhang, Z.; Wang, J.; Ma, L.; Zhao, J.; Wang, J.; Fang, Z.; Hou, W.; Guo, H. Neuronal GPER Participates in Genistein-Mediated Neuroprotection in Ischemic Stroke by Inhibiting NLRP3 Inflammasome Activation in Ovariectomized Female Mice. Mol. Neurobiol. 2022, 59, 5024–5040. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.P.; Yan, H.X.; Peng, F.; Feng, W.; Chen, F.F.; Huang, X.Y.; Zhang, X.; Zhou, Y.Y.; Chen, Y.S. Genistein protects epilepsy-induced brain injury through regulating the JAK2/STAT3 and Keap1/Nrf2 signaling pathways in the developing rats. Eur. J. Pharmacol. 2021, 912, 174620. [Google Scholar] [CrossRef]
- Munekawa, C.; Okamura, T.; Majima, S.; River, B.; Kawai, S.; Kobayashi, A.; Nakajima, H.; Kitagawa, N.; Okada, H.; Senmaru, T.; et al. Daidzein Inhibits Muscle Atrophy by Suppressing Inflammatory Cytokine- and Muscle Atrophy-Related Gene Expression. Nutrients 2024, 16, 3084. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zheng, Z.; Yin, Y.; Jiang, Z. Genistein prevents bone loss in type 2 diabetic rats induced by streptozotocin. Food Nutr. Res. 2020, 64, 10–29219. [Google Scholar] [CrossRef]
- Harahap, I.A.; Kuligowski, M.; Schmidt, M.; Kołodziejski, P.A.; Suliburska, J. Effects of isoflavone and probiotic intake on calcium transport and bone metabolism biomarkers in female rats. Food Sci. Nutr. 2023, 11, 6324–6335. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bielecki, W.; Szterk, A.; Ofiara, K.; Bobrowska-Korczak, B. Genistein Supplementation and Bone Health in Breast Cancer in Rats. Nutrients 2024, 16, 912. [Google Scholar] [CrossRef] [PubMed]
- Poasakate, A.; Maneesai, P.; Rattanakanokchai, S.; Bunbupha, S.; Tong-Un, T.; Pakdeechote, P. Genistein Prevents Nitric Oxide Deficiency-Induced Cardiac Dysfunction and Remodeling in Rats. Antioxidants 2021, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Akhmad, S.; Achmad, H.; Wening, H. Effect of Isoflavon Genestein Daidzein From Pueraria Lobata Extract on Vascular Endothelial Growth Factor Expression in the Aorta of Hypoestrogenic Rat. Asian J. Health Res. 2022, 1, 29–34. [Google Scholar] [CrossRef]
- Na Takuathung, M.; Klinjan, P.; Sakuludomkan, W.; Dukaew, N.; Inpan, R.; Kongta, R.; Chaiyana, W.; Teekachunhatean, S.; Koonrungsesomboon, N. Efficacy and Safety of the Genistein Nutraceutical Product Containing Vitamin E, Vitamin B3, and Ceramide on Skin Health in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Clin. Med. 2023, 12, 1326. [Google Scholar] [CrossRef]
- Nakano, H.; Ogura, K.; Takahashi, E.; Harada, T.; Nishiyama, T.; Muro, K.; Hiratsuka, A.; Kadota, S.; Watabe, T. Regioselective monosulfation and disulfation of the phytoestrogens daidzein and genistein by human liver sulfotransferases. Drug Metab. Pharmacokinet. 2004, 19, 216–226. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Busby, M.G.; Jeffcoat, A.R.; Bloedon, L.T.; Koch, M.A.; Black, T.; Dix, K.J.; Heizer, W.D.; Thomas, B.F.; Hill, J.M.; Crowell, J.A.; et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Single-dose administration to healthy men. Am. J. Clin. Nutr. 2002, 75, 126–136. [Google Scholar] [CrossRef]
- Yamagata, K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2019, 74, 201–209. [Google Scholar] [CrossRef]
- Ahmed, M.; Mäkinen, V.P.; Mulugeta, A.; Shin, J.; Boyle, T.; Hyppönen, E.; Lee, S.H. Considering hormone-sensitive cancers as a single disease in the UK biobank reveals shared aetiology. Commun. Biol. 2022, 5, 614. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Landete, J.M. Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. Int. J. Food Sci. Nutr. 2016, 67, 117–124. [Google Scholar] [CrossRef]
- Hosoda, K.; Furuta, T.; Ishii, K. Metabolism and disposition of isoflavone conjugated metabolites in humans after ingestion of kinako. Drug Metab. Dispos. 2011, 39, 1762–1767. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.J.; Song, T.T.; Murphy, P.A.; Hendrich, S. Urinary disposition of the soybean isoflavones daidzein, genistein and glycitein differs among humans with moderate fecal isoflavone degradation activity. J. Nutr. 1999, 129, 957–962. [Google Scholar] [CrossRef]
- Kunisue, T.; Tanabe, S.; Isobe, T.; Aldous, K.M.; Kannan, K. Profiles of phytoestrogens in human urine from several Asian countries. J. Agric. Food Chem. 2010, 58, 9838–9846. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.L.; Williamson, G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr. Cancer 2007, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J.M. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef]
- Clarke, D.B.; Lloyd, A.S. Dietary exposure estimates of isoflavones from the 1998 UK Total Diet Study. Food Addit. Contam. 2004, 21, 305–316. [Google Scholar] [CrossRef]
- Ferriere, F.; Aasi, N.; Flouriot, G.; Pakdel, F. Exploring the Complex Mechanisms of Isoflavones: From Cell Bioavailability, to Cell Dynamics and Breast Cancer. Phytother. Res. 2025, 39, 957–979. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Santell, R.C.; Haslam, S.Z.; Helferich, W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar]
- Atkinson, C.; Compston, J.E.; Day, N.E.; Dowsett, M.; Bingham, S.A. The effects of phytoestrogen isoflavones on bone density in women: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2004, 79, 326–333. [Google Scholar] [CrossRef]
- Squadrito, F.; Altavilla, D.; Morabito, N.; Crisafulli, A.; D’Anna, R.; Corrado, F.; Ruggeri, P.; Campo, G.M.; Calapai, G.; Caputi, A.P.; et al. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis 2002, 163, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Halm, B.M.; Custer, L.J.; Tatsumura, Y.; Hebshi, S. Isoflavones in breastfed infants after mothers consume soy. Am. J. Clin. Nutr. 2006, 84, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Khodadadi, I. Antidiabetic Effects of Genistein: Mechanism of Action. Endocr. Metab. Immune Disord.-Drug Targets 2023, 23, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Jefferson, W.N.; Padilla-Banks, E.; Newbold, R.R. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod. Toxicol. 2007, 23, 308–316. [Google Scholar] [CrossRef]
- Mersereau, J.E.; Levy, N.; Staub, R.E.; Baggett, S.; Zogric, T.; Chow, S.; Ricke, W.A.; Tagliaferri, M.; Cohen, I.; Bjeldanes, L.F. Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Mol. Cell. Endocrinol. 2008, 283, 49–57. [Google Scholar] [CrossRef]
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Xiao, C.W. Health effects of soy protein and isoflavones in humans. J. Nutr. 2008, 138, 1244S–1249S. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Risk assessment for peri-and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015, 13, 4246. [Google Scholar] [CrossRef]
- Cassidy, A.; Brown, J.E.; Hawdon, A.; Faughnan, M.S.; King, L.J.; Millward, J.; Zimmer-Nechemias, L.; Wolfe, B.; Setchell, K.D. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J. Nutr. 2006, 136, 45–51. [Google Scholar] [CrossRef]
- Messina, M.J.; Wood, C.E. Soy isoflavones, estrogen therapy, and breast cancer risk: Analysis and commentary. Nutr. J. 2008, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Kuhnle, G.G.; Mulligan, A.A.; Touillaud, M.; Slimani, N.; Romieu, I.; Powell, N.; Tumino, R.; et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-hour dietary recall cohort. Eur. J. Clin. Nutr. 2012, 66, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Anderson, G.L.; Gass, M.; Lane, D.S.; Aragaki, A.K.; Kuller, L.H.; Manson, J.E.; Stefanick, M.L.; Ockene, J.; Sarto, G.E. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. Jama 2010, 304, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Grady, D.; Gebretsadik, T.; Kerlikowske, K.; Ernster, V.; Petitti, D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet. Gynecol. 1995, 85, 304–313. [Google Scholar] [CrossRef]
- Kuang, Y.; Shen, P.; Ye, J.; Raj, R.; Ge, H.; Yu, B.; Zhang, J. Probing the interactions of genistein with HMGB1 through multi-spectroscopic and in-silico approaches. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 327, 125385. [Google Scholar] [CrossRef]
- Zefzoufi, M.; Fdil, R.; Bouamama, H.; Gadhi, C.; Katakura, Y.; Mouzdahir, A.; Sraidi, K. Effect of extracts and isolated compounds derived from Retama monosperma (L.) Boiss. on anti-aging gene expression in human keratinocytes and antioxidant activity. J. Ethnopharmacol. 2021, 280, 114451. [Google Scholar] [CrossRef]
- Márquez-Flores, Y.K.; Martínez-Galero, E.; Correa-Basurto, J.; Sixto-López, Y.; Villegas, I.; Rosillo, M.Á.; Cárdeno, A.; Alarcón-de-la-Lastra, C. Daidzein and Equol: Ex Vivo and In Silico Approaches Targeting COX-2, iNOS, and the Canonical Inflammasome Signaling Pathway. Pharmaceuticals 2024, 17, 647. [Google Scholar] [CrossRef]

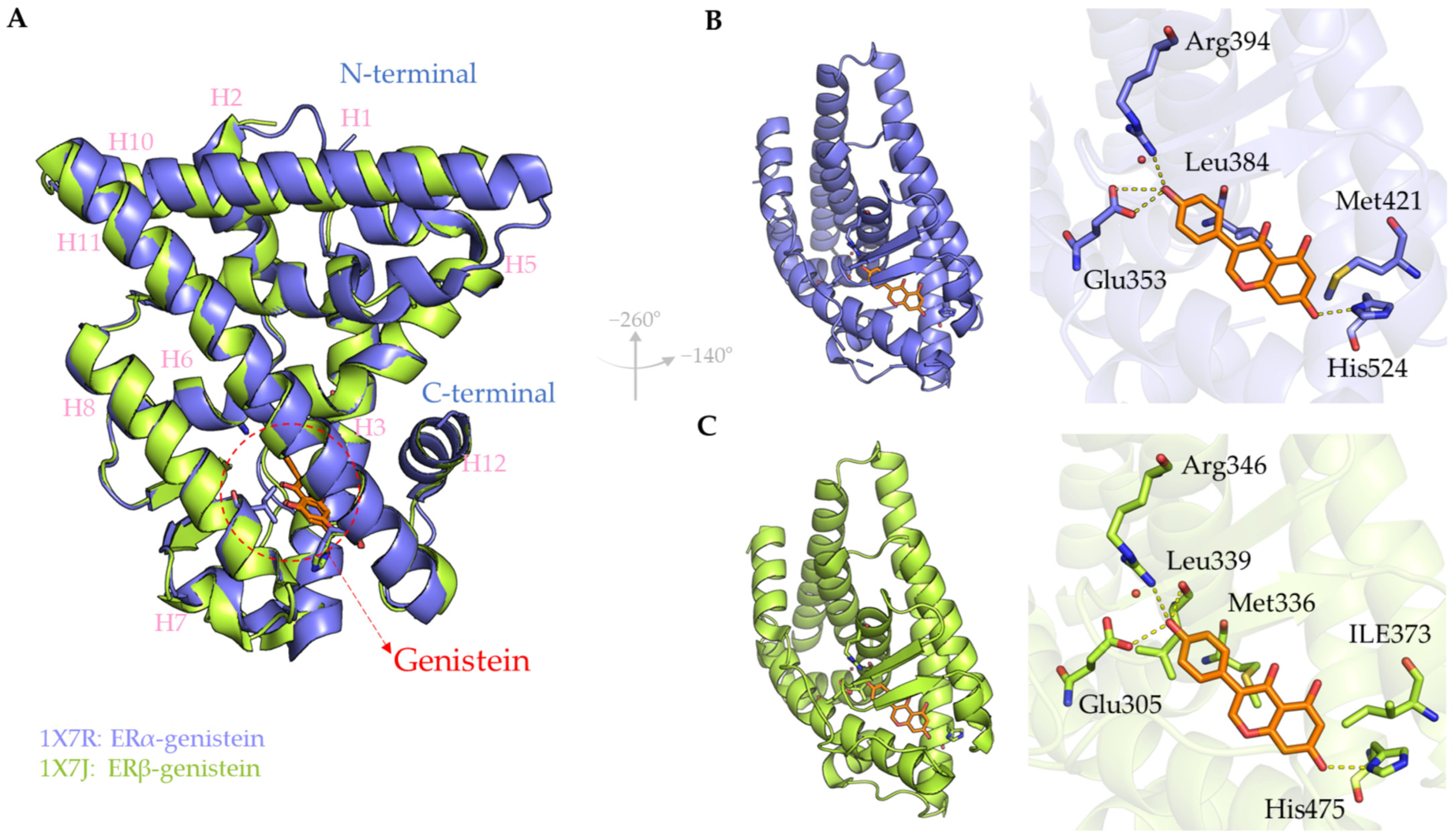
| Biological Activities | Study Model/ Assay | Effective Dose/ Concentration | Key Findings | Reference |
|---|---|---|---|---|
| In silico | ||||
| Neurological | Molecular docking, Molecular dynamic simulations, and ADME properties | - | Exhibited the score of best binding pose for the complex at −5.3 Kcal/ Mol and predicted to have ability to cross the blood-brain barrier | [145] |
| Molecular docking against FAAH | - | Demonstrated a binding energy of −64.77 Kcal/mol and a binding affinity of −11.77 Kcal/mol | [146] | |
| Anti-cancer | Molecular docking targeting ERα | - | Displayed strong binding to ERα but less than genistein (docking score −8.47 Kcal/mol) and formed two hydrogen bonds with critical amino acids, specifically His-524 and Gly-521. | [147] |
| Molecular docking targeting human ER | - | Exhibited a strong interaction with Human Estrogen Receptor (PDB ID: 2IOK) with binding energy of −8.82 kcal/mol and interacted with Leu346, Leu384, Leu387, Phe404, and Leu525. | [148] | |
| In vitro | ||||
| Neurological | ThT fluorescence assay, 90° Light scattering studies, TEM, ANS fluorescence assay, tyrosine fluorescence quenching studies, and CD spectroscopy | α-Synuclein (α-syn)/Daidzein molar ratios (1:0, 1:1, 1:3, and 1:5) | Inhibited α-syn fibrillation in a concentration dependent manner via modulation of hydrophobic and hydrogen bonding interactions and delaying β-rich structure formation | [145] |
| FAAH enzyme inhibitory assay | - | Inhibited FAAH activity (IC50 = 1.3 ± 0.13 μM) | [146] | |
| Anti-cancer | MDA-MB-231 and MCF-7 breast cancer cell lines | - | Exhibited cytotoxicity in MDA-MB-231 (IC50 = 25.36 ± 0.962 μM) and MCF-7 (IC50 = 33.23 ± 1.043 μM). However, its effect was found associated with ferroptosis only in MDA-MB-231 cells, characterized by elevated LPO, reduced GSH/GSSG ratio, and downregulated mRNA expression of ferroptosis-regulatory genes Gpx4 and Fsp-1 | [149] |
| MCF-7 and T47D human ERα-positive breast cancer cells | 1 μM | Suppressed estrogen-induced neuroglobin expression and enhanced the pro-apoptotic effects of paclitaxel in ERα-positive breast cancer cells by activating p38 MAPK signaling | [150] | |
| A549 and 95D human NSCLC cells | 25 μM DRIA | Inhibited proliferation and colony formation of lung cancer cells by downregulating NF-κB signaling and suppressing IL-6 and IL-8 | [151] | |
| MIA PaCa-2 pancreatic carcinoma cells and HT-29 colon adenocarcinoma cells | 200 μM | Inhibited proliferation and induced DNA damage in MIA PaCa-2 and HT-29 cancer cells in a dose-dependent manner | [152] | |
| SW620 colorectal cancer cells | - | Inhibited cell proliferation (IC50 = 23.5 ± 0.8 μM) and reduced activation of oncogenic pathways by downregulating phosphorylated ERK and AKT | [153] | |
| Anti-osteoporotic | ATDC5 mouse chondrogenic cells | 10 μM | Inhibited chondrogenic differentiation in ATDC5 cells (less potent than Genistein) and suppressed proteoglycan production and chondrogenic gene expression. | [154] |
| Caco-2 intestinal epithelial cells and Saos-2 human osteoblast-like cells | 0.05–1.0 mg/mL | Promoted Saos-2 cell proliferation and enhancing intracellular calcium content during osteogenic induction | [155] | |
| Cardioprotective | Human platelets isolated from platelet-rich plasma (PRP) | 12.5–50 μM | Inhibited collagen-induced human platelet aggregation by suppressing granule release (ATP, serotonin, P-selectin), TXA2 production, integrin αIIbβ3 activation, and key signaling pathways (PI3K/PDK1/Akt/GSK3αβ/p38, and ERK) | [156] |
| In vivo | ||||
| Neurological | Male and female Balb/c mice | 20 mg/kg/day for 14 days, IP | Alleviated depressive-like behavior by reducing immobility time in forced swim test and lowering plasma corticosterone level | [146] |
| CUMS-induced male Swiss albino mice—a model of depression | 1 mg/kg/day for 21 days, PO | Reduced depressive- and anxiety-like behaviors, and improved motor coordination and memory via upregulating ERβ-dependent ERK/mTOR signaling | [157] | |
| Aβ42 transgenic Drosophila flies—a model of AD | 1 mM in standard food for 96 h | Prolonged the lifespan of Aβ42 transgenic flies | [158] | |
| I/R injury in male ICR rats—a model of ischemic stroke | 20 and 30 mg/kg/day for 2 weeks, IG | Improved neurological deficits, reduced infarct size and brain edema, and restored dopamine levels, mediated by inhibiting expression of cleaved caspase-3, while activating Akt/mTOR, Akt/BAD, and BDNF/CREB signaling pathways | [159] | |
| TBI model in male albino BALB/c mice | 10 mg/kg/day for 14 days, IP | Improved neurological function, enhanced motor coordination, reduced anxiety-like behavior, alleviated mechanical allodynia, and restored blood–brain barrier integrity. | [160] | |
| Anti-cancer | Male albino rats with DMH and DSS- induced colorectal cancer | 5 and 10 mg/kg, three times/week for 8 weeks, PO | Reduced tumor progression by lowering CXCL1, AREG, MMP-9 which involved in tumor progression and metastasis, and oxidative stress markers and improved colon tissue. | [153] |
| DENA and CCl4-induced male Wistar rats—a model of HCC | 20 and 40 mg/kg/day for 8 weeks (pre-treatment), PO | Protected against HCC by improving liver function markers (ALP, ALT, AST), reducing oxidative stress and IL-6, TNF-α, CRP, lowering tumor markers (AFP, GPC3, VEGF), and restoring near-normal liver histology | [161] | |
| Anti-osteoporotic | OVX C57BL/6 female mice—a model of postmenopausal osteoporosis | 25 mg/kg, 5 days/week for 8 weeks, IG | Enhanced bone formation, inhibited osteoclast activity, and promoted H-type vessel formation via suppression of Caveolin-1 and activation of EGFR/PI3K/AKT signaling in endothelial cells | [125] |
| OVX female Wistar rats—a model of postmenopausal osteoporosis | 10 mg/kg/day for 6 weeks, PO | Improved bone microarchitecture, increased femoral calcium content, enhanced intestinal calcium transporter expression (TRPV5 and TRPV6 mRNA), and favorably modulated bone metabolism markers | [162] | |
| Clinical trial | ||||
| Anti-cancer | Chinese equol-producing postmenopausal women | 63 mg/day for 6 months, PO | no significant effect on bone turnover markers or inflammation compared to placebo. | [163] |
| Biological Activities | Study Model/ Assay | Effective Dose/ Concentration | Key Findings | Reference |
|---|---|---|---|---|
| In silico | ||||
| Neurological | Molecular docking studies against therapeutic targets for AD | - | Exhibited high binding affinities against human AChE, β-secretase, TACE, GSK3, and APP. It was also confirmed for its favorable drug-likeness profiles, although less likely to penetrate the CNS. | [164] |
| Anti-cancer | Molecular docking targeting ERα | - | Exhibited strong binding affinity toward ERα (−8.5 kcal/mol) and formed 5 hydrogen bonds with Leu-387, Glu-353, Arg-394, Glu-419, and His-524 | [147] |
| Molecular docking targeting human ER | - | Showed a favorable binding (−8.36 kcal/mol) and interacted with Leu346, Leu384, Leu387, and Phe404 | [148] | |
| In vitro | ||||
| Neurological | OGD/R-induced rat pheochromocytoma PC12 cells | 30 µM | Reduced the levels of Ca2+, ROS, apoptosis as well as inhibited the Wnt/Ca2+ signaling pathway | [165] |
| OGD/R-induced N9 primary microglia and the cocultured N9 with HT22 hippocampal neuronal cells | 5 μg/mL | Reduced inflammatory responses (TNF-α, IL-1β, IL-18, IL-6 and cleaved caspase-1) and microglial expression of NLRP3 inflammasome | [166] | |
| Anti-cancer | MDA-MB-231 and MCF-7 breast cancer cells | - | Exhibited cytotoxicity in MDA-MB-231 (IC50 = 26.72 ± 1.261 μM) and MCF-7 (IC50 = 45.02 ± 1.064 μM). However, its effect was found associated with ferroptosis only in MDA-MB-231 cells, characterized by elevated LPO, reduced GSH/GSSG ratio, and downregulated mRNA expression of ferroptosis-regulatory genes Gpx4 and Fsp-1 | [149] |
| Human prostate cancer cell line DU145 and Normal prostate epithelial cells HPrEC | 50–100 μM | Inhibited DU145 proliferation by inducing p53-mediated, caspase-dependent apoptosis and suppressing oncogenic STAT3, Akt, ERK, and p38 signaling pathways, with minimal cytotoxicity to normal prostate cells. | [167] | |
| HAG/src3-1 human gallbladder carcinoma cells (v-Src-transfected) and HAG/neo3-5 control cells | 50 μM | Inhibited Src-driven gallbladder cancer cell proliferation by inducing G2/M cell cycle arrest through upregulation of p53 and p21while reducing phosphorylated p21 | [168] | |
| Human colon cancer SW1116, DLD-1, and SW480 cell lines | 75 μM | Suppressed proliferation of colon cancer cells and reactivated WNT5a expression in SW1116 cells by promoter demethylation, suggesting an epigenetic mechanism | [169] | |
| Anti-osteoporotic | Mouse chondrogenic ATDC5 cells | 10 μM | Suppressed chondrogenic differentiation in ATDC5 cells by reducing sulfated proteoglycans, collagen fibers, and calcium deposition, and downregulating genes related to chondrocyte differentiation, while promoting osteogenic marker expression | [154] |
| In vivo | ||||
| Neurological | I/R injury in OVX female C57BL/6 J mice—a model of postmenopausal stroke | 10 mg/kg/day for 2 days, IP | Enhanced the neuronal GPER/PGC-1α pathway and inhibited NLRP3 inflammasome activation | [170] |
| I/R injury in male Sprague–Dawley rats—a model of ischemic stroke | 100 mg/kg/day for 21 days, PO | Alleviated CIRI by reduced infarct size, improved neurological function. It also mitigated Ca2+ overload, oxidative stress, and apoptosis via inhibition of the Wnt/Ca2+ signaling pathway. | [165] | |
| I/R injury in reproductively senescent female C57BL/6 J mice—a model of postmenopausal stroke | 10 mg/kg/day for 2 weeks, IP | Alleviated cerebral ischemic injury by improving neurological deficit scores and reducing inflammatory responses (TNF-α, IL-1β, IL-18, IL-6, and cleaved caspase-1) as well as microglial expression of NLRP3 inflammasome | [166] | |
| PTZ-induced male Sprague–Dawley rats—a model of epilepsy | 5 and 15 mg/kg for 30 min (pre-treatment), IP | Reduced the intensity and duration of seizures and promoted neuronal survival while inhibited microglial and astrocytic activation. The effects are mediated through the inhibition of JAK2/STAT3 signaling pathway and activation of the Keap1/Nrf2 oxidative stress pathway. | [171] | |
| Anti-osteoporotic | Male Sprague–Dawley rats with orchiectomy-induced osteoporosis | 1 g/kg in food (~20.7 mg/kg/day) for 95, 102 and 151 days, PO | Demonstrated short-term improvement in cortical bone thickness via the estrogen pathway but had limited long-term osteoprotective effects and no significant benefit on trabecular bone | [172] |
| Male Sprague–Dawley rats with T2DM | 30 mg/kg/day for 8 weeks, PO | Improved bone density, enhanced bone microarchitecture, promoted osteogenesis, suppressed bone resorption, and reduced inflammation in diabetic osteoporotic rats by modulating the OPG/RANKL, PPAR-γ, and β-catenin/Runx-2 pathways | [173] | |
| Female Wistar rats | 100 mg/kg/day in combination with 10 mg daidzein/kg/day for 8 weeks, PO | Upregulated Trpv6 expression, promoting intestinal calcium transport, and decreased serum pyridinoline, a marker of bone resorption | [174] | |
| Female Sprague–Dawley rats with DMBA-induced mammary gland cancer | 0.2 mg/kg/day for 10 weeks, PO | Disrupted bone structure, increased calcium accumulation, and altered mineral composition in rats with breast cancer, leading to fragile and structurally compromised bones. | [175] | |
| Cardioprotective | Male Wistar rats with Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME)-induced NO deficiency hypertension and cardiac dysfunction | 80 mg/kg/day for 5 weeks, PO | Prevented NO deficiency-induced hypertension, oxidative stress, cardiac hypertrophy, and fibrosis in rats by suppressing RAS activation and the Ang II/AT1R/NADPH oxidase/TGF-β1 pathway | [176] |
| OVX female Wistar rats—a model of menopause hypoestrogenism | 15, 30 and 60 mg IGD/kg/day for 3 weeks, PO | Enhanced aortic VEGF expression, suggesting a potential cardioprotective effect through promoting vascular endothelial repair and angiogenesis | [177] | |
| Clinical trial | ||||
| Anti-aging | Randomized, double-blind, placebo-controlled clinical trial in postmenopausal women (n = 50) | Product consisted of 4% genistein, TOP on facial skin twice daily for 6 weeks | Increased skin hydration, reduced fine pores and pore area, decreased wrinkles, and improved overall facial skin quality | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intharuksa, A.; Arunotayanun, W.; Na Takuathung, M.; Chaichit, S.; Prasansuklab, A.; Chaikhong, K.; Sirichanchuen, B.; Chupradit, S.; Koonrungsesomboon, N. Daidzein and Genistein: Natural Phytoestrogens with Potential Applications in Hormone Replacement Therapy. Int. J. Mol. Sci. 2025, 26, 6973. https://doi.org/10.3390/ijms26146973
Intharuksa A, Arunotayanun W, Na Takuathung M, Chaichit S, Prasansuklab A, Chaikhong K, Sirichanchuen B, Chupradit S, Koonrungsesomboon N. Daidzein and Genistein: Natural Phytoestrogens with Potential Applications in Hormone Replacement Therapy. International Journal of Molecular Sciences. 2025; 26(14):6973. https://doi.org/10.3390/ijms26146973
Chicago/Turabian StyleIntharuksa, Aekkhaluck, Warunya Arunotayanun, Mingkwan Na Takuathung, Siripat Chaichit, Anchalee Prasansuklab, Kamonwan Chaikhong, Buntitabhon Sirichanchuen, Suthunya Chupradit, and Nut Koonrungsesomboon. 2025. "Daidzein and Genistein: Natural Phytoestrogens with Potential Applications in Hormone Replacement Therapy" International Journal of Molecular Sciences 26, no. 14: 6973. https://doi.org/10.3390/ijms26146973
APA StyleIntharuksa, A., Arunotayanun, W., Na Takuathung, M., Chaichit, S., Prasansuklab, A., Chaikhong, K., Sirichanchuen, B., Chupradit, S., & Koonrungsesomboon, N. (2025). Daidzein and Genistein: Natural Phytoestrogens with Potential Applications in Hormone Replacement Therapy. International Journal of Molecular Sciences, 26(14), 6973. https://doi.org/10.3390/ijms26146973











