The Role of Heavy Metals in the Biology of Female Cancers
Abstract
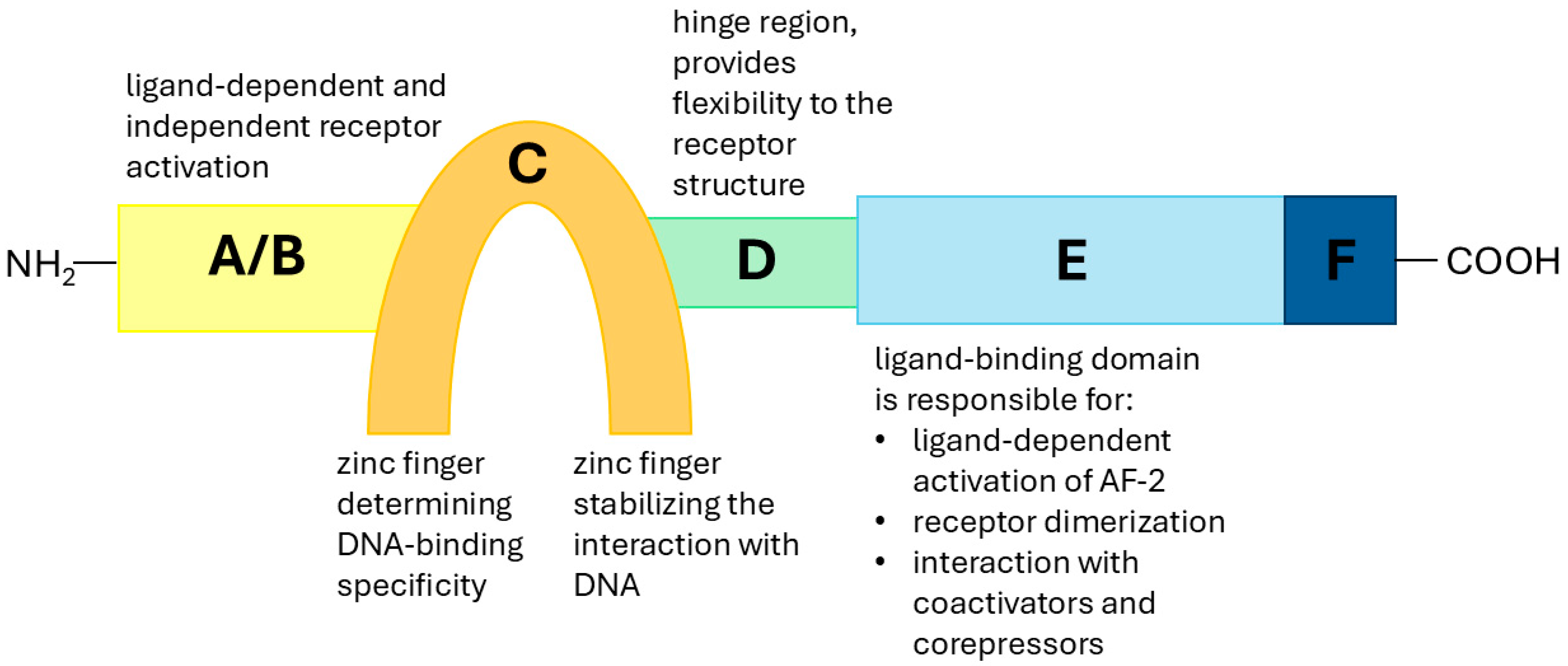
1. Introduction
1.1. Heavy Metals
1.2. Heavy Metals and Female Cancers
1.3. Breast Cancer
1.4. Ovarian Cancer
1.5. Endometrial Cancer
1.6. Cervical Cancer
1.7. Other Female Cancers
1.8. Conclusions
Funding
Conflicts of Interest
References
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy Metal Contaminations in Herbal Medicines: Determination, Comprehensive Risk Assessments, and Solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef] [PubMed]
- Ghorani-Azam, A.; Riahi-Zanjani, B.; Balali-Mood, M. Effects of Air Pollution on Human Health and Practical Measures for Prevention in Iran. J. Res. Med. Sci. 2016, 21, 65. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in Drinking Water: Sources, Metabolism, and Cancer Risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Strużyńska, L.; Dąbrowska-Bouta, B.; Koza, K.; Sulkowski, G. Inflammation-Like Glial Response in Lead-Exposed Immature Rat Brain. Toxicol. Sci. 2007, 95, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Niedzielski, P.; Rzymski, P.; Tomczyk, K.; Kozak, L.; Poniedziałek, B. Metal Accumulation in the Human Uterus Varies by Pathology and Smoking Status. Fertil. Steril. 2016, 105, 1511–1518.e3. [Google Scholar] [CrossRef]
- Rzymski, P.; Rzymski, P.; Tomczyk, K.; Niedzielski, P.; Jakubowski, K.; Poniedziałek, B.; Opala, T. Metal Status in Human Endometrium: Relation to Cigarette Smoking and Histological Lesions. Environ. Res. 2014, 132, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Bottino, C.; Vázquez, M.; Devesa, V.; Laforenza, U. Impaired Aquaporins Expression in the Gastrointestinal Tract of Rat after Mercury Exposure. J. Appl. Toxicol. 2016, 36, 113–120. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volumn 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Baan, R.A.; Stewart, B.W.; Straif, K. (Eds.) Tumour Site Concordance and Mechanisms of Carcinogenesis; IARC Scientific Publications; International Agency for Research on Cancer: Lyon, France, 2019; ISBN 978-92-832-2217-0. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Amirmostofian, M.; Akbari, F.; Hashemzaei, M.; Tabrizian, K.; Arbab, H.; Rezaee, R.; Hemat, S.; Ghorani, V.; Shahraki, J. Hormetic Effects of Curcumin on Oxidative Stress Injury Induced by Trivalent Arsenic in Isolated Rat Hepatocytes. Avicenna J. Phytomedicine 2023, 13, 641. [Google Scholar]
- Hu, G.; Li, P.; Li, Y.; Wang, T.; Gao, X.; Zhang, W.; Jia, G. Methylation Levels of P16 and TP53 That Are Involved in DNA Strand Breakage of 16HBE Cells Treated by Hexavalent Chromium. Toxicol. Lett. 2016, 249, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, P.; Cui, X.; Li, Y.; Zhang, J.; Zhai, X.; Yu, S.; Tang, S.; Zhao, Z.; Wang, J.; et al. Cr(VI)-Induced Methylation and down-Regulation of DNA Repair Genes and Its Association with Markers of Genetic Damage in Workers and 16HBE Cells. Environ. Pollut. 2018, 238, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Divekar, S.D.; Li, H.-H.; Parodi, D.A.; Ghafouri, T.B.; Chen, R.; Cyrus, K.; Foxworth, A.E.; Fornace, A.J.; Byrne, C.; Martin, M.B. Arsenite and Cadmium Promote the Development of Mammary Tumors. Carcinogenesis 2020, 41, 1005–1014. [Google Scholar] [CrossRef]
- Garcia-Morales, P.; Saceda, M.; Kenney, N.; Kim, N.; Salomon, D.S.; Gottardis, M.M.; Solomon, H.B.; Sholler, P.F.; Jordan, V.C.; Martin, M.B. Effect of Cadmium on Estrogen Receptor Levels and Estrogen-Induced Responses in Human Breast Cancer Cells. J. Biol. Chem. 1994, 269, 16896–16901. [Google Scholar] [CrossRef]
- Darbre, P.D. Metalloestrogens: An Emerging Class of Inorganic Xenoestrogens with Potential to Add to the Oestrogenic Burden of the Human Breast. J. Appl. Toxicol. 2006, 26, 191–197. [Google Scholar] [CrossRef]
- Antoniou, A.; Pharoah, P.D.P.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, Å.; et al. Average Risks of Breast and Ovarian Cancer Associated with BRCA1 or BRCA2 Mutations Detected in Case Series Unselected for Family History: A Combined Analysis of 22 Studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Sukumar, J.; Kassem, M.; Agnese, D.; Pilarski, R.; Ramaswamy, B.; Sweet, K.; Sardesai, S. Concurrent Germline BRCA1, BRCA2, and CHEK2 Pathogenic Variants in Hereditary Breast Cancer: A Case Series. Breast Cancer Res. Treat. 2021, 186, 569–575. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Lingle, W.L.; Degnim, A.C.; Ghosh, K.; Vierkant, R.A.; Maloney, S.D.; Pankratz, V.S.; Hillman, D.W.; et al. Benign Breast Disease and the Risk of Breast Cancer. N. Engl. J. Med. 2005, 353, 229–237. [Google Scholar] [CrossRef]
- Parodi, D.A.; Greenfield, M.; Evans, C.; Chichura, A.; Alpaugh, A.; Williams, J.; Martin, M.B. Alteration of Mammary Gland Development and Gene Expression by in Utero Exposure to Arsenic. Reprod. Toxicol. 2015, 54, 66–75. [Google Scholar] [CrossRef]
- Divekar, S.D.; Storchan, G.B.; Sperle, K.; Veselik, D.J.; Johnson, E.; Dakshanamurthy, S.; Lajiminmuhip, Y.N.; Nakles, R.E.; Huang, L.; Martin, M.B. The Role of Calcium in the Activation of Estrogen Receptor-Alpha. Cancer Res. 2011, 71, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Green, S.; Stack, G.; Berry, M.; Jin, J.-R.; Chambon, P. Functional Domains of the Human Estrogen Receptor. Cell 1987, 51, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Bourguet, W.; Germain, P.; Gronemeyer, H. Nuclear Receptor Ligand-Binding Domains: Three-Dimensional Structures, Molecular Interactions and Pharmacological Implications. Trends Pharmacol. Sci. 2000, 21, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S.; et al. Estrogen-Like Activity of Metals in Mcf-7 Breast Cancer Cells. Endocrinology 2003, 144, 2425–2436. [Google Scholar] [CrossRef]
- Mosselman, S.; Polman, J.; Dijkema, R. ERβ: Identification and Characterization of a Novel Human Estrogen Receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef]
- Lecce, G.; Meduri, G.; Ancelin, M.; Bergeron, C.; Perrot-Applanat, M. Presence of Estrogen Receptor β in the Human Endometrium through the Cycle: Expression in Glandular, Stromal, and Vascular Cells. J. Clin. Endocrinol. Metab. 2001, 86, 1379–1386. [Google Scholar] [CrossRef]
- Kim, J.; Bang, H.; Seong, C.; Kim, E.-S.; Kim, S. Transcription Factors and Hormone Receptors: Sex-specific Targets for Cancer Therapy. Oncol. Lett. 2024, 29, 93. [Google Scholar] [CrossRef]
- Huang, B.; Omoto, Y.; Iwase, H.; Yamashita, H.; Toyama, T.; Coombes, R.C.; Filipovic, A.; Warner, M.; Gustafsson, J.-Å. Differential Expression of Estrogen Receptor α, Β1, and Β2 in Lobular and Ductal Breast Cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 1933–1938. [Google Scholar] [CrossRef]
- Martin, E.C.; Conger, A.K.; Yan, T.J.; Hoang, V.T.; Miller, D.F.B.; Buechlein, A.; Rusch, D.B.; Nephew, K.P.; Collins-Burow, B.M.; Burow, M.E. MicroRNA-335-5p and -3p Synergize to Inhibit Estrogen Receptor Alpha Expression and Promote Tamoxifen Resistance. FEBS Lett. 2017, 591, 382–392. [Google Scholar] [CrossRef]
- Langley, R.E.; Godsland, I.F.; Kynaston, H.; Clarke, N.W.; Rosen, S.D.; Morgan, R.C.; Pollock, P.; Kockelbergh, R.; Lalani, E.; Dearnaley, D.; et al. Early Hormonal Data from a Multicentre Phase II Trial Using Transdermal Oestrogen Patches as First-line Hormonal Therapy in Patients with Locally Advanced or Metastatic Prostate Cancer. BJU Int. 2008, 102, 442–445. [Google Scholar] [CrossRef]
- Park, S.-H.; Cheung, L.W.T.; Wong, A.S.T.; Leung, P.C.K. Estrogen Regulates Snail and Slug in the Down-Regulation of E-Cadherin and Induces Metastatic Potential of Ovarian Cancer Cells through Estrogen Receptor α. Mol. Endocrinol. 2008, 22, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; Vandeusen, J.; et al. Estrogen Receptor Beta (ERβ): A Ligand Activated Tumor Suppressor. Front. Oncol. 2020, 10, 587386. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G. Estrogen Receptor Beta, a Possible Tumor Suppressor Involved in Ovarian Carcinogenesis. Cancer Lett. 2006, 231, 151–157. [Google Scholar] [CrossRef]
- Hwang, N.M.; Stabile, L.P. Estrogen Receptor ß in Cancer: To ß(e) or Not to ß(e)? Endocrinology 2021, 162, bqab162. [Google Scholar] [CrossRef] [PubMed]
- Dalal, H.; Dahlgren, M.; Gladchuk, S.; Brueffer, C.; Gruvberger-Saal, S.K.; Saal, L.H. Clinical Associations of ESR2 (Estrogen Receptor Beta) Expression across Thousands of Primary Breast Tumors. Sci. Rep. 2022, 12, 4696. [Google Scholar] [CrossRef]
- Christoforou, P.; Christopoulos, P.F.; Koutsilieris, M. The Role of Estrogen Receptor β in Prostate Cancer. Mol. Med. 2014, 20, 427–434. [Google Scholar] [CrossRef]
- Lim, W.; Park, Y.; Cho, J.; Park, C.; Park, J.; Park, Y.-K.; Park, H.; Lee, Y. Estrogen Receptor Beta Inhibits Transcriptional Activity of Hypoxia Inducible Factor-1 through the Downregulation of Arylhydrocarbon Receptor Nuclear Translocator. Breast Cancer Res. 2011, 13, R32. [Google Scholar] [CrossRef]
- Mak, P.; Leav, I.; Pursell, B.; Bae, D.; Yang, X.; Taglienti, C.A.; Gouvin, L.M.; Sharma, V.M.; Mercurio, A.M. ERβ Impedes Prostate Cancer EMT by Destabilizing HIF-1α and Inhibiting VEGF-Mediated Snail Nuclear Localization: Implications for Gleason Grading. Cancer Cell 2010, 17, 319–332. [Google Scholar] [CrossRef]
- Bossard, C.; Busson, M.; Vindrieux, D.; Gaudin, F.; Machelon, V.; Brigitte, M.; Jacquard, C.; Pillon, A.; Balaguer, P.; Balabanian, K.; et al. Potential Role of Estrogen Receptor Beta as a Tumor Suppressor of Epithelial Ovarian Cancer. PLoS ONE 2012, 7, e44787. [Google Scholar] [CrossRef]
- Hartman, J.; Edvardsson, K.; Lindberg, K.; Zhao, C.; Williams, C.; Ström, A.; Gustafsson, J.-Å. Tumor Repressive Functions of Estrogen Receptor β in SW480 Colon Cancer Cells. Cancer Res. 2009, 69, 6100–6106. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Y.; Xu, Y.; Xu, L.; Zheng, W.; Wu, Y.; Li, L.; Shen, P. Estrogen Represses Hepatocellular Carcinoma (HCC) Growth via Inhibiting Alternative Activation of Tumor-Associated Macrophages (TAMs). J. Biol. Chem. 2012, 287, 40140–40149. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for Estrogen Receptor Expression in Human Cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.-K.; Lam, H.-M.; Wu, S.; Song, D.; Levin, L.; Cheng, L.; Wu, C.-L.; Ho, S.-M. Estrogen Receptor Β2 and Β5 Are Associated with Poor Prognosis in Prostate Cancer, and Promote Cancer Cell Migration and Invasion. Endocr.-Relat. Cancer 2010, 17, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.; Pentecost, E.; Martin, M.B. Effects of Arsenite on Estrogen Receptor-α Expression and Activity in MCF-7 Breast Cancer Cells. Endocrinology 2000, 141, 3595–3602. [Google Scholar] [CrossRef]
- Lappano, R.; Malaguarnera, R.; Belfiore, A.; Maggiolini, M. Recent Advances on the Stimulatory Effects of Metals in Breast Cancer. Mol. Cell. Endocrinol. 2017, 457, 49–56. [Google Scholar] [CrossRef]
- Freni-Titulaer, L.W.; Cordero, J.F.; Haddock, L.; Lebrón, G.; Martínez, R.; Mills, J.L. Premature Thelarche in Puerto Rico. A Search for Environmental Factors. Am. J. Dis. Child. 1986, 140, 1263–1267. [Google Scholar] [CrossRef]
- Durmaz, E.; Asci, A.; Erkekoglu, P.; Balcı, A.; Bircan, I.; Koçer-Gumusel, B. Urinary Bisphenol A Levels in Turkish Girls with Premature Thelarche. Hum. Exp. Toxicol. 2018, 37, 1007–1016. [Google Scholar] [CrossRef]
- Supornsilchai, V.; Jantarat, C.; Nosoognoen, W.; Pornkunwilai, S.; Wacharasindhu, S.; Soder, O. Increased Levels of Bisphenol A (BPA) in Thai Girls with Precocious Puberty. J. Pediatr. Endocrinol. Metab. 2016, 29, 1233–1239. [Google Scholar] [CrossRef]
- Kozak, J.; Jonak, K.; Maciejewski, R. The Function of miR-200 Family in Oxidative Stress Response Evoked in Cancer Chemotherapy and Radiotherapy. Biomed. Pharmacother. 2020, 125, 110037. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog 2006, 5, 14. [Google Scholar] [CrossRef]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Effects of Cadmium Chloride on Some Mitochondria-Related Activity and Gene Expression of Human MDA-MB231 Breast Tumor Cells. J. Inorg. Biochem. 2008, 102, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Casano, C.; Agnello, M.; Sirchia, R.; Luparello, C. Cadmium Effects on P38/MAPK Isoforms in MDA-MB231 Breast Cancer Cells. Biometals 2010, 23, 83–92. [Google Scholar] [CrossRef]
- Kozak, J.; Jonak, K. Association between the Antioxidant Properties of SESN Proteins and Anti-Cancer Therapies. Amino Acids 2023, 55, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, X.; Shaikh, Z.A. Rapid Activation of ERK1/2 and AKT in Human Breast Cancer Cells by Cadmium. Toxicol. Appl. Pharmacol. 2008, 228, 286–294. [Google Scholar] [CrossRef]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK Signalling: A Master Regulator of Cell Behaviour, Life and Fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.; Katzenellenbogen, B.S.; Martin, M.B. Activation of Estrogen Receptor-α by the Heavy Metal Cadmium. Mol. Endocrinol. 2000, 14, 545–553. [Google Scholar] [CrossRef]
- Xu, Y.; Tokar, E.J.; Waalkes, M.P. Arsenic-Induced Cancer Cell Phenotype in Human Breast Epithelia Is Estrogen Receptor-Independent but Involves Aromatase Activation. Arch. Toxicol. 2014, 88, 263–274. [Google Scholar] [CrossRef]
- Fadare, O.; Tavassoli, F.A. Clinical and Pathologic Aspects of Basal-like Breast Cancers. Nat. Rev. Clin. Oncol. 2008, 5, 149–159. [Google Scholar] [CrossRef]
- Du, J.; Zhou, N.; Liu, H.; Jiang, F.; Wang, Y.; Hu, C.; Qi, H.; Zhong, C.; Wang, X.; Li, Z. Arsenic Induces Functional Re-Expression of Estrogen Receptor α by Demethylation of DNA in Estrogen Receptor-Negative Human Breast Cancer. PLoS ONE 2012, 7, e35957. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Adams, S.V.; Quraishi, S.M.; Shafer, M.M.; Passarelli, M.N.; Freney, E.P.; Chlebowski, R.T.; Luo, J.; Meliker, J.R.; Mu, L.; Neuhouser, M.L.; et al. Dietary Cadmium Exposure and Risk of Breast, Endometrial, and Ovarian Cancer in the Women’s Health Initiative. Environ. Health Perspect 2014, 122, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Newcomb, P.A.; White, E. Dietary Cadmium and Risk of Invasive Postmenopausal Breast Cancer in the VITAL Cohort. Cancer Causes Control. 2012, 23, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.T.; Halkjær, J.; Sørensen, M.; Meliker, J.R.; McElroy, J.A.; Tjønneland, A.; Raaschou-Nielsen, O. Dietary Cadmium Intake and Risk of Breast, Endometrial and Ovarian Cancer in Danish Postmenopausal Women: A Prospective Cohort Study. PLoS ONE 2014, 9, e100815. [Google Scholar] [CrossRef]
- Sawada, N.; Iwasaki, M.; Inoue, M.; Takachi, R.; Sasazuki, S.; Yamaji, T.; Shimazu, T.; Endo, Y.; Tsugane, S. Long-Term Dietary Cadmium Intake and Cancer Incidence. Epidemiology 2012, 23, 368–376. [Google Scholar] [CrossRef]
- Itoh, H.; Iwasaki, M.; Sawada, N.; Takachi, R.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; Kusama, R.; Yokoyama, K.; et al. Dietary Cadmium Intake and Breast Cancer Risk in Japanese Women: A Case–Control Study. Int. J. Hyg. Environ. Health 2014, 217, 70–77. [Google Scholar] [CrossRef]
- Franjić, S. Ovarian Cancer Is the Deadliest of All Gynecological Tumors. Mathews J. Gynecol. Obstet. 2023, 7, 22. [Google Scholar] [CrossRef]
- Sawicka, E.; Saczko, J.; Kulbacka, J.; Szydełko, M.; Szymańska, B.; Piwowar, A. The Influence of Interaction between Cadmium with 17β-Estradiol, 2-Methoxyestradiol and 16α-Hydroxyestrone on Viability and p-Glycoprotein in Ovarian Cancer Cell Line. Int. J. Mol. Sci. 2022, 23, 2628. [Google Scholar] [CrossRef]
- García-Pérez, J.; Lope, V.; López-Abente, G.; González-Sánchez, M.; Fernández-Navarro, P. Ovarian Cancer Mortality and Industrial Pollution. Environ. Pollut. 2015, 205, 103–110. [Google Scholar] [CrossRef]
- Temkin, S.M.; Mallen, A.; Bellavance, E.; Rubinsak, L.; Wenham, R.M. The Role of Menopausal Hormone Therapy in Women with or at Risk of Ovarian and Breast Cancers: Misconceptions and Current Directions. Cancer 2019, 125, 499–514. [Google Scholar] [CrossRef]
- Adams, S.V.; Passarelli, M.N.; Newcomb, P.A. Cadmium Exposure and Cancer Mortality in the Third National Health and Nutrition Examination Survey Cohort. Occup. Environ. Med. 2012, 69, 153–156. [Google Scholar] [CrossRef]
- Julin, B.; Wolk, A.; Åkesson, A. Dietary Cadmium Exposure and Risk of Epithelial Ovarian Cancer in a Prospective Cohort of Swedish Women. Br. J. Cancer 2011, 105, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Canaz, E.; Kilinc, M.; Sayar, H.; Kiran, G.; Ozyurek, E. Lead, Selenium and Nickel Concentrations in Epithelial Ovarian Cancer, Borderline Ovarian Tumor and Healthy Ovarian Tissues. J. Trace Elem. Med. Biol. 2017, 43, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Parada-Bustamante, A.; Valencia, C.; Reuquen, P.; Diaz, P.; Rincion-Rodriguez, R.; Orihuela, P.A. Role of 2-Methoxyestradiol, an Endogenous Estrogen Metabolite, in Health and Disease. Mini-Rev. Med. Chem. 2015, 15, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Kurzer, M.S. Estrogen Metabolism and Breast Cancer. Cancer Lett. 2015, 356, 231–243. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Dai, F.; Zhang, L.; Yuan, M.; Yang, D.; Liu, S.; Cheng, Y. miR-21 Induces Chemoresistance in Ovarian Cancer Cells via Mediating the Expression and Interaction of CD44v6 and P-Gp. OncoTargets Ther. 2021, 14, 325–336. [Google Scholar] [CrossRef]
- Wieder-Huszla, S.; Chudecka-Głaz, A.; Cymbaluk-Płoska, A.; Karakiewicz, B.; Bosiacki, M.; Chlubek, D.; Jurczak, A. Evaluation of the Concentration of Selected Elements in Patients with Cancer of the Reproductive Organs with Respect to Treatment Stage—Preliminary Study. Nutrients 2022, 14, 2368. [Google Scholar] [CrossRef]
- Shih, I.-M.; Wang, Y.; Wang, T.-L. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am. J. Pathol. 2021, 191, 26–39. [Google Scholar] [CrossRef]
- Kozak, J.; Wdowiak, P.; Maciejewski, R.; Torres, A. Interactions between microRNA-200 Family and Sestrin Proteins in Endometrial Cancer Cell Lines and Their Significance to Anoikis. Mol. Cell Biochem. 2019, 459, 21–34. [Google Scholar] [CrossRef]
- Olarewaju, E.; Obeng-Gyasi, E. Cadmium, Lead, Chronic Physiological Stress and Endometrial Cancer: How Environmental Policy Can Alter the Exposure of At-Risk Women in the United States. Healthcare 2023, 11, 1278. [Google Scholar] [CrossRef]
- Skok, K.; Maver, U.; Gradišnik, L.; Kozar, N.; Takač, I.; Arko, D. Endometrial Cancer and Its Cell Lines. Mol. Biol. Rep. 2020, 47, 1399–1411. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Limbergen, E.V.; Vergote, I. Endometrial Cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Tempest, N.; Parkes, C.; Alnafakh, R.; Makrydima, S.; Adishesh, M.; Hapangama, D.K. Hormones and Endometrial Carcinogenesis. Horm. Mol. Biol. Clin. Investig. 2016, 25, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk Factors for Endometrial Cancer: An Umbrella Review of the Literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- McElroy, J.A.; Kruse, R.L.; Guthrie, J.; Gangnon, R.E.; Robertson, J.D. Cadmium Exposure and Endometrial Cancer Risk: A Large Midwestern U.S. Population-Based Case-Control Study. PLoS ONE 2017, 12, e0179360. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial Cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- McElroy, J.A.; Hunter, M.I. Cadmium: A New Risk Factor for Endometrial Cancer? Expert Rev. Anticancer. Ther. 2019, 19, 355–358. [Google Scholar] [CrossRef]
- Razumova, Z.; Govorov, I.; Östensson, E.; Mints, M. Cadmium Intake as a Prognostic Factor in Endometrial Cancer: A Swedish Cohort-Based Study. Nutr. Cancer 2022, 74, 175–184. [Google Scholar] [CrossRef]
- Furtak, G.; Kozłowski, M.; Kwiatkowski, S.; Cymbaluk-Płoska, A. The Role of Lead and Cadmium in Gynecological Malignancies. Antioxidants 2022, 11, 2468. [Google Scholar] [CrossRef]
- Michalczyk, K.; Kupnicka, P.; Witczak, G.; Tousty, P.; Bosiacki, M.; Kurzawski, M.; Chlubek, D.; Cymbaluk-Płoska, A. Assessment of Cadmium (Cd) and Lead (Pb) Blood Concentration on the Risk of Endometrial Cancer. Biology 2023, 12, 717. [Google Scholar] [CrossRef]
- Guyot, E.; Solovyova, Y.; Tomkiewicz, C.; Leblanc, A.; Pierre, S.; El Balkhi, S.; Le Frère-Belda, M.-A.; Lecuru, F.; Poupon, J.; Barouki, R.; et al. Determination of Heavy Metal Concentrations in Normal and Pathological Human Endometrial Biopsies and In Vitro Regulation of Gene Expression by Metals in the Ishikawa and Hec-1b Endometrial Cell Line. PLoS ONE 2015, 10, e0142590. [Google Scholar] [CrossRef]
- Wang, Y.; Mandal, A.K.; Son, Y.-O.; Pratheeshkumar, P.; Wise, J.T.F.; Wang, L.; Zhang, Z.; Shi, X.; Chen, Z. Roles of ROS, Nrf2, and Autophagy in Cadmium-Carcinogenesis and Its Prevention by Sulforaphane. Toxicol. Appl. Pharmacol. 2018, 353, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-O.; Wang, L.; Poyil, P.; Budhraja, A.; Hitron, J.A.; Zhang, Z.; Lee, J.-C.; Shi, X. Cadmium Induces Carcinogenesis in BEAS-2B Cells through ROS-Dependent Activation of PI3K/AKT/GSK-3β/β-Catenin Signaling. Toxicol. Appl. Pharmacol. 2012, 264, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical Cancer Epidemiology, Risk Factors and Screening. Chin. J. Cancer Res. 2020, 32, 720–728. [Google Scholar] [CrossRef]
- Zhang, J.; Nazeri, S.A.; Sohrabi, A. Lead (Pb) Exposure from Outdoor Air Pollution: A Potential Risk Factor for Cervical Intraepithelial Neoplasia Related to HPV Genotypes. Environ. Sci. Pollut. Res. 2022, 29, 26969–26976. [Google Scholar] [CrossRef] [PubMed]
- Motlhale, M.; Sitas, F.; de Villiers, C.B.; Simba, H.; Feliu, A.; Chen, W.C.; Schüz, J.; Muchengeti, M.; McCormack, V. Smokeless Tobacco (Snuff) and Site-Specific Cancer Risks in Adult Black South African Women: Findings from the Johannesburg Cancer Study. Int. J. Cancer 2025, 156, 1916–1925. [Google Scholar] [CrossRef]
- Yang, X.; Cai, M.; Li, N. Complete Remission of Vulvar Squamous Cell Carcinoma After Volumetric Modulated Arc Therapy in Copper Smelting and Purification Workers: A Case Report. Clin. Cosmet. Investig. Dermatol. 2023, 16, 185–192. [Google Scholar] [CrossRef]
- Kummu, M.; Sieppi, E.; Wallin, K.; Rautio, A.; Vähäkangas, K.; Myllynen, P. Cadmium Inhibits ABCG2 Transporter Function in BeWo Choriocarcinoma Cells and MCF-7 Cells Overexpressing ABCG2. Placenta 2012, 33, 859–865. [Google Scholar] [CrossRef]
- Ronco, A.M.; Llaguno, E.; Epuñan, M.J.; Llanos, M.N. Effect of Cadmium on Cortisol Production and 11β-Hydroxysteroid Dehydrogenase 2 Expression by Cultured Human Choriocarcinoma Cells (JEG-3). Toxicol. Vitr. 2010, 24, 1532–1537. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, J. The Role of Heavy Metals in the Biology of Female Cancers. Int. J. Mol. Sci. 2025, 26, 5155. https://doi.org/10.3390/ijms26115155
Kozak J. The Role of Heavy Metals in the Biology of Female Cancers. International Journal of Molecular Sciences. 2025; 26(11):5155. https://doi.org/10.3390/ijms26115155
Chicago/Turabian StyleKozak, Joanna. 2025. "The Role of Heavy Metals in the Biology of Female Cancers" International Journal of Molecular Sciences 26, no. 11: 5155. https://doi.org/10.3390/ijms26115155
APA StyleKozak, J. (2025). The Role of Heavy Metals in the Biology of Female Cancers. International Journal of Molecular Sciences, 26(11), 5155. https://doi.org/10.3390/ijms26115155







