Integrative Analyses of Metabolome and Transcriptome Reveal Scion–Stock Asymmetry Reduction and Shift of Sugar Metabolism During Graft Junction Formation in Malus Domestica (‘Hanfu’) Homograft
Abstract
1. Introduction
2. Results
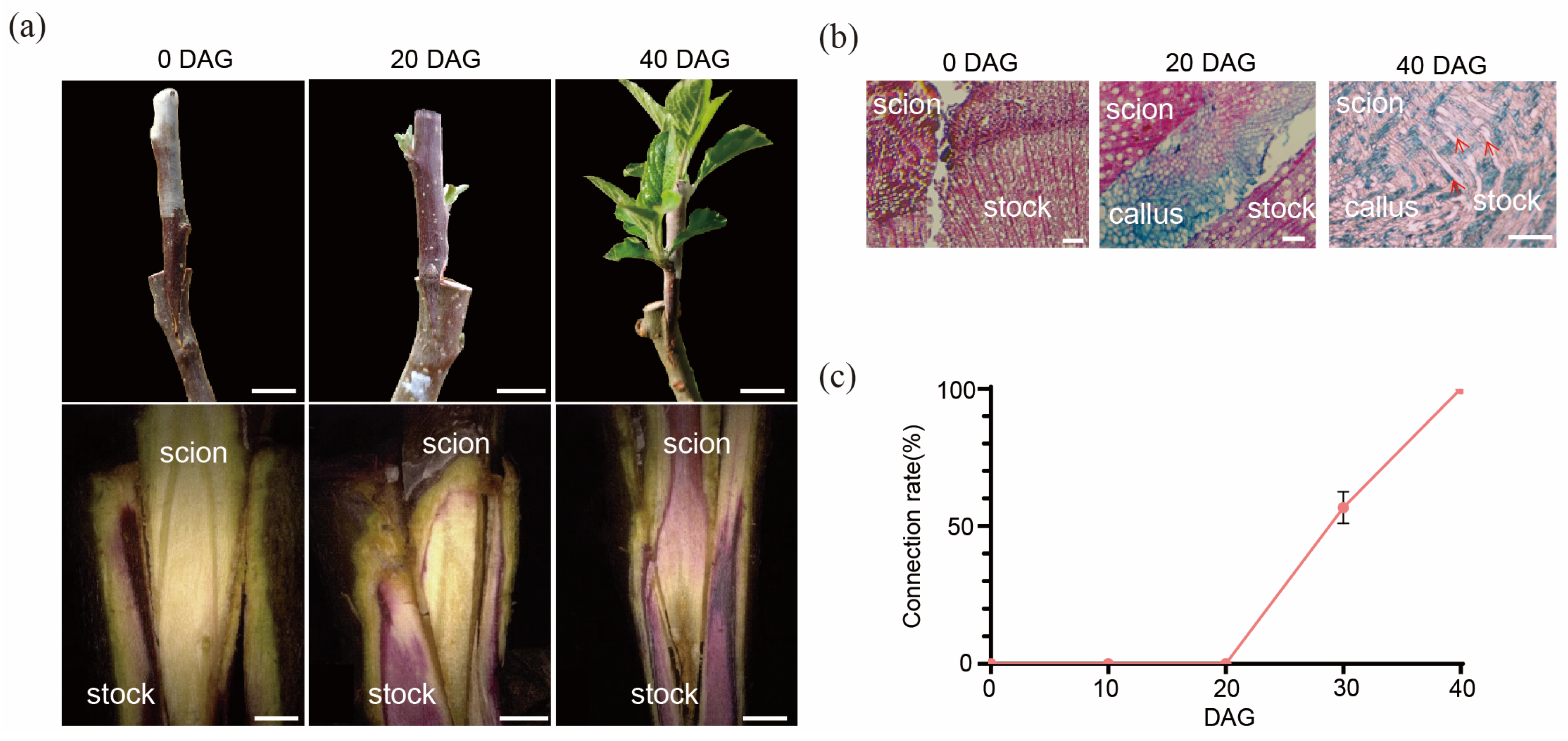
2.1. The Process of the Graft Junction Formation
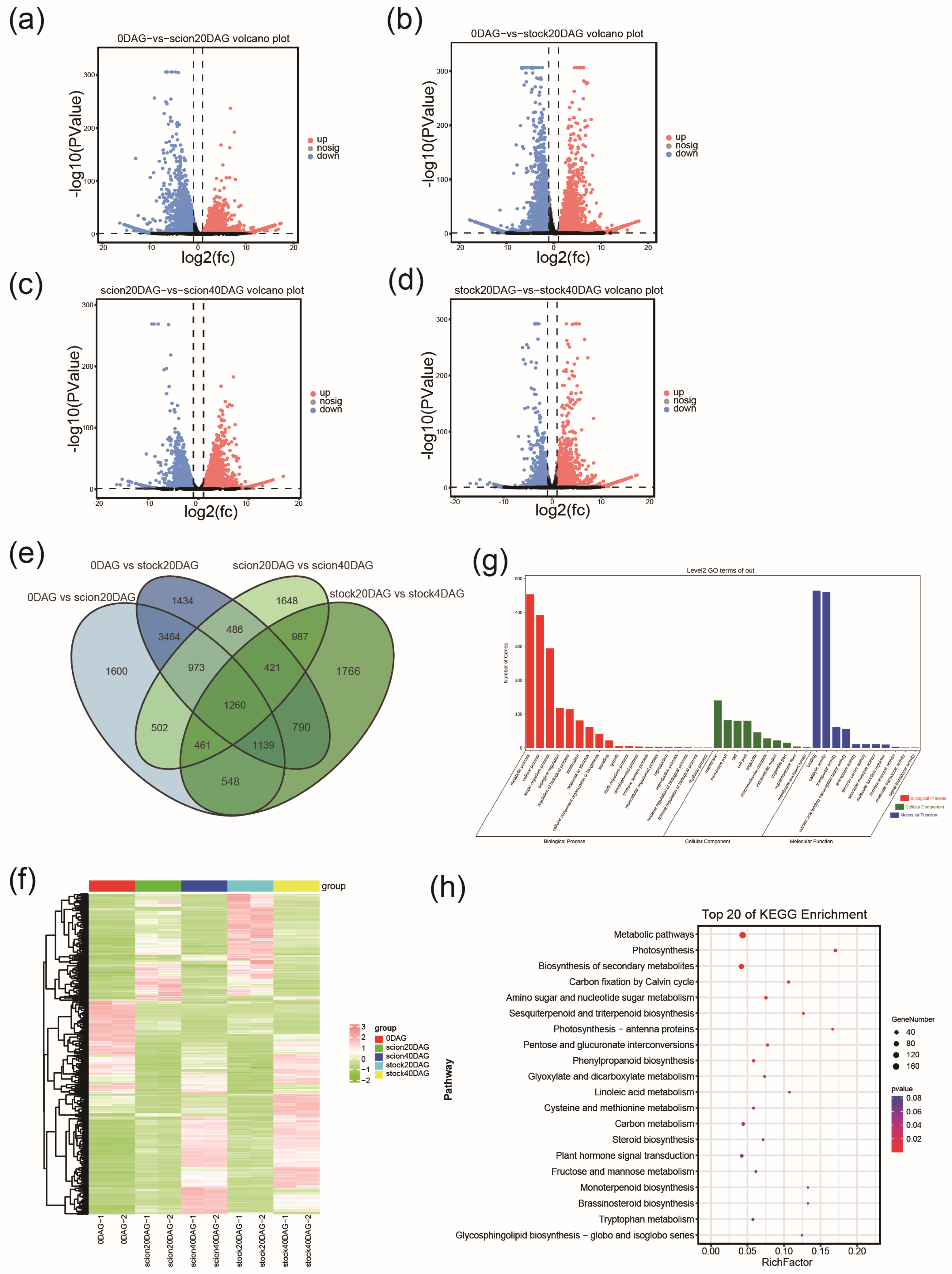
2.2. RNA Sequencing and Functional Annotation of DEGs
2.3. Weighted Gene Co-Expression Network Analysis (WGCNA)
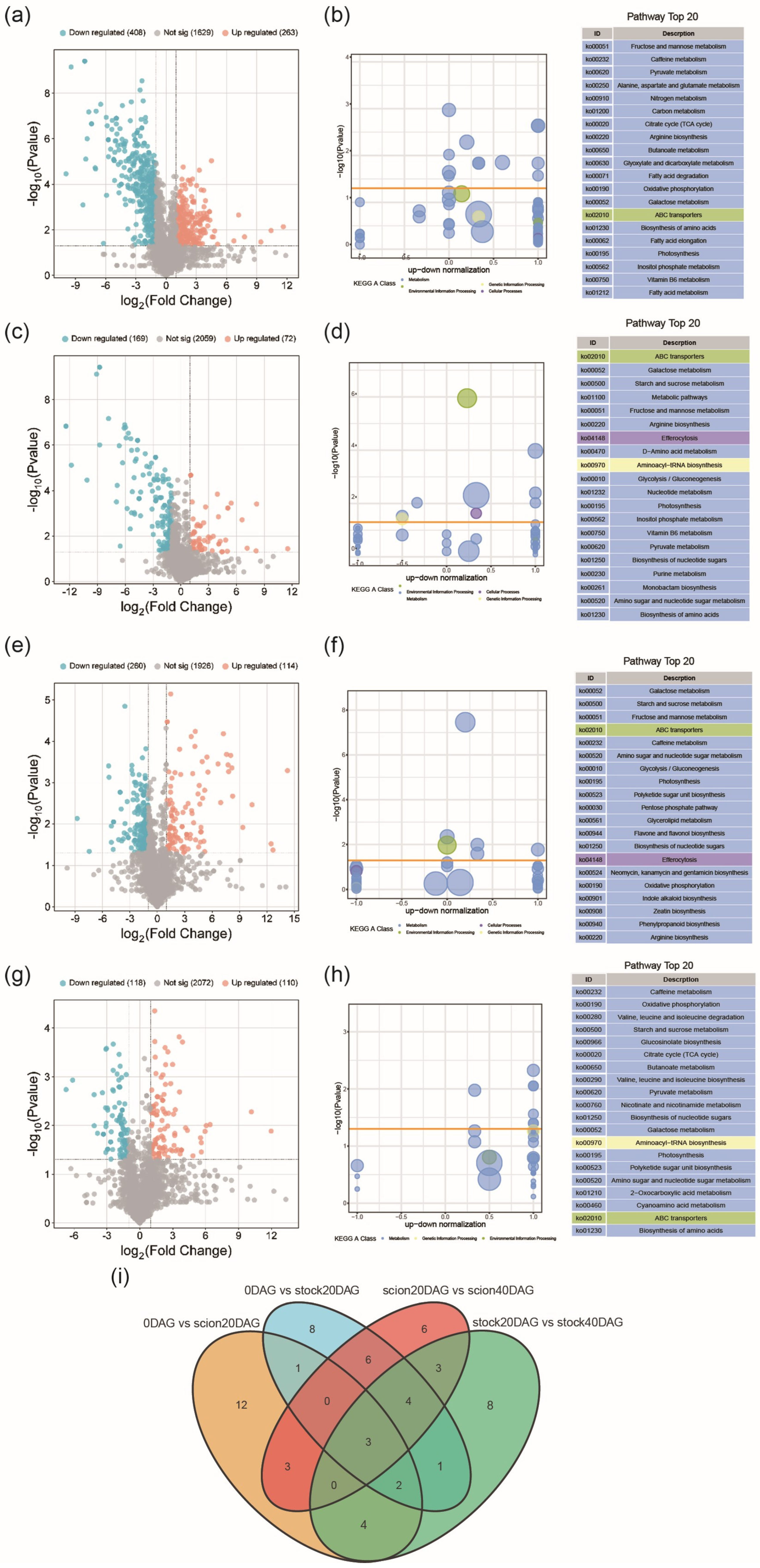
2.4. Pairwise Transcriptomic Comparison Between Stages of Graft Junction Formation
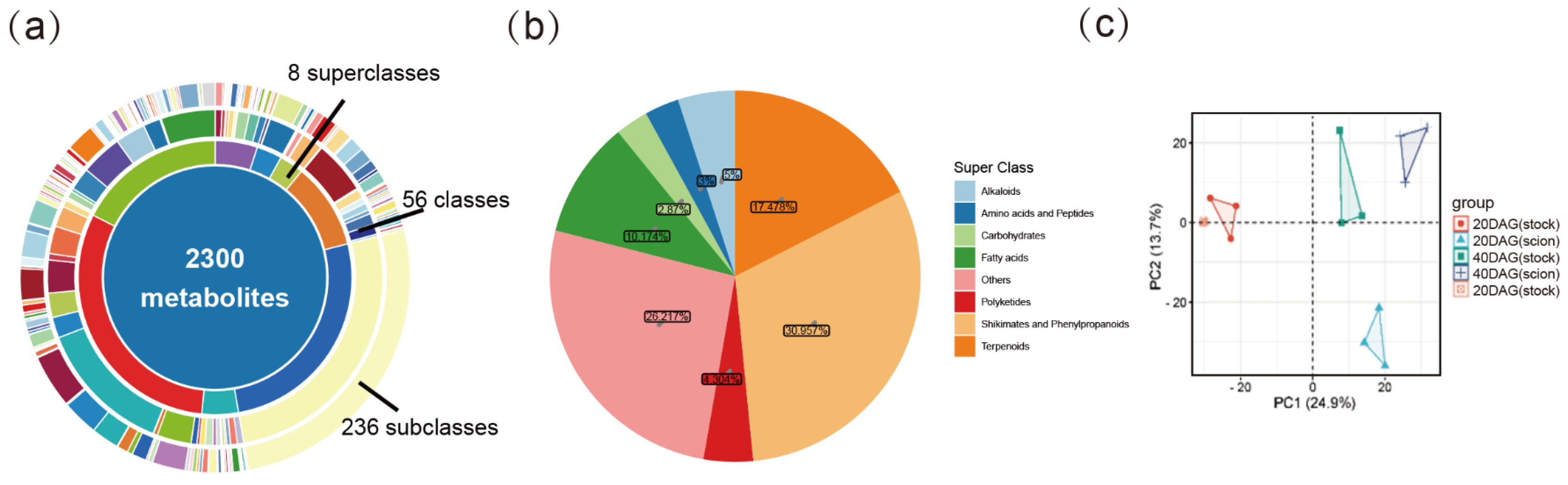
2.5. Changes in Metabolite Profiling During Graft Junction Formation
2.6. Sugar Metabolism Shift During Graft Junction Formation
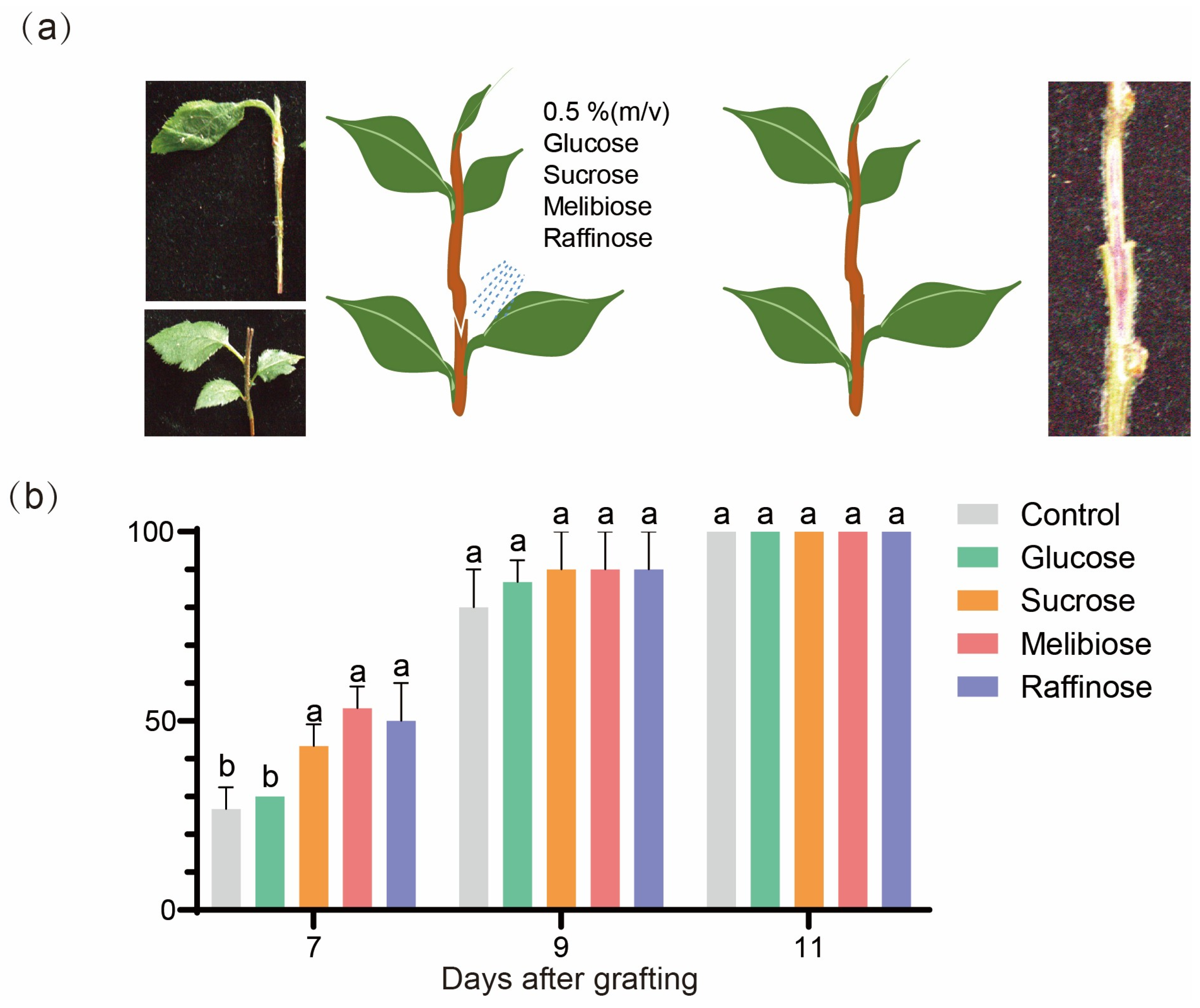
2.7. Effects of Sugar Treatments on Graft Junction Formation
3. Discussion
3.1. Graft Junction Formation Occurs in Phases
3.2. Scion vs. Stock Asymmetry Response to Grafting Is More Pronounced in Phase I
3.3. Graft Junction Formation Involves Shift in Sugar Metabolism and Is Dependent on Sugar Supply
3.4. Exogenous Sugars Promoted Graft Junction Formation
4. Materials and Methods
4.1. Plant Materials and Grafting Methods
4.2. Anatomical Observation for Timeline of Graft Junction Formation
4.3. Transcriptome Sequencing and Analyses
4.4. Metabolomic Analysis
4.5. RT–qPCR Validation
4.6. Exogenous Soluble Sugars Treatments on Grafted Plantlets
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Kong, Q.S.; Chen, F.; Bie, Z.L. The history, current status and future prospects of vegetable grafting in China. In Acta Horticulturae 1086, Proceedings of the I International Symposium on Vegetable Grafting, Wuhan, China, 17–21 March 2014; ISHS: Leuven, Belgium, 2014; Volume 1086, pp. 31–39. [Google Scholar] [CrossRef]
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A history of grafting. Hortic. Rev. 2009, 35, 437–493. [Google Scholar] [CrossRef]
- Tedesco, S.; Pina, A.; Fevereiro, P.; Kragler, F. A phenotypic search on graft compatibility in grapevine. Agronomy 2020, 10, 706. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms Underlying Graft Union Formation and Rootstock Scion Interaction in Horticultural Plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Gur, A.; Samish, R.M.; Lifshitz, E. Role of cyanogenic glycoside of quince in incompatibility between pear cultivars and quince rootstocks. Hortic. Res. 1968, 8, 113–134. [Google Scholar]
- Kollmann, R.; Yang, S.; Glockmann, C. Studies on graft unions. II. Continuous and half plasmodesmata in different regions of the graft interface. Protoplasma 1985, 126, 19–29. [Google Scholar] [CrossRef]
- Kurotani, K.I.; Notaguchi, M. Cell-to-cell connection in plant grafting—Molecular insights into symplasmic reconstruction. Plant Cell Physiol. 2021, 62, 1362–1371. [Google Scholar] [CrossRef]
- Jeffree, C.E.; Yeoman, M.M. Development of intercellular connections between opposing cells in a graft union. New Phytol. 1983, 93, 491–509. [Google Scholar] [CrossRef]
- Flaishman, M.A.; Loginovsky, K.; Golobowich, S.; Lev-Yadun, S. Arabidopsis thaliana as a model system for graft union development in homografts and heterografts. J. Plant Growth Regul. 2008, 27, 231–239. [Google Scholar] [CrossRef]
- Cookson, S.J.; Moreno, M.J.C.; Hevin, C.; Mendome, L.Z.N.; Delrot, S.; Trossat-Magnin, C.; Ollat, N. Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signalling, and secondary metabolism. J. Exp. Bot. 2013, 64, 2997–3008. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Hu, F.; Qin, Y.; Wang, X.; Hu, G. Transcriptome changes between compatible and incompatible graft combination of litchi chinensis by digital gene expression profile. Sci. Rep. 2017, 7, 3954. [Google Scholar] [CrossRef]
- Zheng, B.S.; Chu, H.L.; Jin, S.H.; Huang, Y.J.; Wang, Z.J.; Chen, M.; Huang, J.Q. cDNA-AFLP analysis of gene expression in hickory (Carya cathayensis) during graft process. Tree Physiol. 2010, 30, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Van den Broeck, L.; Spurney, R.; Sozzani, R.; Frank, M. Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. Plant Cell 2021, 34, 535–556. [Google Scholar] [CrossRef] [PubMed]
- Mazur, E.; Benková, E.; Friml, J. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Sci. Rep. 2016, 6, 33754. [Google Scholar] [CrossRef]
- Yin, H.; Yan, B.; Sun, J.; Jia, P.; Zhang, Z.; Yan, X.; Chai, J.; Ren, Z.; Zheng, G.; Liu, H. Graft-union development: A delicate process that involves cell–cell communication between scion and stock for local auxin accumulation. J. Exp. Bot. 2012, 63, 4219–4232. [Google Scholar] [CrossRef] [PubMed]
- Notaguchi, M.; Kurotani, K.I.; Sato, Y.; Tabata, R.; Kawakatsu, Y.; Okayasu, K.; Sawai, Y.; Okada, R.; Asahina, M.; Ichihashi, Y.; et al. Cell-cell adhesion in plant grafting is facilitated by β-1,4-glucanases. Science 2020, 369, 698–702. [Google Scholar] [CrossRef]
- Wang, Y.; Kollmann, R. Vascular differentiation in the graft union of in-vitro grafts with different compatibility.—Structural and functional aspects. J. Plant Physiol. 1996, 147, 521–533. [Google Scholar] [CrossRef]
- Aloni, R. Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 1980, 150, 255–263. [Google Scholar] [CrossRef]
- Marsch-Martinez, N.; Franken, J.; Gonzalez-Aguilera, K.L.; Folter, S.; Angenent, G.C.; Alvarez-Buylla, E.R. An efficient flat-surface collar-free grafting method for Arabidopsis thaliana seedlings. Plant Methods 2013, 9, 14. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Han, X.; Li, J.; Gao, Y.; Richards, C.M.; Zhang, C.; Tian, Y.; Liu, G.; Gul, H.; et al. A high-quality apple genome assembly reveals the association of a retrotransposon and red fruit colour. Nat. Commun. 2019, 10, 1494. [Google Scholar] [CrossRef]
- Serivichyaswat, P.T.; Bartusch, K.; Leso, M.; Musseau, C.; Iwase, A.; Chen, Y.; Sugimoto, K.; Quint, M.; Melnyk, C.W. High temperature perception in leaves promotes vascular regeneration and graft formation in distant tissues. Development 2022, 149, dev200079. [Google Scholar] [CrossRef] [PubMed]
- Bartusch, K.; Trenner, J.; Melnyk, C.W.; Quint, M. Cut and paste: Temperature-enhanced cotyledon micrografting for Arabidopsis thaliana seedlings. Plant Methods 2020, 16, 12. [Google Scholar] [CrossRef]
- Cookson, S.J.; Clemente Moreno, M.J.; Hevin, C.; Nyamba Mendome, L.Z.; Delrot, S.; Magnin, N.; Trossat-Magnin, C.; Ollat, N. Heterografting with nonself rootstocks induces genes involved in stress responses at the graft interface when compared with autografted controls. J. Exp. Bot. 2014, 65, 2473–2481. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P. A review of new advances in mechanism of graft compatibility–incompatibility. Sci. Hortic. 2005, 106, 1–11. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P.; Martens, H.J. Graft union formation and cell-to-cell communication via plasmodesmata in compatible and incompatible stem unions of Prunus spp. Sci. Hortic. 2012, 143, 144–150. [Google Scholar] [CrossRef]
- Tiedemann, R. Graft union development and symplastic phloem contact in the heterograft Cucumis sativus on Cucurbita ficifolia. J. Plant Physiol. 1989, 134, 427–440. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Gabel, A.; Hardcastle, T.J.; Robinson, S.; Miyashima, S.; Grosse, I.; Meyerowitz, E.M. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2447–E2456. [Google Scholar] [CrossRef]
- Harrison, M.A.; Pickard, B.G. Auxin asymmetry during gravitropism by tomato hypocotyls. Plant Physiol. 1989, 89, 652–657. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Zhao, Y.; Xiao, F.; Yang, Y. Transcriptome and physiological analyses reveal new insights into delayed incompatibility formed by interspecific grafting. Sci. Rep. 2023, 13, 4574. [Google Scholar] [CrossRef]
- Zhu, T.F.; Wang, Y.; Su, X.L.; Li, X.; Wang, L.C. Physiological characters in the stock and the scion during graft healing and their relation to graft compatibility in grapefruit. Jiangsu Agric. Sci. 2022, 50, 141–147. [Google Scholar]
- Wang, L.; Ruan, Y.L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef]
- Lopes, M.A.; Larkins, B.A. Endosperm origin, development, and function. Plant Cell 1993, 5, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hong, J.; Xia, G.; Huang, J. Cytological observation on healing responses in grafting of Carya cathayensis. Sci. Silvae Sin. 2009, 45, 34–38. [Google Scholar]
- Miao, L.; Li, Q.; Sun, T.S.; Chai, S.; Wang, C.L.; Bai, L.Q.; Sun, M.T.; Li, Y.S.; Qin, X.; Zhang, Z.H.; et al. Sugars promote graft union development in the heterograft of cucumber onto pumpkin. Hortic. Res. 2021, 8, 146. [Google Scholar] [CrossRef]
- Hoermayer, L.; Montesinos, J.C.; Marhava, P.; Benková, E.; Yoshida, S.; Friml, J. Wounding-induced changes in cellular pressure and localized auxin signalling spatially coordinate restorative divisions in roots. Proc. Natl. Acad. Sci. USA 2020, 117, 15322–15331. [Google Scholar] [CrossRef]
- Cohen, R.; Karni, L.; Aktas, H.; Edelstein, M. Hormonal signaling in rootstock-scion interactions. Sci. Hortic. 2010, 127, 119–126. [Google Scholar] [CrossRef]
- Button, J. The Effects of some Carbohydrates on the Growth and Organization of Citrus Ovular Callus. Z. Für Pflanzenphysiol. 1978, 88, 61–68. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.F. Advances in the Metabolism and Regulatory Key Enzymes of Raffinose Series Oligosaccharides in Plants. Acta Bot. Boreali-Occident. Sin. 2008, 28, 8. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.-Y.; Dillies, M.-A. SARTools: A DESeq2-and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.A.; van Velzen, E.J.; Hoefsloot, H.C.; Smilde, A.K. Multivariate paired data analysis: Multilevel PLSDA versus OPLSDA. Metabolomics 2010, 6, 119–128. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Wang, S.; Mao, C.; Xiang, L.; Zhang, X.; Jiang, F.; Cheng, Y.; Li, T. Integrative Analyses of Metabolome and Transcriptome Reveal Scion–Stock Asymmetry Reduction and Shift of Sugar Metabolism During Graft Junction Formation in Malus Domestica (‘Hanfu’) Homograft. Int. J. Mol. Sci. 2025, 26, 5290. https://doi.org/10.3390/ijms26115290
Huang W, Wang S, Mao C, Xiang L, Zhang X, Jiang F, Cheng Y, Li T. Integrative Analyses of Metabolome and Transcriptome Reveal Scion–Stock Asymmetry Reduction and Shift of Sugar Metabolism During Graft Junction Formation in Malus Domestica (‘Hanfu’) Homograft. International Journal of Molecular Sciences. 2025; 26(11):5290. https://doi.org/10.3390/ijms26115290
Chicago/Turabian StyleHuang, Wenting, Shengyuan Wang, Chong Mao, Ling Xiang, Xiao Zhang, Feng Jiang, Yuqin Cheng, and Tianzhong Li. 2025. "Integrative Analyses of Metabolome and Transcriptome Reveal Scion–Stock Asymmetry Reduction and Shift of Sugar Metabolism During Graft Junction Formation in Malus Domestica (‘Hanfu’) Homograft" International Journal of Molecular Sciences 26, no. 11: 5290. https://doi.org/10.3390/ijms26115290
APA StyleHuang, W., Wang, S., Mao, C., Xiang, L., Zhang, X., Jiang, F., Cheng, Y., & Li, T. (2025). Integrative Analyses of Metabolome and Transcriptome Reveal Scion–Stock Asymmetry Reduction and Shift of Sugar Metabolism During Graft Junction Formation in Malus Domestica (‘Hanfu’) Homograft. International Journal of Molecular Sciences, 26(11), 5290. https://doi.org/10.3390/ijms26115290





