Mandragora autumnalis: Phytochemical Composition, Antioxidant and Anti-Cancerous Bioactivities on Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Phytochemical Composition and Total Phenolic and Total Flavonoid Content (TPC and TFC) Assays
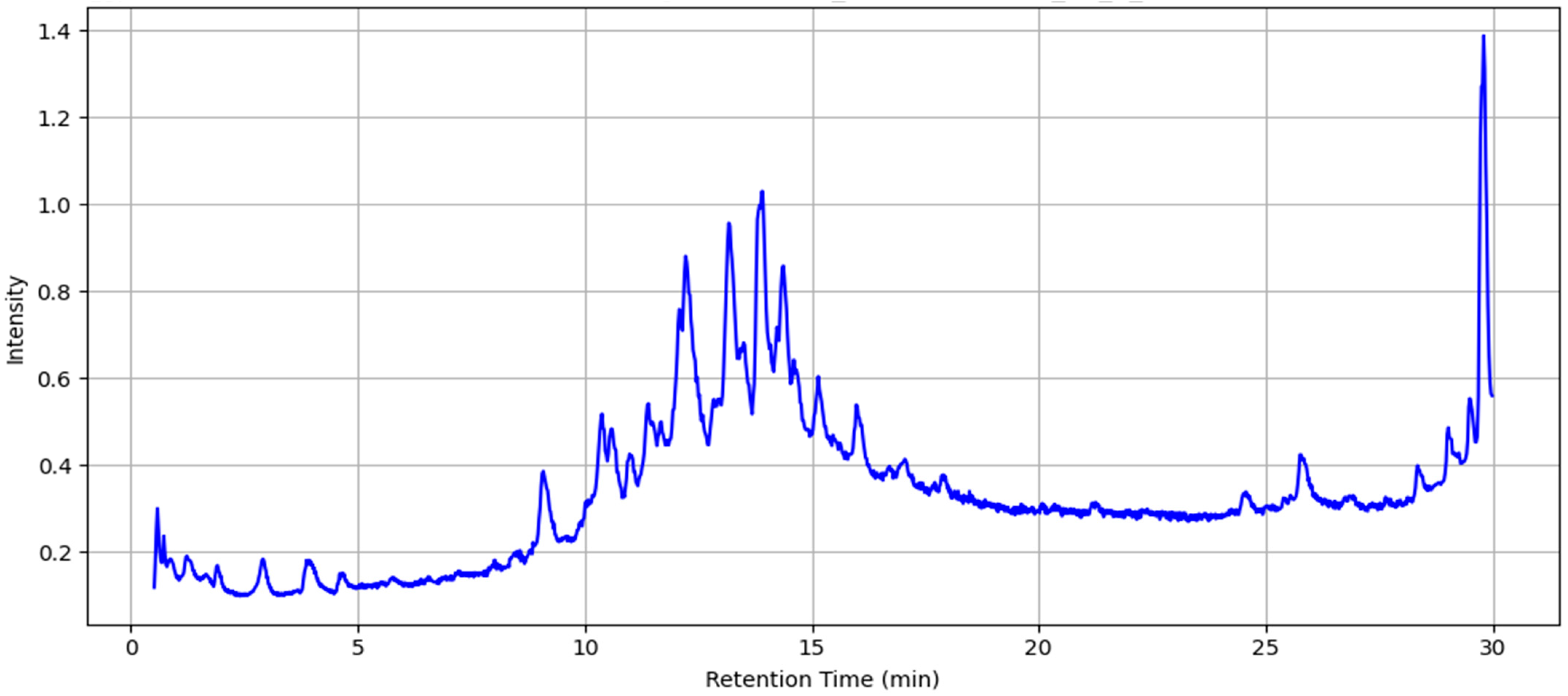
2.2. LC-MS Analysis of MAE
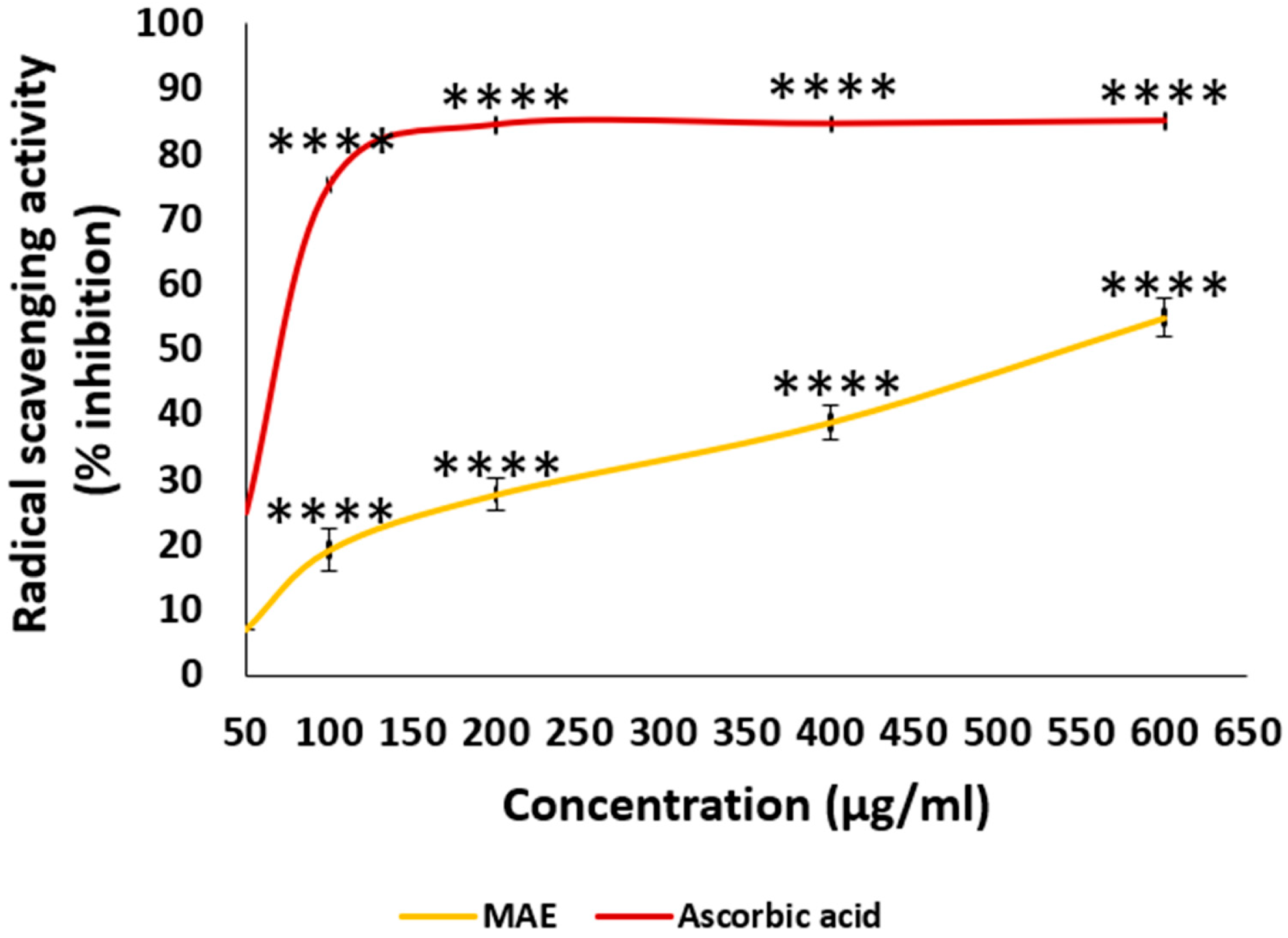
2.3. MAE Has a Potent Radical Scavenging Potential
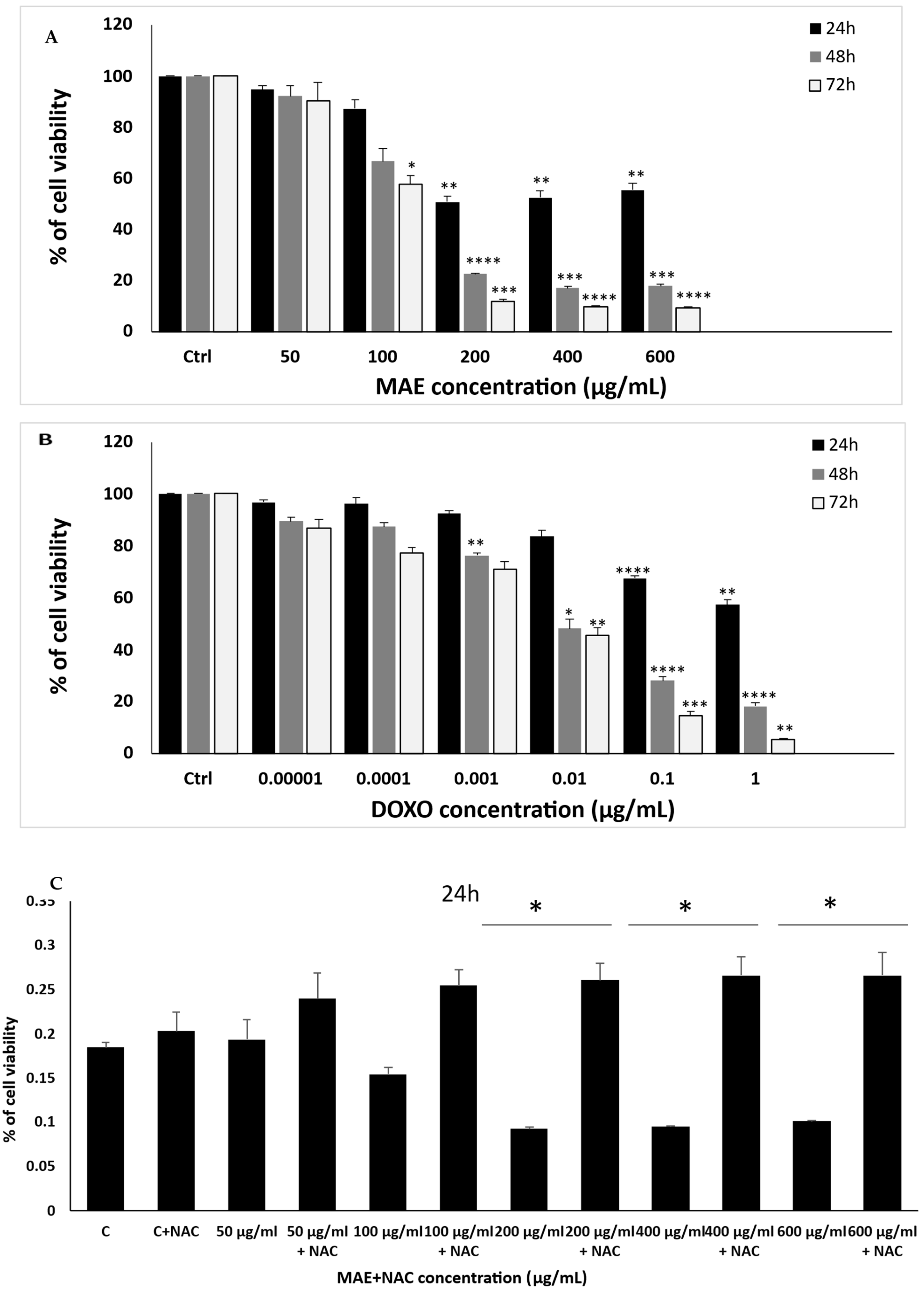
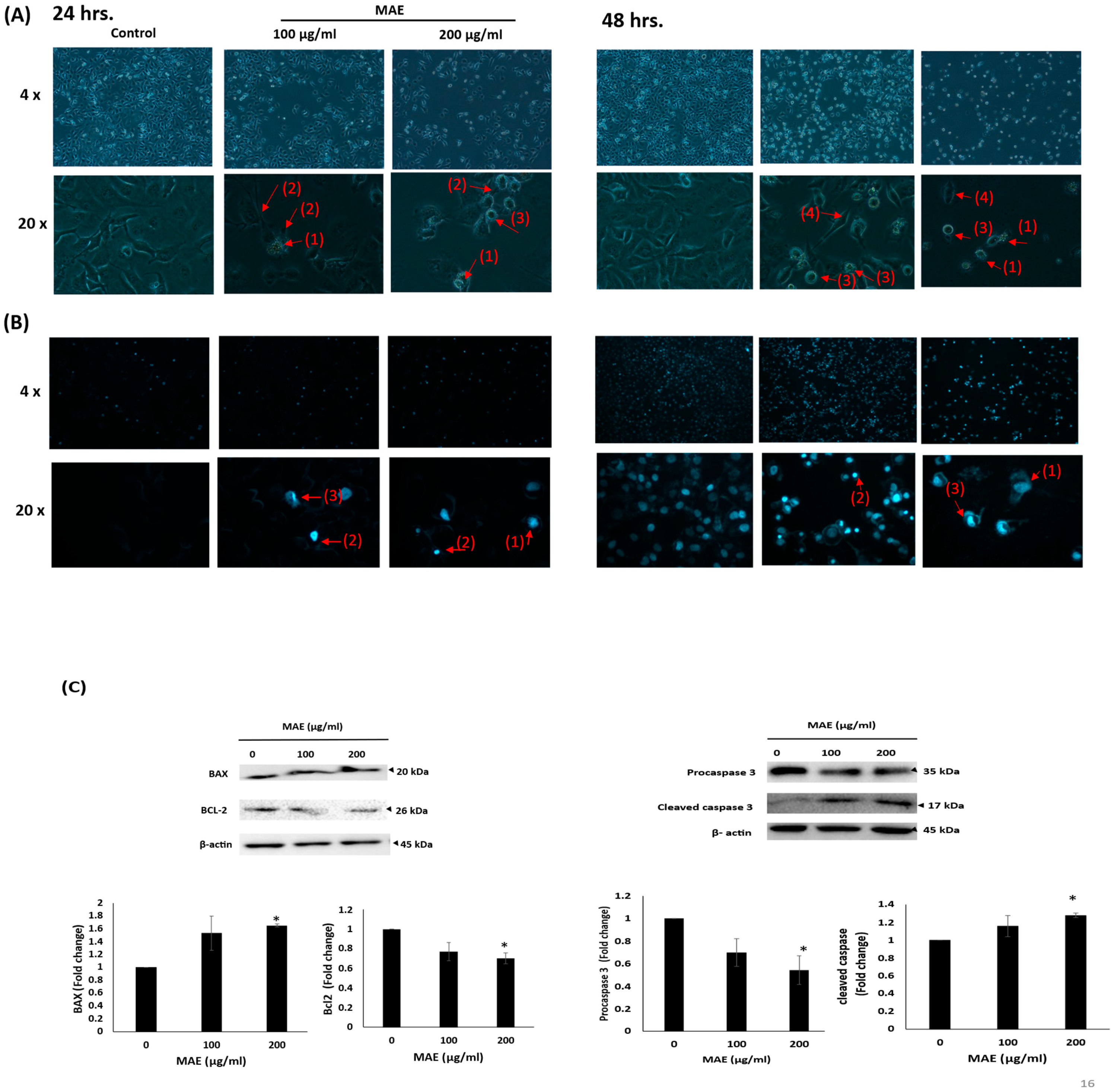
2.4. MDA-MB-231 Cell Proliferation Is Markedly Inhibited by MAE
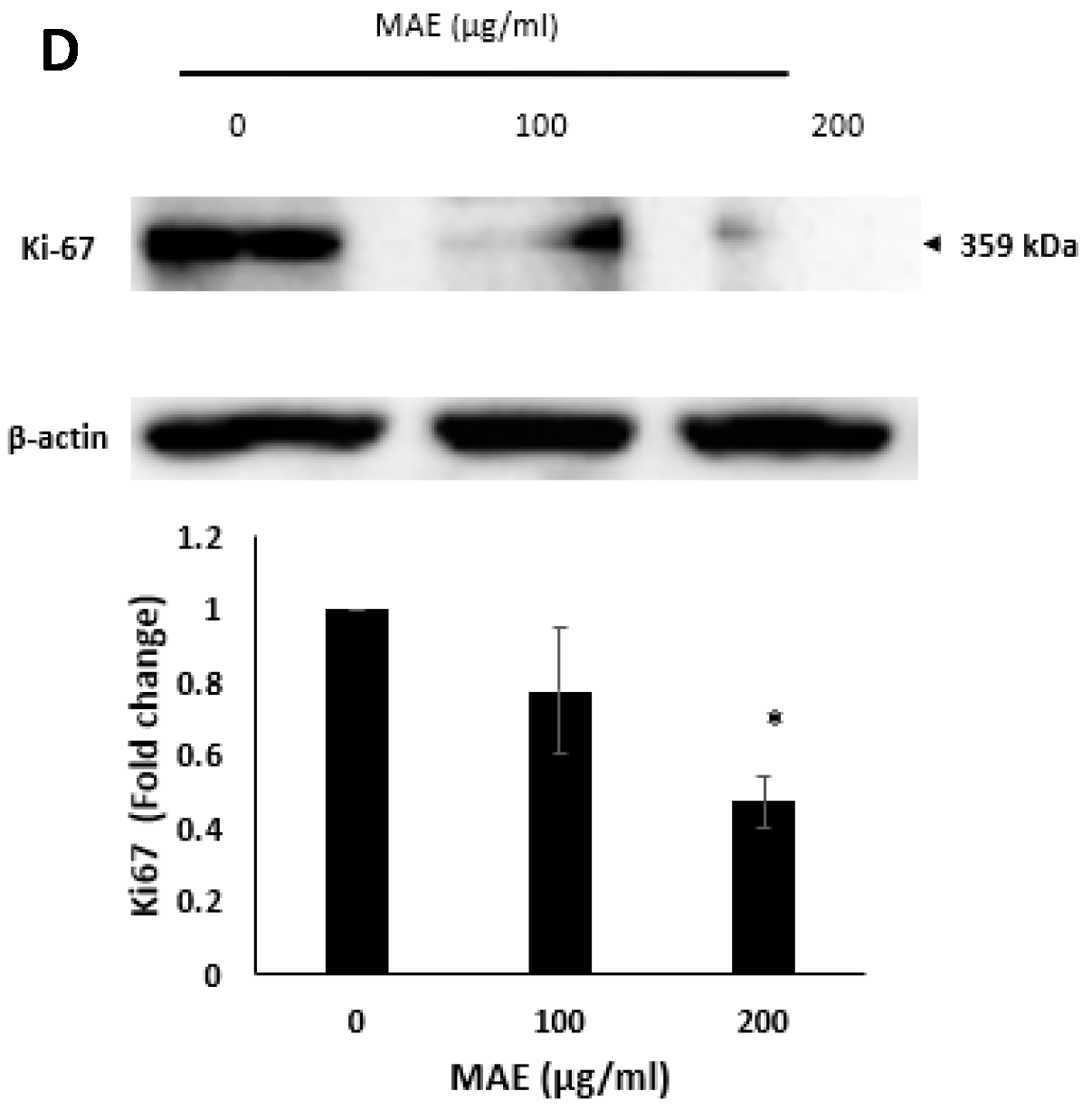
2.5. MAE Triggers Intrinsic Apoptosis in MDA-MB-231 Cells
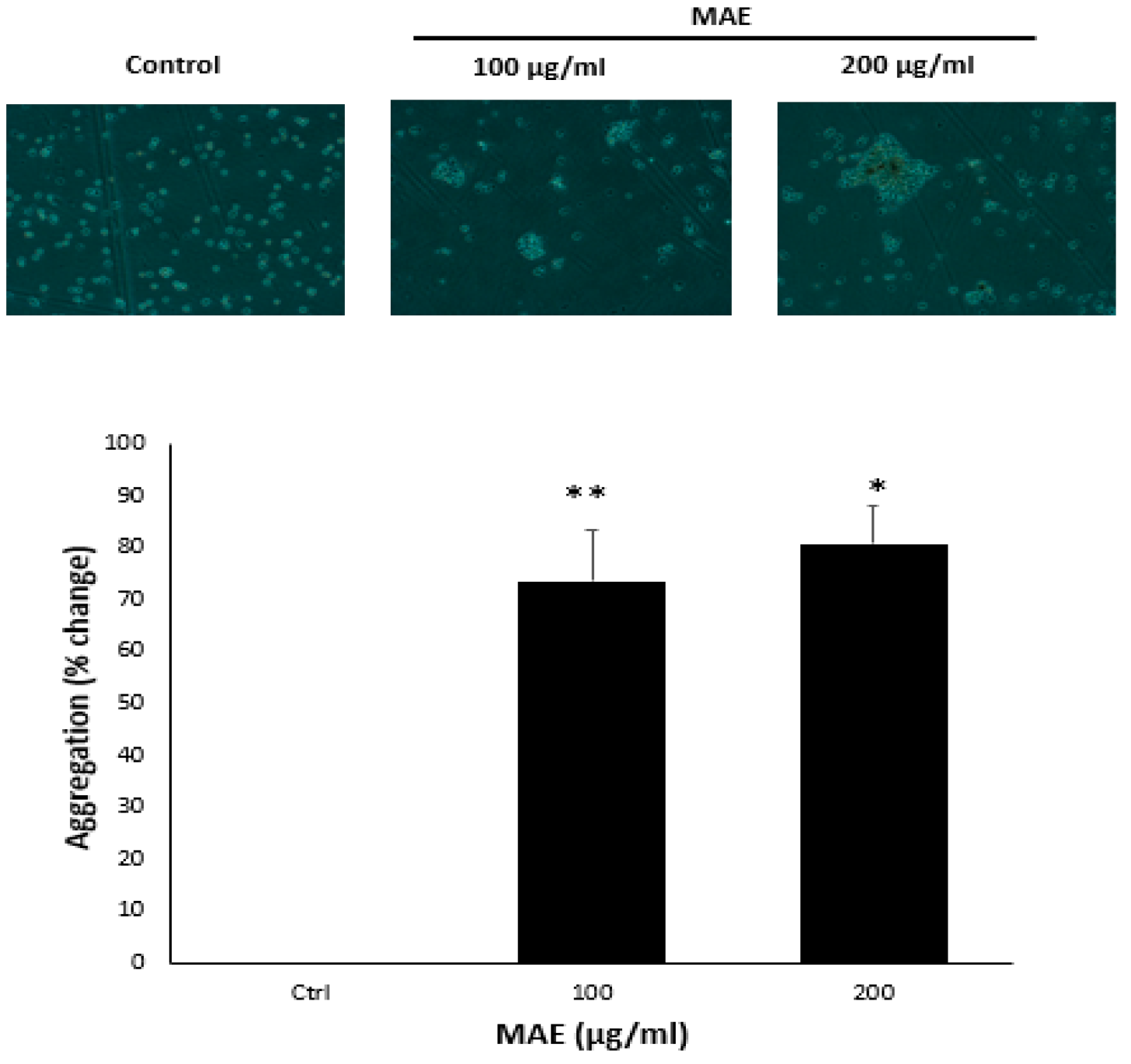
2.6. MAE Enhances the Aggregation of MDA-MB-231 Cells
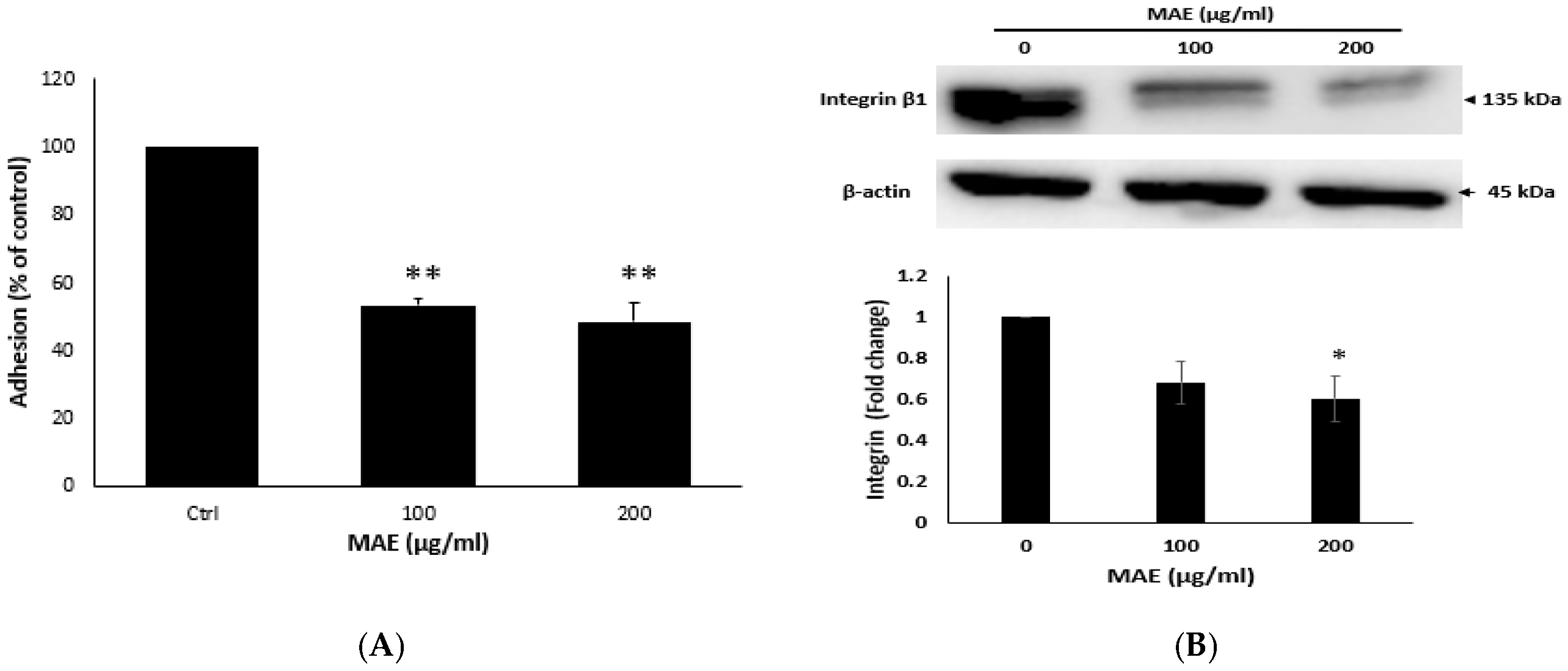
2.7. MAE Lowers the Adhesion Potential of MDA Cells
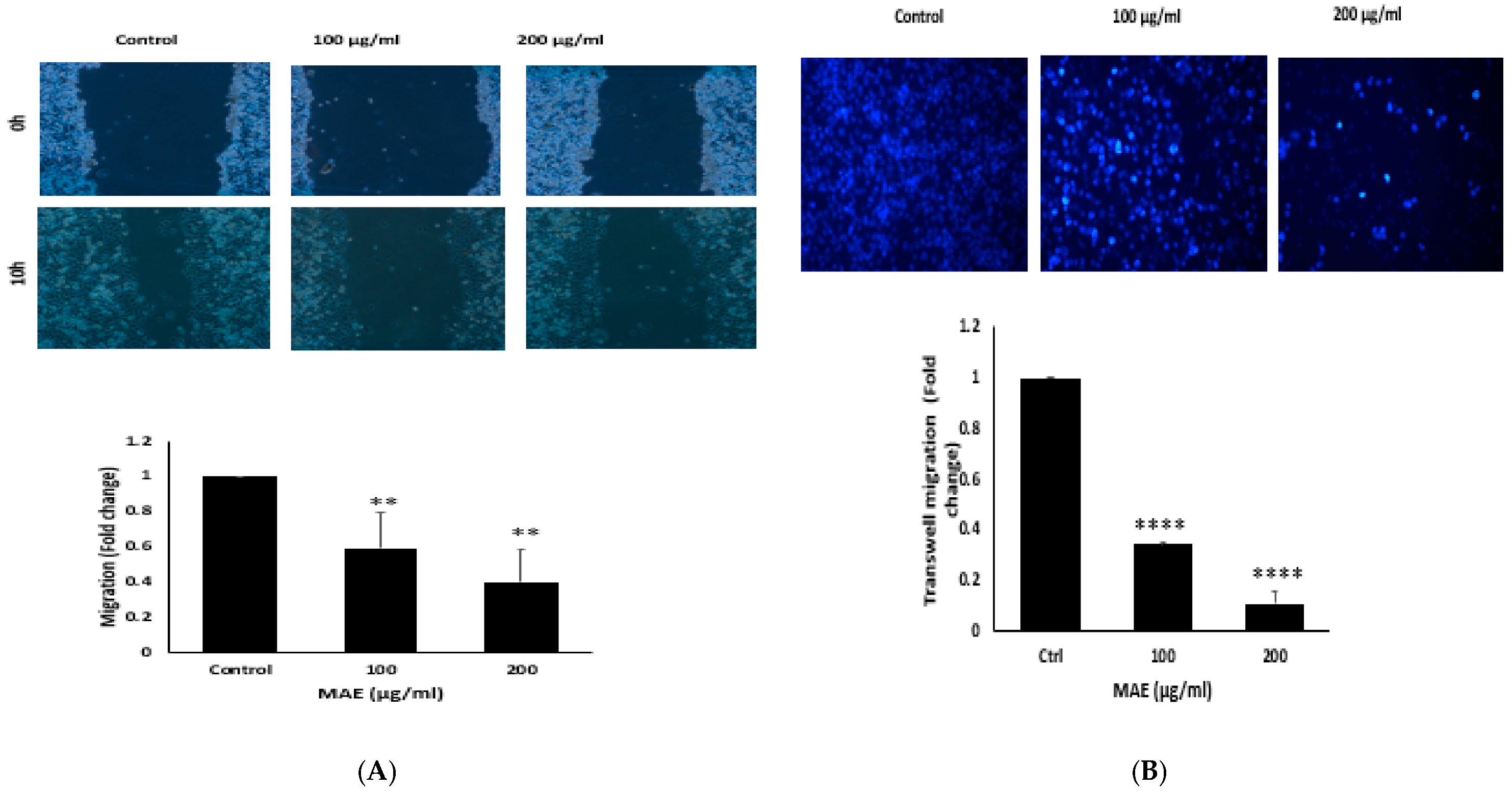
2.8. MAE Decreases MDA Cells’ Ability to Migrate
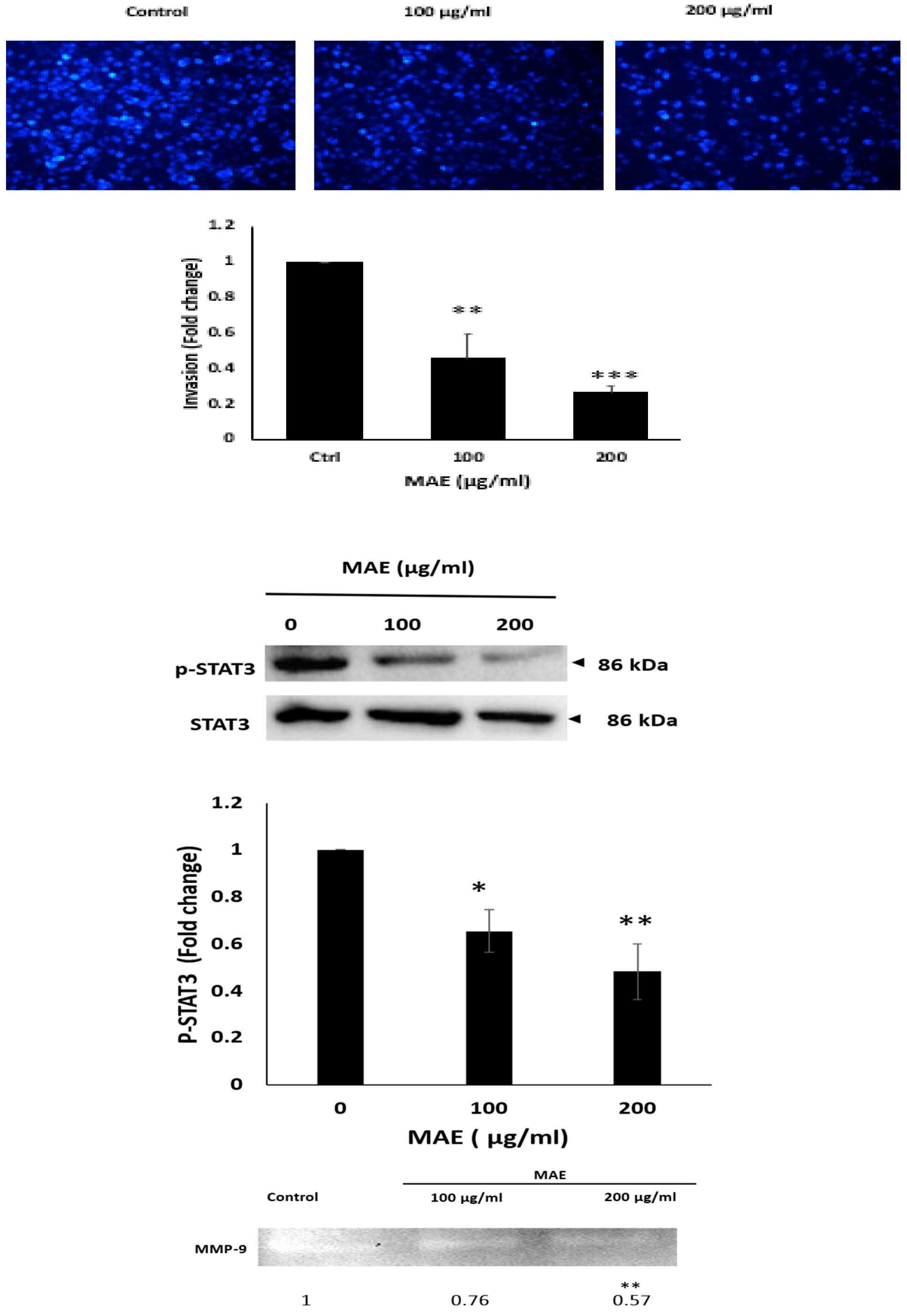
2.9. MAE Inhibits the Invasive Properties of MDA Cells
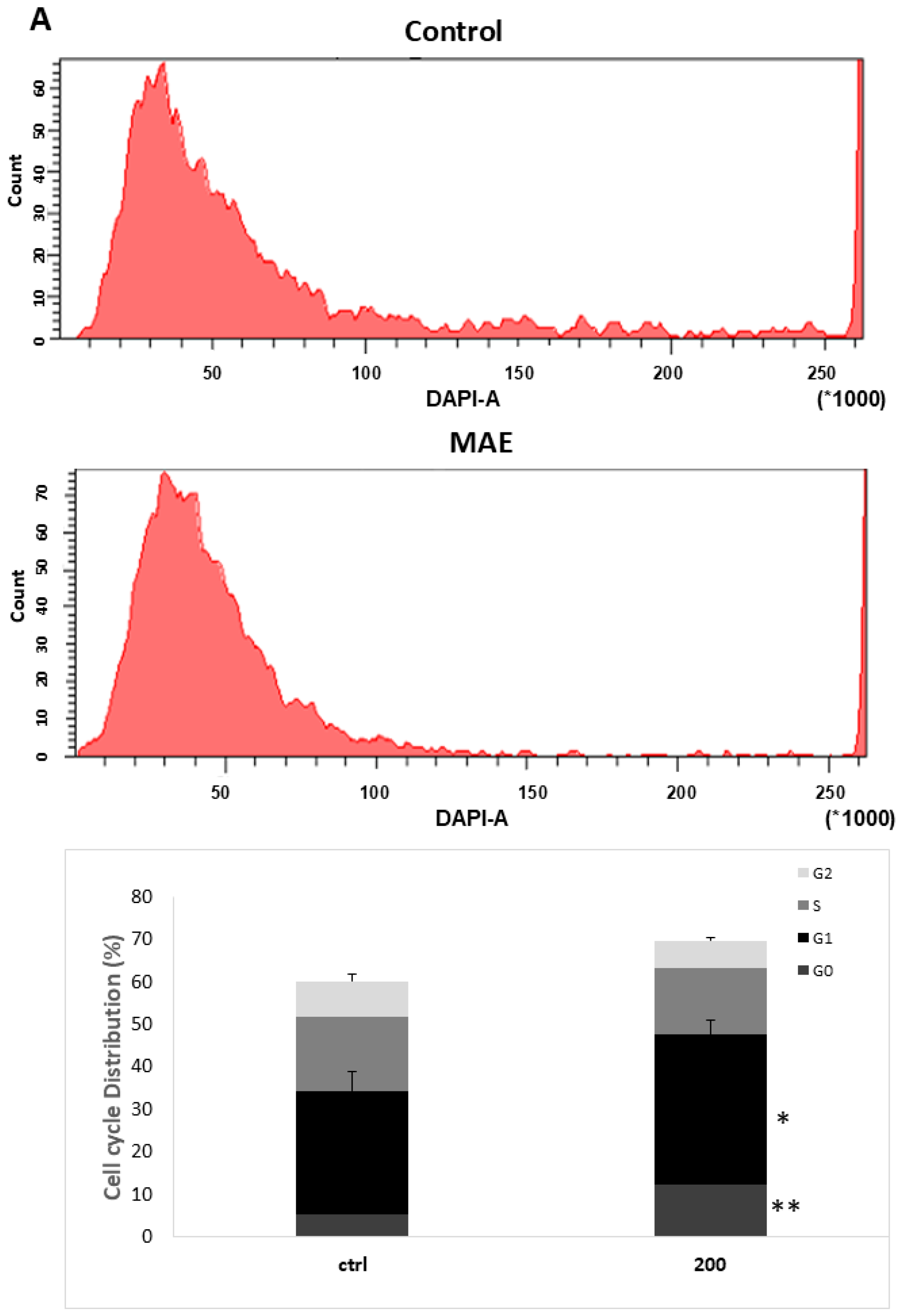
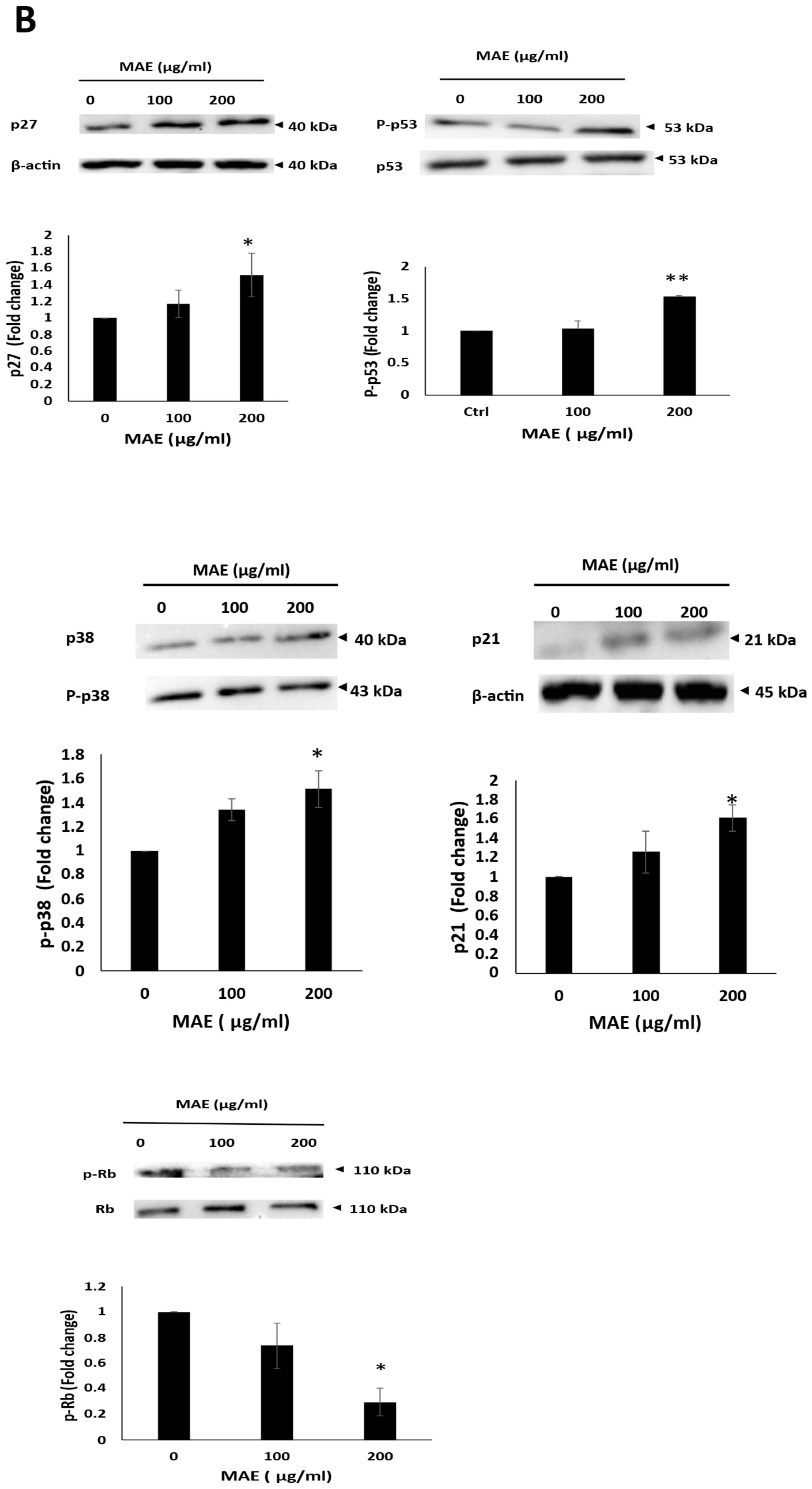
2.10. MAE Causes MDA-MB-231 Cells to Arrest During the Cell Cycle’s G0/G1 Phase
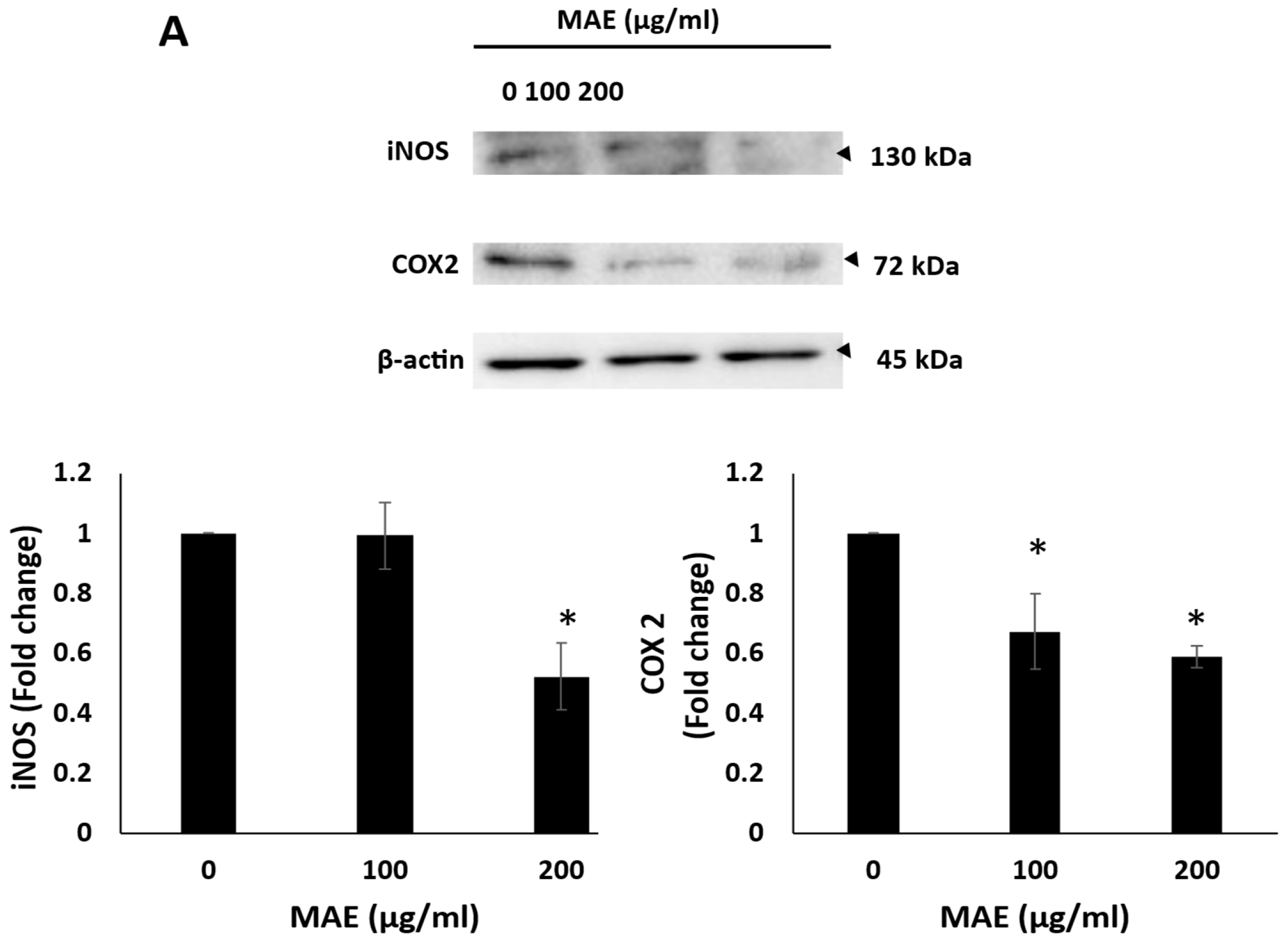
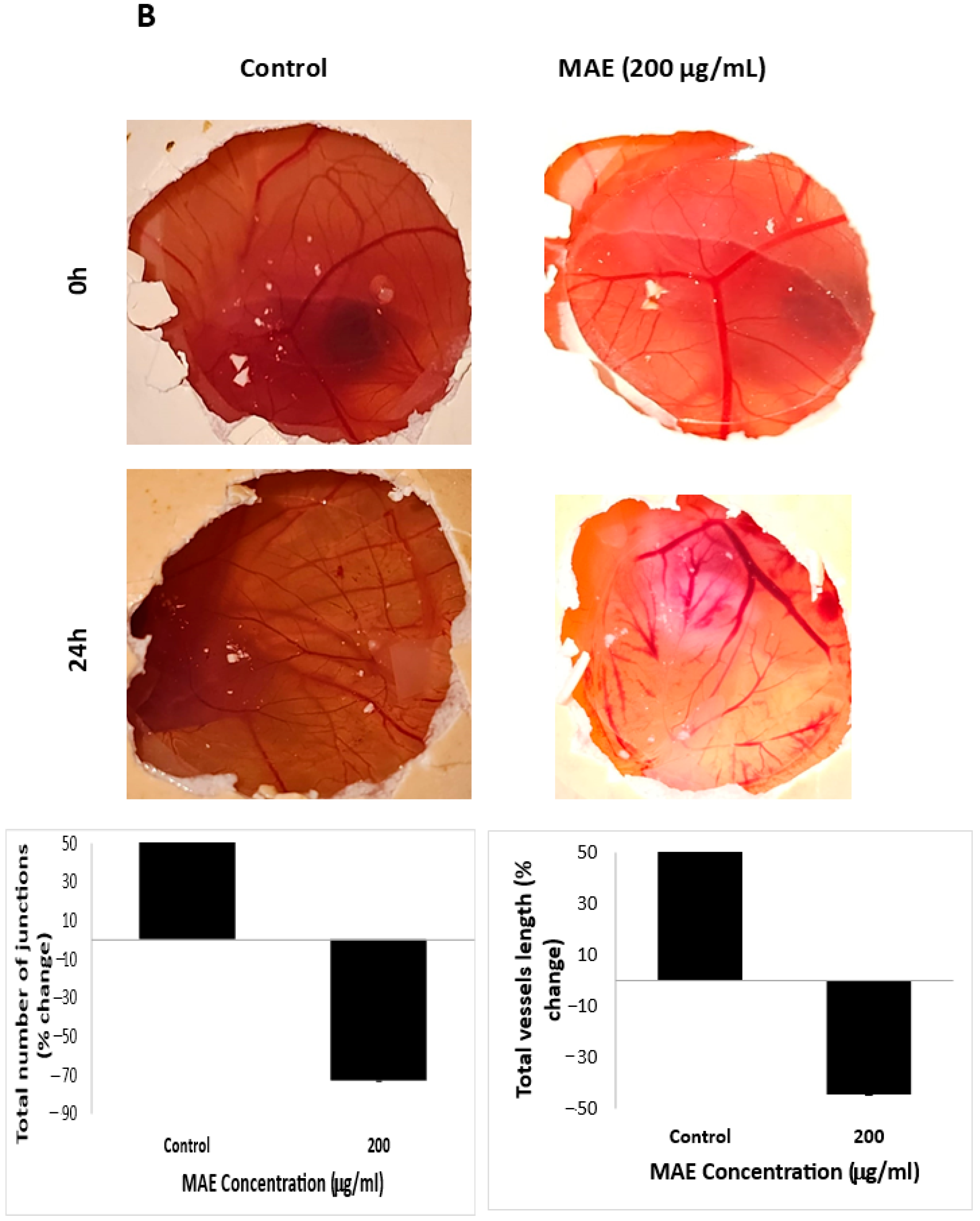
2.11. MAE Reduces iNOS and COX-2 Levels and Inhibits Angiogenesis In Ovo
3. Discussion
4. Materials and Methods
4.1. Collection of Mandragora Autumnalis Leaves and Preparation of Their Ethanolic Extract MAE
4.2. Phytochemical Analysis
4.3. Total Phenolic Content (TPC)
4.4. Total Flavonoid Content (TFC)
4.5. The Antioxidant Activity (DPPH) of Mandragora Autumnalis Ethanolic Extract
4.6. Liquid Chromatography-Mass Spectrometry
4.7. Cell Culture
4.8. MTT Cell Availability Assay
4.9. Migration (Scratch) Assay
4.10. Trans-Well Migration Assay
4.11. Matrigel Invasion Assay
4.12. Aggregation Assay
4.13. Adhesion Assay
4.14. Analysis of Apoptotic Morphological Changes
4.15. Western Blot Analysis
4.16. Gelatin Zymography
4.17. Flow Cytometry Analysis of Cell Cycle
4.18. Chorioallantoic Membrane
4.19. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAE | Mandragora autumnalis ethanolic extract |
| TNBC | Triple-negative breast cancer |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| DOXO | Doxorubicin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EMT | Epithelial–Mesenchymal Transition |
| ECM | Extracellular matrix |
| NAC | N-acetylcysteine |
| ROS | Reactive oxygen species |
| iNOS | Inducible nitric oxide synthase |
| MAPK | Mitogen-Activated Protein Kinase |
| NO | Nitric oxide |
| COX-2 | Cyclooxygenase 2 |
| Rb | Retinoblastoma protein |
| MMP | Matrix metalloproteinases |
| PGE2 | prostaglandin E2 |
| Bcl-2 | B-cell lymphoma 2 |
| Bax | Bcl-2-associated X-protein |
References
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Babar, Q.; Vincent, C.C.N.; Udenze, C.L.; Eze, R.; Okafor, C.J.; Ifionu, B.I.; Amaeze, A.A.; Amaeze, F.N. Therapeutic Targets in Breast Cancer Signaling: A Review. JPRI 2021, 33, 82–99. [Google Scholar] [CrossRef]
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.G.; Hernández Hernández, J.M.; Rotello, V.M.; Ramirez, J.T. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. IJERPH 2020, 17, 2078. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Numan, A.; Agrawal, N.; Tambuwala, M.M.; Singh, V.; Kesharwani, P. Role of Immune Checkpoint Inhibitors in the Revolutionization of Advanced Melanoma Care. Int. Immunopharmacol. 2020, 83, 106417. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Bertoni, D.; Hernandez-Boussard, T.; Telli, M.L.; Wapnir, I.L. Lymph Node Ratio Analysis After Neoadjuvant Chemotherapy Is Prognostic in Hormone Receptor-Positive and Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2016, 23, 3310–3316. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer Statistics in China and United States, 2022: Profiles, Trends, and Determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Korn, A.R.; Reedy, J.; Brockton, N.T.; Kahle, L.L.; Mitrou, P.; Shams-White, M.M. The 2018 World Cancer Research Fund/American Institute for Cancer Research Score and Cancer Risk: A Longitudinal Analysis in the NIH-AARP Diet and Health Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1983–1992. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The Clonal and Mutational Evolution Spectrum of Primary Triple-Negative Breast Cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massagué, J. Genes That Mediate Breast Cancer Metastasis to Lung. Nature 2005, 436, 518–524. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.Z.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The Fate of Chemoresistance in Triple Negative Breast Cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Ávalos, Y.; Canales, J.; Bravo-Sagua, R.; Criollo, A.; Lavandero, S.; Quest, A.F.G. Tumor Suppression and Promotion by Autophagy. BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular Targets of Dietary Agents for Prevention and Therapy of Cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Peto, R.; Boreham, J.; Clarke, M.; Davies, C.; Beral, V. UK and USA Breast Cancer Deaths down 25% in Year 2000 at Ages 20–69 Years. Lancet 2000, 355, 1822. [Google Scholar] [CrossRef]
- Jenča, A.; Mills, D.; Ghasemi, H.; Saberian, E.; Jenča, A.; Karimi Forood, A.M.; Petrášová, A.; Jenčová, J.; Jabbari Velisdeh, Z.; Zare-Zardini, H.; et al. Herbal Therapies for Cancer Treatment: A Review of Phyto-therapeutic Efficacy. BTT 2024, 18, 229–255. [Google Scholar] [CrossRef]
- Mahmod, A.I.; Talib, W.H. Anticancer Activity of Mandragora Autumnalis: An in Vitro and in Vivo Study. PHAR 2021, 68, 827–835. [Google Scholar] [CrossRef]
- Benítez, G.; Leonti, M.; Böck, B.; Vulfsons, S.; Dafni, A. The Rise and Fall of Mandrake in Medicine. J. Ethnopharmacol. 2023, 303, 115874. [Google Scholar] [CrossRef]
- Monadi, T.; Azadbakht, M.; Ahmadi, A.; Chabra, A. A Comprehensive Review on the Ethnopharmacology, Phytochemistry, Pharmacology, and Toxicology of the Mandragora Genus; from Folk Medicine to Modern Medicine. CPD 2021, 27, 3609–3637. [Google Scholar] [CrossRef] [PubMed]
- Albahri, G.; Badran, A.; Baki, Z.A.; Alame, M.; Hijazi, A.; Daou, A.; Mesmar, J.E.; Baydoun, E. Mandragora Autumnalis Distribution, Phytochemical Characteristics, and Pharmacological Bioactivities. Pharmaceuticals 2025, 18, 328. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. ROS Inhibitor N-Acetyl-L-Cysteine Antagonizes the Activity of Proteasome Inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Boudreau, M.W.; Peh, J.; Hergenrother, P.J. Procaspase-3 Overexpression in Cancer: A Paradoxical Observation with Therapeutic Potential. ACS Chem. Biol. 2019, 14, 2335–2348. [Google Scholar] [CrossRef]
- Banta, K.L.; Wang, X.; Das, P.; Winoto, A. B Cell Lymphoma 2 (Bcl-2) Residues Essential for Bcl-2′s Apoptosis-Inducing Interaction with Nur77/Nor-1 Orphan Steroid Receptors. J. Biol. Chem. 2018, 293, 4724–4734. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Troyanovsky, S.M. Adherens Junction: The Ensemble of Specialized Cadherin Clusters. Trends Cell Biol. 2023, 33, 374–387. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Krakhmal, N.V.; Zavyalova, M.V.; Denisov, E.V.; Vtorushin, S.V.; Perelmuter, V.M. Cancer Invasion: Patterns and Mechanisms. Acta Naturae 2015, 7, 17–28. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Sig. Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Man, Q.; Huo, F.; Gao, X.; Lin, H.; Li, S.; Wang, J.; Su, F.; Cai, L.; Shi, Y.; et al. STAT3 Pathway in Cancers: Past, Present, and Future. MedComm 2022, 3, e124. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and Its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the P53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. IJMS 2020, 21, 2346. [Google Scholar] [CrossRef]
- Cress, D.; Engel, B.; Santiago-Cardona, P. The Retinoblastoma Protein: A Master Tumor Suppressor Acts as a Link between Cell Cycle and Cell Adhesion. CHC 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Njau, M.N.; Jacob, J. Inducible Nitric Oxide Synthase Is Crucial for Plasma Cell Survival. Nat. Immunol. 2014, 15, 219–221. [Google Scholar] [CrossRef][Green Version]
- Nørregaard, R.; Kwon, T.-H.; Frøkiær, J. Physiology and Pathophysiology of Cyclooxygenase-2 and Prostaglandin E2 in the Kidney. Kidney Res. Clin. Pract. 2015, 34, 194–200. [Google Scholar] [CrossRef]
- Aarland, R.C.; Bañuelos-Hernández, A.E.; Fragoso-Serrano, M.; Sierra-Palacios, E.D.C.; Díaz De León-Sánchez, F.; Pérez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. Studies on Phytochemical, Antioxidant, Anti-Inflammatory, Hypoglycaemic and Antiproliferative Activities of Echinacea Purpurea and Echinacea Angustifolia Extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A. Effect of High-Temperature Stress on Crop Productivity. In Effect of High Temperature on Crop Productivity and Metabolism of Macro Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–114. ISBN 978-0-12-817562-0. [Google Scholar]
- Jiménez-González, V.; Benítez, G.; Pastor, J.E.; López-Lázaro, M.; Calderón-Montaño, J.M. Evaluation of Anticancer Activity of 76 Plant Species Collected in Andalusia (Spain) against Lung Cancer Cells. Plants 2023, 12, 3275. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural Phenolic Compounds From Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N.; Jaradat, N.; Bassalat, N.; Hawash, M.; Zaid, H. Isolation, Identification and Pharmacological Effects of Mandragora autumnalis Fruit Flavonoids Fraction. Molecules 2022, 27, 1046. [Google Scholar] [CrossRef]
- Ammendola, M.; Haponska, M.; Balik, K.; Modrakowska, P.; Matulewicz, K.; Kazmierski, L.; Lis, A.; Kozlowska, J.; Garcia-Valls, R.; Giamberini, M.; et al. Stability and Anti-Proliferative Properties of Biologically Active Compounds Extracted from Cistus L. after Sterilization Treatments. Sci. Rep. 2020, 10, 6521. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Liang, J.; Wen, T.; Zhang, X.; Luo, X. Chlorogenic Acid as a Potential Therapeutic Agent for Cholangiocarcinoma. Pharmaceuticals 2024, 17, 794. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic Acid Induces Apoptosis, Inhibits Metastasis and Improves Antitumor Immunity in Breast Cancer via the NF-κB Signaling Pathway. Oncol. Rep. 2020, 45, 717–727. [Google Scholar] [CrossRef]
- Ovadje, P.; Chochkeh, M.; Akbari-Asl, P.; Hamm, C.; Pandey, S. Selective Induction of Apoptosis and Autophagy Through Treatment With Dandelion Root Extract in Human Pancreatic Cancer Cells. Pancreas 2012, 41, 1039–1047. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Yoshida, T.; Ozawa, Y.; Kimura, T.; Sato, Y.; Kuznetsov, G.; Xu, S.; Uesugi, M.; Agoulnik, S.; Taylor, N.; Funahashi, Y.; et al. Eribulin Mesilate Suppresses Experimental Metastasis of Breast Cancer Cells by Reversing Phenotype from Epithelial–Mesenchymal Transition (EMT) to Mesenchymal–Epithelial Transition (MET) States. Br. J. Cancer 2014, 110, 1497–1505. [Google Scholar] [CrossRef]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Mrouj, K.; Andrés-Sánchez, N.; Dubra, G.; Singh, P.; Sobecki, M.; Chahar, D.; Al Ghoul, E.; Aznar, A.B.; Prieto, S.; Pirot, N.; et al. Ki-67 Regulates Global Gene Expression and Promotes Sequential Stages of Carcinogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2026507118. [Google Scholar] [CrossRef]
- Yuan, L.; Cai, Y.; Zhang, L.; Liu, S.; Li, P.; Li, X. Promoting Apoptosis, a Promising Way to Treat Breast Cancer With Natural Products: A Comprehensive Review. Front. Pharmacol. 2022, 12, 801662. [Google Scholar] [CrossRef] [PubMed]
- Kamalabadi-Farahani, M.; Najafabadi, M.R.H.; Jabbarpour, Z. Apoptotic Resistance of Metastatic Tumor Cells in Triple Negative Breast Cancer: Roles of Death Receptor-5. Asian Pac. J. Cancer Prev. 2019, 20, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, R.; Shaito, A.A.; Badran, A.; Baydoun, S.; Sobeh, M.; Ouchari, W.; Sahri, N.; Eid, A.H.; Mesmar, J.E.; Baydoun, E. Fractionation and Phytochemical Composition of an Ethanolic Extract of Ziziphus nummularia Leaves: Antioxidant and Anticancerous Properties in Human Triple Negative Breast Cancer Cells. Front. Pharmacol. 2024, 15, 1331843. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, N.; Badran, A.; Baydoun, S.; Al-Sawalmih, A.; Maresca, M.; Baydoun, E.; Mesmar, J.E. The Antioxidant Potential and Anticancer Activity of Halodule uninervis Ethanolic Extract against Triple-Negative Breast Cancer Cells. Antioxidants 2024, 13, 726. [Google Scholar] [CrossRef]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Jain, A.K.; Barton, M.C. P53: Emerging Roles in Stem Cells, Development and Beyond. Development 2018, 145, dev158360. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Berke, T.P.; Slight, S.H.; Hyder, S.M. Role of Reactivating Mutant P53 Protein in Suppressing Growth and Metastasis of Triple-Negative Breast Cancer. OTT 2022, 15, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dumaz, N.; Milne, D.M.; Meek, D.W. Protein Kinase CK1 Is a P53-threonine 18 Kinase Which Requires Prior Phosphorylation of Serine 15. FEBS Lett. 1999, 463, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Meek, D.W.; Anderson, C.W. Posttranslational Modification of P53: Cooperative Integrators of Function. Cold Spring Harb. Perspect. Biol. 2009, 1, a000950. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, L.; García-Ortega, M.B.; Ruiz-Alcalá, G.; Carrillo, E.; Marchal, J.A.; García, M.Á. The P38 MAPK Components and Modulators as Biomarkers and Molecular Targets in Cancer. IJMS 2021, 23, 370. [Google Scholar] [CrossRef]
- Phong, M.S.; Van Horn, R.D.; Li, S.; Tucker-Kellogg, G.; Surana, U.; Ye, X.S. P38 Mitogen-Activated Protein Kinase Promotes Cell Survival in Response to DNA Damage but Is Not Required for the G2 DNA Damage Checkpoint in Human Cancer Cells. Mol. Cell. Biol. 2010, 30, 3816–3826. [Google Scholar] [CrossRef]
- Whitaker, R.H.; Cook, J.G. Stress Relief Techniques: P38 MAPK Determines the Balance of Cell Cycle and Apoptosis Pathways. Biomolecules 2021, 11, 1444. [Google Scholar] [CrossRef]
- Gubern, A.; Joaquin, M.; Marquès, M.; Maseres, P.; Garcia-Garcia, J.; Amat, R.; González-Nuñez, D.; Oliva, B.; Real, F.X.; de Nadal, E.; et al. The N-Terminal Phosphorylation of RB by P38 Bypasses Its Inactivation by CDKs and Prevents Proliferation in Cancer Cells. Mol. Cell 2016, 64, 25–36. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.A.; Terluk, M.R.; Kartha, R.V. N-Acetylcysteine Pharmacology and Applications in Rare Diseases—Repurposing an Old Antioxidant. Antioxidants 2023, 12, 1316. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Y.; Lu, G.; Xie, S.; Ma, Z.; Chen, Z.; Shen, H.-M.; Xia, D. Importance of ROS-Mediated Autophagy in Determining Apoptotic Cell Death Induced by Physapubescin B. Redox Biol. 2017, 12, 198–207. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Fortier, A.-M.; Asselin, E.; Cadrin, M. Keratin 8 and 18 Loss in Epithelial Cancer Cells Increases Collective Cell Migration and Cisplatin Sensitivity through Claudin1 Up-Regulation. J. Biol. Chem. 2013, 288, 11555–11571. [Google Scholar] [CrossRef]
- Haeger, A.; Wolf, K.; Zegers, M.M.; Friedl, P. Collective Cell Migration: Guidance Principles and Hierarchies. Trends Cell Biol. 2015, 25, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, H. Prognostic Values of Tumoral MMP2 and MMP9 Overexpression in Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2021, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- El Hasasna, H.; Saleh, A.; Samri, H.A.; Athamneh, K.; Attoub, S.; Arafat, K.; Benhalilou, N.; Alyan, S.; Viallet, J.; Dhaheri, Y.A.; et al. Rhus Coriaria Suppresses Angiogenesis, Metastasis and Tumor Growth of Breast Cancer through Inhibition of STAT3, NFκB and Nitric Oxide Pathways. Sci. Rep. 2016, 6, 21144. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Jorjani, M.; Pourgholami, M.H. The Effects of Anticancer Medicinal Herbs on Vascular Endothelial Growth Factor Based on Pharmacological Aspects: A Review Study. Nutr. Cancer 2021, 73, 1–15. [Google Scholar] [CrossRef]
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Mbaria, J.M.; Kiama, S.G. Medicinal Plants Used in Treatment and Management of Cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014, 151, 1040–1055. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional Control and Biological Impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Mohammad, I.; Liu, Z. Overview of the STAT-3 Signaling Pathway in Cancer and the Development of Specific Inhibitors. Oncol. Lett. 2020, 19, 2585–2594. [Google Scholar] [CrossRef]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in Mediating the Cell Growth, Differentiation and Survival Signals Relayed through the IL-6 Family of Cytokine Receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically Exploiting STAT3 Activity in Cancer—Using Tissue Repair as a Road Map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in Cancer Inflammation and Immunity: A Leading Role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Zhu, S.; He, J.; Yin, L.; Zhou, J.; Lian, J.; Ren, Y.; Zhang, X.; Yuan, J.; Wang, G.; Li, X. Matrix Metalloproteinases Targeting in Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2024, 42, 275–287. [Google Scholar] [CrossRef]
- Barillari, G. The Impact of Matrix Metalloproteinase-9 on the Sequential Steps of the Metastatic Process. IJMS 2020, 21, 4526. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Y. P53, Oxidative Stress, and Aging. Antioxid. Redox Signal. 2011, 15, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, D.; Yao, Z.; Lin, X.; Liu, J.; Gu, Q.; Dong, X.; Liu, F.; Wang, Y.; Yao, N.; et al. Anti-Angiogenic Treatment Promotes Triple-Negative Breast Cancer Invasion via Vasculogenic Mimicry. Cancer Biol. Ther. 2017, 18, 205–213. [Google Scholar] [CrossRef]
- Chen, T. Unveiling the Significance of Inducible Nitric Oxide Synthase: Its Impact on Cancer Progression and Clinical Implications. Cancer Lett. 2024, 592, 216931. [Google Scholar] [CrossRef]
- Mesmar, J.; Abdallah, R.; Hamade, K.; Baydoun, S.; Al-Thani, N.; Shaito, A.; Maresca, M.; Badran, A.; Baydoun, E. Ethanolic Extract of Origanum syriacum L. Leaves Exhib. Potent Anti-Breast Cancer Potential Robust Antioxid. Properties. Front. Pharmacol. 2022, 13, 994025. [Google Scholar] [CrossRef]

 : inhibits.
: inhibits.
 : inhibits.
: inhibits.
| Metabolite | MAE |
|---|---|
| Anthraquinones | - |
| Tannins | + |
| Resins | - |
| Terpenoids | + |
| Flavonoids | + |
| Quinones | - |
| Anthocyanins | - |
| Saponins | - |
| Phenols | + |
| Steroids | + |
| Cardiac glycosides | - |
| Fixed oils and fatty acids | + |
| Assay Type | MAE |
|---|---|
| TPC (mg GAE/g) | 58.98 ± 7.40 |
| TFC (mg QE/g) | 36.47 ± 0.87 |
| A. Positive Ionization Mode | ||||||
|---|---|---|---|---|---|---|
| Number | m/z | RT [min] | Ions | Compound Name | Molecular Formula | Intensity |
| 1 | 127.0389 | 0.58 | [M + H]+ | 5-Hydroxymethyl-2-furancarboxaldehyde | C6H6O3 | 49,130.136 |
| 2 | 133.0827 | 0.71 | [M + H]+ | Ethyl 3-hydroxy-butanoate | C6H12O3 | 72,955.974 |
| 3 | 140.1066 | 0.86 | [M + H]+ | Tropinone | C8H13NO | 21,053.859 |
| 4 | 149.0596 | 1.23 | [M + H]+ | 3-(Methylthio)propyl acetate | C6H12O2S | 8790.512 |
| 5 | 117.0542 | 1.3 | [M + H]+ | 1-Hydroxy-2-propanone acetate | C5H8O3 | 8646.324 |
| 6 | 193.0492 | 2.91 | [M + H-C6H10O5]+ | Chlorogenic acid | C16H18O9 | 669,015.883 |
| 355.1018 | [M + H]+ | 221,652.966 | ||||
| 445.0708 | [M + Na + NaCOOH]+ | 20,681.809 | ||||
| 7 | 619.2479 | 2.91 | [M + H]+ | Simulanoquinoline | C37H34N2O7 | 11,326.110 |
| 8 | 641.2302 | [M + Na]+ | 90,245.22 | |||
| 9 | 290.1745 | 3.89 | [M + H]+ | Hyoscyamine | C17H23NO3 | 5,938,808.003 |
| 10 | 303.0494 | 9.16 | [M + H]+ | Quercetin | C15H10O7 | 20,868.344 |
| 11 | 179.1178 | 9.6 | [M + H]+ | Ethyl hydrocinnamate | C11H14O2 | 24,858.923 |
| 12 | 255.0862 | 13.43 | [M + H]+ | Chrysin | C15H10O4 | 11,043.2 |
| 13 | 281.266 | 26.76 | [M + H]+ | Linoleic acid | C18H32O2 | 70,427.883 |
| 14 | 311.2933 | 27.7 | [M + H]+ | Ethyl oleate | C20H38O2 | 4151.762 |
| 15 | 243.2505 | 28.62 | [M + H]+ | n-Pentadecanoic acid | C15H30O2 | 13,652.643 |
| 16 | 193.1581 | 29.04 | [M + H]+ | Ionone (β-Ionone) | C13H20O | 10,667.398 |
| 17 | 307.266 | 29.32 | [M + H]+ | Ethyl linolenate | C20H34O2 | 10,224.308 |
| 18 | 156.138 | 29.41 | [M + H]+ | Methylisopelletierine | C9H17NO | 48,479.510 |
| 19 | 114.0911 | 29.41 | [M + H-C2H4]+ | Tropine | C8H15NO | 42,742.76 |
| 142.1224 | [M + H]+ | 57,239.581 | ||||
| 20 | 336.2868 | 29.49 | [M + Na]+ | Solacaproine | C18H39N3O | 8765.329 |
| 21 | 256.2629 | 29.51 | [M + H]+ | Hexadecanamide (Palmitic amide) | C16H33NO | 7,806,388.659 |
| 278.2449 | [M + Na]+ | 1,763,628.958 | ||||
| 511.5185 | 29.52 | [2M + H]+ | 449,241.592 | |||
| 533.5006 | [2M + Na]+ | 363,277.043 | ||||
| 294.2182 | [M + K]+ | 35,062.909 | ||||
| 22 | 297.2893 | [M + H-NH3]+ | Solacaproine | C18H39N3O | 86,740.906 | |
| 314.3049 | [M + H]+ | 52,611.286 | ||||
| 23 | 285.2879 | 29.77 | [M + H]+ | Ethyl palmitate | C18H36O2 | 58,073.530 |
| B. Negative ionization mode | ||||||
| 24 | 111.0088 | 0.75 | [M-H]- | 3-Methyl-2-5-furandione | C5H4O3 | 100.285 |
| 25 | 117.01932 | 0.83 | [M-H]- | Succinic acid | C4H6O4 | 12,012 |
| 26 | 353.08783 | 2.21 | [M-H]- | Chlorogenic acid | C16H18O9 | 258,912 |
| 27 | 207.050913 | 3.29 | [M-H]- | 4-O-Methylglucuronic acid | C7H12O7 | 4598.659 |
| 28 | 131.07127 | 3.33 | [M-H]- | Ethyl 3-hydroxy-butanoate | C6H12O3 | 2582 |
| 29 | 179.03492 | 3.86 | [M-H]- | Caffeic Acid | C9H8O4 | 5934 |
| 30 | 175.04000 | 4.23 | [M-H-COCH2]- | 4-Methylumbelliferyl acetate | C12H10O4 | 27,106.992 |
| 217.05106 | [M-H]- | 4492.950 | ||||
| 31 | 176.01133 | 6.56 | [M-H-CH3]- | Scopoletin | C10H8O4 | 26,388.932 |
| 191.03486 | [M-H]- | 43,250.386 | ||||
| 259.02198 | [M-H + NaCOOH]- | 9135.296 | ||||
| 32 | 609.1457 | 9.19 | [M-H]- | Rutin | C27H30O16 | 25,850 |
| 33 | 463.08799 | 10.39 | [M-H]- | Hyperoside | C21H20O12 | 18,198 |
| 34 | 277.21675 | 29.84 | [M-H]- | Linolenic acid | C18H30O2 | 51,774.794 |
| 345.20465 | [M-H + NaCOOH]- | 4077.093 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albahri, G.; Badran, A.; Hellany, H.; Baydoun, S.; Abdallah, R.; Alame, M.; Hijazi, A.; Maresca, M.; Baydoun, E. Mandragora autumnalis: Phytochemical Composition, Antioxidant and Anti-Cancerous Bioactivities on Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 8506. https://doi.org/10.3390/ijms26178506
Albahri G, Badran A, Hellany H, Baydoun S, Abdallah R, Alame M, Hijazi A, Maresca M, Baydoun E. Mandragora autumnalis: Phytochemical Composition, Antioxidant and Anti-Cancerous Bioactivities on Triple-Negative Breast Cancer Cells. International Journal of Molecular Sciences. 2025; 26(17):8506. https://doi.org/10.3390/ijms26178506
Chicago/Turabian StyleAlbahri, Ghosoon, Adnan Badran, Heba Hellany, Serine Baydoun, Rola Abdallah, Mohamad Alame, Akram Hijazi, Marc Maresca, and Elias Baydoun. 2025. "Mandragora autumnalis: Phytochemical Composition, Antioxidant and Anti-Cancerous Bioactivities on Triple-Negative Breast Cancer Cells" International Journal of Molecular Sciences 26, no. 17: 8506. https://doi.org/10.3390/ijms26178506
APA StyleAlbahri, G., Badran, A., Hellany, H., Baydoun, S., Abdallah, R., Alame, M., Hijazi, A., Maresca, M., & Baydoun, E. (2025). Mandragora autumnalis: Phytochemical Composition, Antioxidant and Anti-Cancerous Bioactivities on Triple-Negative Breast Cancer Cells. International Journal of Molecular Sciences, 26(17), 8506. https://doi.org/10.3390/ijms26178506









