Hyperthermia Augments the H1-Histamine Receptor-Mediated Force in the Human Atrium
Abstract
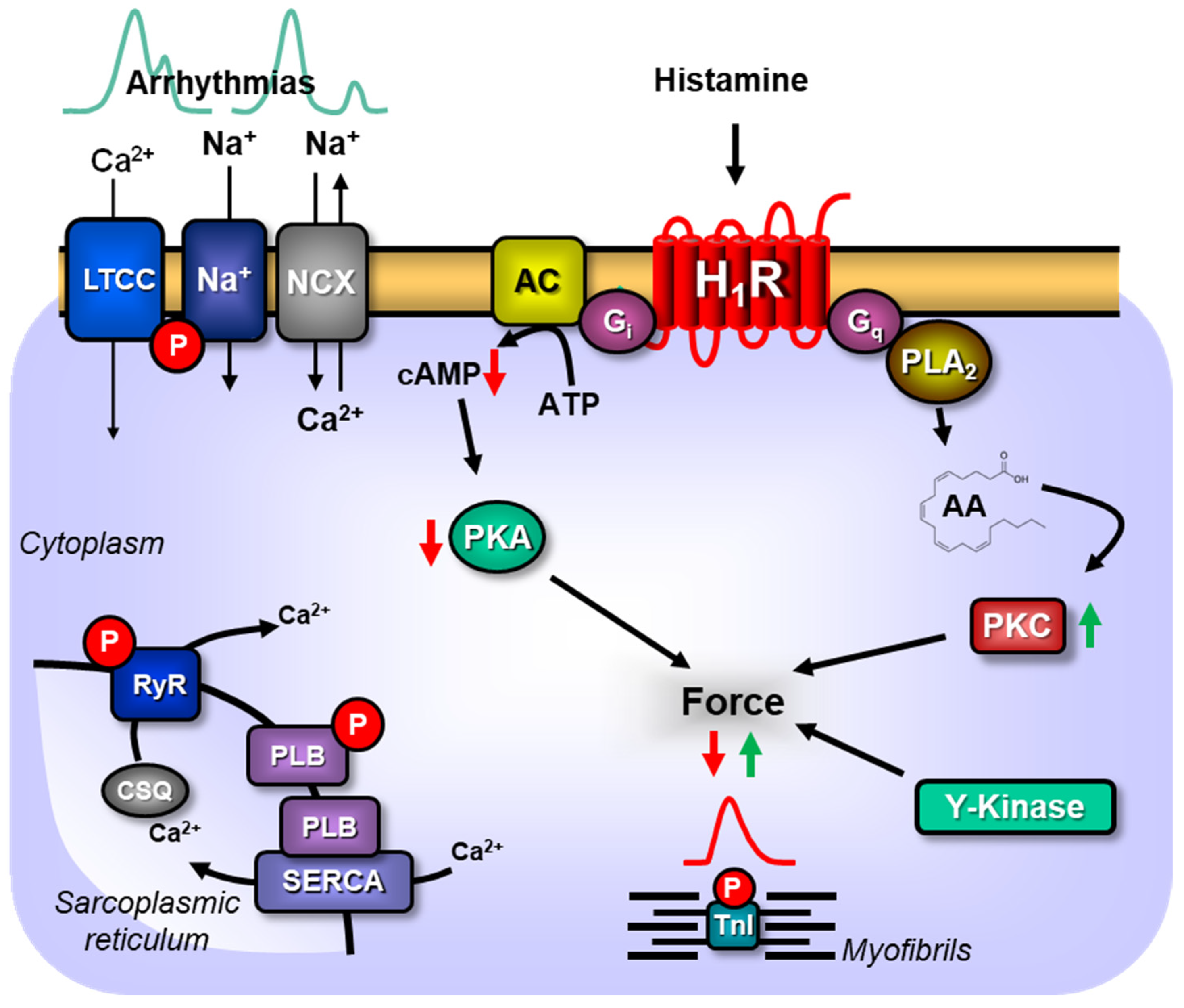
1. Introduction
2. Results
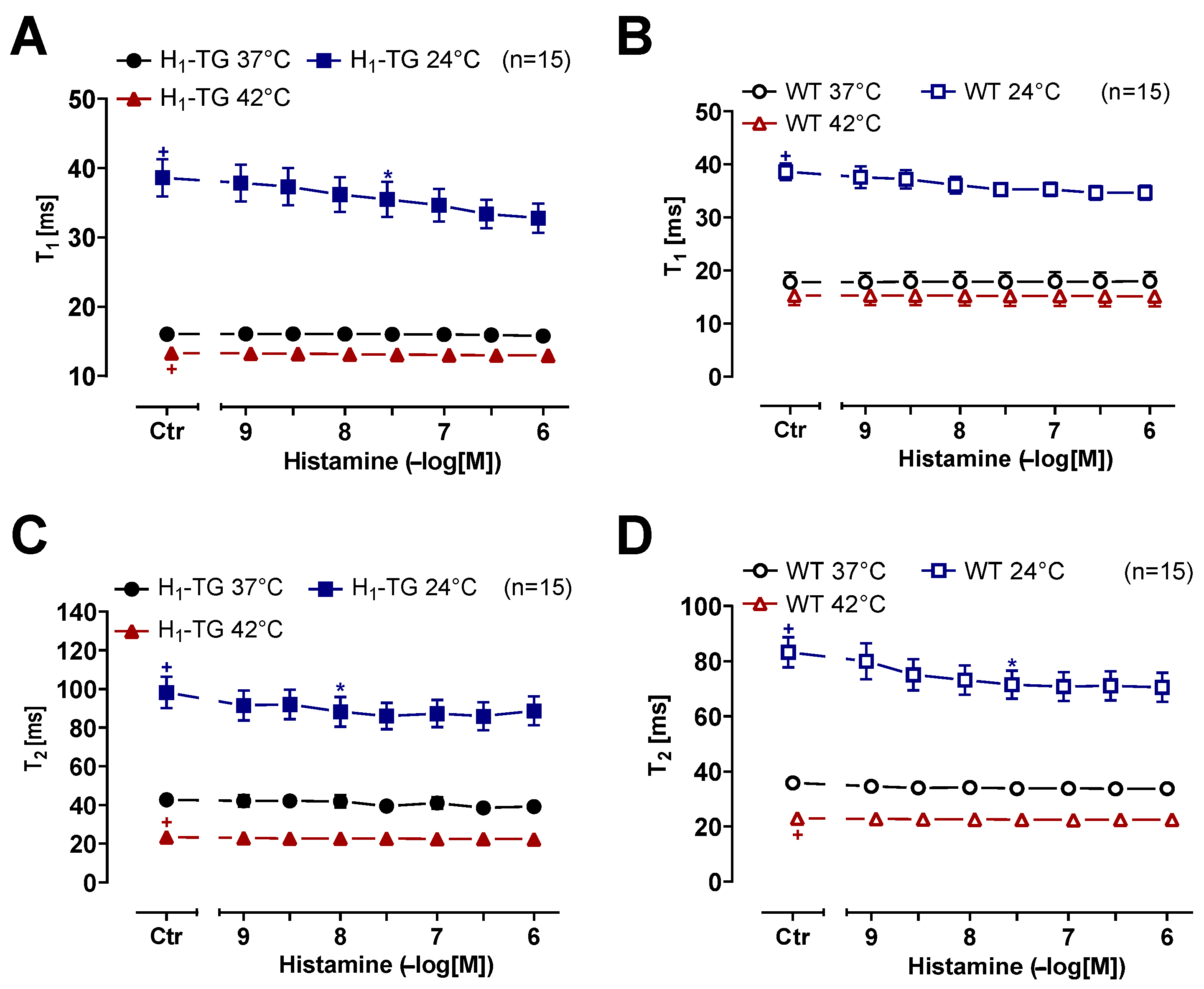
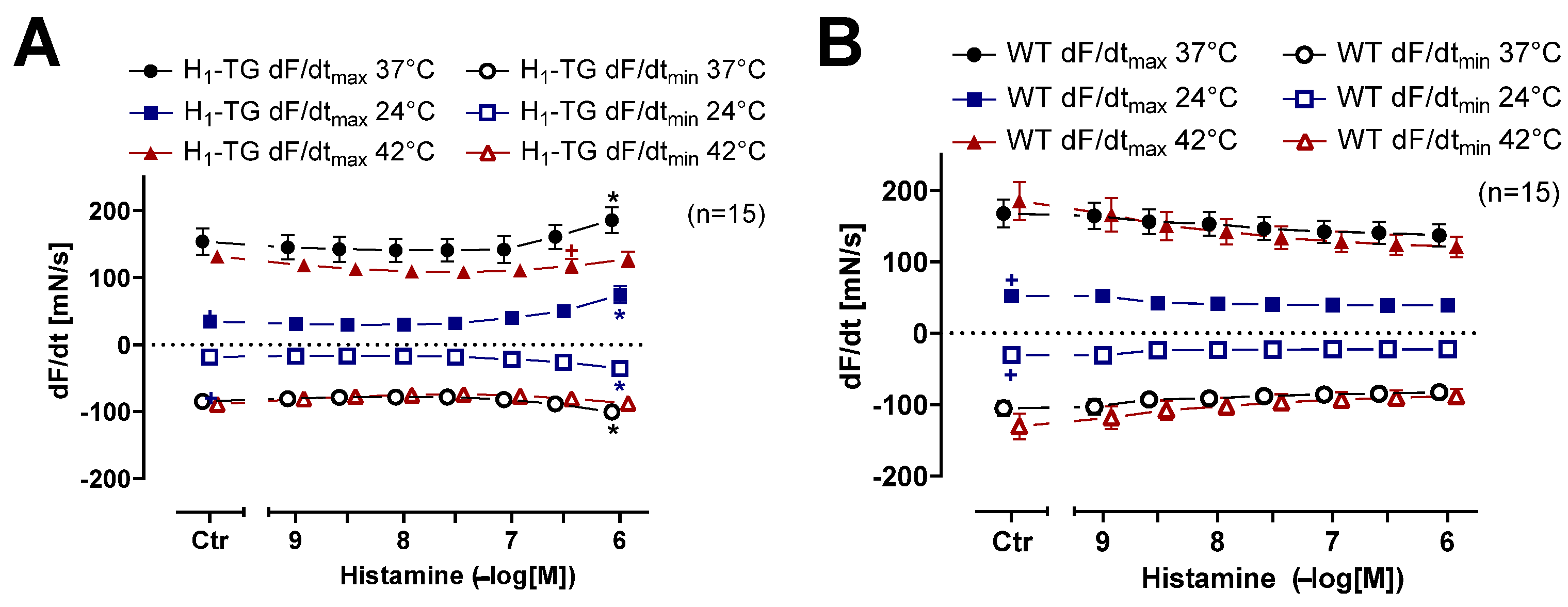
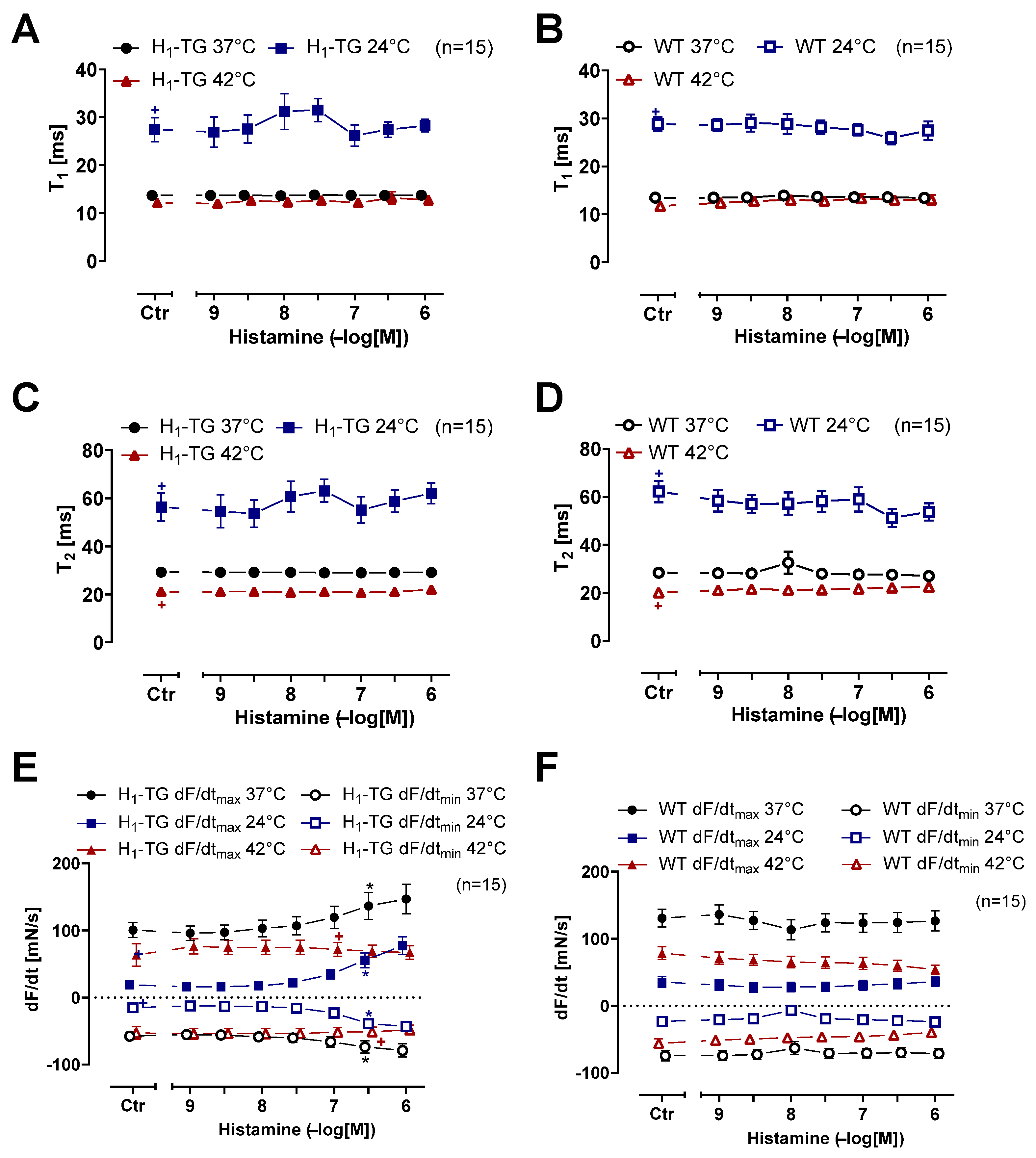
2.1. Mouse Atrial Preparations
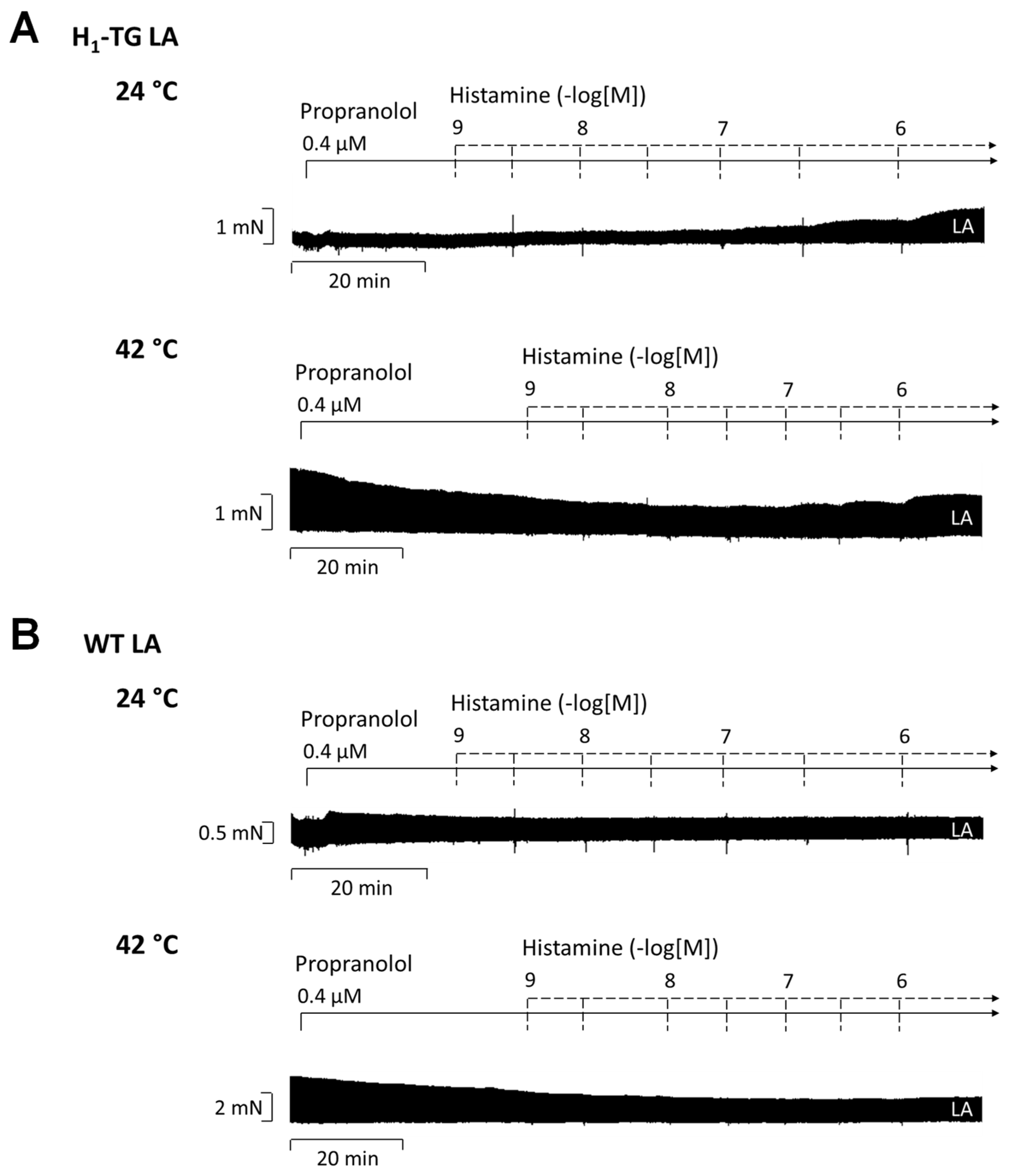
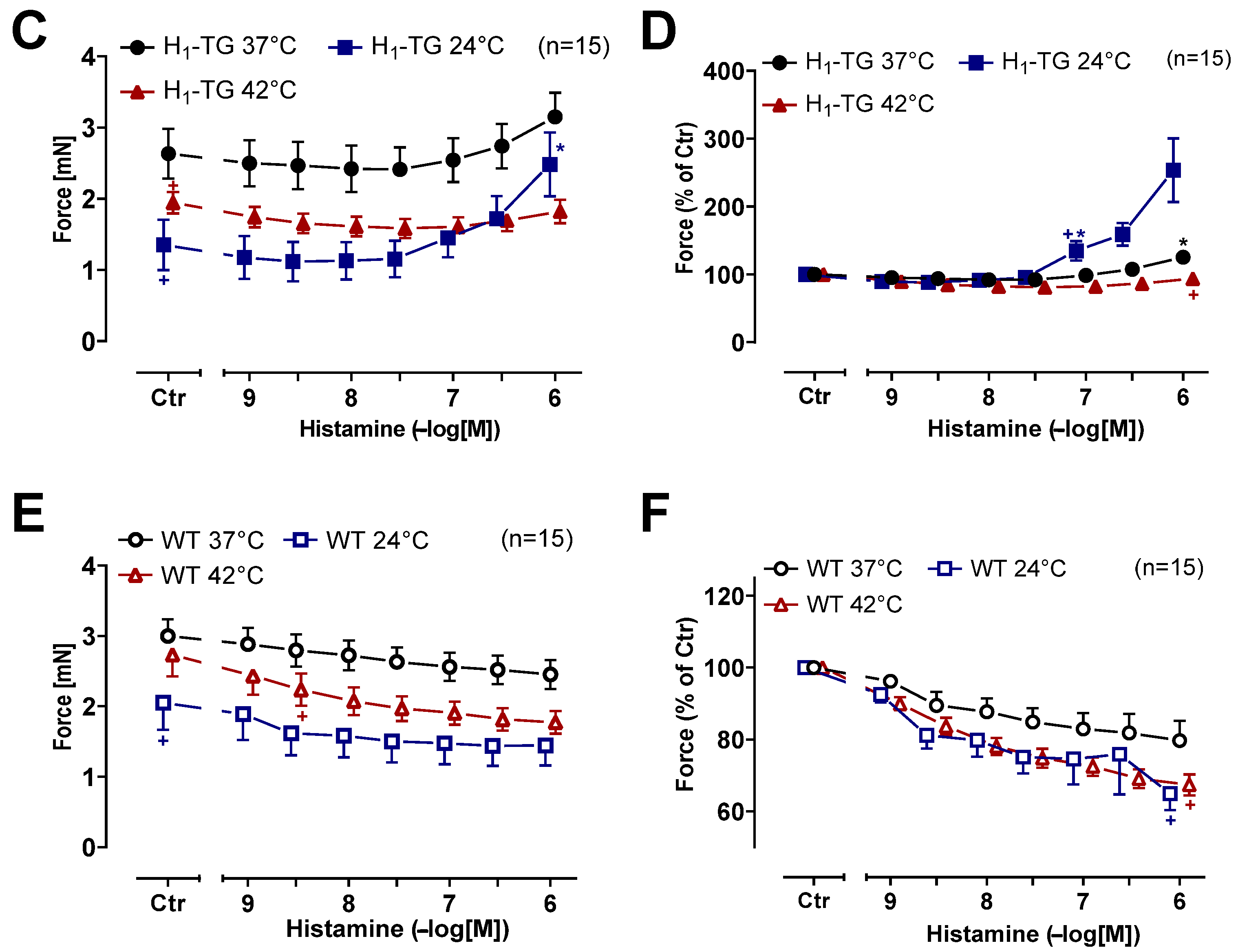
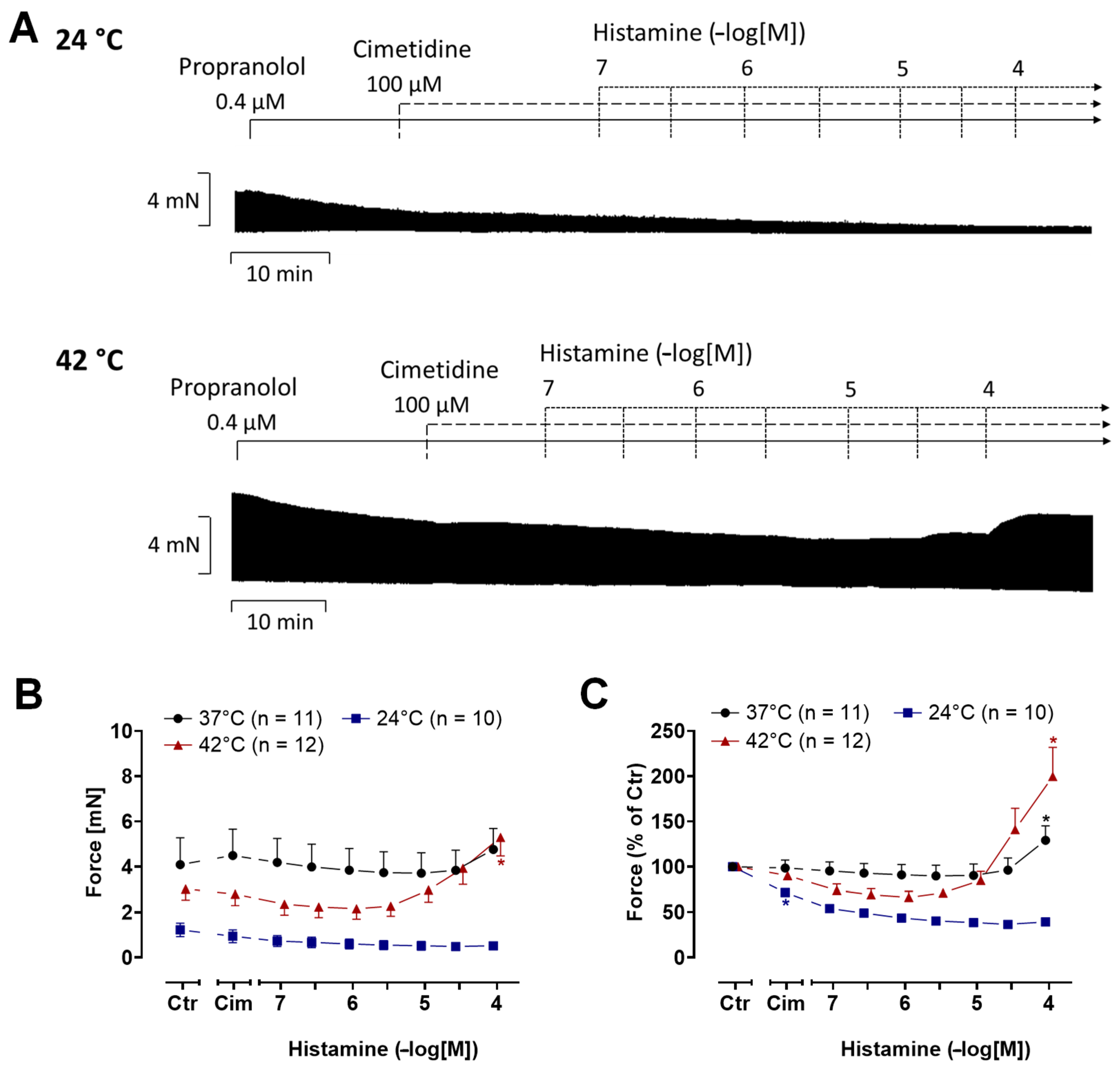
2.2. Human Atrial Preparations
3. Discussion
3.1. Clinical Relevance
3.2. Study Limitations
4. Materials and Methods
4.1. Transgenic Mice
4.2. Contractility in Isolated Mouse Atria
4.3. Contractility in Isolated Human Atria
4.4. Data Analysis
4.5. Drugs and Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, T.H.; Abella, L.M.R.; Hadova, K.; Klimas, J.; Dhein, S.; Pockes, S.; Schlicht, J.M.A.; Hofmann, B.; Kirchhefer, U.; Neumann, J.; et al. Stimulation of histamine H1-receptors produces a positive inotropic effect in the human atrium. Naunyn Schmiedebergs Arch. Pharmacol. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, U.; Lewartowski, B. Temperature dependent contribution of Ca2+ transporters to relaxation in cardiac myocytes: Important role of sarcolemmal Ca2+-ATPase. J. Physiol. Pharmacol. 2006, 57, 3–15. [Google Scholar] [PubMed]
- Antzelevitch, C.; Yan, G.X. J wave syndromes. Heart Rhythm. 2010, 7, 549–558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukaya, H.; Piktel, J.S.; Wan, X.; Plummer, B.N.; Laurita, K.R.; Wilson, L.D. Arrhythmogenic delayed afterdepolarizations are promoted by severe hypothermia but not therapeutic hypothermia. Circ. J. 2017, 82, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.J.R.; Gergs, U.; Hofmann, B.; Kirchhefer, U.; Neumann, J. Temperature alters the inotropic, chronotropic and proarrhythmic effects of histamine in atrial muscle preparations from humans and H2-receptor overexpressing mice. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2137–2150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akaishi, Y.; Hattori, Y.; Yoshimoto, K.; Kitabatake, A.; Yasuda, K.; Kanno, M. Involvement of tyrosine phosphorylation in the positive inotropic effect produced by H(1)-receptors with histamine in guinea-pig left atrium. Br. J. Pharmacol. 2000, 130, 907–915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tenner, T.E., Jr.; McNeill, J.H. Characterization of the inotropic response induced by stimulation of beta-adrenergic and H1 histaminergic receptors in guinea pig left atria. Can. J. Physiol. Pharmacol. 1978, 56, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, P.A.; McNeill, J.H. Guinea-pig and rabbit papillary muscles differ in their response to histamine. Gen. Pharmacol. 1983, 14, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.I.; Huang, H.; Zhao, J.; Frank, R.; Suarez, V.; Delacrétaz, E.; Brink, M.; Osswald, S.; Schwick, N.; Chahine, M. A novel SCN5A mutation, F1344S, identified in a patient with Brugada syndrome and fever-induced ventricular fibrillation. Cardiovasc. Res. 2006, 70, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Zipes, D.P.; Morita, S.T.; Wu, J. Temperature modulation of ventricular arrhythmogenicity in a canine tissue model of Brugada syndrome. Heart Rhythm. 2007, 4, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Abdelsayed, M.; Peters, C.H.; Ruben, P.C. Differential thermosensitivity in mixed syndrome cardiac sodium channel mutants. J. Physiol. 2015, 593, 4201–4223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Battrawy, I.; Lang, S.; Zhao, Z.; Akin, I.; Yücel, G.; Meister, S.; Patocskai, B.; Behnes, M.; Rudic, B.; Tülümen, E.; et al. Hyperthermia influences the effects of sodium channel blocking drugs in human-induced pluripotent stem cell-derived cardiomyocytes. PLoS ONE 2016, 11, e0166143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Wang, T.; Guo, J.; Yang, T.; Li, W.; Koichopolos, J.; Lamothe, S.M.; Kang, Y.; Ma, A.; Zhang, S. Febrile temperature facilitates hERG/IKr degradation through an altered K(+) dependence. Heart Rhythm. 2016, 13, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Gregory, H.; Weant, K.A. Pathophysiology and treatment of malignant hyperthermia. Adv. Emerg. Nurs. J. 2021, 43, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Grundig, P.; Pham, T.H.; Hofmann, B.; Neumann, J.; Gergs, U. Temperature alters the inotropic and chronotropic effect of D1-dopamine receptor stimulation in the mammalian atrium. Naunyn Schmiedebergs Arch. Pharmacol. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Hofmann, B.; Kirchhefer, U.; Dhein, S.; Gergs, U. Function and role of histamine H1 Receptor in the mammalian heart. Pharmaceuticals 2023, 16, 734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gergs, U.; Brückner, T.; Hofmann, B.; Neumann, J. The proarrhythmic effects of hypothermia in atria isolated from 5-HT4-receptor-overexpressing mice. Eur. J. Pharmacol. 2021, 906, 174206. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Ross, J., Jr.; Sonnenblick, E.H. Mechanisms of contraction of the normal and failing heart. N. Engl. J. Med. 1967, 277, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]



| Mouse left atrium | ||||
| basal tension [mN] | WT | 3.15 ± 0.29 (n = 16) | 2.16 ± 0.36 (n = 16) | 2.73 ± 0.32 # (n = 16) |
| H1-TG | 2.63 ± 0.35 (n = 14) | 1.35 ± 0.35 + (n = 14) | 1.94 ± 0.15 (n = 14) | |
| basal time-to-peak tension [ms] | WT | 17.8 ± 1.92 (n = 16) | 38.62 ± 1.67 (n = 16) | 15.31 ± 2.01 (n = 16) |
| H1-TG | 16.04 ± 0.32 (n = 14) | 38.6 ± 2.67 + (n = 14) | 13.31 ± 0.26 + (n = 14) | |
| basal relaxation time [ms] | WT | 38.52 ± 1.85 # (n = 16) | 83.21 ± 5.75 # (n = 16) | 22.99 ± 1.61 (n = 16) |
| H1-TG | 42.7 ± 3.06 (n = 14) | 98.18 ± 8.14 + (n = 14) | 23.35 ± 0.91 + (n = 14) | |
| Mouse right atrium | 37 °C | 24 °C | 42 °C | |
| basal tension [mN] | WT | 1.88 ± 0.23 (n = 14) | 0.8 ± 0.19 # (n = 14) | 0.97 ± 0.13 (n = 14) |
| H1-TG | 1.41 ± 0.2 (n = 15) | 0.38 ± 0.09 + (n = 12) | 0.99 ± 0.18 (n = 14) | |
| basal time-to-peak tension [ms] | WT | 13.47 ± 0.21 (n = 14) | 28.83 ± 1.47 (n = 14) | 11.7 ± 0.31 (n = 14) |
| H1-TG | 13.71 ± 0.23 (n = 15) | 24.93 ± 3.36 + (n = 15) | 12.16 ± 0.32 (n = 15) | |
| basal relaxation time [ms] | WT | 28.34 ± 0.71 (n = 14) | 62.21 ± 4.51 (n = 14) | 20.06 ± 0.69 (n = 14) |
| H1-TG | 29.31 ± 1.23 (n = 15) | 51.25 ± 7.34 + (n = 15) | 21.16 ± 0.74 + (n = 15) | |
| basal beating rate [bpm] | WT | 274.03 ± 30.25 (n = 14) | 170.6 ± 72.52 (n = 13) | 344.15 ± 39.61 (n = 14) |
| H1-TG | 284.43 ± 31.55 (n = 14) | 58.16 ± 10.45 + (n = 10) | 348.21 ± 22.03 (n = 10) | |
| Human Right Atrium | 37 °C | 24 °C | 42 °C |
| basal tension [mN] | 4.12 ± 1.13 (n = 11) | 1.82 ± 0.5 + (n = 4) | 3.69 ± 0.66 (n = 9) |
| basal time-to-peak tension [ms] | 67.05 ± 5.88 (n = 11) | 91.76 ± 6.37 + (n = 4) | 47.95 ± 2.44 + (n = 9) |
| basal relaxation time [ms] | 134.26 ± 4.54 (n = 11) | 290.38 ± 11.48 + (n = 4) | 103.74 ± 4.72 + (n = 9) |
| Tissue | HAP: H1 | HAP: H2 | H1-TG | H2-TG |
| Hypothermia | No effect | Small PIE | PIE | Large PIE |
| Normothermia | Small PIE | PIE | PIE | PIE |
| Hyperthermia | Large PIE | Small PIE | No effect | Small PIE |
| Reference | This study | [5] | This study | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.H.; Grundig, P.; Hofmann, B.; Kirchhefer, U.; Neumann, J.; Gergs, U. Hyperthermia Augments the H1-Histamine Receptor-Mediated Force in the Human Atrium. Int. J. Mol. Sci. 2025, 26, 6842. https://doi.org/10.3390/ijms26146842
Pham TH, Grundig P, Hofmann B, Kirchhefer U, Neumann J, Gergs U. Hyperthermia Augments the H1-Histamine Receptor-Mediated Force in the Human Atrium. International Journal of Molecular Sciences. 2025; 26(14):6842. https://doi.org/10.3390/ijms26146842
Chicago/Turabian StylePham, Thanh Hoai, Peter Grundig, Britt Hofmann, Uwe Kirchhefer, Joachim Neumann, and Ulrich Gergs. 2025. "Hyperthermia Augments the H1-Histamine Receptor-Mediated Force in the Human Atrium" International Journal of Molecular Sciences 26, no. 14: 6842. https://doi.org/10.3390/ijms26146842
APA StylePham, T. H., Grundig, P., Hofmann, B., Kirchhefer, U., Neumann, J., & Gergs, U. (2025). Hyperthermia Augments the H1-Histamine Receptor-Mediated Force in the Human Atrium. International Journal of Molecular Sciences, 26(14), 6842. https://doi.org/10.3390/ijms26146842






