Structural, Computational, and Biomolecular Interaction Study of Europium(III) and Iron(III) Complexes with Pyridoxal-Semicarbazone Ligand
Abstract
1. Introduction
2. Results and Discussion
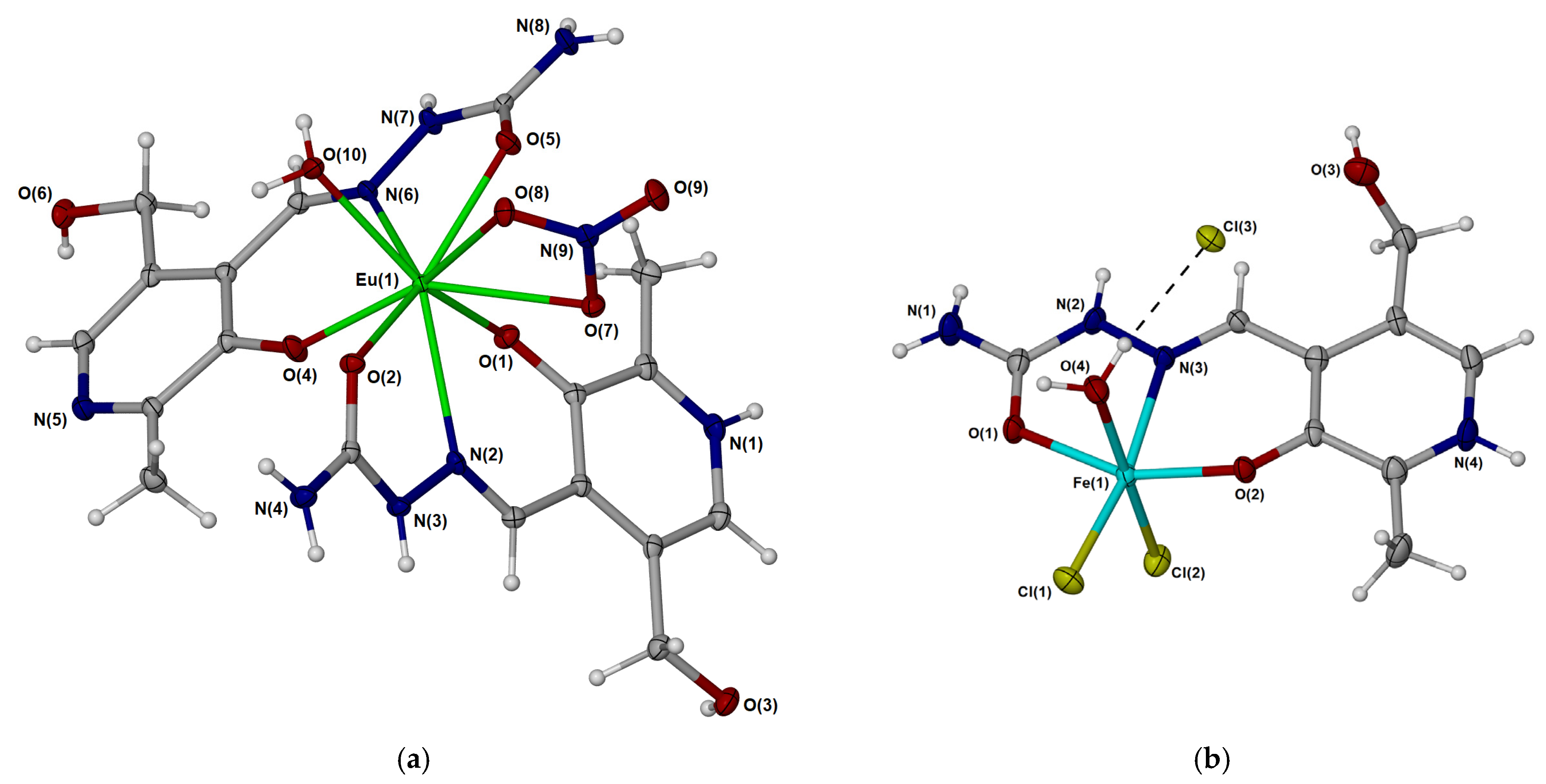
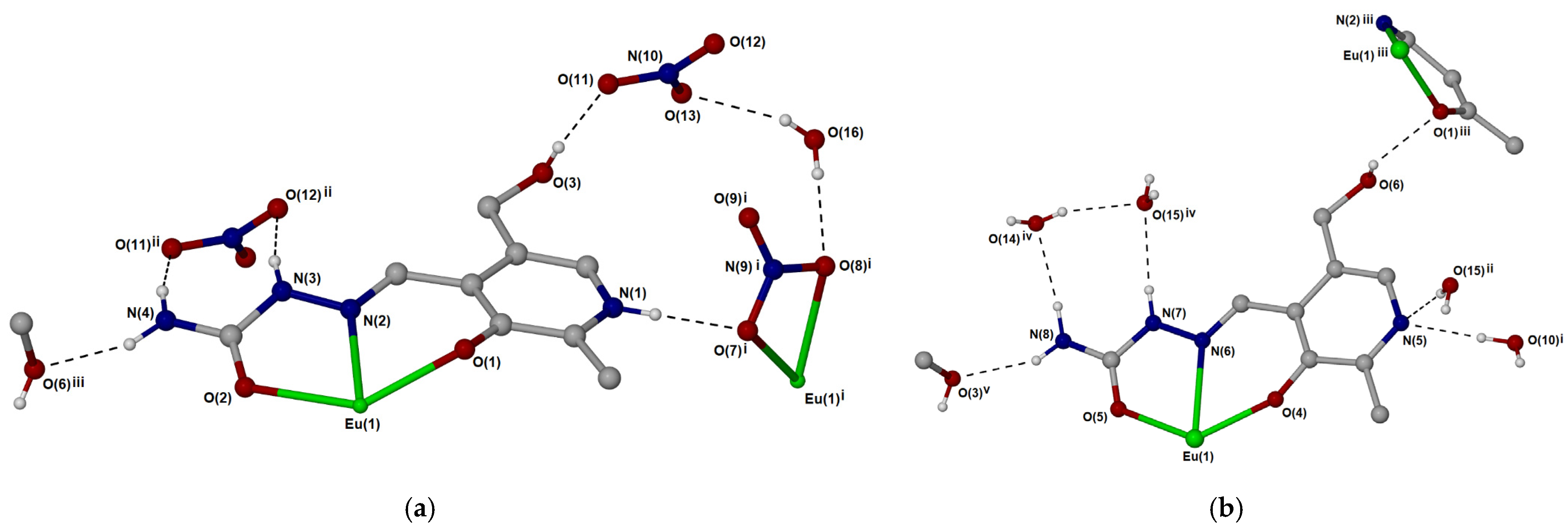
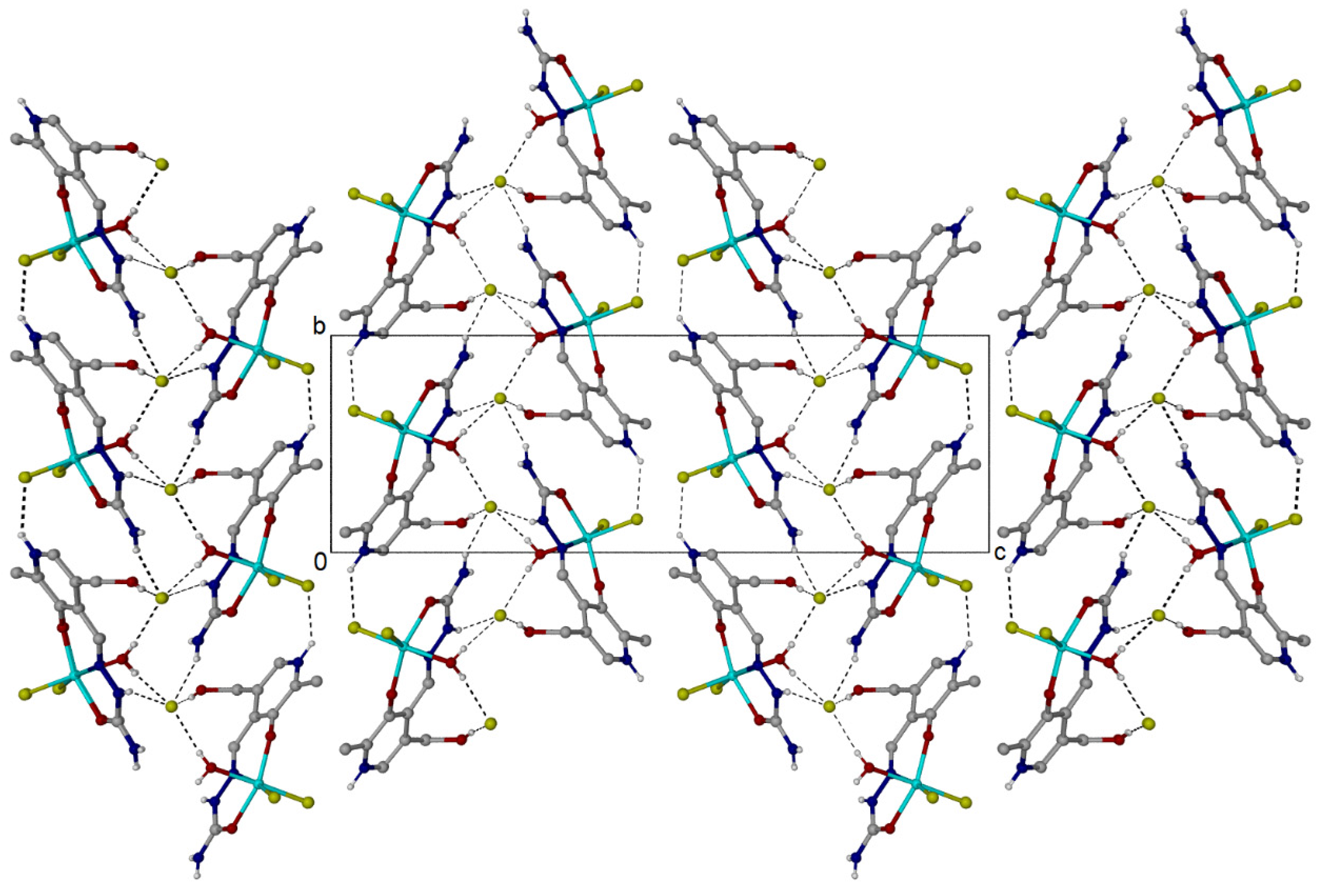
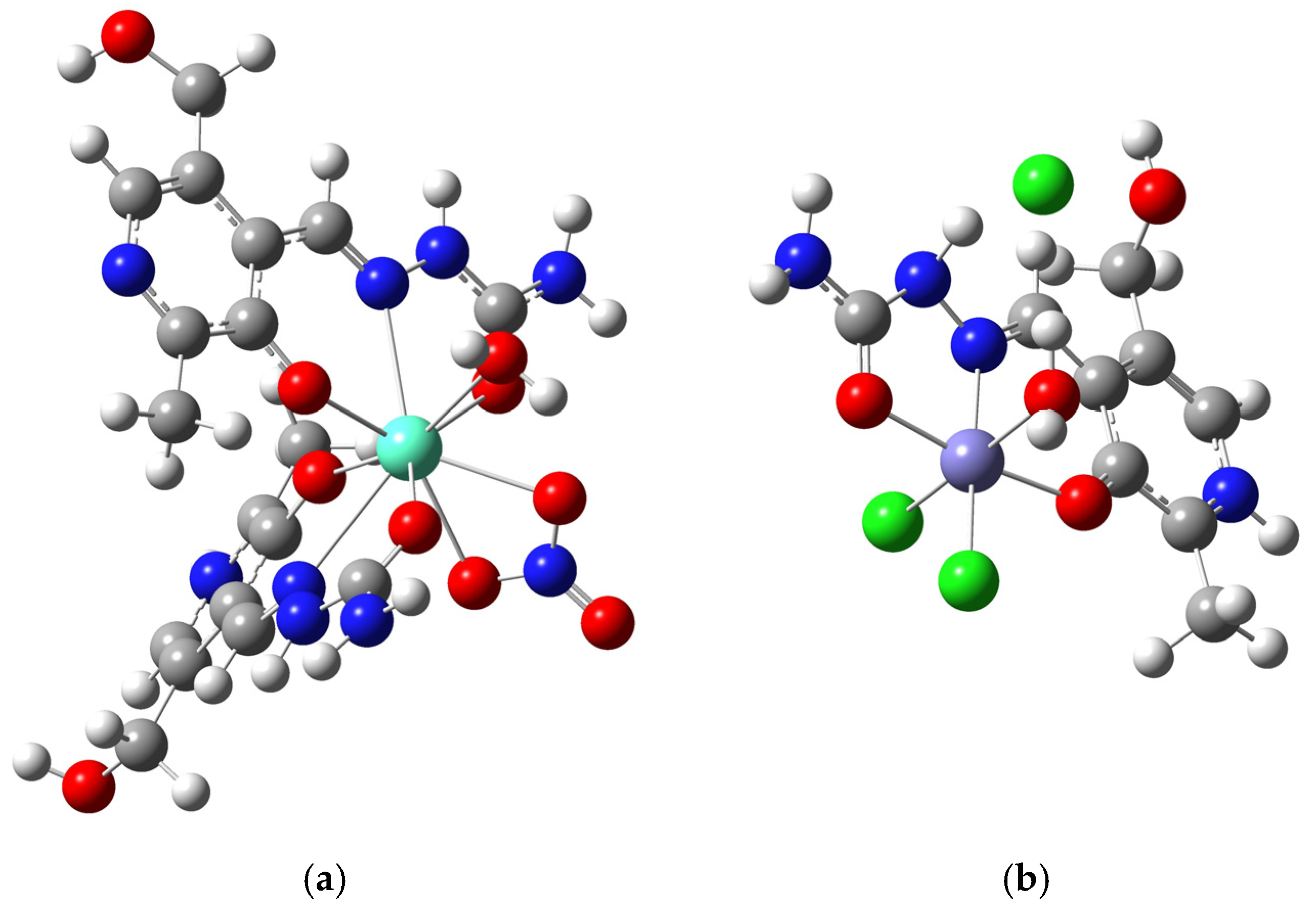
2.1. Crystallographic Structural Analysis
2.2. Spectroscopic FTIR Analysis
2.3. Hirshfeld Surface Analysis
2.4. Theoretical Structural Analysis and Intramolecular Interactions
2.5. QTAIM Analysis of Interactions
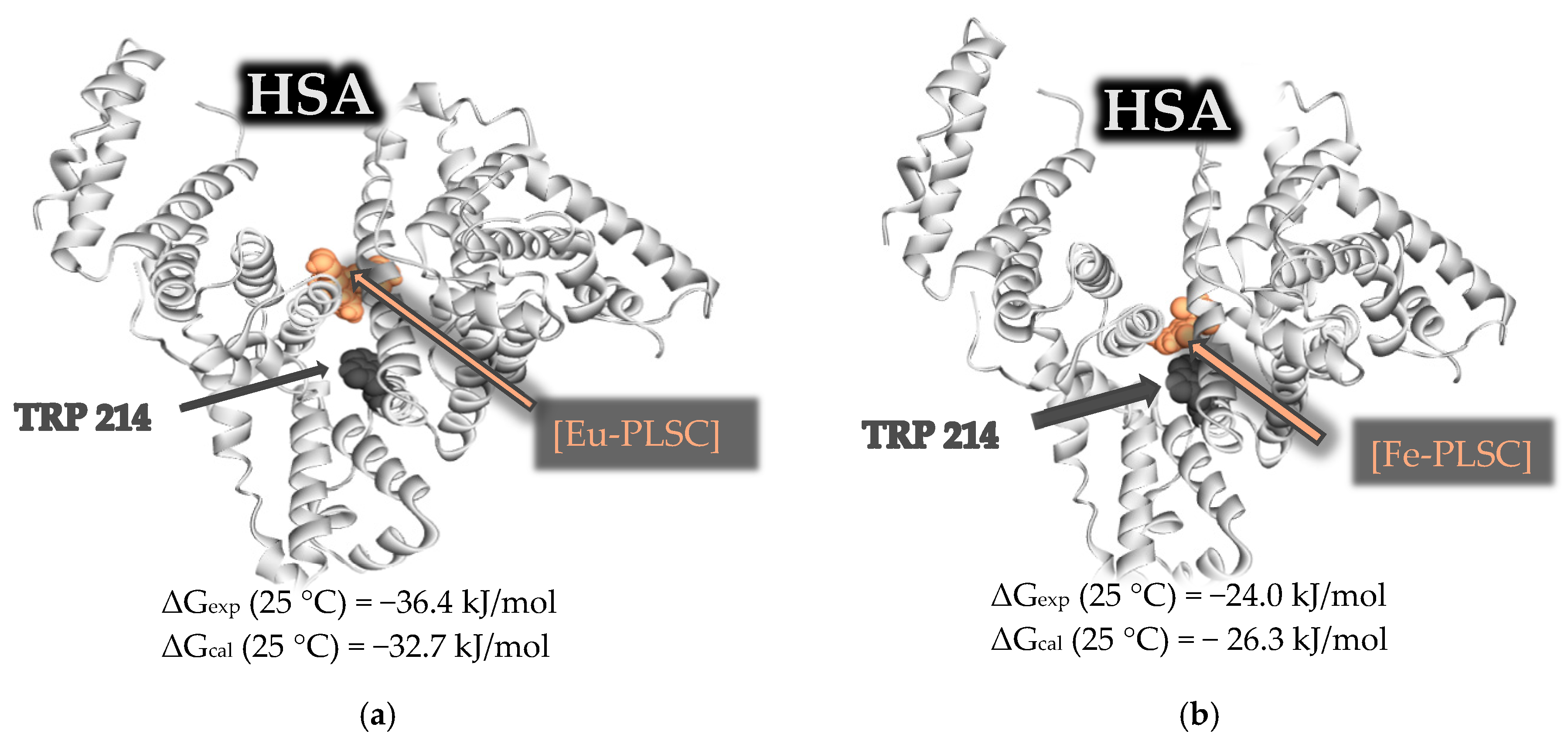
2.6. Experimental and Theoretical HSA-Binding Affinity
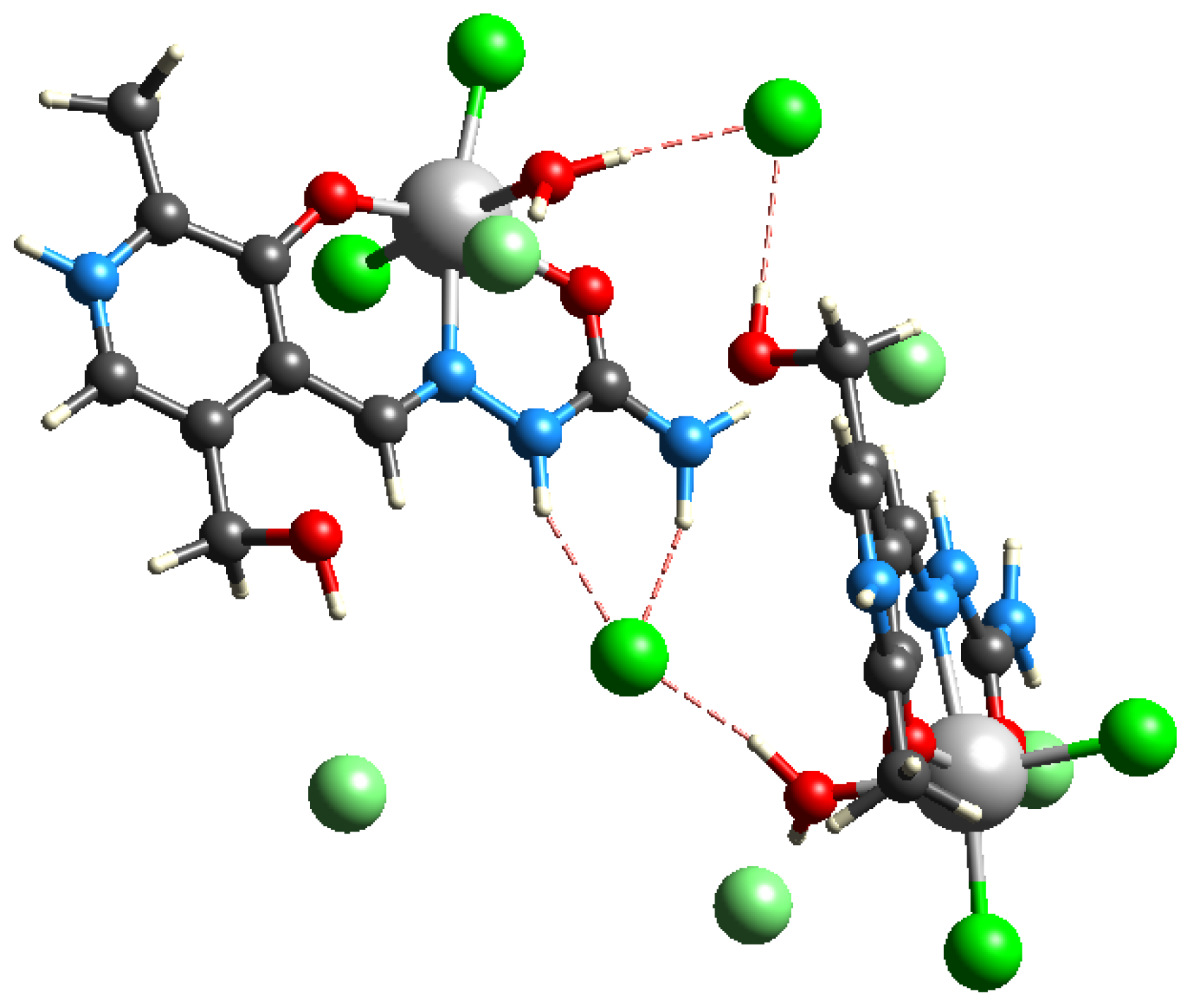
2.7. Experimental and Theoretical DNA-Binding Affinity
2.7.1. Ethidium Bromide Displacement Studies
2.7.2. Molecular Docking Simulations of the Binding Mechanism to DNA
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of [Fe(PLSC)Cl2(H2O)][Cl] (Fe-PLSC)
3.3. Synthesis of [Eu(PLSC)(PLSC-H)(NO3)(H2O)][NO3]·3(H2O) (Eu-PLSC)
3.4. Spectral Characterization
3.5. X-Ray Crystallographic Analysis
3.6. Hirshfeld Surface Analysis
3.7. Theoretical Structural Analysis
3.8. Spectrofluorimetric Determination of HSA Binding Affinity
3.9. Spectrofluorimetric Determination of DNA Binding Affinity
3.10. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Guo, Z.; Lei, J.; Yu, J. Scutellarin Synergistically Enhances Cisplatin Effect against Ovarian Cancer Cells through Enhancing the Ability of Cisplatin Binding to DNA. Eur. J. Pharmacol. 2019, 844, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Zhang, Q.-Y.; Zheng, G.-J.; Feng, B. Phytochemicals: Current Strategy to Sensitize Cancer Cells to Cisplatin. Biomed. Pharmacother. 2019, 110, 518–527. [Google Scholar] [CrossRef]
- Casas, J.S.; García-Tasende, M.S.; Sordo, J. Main Group Metal Complexes of Semicarbazones and Thiosemicarbazones. A Structural Review. Coord. Chem. Rev. 2000, 209, 197–261. [Google Scholar] [CrossRef]
- Mustafa, Y.F. Modern Developments in the Application and Function of Metal/Metal Oxide Nanocomposite–Based Antibacterial Agents. BioNanoScience 2023, 13, 840–852. [Google Scholar] [CrossRef]
- Pal, R.; Kumar, V.; Gupta, A.K.; Beniwal, V. Synthesis, Characterization and DNA Photocleavage Study of a Novel Dehydroacetic Acid Based Hydrazone Schiff’s Base and Its Metal Complexes. Med. Chem. Res. 2014, 23, 3327–3335. [Google Scholar] [CrossRef]
- Padhyé, S.; Kauffman, G.B. Transition Metal Complexes of Semicarbazones and Thiosemicarbazones. Coord. Chem. Rev. 1985, 63, 127–160. [Google Scholar] [CrossRef]
- Alsoliemy, A.; Alrefaei, A.F.; Almehmadi, S.J.; Almehmadi, S.J.; Hossan, A.; Khalifa, M.E.; El-Metwaly, N.M. Synthesis, Characterization and Self-Assembly of New Cholesteryl-Substitued Sym-Tetrazine: Fluorescence, Gelation and Mesogenic Properties. J. Mol. Liq. 2021, 342, 117543. [Google Scholar] [CrossRef]
- Cordes, E.H.; Jencks, W.P. Semicarbazone Formation from Pyridoxal, Pyridoxal Phosphate, and Their Schiff Bases. Biochemistry 1962, 1, 773–778. [Google Scholar] [CrossRef]
- Jevtovic, V.; Rakić, A.; Živković, J.M.; Alrashidi, A.A.; Alreshidi, M.A.; Alzahrani, E.A.; Alshammari, O.A.O.; Rajeh, S.Y.; Hussien, M.A.; Dimić, D. Synthesis, Crystallographic Structure, Stability, and HSA-Binding Affinity of a Novel Copper(II) Complex with Pyridoxal-Semicarbazone Ligand. Crystals 2024, 14, 1106. [Google Scholar] [CrossRef]
- Cotton, S.A.; Raithby, P.R. Systematics and Surprises in Lanthanide Coordination Chemistry. Coord. Chem. Rev. 2017, 340, 220–231. [Google Scholar] [CrossRef]
- Allis, D.G.; Torvisco, A.; Webb, C.C.; Gillett-Kunnath, M.M.; Ruhlandt-Senge, K. Azobenzene as an Effective Ligand in Europium Chemistry—A Synthetic and Theoretical Study. Molecules 2024, 29, 5187. [Google Scholar] [CrossRef]
- Thor, W.; Carneiro Neto, A.N.; Moura, R.T.; Wong, K.-L.; Tanner, P.A. Europium(III) Coordination Chemistry: Structure, Spectra and Hypersensitivity. Coord. Chem. Rev. 2024, 517, 215927. [Google Scholar] [CrossRef]
- Daumann, L.J. Essential and Ubiquitous: The Emergence of Lanthanide Metallobiochemistry. Angew. Chem. Int. Ed. 2019, 58, 12795–12802. [Google Scholar] [CrossRef]
- Qin, X.; Xu, J.; Wu, Y.; Liu, X. Energy-Transfer Editing in Lanthanide-Activated Upconversion Nanocrystals: A Toolbox for Emerging Applications. ACS Cent. Sci. 2019, 5, 29–42. [Google Scholar] [CrossRef]
- Jiang, J.; Higashiyama, N.; Machida, K.; Adachi, G. The Luminescent Properties of Divalent Europium Complexes of Crown Ethers and Cryptands. Coord. Chem. Rev. 1998, 170, 1–29. [Google Scholar] [CrossRef]
- Butler, S.J.; Delbianco, M.; Lamarque, L.; McMahon, B.K.; Neil, E.R.; Pal, R.; Parker, D.; Walton, J.W.; Zwier, J.M. EuroTracker® Dyes: Design, Synthesis, Structure and Photophysical Properties of Very Bright Europium Complexes and Their Use in Bioassays and Cellular Optical Imaging. Dalton Trans. 2015, 44, 4791–4803. [Google Scholar] [CrossRef]
- Dalal, A.; Nehra, K.; Hooda, A.; Singh, D.; Kumar, S.; Malik, R.S. Red Emissive Ternary Europium Complexes: Synthesis, Optical, and Luminescence Characteristics. Luminescence 2022, 37, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, T.; Wu, J. Luminescence of Europium(III) and Terbium(III) Complexes Incorporated in Poly(Vinyl Pyrrolidone) Matrix. J. Phys. Chem. B 2001, 105, 12293–12296. [Google Scholar] [CrossRef]
- Kido, J.; Okamoto, Y. Organo Lanthanide Metal Complexes for Electroluminescent Materials. Chem. Rev. 2002, 102, 2357–2368. [Google Scholar] [CrossRef]
- Felício, M.R.; Vaz, P.D.; Nunes, C.D.; Nolasco, M.M. Novel Versatile Europium and Terbium Complexes as Bioprobes and Anticancer Agents. New J. Chem. 2022, 46, 21311–21323. [Google Scholar] [CrossRef]
- Rocha, J.S.; Pereira, G.B.S.; Palmeira-Mello, M.V.; Oliveira, W.F.; Dutra, J.L.; Teixeira, T.; Da Silva, N.N.P.; Oliveira, G.P.; Gomes-Junior, P.C.; Passaes, A.C.S.J.; et al. Synthesis, Characterization of Europium(III) and Terbium(III) Complexes Containing Lawsone Ligand and Their Interaction with DNA, HSA, and Topoisomerases. J. Mol. Struct. 2025, 1333, 141790. [Google Scholar] [CrossRef]
- Sogukomerogullari, H.G.; Başaran, E.; Kepekçi, R.A.; Türkmenoğlu, B.; Sarıoğlu, A.O.; Köse, M. Novel Europium(III), Terbium(III), and Gadolinium(III) Schiff Base Complexes: Synthesis, Structural, Photoluminescence, Antimicrobial, Antioxidant, and Molecular Docking Studies. Polyhedron 2025, 265, 117275. [Google Scholar] [CrossRef]
- Mosbah, H.K.; Ibrahim, A.B.M.; Zidan, A.S.A.; Aly, A.A.M.; Saber, S.H. La(III), Ce(III), Pr(III) and Eu(III) Complexes with Fenamic Acid Based Ligands: Preparation, Spectral and Thermal Analysis and Evaluation of Their Cytotoxicity in MDA-MB-231 Breast Cancer Cells. J. Iran. Chem. Soc. 2024, 21, 1681–1689. [Google Scholar] [CrossRef]
- Gamov, G.A.; Zavalishin, M.N. La3+, Ce3+, Eu3+, and Gd3+ Complex Formation with Hydrazones Derived from Pyridoxal 5’-Phosphate in a Neutral Tris–HCl Buffer. Russ. J. Inorg. Chem. 2021, 66, 1561–1568. [Google Scholar] [CrossRef]
- Ayers, K.M.; Schley, N.D.; Ung, G. Monometallic Lanthanide Salicylhydrazone Complexes Exhibiting Strong Near-Infrared Luminescence. Chem. Commun. 2019, 55, 8446–8449. [Google Scholar] [CrossRef] [PubMed]
- Lekha, L.; Raja, K.K.; Rajagopal, G.; Easwaramoorthy, D. Synthesis, Spectroscopic Characterization and Antibacterial Studies of Lanthanide(III) Schiff Base Complexes Containing N, O Donor Atoms. J. Mol. Struct. 2014, 1056–1057, 307–313. [Google Scholar] [CrossRef]
- Jevtovic, V.; Jovanovic, L.; Leovac, V.; Bjelica, L. Transition Metal Complexes with Thiosemicarbazide-Based Ligands, Part 47: Synthesis, Physicochemical and Voltammetric Characterization of Iron(III) Complexes with Pyridoxal Semi-, Thiosemi- and S-Meth. J. Serb. Chem. Soc. 2003, 68, 929–942. [Google Scholar] [CrossRef]
- Jevtovic, V.; Alenezi, K.M.; Moll, H.E.; Haque, A.; Humaidi, J.; Al-Zahrani, S.A.; Vidovic, D. Pydridoxal (Thio) Semicarbazone Ligands And Their Fe(Iii) Complexes As Potential Electrocatalysts For Hydrogen Evolution Raction. Int. J. Electrochem. Sci. 2021, 16, 210731. [Google Scholar] [CrossRef]
- Jaćimović, Ž.K.; Leovac, V.M.; Giester, G.; Tomić, Z.D.; Szécsényi, K.M. Structural and Thermal Characterization of Fe(III) and Fe(II) Complexes with Tridentate ONO Pyridoxal Semicarbazone. J. Therm. Anal. Calorim. 2007, 90, 549–555. [Google Scholar] [CrossRef]
- Jevtovic, V.; Cvetkovic, D.; Vidovic, D. Synthesis, X-Ray Characterization and Antimicrobial Activity of Iron(II) and Cobalt(III) Complexes with the Schiff Base Derived from Pyridoxal and Semicarbazide or S-Methylisothiosemicarbazide. J. Iran. Chem. Soc. 2011, 8, 727–733. [Google Scholar] [CrossRef]
- Leovac, V.; Jevtovic, V.; Jovanovic, L.; Bogdanovic, G. Metal Complexes with Schiff-Base Ligands—Pyridoxal and Semicarbazide-Based Derivatives. J. Serb. Chem. Soc. 2005, 70, 393–422. [Google Scholar] [CrossRef]
- Jevtovic, V.; Golubović, L.; Alshammari, O.A.O.; Alhar, M.S.; Alanazi, T.Y.A.; Radulović, A.; Nakarada, Đ.; Marković, J.D.; Rakić, A.; Dimić, D. Structural, Antioxidant, and Protein/DNA-Binding Properties of Sulfate-Coordinated Ni(II) Complex with Pyridoxal-Semicarbazone (PLSC) Ligand. Inorganics 2024, 12, 280. [Google Scholar] [CrossRef]
- Osman, U.M.; Silvarajoo, S.; Kamarudin, K.H.; Tahir, M.I.M.; Kwong, H.C. Ni(II) Complex Containing a Thiosemicarbazone Ligand: Synthesis, Spectroscopy, Single-Crystal X-Ray Crystallographic and Conductivity Studies. J. Mol. Struct. 2021, 1223, 128994. [Google Scholar] [CrossRef]
- Devi, J.; Yadav, M.; Jindal, D.K.; Kumar, D.; Poornachandra, Y. Synthesis, Spectroscopic Characterization, Biological Screening and in Vitro Cytotoxic Studies of 4-Methyl-3-Thiosemicarbazone Derived Schiff Bases and Their Co(II), Ni(II), Cu(II) and Zn(II) Complexes. Appl. Organomet. Chem. 2019, 33, e5154. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Leovac, V.M.; Jevtović, V.S.; Grgurić-Šipka, S.; Sabo, T.J. Platinum(IV) Complex with Pyridoxal Semicarbazone. Inorg. Chem. Commun. 2003, 6, 561–564. [Google Scholar] [CrossRef]
- Belicchi, M.F.; Gasparri, G.F.; Leporati, E.; Pelizzi, C.; Tarasconi, P.; Tosi, G. Thiosemicarbazones as Co-Ordinating Agents. Solution Chemistry and X-Ray Structure of Pyridoxal Thiosemicarbazone Trihydrate and Spectroscopic Properties of Its Metal Complexes. J. Chem. Soc. Dalton Trans. 1986, 3, 2455–2461. [Google Scholar] [CrossRef]
- Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Munawar, K.S.; Ashfaq, M.; Tahir, M.N. Diverse Coordination of Isoniazid Hydrazone Schiff Base Ligand towards Iron(III): Synthesis, Characterization, SC-XRD, HSA, QTAIM, MEP, NCI, NBO and DFT Study. J. Mol. Struct. 2022, 1250, 131691. [Google Scholar] [CrossRef]
- Jevtovic, V.; Alshammari, N.; Latif, S.; Alsukaibi, A.K.D.; Humaidi, J.; Alanazi, T.Y.A.; Abdulaziz, F.; Matalka, S.I.; Pantelić, N.Đ.; Marković, M.; et al. Synthesis, Crystal Structure, Theoretical Calculations, Antibacterial Activity, Electrochemical Behavior, and Molecular Docking of Ni(II) and Cu(II) Complexes with Pyridoxal-Semicarbazone. Molecules 2022, 27, 6322. [Google Scholar] [CrossRef]
- Hueso-Ureña, F.; Jiménez-Pulido, S.B.; Fernández-Liencres, M.P.; Fernández-Gómez, M.; Moreno-Carretero, M.N. A New Five-Coordinated CuIP2NO2 System: XRD Structure of 6-Acetyl-1,3,7-Trimethyl-Pteridine-2,4(1H,3H)-Dione and Its Cu(i) (N5,O61,O4)-Tridentate Complex with Triphenylphosphine. An AIM Study of the Nature of Metal–Ligand Bonds. Dalton Trans. 2008, 45, 6461. [Google Scholar] [CrossRef]
- Fabijanić, I.; Matković-Čalogović, D.; Pilepić, V.; Ivanišević, I.; Mohaček-Grošev, V.; Sanković, K. New Investigations of the Guanine Trichloro Cuprate(II) Complex Crystal. J. Mol. Struct. 2017, 1128, 317–324. [Google Scholar] [CrossRef]
- Dognon, J.-P. Theoretical Insights into the Chemical Bonding in Actinide Complexes. Coord. Chem. Rev. 2014, 266–267, 110–122. [Google Scholar] [CrossRef]
- Kasalović, M.P.; Jelača, S.; Milanović, Ž.; Maksimović-Ivanić, D.; Mijatović, S.; Lađarević, J.; Božić, B.; Marković, Z.; Dunđerović, D.; Rüffer, T.; et al. Novel Triphenyltin(iv) Compounds with Carboxylato N-Functionalized 2-Quinolones as Promising Potential Anticancer Drug Candidates: In Vitro and in Vivo Evaluation. Dalton Trans. 2024, 53, 8298–8314. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.; Albering, J.; Abu-Youssef, M.A.M. Structural Analyses of Two New Highly Distorted Octahedral Copper(II) Complexes with Quinoline-Type Ligands; Hirshfeld, AIM and NBO Studies. Polyhedron 2017, 127, 36–50. [Google Scholar] [CrossRef]
- Lepetit, C.; Vabre, B.; Canac, Y.; Alikhani, M.E.; Zargarian, D. Pentacoordinated, Square Pyramidal Cationic PCP Ni(II) Pincer Complexes: ELF and QTAIM Topological Analyses of Nickel–Triflate Interactions. Theor. Chem. Acc. 2018, 137, 141. [Google Scholar] [CrossRef]
- Bianchi, R.; Gervasio, G.; Marabello, D. Experimental Electron Density Analysis of Mn2(CO)10: Metal−Metal and Metal−Ligand Bond Characterization. Inorg. Chem. 2000, 39, 2360–2366. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Li, A.Y. Theoretical Insight on Cm(III) and Eu(III) Competing with Am(III) for Binding to N-Donor Extractants with Different Dentate Numbers. Int. J. Quantum. Chem. 2023, 123, e27220. [Google Scholar] [CrossRef]
- Fernández-Sainz, J.; Pacheco-Liñán, P.J.; Granadino-Roldán, J.M.; Bravo, I.; Garzón, A.; Rubio-Martínez, J.; Albaladejo, J. Binding of the Anticancer Drug BI-2536 to Human Serum Albumin. A Spectroscopic and Theoretical Study. J. Photochem. Photobiol. B Biol. 2017, 172, 77–87. [Google Scholar] [CrossRef]
- Guo, J.-L.; Liu, G.-Y.; Wang, R.-Y.; Sun, S.-X. Synthesis and Structure Elucidation of Two Essential Metal Complexes: In-Vitro Studies of Their BSA/HSA-Binding Properties, Docking Simulations, and Anticancer Activities. Molecules 2022, 27, 1886. [Google Scholar] [CrossRef]
- Song, X.-Q.; Wang, Z.-G.; Wang, Y.; Huang, Y.-Y.; Sun, Y.-X.; Ouyang, Y.; Xie, C.-Z.; Xu, J.-Y. Syntheses, Characterization, DNA/HSA Binding Ability and Antitumor Activities of a Family of Isostructural Binuclear Lanthanide Complexes Containing Hydrazine Schiff Base. J. Biomol. Struct. Dyn. 2020, 38, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Nejat Dehkordi, M.; Lincoln, P. Comprehensive Study on the Binding of Iron Schiff Base Complex with DNA and Determining the Binding Mode. J. Fluoresc. 2013, 23, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Kashanian, S.; Mahdavi, M.; Sourinejad, N. DNA Interaction and DNA Cleavage Studies of a New Platinum(II) Complex Containing Aliphatic and Aromatic Dinitrogen Ligands. Bioinorg. Chem. Appl. 2011, 2011, 525794. [Google Scholar] [CrossRef]
- Parsekar, S.U.; Haldar, P.; Antharjanam, P.K.S.; Kumar, M.; Koley, A.P. Synthesis, Characterization, Crystal Structure, DNA and Human Serum Albumin Interactions, as Well as Antiproliferative Activity of a Cu(II) Complex Containing a Schiff Base Ligand Formed in Situ from the Cu(II)-induced Cyclization of 1,5-bis(Salicylidene). Appl. Organomet. Chem. 2021, 35, e6152. [Google Scholar] [CrossRef]
- Psomas, G. Mononuclear Metal Complexes with Ciprofloxacin: Synthesis, Characterization and DNA-Binding Properties. J. Inorg. Biochem. 2008, 102, 1798–1811. [Google Scholar] [CrossRef]
- OriginLab Corporation. Origin (Version 2018) [Computer Software]; OriginLab Corporation: Northampton, MA, USA, 2018. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Jevtovic, V.; Alhar, M.S.O.; Milenković, D.; Marković, Z.; Marković, J.D.; Dimić, D. Synthesis, Structural Characterization, Cytotoxicity, and Protein/DNA Binding Properties of Pyridoxylidene-Aminoguanidine-Metal (Fe, Co, Zn, Cu) Complexes. Int. J. Mol. Sci. 2023, 24, 14745. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Byrom, P.G. A Novel Definition of a Molecule in a Crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian, 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K to Au Including the Outermost Core Orbitale. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Slee, T.S.; Cremer, D.; Kraka, E. Description of Conjugation and Hyperconjugation in Terms of Electron Distributions. J. Am. Chem. Soc. 1983, 105, 5061–5068. [Google Scholar] [CrossRef]
- Todd, A. AIMAll; Version 19.10.12; Keith, T.K., Ed.; Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]
- Caramori, G.F.; Parreira, R.L.T.; Ferreira, A.M.D.C. Isatin-Schiff Base Copper(II) Complexes—A DFT Study of the Metal-ligand Bonding Situation. Int. J. Quantum Chem. 2012, 112, 625–646. [Google Scholar] [CrossRef]
- Dennington, R.; Todd, K.; Millam, J. GausView 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Yan, J.; Zhang, G.; Pan, J.; Wang, Y. α-Glucosidase Inhibition by Luteolin: Kinetics, Interaction and Molecular Docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef]
- Suo, Z.; Sun, Q.; Yang, H.; Tang, P.; Gan, R.; Xiong, X.; Li, H. Combined Spectroscopy Methods and Molecular Simulations for the Binding Properties of Trametinib to Human Serum Albumin. RSC Adv. 2018, 8, 4742–4749. [Google Scholar] [CrossRef]
- Wu, D.; Liu, D.; Zhang, Y.; Zhang, Z.; Li, H. Unravelling the Binding Mechanism of Benproperine with Human Serum Albumin: A Docking, Fluorometric, and Thermodynamic Approach. Eur. J. Med. Chem. 2018, 146, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Wenskowsky, L.; Schreuder, H.; Derdau, V.; Matter, H.; Volkmar, J.; Nazaré, M.; Opatz, T.; Petry, S. Identification and Characterization of a Single High-Affinity Fatty Acid Binding Site in Human Serum Albumin. Angew. Chem. Int. Ed. 2018, 57, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA Dodecamer: Conformation and Dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.K.; Rees, D.C.; Kielkopf, C.L.; Erkkila, K.E.; Hudson, B.P. Structure of a Photoactive Rhodium Complex Intercalated into DNA. Nat. Struct. Biol. 2000, 7, 117–121. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment (Release 2017); Dassault Systèmes: San Diego, CA, USA, 2016. [Google Scholar]
- Fuhrmann, J.; Rurainski, A.; Lenhof, H.-P.; Neumann, D. A New Lamarckian Genetic Algorithm for Flexible Ligand-Receptor Docking. J. Comput. Chem. 2010, 31, 1911–1918. [Google Scholar] [CrossRef]

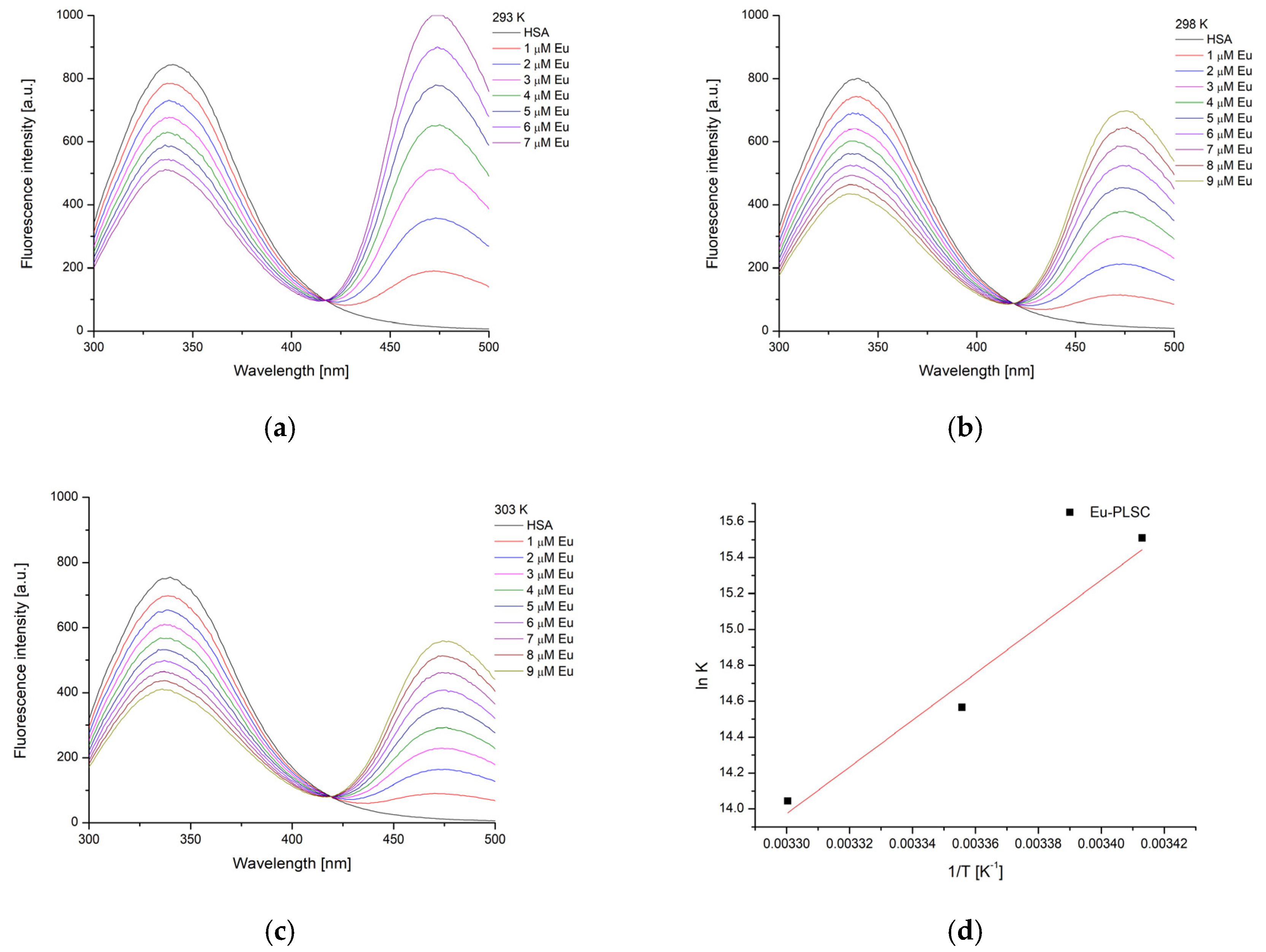
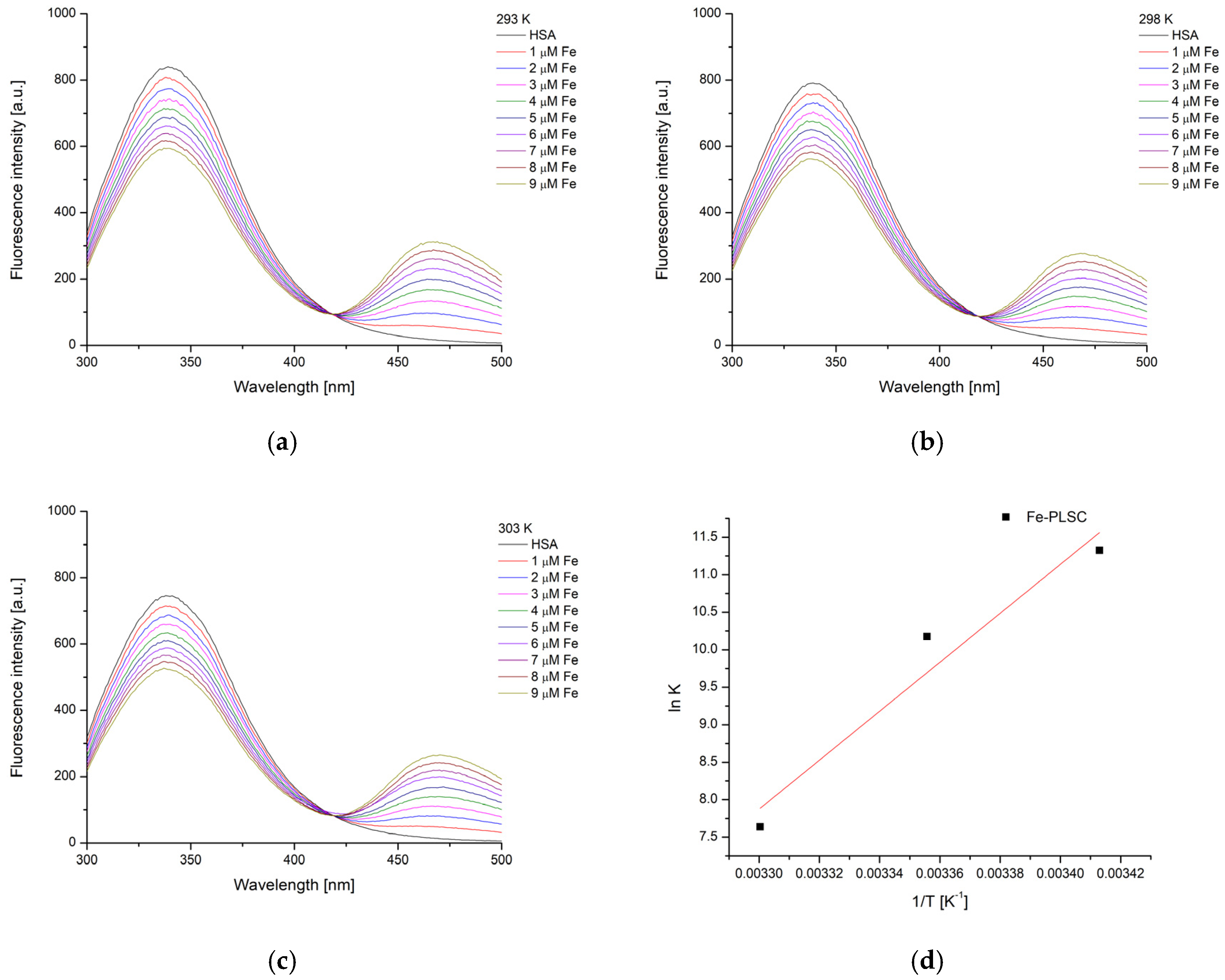
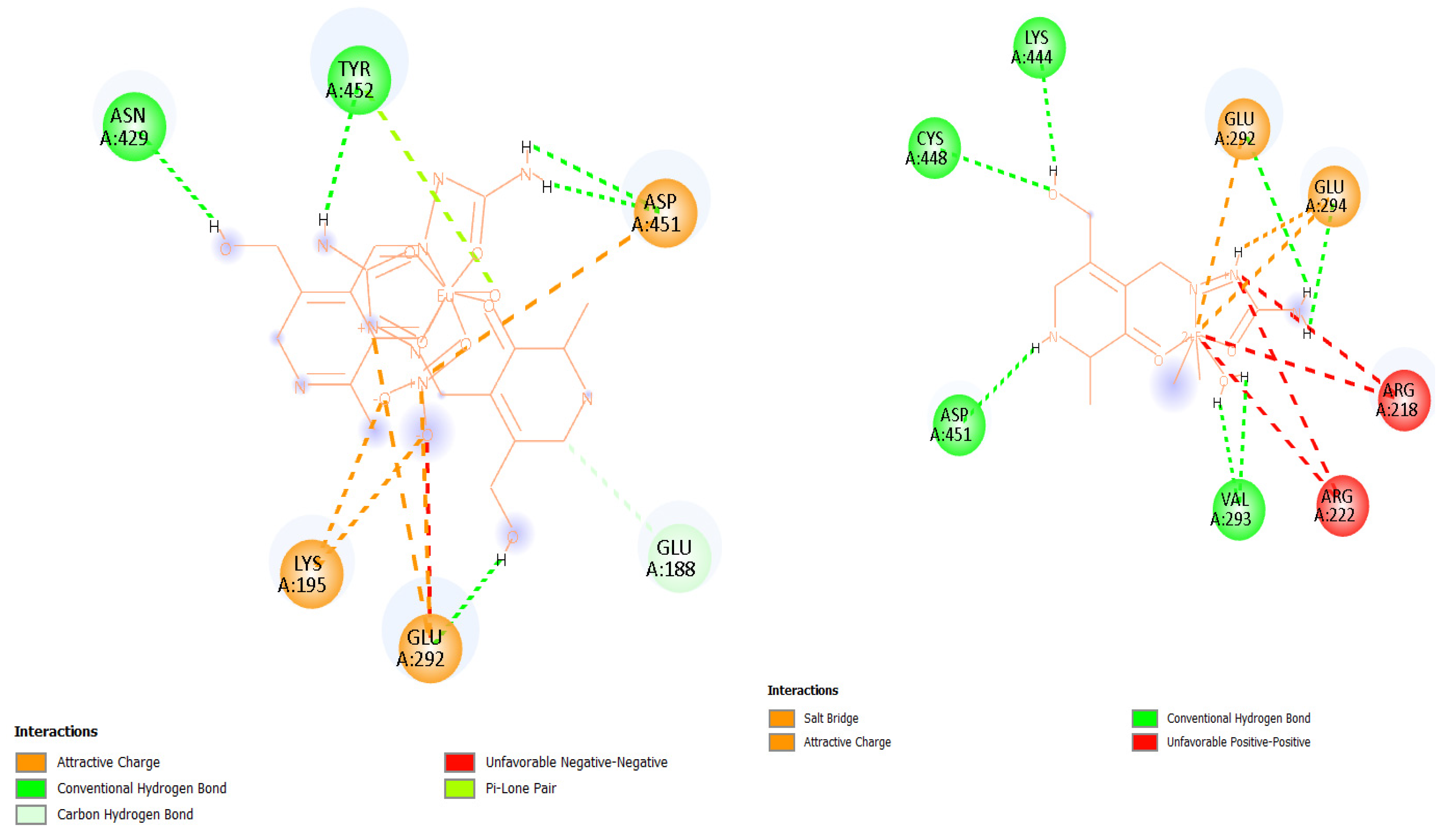
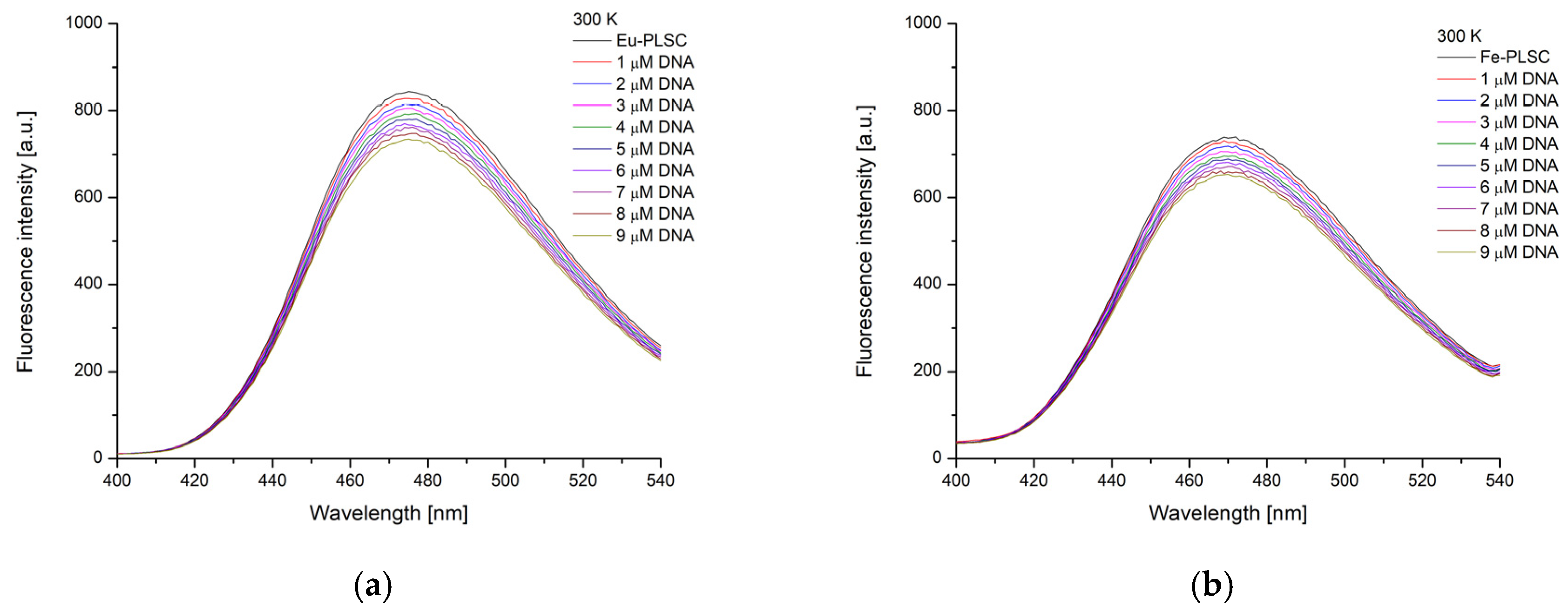
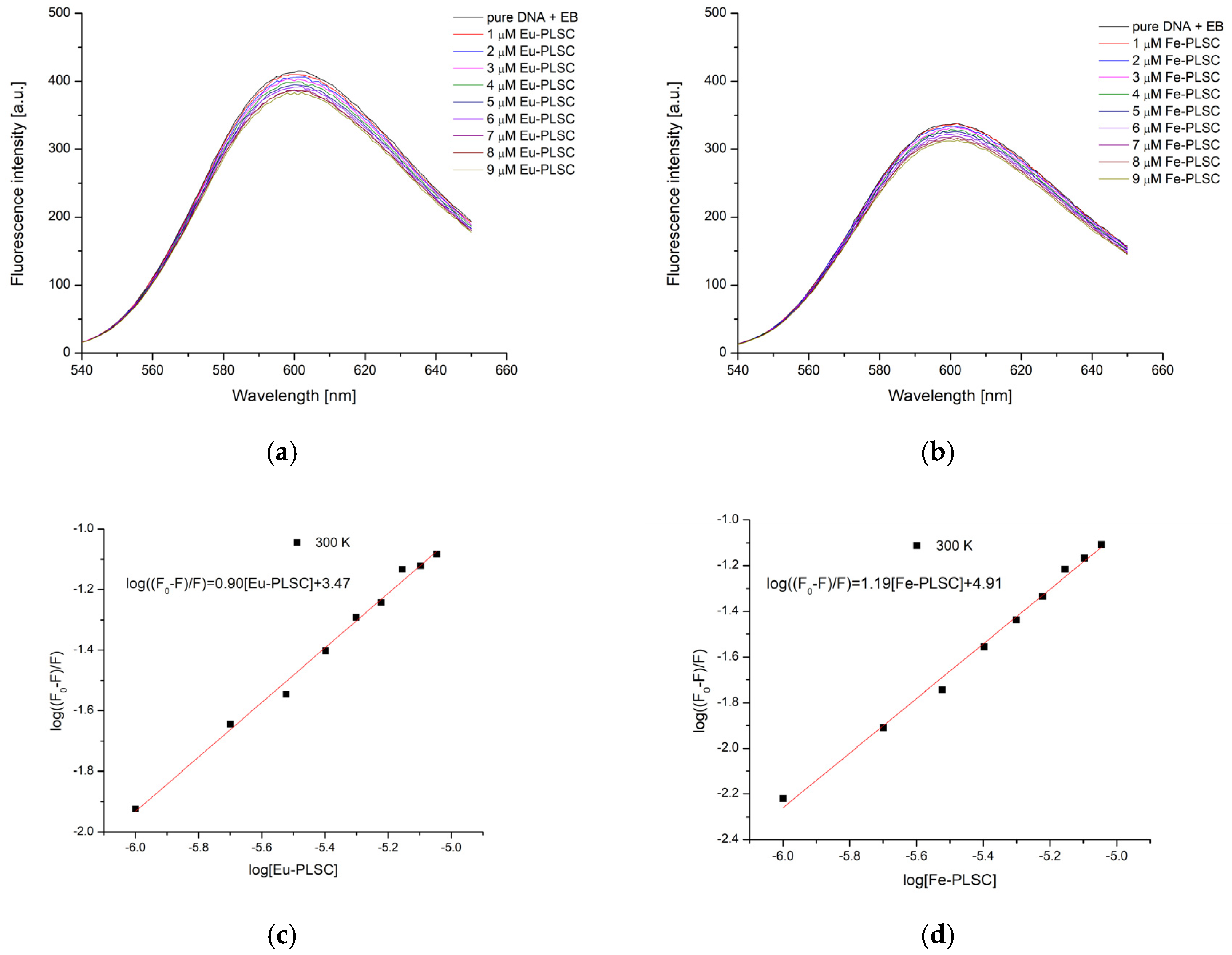

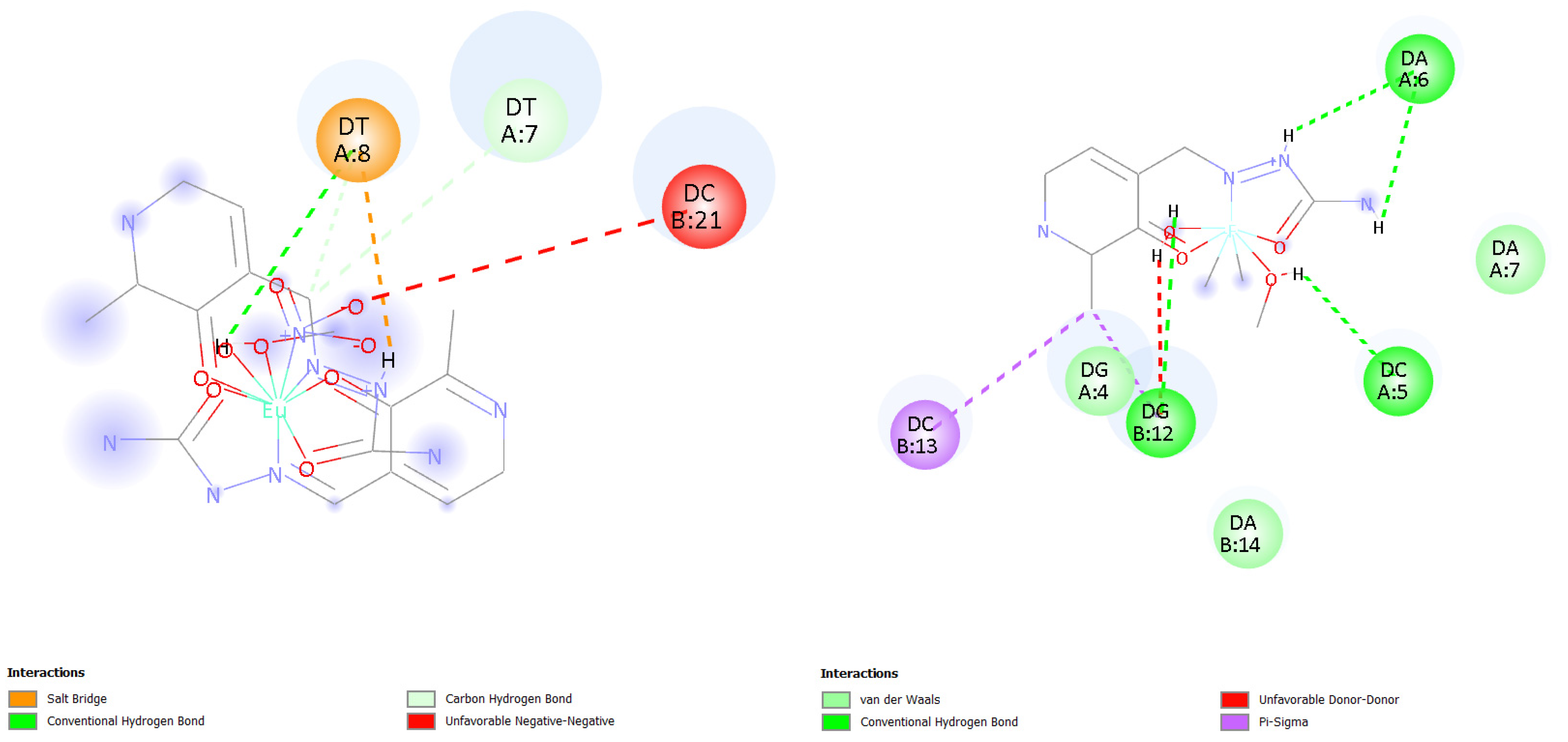
| Complex | T [K] | Kb [M−1] | ∆Hb [kJ mol−1] | ∆Sb [J mol−1 K−1] | ∆Gb [kJ mol−1] | n | R2 |
|---|---|---|---|---|---|---|---|
| Eu-PLSC–HSA | 293 | 5.44 × 106 | −108.3 | −241.1 | −37.6 | 1.39 | 0.994 |
| 298 | 2.12 × 106 | −36.4 | 1.31 | 0.995 | |||
| 303 | 1.26 × 106 | −35.2 | 1.27 | 0.998 | |||
| Fe-PLSC–HSA | 293 | 8.29 × 104 | −271.4 | −830.2 | −28.2 | 1.08 | 0.998 |
| 298 | 2.62 × 104 | −24.0 | 0.99 | 0.998 | |||
| 303 | 2.08 × 103 | −19.9 | 0.76 | 0.991 |
| Complex | T [K] | KSV [M−1] | Kb [M−1] | ∆Gb [kJ mol−1] | n | R2 |
|---|---|---|---|---|---|---|
| Eu-PLSC–CT-DNA | 300 | 16.22 × 103 | 6.05 × 103 | −21.72 | 0.92 | 0.997 |
| Fe-PLSC–CT-DNA | 14.55 × 103 | 3.60 × 104 | −26.17 | 1.07 | 0.994 |
| Empirical Formula | [Eu(PLSC)(PLSC-H)(NO3)(H2O)][NO3] × 3(H2O) C18H31EuN10O16 | [Fe(PLSC)Cl2(H2O)][Cl] C9H14FeN4O4 |
|---|---|---|
| Formula weight | 795.49 | 404.44 |
| Temperature (K) | 123(2) | 123(2) |
| Crystal system | Triclinic | Orthorhombic |
| Space group | P−1 | P212121 |
| Radiation/wavelength (Å) | MoKα/0.71073 Å | CuKα/1.54184 Å |
| Volume (Å3) | 1414.16(3) | 1514.67(4) |
| Unit cell dimension (Å/°) | a = 9.03060(10) b = 11.97860(10) c = 13.8857(2) α = 73.8600(10) β = 78.7810(10) β = 80.0940(10) | a = 7.07760(10) b = 8.40230(10) c = 25.4703(2) α = 90(10) β = 90(10) β = 90(10) |
| Z | 2 | 4 |
| Volume (Å3) | 1442.02(3) | 1514.67(4) |
| Calculated density (g cm−3) | 1.881 | 1.774 |
| Goodness-of-fit on F2 | 1.040 | 1.078 |
| h, k, lmax | 13, 17, 20 | 8, 7, 32 |
| Data completeness | 0.998 | 0.999 |
| Θ range (°) | 3.421–32.329 | 5.544–80.179 |
| R1 [I>2s(I)], R1 (all) | 0.0266 | 0.0335 |
| wR2 [I>2s(I)], wR2 (all) | 0.0539 | 0.0844 |
| CCDC no. | 2,420,972 | 2,420,971 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevtovic, V.; Perendija, S.; Alrashidi, A.A.; Alreshidi, M.A.; Alzahrani, E.A.; Alshammari, O.A.O.; Hussien, M.A.; Dimitrić Marković, J.; Dimić, D. Structural, Computational, and Biomolecular Interaction Study of Europium(III) and Iron(III) Complexes with Pyridoxal-Semicarbazone Ligand. Int. J. Mol. Sci. 2025, 26, 5289. https://doi.org/10.3390/ijms26115289
Jevtovic V, Perendija S, Alrashidi AA, Alreshidi MA, Alzahrani EA, Alshammari OAO, Hussien MA, Dimitrić Marković J, Dimić D. Structural, Computational, and Biomolecular Interaction Study of Europium(III) and Iron(III) Complexes with Pyridoxal-Semicarbazone Ligand. International Journal of Molecular Sciences. 2025; 26(11):5289. https://doi.org/10.3390/ijms26115289
Chicago/Turabian StyleJevtovic, Violeta, Stefan Perendija, Aljazi Abdullah Alrashidi, Maha Awjan Alreshidi, Elham A. Alzahrani, Odeh A. O. Alshammari, Mostafa Aly Hussien, Jasmina Dimitrić Marković, and Dušan Dimić. 2025. "Structural, Computational, and Biomolecular Interaction Study of Europium(III) and Iron(III) Complexes with Pyridoxal-Semicarbazone Ligand" International Journal of Molecular Sciences 26, no. 11: 5289. https://doi.org/10.3390/ijms26115289
APA StyleJevtovic, V., Perendija, S., Alrashidi, A. A., Alreshidi, M. A., Alzahrani, E. A., Alshammari, O. A. O., Hussien, M. A., Dimitrić Marković, J., & Dimić, D. (2025). Structural, Computational, and Biomolecular Interaction Study of Europium(III) and Iron(III) Complexes with Pyridoxal-Semicarbazone Ligand. International Journal of Molecular Sciences, 26(11), 5289. https://doi.org/10.3390/ijms26115289











