Electromagnetic Transduction Therapy (EMTT) Enhances Tenocyte Regenerative Potential: Evidence for Senolytic-like Effects and Matrix Remodeling
Abstract
1. Introduction
2. Results
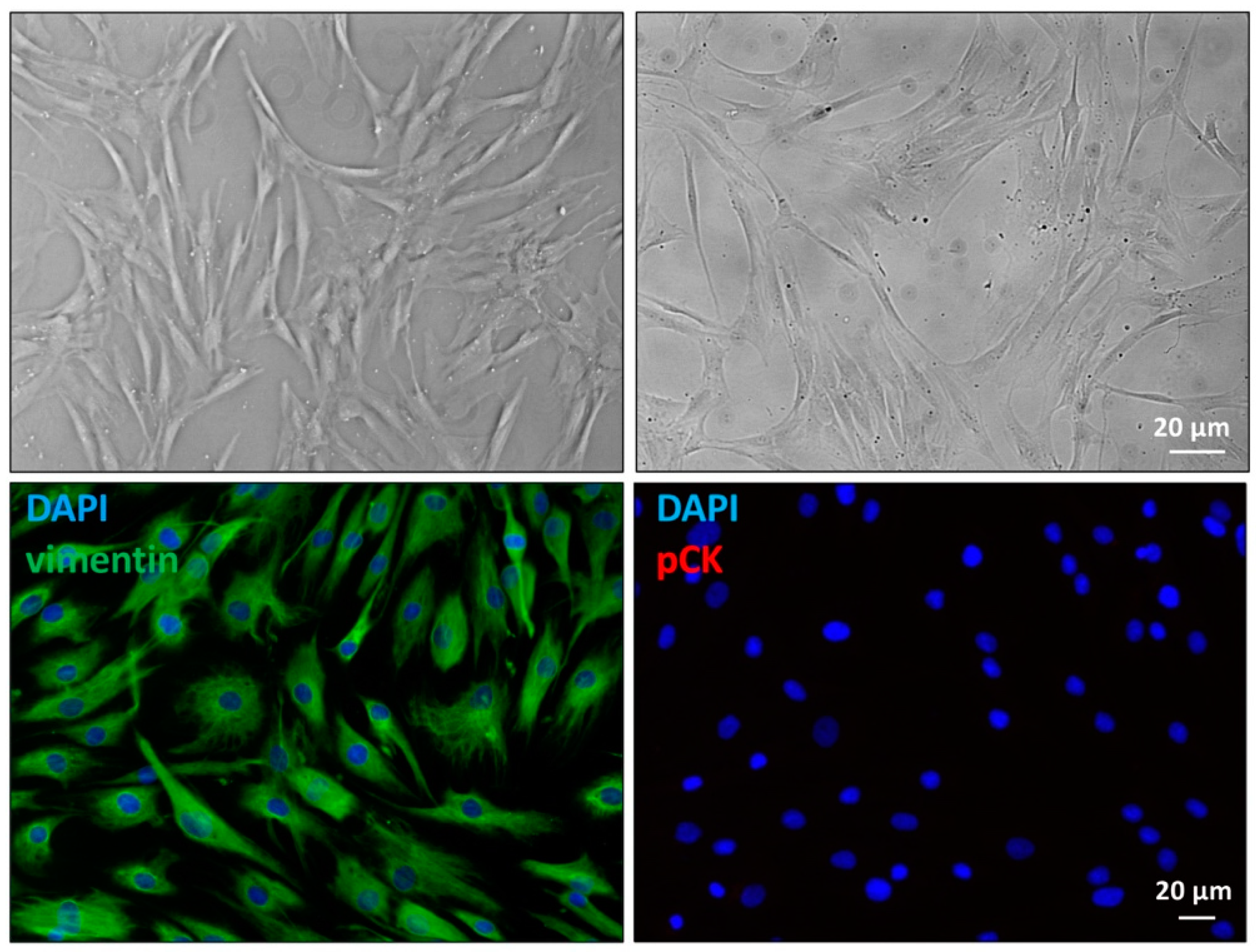
2.1. Primary Cultures Characterization
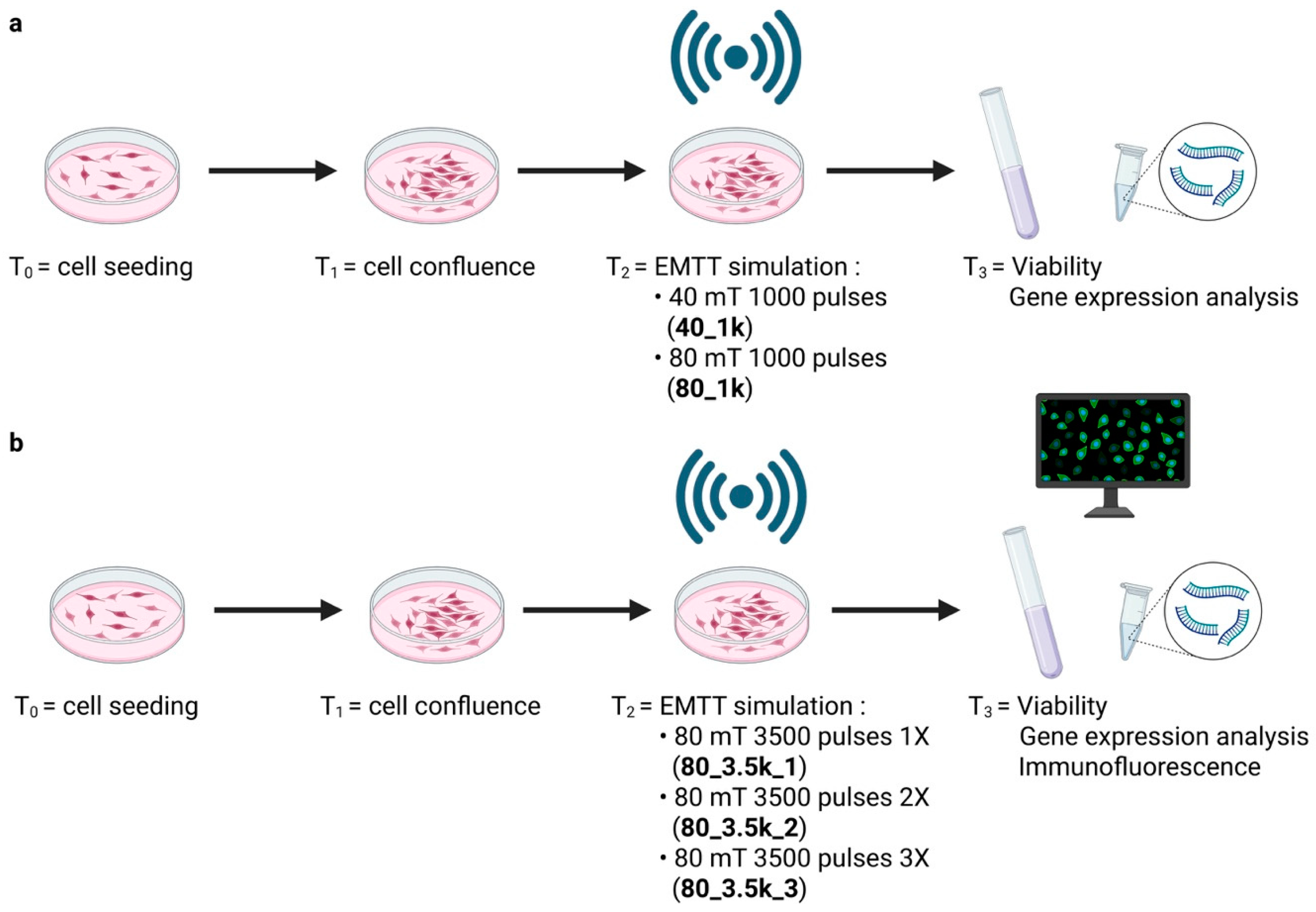
2.2. Experimental Design
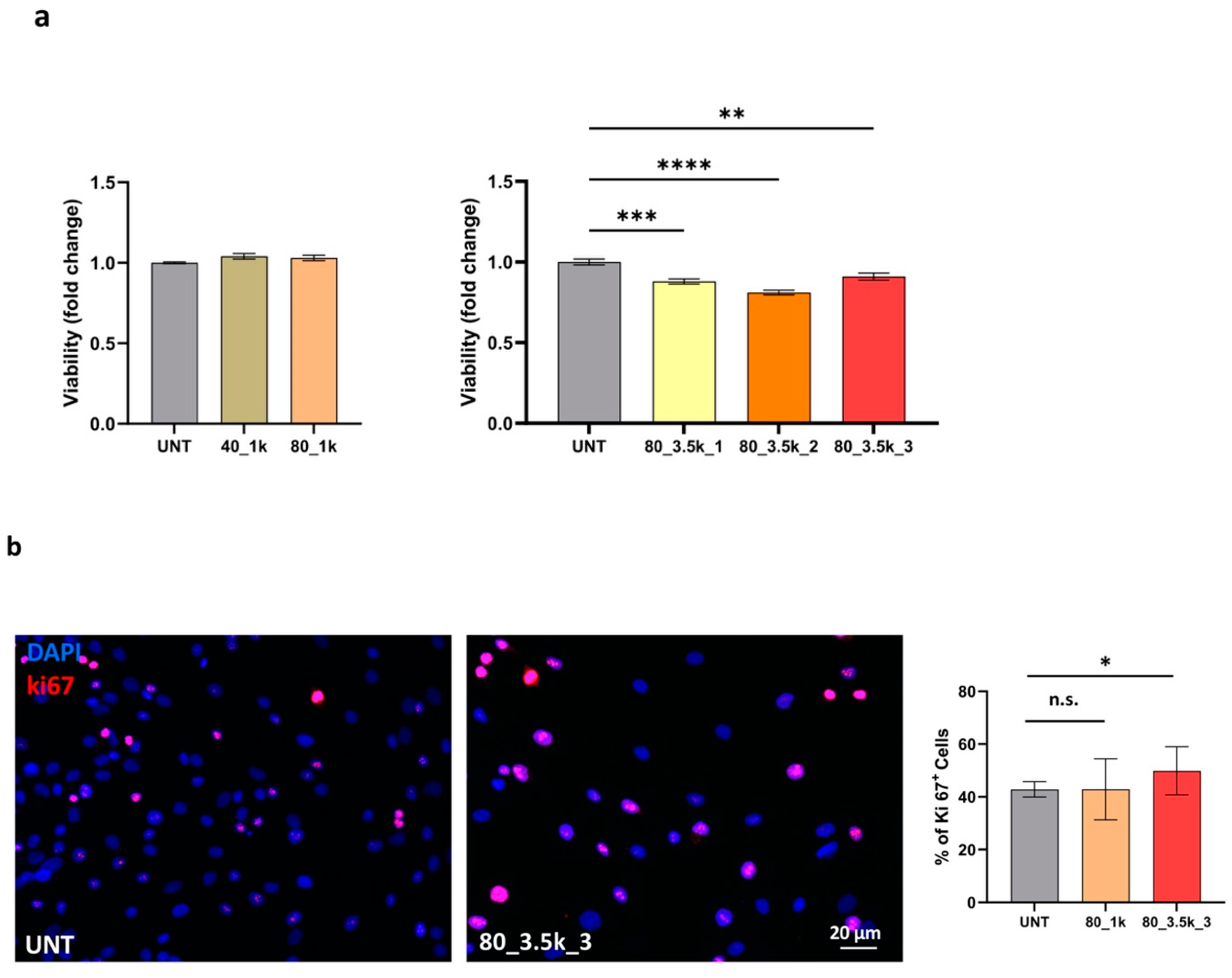
2.3. Effects of EMTT on Cell Viability and Proliferation
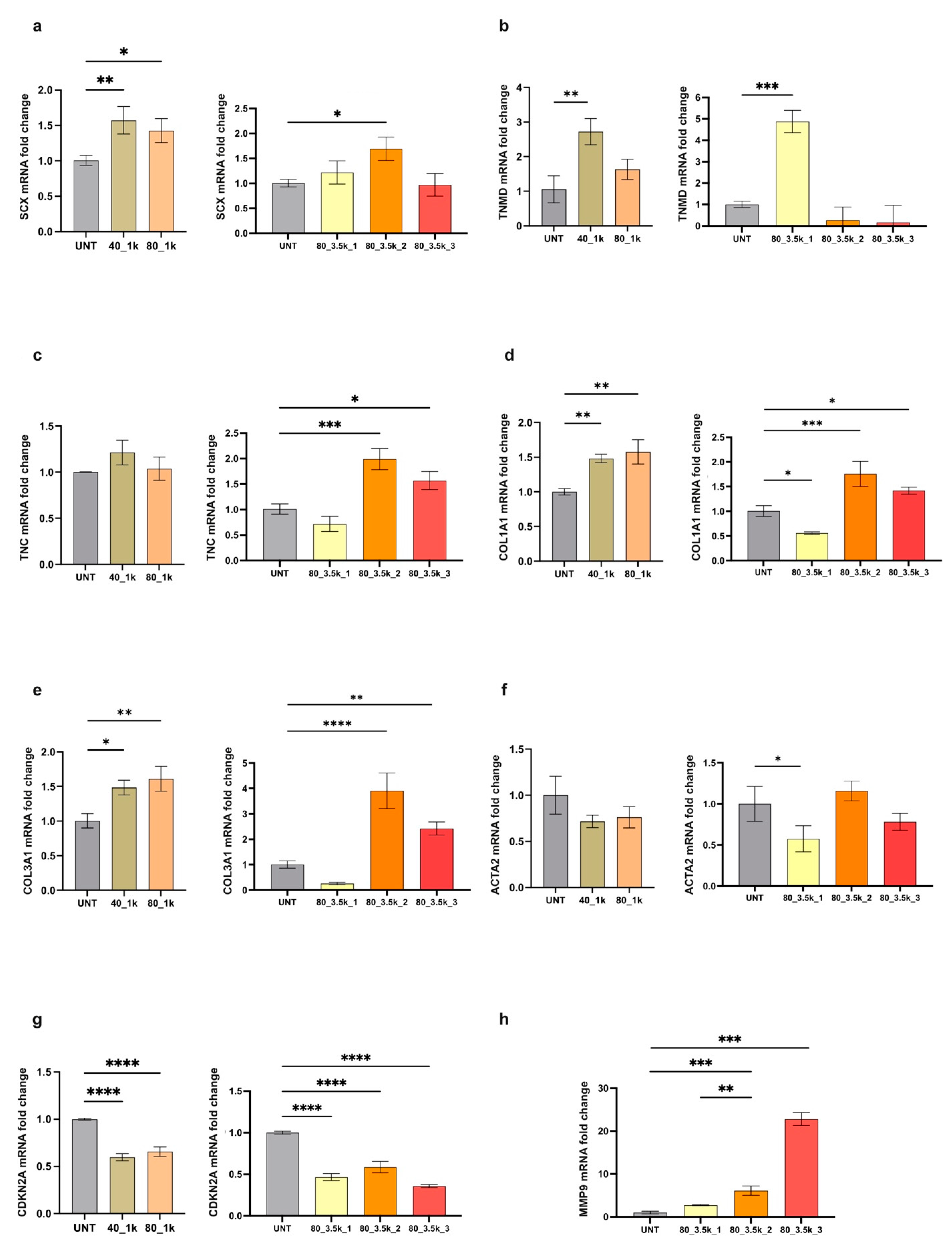
2.4. Molecular Analysis of EMTT-Exposed Tenocytes
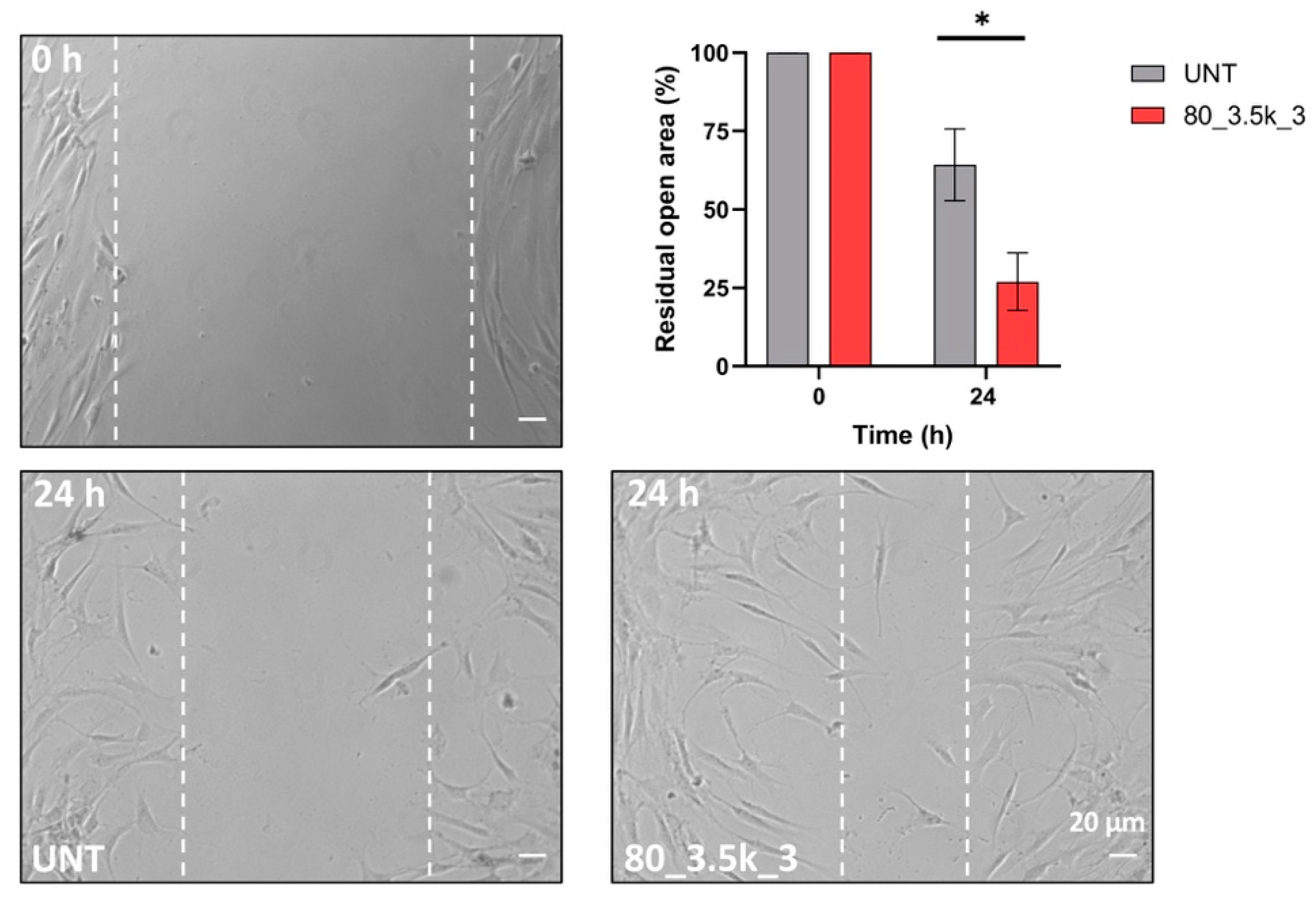
2.5. Scratch Test of EMTT-Treated Tenocytes
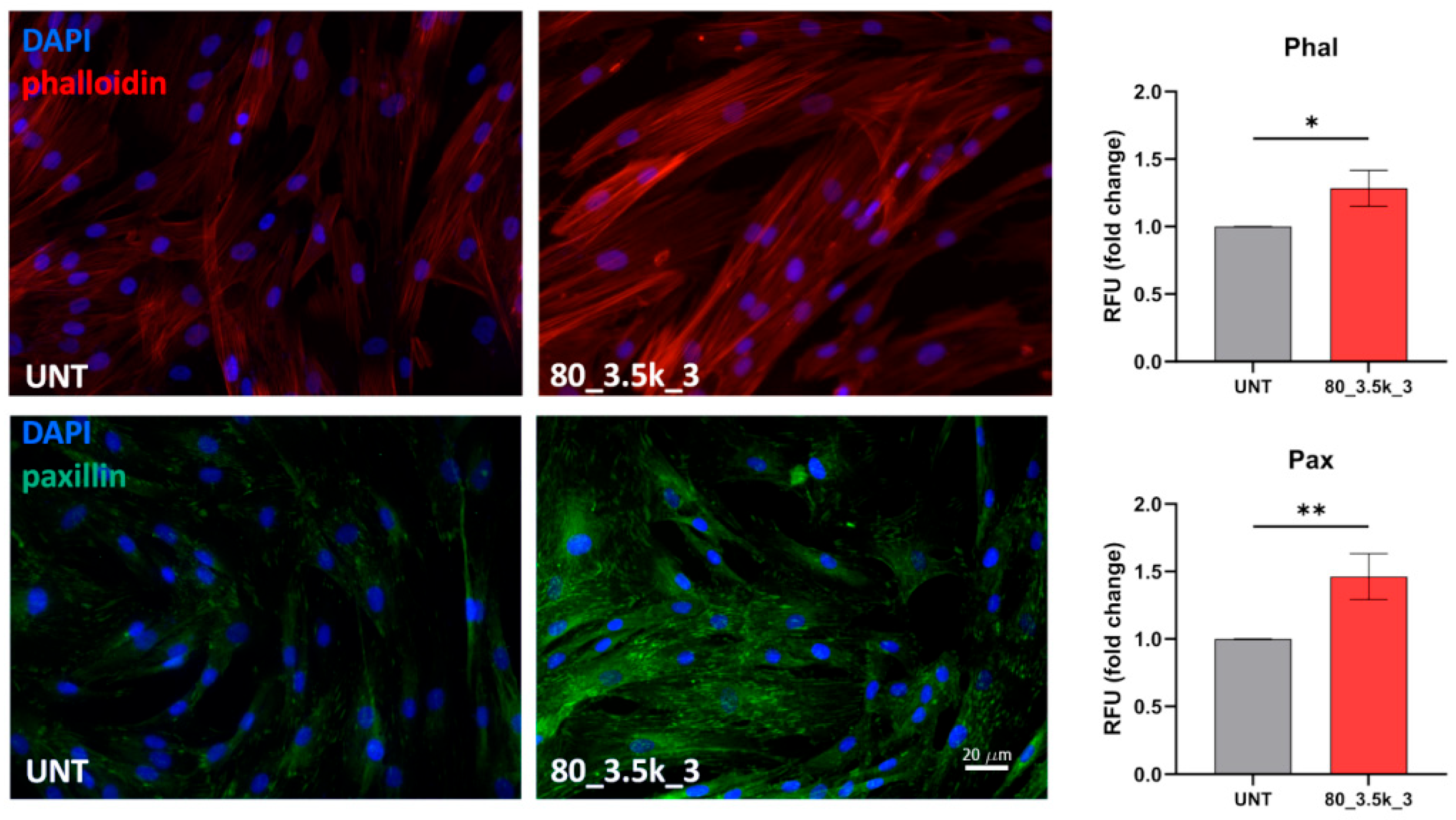
2.6. Actin Cytoskeleton Reorganization and Paxillin Localization
3. Discussion
4. Materials and Methods
4.1. Cells and Treatments
4.2. Immunofluorescence
4.3. Colorimetric MTT Assay
4.4. Scratch Assay
4.5. RNA Extraction and cDNA Synthesis
4.6. Primers
4.7. RT-PCR Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sharma, P.; Maffulli, N. Tendinopathy and tendon injury: The future. Disabil. Rehabil. 2008, 30, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Fu, S.C.; Chua, E.; Hu, X.; Rolf, C.; Mattila, V.M.; Qin, L.; Yung, P.S.-H.; Chan, K.-M. Critical review on the socioeconomic impact of tendinopathy. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2016, 4, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Khan, K.M.; Puddu, G. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy 1998, 14, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Squier, K.; Alfredson, H.; Bahr, R.; Cook, J.L.; Coombes, B.; de Vos, R.-J.; Fu, S.N.; Grimaldi, A.; Lewis, J.S.; et al. ICON 2019: International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br. J. Sports Med. 2020, 54, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. Tendon healing in presence of chronic low-level inflammation: A systematic review. Br. Med. Bull. 2019, 132, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Murrell, G.A.C.; McInnes, I.B. Inflammatory mechanisms in tendinopathy—Towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. In Vitro Innovation of Tendon Tissue Engineering Strategies. Int. J. Mol. Sci. 2020, 21, 6726. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jones, I.A.; Park, C.; Vangsness, C.T., Jr. The Efficacy of Platelet-Rich Plasma on Tendon and Ligament Healing: A Systematic Review and Meta-analysis with Bias Assessment. Am. J. Sports Med. 2018, 46, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [PubMed]
- Moya, D.; Ramón, S.; Schaden, W.; Wang, C.-J.; Guiloff, L.; Cheng, J.-H. The Role of Extracorporeal Shockwave Treatment in Musculoskeletal Disorders. J. Bone Jt. Surg. Am. 2018, 100, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, M.; d’Alessandro, F.; Torrisi, M.R.; Ferretti, A.; Vulpiani, M.C.; Visco, V. Extracorporeal shock wave therapy promotes cell proliferation and collagen synthesis of primary cultured human tenocytes. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; Vetrano, M.; Ranieri, D.; Raffa, S.; Vulpiani, M.C.; Ferretti, A.; Torrisi, M.R.; Visco, V.; Yue, J. Extracorporeal Shock Wave Treatment (ESWT) improves in vitro functional activities of ruptured human tendon-derived tenocytes. PLoS One 2012, 7, e49759. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, M.; Ranieri, D.; Nanni, M.; Pavan, A.; Malisan, F.; Vulpiani, M.C.; Visco, V. Hyaluronic Acid (HA), Platelet-Rich Plasm and Extracorporeal Shock Wave Therapy (ESWT) promote human chondrocyte regeneration in vitro and ESWT-mediated increase of CD44 expression enhances their susceptibility to HA treatment. PLoS ONE 2019, 14, e0218740. [Google Scholar] [CrossRef] [PubMed]
- Gerdesmeyer, L.; Saxena, A.; Klueter, T.; Harrasser, N.; Fullem, B.; Krath, A. Electromagnetic Transduction Therapy for Achilles Tendinopathy: A Preliminary Report on a New Technology. J. Foot Ankle Surg. 2017, 56, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Gerdesmeyer, L.; Tubel, J.; Obermeier, A.; Harrasser, N.; Glowalla, C.; von Eisenhart-Rothe, R.; Burgkart, R. Extracorporeal Magnetotransduction Therapy as a New Form of Electromagnetic Wave Therapy: From Gene Upregulation to Accelerated Matrix Mineralization in Bone Healing. Biomedicines 2024, 12, 2269. [Google Scholar] [CrossRef] [PubMed]
- Klüter, T.; Krath, A.; Stukenberg, M.; Zielhardt, P.; Gollwitzer, H.; Harrasser, N.; Hausdorf, J.; Ringeisen, M.; Gerdesmeyer, L. Electromagnetic transduction therapy in non-specific low back pain: A prospective randomized controlled trial. J. Orthop. 2017, 14, 410–415. [Google Scholar]
- Uzunca, K.; Birtane, M.; Taştekin, N. Effectiveness of pulsed electromagnetic field therapy in lateral epicondylitis. Clin. Rheumatol. 2007, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Klüter, T.; Krath, A.; Stukenberg, M.; Gollwitzer, H.; Harrasser, N.; Knobloch, K.; Maffulli, N.; Hausdorf, J.; Gerdesmeyer, L. Electromagnetic transduction and shockwave therapy in 86 patients with rotator cuff tendinopathy: A prospectiverandomizedd controlled trial. Electromagn. Biol. Med. 2018, 37, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Yau, D.K.W.; Li, A.C.Y.; Cheung, H.Y.; Cheung, N.; Lee, A. The use of electromagnetic transduction therapy in patients with chronic myofascial pain: A pilot double-blinded randomised controlled trial. Electromagn. Biol. Med. 2025, 44, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gerdesmeyer, L.; Zielhardt, P.; Klüter, T.; Wang, F.; Gollwitzer, H.; Gerdesmeyer, L.; Hausdorf, J.; Ringeisen, M.; Knobloch, K.; Saxena, A.; et al. Stimulation of human bone marrow mesenchymal stem cells by electromagnetic transduction therapy—EMTT. Electromagn. Biol. Med. 2022, 41, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kumagai, K. Molecular dissection of tendon development and healing: Insights into tenogenic phenotypes and functions. J. Biol. Chem. 2025, 301, 108353. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Ewen, S.W.; Waterston, S.W.; Reaper, J.; Barrass, V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am. J. Sports Med. 2000, 28, 499–505. [Google Scholar]
- Ashraf, S.; Cha, B.H.; Kim, J.S.; Ahn, J.; Han, I.; Park, H.; Lee, S.-H. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr. Cartil. 2016, 24, 196–205. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.H.; Kim, C.; Laberge, R.-M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, A.; Oliva, F.; Osti, L.; Maffulli, N. Metalloproteases and tendinopathy. Muscles Ligaments Tendons J. 2013, 3, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Fernandes, T.; Godoy-Santos, A.L.; Santos, M.C.; Pontin, P.; Pereira, C.A.A.; Jardim, Y.J.; Velosa, A.P.P.; Maffulli, N.; Teodoro, W.R.; Capelozzi, V.L. Matrix metalloproteinase-1 (MMP-1) and (MMP-8) gene polymorphisms promote increase and remodeling of the collagen III and V in posterior tibial tendinopathy. Histol. Histopathol. 2018, 33, 929–936. [Google Scholar] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, C.; Chen, R.; Jin, C.; Li, H.; Yan, H.; Wang, X. Focal adhesion kinase and paxillin promote migration and adhesion to fibronectin by swine skeletal muscle satellite cells. Oncotarget 2016, 7, 30845–30854. [Google Scholar] [CrossRef] [PubMed]
- Laukaitis, C.M.; Webb, D.J.; Donais, K.; Horwitz, A.F. Differential Dynamics of alpha-5 Integrin, Paxillin, and alpha-Actinin during Formation and Disassembly of Adhesions in Migrating Cells. J. Cell Biol. 2001, 153, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tondravi, M.; Liu, J.; Smith, E.; Haudenschild, C.C.; Kaczmarek, M.; Zhan, X. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 2001, 61, 6906–6911. [Google Scholar] [PubMed]
- Rajalekshmi, R.; Agrawal, D.K. Energizing Healing with Electromagnetic Field Therapy in Musculoskeletal Disorders. J. Orthop. Sports Med. 2024, 6, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.G.; Delgado-Calcares, M.; Stange, R.; Wildemann, B.; Docheva, D. Tendon healing: A concise review on cellular and molecular mechanisms with a particular focus on the Achilles tendon. Bone Jt. Res. 2022, 11, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Yang, R.; Li, J. Single-cell and spatial transcriptomics reveal changes in cell heterogeneity during progression of human tendinopathy. BMC Biol. 2023, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Stowe, E.J.; Keller, M.R.; Connizzo, B.K. Cellular senescence impairs tendon extracellular matrixremodeling in response to mechanical unloading. Aging Cell 2024, 23, e14278. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, G.-C.; Li, Y.-J.; Chen, M.-H.; Lu, P.-P.; Zhang, Y.-W.; Zhang, M.; Cao, M.-M.; Rui, Y.-F. Targeting senescent tendon stem/progenitor cells to prevent or treat age-related tendon disorders. Stem Cell Rev. Rep. 2023, 19, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, Q.; Song, W.; He, Y. Platelet-derived exosomes alleviate tendon stem/progenitor cell senescence and ferroptosis by regulating AMPK/Nrf2/GPX4 signaling and improve tendon-bone junction regeneration in rats. J. Orthop. Surg. Res. 2024, 19, 382. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Jiang, Y.; Backman, L.J.; Zhang, W.; Chen, J. The Application of Mechanical Stimulations in Tendon Tissue Engineering. Stem Cells Int. 2020, 2020, 8824783. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, A.E.; Frisch, B.J.; Wolenski, M.; Jacobson, J.A.; Calvi, L.M.; Schwarz, E.M.; Awad, H.A.; O’Keefe, R.J. Bone marrow-derived matrix metalloproteinase-9 is associated with fibrous adhesion formation after murine flexor tendon injury. PLoS One 2012, 7, e40602. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Cesari, E.; Garofalo, R.; Gigante, A.; Conti, M.; Markopoulos, N.; Maffulli, N. Matrix metalloproteases and their inhibitors are altered in torn rotator cuff tendons, but also in the macroscopically and histologically intact portion of those tendons. Muscles Ligaments Tendons J. 2013, 3, 132–138. [Google Scholar] [PubMed]
- Gaut, L.; Duprez, D. Tendon development and diseases. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.C.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology -a minireview of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.C. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- von Rickenbach, K.J.; Borgstrom, H.; Tenforde, A.; Borg-Stein, J.; McInnis, K.C. Achilles Tendinopathy: Evaluation, Rehabilitation, and Prevention. Curr. Sports Med. Rep. 2021, 20, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Morya, V.K.; Shahid, H.; Lang, J.; Kwak, M.K.; Park, S.H.; Noh, K.C. Advancements in Therapeutic Approaches for Degenerative Tendinopathy: Evaluating Efficacy and Challenges. Int. J. Mol. Sci. 2024, 25, 11846. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, D.; Rosato, B.; Nanni, M.; Belleudi, F.; Torrisi, M.R. Expression of the FGFR2c mesenchymal splicing variant in human keratinocytes inhibits differentiation and promotes invasion. Mol. Carcinog. 2018, 57, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Traversa, A.; Mari, E.; Pontecorvi, P.; Gerini, G.; Romano, E.; Megiorni, F.; Amedei, A.; Marchese, C.; Ranieri, D.; Ceccarelli, S. Polyethylene Micro/Nanoplastics Exposure Induces Epithelial-Mesenchymal Transition in Human Bronchial and Alveolar Epithelial Cells. Int. J. Mol. Sci. 2024, 25, 10168. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T. Primer-BLAST: A tool to design target-specific primersfor polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, D.; Avitabile, D.; Shiota, M.; Yokomizo, A.; Naito, S.; Bizzarri, M.; Torrisi, M.R. Nuclear redox imbalance affects circadian oscillation in HaCaT keratinocytes. Int. J. Biochem. Cell Biol. 2015, 65, 113–124. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primers |
|---|---|
| Col I | 5′–ACATGTTCAGCTTTGTGGACCTCCG-3′/5′–ACGCAGGTGATTGGTGGGAGTTCT-3′ |
| Col III | 5′–AGGGTGTCAAGGGTGAAAGTGGGA-3′/5′–ACCAGCCAGACCAGGAAGACCC-3′ |
| Scx | 5′–CGAGCGAGACCGCACCAACA-3′/5′–CGTTGCCCAGGTGCGAGATGTAG-3′ |
| Tnm | 5′–CCGCCGCGTCTGTGAACCTT-3′/5′–GCGGGCCACCCACCAGTTAC-3′ |
| Tn-C | 5′–GGAGGGGACCACGCTAGGT-3′/5′–TCCCGGCCTAGACCTGTGAG-3′ |
| 18 s | 5′–CGAGCCGCCTGGATACC-3′/5′–CATGGCCTCAGTTCCGAAAA-3′ |
| CDKN2A | 5′–CGTGGACCTGGCTGAGGA-3′/5′–AATCGGGGATGTCTGAGGGA-3′ |
| Alpha-SMA | 5′–GCACCCCTAGAACCCCAAG-3′/5′–ACGATGCCAGTTGTGCGT-3′ |
| MMP9 | 5′–CGCGCTGGGCTTAGATCATT-3ʹ/5′–GGGCGAGGACCATAGAGGT-3ʹ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, M.; Vetrano, M.; Traversa, A.; Cauli, C.; Ceccarelli, S.; Malisan, F.; Vulpiani, M.C.; Maffulli, N.; Marchese, C.; Visco, V.; et al. Electromagnetic Transduction Therapy (EMTT) Enhances Tenocyte Regenerative Potential: Evidence for Senolytic-like Effects and Matrix Remodeling. Int. J. Mol. Sci. 2025, 26, 7122. https://doi.org/10.3390/ijms26157122
Mancini M, Vetrano M, Traversa A, Cauli C, Ceccarelli S, Malisan F, Vulpiani MC, Maffulli N, Marchese C, Visco V, et al. Electromagnetic Transduction Therapy (EMTT) Enhances Tenocyte Regenerative Potential: Evidence for Senolytic-like Effects and Matrix Remodeling. International Journal of Molecular Sciences. 2025; 26(15):7122. https://doi.org/10.3390/ijms26157122
Chicago/Turabian StyleMancini, Matteo, Mario Vetrano, Alice Traversa, Carlo Cauli, Simona Ceccarelli, Florence Malisan, Maria Chiara Vulpiani, Nicola Maffulli, Cinzia Marchese, Vincenzo Visco, and et al. 2025. "Electromagnetic Transduction Therapy (EMTT) Enhances Tenocyte Regenerative Potential: Evidence for Senolytic-like Effects and Matrix Remodeling" International Journal of Molecular Sciences 26, no. 15: 7122. https://doi.org/10.3390/ijms26157122
APA StyleMancini, M., Vetrano, M., Traversa, A., Cauli, C., Ceccarelli, S., Malisan, F., Vulpiani, M. C., Maffulli, N., Marchese, C., Visco, V., & Ranieri, D. (2025). Electromagnetic Transduction Therapy (EMTT) Enhances Tenocyte Regenerative Potential: Evidence for Senolytic-like Effects and Matrix Remodeling. International Journal of Molecular Sciences, 26(15), 7122. https://doi.org/10.3390/ijms26157122









