Development of Cytisus Flower Extracts with Antioxidant and Anti-Inflammatory Properties for Nutraceutical and Food Uses
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Cytisus Striatus Flowers
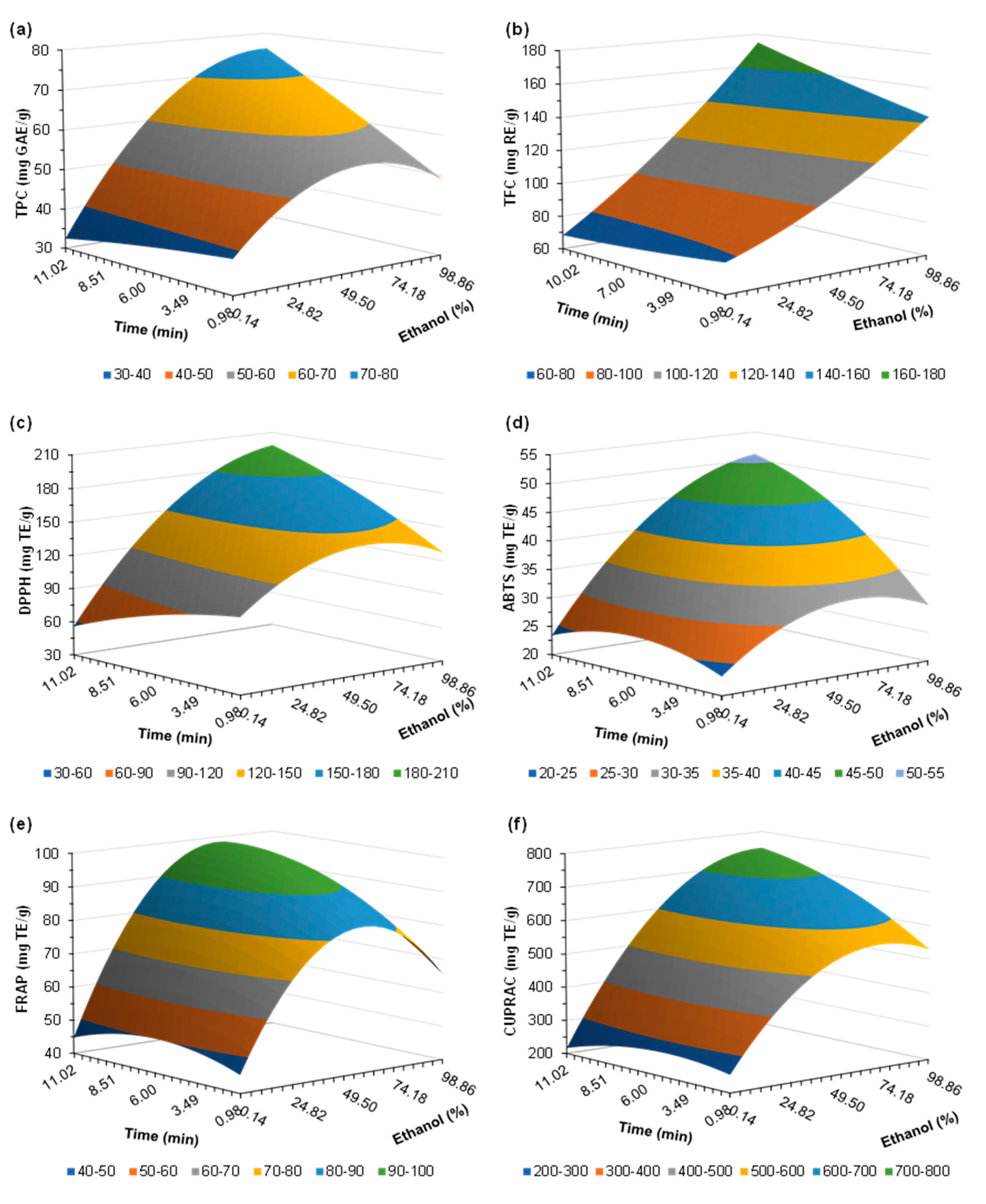
2.2. Extraction Conditions Optimization
2.2.1. Total Phenolic Content (TPC)
2.2.2. Total Flavonoid Content (TFC)
2.2.3. Antioxidant Activity of Extracts
2.2.4. Optimized Conditions
2.3. Phenolic Compounds of Cytisus Flower Extract
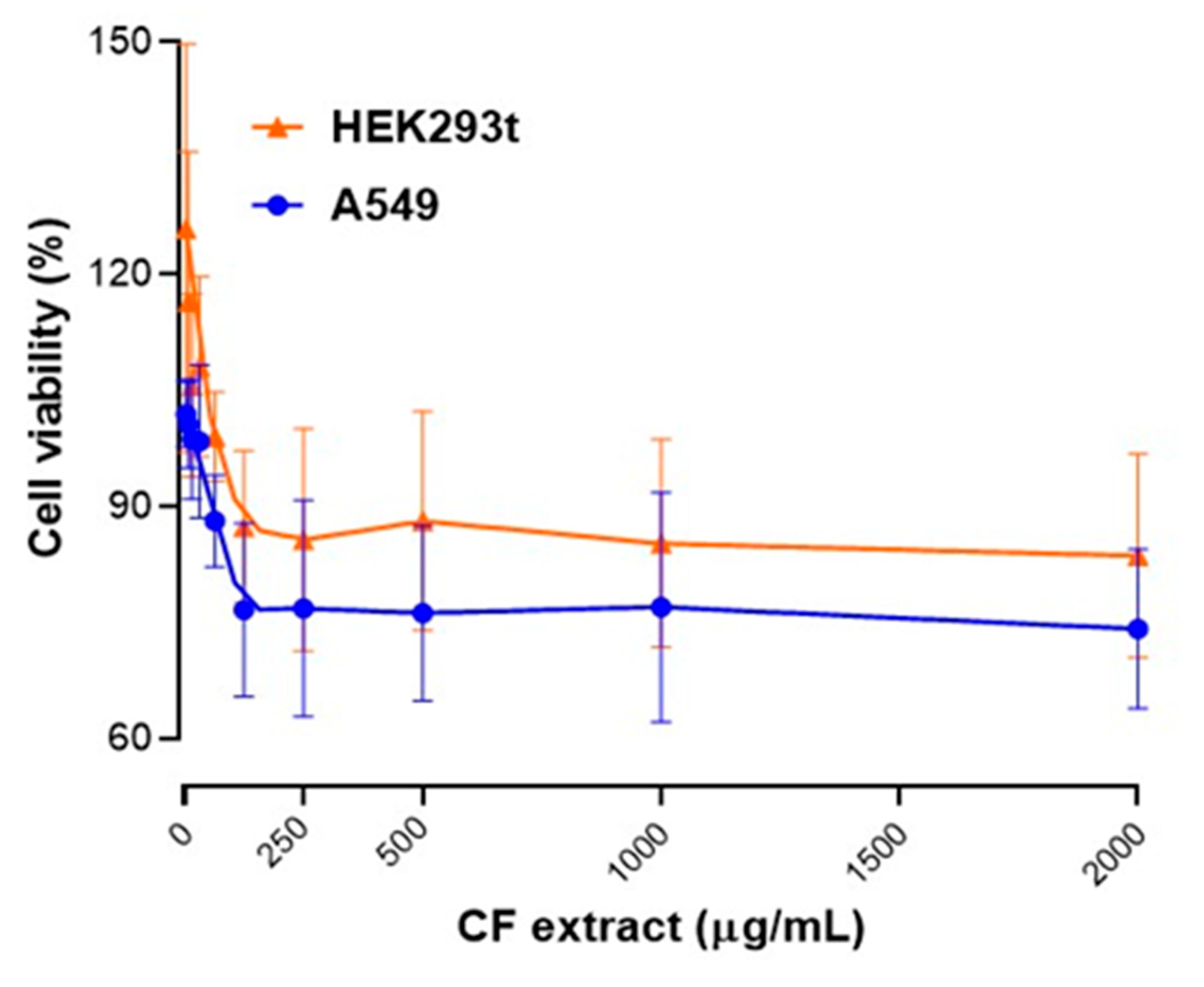
2.4. Cytotoxicity of Cytisus Flower Extract
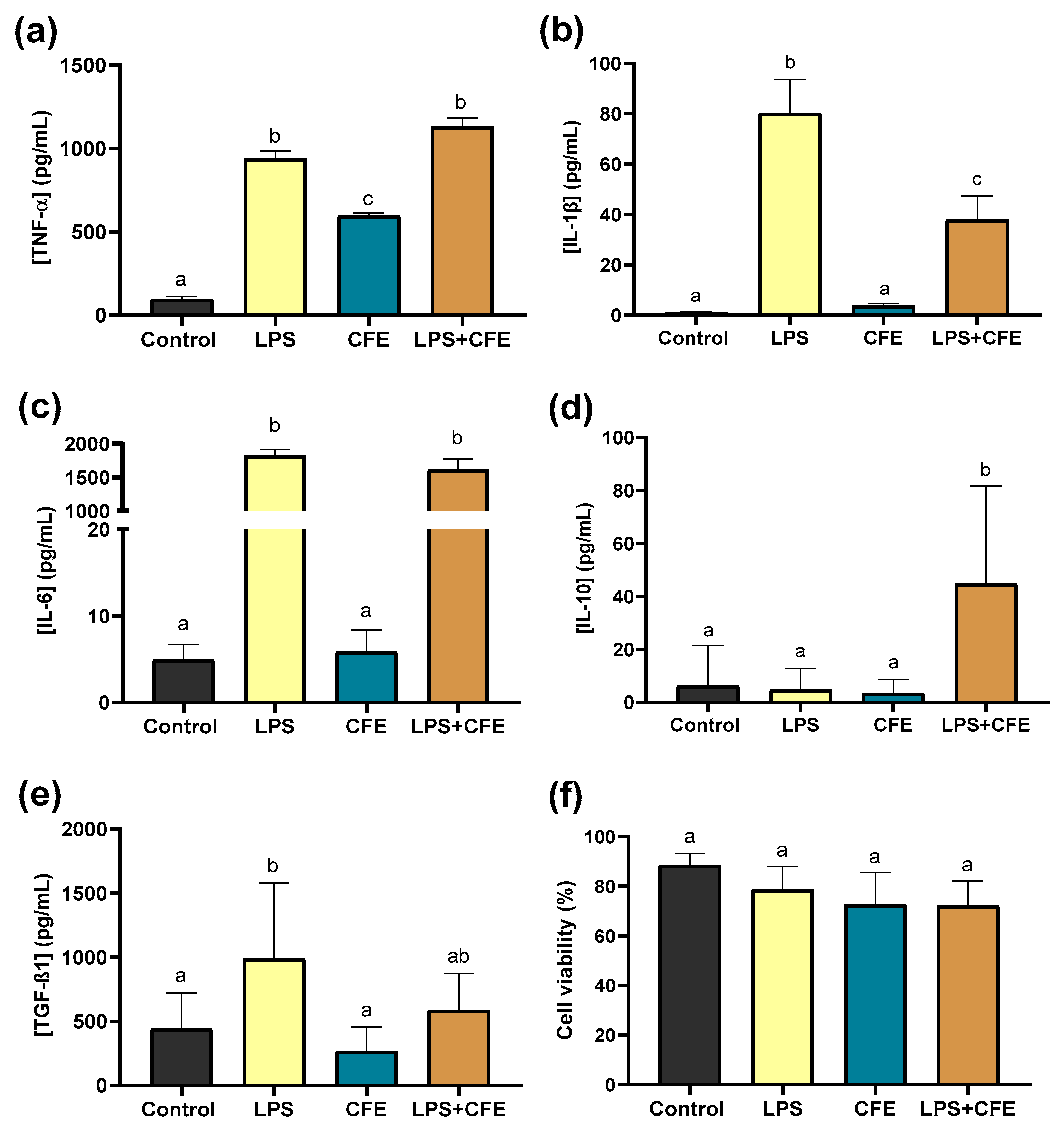
2.5. Anti-Inflammatory Response of Cytisus Flower Extract
3. Materials and Methods
3.1. Chemicals
3.2. Chemical Characterization of Plant Material
3.3. Optimization of Extraction Conditions and Extract Preparation
3.4. HPLC-TOF-MS Analyses of the Extracts
3.5. Antioxidant Activity
3.6. Cell Viability
3.7. Anti-Inflammatory Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cytisus striatus flowers |
| MAE | Microwave-assisted extraction |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP | Ferric reducing antioxidant power |
| CUPRAC | Cupric ion reducing antioxidant capacity |
| GAE | Gallic acid equivalents |
| RE | Rutin equivalents |
| TE | Trolox equivalents |
| HPLC-TOF-MS | High-performance liquid chromatography–time of flight–mass spectrometry |
| RSM | Response surface methodology |
| DOE | Design of experiments |
| HEK293T | Human embryonic kidney 293T cells |
| A549 | Human lung adenocarcinoma cell line |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | Dimethyl sulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| TNF-α | Tumor necrosis factor alpha |
| IL-1β | Interleukin-1 beta |
| IL-6/IL-10 | Interleukins 6 and 10 |
| TGF-β1 | Transforming growth factor beta 1 |
| SD | Standard deviation |
| AIR | Acid-insoluble residue |
| LOQ | Limit of quantification |
| ICP-OES | Inductively coupled plasma—optical emission spectrometry |
References
- Population|United Nations. Available online: https://www.un.org/en/global-issues/population (accessed on 19 January 2025).
- Puig, M.; Azarkamand, S.; Wooldridge, C.; Selén, V.; Darbra, R.M. Insights on the Environmental Management System of the European Port Sector. Sci. Total Environ. 2022, 806, 150550. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Salgado, I.; Pérez-Rodríguez, P.; Campillo-Cora, C.; Gómez-Armesto, A.; Arias-Estévez, M.; Díaz-Raviña, M.; Nóvoa-Muñoz, J.C.; Fernández-Calviño, D. Nitrogen Mineralization Dynamics in Acid Vineyard Soils Amended with Bentonite Winery Waste. Arch. Agron. Soil Sci. 2018, 64, 805–818. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Miranda, S.M.; Belo, I.; Spigno, G.; Teixeira, J.A.; Rocha, C.M.R. Sequential Multi-Stage Extraction of Biocompounds from Spirulina Platensis: Combined Effect of Ohmic Heating and Enzymatic Treatment. Innov. Food Sci. Emerg. Technol. 2021, 71, 102707. [Google Scholar] [CrossRef]
- Sánchez, M.; Ferreira-Santos, P.; Gomes-Dias, J.S.; Laca, A.; Rocha, C.M.R. Cascading Recovery of Added-Value Cocoa Bean Shell Fractions Through Autohydrolysis Treatments. Food Bioprocess Technol. 2025, 18, 965–978. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nogueira, A.; Rocha, C.M.R.; Wilson, C.P.; Teixeira, J.A.; Botelho, C. Sambucus Nigra Flower and Berry Extracts for Food and Therapeutic Applications: Effect of Gastrointestinal Digestion on in Vitro and in vivo Bioactivity and Toxicity. Food Funct. 2022, 13, 6762–6776. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rivera, L.; Santos, P.F.; Miralles, B.; Carrón, R.; José Montero, M.; Recio, I. Peptide Fragments from β-Casein f(134–138), HLPLP, Generated by the Action of Rat Blood Plasma Peptidases Show Potent Antihypertensive Activity. Food Res. Int. 2016, 88, 348–353. [Google Scholar] [CrossRef]
- Botelho, C.M.; Ferreira-Santos, P.; Toubarro, D.; Dinis, H.; Osório, H.; Costa-Barbosa, A.; Sampaio, P.; Simões, N.; Teixeira, J.A. Chicken Feather Keratin Peptides for the Control of Keratinocyte Migration. Appl. Sci. 2021, 11, 6779. [Google Scholar] [CrossRef]
- Yusoff, M.A.; Mohammadi, P.; Ahmad, F.; Sanusi, N.A.; Hosseinzadeh-Bandbafha, H.; Vatanparast, H.; Aghbashlo, M.; Tabatabaei, M. Valorization of Seafood Waste: A Review of Life Cycle Assessment Studies in Biorefinery Applications. Sci. Total Environ. 2024, 952, 175810. [Google Scholar] [CrossRef] [PubMed]
- Lores, M.; Pájaro, M.; Álvarez-Casas, M.; Domínguez, J.; García-Jares, C. Use of Ethyl Lactate to Extract Bioactive Compounds from Cytisus Scoparius: Comparison of Pressurized Liquid Extraction and Medium Scale Ambient Temperature Systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bouziane, A.; Bakchiche, B.; Dias, M.; Barros, L.; Ferreira, I.; AlSalamat, H.; Bardaweel, S. Phenolic Compounds and Bioactivity of Cytisus villosus Pourr. Molecules 2018, 23, 1994. [Google Scholar] [CrossRef] [PubMed]
- Daghbouche, S.; Ammar, I.; Rekik, D.M.; Djazouli, Z.-E.; Zebib, B.; Merah, O. Effect of Phenological Stages on Essential Oil Composition of Cytisus Triflorus L’Her. J. King Saud Univ.—Sci. 2020, 32, 2383–2387. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Influence of the Drying Method in the Antioxidant Potential and Chemical Composition of Four Shrubby Flowering Plants from the Tribe Genisteae (Fabaceae). Food Chem. Toxicol. 2011, 49, 2983–2989. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Ribeiro, D.; Fernandes, E.; Nogueira, D.R.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential Use of Cytisus Scoparius Extracts in Topical Applications for Skin Protection against Oxidative Damage. J. Photochem. Photobiol. B Biol. 2013, 125, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Sousa, D.; Genisheva, Z.; Rodríguez-Yoldi, M.J.; Gullón, B.; Costa, C.E.; Teixeira, J.A.; Botelho, C.M.; Ferreira-Santos, P. Exploration of Polyphenols Extracted from Cytisus Plants and Their Potential Applications: A Review. Antioxidants 2024, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cruz, I.; Contreras, M.d.M.; Romero, I.; Castro, E. Optimization of Microwave-Assisted Water Extraction to Obtain High Value-Added Compounds from Exhausted Olive Pomace in a Biorefinery Context. Foods 2022, 11, 2002. [Google Scholar] [CrossRef] [PubMed]
- Del-Castillo-Llamosas, A.; Eibes, G.; Ferreira-Santos, P.; Pérez-Pérez, A.; Del-Río, P.G.; Gullón, B. Microwave-Assisted Autohydrolysis of Avocado Seed for the Recovery of Antioxidant Phenolics and Glucose. Bioresour. Technol. 2023, 385, 129432. [Google Scholar] [CrossRef] [PubMed]
- Jaouhari, Y.; Bordiga, M.; Travaglia, F.; Coisson, J.-D.; Costa-Barbosa, A.; Sampaio, P.; Botelho, C.; Gullón, B.; Ferreira-Santos, P. Microwave-Assisted Extraction of Raspberry Pomace Phenolic Compounds, and Their Bioaccessibility and Bioactivity. Food Chem. 2025, 478, 143641. [Google Scholar] [CrossRef] [PubMed]
- Rubira, A.; Rodríguez-Rebelo, F.; Del-Río, P.G.; Romaní, A.; Gullón, B. Valorization of Hop (Humulus lupulus L.) Pruning to Produce Valuable Compounds Using Two Biorefinery Strategies: Conventional Processing and Microwave-Assisted Autohydrolysis. Ind. Crops Prod. 2024, 219, 119174. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Gullón, B.; Lobato-Rodríguez, Á.; Garrote, G.; del Río, P.G. Microwave-Assisted Extraction of Hemicellulosic Oligosaccharides and Phenolics from Robinia Pseudoacacia Wood. Carbohydr. Polym. 2023, 301, 120364. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.V.; Majahar Ali, M.K.; Wan Ishak, W.R. Optimization of Aqueous Extraction Conditions for Bioactive Compounds and Antioxidant Properties of Overripe Banana (Musa Acuminata) Using Response Surface Methodology. J. Agric. Food Res. 2025, 20, 101775. [Google Scholar] [CrossRef]
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. Dietary Supplement Fact Sheets. Available online: https://ods.od.nih.gov/factsheets/list-all/ (accessed on 3 March 2025).
- Caramelo, D.; Pitacas, I.; Vitória, C.; Gonçalves, J.; Gominho, J.; Gallardo, E.; Anjos, O. Chemical Composition and Nutritional Value of Flowers and Fruits of Cytisus Striatus (Hill) Rothm. Plants 2024, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Genisheva, Z.; Botelho, C.; Santos, J.; Ramos, C.; Teixeira, J.A.; Rocha, C.M.R. Unravelling the Biological Potential of Pinus Pinaster Bark Extracts. Antioxidants 2020, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Gullón, P.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Optimization of Solvent Extraction of Antioxidants from Eucalyptus Globulus Leaves by Response Surface Methodology: Characterization and Assessment of Their Bioactive Properties. Ind. Crops Prod. 2017, 108, 649–659. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Ethanolic Extraction of Flavonoids, Phenolics and Antioxidants from Vernonia Amygdalina Leaf Using Two-Level Factorial Design. J. King Saud Univ.—Sci. 2020, 32, 7–16. [Google Scholar] [CrossRef]
- Lohvina, H.; Sándor, M.; Wink, M. Effect of Ethanol Solvents on Total Phenolic Content and Antioxidant Properties of Seed Extracts of Fenugreek (Trigonella foenum-graecum L.) Varieties and Determination of Phenolic Composition by HPLC-ESI-MS. Diversity 2022, 14, 7. [Google Scholar] [CrossRef]
- Yusof, N.; Abdul Munaim, M.S.; Kutty, R.V. The Effects of Different Ethanol Concentration on Total Phenolic and Total Flavonoid Content In Malaysian Propolis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012033. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive Compounds, RP-HPLC Analysis of Phenolics, and Antioxidant Activity of Some Portuguese Shrub Species Extracts. Nat. Prod. Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Touami, Y.; Marir, R.; Merouane, F. Improvement of Ultrasound-Assisted Extraction of Polyphenolic Content of the Plant Cytisus Triflorus L’Her as a Natural Resource Using Artificial Neural Network Modeling and Multi-Objective Optimization. Sustain. Chem. Pharm. 2023, 32, 101032. [Google Scholar] [CrossRef]
- Del-Castillo-Llamosas, A.; Rodríguez-Rebelo, F.; Rodríguez-Martínez, B.; Mallo-Fraga, A.; Del-Río, P.G.; Gullón, B. Valorization of Avocado Seed Wastes for Antioxidant Phenolics and Carbohydrates Recovery Using Deep Eutectic Solvents (DES). Antioxidants 2023, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Gullón, B.; Teixeira, J.A.; Botelho, C.M.; Yáñez, R. Exploiting the Potential of Bioactive Molecules Extracted by Ultrasounds from Avocado Peels—Food and Nutraceutical Applications. Antioxidants 2021, 10, 1475. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.Q.; Nguyen, M.T.; Nguyen, V.T.; Le, V.M.; Trieu, L.H.; Le, X.T.; Khang, T.V.; Giang, N.T.L.; Thach, N.Q.; Hung, T.T. The Effects of Different Extraction Conditions on the Polyphenol, Flavonoids Components and Antioxidant Activity of Polyscias Fruticosa Roots. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022067. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Pintado, M.E.; Aguilar, C.N. Process Optimization of Microwave-Assisted Extraction of Bioactive Molecules from Avocado Seeds. Ind. Crops Prod. 2020, 154, 112623. [Google Scholar] [CrossRef]
- Ismail-Suhaimy, N.W.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E.; Bawon, P. Optimizing Conditions for Microwave-Assisted Extraction of Polyphenolic Content and Antioxidant Activity of Barleria lupulina Lindl. Plants 2021, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, M.D.; Buntić, A.V.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Ethanol Influenced Fast Microwave-Assisted Extraction for Natural Antioxidants Obtaining from Spent Filter Coffee. Sep. Purif. Technol. 2013, 118, 503–510. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, Y.; Liu, P. Optimization of Microwave-Assisted Extraction, Antioxidant Capacity, and Characterization of Total Flavonoids from the Leaves of Alpinia Oxyphylla Miq. Prep. Biochem. Biotechnol. 2020, 50, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Ma, Q.; Zhang, Y.; Peng, Z. Phenolic Compounds with Antioxidant Activity from Strawberry Leaves: A Study on Microwave-Assisted Extraction Optimization. Prep. Biochem. Biotechnol. 2020, 50, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Kayahan, S.; Saloglu, D. Optimization and Kinetic Modelling of Microwave-Assisted Extraction of Phenolic Contents and Antioxidants from Turkish Artichoke. CyTA—J. Food 2020, 18, 635–643. [Google Scholar] [CrossRef]
- Sundararajan, R.; Haja, N.A.; Venkatesan, K.; Mukherjee, K.; Saha, B.P.; Bandyopadhyay, A.; Mukherjee, P.K. Cytisus Scoparius Link—A Natural Antioxidant. BMC Complement. Altern. Med. 2006, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Muras, M.; Puig, C.G.; Souto, X.C.; Pedrol, N. Water-Soluble Phenolic Acids and Flavonoids Involved in the Bioherbicidal Potential of Ulex Europaeus and Cytisus Scoparius. S. Afr. J. Bot. 2020, 133, 201–211. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Lai, H.; Liang, G.; Huang, J.; Zhao, F.; Xie, X.; Peng, C. Erianin: A Phytoestrogen with Therapeutic Potential. Front. Pharmacol. 2023, 14, 1197056. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, X.; Li, Y.; Kong, Y.; Lan, J.; Huang, Y.; Liu, Y. Chemical Profiling and Rapid Discrimination of Blumea Riparia and Blumea Megacephala by UPLC-Q-Exactive-MS/MS and HPLC. Chin. Herb. Med. 2023, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.-J. Pharmacological and Therapeutic Applications of Esculetin. Int. J. Mol. Sci. 2022, 23, 12643. [Google Scholar] [CrossRef] [PubMed]
- Karağaç, M.S.; Yeşilkent, E.N.; Kizir, D.; Öztürk, N.; Isıyel, M.; Karadaş, H.; Tosun, H.; Karaman, M.; Ceylan, H.; Demir, Y. Esculetin Improves Inflammation of the Kidney via Gene Expression against Doxorubicin-Induced Nephrotoxicity in Rats: In Vivo and in Silico Studies. Food Biosci. 2024, 62, 105159. [Google Scholar] [CrossRef]
- Del Fabbro, L.; Bortolotto, V.C.; Ferreira, L.M.; Sari, M.H.M.; Furian, A.F. Chrysin’s Anti-Inflammatory Action in the Central Nervous System: A Scoping Review and an Evidence-Gap Mapping of Its Mechanisms. Eur. J. Pharmacol. 2025, 997, 177602. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Espinosa, J.; Saldaña-Ríos, J.; García-Jiménez, S.; Villalobos-Molina, R.; Ávila-Villarreal, G.; Rodríguez-Ocampo, A.; Bernal-Fernández, G.; Estrada-Soto, S. Chrysin Induces Antidiabetic, Antidyslipidemic and Anti-Inflammatory Effects in Athymic Nude Diabetic Mice. Molecules 2017, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Natesan, V. Chrysin: Sources, Beneficial Pharmacological Activities, and Molecular Mechanism of Action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Chrysin, an Anti-Inflammatory Molecule, Abrogates Renal Dysfunction in Type 2 Diabetic Rats. Toxicol. Appl. Pharmacol. 2014, 279, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-J.; Kang, H.-J.; Jung, H.-J.; Kim, K.; Lim, C.-J.; Park, E.-H. Anti-Inflammatory, Anti-Angiogenic and Anti-Nociceptive Activities of 4-Hydroxybenzaldehyde. Biomol. Ther. 2008, 16, 231–236. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Santos-Buelga, C. Characterization of Phenolic Compounds in Flowers of Wild Medicinal Plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.R.; Macias, R.I.R.; Perez, M.J.; Marin, J.J.G.; Cardoso, S.M. Protective Effects of Phenolic Constituents from Cytisus Multiflorus, Lamium album L. and Thymus Citriodorus on Liver Cells. J. Funct. Foods 2013, 5, 1170–1179. [Google Scholar] [CrossRef]
- Larit, F.; Nael, M.A.; Benyahia, S.; Radwan, M.M.; León, F.; Jasicka-Misiak, I.; Poliwoda, A.; Wieczorek, D.; Benayache, F.; Benayache, S.; et al. Secondary Metabolites from the Aerial Parts of Cytisus villosus Pourr. Phytochem. Lett. 2018, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, F.; Wang, Y.; Li, B.; Prabhakaran, P.; Zhou, W.; Han, Y.; Sun-Waterhouse, D.; Li, D.; Li, F. Sesamin Alleviates Allergen-Induced Diarrhea by Restoring Gut Microbiota Composition and Intestinal Barrier Function. J. Agric. Food Chem. 2025, 73, 1965–1981. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Xu, L.; Tan, X.; Li, D.; Sun-Waterhouse, D.; Li, F. Sesamin Is an Effective Spleen Tyrosine Kinase Inhibitor against IgE-Mediated Food Allergy in Computational, Cell-Based and Animal Studies. Food Sci. Hum. Wellness 2025, 14, 9250081. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-Shaped Dose Responses and Their Centrality in Toxicology. Trends Pharmacol. Sci. 2001, 22, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural Compounds for Cancer Treatment and Prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic Compounds: Natural Alternative in Inflammation Treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Babla, M.; Katwal, U.; Yong, M.-T.; Jahandari, S.; Rahme, M.; Chen, Z.-H.; Tao, Z. Value-Added Products as Soil Conditioners for Sustainable Agriculture. Resour. Conserv. Recycl. 2022, 178, 106079. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham-Ul-Haq; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and Therapeutic Properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.A.; Akhtar, T.; Naseem, N.; Aftab, U.; Zafar, M.S.; Hussain, S.; Shahzad, M.; Gobe, G.C. Chrysin Is Immunomodulatory and Anti-Inflammatory against Complete Freund’s Adjuvant-Induced Arthritis in a Pre-Clinical Rodent Model. Pharmaceutics 2023, 15, 1225. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.H.; Babu, R.L.; Naveen Kumar, M.; Kiran Kumar, K.M.; Hegde, S.M.; Nagesh, R.; Ramesh, G.T.; Sharma, S.C. Anti-Inflammatory Effect of Apigenin on LPS-Induced Pro-Inflammatory Mediators and AP-1 Factors in Human Lung Epithelial Cells. Inflammation 2016, 39, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Moderate Electric Fields as a Potential Tool for Sustainable Recovery of Phenolic Compounds from Pinus Pinaster Bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent Effects on the Antioxidant Capacity of Lipophilic and Hydrophilic Antioxidants Measured by CUPRAC, ABTS/Persulphate and FRAP Methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of Phenolic Compounds from Grape Pomace Using Ohmic Heating: Chemical Composition, Bioactivity and Bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef] [PubMed]
- American Type Culture Collection. Components. In MTT Cell Proliferation Assay Instruction Guide; ATCC 30-1010K; American Type Culture Collection: Manassas, VA, USA, 2011. [Google Scholar]

| Component | Mean | SD |
|---|---|---|
| Moisture | 6.9 | 0.14 |
| Ash | 3.94 | 0.11 |
| AIR | 6.22 | 0.70 |
| Glucan | 13.92 | 1.69 |
| Xylan | 4.69 | 1.04 |
| Arabinan | 3.52 | 0.44 |
| Acetic acid | 1.01 | 0.11 |
| Uronic acid | 4.45 | 0.93 |
| Aqueous extractives | 38.01 | 0.1 |
| Ethanolic extractives | 26.84 | 0.02 |
| Protein | 22.35 | 0.45 |
| Fat | 1.67 | 0.01 |
| Minerals | LOQ | Mean | SD |
|---|---|---|---|
| K | 12.50 | 15,136.6 | 202.4 |
| P | 2.50 | 3021.7 | 2.2 |
| Ca | 2.50 | 2888.9 | 95.8 |
| Mg | 5.00 | 1719.0 | 28.1 |
| Na | 12.50 | 844.6 | 10.1 |
| Mn | 0.25 | 477.6 | 4.9 |
| Fe | 1.00 | 64.9 | 0.9 |
| Zn | 1.00 | 35.1 | 0.2 |
| Cu | 1.00 | 7.7 | 0.2 |
| R | X1-EtOH (%) | X2-T (°C) | X3-t (min) | Y1-TPC (mgGAE/g CF) | Y2-TFC (mg RE/g CF) | Y3-DPPH (mg TE/g CF) | Y4-ABTS (mg TE/g CF) | Y5-FRAP (mg TE/g CF) | Y6-CUPRAC (mg TE/g CF) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 (20) | −1 (110) | −1 (3) | 49.27 | 78.68 | 123.28 | 29.24 | 57.06 | 437.78 |
| 2 | 1 (79) | 1 (150) | −1 (3) | 57.37 | 128.72 | 145.71 | 37.64 | 83.41 | 587.17 |
| 3 | 0 (49.5) | 0 (130) | 0 (6) | 51.61 | 97.03 | 143.70 | 33.17 | 82.02 | 552.92 |
| 4 | 1 (79) | −1 (110) | 1 (9) | 40.37 | 103.33 | 138.91 | 28.43 | 54.16 | 429.40 |
| 5 | −1 (20) | 1 (150) | 1 (9) | 45.04 | 87.01 | 104.38 | 31.82 | 71.48 | 413.73 |
| 6 | 1 (79) | −1 (110) | −1 (3) | 43.94 | 100.69 | 111.94 | 27.03 | 60.67 | 391.50 |
| 7 | −1 (20) | 1 (150) | −1 (3) | 50.28 | 93.31 | 114.46 | 30.26 | 76.33 | 444.70 |
| 8 | 0 (49.5) | 0 (130) | 0 (6) | 52.36 | 96.22 | 140.42 | 35.06 | 82.48 | 547.09 |
| 9 | −1 (20) | −1 (110) | 1 (9) | 43.61 | 79.49 | 116.98 | 26.38 | 60.25 | 435.59 |
| 10 | 1 (79) | 1 (150) | 1 (9) | 74.74 | 150.05 | 182.51 | 48.05 | 98.80 | 745.84 |
| 11 | −1.67 (0) | 0 (130) | 0 (6) | 39.03 | 67.30 | 99.59 | 25.73 | 49.57 | 300.41 |
| 12 | 1.67 (98.9) | 0 (130) | 0 (6) | 42.39 | 136.03 | 137.56 | 35.86 | 61.80 | 515.75 |
| 13 | 0 (49.5) | −1.67 (98.5) | 0 (6) | 48.84 | 94.93 | 148.74 | 31.98 | 64.83 | 504.09 |
| 14 | 0 (49.5) | 0 (130) | 0 (6) | 52.71 | 95.88 | 144.45 | 32.79 | 80.91 | 517.94 |
| 15 | 0 (49.5) | 1.67 (163.4) | 0 (6) | 68.20 | 112.81 | 178.98 | 49.34 | 99.77 | 670.54 |
| 16 | 0 (49.5) | 0 (130) | −1.67 (1) | 51.34 | 94.09 | 141.43 | 30.15 | 72.87 | 486.97 |
| 17 | 0 (49.5) | 0 (130) | 1.67 (11) | 51.01 | 98.11 | 143.19 | 33.12 | 83.13 | 540.16 |
| Coefficient | Y1-TPC | Y2-TFC | Y3-DPPH | Y4-ABTS | Y5-FRAP | Y6-CUPRAC |
|---|---|---|---|---|---|---|
| x0 | 52.18 a | 96.24 a | 143.73 a | 33.83 a | 81.88 a | 540.41 a |
| x1 | 2.49 b | 19.07 a | 13.49 a | 2.97 a | 3.85 b | 57.53 a |
| x2 | 6.08 a | 9.32 a | 7.84 b | 4.83 a | 11.50 a | 57.04 a |
| x3 | 0.17 | 1.86 | 3.70 | 1.14 b | 1.80 | 18.56 b |
| x12 | 5.67 a | 6.58 b | 12.35 a | 2.97 a | 4.61 b | 65.88 a |
| x13 | 3.09 b | 3.68 | 10.02 b | 1.64 b | 1.32 | 28.72 b |
| x23 | 2.67 c | 1.45 | 0.76 | 1.68 b | 1.73 | 11.50 |
| x11 | −3.98 a | 2.30 | −11.33 a | −1.50 b | −9.56 a | −50.21 a |
| x22 | 2.38 b | 3.09 | 4.84 | 2.03 a | −0.06 | 13.81 |
| x33 | −0.24 | 0.31 | −2.86 | −1.20 b | −1.59 | −12.53 |
| R2 | 0.945 | 0.962 | 0.926 | 0.971 | 0.959 | 0.971 |
| F-exp | 13.23 | 19.55 | 9.80 | 25.94 | 18.29 | 26.12 |
| Significance level (%) | 99.87 | 99.96 | 99.67 | 99.99 | 99.95 | 99.99 |
| Y1-TPC | Y2-TFC | Y3-DPPH | Y4-ABTS | Y5-FRAP | Y6-CUPRAC | |
|---|---|---|---|---|---|---|
| Predicted value | 82.46 | 168.02 | 203.52 | 57.57 | 101.88 | 838.85 |
| Experimental value | 85.9 ± 1.8 | 120.3 ± 8.0 | 260.1 ± 12.7 | 62.9 ± 2.2 | 105.1 ± 3.5 | 907.3 ± 9.6 |
| Tentative Name | CAS IDs | Molecular Formula | RT [min] | m/z Meas. | Δm/z [ppm] | M Meas. | Ions | mSigma | Peak Intensity |
|---|---|---|---|---|---|---|---|---|---|
| Clorogenic acid | 327-97-9 | C16H18O9 | 0.82 | 353.08779 | 0.36 | 354.09507 | [M-H]- | 20.7 | 1097 |
| Erigeside C | 112667-09-1 | C15H20O10 | 0.81 | 359.0988 | 1.198 | 360.10608 | [M-H]- | 24.5 | 1316 |
| Pyrogallol | 87-66-1 | C6H6O3 | 0.83 | 125.02401 | −3.061 | 126.03129 | [M-H]- | 7.2 | 31,854 |
| Quercetin 3-galactoside | 482-36-0 | C21H20O12 | 0.86 | 463.08848 | 0.127 | 464.09575 | [M-H]- | 28.2 | 13,145 |
| 3,4-Dihydroxybenzoic acid | 99-50-3 | C7H6O4 | 0.87 | 153.0187 | −4.201 | 154.02598 | [M-H]- | 6.9 | 120,796 |
| Esculetin | 305-01-1 | C9H6O4 | 0.88 | 177.01891 | −2.373 | 178.02619 | [M-H]- | 7.3 | 248,808 |
| 1,2-Benzenediol | 120-80-9 | C6H6O2 | 0.89 | 109.02938 | −0.851 | 110.03666 | [M-H]- | 2.5 | 63,753 |
| 2,5-Dihydroxybenzaldehyde | 1194-98-5 | C7H6O3 | 0.91 | 137.02388 | −3.952 | 138.03115 | [M-H]- | 5.0 | 131,780 |
| 2,4-Dihydroxyacetophenone | 89-84-9 | C8H8O3 | 0.92 | 151.03947 | −3.981 | 152.04674 | [M-H]- | 5.8 | 19,259 |
| Gallic acid | 149-91-7 | C7H6O5 | 1 | 169.01376 | −2.882 | 170.02104 | [M-H]- | 9.2 | 5246 |
| 4-Hydroxybenzaldehyde | 123-08-0 | C7H6O2 | 3.72 | 121.02904 | −3.845 | 122.03632 | [M-H]- | 30.3 | 1,568,340 |
| trans-o-Coumaric acid 2-glucoside | 614-60-8 | C15H18O8 | 6.43 | 325.09302 | 0.959 | 326.10029 | [M-H]- | 17.7 | 60,382 |
| Rutin | 153-18-4 | C27H30O16 | 9.12 | 609.1453 | −1.24 | 610.15258 | [M-H]- | 29.3 | 17,286 |
| 2,5-Dihydroxybenzaldehyde | 1194-98-5 | C7H6O3 | 9.17 | 137.02383 | −4.283 | 138.03111 | [M-H]- | 0.8 | 1,312,766 |
| Apigenin-7-glucoside | 578-74-5 | C21H20O10 | 9.23 | 431.09847 | −0.301 | 432.10575 | [M-H]- | 13.6 | 232,042 |
| Quercetin 3-galactoside | 482-36-0 | C21H20O12 | 9.26 | 463.08816 | −0.167 | 464.09543 | [M-H]- | 7.0 | 116,170 |
| 2,5-Dihydroxybenzaldehyde | 1194-98-5 | C7H6O3 | 9.81 | 137.02383 | −4.35 | 138.03111 | [M-H]- | 2.5 | 666,716 |
| 2-Cinnamoyl-1-galloylglucose | 56994-83-3 | C22H22O11 | 9.86 | 461.10907 | 0.208 | 462.11635 | [M-H]- | 4.7 | 199,241 |
| Kaempferol | 520-18-3 | C15H10O6 | 10.56 | 285.04042 | −0.143 | 286.0477 | [M-H]- | 17.6 | 664,234 |
| Quercetin | 6151-25-3 | C15H10O7 | 10.63 | 301.03529 | −0.215 | 302.04257 | [M-H]- | 3.2 | 97,502 |
| 9-Dehydroxyeurotinone | 1360606-85-4 | C15H12O5 | 11.3 | 271.06124 | 0.175 | 272.06852 | [M-H]- | 15.2 | 19,319 |
| Apigenin | 520-36-5 | C15H10O5 | 11.31 | 269.0455 | −0.543 | 270.05278 | [M-H]- | 13.4 | 736,030 |
| Chrysin 7-(4″-acetylglucoside) | 674299-89-9 | C23H22O10 | 11.56 | 459.12858 | 0.001 | 458.1213 | [M+H]+ | 1.5 | 16,413,959 |
| Amentoflavone | 1617-53-4 | C30H18O10 | 11.93 | 537.0826 | 0.245 | 538.08987 | [M-H]- | 15.8 | 11,780 |
| Oroxylin A | 480-11-5 | C16H12O5 | 13.05 | 283.06126 | −0.494 | 284.06854 | [M-H]- | 17.9 | 42,832 |
| Erianin | 95041-90-0 | C18H22O5 | 14.7 | 317.13942 | 2.126 | 318.1467 | [M-H]- | 7.3 | 13,100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvaredo-López-Vizcaíno, A.; Costa-Barbosa, A.; Sampaio, P.; del Río, P.G.; Botelho, C.; Ferreira-Santos, P. Development of Cytisus Flower Extracts with Antioxidant and Anti-Inflammatory Properties for Nutraceutical and Food Uses. Int. J. Mol. Sci. 2025, 26, 7100. https://doi.org/10.3390/ijms26157100
Alvaredo-López-Vizcaíno A, Costa-Barbosa A, Sampaio P, del Río PG, Botelho C, Ferreira-Santos P. Development of Cytisus Flower Extracts with Antioxidant and Anti-Inflammatory Properties for Nutraceutical and Food Uses. International Journal of Molecular Sciences. 2025; 26(15):7100. https://doi.org/10.3390/ijms26157100
Chicago/Turabian StyleAlvaredo-López-Vizcaíno, Adela, Augusto Costa-Barbosa, Paula Sampaio, Pablo G. del Río, Claudia Botelho, and Pedro Ferreira-Santos. 2025. "Development of Cytisus Flower Extracts with Antioxidant and Anti-Inflammatory Properties for Nutraceutical and Food Uses" International Journal of Molecular Sciences 26, no. 15: 7100. https://doi.org/10.3390/ijms26157100
APA StyleAlvaredo-López-Vizcaíno, A., Costa-Barbosa, A., Sampaio, P., del Río, P. G., Botelho, C., & Ferreira-Santos, P. (2025). Development of Cytisus Flower Extracts with Antioxidant and Anti-Inflammatory Properties for Nutraceutical and Food Uses. International Journal of Molecular Sciences, 26(15), 7100. https://doi.org/10.3390/ijms26157100









