Co-Occurrence of Endometriosis with Systemic Lupus Erythematosus: Genetic Aspects
Abstract
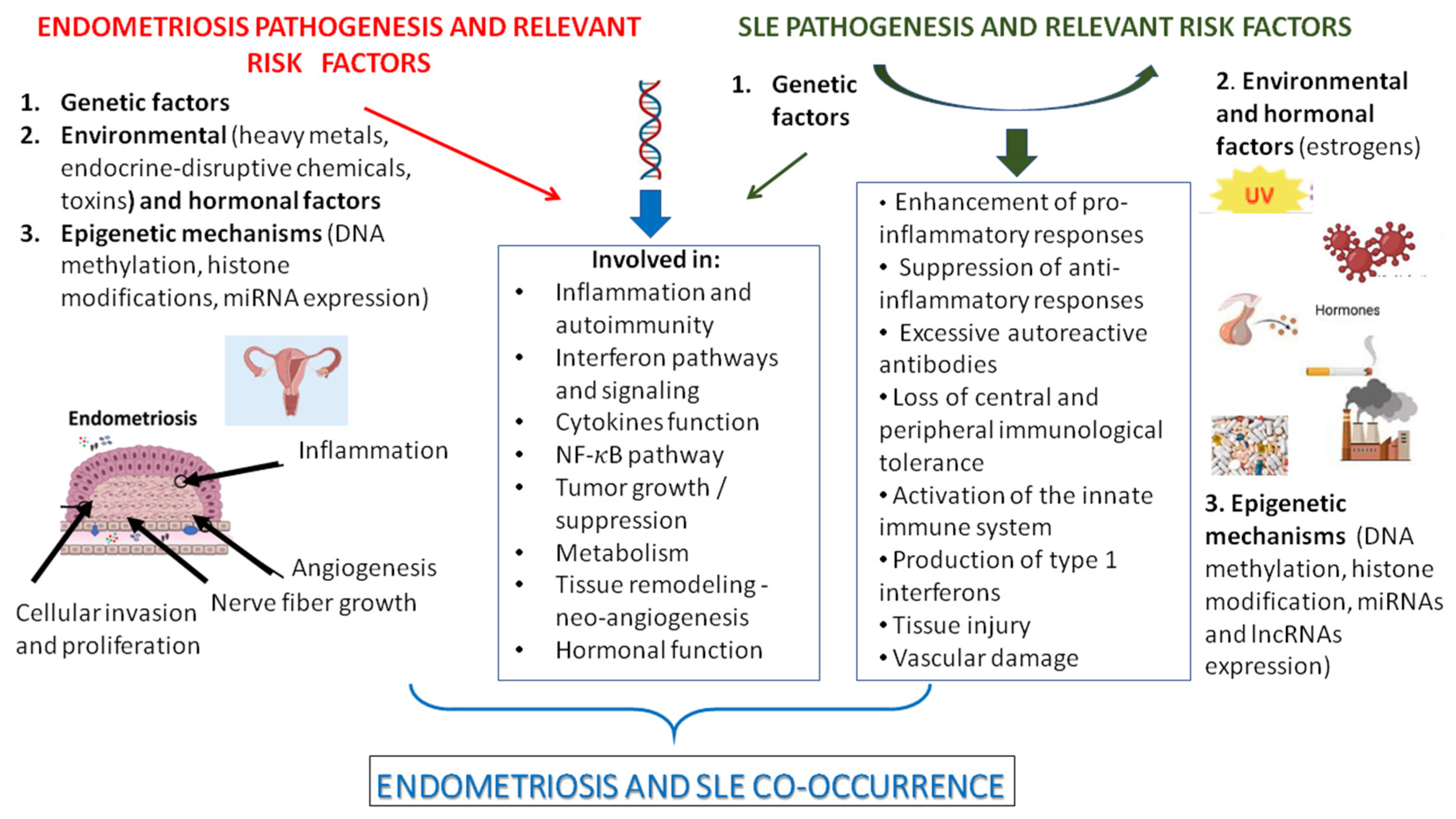
1. Introduction
2. Genetics of Endometriosis and SLE
3. Shared Susceptibility Loci Between Endometriosis and SLE
4. Biological Mechanisms Related to the Co-Occurrence of Endometriosis and SLE
4.1. Polymorphisms in Genes Associated with Interferon Pathways and Signaling
4.2. Polymorphisms in Inflammation and Autoimmunity-Related Genes
4.3. Polymorphisms in Cytokine Genes
4.4. Polymorphisms in Genes Involved in the NF-κB Pathways
4.5. Polymorphisms in Tumor Growth/Suppression and Metabolism-Related Genes
4.6. Polymorphisms in Genes Involved in Hormonal Function
5. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEA | Autoantibodies to endometrial antigens |
| ANA | Antinuclear antibodies |
| AS | Ankylosing spondylitis |
| aPL | Antiphospholipid antibodies |
| CeD | Coeliac disease |
| CD | Crohn’s disease |
| GWAS | Genome wide association studies |
| lncRNAs | Long non-coding RNAs |
| miRs | microRNAS |
| MS | Multiple sclerosis |
| NIK | NF-κB-inducing kinase |
| PS | Psoriatic arthritis |
| RA | Rheumatoid arthritis |
| SLE | Systemic lupus erythematosus |
| SNPs | Single nucleotide polymorphisms |
| SS | Sjogren’s disease |
| Th1 | T-helper 1 cells |
| Th2 | T-helper 2 cells |
| Th17 | T-helper 17 cells |
| Tregs | T-regulatory cells |
| UC | Ulcerative colitis |
| WES | Whole exome sequencing |
References
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The immunopathophysiology of endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Menzhinskaya, I.V.; Pavlovich, S.V.; Melkumyan, A.G.; Chuprynin, V.D.; Yarotskaya, E.; Sukhikh, G.T. Potential Significance of Serum Autoantibodies to Endometrial Antigens, α-Enolase and Hormones in Non-Invasive Diagnosis and Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 15578. [Google Scholar] [CrossRef] [PubMed]
- Zervou, M.I.; Vlachakis, D.; Papageorgiou, L.; Eliopoulos, E.; Goulielmos, G.N. Increased risk of rheumatoid arthritis in patients with endometriosis: Genetic aspects. Rheumatology 2022, 61, 4252–4262. [Google Scholar] [CrossRef] [PubMed]
- Zervou, M.I.; Papageorgiou, L.; Vlachakis, D.; Spandidos, D.A.; Eliopoulos, E.; Goulielmos, G.N. Genetic factors involved in the co-occurrence of endometriosis with ankylosing spondylitis (AS). Mol. Med. Rep. 2023, 27, 96. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Isenberg, D.A.L. Systemic lupus erythematosus. N. Engl. J. Med. 2008, 358, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Harley, J.B.; Kelly, J.A.; Kaufman, K.M. Unraveling the genetics of systemic lupus erythematosus. Springer Semin. Immunopathol. 2006, 28, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Sinaii, N.; Cleary, S.D.; Ballweg, M.L.; Nieman, L.K.; Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum. Reprod. 2002, 17, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Rafi, U.; Ahmad, S.; Bokhari, S.S.; Iqbal, M.A.; Zia, A.; Khan, M.A.; Roohi, N. Association of inflammatory markers/cytokines with cardiovascular risk manifestation in patients with endometriosis. Mediators Inflamm. 2021, 2021, 3425560. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Low, H.Y.; Chen, B.S.; Huang, K.S.; Zhang, Y.; Wang, Y.H.; Ye, Z.; Wei, J.C. Risk of ankylosing spondylitis in patients with endometriosis: A population-based retrospective cohort study. Front. Immunol. 2022, 13, 877942. [Google Scholar] [CrossRef] [PubMed]
- Santoro, L.; Campo, S.; D’Onofrio, F.; Gallo, A.; Covino, M.; Campo, V.; Palombini, G.; Santoliquido, A.; Gasbarrini, G.; Montalto, M. Looking for celiac disease in Italian women with endometriosis: A case control study. BioMed Res. Int. 2014, 2014, 236821. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Frisch, M.; Jørgensen, K.T.; Pedersen, B.V.; Nielsen, N.M. Increased risk of inflammatory bowel disease in women with endometriosis: A nationwide Danish cohort study. Gut 2012, 61, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Korkes, K.M.N.; Li, T.; Kvaskoff, M.; Cho, E.; Carvalho, L.F.; Qureshi, A.A.; Abrao, M.; Missmer, S.A. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am. J. Epidemiol. 2022, 191, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-H.; Liu, C.-H.; Pan, Y.-A.; Yen, F.-S.; Chiou, J.-Y.; Wei, J.C.-C. Association Between Endometriosis and Subsequent Risk of Sjögren’s Syndrome: A Nationwide Population-Based Cohort Study. Front. Immunol. 2022, 13, 845944. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Costenbader, K.H.; Mu, F.; Kvaskoff, M.; Malspeis, S.; Karlson, E.W.; Missmer, S.A. Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses’ Health Study II. Ann. Rheum. Dis. 2016, 75, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Zervou, M.I.; Tarlatzis, B.C.; Grimbizis, G.F.; Spandidos, D.A.; Niewold, T.B.; Goulielmos, G.N. Association of endometriosis with Sjögren’s syndrome: Genetic insights (Review). Int. J. Mol. Med. 2024, 53, 20. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Pettersson, H.J.; Svedberg, P.; Olovsson, M.; Bergqvist, A.; Marions, L.; Tornvall, P.; Kuja-Halkola, R. Heritability of endometriosis. Fertil. Steril. 2015, 104, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Matalliotakis, M.; Zervou, M.I.; Matalliotaki, C.; Rahmioglu, N.; Koumantakis, G.; Kalogiannidis, I.; Prapas, I.; Zondervan, K.; Spandidos, D.A.; Matalliotakis, I.; et al. The role of gene polymorphisms in endometriosis. Mol. Med. Rep. 2017, 16, 5881–5886. [Google Scholar] [CrossRef] [PubMed]
- Matalliotakis, M.; Zervou, M.I.; Eliopoulos, E.; Matalliotaki, C.; Rahmioglu, N.; Kalogiannidis, I.; Zondervan, K.; Spandidos, D.A.; Matalliotakis, I.; Goulielmos, G.N. The role of IL16 gene polymorphisms in endometriosis. Int. J. Mol. Med. 2018, 41, 1469–1476. [Google Scholar] [PubMed]
- Rahmioglu, N.; Nyholt, D.R.; Morris, A.P.; Missmer, S.A.; Montgomery, G.W.; Zondervan, K.T. Genetic variants underlying risk of endometriosis: Insights from meta-analysis of eight genome-wide association and replication datasets. Hum. Reprod. Update 2014, 20, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, Y.; Steinthorsdottir, V.; Morris, A.P.; Fassbender, A.; Rahmioglu, N.; De Vivo, I.; Buring, J.E.; Zhang, F.; Edwards, T.L.; Jones, S.; et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat. Commun. 2017, 8, 15539. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, L.; Matalliotakis, M.; Zervou, M.I.; Matalliotaki, C.; Krithinakis, K.; Matalliotakis, I.; Spandidos, D.A.; Goulielmos, G.N. Defining the genetic profile of endometriosis. Exp. Therap Med. 2019, 17, 3267–3281. [Google Scholar]
- Rahmioglu, N.; Mortlock, S.; Ghiasi, M.; Møller, P.L.; Stefansdottir, L.; Galarneau, G.; Turman, C.; Danning, R.; Law, M.H.; Sapkota, Y.; et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat. Genet. 2023, 55, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Koukoura, O.; Sifakis, S.; Spandidos, D.A. DNA methylation in endometriosis (Review). Mol. Med. Rep. 2016, 13, 939–2948. [Google Scholar] [CrossRef] [PubMed]
- Goulielmos, G.N.; Matalliotakis, M.; Matalliotaki, C.; Eliopoulos, E.; Matalliotakis, I.; Zervou, M.I. Endometriosis research in the -omics era. Gene 2020, 741, 144545. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.A.; Nolte, I.M.; van der Steege, G.; Schipper, M.; Kallenberg, C.G.; Te Meerman, G.J.; Bijl, M. An extensive screen of the HLA region reveals an independent association of HLA class I and class II with susceptibility for systemic lupus erythematosus. Scand. J. Rheumatol. 2009, 38, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Remmers, E.F.; Plenge, R.M.; Lee, A.T.; Graham, R.R.; Hom, G.; Behrens, T.W.; de Bakker, P.I.; Le, J.M.; Lee, H.S.; Batliwalla, F.; et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 2007, 357, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Cunningham Graham, D.S.; Morris, D.L.; Bhangale, T.R.; Criswell, L.A.; Syvänen, A.-C.; Rönnblom, L.; Behrens, T.W.; Graham, R.; Vyse, T.L. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 2011, 7, e1002341. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Sheng, Y.; Zhang, Y.; Wang, Y.F.; Zhu, Z.; Tombleson, P.; Chen, L.; Cunninghame Graham, D.S.; Bentham, J.; Roberts, A.L.; et al. Genome-Wide association meta-analysis in Chinese and European individuals identifies ten new loci associated with systemic lupus erythematosus. Nat. Genet. 2016, 48, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.C.; Chun, S.; Kim, K.; Mak, A. Update on the Genetics of Systemic Lupus Erythematosus: Genome-Wide Association Studies and Beyond. Cells 2019, 8, E1180. [Google Scholar] [CrossRef] [PubMed]
- Julià, A.; López-Longo, F.J.; Pérez Venegas, J.J.; Bonàs-Guarch, S.; Olivé, À.; Andreu, J.L.; Aguirre-Zamorano, M.Á.; Vela, P.; Nolla, J.M.; de la Fuente, J.L.M.; et al. Genome-wide association study meta-analysis identifies five new loci for systemic lupus erythematosus. Arthritis Res. Ther. 2018, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zuo, X. Epigenetics in systemic lupus erythematosus. Biomed. Rep. 2016, 4, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, M.A.; Dozmorov, M.; Tang, Y.; Merrill, J.T.; Wren, J.D.; Sawalha, A.H. Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics 2011, 6, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, F.; Ma, J.; Zhang, X.; Wu, L.; Qu, B.; Xia, S.; Chen, S.; Tang, Y.; Shen, N. Association of large intergenic noncoding RNA expression with disease activity and organ damage in systemic lupus erythematosus. Arthritis Res. Ther. 2015, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Eldafira, E.; Prasasty, V.D.; Abinawanto, A.; Syahfirdi, L.; Pujianto, D.A. Polymorphisms of estrogen receptor-α and estrogen receptor-β genes and its expression in endometriosis. Turk. J. Pharm. Sci. 2021, 18, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nuite, M.; McAlindon, T.E. Association of estrogen and aromatase gene polymorphisms with systemic lupus erythematosus. Lupus 2010, 19, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, C.; Huang, K.; Fan, W.; Li, L.; Ye, M.; Han, W.; Meng, Y. Association between oestrogen receptor alpha (ESR1) gene polymorphisms and endometriosis: A meta-analysis of 24 case-control studies. Reprod. Biomed. Online 2016, 33, 335–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdel-Monem, S.M.; El-Brashy, A.W.S.E.; Hassan, W.A.; Abdullah, O.A.; Almallah, D.H. Determination of estrogen receptor alpha gene (ESR1) polymorphism and its relation to systemic lupus erythematosus disease status. Egypt. Rheumatol. Rehabil. 2022, 49, 19. [Google Scholar] [CrossRef]
- Barbosa, C.P.; Teles, J.S.; Lerner, T.G.; Peluso, C.; Mafra, F.A.; Vilarino, F.L.; Christofolini, D.M.; Bianco, B. Genetic association study of polymorphisms FOXP3 and FCRL3 in women with endometriosis. Fertil. Steril. 2012, 97, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Kochi, Y.; Yamada, R.; Suzuki, A.; Harley, J.B.; Shirasawa, S.; Sawada, T.; Bae, S.-C.; Tokuhiro, S.; Chang, X.; Sekine, A.; et al. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet. 2005, 37, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Birjan, Z.; Khashei Varnamkhasti, K.; Parhoudeh, S.; Naeimi, L.; Naeimi, S. Crucial Role of Foxp3 Gene Expression and Mutation in Systemic Lupus Erythematosus, Inferred from Computational and Experimental Approaches. Diagnostics 2023, 13, 3442. [Google Scholar] [CrossRef] [PubMed]
- Kublinsky, K.S.; Urazova, O.I.; Novitsky, V.V.; Kutsenko, I.G. Polymorphisms of Cytokine Genes in Genital Endometriosis. Neurosci. Behav. Physi 2019, 49, 962–971. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, Y.; Qin, B.; Zhong, R. A meta-analysis of the association of IL-6 −174 G/C and −572 G/C polymorphisms with systemic lupus erythematosus risk. Rheumatol. Int. 2014, 34, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Juo, S.-H.H.; Wu, R.; Lin, C.-S.; Wu, M.-T.; Lee, J.N.; Tsai, E.M. A functional promoter polymorphism in interleukin-10 gene influences susceptibility to endometriosis. Fertil. Steril. 2009, 92, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Zuo, T.; Zuo, Z.C. Impact of IL-10 gene polymorphisms and its interaction with environment on susceptibility to systemic lupus erythematosus. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420945916. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, I.; Jahan, N.; Lone, K.P.; Pakstis, A.; Taylor, H.S. Genetic Polymorphisms Associated with Endometriosis in Pakistani Women. J. Endometr. Pelvic Pain Disord. 2013, 5, 134–143. [Google Scholar] [CrossRef]
- Mohammadi, S.; Saghaeian Jazi, M.; Zare Ebrahimabad, M.; Eghbalpour, F.; Abdolahi, N.; Tabarraei, A.; Yazdani, Y. Interleukin 10 gene promoter polymorphisms (rs1800896, rs1800871 and rs1800872) and haplotypes are associated with the activity of systemic lupus erythematosus and IL10 levels in an Iranian population. Int. J. Immunogenet. 2019, 46, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Zhao, J.; Kang, S. A functional promoter polymorphism in interleukin 12B gene is associated with an increased risk of ovarian endometriosis. Gene 2018, 666, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Gorycka, A.; Sowinska, A.; Stypinska, B.; Grobelna, M.K.; Walczyk, M.; Olesinska, M.; Piotrowski, P.; Jagodzinski, P.P. Genetic Variants in IL-12B and IL-27 in the Polish Patients with Systemic Lupus Erythematosus. Scand. J. Immunol. 2016, 84, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Gao, L.; Wu, Y.; Fang, W.; Wang, L.; Li, C.; Li, Y.; Liang, W.; Zhang, L. The IL-16 gene polymorphisms and the risk of the systemic lupus erythematosus. Clin. Chim. Acta. 2009, 403, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Zervou, M.I.; Dorschner, J.M.; Ghodke-Puranik, Y.; Boumpas, D.T.; Niewold, T.B.; Goulielmos, G.N. Association of IRF5 polymorphisms with increased risk for systemic lupus erythematosus in population of Crete, a southern-eastern European Greek island. Gene 2017, 610, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bianco, B.; De Camargo, C.R.; Christofolini, D.M.; Barbosa, C.P. Involvement of Interferon Regulatory Factor 5 (IRF5) Gene Polymorphisms and Haplotype in Endometriosis-related Infertility. J. Endometr. Pelvic Pain Disord. 2017, 9, 188–192. [Google Scholar] [CrossRef]
- Delli Carpini, G.; Giannella, L.; Di Giuseppe, J.; Montik, N.; Montanari, M.; Fichera, M.; Crescenzi, D.; Marzocchini, C.; Meccariello, M.L.; Di Biase, D.; et al. Homozygous C677T Methylenetetrahydrofolate Reductase (MTHFR) Polymorphism as a Risk Factor for Endometriosis: A Retrospective Case–Control Study. Int. J. Mol. Sci. 2023, 24, 15404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Yuan, M. MTHFR polymorphisms (rs1801133) and systemic lupus erythematosus risk: A meta-analysis. Medicine 2020, 99, e22614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Rao, L.; Peng, Y.; Wang, Y.; Qie, M.; Zhang, Z.; Song, Y.; Zhang, L. A functional promoter polymorphism in NF-kB increases susceptibility to endometriosis. DNA Cell Biol. 2010, 29, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Zhou, M.; Leng, R.X.; Wang, W.; Feng, C.C.; Li, B.Z.; Zhu, Y.; Yang, X.K.; Yang, M.; Zhai, Y.; et al. Genetic interaction between genes involved in NF-κB signaling pathway in systemic lupus erythematosus. Mol. Immunol. 2013, 56, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.M.; Bianco, B.; Teles, J.S.; Christofolini, D.M.; de Souza, A.M.; Guedes, A.D.; Barbosa, C.P. PTPN22 C1858T polymorphism in women with endometriosis. Am. J. Reprod. Immunol. 2010, 63, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, E.; Zervou, M.I.; Andreou, A.; Dimopoulou, K.; Voloudakis, G.; Cosmidis, N.; Mysirlaki, H.; Vazgiourakis, V.P.; Sidiropoulos, P.; Newold, T.B.; et al. Association of the PTPN22 R620W polymorphism with increased risk for SLE in the genetic homogeneous population of Crete. Lupus 2011, 10, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Bianco, B.; Fernandes, R.F.M.; Trevisan, C.M.; Christofolini, D.M.; Sanz-Lomana, C.M.; de Bernabe, J.V.; Barbosa, C.P. Influence of STAT4 gene polymorphisms in the pathogenesis of endometriosis. Ann. Hum. Genet. 2019, 83, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.R.; Salmaninejad, A.; Akbari Asbagh, F.; Masoud, A.; Rezaei, N. STAT4 single nucleotide gene polymorphisms and susceptibility to endometriosis-related infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, P.; Lianeri, M.; Wudarski, M.; Olesińska, M.; Jagodziński, P.P. Contribution of STAT4 gene single-nucleotide polymorphism to systemic lupus erythematosus in the Polish population. Mol. Biol. Rep. 2012, 39, 8861–8866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mier-Cabrera, J.; Cruz-Orozco, O.; de la Jara-Díaz, J.; Galicia-Castillo, O.; Buenrostro-Jáuregui, M.; Parra-Carriedo, A.; Hernández-Guerrero, C. Polymorphisms of TNF-alpha (−308), IL-1beta (+3954) and IL1-Ra (VNTR) are associated to severe stage of endometriosis in Mexican women: A case control study. BMC Women’s Health 2022, 22, 356. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Z.; Liao, Y.; Yang, B.; Zhang, J. Association between tumor necrosis factor polymorphisms and rheumatoid arthritis as well as systemic lupus erythematosus: A meta-analysis. Braz. J. Med. Biol. Res. 2019, 52, e7927. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wu, R.; Li, S.; He, J. Meta-analysis of association between the TP53 Arg72Pro polymorphism and risk of endometriosis based on case-control studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 189, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, J.M.; Wu, S.; Li, J.; Wang, M.R.; Wang, T.T.; Lu, Y.W. Association study between the TP53 Rs1042522G/C polymorphism and susceptibility to systemic lupus erythematosus in a Chinese Han population. Rheumatol. Int. 2017, 37, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Mahale, S.D. Endometriosis Knowledgebase: A gene-based resource on endometriosis. Database 2019, 2019, baz062. [Google Scholar] [CrossRef] [PubMed]
- Wun, C.M.; Piao, Z.; Hong, K.T.; Choi, J.Y.; Hong, C.R.; Park, J.D.; Park, K.D.; Shin, H.Y.; Kang, H.J. Effect of donor STAT4 polymorphism rs7574865 on clinical outcomes of pediatric acute leukemia patients after hematopoietic stem cell transplant. Int. Immunopharmacol. 2017, 43, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Podgaec, S.; Dias Junior, J.A.; Chapron, C.; Oliveira, R.M.; Baracat, E.C.; Abrão, M.S. Th1 and Th2 immune responses related to pelvic endometriosis. Rev. Assoc. Med. Bras. 2010, 56, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Yusoff, F.; Wong, K.K.; Mohd Redzwan, N. Th1, Th2, and Th17 cytokines in systemic lupus erythematosus. Autoimmunity 2020, 53, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Y.; Zhang, N.; Xian, Y.; Tang, Y.; Ye, J.; Reza, F.; He, G.; Wen, X.; Jiang, X. The multiple roles of interferon regulatory factor family in health and disease. Sig. Transduct. Target. Ther. 2024, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Sabry, A.; El-husseini, A.; Mahmoud, K.; Eldahshan, K.F.; George, S.K.; Abdel-Khalek, E.; El-Shafey, E.M.; Abo-Zenah, H. Proinflammatory cytokines (TNF-alpha and IL-6) in Egyptian patients with SLE: Its correlation with disease activity. Cytokine 2006, 35, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.G.; Symons, J.A.; McDowell, T.L.; McDevitt, H.O.; Duff, G.W. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc. Natl. Acad. Sci. USA 1997, 94, 3195–3199. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.C.; Xu, F.; Tang, M.; Xiong, X. Association Between TNF-α Promoter -308 A/G Polymorphism and Systemic Lupus Erythematosus Susceptibility: A Case-Control Study and Meta-Analysis. Scand. J. Immunol. 2017, 85, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Stanford, S.; Bottini, N. PTPN22: The archetypal non-HLA autoimmunity gene. Nat. Rev. Rheumatol. 2014, 10, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, J.F.; Veillette, A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 1999, 189, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bottini, N.; Musumeci, L.; Alonso, A.; Rahmouni, S.; Nika, K.; Rostamkhani, M.; MacMurray, J.; Meloni, G.F.; Lucarelli, P.; Pellecchia, M.; et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 2004, 36, 337–338. [Google Scholar] [CrossRef] [PubMed]
- van der Veeken, J.; Glasner, A.; Zhong, Y.; Hu, W.; Wang, Z.M.; Bou-Puerto, R.; Charbonnier, L.-M.; Chatila, T.A.; Leslie, C.S.; Rudensky, A.Y.; et al. The Transcription Factor Foxp3 Shapes Regulatory T Cell Identity by Tuning the Activity of trans-Acting Intermediaries. Immunity 2020, 3, 971–984.e5. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.S.; Jang, S.W.; Kim, M.K.; Kim, L.K.; Kim, B.S.; Kim, H.S.; Kim, K.; Lee, W.; Flavell, R.A.; Lee, G.R. YY1 inhibits differentiation and function of regulatory T cells by blocking Foxp3 expression and activity. Nat. Commun. 2016, 7, 10789. [Google Scholar] [CrossRef] [PubMed]
- Solus, J.F.; Chung, C.P.; Oeser, A.; Li, C.; Rho, Y.H.; Bradley, K.M.; Kawai, V.K.; Smith, J.R.; Stein, C.M. Genetics of serum concentration of IL-6 and TNF alpha in systemic lupus erythematosus and rheumatoid arthritis: A candidate gene analysis. Clin. Rheumatol. 2015, 34, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.X.; Fu, C.W.; Jiang, F.; Ye, L.X.; Meng, W. Association of the interleukin-6 polymorphisms with systemic lupus erythematosus: A meta-analysis. Lupus 2015, 24, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, Y.; Wang, Y.; Hu, J.; Di, W.; Liu, S.; Zeng, X.; Yu, G.; Wang, Y.; Wang, Z. The IL-6 rs1800795 and rs1800796 polymorphisms are associated with coronary artery disease risk. J. Cell Mol. Med. 2020, 24, 6191–6207. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Jeung, I.C.; Park, A.; Park, Y.J.; Jung, H.; Kim, T.D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Illei, G.G.; Shirota, Y.; Yarboro, C.H.; Daruwalla, J.; Tackey, E.; Takada, K.; Fleisher, T.; Balow, J.E.; Lipsky, P.E. Tocilizumab in systemic lupus erythematosus: Data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010, 62, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, T.; Liu, S.; Zou, H.; Sun, X.; Shi, X.; Li, Y.; Shan, Z.; Teng, W. Interleukin-10 influences susceptibility to experimental autoimmune thyroiditis independently of the H-2 gene. Int. J. Mol. Med. 2015, 35, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hei, P.; Deng, L.; Lin, J. Interleukin-10 gene promoter polymorphisms and their protein production in peritoneal fluid in patients with endometriosis. Mol. Hum. Reprod. 2007, 13, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.C.; Zheng, L.; Han, M.Y.; Hu, L.Y.; Zhao, P.P.; Bai, W.Y.; Zhu, X.W.; Xia, J.W.; Wang, X.B.; et al. Comprehensive assessment of the association between genes on JAK-STAT pathway (IFIH1, TYK2, IL-10) and systemic lupus erythematosus: A meta-analysis. Arch. Dermatol. Res. 2018, 310, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, K.A.; Schulze, L.L.; Paap, E.M.; Müller, T.M.; Neurath, M.F.; Zundler, S. Immunology of IL-12: An update on functional activities and implications for disease. EXCLI J. 2020, 19, 1563–1589. [Google Scholar] [PubMed]
- Shimokawa, N.; Nishiyama, C.; Hirota, T.; Tamari, M.; Hara, M.; Ikeda, S.; Okumura, K.; Ogawa, H. Functional analysis of a polymorphism in the promoter region of the IL-12/23p40 gene. Clin. Exp. Allergy 2009, 39, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Muller-Berghaus, J.; Kern, K.; Paschen, A.; Nguyen, X.D.; Klüter, H.; Morahan, G.; Schadendorf, D. Deficient IL-12p70 secretion by dendritic cells based on IL12B promoter genotype. Genes. Immun. 2004, 5, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Osuga, Y.; Yoshino, O.; Hirota, Y.; Yano, T.; Tsutsumi, O.; Taketani, Y. Elevated interleukin-16 levels in the peritoneal fluid of women with endometriosis may be a mechanism for inflammatory reactions associated with endometriosis. Fertil. Steril. 2005, 83, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Sig Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of cytokines in endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rostamzadeh, D.; Kazemi, T.; Amirghofran, Z.; Shabani, M. Update on Fc receptor-like (FCRL) family: New immunoregulatory players in health and diseases. Expert. Opin. Ther. Targets 2018, 22, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.H.; Cohen, P.L. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Mo, Z.; Li, L. TP53 Arg72Pro polymorphism (rs1042522) and risk of endometriosis among Asian and Caucasian populations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 189, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Rosenblatt, D.S. Inherited disorders of folate and cobalamin transport and Metabolism. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Vander Put, N.M. A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects. Am. J. Hum. Genet. 1998, 62, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Casazza, K.; Page, G.P.; Fernandez, J.R. The association between the rs2234693 and rs9340799 estrogen receptor alpha gene polymorphisms and risk factors for cardiovascular disease: A review. Biol. Res. Nurs. 2010, 12, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, T.H.; Lu, W.S.; Mu, P.W.; Yang, Y.F.; Liang, W.W.; Li, C.X.; Lin, G.P. Estrogen receptor alpha gene polymorphism associated concentration in Chinese women in Guangzhou. Chin. Med. J. 2006, 119, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Costenbader, K.H.; Feskanich, D.; Stampfer, M.J.; Karlson, E.W. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007, 56, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Abisror, N.; Kolanska, K.; Cheloufi, M.; Selleret, L.; d’Argent, E.; Kayem, G.; Mekinian, A. Endometriosis and autoimmunity. Explor. Immunol. 2022, 2, 25–31. [Google Scholar] [CrossRef]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.H.; Leong, P.Y.; Chiou, J.Y.; Wang, Y.H.; Ku, M.H.; Wei, J.C.C. Association between endometriosis and risk of systemic lupus erythematosus. Sci. Rep. 2021, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhong, Y.; Xu, S.; Yu, H. Causal effects of endometriosis on SLE, RA and SS risk: Evidence from meta-analysis and Mendelian randomization. BMC Pregnancy Childbirth 2024, 24, 162. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Leong, P.-Y.; Huang, J.-Y.; Wei, J.C.-C. Increased risk of being diagnosed with endometriosis in patients with Systemic lupus erythematosus: A population-based cohort study in Taiwan. Sci. Rep. 2022, 12, 13336. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Y.; Chen, Q.; Shi, Y.Z.; Li, L.W.; Hua, C.; Zheng, H. Risk factors of systemic lupus erythematosus: An overview of systematic reviews and Mendelian randomization studies. Adv. Rheumatol. 2023, 63, 42. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Su, S.; You, T.; Xia, T.; Lin, X.; Chen, Z.; Zhang, L. Serum interleukin-6 level is correlated with the disease activity of systemic lupus erythematosus: A meta-analysis. Clinics 2020, 75, e1801. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, V.; Balakrishnan, C. Tocilizumab Therapy for Treatment-Resistant Systemic Lupus Erythematosus with Elevated IL-6 and CRP Levels: A Case Report. SN Compr. Clin. Med. 2023, 5, 199. [Google Scholar] [CrossRef]
- El-Zayadi, A.A.; Mohamed, S.A.; Arafa, M.; Mohammed, S.M.; Zayed, A.; Abdelhafez, M.S.; Badawy, A.M. Anti-IL-6 receptor monoclonal antibody as a new treatment of endometriosis. Immunol. Res. 2020, 68, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Shirota, Y.; Yarboro, C.; Fischer, R.; Pham, T.-H.; Lipsky, P.; Illei, G.G. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2013, 72, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Nuclear factor-kappab (NF-kappaB): An unsuspected major culprit in the pathogenesis of endometriosis that is still at large? Gynecol. Obstet. Invest. 2007, 63, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Brightbill, H.D.; Suto, E.; Blaquiere, N.; Ramamoorthi, N.; Sujatha-Bhaskar, S.; Gogol, E.B.; Castanedo, G.M.; Jackson, B.T.; Kwon, Y.C.; Haller, S.; et al. NF-κB inducing kinase is a therapeutic target for systemic lupus erythematosus. Nat. Commun. 2018, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Vanni, V.S.; Villanacci, R.; Salmeri, N.; Papaleo, E.; Delprato, D.; Ottolina, J.; Rovere-Querini, P.; Ferrari, S.; Viganò, P.; Candiani, M. Concomitant autoimmunity may be a predictor of more severe stages of endometriosis. Sci. Rep. 2021, 11, 15372. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, R.; Bendigeri, T.; Ghuge, A.; Bhusane, K.; Begum, S.; Warty, N.; Sawant, R.; Padte, K.; Humane, A.; Dasmahapatra, P.; et al. Panel of Autoimmune Markers for Noninvasive Diagnosis of Minimal-Mild Endometriosis. Reprod. Sci. 2017, 24, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, L.; Andreou, A.; Zervou, M.; Vlachakis, D.; Goulielmos, G.N.; Eliopoulos, E. A global population genomic analysis shows novel insights into the genetic characteristics of endometriosis. World Acad. Sci. J. 2023, 5, 12. [Google Scholar] [CrossRef]
- Goulielmos, G.N.; Zervou, M.I.; Vazgiourakis, V.M.; Ghodke-Puranik, Y.; Garyfallos, A.; Niewold, T.B. The genetics and molecular pathogenesis of SLE in populations of different ancestral background. Gene 2018, 668, 59–72. [Google Scholar] [CrossRef] [PubMed]
| dbSNP ID | Endometriosis and SLE-Associated Gene | Function | Association with Endometriosis | Association with SLE | References |
|---|---|---|---|---|---|
| rs9340799 rs2234693 | ESR1 | An estrogen receptor and ligand-activated transcription factor | Allele “G”, OR 2.54 Allele “T”, OR = 1.53 (95% CI: 1.05–2.21; p = 0.025) | XX vs. xx, OR: 3.4 (95% CI: 1.1–10.5) PP vs. pp, OR: 3.1 (95% CI: 1.1–9.3) | [38,39,40,41] |
| rs7528684 | FCRL3 | An Ig receptor, mediating plasma B cell maturation and antibody production | Allele “C”, OR = 1.58 (95% CI: 1.17–2.12; p = 0.03) | Allele “C”, OR = 1.49 (95% CI: 1.16–1.92; p = 0.0017) | [42,43] |
| rs3761549 | FOXP3 | A regulator of T cell activation; down regulates cytokine production in T cells | Allele “T”, OR = 2.05 (95% CI: 1.22–3.45); p = 0.08) | Allele “T”, OR = 2.2 (95% CI: 1.4–3.3; p < 0.007) | [42,44] |
| rs1800796 | IL-6 | A pro-inflammatory cytokine; stimulator of osteoclast formation | Allele “C”, OR = 2.17 (p < 0.001) | Allele “C”, OR = 1.49 (95% CI: 1.10–2.01, p = 0.009 | [45,46] |
| rs1800871 rs1800896 | IL-10 | An anti-inflammatory cytokine; inhibitor of Th1 differentiation | TT genotype, OR = 0.52 (p = 0.006) GG genotype, OR = 2.22 (95% CI: 1.25–3.94, p = 0.009) | Allele “T”, OR = 1.47 (95% CI: 1.12–1.94, p < 0.05) GG genotype, OR = 2.65 (95% CI: 1.21–5.82, p = 0.046) | [47,48,49,50] |
| rs17860508 | IL-12B | A cytokine acting on T and natural killer cells | Allele “GC”, OR = 1.25 (95% CI: 1.09–1.44, p = 0.01) | Allele “GC”, p < 0.001 | [51,52] |
| rs11556218 | IL-16 | A pleiotropic pro-inflammatory cytokine | Allele “G”, OR = 3.02 (95% CI: 2.17–4.20, p < 0.0001) | Allele “G”, OR = 2.25 (95% CI: 1.64–3.13, p < 0.001) | [22,53] |
| rs10488631 | IRF5 | A pleiotropic transcription factor involved in virus-mediated activation of IFN | Allele “C”, OR = 1.79 (95% CI: 1.09–2.94, p = 0.028) | Allele “C”, OR = 0.54 (95% CI: 0.37–0.79, p = 0.0012) | [54,55] |
| rs1801133 | MTHFR | A key regulatory enzyme in folate and homocysteine metabolism | Allele “T”, OR = 1.899 (95% CI: 1.076–3.318, p = 0.0269) | Allele “T”, OR = 1.766 (95% CI: 1.014–3.075, p = 0.04) | [56,57] |
| rs28362491 | NF-kB | A major transcription factor of genes involved in both the innate and adaptive immunity | −94 insertion/ deletion ATTG polymorphism, OR = 1.968 (95% CI: 1.442–2.686, p < 0.0001) | −94 insertion/ deletion ATTG polymorphism, OR = 1.14 (95% CI: 1.00–1.31, p = 0.047) | [58,59] |
| rs2476601 | PTPN22 | A lymphoid-specific phosphatase; down-regulator of T cell activation | Allele “T”, OR = 2.05 (95% CI: 1.28–3.29, p = 0.004) | Allele “T”, OR = 1.91 (95% CI: 1.11–3.90, p = 0.017) | [60,61] |
| rs7574865 rs7582694 | STAT4 | A transcription factor involved in Th17 differentiation and monocyte activation | TT genotype, OR = 1.03 (95% CI: 0.68–1.58, p = 0.047) Allele “C”, OR = 1.986 (95% CI: 1.262–3.126, p = 0.002) | Allele “T’, OR = 1.55 (95% CI: 1.34–1.79, p = 1.87 × 10−9) Allele “C”. OR = 1.539 (95% CI: 1.209–1.969, p = 0.0004) | [30,62,63,64] |
| rs1800629 | TNF-α | A multifunctional pro-inflammatory cytokine | Allele “A”, OR = 3.4 (95% CI: 1.25 = 9.23, p = 0.029) | Allele “A”, OR = 1.78 (95% CI: 1.45–2.19, p < 0.001) | [65,66] |
| rs1042522 | TP53 | A tumor suppressor protein | Allele “G”, OR = 1.32 (95% CI: 1.14–1.53, p < 0.001) | Allele “G”, OR = 0.89 (95% CI: 0.81–0.97, p = 0.01) | [67,68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zervou, M.I.; Tarlatzi, T.B.; Grimbizis, G.F.; Niewold, T.B.; Tarlatzis, B.C.; Bertsias, G.; Goulielmos, G.N. Co-Occurrence of Endometriosis with Systemic Lupus Erythematosus: Genetic Aspects. Int. J. Mol. Sci. 2025, 26, 6841. https://doi.org/10.3390/ijms26146841
Zervou MI, Tarlatzi TB, Grimbizis GF, Niewold TB, Tarlatzis BC, Bertsias G, Goulielmos GN. Co-Occurrence of Endometriosis with Systemic Lupus Erythematosus: Genetic Aspects. International Journal of Molecular Sciences. 2025; 26(14):6841. https://doi.org/10.3390/ijms26146841
Chicago/Turabian StyleZervou, Maria I., Theoni B. Tarlatzi, Grigoris F. Grimbizis, Timothy B. Niewold, Basil C. Tarlatzis, George Bertsias, and George N. Goulielmos. 2025. "Co-Occurrence of Endometriosis with Systemic Lupus Erythematosus: Genetic Aspects" International Journal of Molecular Sciences 26, no. 14: 6841. https://doi.org/10.3390/ijms26146841
APA StyleZervou, M. I., Tarlatzi, T. B., Grimbizis, G. F., Niewold, T. B., Tarlatzis, B. C., Bertsias, G., & Goulielmos, G. N. (2025). Co-Occurrence of Endometriosis with Systemic Lupus Erythematosus: Genetic Aspects. International Journal of Molecular Sciences, 26(14), 6841. https://doi.org/10.3390/ijms26146841








