Application of Hansen Solubility Parameters in the Aqueous-Ethanol Extraction of Genistein-7-O-[α-rhamnopyranosyl-(1→6)]-β-glucopyranoside from Derris scandens and Its Molecular Orbital Study on Antioxidant Activity
Abstract
1. Introduction
2. Results
2.1. Preparation of In-House GTG from Derris scandens
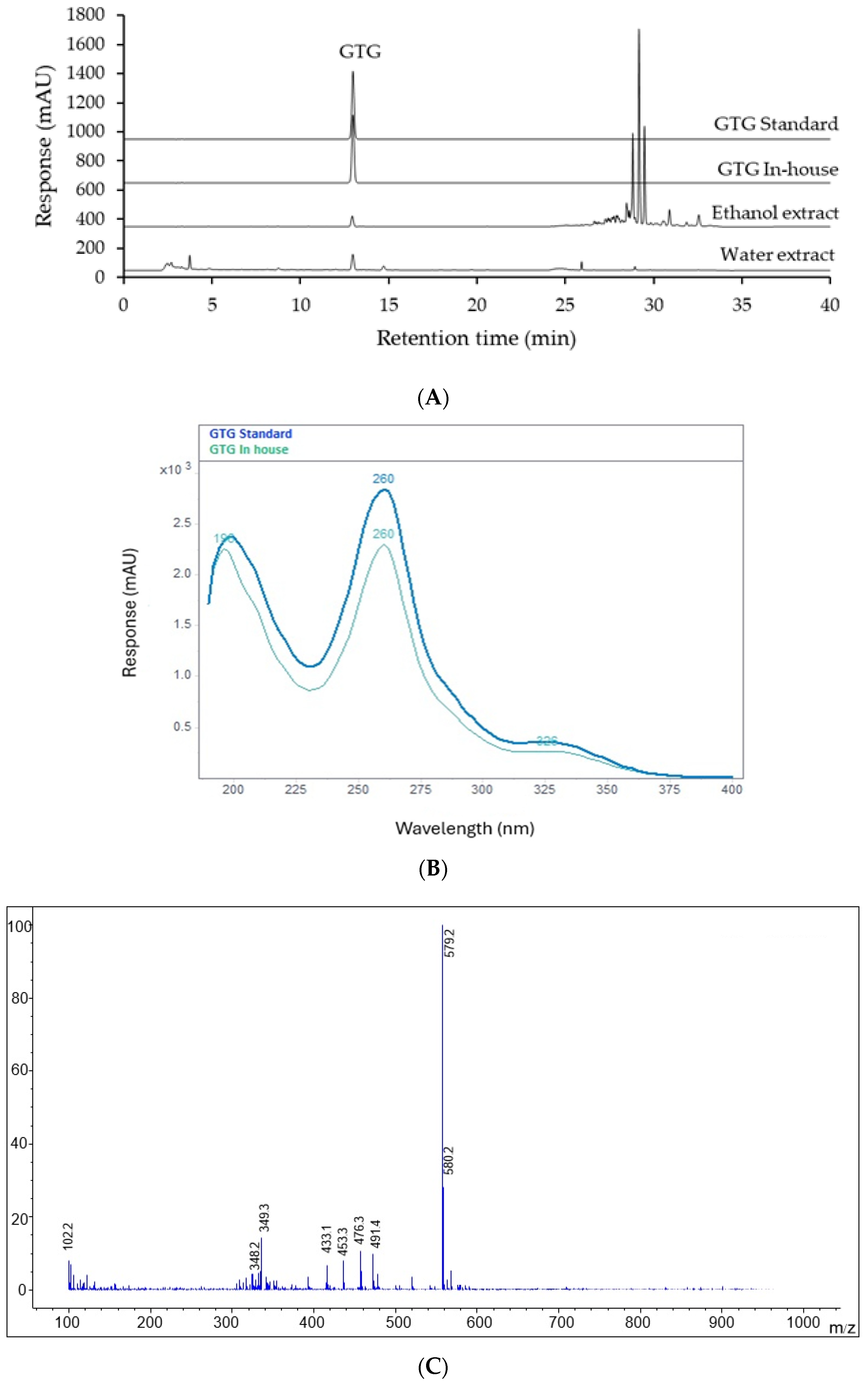
2.2. Identification of GTG in Derris scandens Extracts
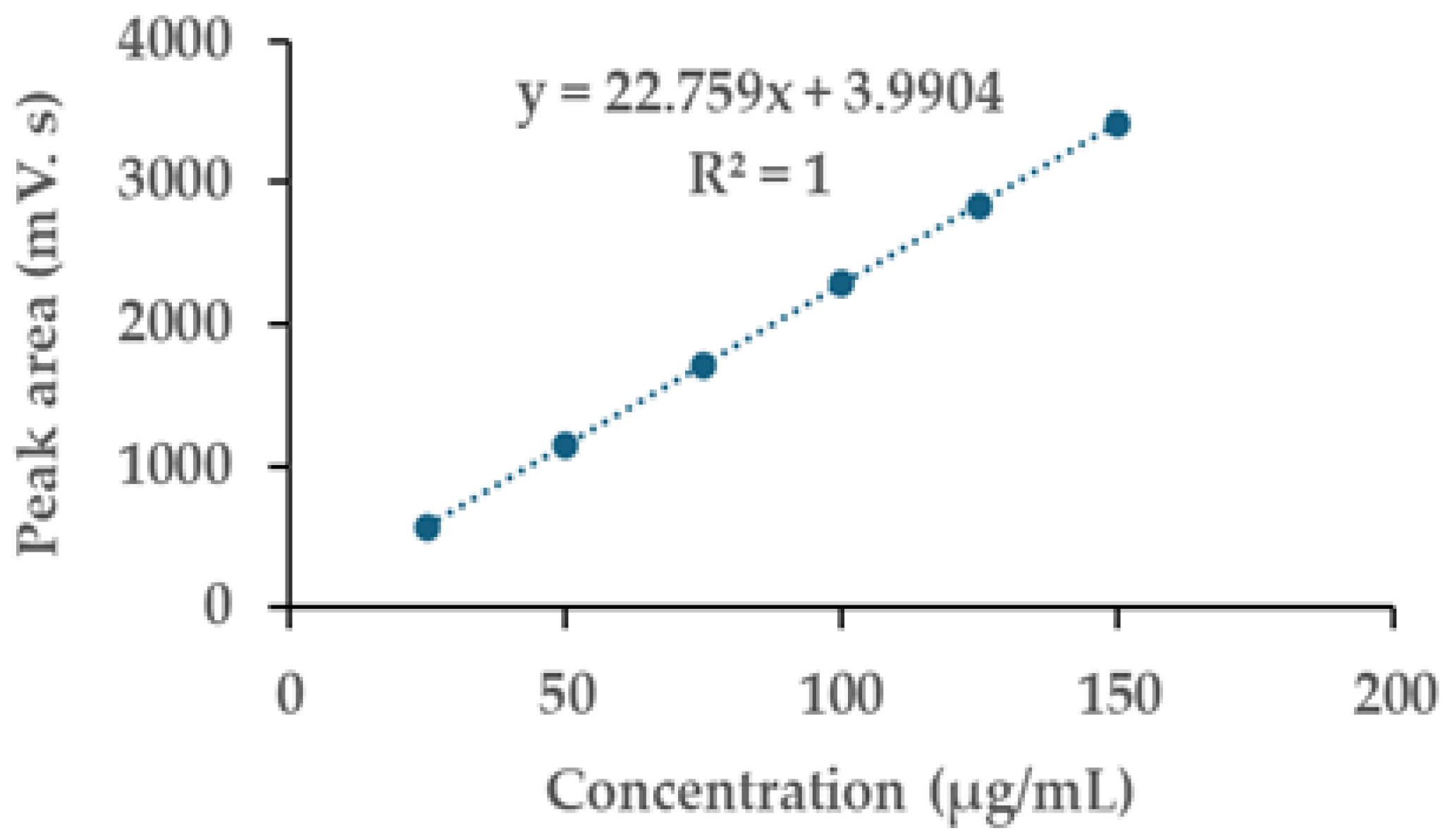
2.3. Validation of HPLC Analytical Method for Determination of GTG in Derris scandens Extracts
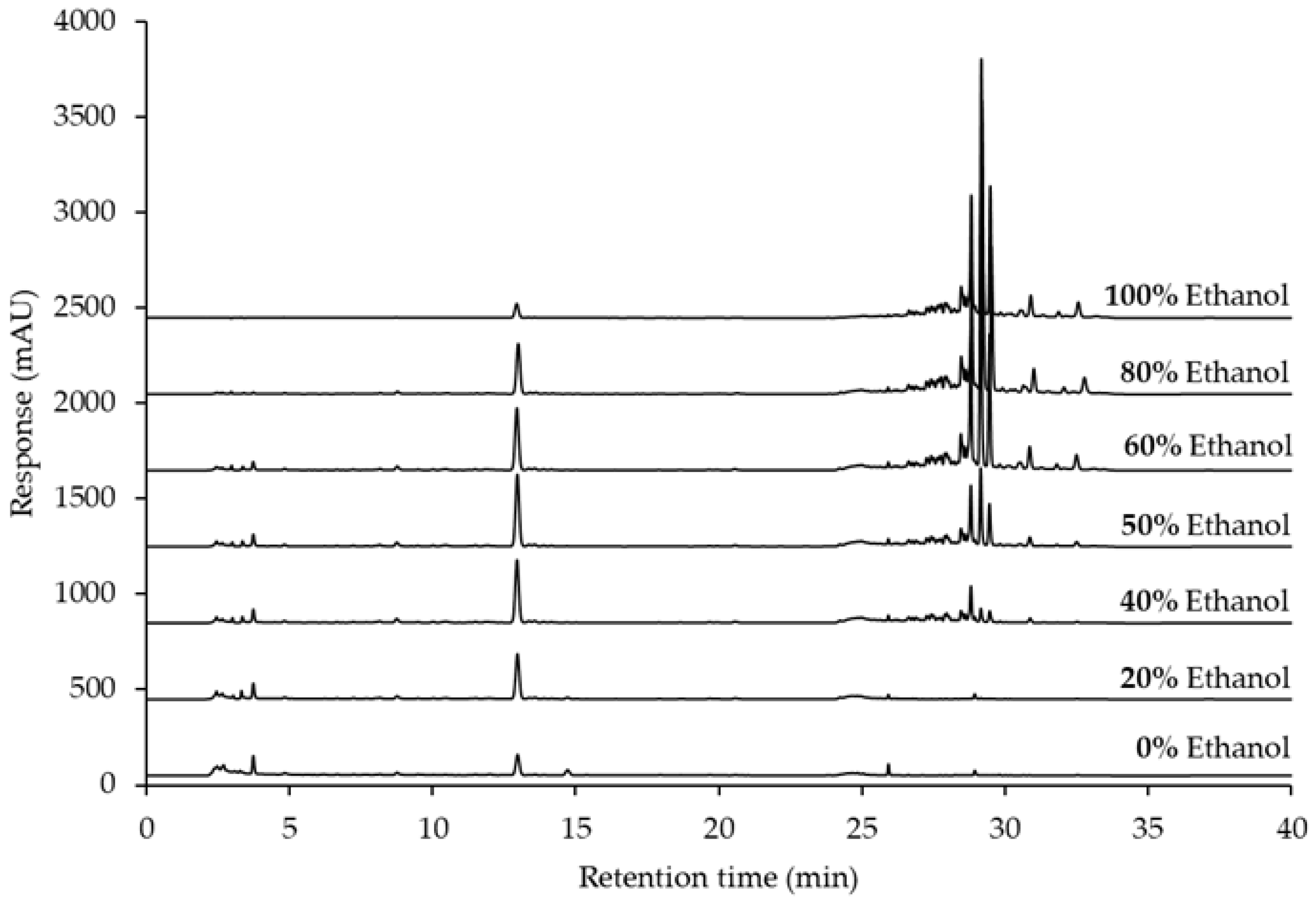
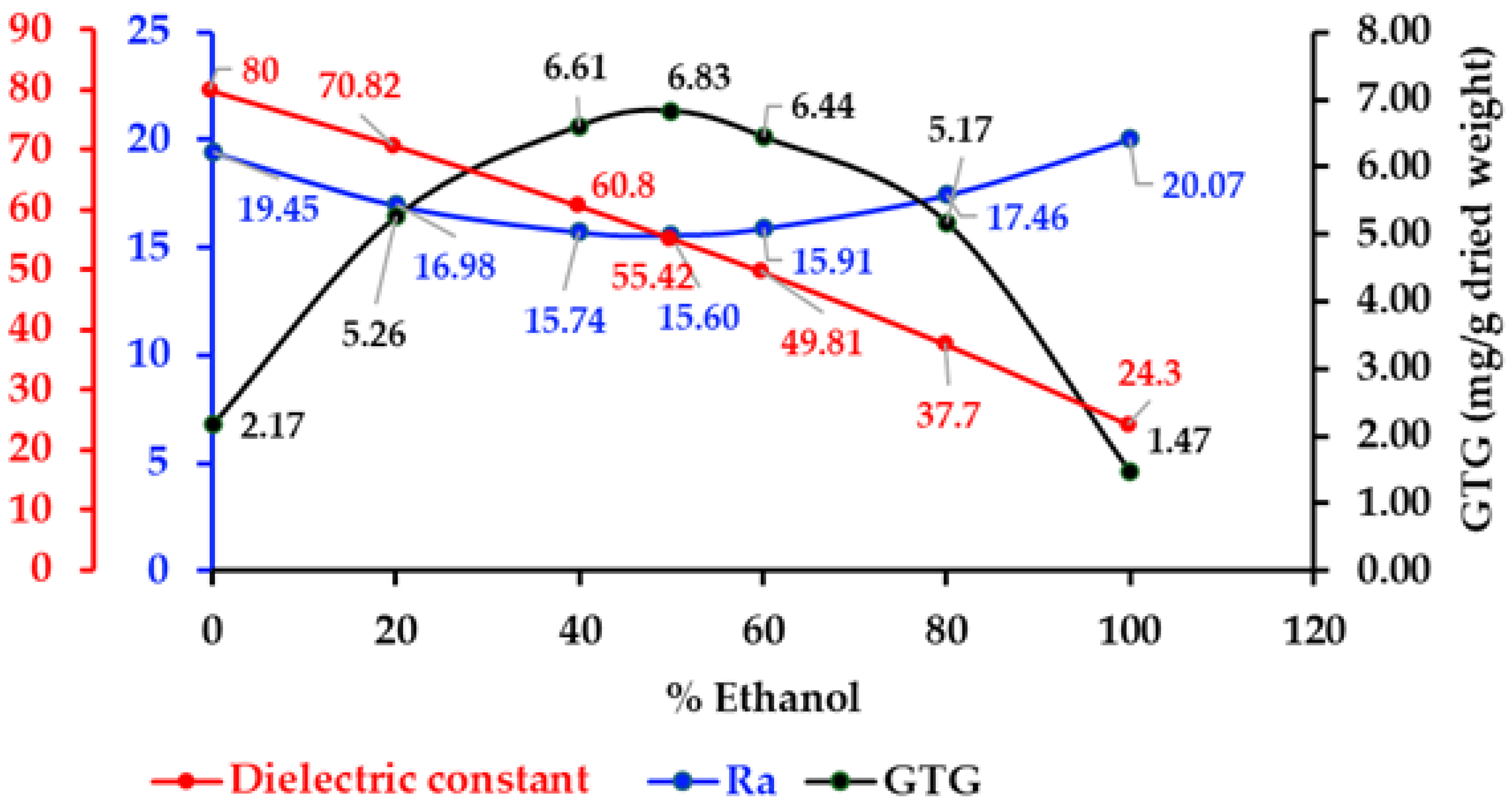
2.4. Solvent Selection for the Extraction of GTG from Derris scandens
2.5. The Relationship of Extracted GTG Content to Hansen Solubility Parameters and Dielectric Constants
2.6. Total Phenolics, Total Flavonoids and Antioxidants Activities of Derris scandens Extracts
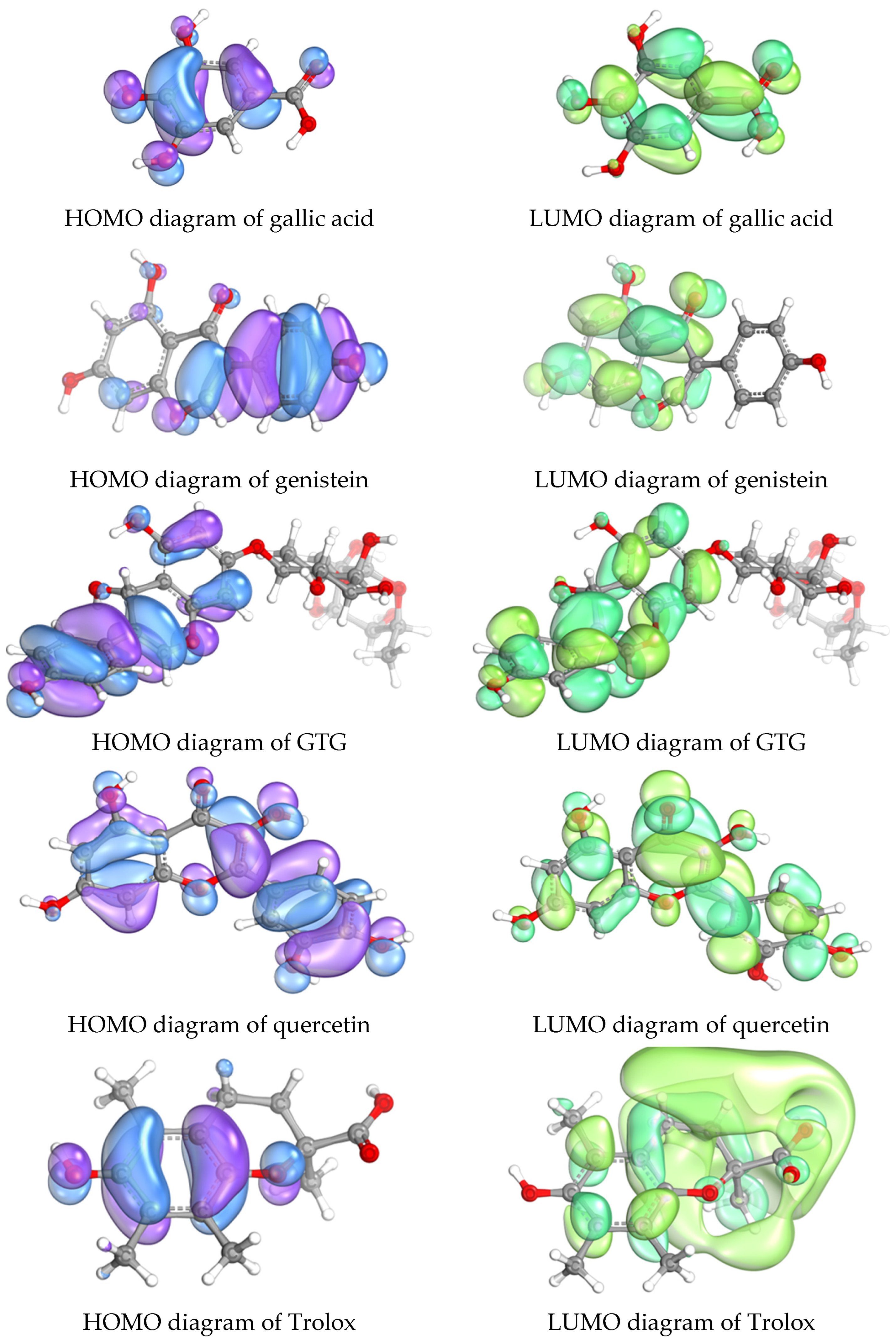
2.7. Relationship Between Molecular Orbitals and Antioxidant Activities of GTG and Established Antioxidant Molecules (Gallic Acid, Genistein, Quercetin, and Trolox)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Calculation of Hansen Solubility Parameters
4.3. Preparation of Crude Extract
4.4. HPLC Analysis of GTG in Derris scandens Extracts
4.4.1. Identification of GTG by HPLC-MS
4.4.2. Quantification of GTG in Derris scandens Extracts by HPLC-PDA
4.5. Method Validation
4.5.1. Specificity
4.5.2. Linearity
4.5.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
4.5.4. Accuracy
4.5.5. Precision
4.6. Preparation of In-House GTG
4.7. Determination of Total Phenolic Content
4.8. Determination of Total Flavonoid Content
4.9. Evaluation of Antioxidant Activity
4.9.1. DPPH Radical Scavenging Activity
4.9.2. Ferric Reduction Ability-Antioxidant Power Test
4.10. Density Functional Theory (DFT) Calculations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AlCl3 | Aluminum chloride |
| AOA | Antioxidant activity |
| AOAC | Association of Official Analytical Collaboration |
| °C | Celsius degree |
| cm | Centimeter |
| Da | Dalton |
| DFT | Density functional theory |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| eV | Electron volt |
| ESI | Electrospray ionization |
| FeSO4 | Ferrous sulfate |
| FMO | Frontier molecular orbital |
| g | Gram |
| GAE | Gallic acid equivalent |
| GC-MS | Gas chromatography-mass spectrometry |
| GTG | Genistein-7-O-[α-rhamnopyranosyl-(1→6)]-β-glucopyranoside |
| HCl | Hydrochloric acid |
| HOMO | Highest occupied molecular orbital |
| HPLC | High Performance Liquid Chromatography |
| HSP | Hansen Solubility Parameters |
| IBO | Intrinsic bond orbital |
| IC50 | Half–maximal inhibitory concentration |
| ICH | The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| LUMO | Lowest unoccupied molecular orbital |
| L | Litter |
| LOD | Limit of Detection |
| LOQ | Limit of Quantitation |
| mL | Milliliter |
| mM | Millimolar |
| Mol | Mole |
| MW | Molecular weight |
| Na2CO3 | Sodium carbonate |
| QE | Quercetin equivalent |
| Ra | Hansen solubility parameter distance |
| SD | Standard deviation |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| UAE | Ultrasonic-assisted extraction |
| UV | Ultraviolet |
| δD | Dispersion force |
| δH | Hydrogen bonding energy |
| δP | Dipolar intermolecular force |
| µg | Micro gram |
| µM | Micro molar |
| %RSD | Percentage relative standard deviation |
References
- Laupattarakasem, P.; Houghton, P.J.; Hoult, J.R.S. Anti-inflammatory isoflavonoids from the stems of Derris scandens. Planta Medica 2004, 70, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Mukit, T.; Ashrafi, S.; Ahsan, M.; Azam, A.Z. Chemical and Biological Profiling of Derris scandens (Roxb.) Benth. Bangladesh J. Bot. 2024, 53, 227–233. [Google Scholar] [CrossRef]
- Sangkaew, W.; Sianglum, W.; Wunnoo, S.; Voravuthikunchai, S.P.; Joycharat, N. Bioactive substance contents and therapeutic potential for skin inflammation of an herbal gel containing Derris reticulata and Glycyrrhiza glabra. Pharm. Biol. 2024, 62, 648–658. [Google Scholar] [CrossRef]
- Ayameang, O.; Rattarom, R.; Mekjaruskul, C.; Caichompoo, W. Anti-inflammatory activity and quantitative analysis of major compounds of the mixtures of Derris scandens (DZSS) formula. Pharmacogn. J. 2020, 12, 828–834. [Google Scholar] [CrossRef]
- Hematulin, A.; Meethang, S.; Ingkaninan, K.; Sagan, D. Derris scandens Benth extract potentiates radioresistance of Hep-2 laryngeal cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 1289–1295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puttarak, P.; Sawangjit, R.; Chaiyakunapruk, N. Efficacy and safety of Derris scandens (Roxb.) Benth. for musculoskeletal pain treatment: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2016, 194, 316–323. [Google Scholar] [CrossRef]
- Sae-Foo, W.; Yusakul, G.; Nualkaew, N.; Putalun, W. Estrogenic Activity of Derris scandens Stem Extract and its Major Compounds Using MCF-7 Cell Proliferation Assay and Estrogen-Related Gene Expression. Planta Medica 2024, 90, 766–773. [Google Scholar] [CrossRef]
- Shahriar, S.; Jaman, A.U.; Saha, S.; Islam, M.S.; Islam, M.R.; Saha, B.; Sikder, M.A.A.; Rashid, M.A. Comparison of antimicrobial and membrane stabilizing properties of leaf and stem extracts of Derris scandens (Roxb.) Benth.(Family: Fabaceae). Bangladesh Pharm. J. 2023, 26, 67–72. [Google Scholar] [CrossRef]
- Sae-Foo, W.; Nualkaew, N.; Yusakul, G.; Putalun, W. Estrogenic activity of isoflavones derived from Derris scandens using MCF-7 cell. Planta Medica 2022, 88, 1475. [Google Scholar] [CrossRef]
- Veitch, N.C. Isoflavonoids of the leguminosae. Nat. Prod. Rep. 2013, 30, 988–1027. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Seo, D.B.; Shin, H.-J.; Lee, S.-J. Effect of Aspergillus oryzae-challenged germination on soybean isoflavone content and antioxidant activity. J. Agric. Food Chem. 2012, 60, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.-H.; Sadat, M.A.; Kim, W.; Park, T.-S.; Park, D.K.; Choi, J. Comprehensive transcriptomic analysis of Cordyceps militaris cultivated on germinated soybeans. Mycobiology 2022, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.A.; Srinivas, P.V.; Tiwari, A.K.; Vanka, U.M.S.; Rao, R.V.S.; Dasari, K.R.; Rao, M.J. Isolation, characterization and chemobiological quantification of α-glucosidase enzyme inhibitory and free radical scavenging constituents from Derris scandens Benth. J. Chromatogr. B 2007, 855, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Hematulin, A.; Ingkaninan, K.; Limpeanchob, N.; Sagan, D. Ethanolic extract from Derris scandens Benth mediates radiosensitzation via two distinct modes of cell death in human colon cancer HT-29 cells. Asian Pac. J. Cancer Prev. 2014, 15, 1871–1877. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Saha, S.; Sil, P.C. An insight into the prophylactic effects of the leguminosae family plants against oxidative stress-induced pathophysiological conditions. React. Oxyg. Species 2018, 6, 220–247. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Zhu, B. Genistein inhibits the proliferation of human multiple myeloma cells through suppression of nuclear factor-κB and upregulation of microRNA-29b. Mol. Med. Rep. 2016, 13, 1627–1632. [Google Scholar] [CrossRef]
- Galamba, N.; Paiva, A.; Barreiros, S.; Simoes, P. Solubility of polar and nonpolar aromatic molecules in subcritical water: The role of the dielectric constant. J. Chem. Theory Comput. 2019, 15, 6277–6293. [Google Scholar] [CrossRef]
- Marczyński, Z.; Zgoda, M.M.; Stańczak, A.; Nowak, S.; Jambor, J.; Skibska, B. Predicted real solubility (–log x2) and the level of hydrophilic-lipophilic balance (HLBRequ) of phytochemicals contained in extracts isolated from linden inflorescence (Tiliae flos) with solvents of diversified polarity (εM). Herba Pol. 2019, 65, 39–50. [Google Scholar] [CrossRef]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Padilla de la Rosa, J.D.; García-Cruz, U.; Lizardi-Jiménez, M.A.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; Pacheco, N. UPLC-PDA-ESI-MS based chemometric analysis for solvent polarity effect evaluation on phytochemical compounds and antioxidant activity in habanero pepper (Capsicum chinense Jacq) fruit extract. J. Food Sci. 2025, 90, e17630. [Google Scholar] [CrossRef]
- Sarkar, T.; Kundu, S.; Bhattacharjee, A. Dielectric Property, AC Conductivity, and Electric Modulus Studies of Pristine and Green-Synthesized ZnO Nanoparticles. Phys. Status Solidi (A) 2024, 221, 2300615. [Google Scholar] [CrossRef]
- Chamchomdao, P.; Tadtong, S.; Samee, W. HPLC analysis and solvent extraction of emodin from Ventilago denticulate Willd. Thai Pharm. Health Sci. J. 2020, 15, 210–215. [Google Scholar]
- Radyukova, V.; Boyko, N.; Zhilyakova, E.; Tsvetkova, Z.; Martceva, D.; Malyutina, A.; Kasakova, V.; Fadeeva, D.; Balloul, G. Study and Modeling the Influence of a Solvent on Extraction of Eugenol from Laurus nobilis L. Leaves. In Proceedings of the 1st International Symposium Innovations in Life Sciences (ISILS 2019), Belgorod, Russia, 10–11 October 2019; pp. 356–359. [Google Scholar]
- Jeon, H.J.; Lee, B.-S.; Park, C. Extraction of Chlorogenic Acid Using Single and Mixed Solvents. Molecules 2025, 30, 481. [Google Scholar] [CrossRef] [PubMed]
- Sumontri, S.; Eiamart, W.; Tadtong, S.; Samee, W. Ultra-high-performance liquid chromatography–tandem mass spectrometry analysis of Δ9-tetrahydrocannabinol and cannabidiol in commercial Suk-Saiyasna herbal remedy: Applying Hansen solubility parameters for sample extraction to ensure regulatory compliance. Pharmaceuticals 2024, 17, 1502. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; de Faria, D.C.; Ferraz, F.Z.; de Aquino Neto, F.R. Hansen solubility parameters applied to the extraction of phytochemicals. Plants 2023, 12, 3008. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fabiano-Tixier, A.S.; Ginies, C.; Chemat, F. Direct green extraction of volatile aroma compounds using vegetable oils as solvents: Theoretical and experimental solubility study. LWT-Food Sci. Technol. 2014, 59, 724–731. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Wang, W.; Yu, Q.; Wang, Q.; Miao, C.; Guo, Y.; Zhuang, X.; Yuan, Z. Screening solvents based on Hansen solubility parameter theory to depolymerize lignocellulosic biomass efficiently under low temperature. ACS Sustain. Chem. Eng. 2019, 7, 8678–8686. [Google Scholar] [CrossRef]
- Motsumi, P.; Qwebani-Ogunleye, T.; Ejidike, I.; Mtunzi, F.; Nate, Z. Teedia lucida root extracts by ultrasonication and maceration techniques: Phytochemical screening, antimicrobial and antioxidant activity. Rasayan J. Chem. 2020, 13, 423–433. [Google Scholar] [CrossRef]
- Tobgay, U.; Boonyanuphong, P.; Meunprasertdee, P. Comparison of hot water and methanol extraction combined with ultrasonic pretreatment on antioxidant properties of two pigmented rice cultivars. Food Res. 2020, 4, 547–556. [Google Scholar] [CrossRef]
- Pham, D.-C.; Nguyen, H.-C.; Nguyen, T.-H.L.; Ho, H.-L.; Trinh, T.-K.; Riyaphan, J.; Weng, C.-F. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Celastrus hindsii Leaves Using Response Surface Methodology and Evaluation of Their Antioxidant and Antitumor Activities. BioMed Res. Int. 2020, 2020, 3497107. [Google Scholar] [CrossRef]
- Mohamed, H.; Al-Hajhoj, M.; Al-Saikhan, M.; Alqahtani, N.; Zayed, M.; Moawad, M.; Alsenaien, W.; Mohamed, M.E. Green extraction of date palm fruits via ultrasonic-assisted approach: Optimizations and antioxidant enrichments. Processes 2022, 10, 1049. [Google Scholar] [CrossRef]
- Soib, H.H.; Ismail, H.F.; Husin, F.; Abu Bakar, M.H.; Yaakob, H.; Sarmidi, M.R. Bioassay-guided different extraction techniques of Carica papaya (Linn.) leaves on in vitro wound-healing activities. Molecules 2020, 25, 517. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Ding, G.; Quan, W. Artificial intelligence assisted ultrasonic extraction of total flavonoids from Rosa sterilis. Molecules 2021, 26, 3835. [Google Scholar] [CrossRef]
- Md Yusof, A.H.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E.; Zainudin, B.H. Optimization of an ultrasound-assisted extraction condition for flavonoid compounds from cocoa shells (Theobroma cacao) using response surface methodology. Molecules 2019, 24, 711. [Google Scholar] [CrossRef]
- Ozalp, L.; Danış, Ö.; Yuce-Dursun, B.; Demir, S.; Gündüz, C.; Ogan, A. Investigation of HMG-CoA reductase inhibitory and antioxidant effects of various hydroxycoumarin derivatives. Arch. Der Pharm. 2020, 353, 1900378. [Google Scholar] [CrossRef]
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of conformational, structural, magnetic, and electronic properties related to antioxidant activity: Revisiting flavan, anthocyanidin, flavanone, flavonol, isoflavone, flavone, and flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Y.; Qiao, M.; Yuan, W.; Li, X.; Wang, X.; Sheng, J.; Zi, C. Structure–antioxidant activity relationships of dendrocandin analogues determined using density functional theory. Struct. Chem. 2022, 33, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Middya, T.R.; Jana, A.D. A QSAR study of radical scavenging antioxidant activity of a series of flavonoids using DFT based quantum chemical descriptors–the importance of group frontier electron density. J. Mol. Model. 2012, 18, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-L.; Zhu, H.-W.; Ma, Y.; Xiong, L.-X.; Li, Y.-Q.; Zhao, Y.; Zhang, J.-F.; Chen, Y.-W.; Zhou, S.; Li, Z.-M. Synthesis, insecticidal activities, and SAR studies of novel pyridylpyrazole acid derivatives based on amide bridge modification of anthranilic diamide insecticides. J. Agric. Food Chem. 2013, 61, 5483–5493. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yi, C.; Zhang, R.; Yang, S.; Liu, T.; Jia, D.; Yang, Q.; Peng, S. Evaluation of antioxidant properties and molecular design of lubricant antioxidants based on QSPR Model. Lubricants 2023, 12, 3. [Google Scholar] [CrossRef]
- ABDELMADJID, A.; Haffar, D.; Benghanem, F.; Ghedjati, S.; Toukal, L.; Dorcet, V.; Bourzami, R. Synthesis, crystal structure, electrochemical, theoretical studies and antioxidant activities of new Schiff base. J. Mol. Struct. 2021, 1227, 129368. [Google Scholar] [CrossRef]
- Deviani, V.; Hardianto, A.; Farabi, K.; Herlina, T. Flavanones from Erythrina crista-galli twigs and their antioxidant properties determined through in silico and in vitro studies. Molecules 2022, 27, 6018. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.S.; Leal, D.H.; Kuca, K.; Ramalho, T.C. Perspectives on the role of the frontier effective-for-reaction molecular orbital (FERMO) in the study of chemical reactivity: An updated review. Curr. Org. Chem. 2020, 24, 314–331. [Google Scholar] [CrossRef]
- Morad, R.; Akbari, M.; Maaza, M. Theoretical study of chemical reactivity descriptors of some repurposed drugs for COVID-19. MRS Adv. 2023, 8, 656–660. [Google Scholar] [CrossRef]
- Gökalp, F. An investigation of the olive phenols activity as a natural medicine. J. Food Drug Anal. 2018, 26, 657–661. [Google Scholar] [CrossRef]
- Sae-Foo, W.; Singkham, S.; Srisongkhram, P.; Yusakul, G.; Masugarut, P.; Putalun, W. Development and characterisation of highly specific monoclonal antibody-based immunoassays for the detection and quantification of genistein-7-O-[α-rhamnopyranosyl-(1→6)]-β-glucopyranoside in Derris scandens (Roxb.) Benth. Phytochem. Anal. 2024, 35, 483–492. [Google Scholar] [CrossRef]
- Guideline, I. Validation of Analytical Procedures Q2 (R2); ICH: Geneva, Switzerland, 2022. [Google Scholar]
- Paez, V.; Barrett, W.B.; Deng, X.; Diaz-Amigo, C.; Fiedler, K.; Fuerer, C.; Hostetler, G.L.; Johnson, P.; Joseph, G.; Konings, E.J. AOAC SMPR® 2016.002. J. AOAC Int. 2016, 99, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Aybastier, Ö.; Demir, C. Optimization of Ultrasonic-Assisted Extraction of Quercetin and Cyanidin from P yracantha Coccinea and Their Scavenging Effect on Free Radicals. J. Food Biochem. 2016, 40, 472–479. [Google Scholar] [CrossRef]
- Sukhonthasilakun, S.; Mahakunakorn, P.; Naladta, A.; Nuankaew, K.; Nualkaew, S.; Yenjai, C.; Nualkaew, N. Anti-inflammatory effects of Derris scandens extract on narrowband-ultraviolet B exposed HaCaT human keratinocytes. J. Ayurveda Integr. Med. 2023, 14, 100693. [Google Scholar] [CrossRef]
- Ozaki, T.; Mikami, K.; Toyomaki, A.; Hashimoto, N.; Ito, Y.M.; Kusumi, I. Assessment of electroencephalography modification by antipsychotic drugs in patients with schizophrenia spectrum disorders using frontier orbital theory: A preliminary study. Neuropsychopharmacol. Rep. 2023, 43, 177–187. [Google Scholar] [CrossRef]
- Cai, W.; Chen, Y.; Xie, L.; Zhang, H.; Hou, C. Characterization and density functional theory study of the antioxidant activity of quercetin and its sugar-containing analogues. Eur. Food Res. Technol. 2014, 238, 121–128. [Google Scholar] [CrossRef]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Syed Mohamad, S.N.A.; Khatib, A.; Latip, J. Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. BioMed Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, E.; Panayiotou, C. Prediction of Hansen solubility parameters with a new group-contribution method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Knizia, G. IboView v.20211019-RevA.: A Program for Chemical Analysis. Available online: http://www.iboview.org/ (accessed on 20 March 2025).



| Parameters | % RSD of Retention Time | % RSD of Peak Area | Resolution | Number of Theoretical Plate | Tailing Factor |
|---|---|---|---|---|---|
| Acceptance criteria | ≤2% | ≤2% | >2 | 2500 | ±1.2 |
| Results | 0.15 | 1.57 | 3.12 | 25,423 | ±1.01 |
| Linearity | Linear Equation | R2 | LOD (µg/mL) | LOQ (µg/mL) | ||
|---|---|---|---|---|---|---|
| Y = 22.783X + 5.213 | 0.9999 | 0.39 | 1.18 | |||
| Y = 22.796X + 5.852 | 0.9999 | |||||
| Y = 22.697X + 0.906 | 0.9999 | |||||
| mean | Y = 22.759X + 3.990 | 1 | ||||
| Day | Concentration (µg/mL) | %Recovery | Mean recovery | SD | %RSD | |
| Actual | Found | |||||
| 1 | 40 | 39.87 | 99.67 | 98.90 | 0.7417 | 0.75 |
| 40 | 39.53 | 98.83 | ||||
| 40 | 39.28 | 98.20 | ||||
| 80 | 79.97 | 99.96 | 99.21 | 0.7175 | 0.72 | |
| 80 | 78.82 | 98.53 | ||||
| 80 | 79.31 | 99.14 | ||||
| 120 | 119.66 | 99.71 | 99.10 | 0.5719 | 0.58 | |
| 120 | 118.82 | 99.02 | ||||
| 120 | 118.30 | 98.58 | ||||
| 2 | 40 | 39.94 | 99.85 | 98.61 | 1.4872 | 1.51 |
| 40 | 39.61 | 99.02 | ||||
| 40 | 38.78 | 96.96 | ||||
| 80 | 79.68 | 99.59 | 99.68 | 0.6894 | 0.69 | |
| 80 | 79.22 | 99.03 | ||||
| 80 | 80.32 | 100.40 | ||||
| 120 | 118.31 | 98.59 | 98.46 | 0.6947 | 0.71 | |
| 120 | 118.90 | 99.09 | ||||
| 120 | 117.26 | 97.71 | ||||
| 3 | 40 | 39.18 | 97.95 | 98.32 | 1.8939 | 1.93 |
| 40 | 40.15 | 100.37 | ||||
| 40 | 38.65 | 96.63 | ||||
| 80 | 79.13 | 98.91 | 98.46 | 0.4691 | 0.48 | |
| 80 | 78.79 | 98.49 | ||||
| 80 | 78.38 | 97.98 | ||||
| 120 | 117.67 | 98.06 | 98.15 | 0.8601 | 0.88 | |
| 120 | 118.86 | 99.05 | ||||
| 120 | 116.80 | 97.34 | ||||
| Inter-Day (n = 9) | Concentration | Mean recovery | SD | %RSD | ||
| 40 | 98.61 | 1.2847 | 1.30 | |||
| 80 | 99.11 | 0.7654 | 0.77 | |||
| 120 | 98.57 | 0.7522 | 0.76 | |||
| Group | Ni | Ci | (NiCi) d | (NiCi) p | (NiCi) h | ||
|---|---|---|---|---|---|---|---|
| 1st-Order | δD | δP | δH | ||||
| CH3 | 1 | −0.9714 | −1.6448 | −0.7813 | −0.9714 | −1.6448 | −0.7813 |
| CH2 | 1 | −0.0269 | −0.3045 | −0.4119 | −0.0269 | −0.3045 | −0.4119 |
| CH | 4 | 0.6450 | 0.6491 | −0.2018 | 2.5800 | 2.5964 | −0.8072 |
| >CHOH | 6 | 0.1123 | 0.2564 | −0.1928 | 0.6738 | 1.5384 | −1.1568 |
| ACOH | 2 | 0.5288 | 1.1010 | 6.9580 | 1.0576 | 2.2020 | 13.9160 |
| AC | 2 | 0.8446 | 0.6187 | 0.0084 | 1.6892 | 1.2374 | 0.0168 |
| ACH | 6 | 0.1105 | −0.5303 | −0.4305 | 0.6630 | −3.1818 | −2.5830 |
| −CH=C< | 1 | 0.5372 | −0.9024 | −1.8872 | 0.5372 | −0.9024 | −1.8872 |
| >C=C< | 1 | 0.3592 | 1.0526 | −15.4659 | 0.3592 | 1.0526 | −15.4659 |
| >C=O | 1 | −0.4343 | 0.7905 | 1.8147 | −0.4343 | 0.7905 | 1.8147 |
| OH | 4 | −0.3462 | 1.1404 | 7.1908 | −1.3848 | 4.5616 | 28.7632 |
| O | 5 | 0.0472 | 3.3432 | 0.9256 | 0.2360 | 16.7160 | 4.6280 |
| ΣNiCi | 4.9786 | 24.6614 | 26.0454 | ||||
| Group | Mj | Dj | (MiDi) d | (MiDi) p | (MiDi) h | ||
| 2nd-Order | δD | δP | δH | ||||
| AC-O-C | 2 | 0.2568 | 0.8153 | 0.6092 | 0.5136 | 1.6306 | 1.2184 |
| ring of 6 carbon | 2 | −0.3874 | −3.6432 | 0.0000 | −0.7748 | −7.2864 | 0.0000 |
| ring of 5 carbon | 3 | −0.6681 | −2.3430 | −0.3079 | −2.0043 | −7.0290 | −0.9237 |
| cyclic-OH | 2 | −0.0876 | −3.5220 | 0.5914 | −0.1752 | −7.0440 | 1.1828 |
| ΣMiDj | −2.4407 | −19.7288 | 1.4775 | ||||
| ΣNiCi | 4.9786 | 24.6614 | 26.0454 | ||||
| ΣMiDj | −2.4407 | −19.7288 | 1.4775 | ||||
| Constant (C) | 17.3231 | 7.3548 | 7.9793 | ||||
| ΣNiCi + ΣMiDj + C | 19.8610 | 12.2874 | 35.5022 | ||||
| Name | Hansen Solubility Parameters | Ra | ||
|---|---|---|---|---|
| Solute | δD | δP | δH | |
| GTG | 19.8610 | 12.2874 | 35.5022 | |
| Solvent | ||||
| 100% Ethanol | 15.8 | 8.8 | 19.4 | 20.07 |
| 80% Ethanol | 15.76 | 10.24 | 23.98 | 17.46 |
| 60% Ethanol | 15.72 | 11.68 | 28.56 | 15.91 |
| 50% Ethanol | 15.74 | 12.40 | 30.85 | 15.60 |
| 40% Ethanol | 15.68 | 13.12 | 33.14 | 15.74 |
| 20% Ethanol | 15.64 | 14.56 | 37.72 | 16.98 |
| 0% Ethanol | 15.5 | 16 | 42.3 | 19.45 |
| Solvents | Ra | Dielectric Constant (ε) | GTG Contents (mg/g Dried Weight) * |
|---|---|---|---|
| 100% Ethanol | 20.07 | 24.30 | 1.47 ± 0.02 |
| 80% Ethanol | 17.46 | 37.70 | 5.17 ± 0.09 |
| 60% Ethanol | 15.91 | 49.81 | 6.44 ± 0.09 |
| 50% Ethanol | 15.60 | 55.42 | 6.83 ± 0.06 |
| 40% Ethanol | 15.74 | 60.80 | 6.61 ± 0.08 |
| 20% Ethanol | 16.98 | 70.82 | 5.26 ± 0.12 |
| 0% Ethanol | 19.45 | 80.00 | 2.17 ± 0.11 |
| Sample | TPC * (mgGAE/g Dried Weight) | TFC * (mgQE/g Dried Weight) | DPPH * IC50 (mg/mL) | FRAP * (µg/mL FeSO4/g Dried Weight) |
|---|---|---|---|---|
| 100% Ethanol extract | 22.44 ± 2.73 | 44.25 ± 1.08 | 6.97 ± 0.08 | 253.04 ± 3.47 |
| 80% Ethanol extract | 28.08 ± 5.57 | 32.81 ± 0.20 | 2.30 ± 0.02 | 422.16 ± 34.05 |
| 60% Ethanol extract | 30.39 ± 0.50 | 25.63 ± 2.11 | 1.41 ± 0.01 | 495.49 ± 16.72 |
| 50% Ethanol extract | 31.53 ± 0.61 | 21.21 ± 1.24 | 1.43 ± 0.03 | 513.97 ± 26.29 |
| 40% Ethanol extract | 26.58 ± 3.78 | 14.58 ± 0.55 | 1.58 ± 0.03 | 550.32 ± 30.71 |
| 20% Ethanol extract | 17.46 ± 1.65 | 12.42 ± 0.40 | 1.88 ± 0.01 | 460.56 ± 29.50 |
| 0% Ethanol extract | 17.99 ± 2.96 | 22.33 ± 1.77 | 1.08 ± 0.01 | 762.46 ± 47.95 |
| Compounds | M.W. | HOMO (eV) | LUMO (eV) | ΔE (eV) | DPPH (IC50) | FRAP FeSO4/mM (µg/mL) | |
|---|---|---|---|---|---|---|---|
| µg/mL | µM | ||||||
| Gallic acid | 170 | −8.3543 | 2.2706 | 10.6249 | 10.54 ± 0.35 | 62.00 ± 2.06 | 774.19 ± 10.49 |
| Genistein | 270 | −7.7018 | 2.8563 | 10.5581 | 15.62 ± 0.54 | 57.85 ± 2.00 | 608.09 ± 17.70 |
| GTG | 578 | −7.9859 | 2.6856 | 10.6715 | 37.32 ± 1.85 | 64.57 ± 3.20 | 623.32 ± 12.68 |
| Quercetin | 302 | −7.8091 | 2.0910 | 9.9001 | 1.81 ± 0.12 | 5.99 ± 0.40 | 1399.88 ± 16.96 |
| Trolox | 250 | −7.5032 | 3.6961 | 11.1993 | 16.51 ± 0.22 | 66.04 ± 0.22 | 404.07 ± 8.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tantinithiphong, T.; Eiamart, W.; Tadtong, S.; Vorarat, S.; Samee, W. Application of Hansen Solubility Parameters in the Aqueous-Ethanol Extraction of Genistein-7-O-[α-rhamnopyranosyl-(1→6)]-β-glucopyranoside from Derris scandens and Its Molecular Orbital Study on Antioxidant Activity. Int. J. Mol. Sci. 2025, 26, 5740. https://doi.org/10.3390/ijms26125740
Tantinithiphong T, Eiamart W, Tadtong S, Vorarat S, Samee W. Application of Hansen Solubility Parameters in the Aqueous-Ethanol Extraction of Genistein-7-O-[α-rhamnopyranosyl-(1→6)]-β-glucopyranoside from Derris scandens and Its Molecular Orbital Study on Antioxidant Activity. International Journal of Molecular Sciences. 2025; 26(12):5740. https://doi.org/10.3390/ijms26125740
Chicago/Turabian StyleTantinithiphong, Thitiporn, Wanna Eiamart, Sarin Tadtong, Suwanna Vorarat, and Weerasak Samee. 2025. "Application of Hansen Solubility Parameters in the Aqueous-Ethanol Extraction of Genistein-7-O-[α-rhamnopyranosyl-(1→6)]-β-glucopyranoside from Derris scandens and Its Molecular Orbital Study on Antioxidant Activity" International Journal of Molecular Sciences 26, no. 12: 5740. https://doi.org/10.3390/ijms26125740
APA StyleTantinithiphong, T., Eiamart, W., Tadtong, S., Vorarat, S., & Samee, W. (2025). Application of Hansen Solubility Parameters in the Aqueous-Ethanol Extraction of Genistein-7-O-[α-rhamnopyranosyl-(1→6)]-β-glucopyranoside from Derris scandens and Its Molecular Orbital Study on Antioxidant Activity. International Journal of Molecular Sciences, 26(12), 5740. https://doi.org/10.3390/ijms26125740







