Molecular Targets in Alveolar Rhabdomyosarcoma: A Narrative Review of Progress and Pitfalls
Abstract
1. Introduction
2. PAX3/7-FOXO1 Fusion Proteins as Master Regulators of ARMS Oncogenesis
3. Molecular Diagnostics and Biomarker Identification in Rhabdomyosarcoma
3.1. Diagnostic Implications of YAP Activation in Alveolar Rhabdomyosarcoma
3.2. The Central Role of PAX3-FOXO1 in ARMS Pathogenesis and Diagnostics
3.3. Myogenin in Rhabdomyosarcoma: A Diagnostic Marker and a Blocked Driver of Differentiation
3.4. TFAP2B as a Downstream Effector of PAX3-FOXO1 and Diagnostic Marker in FP-RMS
3.5. P-Cadherin as a Mediator of ARMS Aggressiveness
4. Transcriptional Repression of Differentiation Pathways in Fusion-Positive Rhabdomyosarcoma
4.1. PAX3-FOXO1-Mediated Repression of ACTA1 via the RhoA–MKL1–SRF Pathway
4.2. TBX2 as a Transcriptional Repressor in Rhabdomyosarcoma
4.3. Myogenin-Driven Targeted Gene Therapy in ARMS
5. The Role of Serine/Threonine-Protein Kinases in the Development and Treatment of ARMS
5.1. Targeting Aurora A (AurA) to Disrupt PAX3-FOXO1 Function and Enhance FP-RMS Therapy
5.2. CDK4 Amplification and—Targeting in FP-RMS
5.3. Polo-like Kinase 1 (PLK1) as a Therapeutic Target in ARMS
5.4. ERK Signaling as a Therapeutic Target in RMS
5.5. ATR Inhibition in ARMS: Exploiting DNA Damage Response Vulnerabilities
5.6. Targeting PAK4 in RMS: Inhibition of Oncogenic Signaling and Metastasis
6. Transcription Factors in Metabolic Reprogramming and Cancer
6.1. FOXF1 as a Therapeutic Target in Rhabdomyosarcoma
6.2. ETS1 as a Cooperative Transcriptional Effector in PAX3-FOXO1-Driven FP-RMS
6.3. Therapeutic Potential and Limitations of NF-κB Inhibition in Alveolar Rhabdomyosarcoma
6.4. The Role of SNAIL in Alveolar Rhabdomyosarcoma: A Key Regulator of Myogenic Blockade and Therapeutic Target
7. Receptor Tyrosine Kinases RTKs
7.1. Anlotinib as a Multi-Target Tyrosine Kinase Inhibitor in FP-RMS
7.2. FGFR Pathways as Therapeutic Targets in Fusion-Positive Rhabdomyosarcoma
7.3. PDGFR Signaling in ARMS: A Molecular Target with Context-Dependent Therapeutic Impact
7.4. MET Receptor in ARMS: A Key Regulator of Metastatic Behavior and Differentiation
7.5. Mechanisms of Resistance to RTK Inhibition in FP-RMS
7.6. TRIB3 in ARMS: A Promising Pseudokinase Target Amidst Therapeutic Challenges

8. Disarming ARMS: Cytokine Crosstalk, Autoantibodies, and Immune-Effector Engineering in Alveolar Rhabdomyosarcoma
8.1. Targeting Cytokine Signaling in ARMS: The Dual Role of IL-4R and IL-24
8.2. Autoantibody Signatures in ARMS: Diagnostic and Therapeutic Implications
8.3. Immunotherapy Strategies Targeting ERBB2 in Alveolar Rhabdomyosarcoma
9. Epigenetic Mechanisms in the Pathogenesis and Therapeutic Targeting of Alveolar Rhabdomyosarcoma
9.1. DNA Methylation in Rhabdomyosarcoma: Mechanistic Insights and Therapeutic Potential
9.2. Chromatin Remodeling and Epigenetic Reprogramming in Fusion-Positive Rhabdomyosarcoma
9.3. Epigenetic Regulation via Histone Modifications in Alveolar Rhabdomyosarcoma
9.3.1. BMI1 and Polycomb-Mediated Histone Modifications in ARMS
9.3.2. Regulation of Histone Lysine Methylation and Demethylation in ARMS
9.3.3. Regulation of Histone Acetylation in Fusion-Positive ARMS
9.4. RNA-Binding Protein as a Context-Dependent Therapeutic Target in ARMS
9.5. MicroRNAs in Alveolar Rhabdomyosarcoma: Functional Roles and Clinical Implications
10. Molecular Targets in ARMS—High Expectations, Limited Translation
10.1. IGF-1R Signaling in ARMS: A Historically Promising but Clinically Elusive Therapeutic Target
10.2. ALK Signaling in ARMS: A Promising Target Undermined by Therapeutic Inconsistency
10.3. EphB4 Signaling in ARMS: A Dual-Edged Target with Therapeutic Ambiguity
10.4. Futibatinib: A Promising FGFR Inhibitor That Falls Short in ARMS
10.5. EGFR Signaling in RMS: High Hopes, Low Yields
10.6. SFK–CRKL–YES Axis in RMS: A Therapeutic Prospect with Clinical Complexities
10.7. FAK–Src Signaling in ARMS: Synergy with Limits
10.8. AKT Signaling in ARMS: A Double-Edged Sword
10.9. mTOR Signaling in ARMS: Therapeutic Promises and Limitations
10.10. NF-Y in ARMS: An Indispensable Oncogenic Driver with Unavoidable Pitfalls
10.11. PODXL in FP-RMS: Promising Yet Risk-Prone
10.12. Lessons from Failed Monotherapies: Implications for Rational Combination Strategies
11. Emerging Tools for Target Discovery and Resistance Monitoring: Liquid Biopsy and CRISPR Screens
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ARMS | Alveolar Rhabdomyosarcoma |
| CAR T | Chimeric Antigen Receptor T-Cells |
| CR TFs | Core Regulatory Transcription Factors |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| ERMS | Embryonal Rhabdomyosarcoma |
| FN-RMS | Fusion-Negative Rhabdomyosarcoma |
| FP-RMS | Fusion-Positive Rhabdomyosarcoma |
| HSMM | Human Skeletal Muscle Myoblasts |
| IAPs | Inhibitor of Apoptosis Proteins |
| IHC | Immunohistochemistry |
| IVA | Ifosfamide, Vincristine, Actinomycin D |
| MTD | Maximum Tolerated Dose |
| PK | Pharmacokinetics |
| PPI | Protein–Protein Interaction |
| PRMS | Pleomorphic Rhabdomyosarcoma |
| RMS | Rhabdomyosarcoma |
| RP2D | Recommended Phase 2 Dose |
| SD | Stable Disease |
| SE | Super-Enhancer |
| SRMS | Spindle Cell/Sclerosing Rhabdomyosarcoma |
| VAC | Vincristine, Actinomycin D, Cyclophosphamide |
| sdAb | Single-Domain Antibody |
| siRNA | Small Interfering RNA |
References
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Recent Advances and Challenges in the Treatment of Rhabdomyosarcoma. Cancers 2020, 12, 1758. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.A.S.; Bisogno, G.; Koscielniak, E. Fifty Years of Rhabdomyosarcoma Studies on Both Sides of the Pond and Lessons Learned. Cancer Treat. Rev. 2018, 68, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.B.; Hicks, M.J.; Roy, A.; Vasudevan, S.A.; Reddy, K.; Venkatramani, R. Congenital Spindle Cell Rhabdomyosarcoma. Pediatr. Blood Cancer 2019, 66, e27935. [Google Scholar] [CrossRef]
- Fletcher, C.D.M.; Bridge, J.; Hogendoorn, P.C.W.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2013; ISBN 978-92-832-2434-1. [Google Scholar]
- Leiner, J.; Le Loarer, F. The Current Landscape of Rhabdomyosarcomas: An Update. Virchows Arch. 2020, 476, 97–108. [Google Scholar] [CrossRef]
- Amer, K.M.; Thomson, J.E.; Congiusta, D.; Dobitsch, A.; Chaudhry, A.; Li, M.; Chaudhry, A.; Bozzo, A.; Siracuse, B.; Aytekin, M.N.; et al. Epidemiology, Incidence, and Survival of Rhabdomyosarcoma Subtypes: SEER and ICES Database Analysis. J. Orthop. Res. 2019, 37, 2226–2230. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Barr, F.G. Therapeutic Approaches Targeting PAX3-FOXO1 and Its Regulatory and Transcriptional Pathways in Rhabdomyosarcoma. Molecules 2018, 23, 2798. [Google Scholar] [CrossRef]
- Jo, V.Y.; Fletcher, C.D.M. WHO Classification of Soft Tissue Tumours: An Update Based on the 2013 (4th) Edition. Pathology 2014, 46, 95–104. [Google Scholar] [CrossRef]
- Ognjanovic, S.; Linabery, A.M.; Charbonneau, B.; Ross, J.A. Trends in Childhood Rhabdomyosarcoma Incidence and Survival in the United States, 1975–2005. Cancer 2009, 115, 4218–4226. [Google Scholar] [CrossRef]
- Dziuba, I.; Kurzawa, P.; Dopierała, M.; Larque, A.B.; Januszkiewicz-Lewandowska, D. Rhabdomyosarcoma in Children—Current Pathologic and Molecular Classification. Pol. J. Pathol. 2018, 69, 20–32. [Google Scholar] [CrossRef]
- El Demellawy, D.; McGowan-Jordan, J.; de Nanassy, J.; Chernetsova, E.; Nasr, A. Update on Molecular Findings in Rhabdomyosarcoma. Pathology 2017, 49, 238–246. [Google Scholar] [CrossRef]
- Parham, D.M.; Barr, F.G. Classification of Rhabdomyosarcoma and Its Molecular Basis. Adv. Anat. Pathol. 2013, 20, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Pruller, J.; Hofer, I.; Ganassi, M.; Heher, P.; Ma, M.T.; Zammit, P.S. A Human Myogenin Promoter Modified to Be Highly Active in Alveolar Rhabdomyosarcoma Drives an Effective Suicide Gene Therapy. Cancer Gene Ther. 2021, 28, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dorado Garcia, H.; Scheer, M.; Henssen, A.G. Current and Future Treatment Strategies for Rhabdomyosarcoma. Front. Oncol. 2019, 9, 1458. [Google Scholar] [CrossRef]
- Dantonello, T.M.; Int-Veen, C.; Schuck, A.; Seitz, G.; Leuschner, I.; Nathrath, M.; Schlegel, P.G.; Kontny, U.; Behnisch, W.; Veit-Friedrich, I.; et al. Survival Following Disease Recurrence of Primary Localized Alveolar Rhabdomyosarcoma. Pediatr. Blood Cancer 2013, 60, 1267–1273. [Google Scholar] [CrossRef]
- Malempati, S.; Hawkins, D.S. Rhabdomyosarcoma: Review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee Experience and Rationale for Current COG Studies. Pediatr. Blood Cancer 2012, 59, 5–10. [Google Scholar] [CrossRef]
- Schöffski, P.; Wozniak, A.; Leahy, M.G.; Aamdal, S.; Rutkowski, P.; Bauer, S.; Richter, S.; Grünwald, V.; Debiec-Rychter, M.; Sciot, R.; et al. The Tyrosine Kinase Inhibitor Crizotinib Does Not Have Clinically Meaningful Activity in Heavily Pre-Treated Patients with Advanced Alveolar Rhabdomyosarcoma with FOXO Rearrangement: European Organisation for Research and Treatment of Cancer Phase 2 Trial. Eur. J. Cancer 2018, 94, 156–167. [Google Scholar] [CrossRef]
- Olanich, M.E.; Barr, F.G. A Call to ARMS: Targeting the PAX3-FOXO1 Gene in Alveolar Rhabdomyosarcoma. Expert. Opin. Ther. Targets 2013, 17, 607–623. [Google Scholar] [CrossRef]
- Williamson, D.; Missiaglia, E.; De Reyniès, A.; Pierron, G.; Thuille, B.; Palenzuela, G.; Thway, K.; Orbach, D.; Laé, M.; Fréneaux, P.; et al. Fusion Gene-Negative Alveolar Rhabdomyosarcoma Is Clinically and Molecularly Indistinguishable from Embryonal Rhabdomyosarcoma. J. Clin. Oncol. 2010, 28, 2151–2158. [Google Scholar] [CrossRef]
- Kubo, T.; Shimose, S.; Fujimori, J.; Furuta, T.; Ochi, M. Prognostic Value of PAX3/7-FOXO1 Fusion Status in Alveolar Rhabdomyosarcoma: Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2015, 96, 46–53. [Google Scholar] [CrossRef]
- Slater, O.; Shipley, J. Clinical Relevance of Molecular Genetics to Paediatric Sarcomas. J. Clin. Pathol. 2007, 60, 1187–1194. [Google Scholar] [CrossRef]
- Olanich, M.E.; Sun, W.; Hewitt, S.M.; Abdullaev, Z.; Pack, S.D.; Barr, F.G. CDK4 Amplification Reduces Sensitivity to CDK4/6 Inhibition in Fusion-Positive Rhabdomyosarcoma. Clin. Cancer Res. 2015, 21, 4947–4959. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Martínez, J.; Rello-Varona, S.; Herrero-Martín, D.; Barrau, I.; García-Monclús, S.; Sáinz-Jaspeado, M.; Lagares-Tena, L.; Núñez-Álvarez, Y.; Mateo-Lozano, S.; Mora, J.; et al. Caveolin-1 Is down-Regulated in Alveolar Rhabdomyosarcomas and Negatively Regulates Tumor Growth. Oncotarget 2014, 5, 9744–9755. [Google Scholar] [CrossRef] [PubMed]
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive Genomic Analysis of Rhabdomyosarcoma Reveals a Landscape of Alterations Affecting a Common Genetic Axis in Fusion-Positive and Fusion-Negative Tumors. Cancer Discov. 2014, 4, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Sumegi, J.; Streblow, R.; Frayer, R.W.; Cin, P.D.; Rosenberg, A.; Meloni-Ehrig, A.; Bridge, J.A. Recurrent t(2;2) and t(2;8) Translocations in Rhabdomyosarcoma without the Canonical PAX-FOXO1 Fuse PAX3 to Members of the Nuclear Receptor Transcriptional Coactivator Family. Genes Chromosomes Cancer 2010, 49, 224–236. [Google Scholar] [CrossRef]
- Barr, F.G.; Qualman, S.J.; Macris, M.H.; Melnyk, N.; Lawlor, E.R.; Strzelecki, D.M.; Triche, T.J.; Bridge, J.A.; Sorensen, P.H.B. Genetic Heterogeneity in the Alveolar Rhabdomyosarcoma Subset without Typical Gene Fusions. Cancer Res. 2002, 62, 4704–4710. [Google Scholar]
- Davis, R.J.; Barr, F.G. Fusion Genes Resulting from Alternative Chromosomal Translocations Are Overexpressed by Gene-Specific Mechanisms in Alveolar Rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 1997, 94, 8047–8051. [Google Scholar] [CrossRef]
- Wachtel, M.; Schäfer, B.W. PAX3-FOXO1: Zooming in on an “Undruggable” Target. Semin. Cancer Biol. 2018, 50, 115–123. [Google Scholar] [CrossRef]
- Marshall, A.D.; Grosveld, G.C. Alveolar Rhabdomyosarcoma—The Molecular Drivers of PAX3/7-FOXO1-Induced Tumorigenesis. Skelet. Muscle 2012, 2, 25. [Google Scholar] [CrossRef]
- Ahn, E.H. Regulation of Target Genes of PAX3-FOXO1 in Alveolar Rhabdomyosarcoma. Anticancer. Res. 2013, 33, 2029–2035. [Google Scholar]
- Linardic, C.M. PAX3-FOXO1 Fusion Gene in Rhabdomyosarcoma. Cancer Lett. 2008, 270, 10. [Google Scholar] [CrossRef]
- De Giovanni, C.; Landuzzi, L.; Nicoletti, G.; Lollini, P.L.; Nanni, P. Molecular and Cellular Biology of Rhabdomyosarcoma. Future Oncol. 2009, 5, 1449–1475. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Arenkiel, B.R.; Coffin, C.M.; El-Bardeesy, N.; DePinho, R.A.; Capecchi, M.R. Alveolar Rhabdomyosarcomas in Conditional Pax3:Fkhr Mice: Cooperativity of Ink4a/ARF and Trp53 Loss of Function. Genes. Dev. 2004, 18, 2614. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Takita, J.; Mizuguchi, M.; Tanaka, K.; Ida, K.; Koh, K.; Igarashi, T.; Hanada, R.; Tanaka, Y.; Park, M.J.; et al. Mutation and Expression Analyses of the MET and CDKN2A in Rhabdomyosarcoma with Emphasis on MET Overexpression. Genes Chromosomes Cancer 2007, 46, 348–358. [Google Scholar] [CrossRef]
- Linardic, C.M.; Naini, S.; Herndon, J.E.; Kesserwan, C.; Qualman, S.J.; Counter, C.M. The PAX3-FKHR Fusion Gene of Rhabdomyosarcoma Cooperates with Loss of P16INK4A to Promote Bypass of Cellular Senescence. Cancer Res. 2007, 67, 6691–6699. [Google Scholar] [CrossRef]
- Kendall, G.C.; Watson, S.; Xu, L.; Lavigne, C.A.; Murchison, W.; Rakheja, D.; Skapek, S.X.; Tirode, F.; Delattre, O.; Amatruda, J.F. PAX3-FOXO1 Transgenic Zebrafish Models Identify HES3 as a Mediator of Rhabdomyosarcoma Tumorigenesis. Elife 2018, 7, e33800. [Google Scholar] [CrossRef]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers 2019, 5, 1. [Google Scholar] [CrossRef]
- Ren, Y.-X.; Finckenstein, F.G.; Abdueva, D.A.; Shahbazian, V.; Chung, B.; Weinberg, K.I.; Triche, T.J.; Shimada, H.; Anderson, M.J. Mouse Mesenchymal Stem Cells Expressing PAX-FKHR Form Alveolar Rhabdomyosarcomas by Cooperating with Secondary Mutations. Cancer Res. 2008, 68, 6587–6597. [Google Scholar] [CrossRef]
- Gryder, B.E.; Yohe, M.E.; Chou, H.C.; Zhang, X.; Marques, J.; Wachtel, M.; Schaefer, B.; Sen, N.; Song, Y.; Gualtieri, A.; et al. PAX3-FOXO1 Establishes Myogenic Super Enhancers and Confers BET Bromodomain Vulnerability. Cancer Discov. 2017, 7, 884–899. [Google Scholar] [CrossRef]
- Asante, Y.; Benischke, K.; Osman, I.; Ngo, Q.A.; Wurth, J.; Laubscher, D.; Kim, H.; Udhayakumar, B.; Khan, M.I.H.; Chin, D.H.; et al. PAX3-FOXO1 Uses Its Activation Domain to Recruit CBP/P300 and Shape RNA Pol2 Cluster Distribution. Nat. Commun. 2023, 14, 8361. [Google Scholar] [CrossRef]
- Linardic, C.M.; Crose, L.E.S. Receptor Tyrosine Kinases as Therapeutic Targets in Rhabdomyosarcoma. Sarcoma 2011, 2011, 756982. [Google Scholar] [CrossRef]
- Bernasconi, M.; Remppis, A.; Fredericks, W.J.; Rauscher, F.J.; Schäfer, B.W. Induction of Apoptosis in Rhabdomyosarcoma Cells through Down-Regulation of PAX Proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 13164–13169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Yoon, H.M.; Koh, K.N.; Jung, A.Y.; Cho, Y.A.; Lee, J.S. Rhabdomyosarcoma in Children and Adolescents: Patterns and Risk Factors of Distant Metastasis. AJR Am. J. Roentgenol. 2017, 209, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, X.Y.; Guo, P.; Wang, D.L. MYBPC2 and MYL1 as Significant Gene Markers for Rhabdomyosarcoma. Technol. Cancer Res. Treat. 2021, 20, 1533033820979669. [Google Scholar] [CrossRef]
- Crose, L.E.S.; Galindo, K.A.; Kephart, J.G.; Chen, C.; Fitamant, J.; Bardeesy, N.; Bentley, R.C.; Galindo, R.L.; Ashley Chi, J.T.; Linardic, C.M. Alveolar Rhabdomyosarcoma-Associated PAX3-FOXO1 Promotes Tumorigenesis via Hippo Pathway Suppression. J. Clin. Investig. 2014, 124, 285–296. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Habeebu, S.S.; Sherman, A.K.; Ye, S.Q.; Wood, N.; Chastain, K.M.; Tsokos, M.G. Potential Value of YAP Staining in Rhabdomyosarcoma. J. Histochem. Cytochem. 2018, 66, 577–584. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo Pathway Regulation. Genes. Dev. 2016, 30, 1. [Google Scholar] [CrossRef]
- Azorsa, D.O.; Bode, P.K.; Wachtel, M.; Cheuk, A.T.C.; Meltzer, P.S.; Vokuhl, C.; Camenisch, U.; Khov, H.L.; Bode, B.; Schäfer, B.W.; et al. Immunohistochemical Detection of PAX-FOXO1 Fusion Proteins in Alveolar Rhabdomyosarcoma Using Breakpoint Specific Monoclonal Antibodies. Mod. Pathol. 2020, 34, 748–757. [Google Scholar] [CrossRef]
- Kikuchi, K.; Tsuchiya, K.; Otabe, O.; Gotoh, T.; Tamura, S.; Katsumi, Y.; Yagyu, S.; Tsubai-Shimizu, S.; Miyachi, M.; Iehara, T.; et al. Effects of PAX3-FKHR on Malignant Phenotypes in Alveolar Rhabdomyosarcoma. Biochem. Biophys. Res. Commun. 2008, 365, 568–574. [Google Scholar] [CrossRef]
- Nakazawa, K.; Shaw, T.; Song, Y.K.; Kouassi-Brou, M.; Molotkova, A.; Tiwari, P.B.; Chou, H.C.; Wen, X.; Wei, J.S.; Deniz, E.; et al. Piperacetazine Directly Binds to the PAX3::FOXO1 Fusion Protein and Inhibits Its Transcriptional Activity. Cancer Res. Commun. 2023, 3, 2030–2043. [Google Scholar] [CrossRef]
- Boudjadi, S.; Pandey, P.R.; Chatterjee, B.; Nguyen, T.H.; Sun, W.; Barr, F.G. A Fusion Transcription Factor⇓driven Cancer Progresses to a Fusion-Independent Relapse via Constitutive Activation of a Downstream Transcriptional Target. Cancer Res. 2021, 81, 2930–2942. [Google Scholar] [CrossRef]
- Fumoto, Y.; Takada, S.; Onodera, Y.; Hatakeyama, S.; Oikawa, T. Development of a Myogenin Minimal Promoter-Based System for Visualizing the Degree of Myogenic Differentiation. Biochem. Biophys. Res. Commun. 2024, 741, 151091. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Perlman, E.; Harris, C.A.; Raffeld, M.; Tsokos, M. Myogenin Is a Specific Marker for Rhabdomyosarcoma: An Immunohistochemical Study in Paraffin-Embedded Tissues. Mod. Pathol. 2000, 13, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Dionyssiou, M.G.; Ehyai, S.; Avrutin, E.; Connor, M.K.; McDermott, J.C. Glycogen Synthase Kinase 3β Represses MYOGENIN Function in Alveolar Rhabdomyosarcoma. Cell Death Dis. 2014, 5, e1094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, C.; Luo, Y.; Liang, Z.; Li, X.; Kołat, D.; Zhao, L.; Xiong, W. Crucial Role of the Transcription Factors Family Activator Protein 2 in Cancer: Current Clue and Views. J. Transl. Med. 2023, 21, 371. [Google Scholar] [CrossRef]
- Raap, M.; Gierendt, L.; Kreipe, H.H.; Christgen, M. Transcription Factor AP-2beta in Development, Differentiation and Tumorigenesis. Int. J. Cancer 2021, 149, 1221–1227. [Google Scholar] [CrossRef]
- Grass, B.; Wachtel, M.; Behnke, S.; Leuschner, I.; Niggli, F.K.; Schäfer, B.W. Immunohistochemical Detection of EGFR, Fibrillin-2, P-Cadherin and AP2beta as Biomarkers for Rhabdomyosarcoma Diagnostics. Histopathology 2009, 54, 873–879. [Google Scholar] [CrossRef]
- Wachtel, M.; Runge, T.; Leuschner, I.; Stegmaier, S.; Koscielniak, E.; Treuner, J.; Odermatt, B.; Behnke, S.; Niggli, F.K.; Schäfer, B.W. Subtype and Prognostic Classification of Rhabdomyosarcoma by Immunohistochemistry. J. Clin. Oncol. 2006, 24, 816–822. [Google Scholar] [CrossRef]
- Vieira, A.F.; Paredes, J. P-Cadherin and the Journey to Cancer Metastasis. Mol. Cancer 2015, 14, 178. [Google Scholar] [CrossRef]
- Thuault, S.; Hayashi, S.; Lagirand-Cantaloube, J.; Plutoni, C.; Comunale, F.; Delattre, O.; Relaix, F.; Gauthier-Rouvière, C. P-Cadherin Is a Direct PAX3-FOXO1A Target Involved in Alveolar Rhabdomyosarcoma Aggressiveness. Oncogene 2013, 32, 1876–1887. [Google Scholar] [CrossRef]
- Clayton, J.S.; Johari, M.; Taylor, R.L.; Dofash, L.; Allan, G.; Monahan, G.; Houweling, P.J.; Ravenscroft, G.; Laing, N.G. An Update on Reported Variants in the Skeletal Muscle α-Actin (ACTA1) Gene. Hum. Mutat. 2024, 2024, 6496088. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, L.; Li, Y.; Zhou, J.; Xu, J. ACTA1 Is Inhibited by PAX3-FOXO1 through RhoA-MKL1-SRF Signaling Pathway and Impairs Cell Proliferation, Migration and Tumor Growth in Alveolar Rhabdomyosarcoma. Cell Biosci. 2021, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Eckenstaler, R.; Hauke, M.; Benndorf, R.A. A Current Overview of RhoA, RhoB, and RhoC Functions in Vascular Biology and Pathology. Biochem. Pharmacol. 2022, 206, 115321. [Google Scholar] [CrossRef] [PubMed]
- Cen, B.; Selvaraj, A.; Burgess, R.C.; Hitzler, J.K.; Ma, Z.; Morris, S.W.; Prywes, R. Megakaryoblastic Leukemia 1, a Potent Transcriptional Coactivator for Serum Response Factor (SRF), Is Required for Serum Induction of SRF Target Genes. Mol. Cell Biol. 2003, 23, 6597–6608. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.A.; Lamon, S.; Russell, A.P. The Regulation and Function of the Striated Muscle Activator of Rho Signaling (STARS) Protein. Front. Physiol. 2012, 3, 469. [Google Scholar] [CrossRef]
- Huang, F.Y.; Li, Y.N.; Mei, W.L.; Dai, H.F.; Zhou, P.; Tan, G.H. Cytochalasin D, a Tropical Fungal Metabolite, Inhibits CT26 Tumor Growth and Angiogenesis. Asian Pac. J. Trop. Med. 2012, 5, 169–174. [Google Scholar] [CrossRef]
- Evelyn, C.R.; Wade, S.M.; Wang, Q.; Wu, M.; Iñiguez-Lluhí, J.A.; Merajver, S.D.; Neubig, R.R. CCG-1423: A Small-Molecule Inhibitor of RhoA Transcriptional Signaling. Mol. Cancer Ther. 2007, 6, 2249–2260. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.P.; Dong, Q.; Kung, H.F.; He, M.L. TBX2 and TBX3: The Special Value for Anticancer Drug Targets. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2010, 1806, 268–274. [Google Scholar] [CrossRef]
- Schiltz, R.L.; Nakatani, Y. The PCAF Acetylase Complex as a Potential Tumor Suppressor. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2000, 1470, M37–M53. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, M.; Byrum, S.D.; Tackett, A.J.; Davie, J.K. TBX2 Blocks Myogenesis and Promotes Proliferation in Rhabdomyosarcoma Cells. Int. J. Cancer J. Int. Du Cancer 2014, 135, 785–797. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Ommer, J.; Selfe, J.L.; Wachtel, M.; O’Brien, E.M.; Laubscher, D.; Roemmele, M.; Kasper, S.; Delattre, O.; Surdez, D.; Petts, G.; et al. Aurora A Kinase Inhibition Destabilizes PAX3-FOXO1 and MYCN and Synergizes with Navitoclax to Induce Rhabdomyosarcoma Cell Death. Cancer Res. 2020, 80, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Dedobbeleer, M.; Digregorio, M.; Lombard, A.; Lumapat, P.N.; Rogister, B. The Functional Diversity of Aurora Kinases: A Comprehensive Review. Cell Div. 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Barr, F.G.; Duan, F.; Smith, L.M.; Gustafson, D.; Pitts, M.; Hammond, S.; Gastier-Foster, J.M. Genomic and Clinical Analyses of 2p24 and 12q13-Q14 Amplification in Alveolar Rhabdomyosarcoma: A Report from the Children’s Oncology Group. Genes Chromosomes Cancer 2009, 48, 661–672. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Do, I.G.; Jang, J.; Rho, K.; Ahn, S.; Maruja, L.; Kim, S.J.; Kim, K.M.; Mao, M.; et al. Aberrant CDK4 Amplification in Refractory Rhabdomyosarcoma as Identified by Genomic Profiling. Sci. Rep. 2014, 4, 3623. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Sicinska, E.; Czaplinski, J.T.; Remillard, S.P.; Moss, S.; Wang, Y.; Brain, C.; Loo, A.; Snyder, E.L.; Demetri, G.D.; et al. Antiproliferative Effects of CDK4/6 Inhibition in CDK4-Amplified Human Liposarcoma in Vitro and in Vivo. Mol. Cancer Ther. 2014, 13, 2184–2193. [Google Scholar] [CrossRef]
- Rader, J.; Russell, M.R.; Hart, L.S.; Nakazawa, M.S.; Belcastro, L.T.; Martinez, D.; Li, Y.; Carpenter, E.L.; Attiyeh, E.F.; Diskin, S.J.; et al. Dual CDK4/CDK6 Inhibition Induces Cell-Cycle Arrest and Senescence in Neuroblastoma. Clin. Cancer Res. 2013, 19, 6173–6182. [Google Scholar] [CrossRef]
- Tripathy, D.; Bardia, A.; Sellers, W.R. Ribociclib (LEE011): Mechanism of Action and Clinical Impact of This Selective Cyclin-Dependent Kinase 4/6 Inhibitor in Various Solid Tumors. Clin. Cancer Res. 2017, 23, 3251–3262. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Ong, S.S.; Chen, T. Cyclin-Dependent Kinase 4 Phosphorylates and Positively Regulates PAX3-FOXO1 in Human Alveolar Rhabdomyosarcoma Cells. PLoS ONE 2013, 8, e58193. [Google Scholar] [CrossRef]
- Geoerger, B.; Bourdeaut, F.; DuBois, S.G.; Fischer, M.; Geller, J.I.; Gottardo, N.G.; Marabelle, A.; Pearson, A.D.J.; Modak, S.; Cash, T.; et al. A Phase I Study of the CDK4/6 Inhibitor Ribociclib (LEE011) in Pediatric Patients with Malignant Rhabdoid Tumors, Neuroblastoma, and Other Solid Tumors. Clin. Cancer Res. 2017, 23, 2433–2441. [Google Scholar] [CrossRef]
- Study of Efficacy and Safety of Ribociclib (LEE011) in Combination with Topotecan and Temozolomide (TOTEM) in Pediatric Patients with Relapsed or Refractory Neuroblastoma and Other Solid Tumors. Available online: https://clinicaltrials.gov/study/NCT05429502 (accessed on 15 May 2025).
- Gatz, S.A.; Aladowicz, E.; Casanova, M.; Chisholm, J.C.; Kearns, P.R.; Fulda, S.; Geoerger, B.; Schäfer, B.W.; Shipley, J.M. A Perspective on Polo-Like Kinase-1 Inhibition for the Treatment of Rhabdomyosarcomas. Front. Oncol. 2019, 9, 1271. [Google Scholar] [CrossRef]
- Hu, K.; Lee, C.; Qiu, D.; Fotovati, A.; Davies, A.; Abu-Ali, S.; Wai, D.; Lawlor, E.R.; Triche, T.J.; Pallen, C.J.; et al. Small Interfering RNA Library Screen of Human Kinases and Phosphatases Identifies Polo-like Kinase 1 as a Promising New Target for the Treatment of Pediatric Rhabdomyosarcomas. Mol. Cancer Ther. 2009, 8, 3024–3035. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.M.; Wang, L.H.; Song, Y.M.; Ou, Y.W.; Jiang, J.; Fan, J.; Wang, J.B.; Shen, J. Esophageal Carcinoma. In Recent Advances in Cancer Research and Therapy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 493–534. ISBN 9780123978332. [Google Scholar]
- Ashwell, S. Checkpoint Kinase and Wee1 Inhibitors as Anticancer Therapeutics. In DNA Repair in Cancer Therapy; Academic Press: Cambridge, MA, USA, 2012; pp. 211–234. ISBN 9780123849991. [Google Scholar]
- Thalhammer, V.; Lopez-Garcia, L.A.; Herrero-Martin, D.; Hecker, R.; Laubscher, D.; Gierisch, M.E.; Wachtel, M.; Bode, P.; Nanni, P.; Blank, B.; et al. PLK1 Phosphorylates PAX3-FOXO1, the Inhibition of Which Triggers Regression of Alveolar Rhabdomyosarcoma. Cancer Res. 2015, 75, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Stehle, A.; Hugle, M.; Fulda, S. Eribulin synergizes with Polo-like kinase 1 inhibitors to induce apoptosis in rhabdomyosarcoma. Cancer Lett. 2015, 365, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. ERK1/2 MAP Kinases: Structure, Function, and Regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef]
- Otabe, O.; Kikuchi, K.; Tsuchiya, K.; Katsumi, Y.; Yagyu, S.; Miyachi, M.; Iehara, T.; Hosoi, H. MET/ERK2 Pathway Regulates the Motility of Human Alveolar Rhabdomyosarcoma Cells. Oncol. Rep. 2017, 37, 98–104. [Google Scholar] [CrossRef]
- Lebedev, T.D.; Vagapova, E.R.; Prassolov, V.S. The Different Impact of ERK Inhibition on Neuroblastoma, Astrocytoma, and Rhabdomyosarcoma Cell Differentiation. Acta Naturae 2021, 13, 69–77. [Google Scholar] [CrossRef]
- Garcia, N.; Del Pozo, V.; Yohe, M.E.; Goodwin, C.M.; Shackleford, T.J.; Wang, L.; Baxi, K.; Chen, Y.; Rogojina, A.T.; Zimmerman, S.M.; et al. Vertical Inhibition of the RAF–MEK–ERK Cascade Induces Myogenic Differentiation, Apoptosis, and Tumor Regression in H/NRASQ61X Mutant Rhabdomyosarcoma. Mol. Cancer Ther. 2022, 21, 170–183. [Google Scholar] [CrossRef]
- Winkler, M.; Friedrich, J.; Boedicker, C.; Dolgikh, N. Co-Targeting MCL-1 and ERK1/2 Kinase Induces Mitochondrial Apoptosis in Rhabdomyosarcoma Cells. Transl. Oncol. 2022, 16, 101313. [Google Scholar] [CrossRef]
- Rundle, S.; Bradbury, A.; Drew, Y.; Curtin, N.J. Targeting the ATR-CHK1 Axis in Cancer Therapy. Cancers 2017, 9, 41. [Google Scholar] [CrossRef]
- Dorado García, H.; Pusch, F.; Bei, Y.; von Stebut, J.; Ibáñez, G.; Guillan, K.; Imami, K.; Gürgen, D.; Rolff, J.; Helmsauer, K.; et al. Therapeutic Targeting of ATR in Alveolar Rhabdomyosarcoma. Nat. Commun. 2022, 13, 4297. [Google Scholar] [CrossRef]
- Wu, M.; Chen, X.; Wang, H.; Li, C.; Liu, W.; Zheng, X.; Yang, J.; Ye, X.; Weng, Y.; Fan, T.; et al. Discovery of the Clinical Candidate YY2201 as a Highly Potent and Selective ATR Inhibitor. J. Med. Chem. 2025, 68, 5292–5311. [Google Scholar] [CrossRef] [PubMed]
- A Study of the Drugs Prexasertib, Irinotecan, and Temozolomide in People with Desmoplastic Small Round Cell Tumor and Rhabdomyosarcoma. Available online: https://clinicaltrials.gov/study/NCT04095221 (accessed on 10 May 2025).
- Mei, L.; Zhang, J.; He, K.; Zhang, J. Ataxia Telangiectasia and Rad3-Related Inhibitors and Cancer Therapy: Where We Stand. J. Hematol. Oncol. 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Wengner, A.M.; Siemeister, G.; Lucking, U.; Lefranc, J.; Wortmann, L.; Lienau, P.; Bader, B.; Bomer, U.; Moosmayer, D.; Eberspacher, U.; et al. The Novel ATR Inhibitor BAY 1895344 Is Efficacious as Monotherapy and Combined with DNA Damage-Inducing or Repair-Compromising Therapies in Preclinical Cancer Models. Mol. Cancer Ther. 2020, 19, 26–38. [Google Scholar] [CrossRef]
- Yap, T.A.; Tan, D.S.P.; Terbuch, A.; Caldwell, R.; Guo, C.; Goh, B.C.; Heong, V.; Noor, N.R.; Bashir, S.; Drew, Y.; et al. First-in-Human Trial of the Oral Ataxia Telangiectasia and RAD3-Related (ATR) Inhibitor BAY 1895344 in Patients with Advanced Solid Tumors. Cancer Discov. 2021, 11, 80–91. [Google Scholar] [CrossRef]
- Ortiz, M.V.; Glade Bender, J.L.; Minard, C.G.; Liu, X.; Denic, K.Z.; Reid, J.M.; Militano, O.; Church, A.J.; Kentsis, A.; Reynolds, C.P.; et al. A Phase 1/2 Study of Bay 18953444 (Elimusertib) in Pediatric Patients with Relapsed or Refractory Solid Tumors: Initial Report of the Phase 1 Results of PEPN2112. J. Clin. Oncol. 2023, 41, e15131. [Google Scholar] [CrossRef]
- Dasgupta, A.; Sierra, L.; Tsang, S.V.; Kurenbekova, L.; Patel, T.; Rajapakse, K.; Shuck, R.L.; Rainusso, N.; Landesman, Y.; Unger, T.; et al. Targeting PAK4 Inhibits Ras-Mediated Signaling and Multiple Oncogenic Pathways in High-Risk Rhabdomyosarcoma. Cancer Res. 2021, 81, 199–212. [Google Scholar] [CrossRef]
- Won, S.Y.; Park, J.J.; Shin, E.Y.; Kim, E.G. PAK4 Signaling in Health and Disease: Defining the PAK4–CREB Axis. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef]
- Kumar, R.; Sanawar, R.; Li, X.; Li, F. Structure, Biochemistry, and Biology of PAK Kinases. Gene 2017, 605, 20–31. [Google Scholar] [CrossRef]
- Kant, R.; Manne, R.K.; Anas, M.; Penugurti, V.; Chen, T.; Pan, B.S.; Hsu, C.C.; Lin, H.K. Deregulated Transcription Factors in Cancer Cell Metabolisms and Reprogramming. Semin. Cancer Biol. 2022, 86, 1158–1174. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Huilgol, D.; Venkataramani, P.; Nandi, S.; Bhattacharjee, S. Transcription Factors That Govern Development and Disease: An Achilles Heel in Cancer. Genes 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Dharmadhikari, A.V.; Szafranski, P.; Kalinichenko, V.V.; Stankiewicz, P. Genomic and Epigenetic Complexity of the FOXF1 Locus in 16q24.1: Implications for Development and Disease. Curr. Genom. 2015, 16, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Armeanu-Ebinger, S.; Bonin, M.; Häbig, K.; Poremba, C.; Koscielniak, E.; Godzinski, J.; Warmann, S.W.; Fuchs, J.; Seitz, G. Differential Expression of Invasion Promoting Genes in Childhood Rhabdomyosarcoma. Int. J. Oncol. 2011, 38, 993–1000. [Google Scholar] [CrossRef][Green Version]
- Milewski, D.; Pradhan, A.; Wang, X.; Cai, Y.; Le, T.; Turpin, B.; Kalinichenko, V.V.; Kalin, T.V. FoxF1 and FoxF2 Transcription Factors Synergistically Promote Rhabdomyosarcoma Carcinogenesis by Repressing Transcription of P21Cip1 CDK Inhibitor. Oncogene 2017, 36, 850–862. [Google Scholar] [CrossRef]
- Milewski, D.; Shukla, S.; Gryder, B.E.; Pradhan, A.; Donovan, J.; Sudha, P.; Vallabh, S.; Pyros, A.; Xu, Y.; Barski, A.; et al. FOXF1 Is Required for the Oncogenic Properties of PAX3-FOXO1 in Rhabdomyosarcoma. Oncogene 2021, 40, 2182–2199. [Google Scholar] [CrossRef]
- Hsieh, J.; Danis, E.P.; Owens, C.R.; Parrish, J.K.; Nowling, N.L.; Wolin, A.R.; Purdy, S.C.; Rosenbaum, S.R.; Ivancevic, A.M.; Chuong, E.B.; et al. Dependence of PAX3-FOXO1 Chromatin Occupancy on ETS1 at Important Disease-Promoting Genes Exposes New Targetable Vulnerability in Fusion-Positive Rhabdomyosarcoma. Oncogene 2024, 44, 19–29. [Google Scholar] [CrossRef]
- Sobral, L.M.; Hicks, H.M.; Parrish, J.K.; McCann, T.S.; Hsieh, J.; Goodspeed, A.; Costello, J.C.; Black, J.C.; Jedlicka, P. KDM3A/Ets1 Epigenetic Axis Contributes to PAX3/FOXO1-Driven and Independent Disease-Promoting Gene Expression in Fusion-Positive Rhabdomyosarcoma. Mol. Oncol. 2020, 14, 2471–2486. [Google Scholar] [CrossRef]
- Skrzypek, K.; Kusienicka, A.; Trzyna, E.; Szewczyk, B.; Ulman, A.; Konieczny, P.; Adamus, T.; Badyra, B.; Kortylewski, M.; Majka, M. SNAIL Is a Key Regulator of Alveolar Rhabdomyosarcoma Tumor Growth and Differentiation through Repression of MYF5 and MYOD Function. Cell Death Dis. 2018, 9, 643. [Google Scholar] [CrossRef]
- Hsu, T.; Trojanowska, M.; Watson, D.K. Ets Proteins in Biological Control and Cancer. J. Cell Biochem. 2004, 91, 896–903. [Google Scholar] [CrossRef]
- Ichikawa, M.K.; Endo, K.; Itoh, Y.; Osada, A.H.; Kimura, Y.; Ueki, K.; Yoshizawa, K.; Miyazawa, K.; Saitoh, M. Ets Family Proteins Regulate the EMT Transcription Factors Snail and ZEB in Cancer Cells. FEBS Open Bio 2022, 12, 1353–1364. [Google Scholar] [CrossRef]
- Sobral, L.M.; Sechler, M.; Parrish, J.K.; McCann, T.S.; Jones, K.L.; Black, J.C.; Jedlicka, P. KDM3A/Ets1/MCAM Axis Promotes Growth and Metastatic Properties in Rhabdomyosarcoma. Genes Cancer 2020, 11, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-ΚB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Charytonowicz, E.; Matushansky, I.; Domingo-Doménech, J.; Castillo-Martín, M.; Ladanyi, M.; Cordon-Cardo, C.; Ziman, M. PAX7-FKHR Fusion Gene Inhibits Myogenic Differentiation via NF-KappaB Upregulation. Clin. Transl. Oncol. 2012, 14, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Randolph, M.E.; Cleary, M.M.; Bajwa, Z.; Svalina, M.N.; Young, M.C.; Mansoor, A.; Kaur, P.; Bult, C.J.; Goros, M.W.; Michalek, J.E.; et al. EphB4/EphrinB2 Therapeutics in Rhabdomyosarcoma. PLoS ONE 2017, 12, e0183161. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Blankenship, J.W.; Wayson, S.M.; Fedorova, A.V.; Kayagaki, N.; Garg, P.; Zobel, K.; Dynek, J.N.; Elliott, L.O.; Wallweber, H.J.A.; et al. IAP Antagonists Induce Autoubiquitination of C-IAPs, NF-ΚB Activation, and TNFα-Dependent Apoptosis. Cell 2007, 131, 669–681. [Google Scholar] [CrossRef]
- Francí, C.; Takkunen, M.; Dave, N.; Alameda, F.; Gómez, S.; Rodríguez, R.; Escrivà, M.; Montserrat-Sentís, B.; Baró, T.; Garrido, M.; et al. Expression of Snail Protein in Tumor-Stroma Interface. Oncogene 2006, 25, 5134–5144. [Google Scholar] [CrossRef]
- Skrzypek, K.; Kot, M.; Konieczny, P.; Nieszporek, A.; Kusienicka, A.; Lasota, M.; Bobela, W.; Jankowska, U.; Kędracka-Krok, S.; Majka, M. SNAIL Promotes Metastatic Behavior of Rhabdomyosarcoma by Increasing EZRIN and AKT Expression and Regulating MicroRNA Networks. Cancers 2020, 12, 1870. [Google Scholar] [CrossRef]
- Zibat, A.; Missiaglia, E.; Rosenberger, A.; Pritchard-Jones, K.; Shipley, J.; Hahn, H.; Fulda, S. Activation of the Hedgehog Pathway Confers a Poor Prognosis in Embryonal and Fusion Gene-Negative Alveolar Rhabdomyosarcoma. Oncogene 2010, 29, 6323–6330. [Google Scholar] [CrossRef]
- Bhushan, B.; Iranpour, R.; Eshtiaghi, A.; da Silva Rosa, S.C.; Lindsey, B.W.; Gordon, J.W.; Ghavami, S. Transforming Growth Factor Beta and Alveolar Rhabdomyosarcoma: A Challenge of Tumor Differentiation and Chemotherapy Response. Int. J. Mol. Sci. 2024, 25, 2791. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.M.; Lin, S.F. The Protein Tyrosine Kinase Family of the Human Genome. Oncogene 2000, 19, 5548–5557. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Miller, W.T. Receptor Tyrosine Kinases: Mechanisms of Activation and Signaling. Curr. Opin. Cell Biol. 2007, 19, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Schuler, M.; Kang, Y.K.; Yen, C.J.; Edeline, J.; Choo, S.P.; Lin, C.C.; Okusaka, T.; Weiss, K.H.; Macarulla, T.; et al. A First-in-Human Phase 1/2 Study of FGF401 and Combination of FGF401 with Spartalizumab in Patients with Hepatocellular Carcinoma or Biomarker-Selected Solid Tumors. J. Exp. Clin. Cancer Res. 2022, 41, 189. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, V.M.; Middleton, M.R.; Eckhardt, S.G.; Rudin, C.M.; Juergens, R.A.; Gedrich, R.; Gogov, S.; McCarthy, S.; Poondru, S.; Stephens, A.W.; et al. Phase I Dose-Escalation Study of Linsitinib (OSI-906) and Erlotinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 2897–2907. [Google Scholar] [CrossRef]
- Parvaresh, H.; Roozitalab, G.; Golandam, F.; Behzadi, P.; Jabbarzadeh Kaboli, P. Unraveling the Potential of ALK-Targeted Therapies in Non-Small Cell Lung Cancer: Comprehensive Insights and Future Directions. Biomedicines 2024, 12, 297. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhou, Q.; Yan, H.H.; Zhang, X.C.; Chen, H.J.; Tu, H.Y.; Wang, Z.; Xu, C.R.; Su, J.; Wang, B.C.; et al. A Phase III Randomised Controlled Trial of Erlotinib vs Gefitinib in Advanced Non-Small Cell Lung Cancer with EGFR Mutations. Br. J. Cancer 2017, 116, 568–574. [Google Scholar] [CrossRef]
- Lu, L.; Saha, D.; Martuza, R.L.; Rabkin, S.D.; Wakimoto, H. Single Agent Efficacy of the VEGFR Kinase Inhibitor Axitinib in Preclinical Models of Glioblastoma. J. Neurooncol. 2014, 121, 91–100. [Google Scholar] [CrossRef]
- Song, Z.; Gong, B.; Qu, T.; Chen, Y.; Zhao, G.; Jin, Y.; Zhao, Q. Anlotinib Destabilizes PAX3-FOXO1 to Induce Rhabdomyosarcoma Cell Death via Upregulating NEK2. Biomed. Pharmacother. 2024, 177, 117126. [Google Scholar] [CrossRef]
- McKinnon, T.; Venier, R.; Yohe, M.; Sindiri, S.; Gryder, B.E.; Shern, J.F.; Kabaroff, L.; Dickson, B.; Schleicher, K.; Chouinard-Pelletier, G.; et al. Functional Screening of FGFR4-Driven Tumorigenesis Identifies PI3K/MTOR Inhibition as a Therapeutic Strategy in Rhabdomyosarcoma. Oncogene 2018, 37, 2630–2644. [Google Scholar] [CrossRef]
- Crose, L.E.S.; Etheridge, K.T.; Chen, C.; Belyea, B.; Talbot, L.J.; Bentley, R.C.; Linardic, C.M. FGFR4 Blockade Exerts Distinct Antitumorigenic Effects in Human Embryonal versus Alveolar Rhabdomyosarcoma. Clin. Cancer Res. 2012, 18, 3780–3790. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Wang, X.; Chen, B.; Wang, Y.; Liu, S.; Xu, J.; Zhao, W.; Wu, J. FGFR4 Transmembrane Domain Polymorphism and Cancer Risk: A Meta-Analysis Including 8555 Subjects. Eur. J. Cancer 2010, 46, 3332–3338. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Li, J.; Zhu, J.; Zhao, C.; Xu, H. Novel Regulatory Factors and Small-Molecule Inhibitors of FGFR4 in Cancer. Front. Pharmacol. 2021, 12, 633453. [Google Scholar] [CrossRef] [PubMed]
- Roidl, A.; Berger, H.J.; Kumar, S.; Bange, J.; Knyazev, P.; Ullrich, A. Resistance to Chemotherapy Is Associated with Fibroblast Growth Factor Receptor 4 Up-Regulation. Clin. Cancer Res. 2009, 15, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Thussbas, C.; Nahrig, J.; Streit, S.; Bange, J.; Kriner, M.; Kates, R.; Ulm, K.; Kiechle, M.; Hoefler, H.; Ullrich, A.; et al. FGFR4 Arg388 Allele Is Associated with Resistance to Adjuvant Therapy in Primary Breast Cancer. J. Clin. Oncol. 2006, 24, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.; Zheng, L.; Winer, D.; Asa, S.L. Targeting N-Cadherin through Fibroblast Growth Factor Receptor-4: Distinct Pathogenetic and Therapeutic Implications. Mol. Endocrinol. 2006, 20, 2965–2975. [Google Scholar] [CrossRef]
- Cao, L.; Yu, Y.; Bilke, S.; Walker, R.L.; Mayeenuddin, L.H.; Azorsa, D.O.; Yang, F.; Pineda, M.; Helman, L.J.; Meltzer, P.S. Genome-Wide Identification of PAX3-FKHR Binding Sites in Rhabdomyosarcoma Reveals Candidate Target Genes Important for Development and Cancer. Cancer Res. 2010, 70, 6497–6508. [Google Scholar] [CrossRef]
- Taylor VI, J.G.; Cheuk, A.T.; Tsang, P.S.; Chung, J.Y.; Song, Y.K.; Desai, K.; Yu, Y.; Chen, Q.R.; Shah, K.; Youngblood, V.; et al. Identification of FGFR4-Activating Mutations in Human Rhabdomyosarcomas That Promote Metastasis in Xenotransplanted Models. J. Clin. Investig. 2009, 119, 3395–3407. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Chen, D.; Xia, Q.; Liu, Z.; Li, F.; Yan, Y.; Cai, Y. Insight into Ponatinib Resistance Mechanisms in Rhabdomyosarcoma Caused by the Mutations in FGFR4 Tyrosine Kinase Using Molecular Modeling Strategies. Int. J. Biol. Macromol. 2019, 135, 294–302. [Google Scholar] [CrossRef]
- Li, S.Q.; Cheuk, A.T.; Shern, J.F.; Song, Y.K.; Hurd, L.; Liao, H.; Wei, J.S.; Khan, J. Targeting Wild-Type and Mutationally Activated FGFR4 in Rhabdomyosarcoma with the Inhibitor Ponatinib (AP24534). PLoS ONE 2013, 8, e76551. [Google Scholar] [CrossRef]
- Alijaj, N.; Moutel, S.; Gouveia, Z.L.; Gray, M.; Roveri, M.; Dzhumashev, D.; Weber, F.; Meier, G.; Luciani, P.; Rössler, J.K.; et al. Novel FGFR4-Targeting Single-Domain Antibodies for Multiple Targeted Therapies against Rhabdomyosarcoma. Cancers 2020, 12, 3313. [Google Scholar] [CrossRef]
- Darvishi, E.; Slemmons, K.; Wan, Z.; Mitra, S.; Hou, X.; Hugues Parmentier, J.; Eddie Loh, Y.H.; Helman, L.J. Molecular Mechanisms of Guadecitabine Induced FGFR4 down Regulation in Alveolar Rhabdomyosarcomas. Neoplasia 2020, 22, 274–282. [Google Scholar] [CrossRef]
- Milton, C.I.; Selfe, J.; Aladowicz, E.; Man, S.Y.K.; Bernauer, C.; Missiaglia, E.; Walters, Z.S.; Gatz, S.A.; Kelsey, A.; Generali, M.; et al. FGF7–FGFR2 Autocrine Signaling Increases Growth and Chemoresistance of Fusion-positive Rhabdomyosarcomas. Mol. Oncol. 2021, 16, 1272–1289. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, M.V.; Kazlauskas, A. Platelet-Derived Growth Factor Receptor Family. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 538–543. [Google Scholar] [CrossRef]
- Varga, J.; Lafyatis, R. Etiology and Pathogenesis of Systemic Sclerosis. In Rheumatology, 6th ed.; Hochberg, M.C., Silman, A.J., Smolen, J.S., Weinblatt, M.E., Weisman, M.H., Eds.; Mosby Ltd., an imprint of Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, pp. 1177–1189. [Google Scholar] [CrossRef]
- Board, R.; Jayson, G.C. Platelet-Derived Growth Factor Receptor (PDGFR): A Target for Anticancer Therapeutics. Drug Resist. Updates 2005, 8, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Tallquist, M.; Kazlauskas, A. PDGF Signaling in Cells and Mice. Cytokine Growth Factor Rev. 2004, 15, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ehnman, M.; Missiaglia, E.; Folestad, E.; Selfe, J.; Strell, C.; Thway, K.; Brodin, B.; Pietras, K.; Shipley, J.; Östman, A.; et al. Distinct Effects of Ligand-Induced PDGFRα and PDGFRβ Signaling in the Human Rhabdomyosarcoma Tumor Cell and Stroma Cell Compartments. Cancer Res. 2013, 73, 2139–2149. [Google Scholar] [CrossRef]
- Blandford, M.C.; Barr, F.G.; Lynch, J.C.; Randall, R.L.; Qualman, S.J.; Keller, C. Rhabdomyosarcomas Utilize Developmental, Myogenic Growth Factors for Disease Advantage: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2006, 46, 329–338. [Google Scholar] [CrossRef]
- Epstein, J.A.; Song, B.; Lakkis, M.; Wang, C.; Lam, P.Y.P.; Sublett, J.E.; Hollenbach, A.D.; Roussel, M.F. Tumor-Specific PAX3-FKHR Transcription Factor, but Not PAX3, Activates the Platelet-Derived Growth Factor Alpha Receptor. Mol. Cell. Biol. 1998, 18, 4118–4130. [Google Scholar] [CrossRef]
- Lasota, M.; Bentke-Imiolek, A.; Skrzypek, K.; Bobrowska, J.; Jagusiak, A.; Bryniarska-Kubiak, N.; Zagajewski, J.; Kot, M.; Szydlak, R.; Lekka, M.; et al. Small-Molecule Inhibitor-Tyrphostin Ag1296 Regulates Proliferation, Survival and Migration of Rhabdomyosarcoma Cells. J. Physiol. Pharmacol. 2021, 72, 1–13. [Google Scholar] [CrossRef]
- Lasota, M.; Klein, A.; Balwierz, W. Cytostatic and Cytotoxic Effects of Tyrphostin AG1296 on RMS Cells. Wspolczesna Onkol. 2012, 16, 1–5. [Google Scholar] [CrossRef]
- Taniguchi, E.; Nishijo, K.; McCleish, A.T.; Michalek, J.E.; Grayson, M.H.; Infante, A.J.; Abboud, H.E.; Legallo, R.D.; Qualman, S.J.; Rubin, B.P.; et al. PDGFR-A Is a Therapeutic Target in Alveolar Rhabdomyosarcoma. Oncogene 2008, 27, 6550–6560. [Google Scholar] [CrossRef]
- Harwardt, M.L.I.E.; Schröder, M.S.; Li, Y.; Malkusch, S.; Freund, P.; Gupta, S.; Janjic, N.; Strauss, S.; Jungmann, R.; Dietz, M.S.; et al. Single-Molecule Super-Resolution Microscopy Reveals Heteromeric Complexes of MET and EGFR upon Ligand Activation. Int. J. Mol. Sci. 2020, 21, 2803. [Google Scholar] [CrossRef]
- Peschard, P.; Park, M. From Tpr-Met to Met, Tumorigenesis and Tubes. Oncogene 2007, 26, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Kusienicka, A.; Szewczyk, B.; Adamus, T.; Lukasiewicz, E.; Miekus, K.; Majka, M. Constitutive Activation of MET Signaling Impairs Myogenic Differentiation of Rhabdomyosarcoma and Promotes Its Development and Progression. Oncotarget 2015, 6, 31378–31398. [Google Scholar] [CrossRef] [PubMed]
- Miekus, K.; Lukasiewicz, E.; Jarocha, D.; Sekula, M.; Drabik, G.; Majka, M. The Decreased Metastatic Potential of Rhabdomyosarcoma Cells Obtained through MET Receptor Downregulation and the Induction of Differentiation. Cell Death Dis. 2013, 4, e459. [Google Scholar] [CrossRef]
- Szewczyk, B.; Skrzypek, K.; Majka, M. Targeting MET Receptor in Rhabdomyosarcoma: Rationale and Progress. Curr. Drug Targets 2016, 18, 98–107. [Google Scholar] [CrossRef]
- Lukasiewicz, E.; Miekus, K.; Kijowski, J.; Drabik, G.; Wilusz, M.; Bobis-Wozowicz, S.; Majka, M. Inhibition of Rhabdomyosarcoma’s Metastatic Behavior through Downregulation of MET Receptor Signaling. Folia Histochem. Cytobiol. 2009, 47, 485–489. [Google Scholar] [CrossRef][Green Version]
- Simpson, A.; Petnga, W.; Macaulay, V.M.; Weyer-Czernilofsky, U.; Bogenrieder, T. Insulin-Like Growth Factor (IGF) Pathway Targeting in Cancer: Role of the IGF Axis and Opportunities for Future Combination Studies. Target. Oncol. 2017, 12, 571–597. [Google Scholar] [CrossRef]
- Shackleford, T.J.; Hariharan, S.; Vaseva, A.V.; Alagoa, K.; Espinoza, M.; Bid, H.K.; Li, F.; Zhong, H.; Phelps, D.A.; Roberts, R.D.; et al. Redundant Signaling as the Predominant Mechanism for Resistance to Antibodies Targeting the Type-I Insulin-like Growth Factor Receptor in Cells Derived from Childhood Sarcoma. Mol. Cancer Ther. 2023, 22, 539–550. [Google Scholar] [CrossRef]
- Searcy, M.B.; Larsen, R.K.; Stevens, B.T.; Zhang, Y.; Jin, H.; Drummond, C.J.; Langdon, C.G.; Gadek, K.E.; Vuong, K.; Reed, K.B.; et al. PAX3-FOXO1 Dictates Myogenic Reprogramming and Rhabdomyosarcoma Identity in Endothelial Progenitors. Nat. Commun. 2023, 14, 7291. [Google Scholar] [CrossRef]
- Devang, N.; Pani, A.; Rajanikant, G.K. Pseudokinases: Prospects for Expanding the Therapeutic Targets Armamentarium. Adv. Protein Chem. Struct. Biol. 2021, 124, 121–185. [Google Scholar] [CrossRef]
- Yu, J.M.; Sun, W.; Wang, Z.; Liang, X.; Hua, F.; Li, K.; Lv, X.; Zhang, X.; Liu, Y.; Yu, J.; et al. TRIB3 Supports Breast Cancer Stemness by Suppressing FOXO1 Degradation and Enhancing SOX2 Transcription. Nat. Commun. 2019, 10, 5720. [Google Scholar] [CrossRef]
- Gallo-Oller, G.; Pons, G.; Sansa-Girona, J.; Navarro, N.; Zarzosa, P.; García-Gilabert, L.; Cabré-Fernandez, P.; Guillén Burrieza, G.; Valero-Arrese, L.; Segura, M.F.; et al. TRIB3 Silencing Promotes the Downregulation of Akt Pathway and PAX3-FOXO1 in High-Risk Rhabdomyosarcoma. Exp. Hematol. Oncol. 2024, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Moga, A.; Zarzosa, P.; Molist, C.; Velasco, P.; Pyczek, J.; Simon-Keller, K.; Giralt, I.; Vidal, I.; Navarro, N.; Segura, M.F.; et al. Ligand-Dependent Hedgehog Pathway Activation in Rhabdomyosarcoma: The Oncogenic Role of the Ligands. Br. J. Cancer 2017, 117, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.A.; Apfelbaum, A.A.; Hawkins, A.G.; Svoboda, L.K.; Kumar, A.; Ruiz, R.O.; Garcia, A.X.; Haarer, E.; Nwosu, Z.C.; Bradin, J.; et al. EWS-FLI1 and Menin Converge to Regulate ATF4 Activity in Ewing Sarcoma. Mol. Cancer Res. 2021, 19, 1182–1195. [Google Scholar] [CrossRef]
- Yang, K.; Li, B.; Xu, X.; Yu, Z.; Lyu, X.; Ren, K.; Liu, X.; Chen, S.; Li, H. TRIB3 Overexpression Predicts Malignant Progression and Poor Prognosis in Human Solid Tumors: Bioinformatics Validation and Clinical Significance. Expert. Rev. Mol. Diagn. 2024, 24, 1159–1170. [Google Scholar] [CrossRef]
- Ji, Y.; Lin, Z.; Li, G.; Tian, X.; Wu, Y.; Wan, J.; Liu, T.; Xu, M. Identification and Validation of Novel Biomarkers Associated with Immune Infiltration for the Diagnosis of Osteosarcoma Based on Machine Learning. Front. Genet. 2023, 14, 1136783. [Google Scholar] [CrossRef]
- Salazar, M.; Lorente, M.; García-Taboada, E.; Pérez Gómez, E.; Dávila, D.; Zúñiga-García, P.; María Flores, J.; Rodríguez, A.; Hegedus, Z.; Mosén-Ansorena, D.; et al. Loss of Tribbles Pseudokinase-3 Promotes Akt-Driven Tumorigenesis via FOXO Inactivation. Cell Death Differ. 2015, 22, 131–144. [Google Scholar] [CrossRef]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal Antibodies: Versatile Platforms for Cancer Immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, L.; Chen, J. Current Advances and Challenges in CAR T-Cell Therapy for Solid Tumors: Tumor-Associated Antigens and the Tumor Microenvironment. Exp. Hematol. Oncol. 2023, 12, 14. [Google Scholar] [CrossRef]
- Sharma, A.; Schmidt-Wolf, I.G.H. 30 Years of CIK Cell Therapy: Recapitulating the Key Breakthroughs and Future Perspective. J. Exp. Clin. Cancer Res. 2021, 40, 388. [Google Scholar] [CrossRef]
- Hurdayal, R.; Brombacher, F. Interleukin-4 Receptor Alpha: From Innate to Adaptive Immunity in Murine Models of Cutaneous Leishmaniasis. Front. Immunol. 2017, 8, 1354. [Google Scholar] [CrossRef]
- Mak, T.W.; Saunders, M.E. Cytokines and Cytokine Receptors. In The Immune Response; Academic Press: Cambridge, MA, USA, 2006; pp. 463–516. ISBN 978-0-12-088451-3. [Google Scholar]
- Hosoyama, T.; Aslam, M.I.; Abraham, J.; Prajapati, S.I.; Nishijo, K.; Michalek, J.E.; Zarzabal, L.A.; Nelon, L.D.; Guttridge, D.C.; Rubin, B.P.; et al. IL-4R Drives Dedifferentiation, Mitogenesis, and Metastasis in Rhabdomyosarcoma. Clin. Cancer Res. 2011, 17, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Su, Z.Z.; Lebedeva, I.V.; Sauane, M.; Gopalkrishnan, R.V.; Valerie, K.; Dent, P.; Fisher, P.B. Mda-7 (IL-24) Mediates Selective Apoptosis in Human Melanoma Cells by Inducing the Coordinated Overexpression of the GADD Family of Genes by Means of P38 MAPK. Proc. Natl. Acad. Sci. USA 2002, 99, 10054–10059. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.E.; Bhatia, S.; Bhoopathi, P.; Das, S.K.; Emdad, L.; Dasgupta, S.; Dent, P.; Wang, X.Y.; Sarkar, D.; Fisher, P.B. MDA-7/IL-24: Multifunctional Cancer Killing Cytokine. Adv. Exp. Med. Biol. 2014, 818, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Su, Z.Z.; Lebedeva, I.V.; Sarkar, D.; Sauane, M.; Emdad, L.; Bachelor, M.A.; Grant, S.; Curiel, D.T.; Dent, P.; et al. Mda-7/IL-24: Multifunctional Cancer-Specific Apoptosis-Inducing Cytokine. Pharmacol. Ther. 2006, 111, 596–628. [Google Scholar] [CrossRef]
- Chada, S.; Sutton, R.B.; Ekmekcioglu, S.; Ellerhorst, J.; Mumm, J.B.; Leitner, W.W.; Yang, H.Y.; Sahin, A.A.; Hunt, K.K.; Fuson, K.L.; et al. MDA-7/IL-24 Is a Unique Cytokine–Tumor Suppressor in the IL-10 Family. Int. Immunopharmacol. 2004, 4, 649–667. [Google Scholar] [CrossRef]
- Davicioni, E.; Finckenstein, F.G.; Shahbazian, V.; Buckley, J.D.; Triche, T.J.; Anderson, M.J. Identification of a PAX-FKHR Gene Expression Signature That Defines Molecular Classes and Determines the Prognosis of Alveolar Rhabdomyosarcomas. Cancer Res. 2006, 66, 6936–6946. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, B.; Davie, J. Alternative Splicing of MEF2C Pre-MRNA Controls Its Activity in Normal Myogenesis and Promotes Tumorigenicity in Rhabdomyosarcoma Cells. J. Biol. Chem. 2015, 290, 310–324. [Google Scholar] [CrossRef]
- Lacey, A.; Hedrick, E.; Cheng, Y.; Mohankumar, K.; Warren, M.; Safe, S. Interleukin-24 (IL-24) Is Suppressed by PAX3-FOXO1 and Is a Novel Therapy for Rhabdomyosarcoma. Mol. Cancer Ther. 2018, 17, 2756–2766. [Google Scholar] [CrossRef]
- Heo, C.K.; Bahk, Y.Y.; Cho, E.W. Tumor-Associated Autoantibodies as Diagnostic and Prognostic Biomarkers. BMB Rep. 2012, 45, 677. [Google Scholar] [CrossRef]
- Poli, E.; Cattelan, M.; Zanetti, I.; Scagnellato, A.; Giordano, G.; Zin, A.; Bisogno, G.; Bonvini, P. Autoantibody Profiling of Alveolar Rhabdomyosarcoma Patients Unveils Tumor-Associated Antigens with Diagnostic and Prognostic Significance. Oncoimmunology 2021, 10, 1954765. [Google Scholar] [CrossRef]
- Merker, M.; Wagner, J.; Kreyenberg, H.; Heim, C.; Moser, L.M.; Wels, W.S.; Bonig, H.; Ivics, Z.; Ullrich, E.; Klingebiel, T.; et al. ERBB2-CAR-Engineered Cytokine-Induced Killer Cells Exhibit Both CAR-Mediated and Innate Immunity Against High-Risk Rhabdomyosarcoma. Front. Immunol. 2020, 11, 581468. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Moser, L.M.; Kreyenberg, H.; Bonig, H.B.; Tonn, T.; Wels, W.S.; Gradhand, E.; Ullrich, E.; Meister, M.T.; Koerkamp, M.G.; et al. ErbB2 (HER2)-CAR-NK-92 Cells for Enhanced Immunotherapy of Metastatic Fusion-Driven Alveolar Rhabdomyosarcoma. Front. Immunol. 2023, 14, 1228894. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, C.; Landuzzi, L.; Palladini, A.; Nicoletti, G.; Nanni, P.; Lollini, P.L. HER Tyrosine Kinase Family and Rhabdomyosarcoma: Role in Onset and Targeted Therapy. Cells 2021, 10, 1808. [Google Scholar] [CrossRef]
- Yoo, J.; Jeon, Y.H.; Cho, H.Y.; Lee, S.W.; Kim, G.W.; Lee, D.H.; Kwon, S.H. Advances in Histone Demethylase KDM3A as a Cancer Therapeutic Target. Cancers 2020, 12, 1098. [Google Scholar] [CrossRef]
- McCann, T.S.; Sobral, L.M.; Self, C.; Hsieh, J.; Sechler, M.; Jedlicka, P. Biology and Targeting of the Jumonji-Domain Histone Demethylase Family in Childhood Neoplasia: A Preclinical Overview. Expert. Opin. Ther. Targets 2019, 23, 267–280. [Google Scholar] [CrossRef]
- Kucinski, J.; Tallan, A.; Taslim, C.; Wang, M.; Cannon, M.V.; Silvius, K.M.; Stanton, B.Z.; Kendall, G.C. Rhabdomyosarcoma Fusion Oncoprotein Initially Pioneers a Neural Signature in Vivo. bioRxiv 2024. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2012, 38, 23–38. [Google Scholar] [CrossRef]
- Sun, W.; Chatterjee, B.; Wang, Y.; Stevenson, H.S.; Edelman, D.C.; Meltzer, P.S.; Barr, F.G. Distinct Methylation Profiles Characterize Fusion-Positive and Fusion-Negative Rhabdomyosarcoma. Mod. Pathol. 2015, 28, 1214–1224. [Google Scholar] [CrossRef]
- Tombolan, L.; Poli, E.; Martini, P.; Zin, A.; Millino, C.; Pacchioni, B.; Celegato, B.; Bisogno, G.; Romualdi, C.; Rosolen, A.; et al. Global DNA Methylation Profiling Uncovers Distinct Methylation Patterns of Protocadherin Alpha4 in Metastatic and Non-Metastatic Rhabdomyosarcoma. BMC Cancer 2016, 16, 886. [Google Scholar] [CrossRef]
- Sun, W.; Chatterjee, B.; Shern, J.F.; Patidar, R.; Song, Y.; Wang, Y.; Walker, R.L.; Pawel, B.R.; Linardic, C.M.; Houghton, P.; et al. Relationship of DNA Methylation to Mutational Changes and Transcriptional Organization in Fusion-Positive and Fusion-Negative Rhabdomyosarcoma. Int. J. Cancer 2019, 144, 2707–2717. [Google Scholar] [CrossRef]
- Sun, W.; Hewitt, S.M.; Wright, H.; Keller, C.; Barr, F.G. DNA Methylation Patterns Are Influenced by Pax3::Foxo1 Expression and Developmental Lineage in Rhabdomyosarcoma Tumours Forming in Genetically Engineered Mouse Models. J. Pathol. 2025, 265, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Stelzl, U.; Worm, U.; Lalowski, M.; Haenig, C.; Brembeck, F.H.; Goehler, H.; Stroedicke, M.; Zenkner, M.; Schoenherr, A.; Koeppen, S.; et al. A Human Protein-Protein Interaction Network: A Resource for Annotating the Proteome. Cell 2005, 122, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, T.; Guo, C.; Tang, T.S.; Liu, H. Small Molecule Modulators of Chromatin Remodeling: From Neurodevelopment to Neurodegeneration. Cell Biosci. 2023, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.G.; Gryder, B.E.; Pavlovic, B.; Chung, Y.; Ngo, Q.A.; Frommelt, F.; Gstaiger, M.; Song, Y.; Benischke, K.; Laubscher, D.; et al. NuRD Subunit CHD4 Regulates Super-Enhancer Accessibility in Rhabdomyosarcoma and Represents a General Tumor Dependency. Elife 2020, 9, e54993. [Google Scholar] [CrossRef]
- Kelly, R.D.W.; Stengel, K.R.; Chandru, A.; Johnson, L.C.; Hiebert, S.W.; Cowley, S.M. Histone Deacetylases Maintain Expression of the Pluripotent Gene Network via Recruitment of RNA Polymerase II to Coding and Noncoding Loci. Genome Res. 2024, 34, 34–46. [Google Scholar] [CrossRef]
- Böhm, M.; Wachtel, M.; Marques, J.G.; Streiff, N.; Laubscher, D.; Nanni, P.; Mamchaoui, K.; Santoro, R.; Schäfer, B.W. Helicase CHD4 Is an Epigenetic Coregulator of PAX3-FOXO1 in Alveolar Rhabdomyosarcoma. J. Clin. Investig. 2016, 126, 4237–4249. [Google Scholar] [CrossRef]
- Sunkel, B.D.; Wang, M.; LaHaye, S.; Kelly, B.J.; Fitch, J.R.; Barr, F.G.; White, P.; Stanton, B.Z. Evidence of Pioneer Factor Activity of an Oncogenic Fusion Transcription Factor. iScience 2021, 24, 102867. [Google Scholar] [CrossRef]
- Danielli, S.G.; Wei, Y.; Dyer, M.A.; Stewart, E.; Sheppard, H.; Wachtel, M.; Schäfer, B.W.; Patel, A.G.; Langenau, D.M. Single Cell Transcriptomic Profiling Identifies Tumor-Acquired and Therapy-Resistant Cell States in Pediatric Rhabdomyosarcoma. Nat. Commun. 2024, 15, 6307. [Google Scholar] [CrossRef]
- Chen, Y.K.; Bonaldi, T.; Cuomo, A.; Del Rosario, J.R.; Hosfield, D.J.; Kanouni, T.; Kao, S.C.; Lai, C.; Lobo, N.A.; Matuszkiewicz, J.; et al. Design of KDM4 Inhibitors with Antiproliferative Effects in Cancer Models. ACS Med. Chem. Lett. 2017, 8, 869–874. [Google Scholar] [CrossRef]
- Singh, S.; Abu-Zaid, A.; Jin, H.; Fang, J.; Wu, Q.; Wang, T.; Feng, H.; Quarni, W.; Shao, Y.; Maxham, L.; et al. Targeting KDM4 for Treating PAX3-FOXO1-Driven Alveolar Rhabdomyosarcoma. Sci. Transl. Med. 2022, 14, eabq2096. [Google Scholar] [CrossRef]
- A Study to Assess the Safety, Pharmacokinetics, and Antitumor Activity of Oral TACH101 in Participants with Advanced or Metastatic Cancer. Available online: https://clinicaltrials.gov/study/NCT05076552 (accessed on 20 May 2025).
- Kim, Y.Y.; Gryder, B.E.; Sinniah, R.; Peach, M.L.; Shern, J.F.; Abdelmaksoud, A.; Pomella, S.; Woldemichael, G.M.; Stanton, B.Z.; Milewski, D.; et al. KDM3B Inhibitors Disrupt the Oncogenic Activity of PAX3-FOXO1 in Fusion-Positive Rhabdomyosarcoma. Nat. Commun. 2024, 15, 1703. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, Q.; Huang, D.; Luo, L. Regulation of Gene Transcription of B Lymphoma Mo-MLV Insertion Region 1 Homolog (Review). Biomed. Rep. 2021, 14, 52. [Google Scholar] [CrossRef]
- Aranda, S.; Mas, G.; Di Croce, L. Regulation of Gene Transcription by Polycomb Proteins. Sci. Adv. 2015, 1, e1500737. [Google Scholar] [CrossRef]
- Shields, C.E.; Potlapalli, S.; Cuya-Smith, S.M.; Chappell, S.K.; Chen, D.; Martinez, D.; Pogoriler, J.; Rathi, K.S.; Patel, S.A.; Oristian, K.M.; et al. Epigenetic Regulator BMI1 Promotes Alveolar Rhabdomyosarcoma Proliferation and Constitutes a Novel Therapeutic Target. Mol. Oncol. 2021, 15, 2156–2171. [Google Scholar] [CrossRef]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, Erasing and Reading Histone Lysine Methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef]
- Ciarapica, R.; De Salvo, M.; Carcarino, E.; Bracaglia, G.; Adesso, L.; Leoncini, P.P.; Dall’Agnese, A.; Walters, Z.S.; Verginelli, F.; De Sio, L.; et al. The Polycomb Group (PcG) Protein EZH2 Supports the Survival of PAX3-FOXO1 Alveolar Rhabdomyosarcoma by Repressing FBXO32 (Atrogin1/MAFbx). Oncogene 2014, 33, 4173–4184. [Google Scholar] [CrossRef]
- Marquez, V.E. 3-Deazaneplanocin A (DZNep): A Drug That Deserves a Second Look. J. Med. Chem. 2024, 67, 17964–17979. [Google Scholar] [CrossRef]
- O’Brien, E.; Tse, C.; Tracy, I.; Reddin, I.; Selfe, J.; Gibson, J.; Tapper, W.; Pengelly, R.J.; Gao, J.; Aladowicz, E.; et al. Pharmacological EZH2 Inhibition Combined with Retinoic Acid Treatment Promotes Differentiation and Apoptosis in Rhabdomyosarcoma Cells. Clin. Epigenetics 2023, 15, 167. [Google Scholar] [CrossRef]
- Segalés, J.; Perdiguero, E.; Muñoz-Cánoves, P. Epigenetic Control of Adult Skeletal Muscle Stem Cell Functions. FEBS J. 2015, 282, 1571–1588. [Google Scholar] [CrossRef]
- Lee, M.H.; Jothi, M.; Gudkov, A.V.; Mal, A.K. Histone Methyltransferase KMT1A Restrains Entry of Alveolar Rhabdomyosarcoma Cells into a Myogenic Differentiated State. Cancer Res. 2011, 71, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, M.; Marona, P.; Kozakowska, M.; Jez, M.; Seczynska, M.; Loboda, A.; Bukowska-Strakova, K.; Szade, A.; Walawender, M.; Kusior, M.; et al. Heme Oxygenase-1 Controls an HDAC4-MIR-206 Pathway of Oxidative Stress in Rhabdomyosarcoma. Cancer Res. 2016, 76, 5707–5718. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Kozakowska, M.; Ciesla, M.; Stefanska, A.; Skrzypek, K.; Was, H.; Jazwa, A.; Grochot-Przeczek, A.; Kotlinowski, J.; Szymula, A.; Bartelik, A.; et al. Heme Oxygenase-1 Inhibits Myoblast Differentiation by Targeting Myomirs. Antioxid. Redox Signal. 2012, 16, 113–127. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a Key Modulator of Skeletal Muscle Development and Disease. Int. J. Biol. Sci. 2015, 11, 345–352. [Google Scholar] [CrossRef]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef]
- Nachiyappan, A.; Soon, J.L.J.; Lim, H.J.; Lee, V.K.M.; Taneja, R. EHMT1 Promotes Tumor Progression and Maintains Stemness by Regulating ALDH1A1 Expression in Alveolar Rhabdomyosarcoma. J. Pathol. 2022, 256, 349–362. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Y.; Yu, X.; Qian, F.; Bian, B.S.J.; Xiao, H.L.; Wang, W.G.; Xu, S.L.; Yang, J.; Cui, W.; et al. ALDH1A1 Defines Invasive Cancer Stem-like Cells and Predicts Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma. Mod. Pathol. 2014, 27, 775–783. [Google Scholar] [CrossRef]
- Alam, M.; Ahmad, R.; Rajabi, H.; Kharbanda, A.; Kufe, D. MUC1-C Oncoprotein Activates ERK→C/EBPβ Signaling and Induction of Aldehyde Dehydrogenase 1A1 in Breast Cancer Cells. J. Biol. Chem. 2013, 288, 30892–30903. [Google Scholar] [CrossRef]
- Gualtieri, A.; Bianconi, V.; Renzini, A.; Pieroni, L.; Licursi, V.; Mozzetta, C. The RNA Helicase DDX5 Cooperates with EHMT2 to Sustain Alveolar Rhabdomyosarcoma Growth. Cell Rep. 2022, 40, 111267. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.V.; Kala, M.P.; Rao, V.K.; Pignata, L.; Lim, H.J.; Suriyamurthy, S.; Chang, K.T.; Lee, V.K.; Guccione, E.; Taneja, R. Epigenetic Regulation of the PTEN-AKT-RAC1 Axis by G9a Is Critical for Tumor Growth in Alveolar Rhabdomyosarcoma. Cancer Res. 2019, 79, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, S.M.; Helin, K. Molecular Mechanisms and Potential Functions of Histone Demethylases. Nat. Rev. Mol. Cell Biol. 2012, 13, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone Methylation: A Dynamic Mark in Health, Disease and Inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef]
- Mellatyar, H.; Talaei, S.; Pilehvar-Soltanahmadi, Y.; Barzegar, A.; Akbarzadeh, A.; Shahabi, A.; Barekati-Mowahed, M.; Zarghami, N. Targeted Cancer Therapy through 17-DMAG as an Hsp90 Inhibitor: Overview and Current State of the Art. Biomed. Pharmacother. 2018, 102, 608–617. [Google Scholar] [CrossRef]
- Wu, Q.; Young, B.; Wang, Y.; Davidoff, A.M.; Rankovic, Z.; Yang, J. Recent Advances with KDM4 Inhibitors and Potential Applications. J. Med. Chem. 2022, 65, 9564–9579. [Google Scholar] [CrossRef]
- Singh, S.; Abu-Zaid, A.; Lin, W.; Low, J.; Abdolvahabi, A.; Jin, H.; Wu, Q.; Cooke, B.; Fang, J.; Bowling, J.; et al. 17-DMAG Dually Inhibits Hsp90 and Histone Lysine Demethylases in Alveolar Rhabdomyosarcoma. iScience 2021, 24, 101996. [Google Scholar] [CrossRef]
- Yamane, K.; Toumazou, C.; Tsukada, Y.; Erdjument-Bromage, H.; Tempst, P.; Wong, J.; Zhang, Y. JHDM2A, a JmjC-Containing H3K9 Demethylase, Facilitates Transcription Activation by Androgen Receptor. Cell 2006, 125, 483–495. [Google Scholar] [CrossRef]
- Sechler, M.; Parrish, J.K.; Birks, D.K.; Jedlicka, P. The Histone Demethylase KDM3A, and Its Downstream Target MCAM, Promote Ewing Sarcoma Cell Migration and Metastasis. Oncogene 2017, 36, 4150–4160. [Google Scholar] [CrossRef]
- Orentas, R.J.; Yang, J.J.; Wen, X.; Wei, J.S.; Mackall, C.L.; Khan, J. Identification of Cell Surface Proteins as Potential Immunotherapy Targets in 12 Pediatric Cancers. Front. Oncol. 2012, 2, 194. [Google Scholar] [CrossRef] [PubMed]
- ETS1 Gene—GeneCards|ETS1 Protein|ETS1 Antibody. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=ETS1 (accessed on 20 April 2025).
- Rezvani, G.; Lui, J.C.K.; Barnes, K.M.; Baron, J. A Set of Imprinted Genes Required for Normal Body Growth Also Promotes Growth of Rhabdomyosarcoma Cells. Pediatr. Res. 2012, 71, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W. HDAC Expression and Clinical Prognosis in Human Malignancies. Cancer Lett. 2009, 280, 168–176. [Google Scholar] [CrossRef]
- Glozak, M.A.; Seto, E. Histone Deacetylases and Cancer. Oncogene 2007, 26, 5420–5432. [Google Scholar] [CrossRef]
- Gryder, B.E.; Wu, L.; Woldemichael, G.M.; Pomella, S.; Quinn, T.R.; Park, P.M.C.; Cleveland, A.; Stanton, B.Z.; Song, Y.; Rota, R.; et al. Chemical Genomics Reveals Histone Deacetylases Are Required for Core Regulatory Transcription. Nat. Commun. 2019, 10, 3004. [Google Scholar] [CrossRef]
- Chauhan, S.; Lian, E.; Habib, I.; Liu, Q.; Anders, N.M.; Bugg, M.M.; Federman, N.C.; Reid, J.M.; Stewart, C.F.; Cates, T.; et al. Entinostat as a Combinatorial Therapeutic for Rhabdomyosarcoma. Sci. Rep. 2024, 14, 18936. [Google Scholar] [CrossRef]
- Yang, X.J.; Seto, E. Lysine Acetylation: Codified Crosstalk with Other Posttranslational Modifications. Mol. Cell 2008, 31, 449–461. [Google Scholar] [CrossRef]
- Ogryzko, V.V.; Schiltz, R.L.; Russanova, V.; Howard, B.H.; Nakatani, Y. The Transcriptional Coactivators P300 and CBP Are Histone Acetyltransferases. Cell 1996, 87, 953–959. [Google Scholar] [CrossRef]
- Fournier, M.; Orpinell, M.; Grauffel, C.; Scheer, E.; Garnier, J.M.; Ye, T.; Chavant, V.; Joint, M.; Esashi, F.; Dejaegere, A.; et al. KAT2A/KAT2B-Targeted Acetylome Reveals a Role for PLK4 Acetylation in Preventing Centrosome Amplification. Nat. Commun. 2016, 7, 13227. [Google Scholar] [CrossRef]
- Sartorelli, V.; Puri, P.L.; Hamamori, Y.; Ogryzko, V.; Chung, G.; Nakatani, Y.; Wang, J.Y.J.; Kedes, L. Acetylation of MyoD Directed by PCAF Is Necessary for the Execution of the Muscle Program. Mol. Cell 1999, 4, 725–734. [Google Scholar] [CrossRef]
- Bharathy, N.; Suriyamurthy, S.; Rao, V.K.; Ow, J.R.; Lim, H.J.; Chakraborty, P.; Vasudevan, M.; Dhamne, C.A.; Chang, K.T.E.; Min, V.L.K.; et al. P/CAF Mediates PAX3-FOXO1-Dependent Oncogenesis in Alveolar Rhabdomyosarcoma. J. Pathol. 2016, 240, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Tomasiak, P.; Janisiak, J.; Rogińska, D.; Perużyńska, M.; Machaliński, B.; Tarnowski, M. Garcinol and Anacardic Acid, Natural Inhibitors of Histone Acetyltransferases, Inhibit Rhabdomyosarcoma Growth and Proliferation. Molecules 2023, 28, 5292. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Yoshida, M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [PubMed]
- Gryder, B.E.; Pomella, S.; Sayers, C.; Wu, X.S.; Song, Y.; Chiarella, A.M.; Bagchi, S.; Chou, H.C.; Sinniah, R.S.; Walton, A.; et al. Histone Hyperacetylation Disrupts Core Gene Regulatory Architecture in Rhabdomyosarcoma. Nat. Genet. 2019, 51, 1714–1722. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hettmer, S.; Aslam, M.I.; Michalek, J.E.; Laub, W.; Wilky, B.A.; Loeb, D.M.; Rubin, B.P.; Wagers, A.J.; Keller, C. Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma. PLoS Genet. 2014, 10, e1004107. [Google Scholar] [CrossRef]
- Bharathy, N.; Berlow, N.E.; Wang, E.; Abraham, J.; Settelmeyer, T.P.; Hooper, J.E.; Svalina, M.N.; Ishikawa, Y.; Zientek, K.; Bajwa, Z.; et al. The HDAC3-SMARCA4-MiR-27a Axis Promotes Expression of the PAX3:FOXO1 Fusion Oncogene in Rhabdomyosarcoma. Sci. Signal 2018, 11, eaau7632. [Google Scholar] [CrossRef]
- Cassandri, M.; Pomella, S.; Rossetti, A.; Petragnano, F.; Milazzo, L.; Vulcano, F.; Camero, S.; Codenotti, S.; Cicchetti, F.; Maggio, R.; et al. MS-275 (Entinostat) Promotes Radio-Sensitivity in PAX3-FOXO1 Rhabdomyosarcoma Cells. Int. J. Mol. Sci. 2021, 22, 10671. [Google Scholar] [CrossRef]
- Bukowinski, A.; Chang, B.; Reid, J.M.; Liu, X.; Minard, C.G.; Trepel, J.B.; Lee, M.J.; Fox, E.; Weigel, B.J. A Phase 1 Study of Entinostat in Children and Adolescents with Recurrent or Refractory Solid Tumors, Including CNS Tumors: Trial ADVL1513, Pediatric Early Phase-Clinical Trial Network (PEP-CTN). Pediatr. Blood Cancer 2021, 68, e28892. [Google Scholar] [CrossRef]
- Hai, Y.; Kawachi, A.; He, X.; Yoshimi, A. Pathogenic Roles of RNA-Binding Proteins in Sarcomas. Cancers 2022, 14, 3812. [Google Scholar] [CrossRef]
- Crawford Parks, T.E.; Marcellus, K.A.; Langill, J.; Ravel-Chapuis, A.; Michaud, J.; Cowan, K.N.; Côté, J.; Jasmin, B.J. Novel Roles for Staufen1 in Embryonal and Alveolar Rhabdomyosarcoma via C-Myc-Dependent and -Independent Events. Sci. Rep. 2017, 7, 42342. [Google Scholar] [CrossRef]
- Almasi, S.; Crawford Parks, T.E.; Ravel-Chapuis, A.; MacKenzie, A.; Côté, J.; Cowan, K.N.; Jasmin, B.J. Differential Regulation of Autophagy by STAU1 in Alveolar Rhabdomyosarcoma and Non-Transformed Skeletal Muscle Cells. Cell. Oncol. 2021, 44, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Almasi, S.; SarmastiEmami, S.; Baird, S.; Ning, Z.; Figeys, D.; Côté, J.; Cowan, K.N.; Jasmin, B.J. Staufen1 Controls Mitochondrial Metabolism via HIF2α in Embryonal Rhabdomyosarcoma and Promotes Tumorigenesis. Cell. Mol. Life Sci. 2023, 80, 328. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, S.K.; Alahari, S.K. MicroRNA Function in Cancer: Oncogene or a Tumor Suppressor? Cancer Metastasis Rev. 2009, 28, 369–378. [Google Scholar] [CrossRef]
- Raza, U.; Zhang, J.D.; Şahin, Ö. MicroRNAs: Master Regulators of Drug Resistance, Stemness, and Metastasis. J. Mol. Med. 2014, 92, 321–336. [Google Scholar] [CrossRef]
- Missiaglia, E.; Shepherd, C.J.; Patel, S.; Thway, K.; Pierron, G.; Pritchard-Jones, K.; Renard, M.; Sciot, R.; Rao, P.; Oberlin, O.; et al. MicroRNA-206 Expression Levels Correlate with Clinical Behaviour of Rhabdomyosarcomas. Br. J. Cancer 2010, 102, 1769–1777. [Google Scholar] [CrossRef]
- Wang, H.; Garzon, R.; Sun, H.; Ladner, K.J.; Singh, R.; Dahlman, J.; Cheng, A.; Hall, B.M.; Qualman, S.J.; Chandler, D.S.; et al. NF-ΚB–YY1–MiR-29 Regulatory Circuitry in Skeletal Myogenesis and Rhabdomyosarcoma. Cancer Cell 2008, 14, 369–381. [Google Scholar] [CrossRef]
- Diao, Y.; Guo, X.; Jiang, L.; Wang, G.; Zhang, C.; Wan, J.; Jin, Y.; Wu, Z. MiR-203, a Tumor Suppressor Frequently Down-Regulated by Promoter Hypermethylation in Rhabdomyosarcoma. J. Biol. Chem. 2013, 289, 529–539. [Google Scholar] [CrossRef]
- Skrzypek, K.; Nieszporek, A.; Badyra, B.; Lasota, M.; Majka, M. Enhancement of Myogenic Differentiation and Inhibition of Rhabdomyosarcoma Progression by MiR-28-3p and MiR-193a-5p Regulated by SNAIL. Mol. Ther. Nucleic Acids 2021, 24, 888–904. [Google Scholar] [CrossRef]
- Di Paolo, V.; Paolini, A.; Galardi, A.; Gasparini, P.; De Cecco, L.; Colletti, M.; Lampis, S.; Raieli, S.; De Stefanis, C.; Miele, E.; et al. Plasma-Derived Extracellular Vesicles MiR-335–5p as Potential Diagnostic Biomarkers for Fusion-Positive Rhabdomyosarcoma. J. Exp. Clin. Cancer Res. 2024, 43, 282. [Google Scholar] [CrossRef]
- Tombolan, L.; Millino, C.; Pacchioni, B.; Cattelan, M.; Zin, A.; Bonvini, P.; Bisogno, G. Circulating MiR-26a as Potential Prognostic Biomarkers in Pediatric Rhabdomyosarcoma. Front. Genet. 2020, 11, 606274. [Google Scholar] [CrossRef]
- Hanna, J.A.; Garcia, M.R.; Lardennois, A.; Leavey, P.J.; Maglic, D.; Fagnan, A.; Go, J.C.; Roach, J.; Wang, Y.D.; Finkelstein, D.; et al. PAX3-FOXO1 Drives MiR-486-5p and Represses MiR-221 Contributing to Pathogenesis of Alveolar Rhabdomyosarcoma. Oncogene 2018, 37, 1991–2007. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; An, Q.; Niu, B.; Lu, X.; Zhang, N.; Cao, X. Role of MiR-221/222 in Tumor Development and the Underlying Mechanism. J. Oncol. 2019, 2019, 7252013. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, F.; Saab, R.; Hussein, N.; Clézardin, P.; Cohen, P.A.; Ghayad, S.E. Non-Coding RNA in Rhabdomyosarcoma Progression and Metastasis. Front. Oncol. 2022, 12, 971174. [Google Scholar] [CrossRef]
- Gasparini, P.; Ferrari, A.; Casanova, M.; Limido, F.; Massimino, M.; Sozzi, G.; Fortunato, O. MiRNAs as Players in Rhabdomyosarcoma Development. Int. J. Mol. Sci. 2019, 20, 5818. [Google Scholar] [CrossRef]
- Zoroddu, S.; Lucariello, A.; De Luca, A.; Bagella, L. Dysregulation of MiRNAs in Soft Tissue Sarcomas. Cells 2024, 13, 1853. [Google Scholar] [CrossRef]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like Growth Factors and Neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef]
- Foulstone, E.; Prince, S.; Zaccheo, O.; Burns, J.L.; Harper, J.; Jacobs, C.; Church, D.; Hassan, A.B. Insulin-like Growth Factor Ligands, Receptors, and Binding Proteins in Cancer. J. Pathol. 2005, 205, 145–153. [Google Scholar] [CrossRef]
- Atzori, F.; Traina, T.A.; Ionta, M.T.; Massidda, B. Targeting Insulin-like Growth Factor Type 1 Receptor in Cancer Therapy. Target. Oncol. 2009, 4, 255–266. [Google Scholar] [CrossRef]
- Wan, X.; Yeung, C.; Heske, C.; Mendoza, A.; Helman, L.J. IGF-1R Inhibition Activates a YES/SFK Bypass Resistance Pathway: Rational Basis for Co-Targeting IGF-1R and Yes/SFK Kinase in Rhabdomyosarcoma. Neoplasia 2015, 17, 358–366. [Google Scholar] [CrossRef]
- EL-Naggar, A.M.; Leprivier, G.; Sorensen, P.H. Soft Tissue Sarcomas. In Cancer Genomics: From Bench to Personalized Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 377–395. [Google Scholar] [CrossRef]
- Ayalon, D.; Glaser, T.; Werner, H. Transcriptional Regulation of IGF-I Receptor Gene Expression by the PAX3-FKHR Oncoprotein. Growth Horm. IGF Res. 2001, 11, 289–297. [Google Scholar] [CrossRef]
- Shipley, J.; Martins, A.S.; Olmos, D.; Missiaglia, E. Targeting the Insulin-like Growth Factor Pathway in Rhabdomyosarcomas: Rationale and Future Perspectives. Sarcoma 2011, 2011, 209736. [Google Scholar] [CrossRef]
- Tollefsen, S.E.; Sadow, J.L.; Rotwein, P. Coordinate Expression of Insulin-like Growth Factor II and Its Receptor during Muscle Differentiation. Proc. Natl. Acad. Sci. USA 1989, 86, 1543. [Google Scholar] [CrossRef] [PubMed]
- El-Badry, O.M.; Minniti, C.; Kohn, E.C.; Houghton, P.J.; Daughaday, W.H.; Helman, L.J. Insulin-like Growth Factor II Acts as an Autocrine Growth and Motility Factor in Human Rhabdomyosarcoma Tumors. Cell Growth Differ. 1990, 1, 325–331. [Google Scholar]
- Minniti, C.P.; Helman, L.J. IGF-II in the Pathogenesis of Rhabdomyosarcoma: A Prototype of IGFs Involvement in Human Tumorigenesis. Adv. Exp. Med. Biol. 1993, 343, 327–343. [Google Scholar]
- Van Gaal, J.C.; Flucke, U.E.; Roeffen, M.H.S.; De Bont, E.S.J.M.; Sleijfer, S.; Mavinkurve-Groothuis, A.M.C.; Suurmeijer, A.J.H.; Van Der Graaf, W.T.A.; Versleijen-Jonkers, Y.M.H. Anaplastic Lymphoma Kinase Aberrations in Rhabdomyosarcoma: Clinical and Prognostic Implications. J. Clin. Oncol. 2012, 30, 308–315. [Google Scholar] [CrossRef]
- Mayeenuddin, L.H.; Yu, Y.; Kang, Z.; Helman, L.J.; Cao, L. Insulin-like Growth Factor 1 Receptor Antibody Induces Rhabdomyosarcoma Cell Death via a Process Involving AKT and Bcl-x L. Oncogene 2010, 29, 6367–6377. [Google Scholar] [CrossRef]
- Houghton, P.J.; Morton, C.L.; Gorlick, R.; Kolb, E.A.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Wu, J.; Smith, M.A. Initial Testing of a Monoclonal Antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2010, 54, 921–926. [Google Scholar] [CrossRef]
- Carboni, J.M.; Wittman, M.; Yang, Z.; Lee, F.; Greer, A.; Hurlburt, W.; Hillerman, S.; Cao, C.; Cantor, G.H.; Dell-John, J.; et al. BMS-754807, a Small Molecule Inhibitor of Insulin-like Growth Factor-1R/IR. Mol. Cancer Ther. 2009, 8, 3341–3349. [Google Scholar] [CrossRef]
- Scotlandi, K.; Manara, M.C.; Nicoletti, G.; Lollini, P.L.; Lukas, S.; Benini, S.; Croci, S.; Perdichizzi, S.; Zambelli, D.; Serra, M.; et al. Antitumor Activity of the Insulin-like Growth Factor-I Receptor Kinase Inhibitor NVP-AEW541 in Musculoskeletal Tumors. Cancer Res. 2005, 65, 3868–3876. [Google Scholar] [CrossRef]
- García-Echeverría, C.; Pearson, M.A.; Marti, A.; Meyer, T.; Mestan, J.; Zimmermann, J.; Gao, J.; Brueggen, J.; Capraro, H.G.; Cozens, R.; et al. In Vivo Antitumor Activity of NVP-AEW541—A Novel, Potent, and Selective Inhibitor of the IGF-IR Kinase. Cancer Cell 2004, 5, 231–239. [Google Scholar] [CrossRef]
- A Study of R1507 in Participants with Recurrent or Refractory Sarcoma. Available online: https://clinicaltrials.gov/study/NCT00642941 (accessed on 20 May 2025).
- Cixutumumab in Treating Patients with Relapsed or Refractory Solid Tumors. Available online: https://clinicaltrials.gov/study/NCT00831844 (accessed on 20 May 2025).
- Weigel, B.; Malempati, S.; Reid, J.M.; Voss, S.D.; Cho, S.Y.; Chen, H.X.; Krailo, M.; Villaluna, D.; Adamson, P.C.; Blaney, S.M. Phase 2 Trial of Cixutumumab in Children, Adolescents, and Young Adults with Refractory Solid Tumors: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2014, 61, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Pappo, A.S.; Vassal, G.; Crowley, J.J.; Bolejack, V.; Hogendoorn, P.C.W.; Chugh, R.; Ladanyi, M.; Grippo, J.F.; Dall, G.; Staddon, A.P.; et al. A Phase 2 Trial of R1507, a Monoclonal Antibody to the Insulin-like Growth Factor-1 Receptor (IGF-1R), in Patients with Recurrent or Refractory Rhabdomyosarcoma, Osteosarcoma, Synovial Sarcoma, and Other Soft Tissue Sarcomas: Results of a Sarcoma Alliance. Cancer 2014, 120, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Heske, C.M.; Yeung, C.; Mendoza, A.; Baumgart, J.T.; Edessa, L.D.; Wan, X.; Helman, L.J. The Role of PDGFR-β Activation in Acquired Resistance to IGF-1R Blockade in Preclinical Models of Rhabdomyosarcoma. Transl. Oncol. 2016, 9, 540–547. [Google Scholar] [CrossRef][Green Version]
- Wan, X.; Harkavy, B.; Shen, N.; Grohar, P.; Helman, L.J. Rapamycin Induces Feedback Activation of Akt Signaling through an IGF-1R-Dependent Mechanism. Oncogene 2007, 26, 1932–1940. [Google Scholar] [CrossRef]
- Kolb, E.A.; Gorlick, R.; Maris, J.M.; Keir, S.T.; Morton, C.L.; Wu, J.; Wozniak, A.W.; Smith, M.A.; Houghton, P.J. Combination Testing (Stage 2) of the Anti-IGF-1 Receptor Antibody IMC-A12 with Rapamycin by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2012, 58, 729–735. [Google Scholar] [CrossRef]
- Cixutumumab and Temsirolimus in Treating Younger Patients with Recurrent or Refractory Sarcoma. Available online: https://clinicaltrials.gov/study/NCT01614795 (accessed on 20 May 2025).
- Wagner, L.M.; Fouladi, M.; Ahmed, A.; Krailo, M.D.; Weigel, B.; Dubois, S.G.; Doyle, L.A.; Chen, H.; Blaney, S.M. Phase II Study of Cixutumumab in Combination with Temsirolimus in Pediatric Patients and Young Adults with Recurrent or Refractory Sarcoma: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2015, 62, 440–444. [Google Scholar] [CrossRef]
- Malempati, S.; Weigel, B.J.; Chi, Y.Y.; Tian, J.; Anderson, J.R.; Parham, D.M.; Teot, L.A.; Rodeberg, D.A.; Yock, T.I.; Shulkin, B.L.; et al. The Addition of Cixutumumab or Temozolomide to Intensive Multiagent Chemotherapy Is Feasible but Does Not Improve Outcome for Patients with Metastatic Rhabdomyosarcoma: A Report from the Children’s Oncology Group. Cancer 2019, 125, 290–297. [Google Scholar] [CrossRef]
- Malempati, S.; Weigel, B.; Anderson, J.R.; Parham, D.; Teot, L.A.; Rodeberg, D.A.; Yock, T.I.; Shulkin, B.L.; Hawkins, D.S. Early Results from Children’s Oncology Group (COG) ARST08P1: Pilot Studies of Cixutumumab or Temozolomide with Intensive Multiagent Chemotherapy for Patients with Metastatic Rhabdomyosarcoma (RMS). J. Clin. Oncol. 2015, 33, 10015. [Google Scholar] [CrossRef]
- Insulin-like Growth Factor 1 Receptor (IGF-1R) Antibody AMG479 (Ganitumab) in Combination with the Src Family Kinase (SFK) Inhibitor Dasatinib in People with Embryonal and Alveolar Rhabdomyosarcoma. Available online: https://clinicaltrials.gov/study/NCT03041701 (accessed on 20 May 2025).
- Akshintala, S.; Sundby, R.T.; Bernstein, D.; Glod, J.W.; Kaplan, R.N.; Yohe, M.E.; Gross, A.M.; Derdak, J.; Lei, H.; Pan, A.; et al. Phase I Trial of Ganitumab plus Dasatinib to Cotarget the Insulin-Like Growth Factor 1 Receptor and Src Family Kinase YES in Rhabdomyosarcoma. Clin. Cancer Res. 2023, 29, 3329–3339. [Google Scholar] [CrossRef]
- Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Fasano, M.; Martinelli, E.; Troiani, T.; Ciardiello, F.; Morgillo, F. Role and Targeting of Anaplastic Lymphoma Kinase in Cancer. Mol. Cancer 2018, 17, 30. [Google Scholar] [CrossRef]
- Corao, D.A.; Biegel, J.A.; Coffin, C.M.; Barr, F.G.; Wainwright, L.M.; Ernst, L.M.; Choi, J.K.; Zhang, P.J.; Pawel, B.R. ALK Expression in Rhabdomyosarcomas: Correlation with Histologic Subtype and Fusion Status. Pediatr. Dev. Pathol. 2011, 12, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Shibata, T.; Wakai, S.; Ushiku, T.; Tsuta, K.; Fukayama, M.; Makimoto, A.; Furuta, K.; Tsuda, H. Anaplastic Lymphoma Kinase Status in Rhabdomyosarcomas. Mod. Pathol. 2013, 26, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Bonvini, P.; Zin, A.; Alaggio, R.; Pawel, B.; Bisogno, G.; Rosolen, A. High ALK MRNA Expression Has a Negative Prognostic Significance in Rhabdomyosarcoma. Br. J. Cancer 2013, 109, 3084–3091. [Google Scholar] [CrossRef]
- Wierdl, M.; Tsurkan, L.; Chi, L.; Hatfield, M.J.; Tollemar, V.; Bradley, C.; Chen, X.; Qu, C.; Potter, P.M. Targeting ALK in Pediatric RMS Does Not Induce Antitumor Activity in Vivo. Cancer Chemother. Pharmacol. 2018, 82, 251–263. [Google Scholar] [CrossRef]
- Schulte, J.H.; Bachmann, H.S.; Brockmeyer, B.; DePreter, K.; Oberthur, A.; Ackermann, S.; Kahlert, Y.; Pajtler, K.; Theissen, J.; Westermann, F.; et al. High ALK Receptor Tyrosine Kinase Expression Supersedes ALK Mutation as a Determining Factor of an Unfavorable Phenotype in Primary Neuroblastoma. Clin. Cancer Res. 2011, 17, 5082–5092. [Google Scholar] [CrossRef]
- Fleuren, E.D.G.; Roeffen, M.H.S.; Leenders, W.P.; Flucke, U.E.; Vlenterie, M.; Schreuder, H.W.; Boerman, O.C.; Van Der Graaf, W.T.A.; Versleijen-Jonkers, Y.M.H. Expression and Clinical Relevance of MET and ALK in Ewing Sarcomas. Int. J. Cancer 2013, 133, 427–436. [Google Scholar] [CrossRef]
- Gerber, D.E.; Minna, J.D. ALK Inhibition for Non-Small Cell Lung Cancer: From Discovery to Therapy in Record Time. Cancer Cell 2010, 18, 548–551. [Google Scholar] [CrossRef]
- Megiorni, F.; McDowell, H.P.; Camero, S.; Mannarino, O.; Ceccarelli, S.; Paiano, M.; Losty, P.D.; Pizer, B.; Shukla, R.; Pizzuti, A.; et al. Crizotinib-Induced Antitumour Activity in Human Alveolar Rhabdomyosarcoma Cells Is Not Solely Dependent on ALK and MET Inhibition. J. Exp. Clin. Cancer Res. 2015, 34, 112. [Google Scholar] [CrossRef]
- CREATE: Cross-tumoral Phase 2 with Crizotinib (CREATE). Available online: https://clinicaltrials.gov/study/NCT01524926 (accessed on 20 May 2025).
- van Erp, A.E.M.; Hillebrandt-Roeffen, M.H.S.; van Houdt, L.; Fleuren, E.D.G.; van der Graaf, W.T.A.; Versleijen-Jonkers, Y.M.H. Targeting Anaplastic Lymphoma Kinase (ALK) in Rhabdomyosarcoma (RMS) with the Second-Generation ALK Inhibitor Ceritinib. Target. Oncol. 2017, 12, 815–826. [Google Scholar] [CrossRef]
- Van Gaal, J.C.; Roeffen, M.H.S.; Flucke, U.E.; Van Der Laak, J.A.W.M.; Van Der Heijden, G.; De Bont, E.S.J.M.; Suurmeijer, A.J.H.; Versleijen-Jonkers, Y.M.H.; Van Der Graaf, W.T.A. Simultaneous Targeting of Insulin-like Growth Factor-1 Receptor and Anaplastic Lymphoma Kinase in Embryonal and Alveolar Rhabdomyosarcoma: A Rational Choice. Eur. J. Cancer 2013, 49, 3462–3470. [Google Scholar] [CrossRef]
- Berardi, A.C.; Marsilio, S.; Rofani, C.; Salvucci, O.; Altavista, P.; Perla, F.M.; Diomedi-Camassei, F.; Uccini, S.; Kokai, G.; Landuzzi, L.; et al. Up-Regulation of EphB and Ephrin-B Expression in Rhabdomyosarcoma. Anticancer Res. 2008, 28, 763–769. [Google Scholar] [PubMed]
- Aslam, M.I.; Abraham, J.; Mansoor, A.; Druker, B.J.; Tyner, J.W.; Keller, C. PDGFRβ Reverses EphB4 Signaling in Alveolar Rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, 6383–6388. [Google Scholar] [CrossRef] [PubMed]
- Gandhy, S.U.; Casak, S.J.; Mushti, S.L.; Cheng, J.; Subramaniam, S.; Zhao, H.; Zhao, M.; Bi, Y.; Liu, G.; Fan, J.; et al. FDA Approval Summary: Futibatinib for Unresectable Advanced or Metastatic, Chemotherapy Refractory Intrahepatic Cholangiocarcinoma with FGFR2 Fusions or Other Rearrangements. Clin. Cancer Res. 2023, 29, 4027–4031. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Cheuk, A.; Isanogle, K.; Robinson, C.; Zhang, X.; Ceribelli, M.; Beck, E.; Shinn, P.; Klumpp-Thomas, C.; Wilson, K.M.; et al. Preclinical Evaluation of the FGFR-Family Inhibitor Futibatinib for Pediatric Rhabdomyosarcoma. Cancers 2023, 15, 4034. [Google Scholar] [CrossRef]
- Niesen, J.; Brehm, H.; Stein, C.; Berges, N.; Pardo, A.; Fischer, R.; ten Haaf, A.; Gattenlöhner, S.; Tur, M.K.; Barth, S. In Vitro Effects and Ex Vivo Binding of an EGFR-Specific Immunotoxin on Rhabdomyosarcoma Cells. J. Cancer Res. Clin. Oncol. 2015, 141, 1049–1061. [Google Scholar] [CrossRef]
- Abraham, J.; Nelon, L.D.; Kubicek, C.B.; Kilcoyne, A.; Hampton, S.T.; Zarzabal, L.A.; Giles, F.J.; Michalek, J.E.; Rubin, B.P.; Keller, C. Preclinical Testing of Erlotinib in a Transgenic Alveolar Rhabdomyosarcoma Mouse Model. Sarcoma 2011, 2011, 130484. [Google Scholar] [CrossRef]
- Herrmann, D.; Seitz, G.; Warmann, S.W.; Bonin, M.; Fuchs, J.; Armeanu-Ebinger, S. Cetuximab Promotes Immunotoxicity against Rhabdomyosarcoma in Vitro. J. Immunother. 2010, 33, 279–286. [Google Scholar] [CrossRef]
- Ganti, R.; Skapek, S.X.; Zhang, J.; Fuller, C.E.; Wu, J.; Billups, C.A.; Breitfeld, P.P.; Dalton, J.D.; Meyer, W.H.; Khoury, J.D. Expression and Genomic Status of EGFR and ErbB-2 in Alveolar and Embryonal Rhabdomyosarcoma. Mod. Pathol. 2006, 19, 1213–1220. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Mikhailova, T.; Li, X.; Porter, B.A.; Bah, A.; Kotula, L. Src Family Kinases, Adaptor Proteins and the Actin Cytoskeleton in Epithelial-to-Mesenchymal Transition. Cell Commun. Signal. 2021, 19, 67. [Google Scholar] [CrossRef]
- Birge, R.B.; Kalodimos, C.; Inagaki, F.; Tanaka, S. Crk and CrkL Adaptor Proteins: Networks for Physiological and Pathological Signaling. Cell Commun. Signal. 2009, 7, 13. [Google Scholar] [CrossRef]
- Yeung, C.L.; Ngo, V.N.; Grohar, P.J.; Arnaldez, F.I.; Asante, A.; Wan, X.; Khan, J.; Hewitt, S.M.; Khanna, C.; Staudt, L.M.; et al. Loss-of-Function Screen in Rhabdomyosarcoma Identifies CRKL-YES as a Critical Signal for Tumor Growth. Oncogene 2013, 32, 5429–5438. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, Z.; Shan, L.; Zeng, H.; Hua, Y.; Cai, Z. The Importance of Src Signaling in Sarcoma (Review). Oncol. Lett. 2015, 10, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rai, K. Personalized Cancer Therapy: Yes1 Is the New Kid on the Block. Cancer Res. 2019, 79, 5702–5703. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.G.; McAndrew, P.C.; Eve, P.D.; Garrett, M.D. Phosphorylation of Cyclin Dependent Kinase 4 on Tyrosine 17 Is Mediated by Src Family Kinases. FEBS J. 2008, 275, 3099–3109. [Google Scholar] [CrossRef]
- Martellucci, S.; Clementi, L.; Sabetta, S.; Mattei, V.; Botta, L.; Angelucci, A. Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned so Far. Cancers 2020, 12, 1448. [Google Scholar] [CrossRef]
- Casini, N.; Forte, I.M.; Mastrogiovanni, G.; Pentimalli, F.; Angelucci, A.; Festuccia, C.; Tomei, V.; Ceccherini, E.; Di Marzo, D.; Schenone, S.; et al. SRC Family Kinase (SFK) Inhibition Reduces Rhabdomyosarcoma Cell Growth in Vitro and in Vivo and Triggers P38 MAP Kinase-Mediated Differentiation. Oncotarget 2015, 6, 12421. [Google Scholar] [CrossRef][Green Version]
- Waters, A.M.; Stafman, L.L.; Garner, E.F.; Mruthyunjayappa, S.; Stewart, J.E.; Mroczek-Musulman, E.; Beierle, E.A. Targeting Focal Adhesion Kinase Suppresses the Malignant Phenotype in Rhabdomyosarcoma Cells. Transl. Oncol. 2016, 9, 263–273. [Google Scholar] [CrossRef]
- Jothi, M.; Nishijo, K.; Keller, C.; Mal, A.K. AKT and PAX3-FKHR Cooperation Enforces Myogenic Differentiation Blockade in Alveolar Rhabdomyosarcoma Cell. Cell Cycle 2012, 11, 895–908. [Google Scholar] [CrossRef]
- Jothi, M.; Mal, M.; Keller, C.; Mal, A.K. Small Molecule Inhibition of PAX3-FOXO1 through AKT Activation Suppresses Malignant Phenotypes of Alveolar Rhabdomyosarcoma. Mol. Cancer Ther. 2013, 12, 2663–2674. [Google Scholar] [CrossRef]
- Shen, J.; Hong, Y.; Zhao, Q.; Zhang, J.-L. Preclinical Evaluation of Perifosine as a Potential Promising Anti-Rhabdomyosarcoma Agent. Tumor Biol. 2016, 37, 1025–1033. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR Signaling Pathway and MTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Shen, N.; Mendoza, A.; Khanna, C.; Helman, L.J. CCI-779 Inhibits Rhabdomyosarcoma Xenograft Growth by an Antiangiogenic Mechanism Linked to the Targeting of MTOR/Hif-1α/VEGF Signaling. Neoplasia 2006, 8, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kaylani, S.Z.; Xu, J.; Srivastava, R.K.; Kopelovich, L.; Pressey, J.G.; Athar, M. Rapamycin Targeting MTOR and Hedgehog Signaling Pathways Blocks Human Rhabdomyosarcoma Growth in Xenograft Murine Model. Biochem. Biophys. Res. Commun. 2013, 435, 557–561. [Google Scholar] [CrossRef]
- Houghton, P.J.; Gorlick, R.; Kolb, E.A.; Lock, R.; Carol, H.; Morton, C.L.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Phelps, D.; et al. Initial Testing (Stage 1) of the MTOR Kinase Inhibitor AZD8055 by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2012, 58, 191–199. [Google Scholar] [CrossRef]
- Preuss, E.; Hugle, M.; Reimann, R.; Schlecht, M.; Fulda, S. Pan-Mammalian Target of Rapamycin (MTOR) Inhibitor AZD8055 Primes Rhabdomyosarcoma Cells for ABT-737- Induced Apoptosis by Down-Regulating Mcl-1 Protein. J. Biol. Chem. 2013, 288, 35287–35296. [Google Scholar] [CrossRef]
- Gupta, A.A.; Xue, W.; Harrison, D.J.; Hawkins, D.S.; Dasgupta, R.; Wolden, S.; Shulkin, B.; Qumseya, A.; Routh, J.C.; MacDonald, T.; et al. Addition of Temsirolimus to Chemotherapy in Children, Adolescents, and Young Adults with Intermediate-Risk Rhabdomyosarcoma (ARST1431): A Randomised, Open-Label, Phase 3 Trial from the Children’s Oncology Group. Lancet Oncol. 2024, 25, 912–921. [Google Scholar] [CrossRef]
- Renshaw, J.; Taylor, K.R.; Bishop, R.; Valenti, M.; De Haven Brandon, A.; Gowan, S.; Eccles, S.A.; Ruddle, R.R.; Johnson, L.D.; Raynaud, F.I.; et al. Dual Blockade of the PI3K/AKT/MTOR (AZD8055) and RAS/MEK/ERK (AZD6244) Pathways Synergistically Inhibits Rhabdomyosarcoma Cell Growth in Vitro and in Vivo. Clin. Cancer Res. 2013, 19, 5940–5951. [Google Scholar] [CrossRef]
- Guenther, M.K.; Graab, U.; Fulda, S. Synthetic Lethal Interaction between PI3K/Akt/MTOR and Ras/MEK/ERK Pathway Inhibition in Rhabdomyosarcoma. Cancer Lett. 2013, 337, 200–209. [Google Scholar] [CrossRef]
- Graab, U.; Hahn, H.; Fulda, S.; Graab, U.; Hahn, H.; Fulda, S. Identification of a Novel Synthetic Lethality of Combined Inhibition of Hedgehog and PI3K Signaling in Rhabdomyosarcoma. Oncotarget 2015, 6, 8722–8735. [Google Scholar] [CrossRef]
- Dolfini, D.; Imbriano, C.; Mantovani, R. The Role(s) of NF-Y in Development and Differentiation. Cell Death Differ. 2024, 32, 195–206. [Google Scholar] [CrossRef]
- Sroka, M.W.; Skopelitis, D.; Vermunt, M.W.; Preall, J.B.; El Demerdash, O.; de Almeida, L.M.N.; Chang, K.; Utama, R.; Gryder, B.; Caligiuri, G.; et al. Myo-Differentiation Reporter Screen Reveals NF-Y as an Activator of PAX3-FOXO1 in Rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 2023, 120, e2303859120. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Okada, S.; Ishikawa, A.; Wang, B.; Waseda, M.; Kaneko, M.K.; Kato, Y.; Kaneko, S. Development of Chimeric Antigen Receptor T Cells Targeting Cancer-Expressing Podocalyxin. Regen. Ther. 2025, 28, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Deel, M.D. Advances in the Management of Pediatric Genitourinary Rhabdomyosarcoma. Transl. Androl. Urol. 2020, 9, 2441. [Google Scholar] [CrossRef]
- Pacenta, H.L.; Allen-Rhoades, W.; Langenau, D.; Houghton, P.J.; Keller, C.; Heske, C.M.; Deel, M.D.; Linardic, C.M.; Shern, J.F.; Stewart, E.; et al. Prioritization of Novel Agents for Patients with Rhabdomyosarcoma: A Report from the Children’s Oncology Group (COG) New Agents for Rhabdomyosarcoma Task Force. J. Clin. Med. 2021, 10, 1416. [Google Scholar] [CrossRef]
- Shern, J.F.; Selfe, J.; Izquierdo, E.; Patidar, R.; Chou, H.C.; Song, Y.K.; Yohe, M.E.; Sindiri, S.; Wei, J.; Wen, X.; et al. Genomic Classification and Clinical Outcome in Rhabdomyosarcoma: A Report From an International Consortium. J. Clin. Oncol. 2021, 39, 2859–2871. [Google Scholar] [CrossRef]
- Macy, M.E.; Mody, R.; Reid, J.M.; Piao, J.; Saguilig, L.; Alonzo, T.A.; Berg, S.L.; Fox, E.; Weigel, B.J.; Hawkins, D.S.; et al. Palbociclib in Solid Tumor Patients with Genomic Alterations in the CyclinD-Cdk4/6-INK4a-Rb Pathway: Results From National Cancer Institute-Children’s Oncology Group Pediatric Molecular Analysis for Therapy Choice Trial Arm I (APEC1621I). JCO Precis. Oncol. 2024, 8, e2400418. [Google Scholar] [CrossRef]
- Barghi, F.; Shannon, H.E.; Saadatzadeh, M.R.; Bailey, B.J.; Riyahi, N.; Bijangi-Vishehsaraei, K.; Just, M.; Ferguson, M.J.; Pandya, P.H.; Pollok, K.E. Precision Medicine Highlights Dysregulation of the CDK4/6 Cell Cycle Regulatory Pathway in Pediatric, Adolescents and Young Adult Sarcomas. Cancers 2022, 14, 3611. [Google Scholar] [CrossRef]
- Mita, M.M.; Mita, A.C. Bromodomain Inhibitors a Decade Later: A Promise Unfulfilled? Br. J. Cancer 2020, 123, 1713–1714. [Google Scholar] [CrossRef]
- Schöffski, P.; Machiels, J.P.; Rottey, S.; Sadrolhefazi, B.; Musa, H.; Marzin, K.; Awada, A. Phase Ia Dose-Escalation Trial with the BET Protein Inhibitor BI 894999 in Patients with Advanced or Metastatic Solid Tumours. Eur. J. Cancer 2023, 191, 112987. [Google Scholar] [CrossRef]
- Mosse, Y.P.; Fox, E.; Teachey, D.T.; Reid, J.M.; Safgren, S.L.; Carol, H.; Lock, R.B.; Houghton, P.J.; Smith, M.A.; Hall, D.; et al. A Phase 2 Study of Alisertib in Children with Recurrent/Refractory Solid Tumors or Leukemia: Children’s Oncology Group Phase 1 and Pilot Consortium (ADVL0921). Clin. Cancer Res. 2019, 25, 3229–3238. [Google Scholar] [CrossRef]
- de Traux de Wardin, H.; Dermawan, J.K.; Merlin, M.S.; Wexler, L.H.; Orbach, D.; Vanoli, F.; Schleiermacher, G.; Geoerger, B.; Ballet, S.; Guillemot, D.; et al. Sequential Genomic Analysis Using a Multisample/Multiplatform Approach to Better Define Rhabdomyosarcoma Progression and Relapse. NPJ Precis. Oncol. 2023, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Eguchi-Ishimae, M.; Tezuka, M.; Kokeguchi, T.; Nagai, K.; Moritani, K.; Yonezawa, S.; Tauchi, H.; Tokuda, K.; Ishida, Y.; Ishii, E.; et al. Early Detection of the PAX3-FOXO1 Fusion Gene in Circulating Tumor-Derived DNA in a Case of Alveolar Rhabdomyosarcoma. Genes Chromosomes Cancer 2019, 58, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Ruhen, O.; Lak, N.S.M.; Stutterheim, J.; Danielli, S.G.; Chicard, M.; Iddir, Y.; Saint-Charles, A.; Di Paolo, V.; Tombolan, L.; Gatz, S.A.; et al. Molecular Characterization of Circulating Tumor DNA in Pediatric Rhabdomyosarcoma: A Feasibility Study. JCO Precis. Oncol. 2022, 6, e2100534. [Google Scholar] [CrossRef]
- Paras, K.I.; Brunner, J.S.; Boyer, J.A.; Montero, A.M.; Jackson, B.T.; Chakraborty, S.; Xie, A.; Guillan, K.; Siddiquee, A.; Torres, L.P.; et al. PAX3-FOXO1 Drives Targetable Cell State-Dependent Metabolic Vulnerabilities in Rhabdomyosarcoma. bioRxiv 2025. [Google Scholar] [CrossRef]
- World Health Organization. Union for International Cancer Control Review of Cancer Medicines on the WHO List of Essential Medicines: Chronic Lymphocytic Leukemia. Available online: https://cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-expert-committee/public-comments/cmwg-advice.pdf?sfvrsn=75c12c3f_5 (accessed on 20 April 2025).
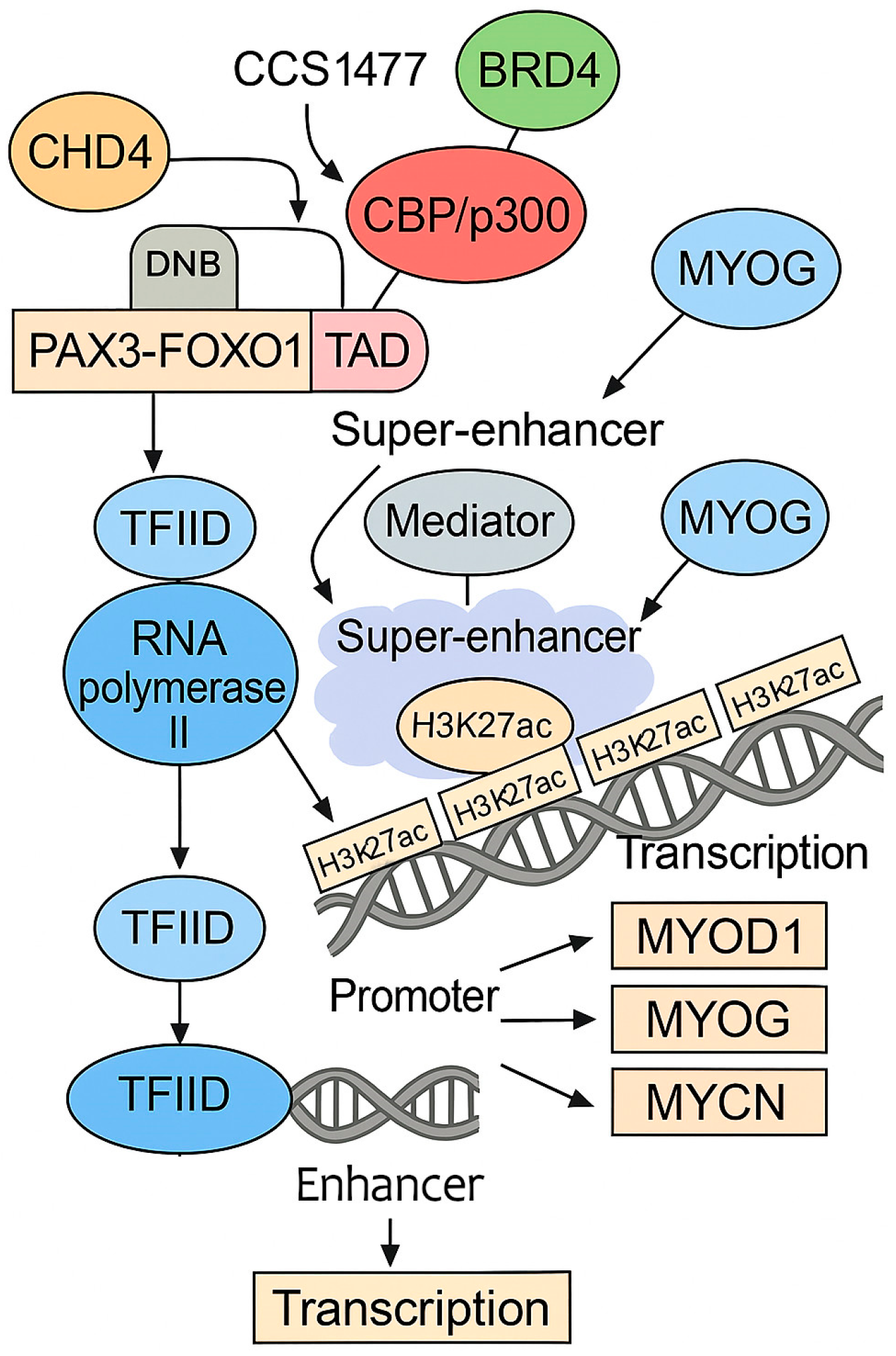
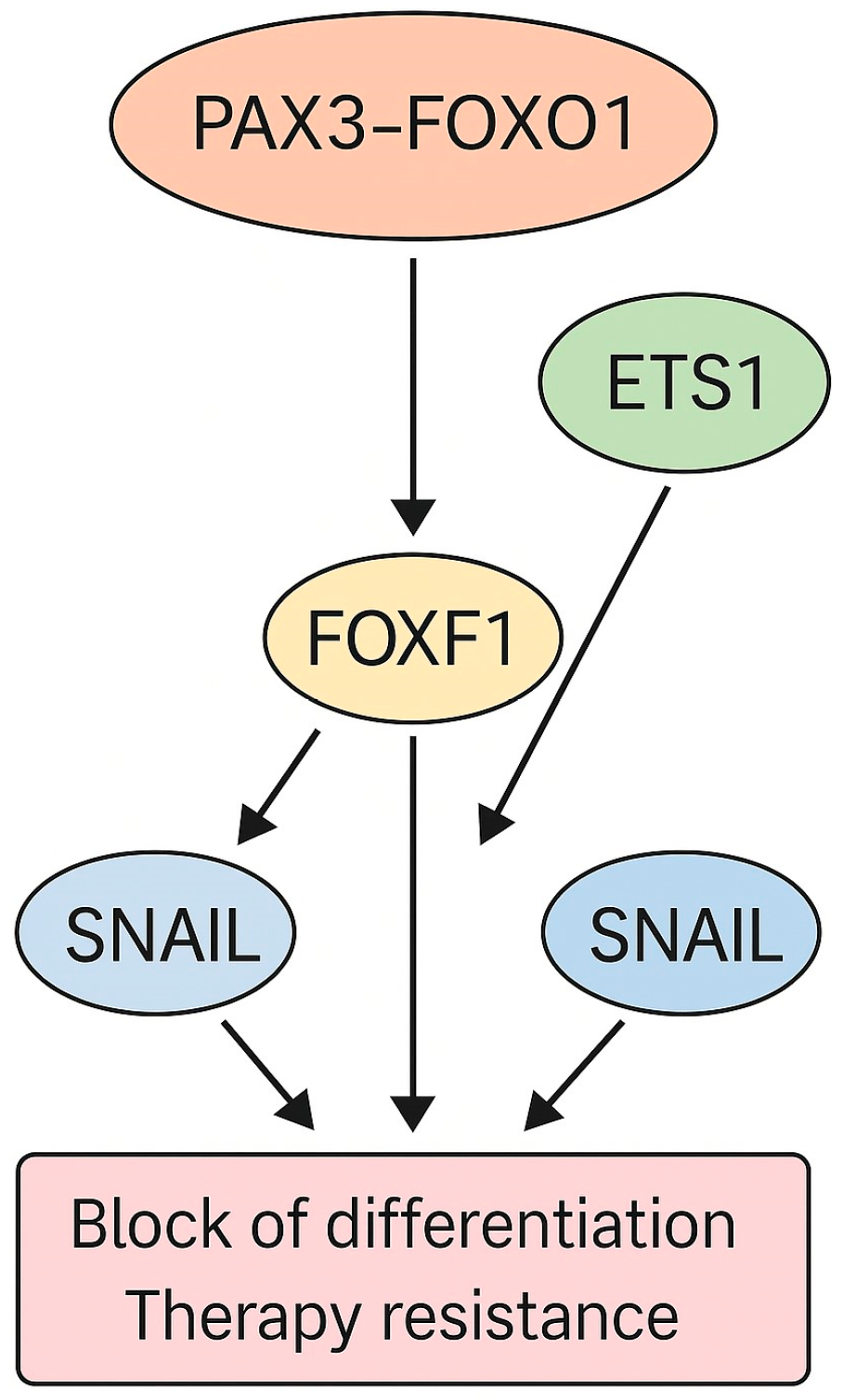
| Molecular Feature | Gene/Protein | Alteration Type | Functional Impact | Therapeutic Implication | Ref. |
|---|---|---|---|---|---|
| Fusion oncogene | PAX3-FOXO1/PAX7-FOXO1 | Gene fusion (t(2;13), t(1;13)) | Potent transcriptional activator, pioneer factor, super-enhancer rewiring | Core oncogenic driver; indirect targeting via co-factors like BRD4 | [12,13,19,20,21,29,38,39] |
| Cell cycle regulator | CDK4 | Amplification (12q13–q14) | Promotes cell cycle progression | CDK4/6 inhibitors (e.g., palbociclib) | [22] |
| Tumor suppressor | CAV1 | Downregulation/silencing | Loss of signaling modulation | Potential diagnostic marker | [23] |
| Oncogene cooperation | CDKN2A, TP53 | Deletion, mutation, promoter methylation | Loss of tumor suppressor function (p16, p53) | Potential sensitization to DNA-damaging agents | [33,34,35,36] |
| RTK signaling | FGFR4, IGF1R, ALK, MET, etc. | Overexpression, mutation | Altered proliferative signaling | Targetable RTKs | [24,36] |
| Epigenetic regulator | BRD4 | Functional dependency via SE recruitment | Maintains transcriptional program driven by PAX3-FOXO1 | BET inhibitors (e.g., JQ1) | [39] |
| Transcription factors | MYOD1, MYCN, MYOG | Amplification, mutation | Cooperative activation of muscle-lineage genes | Targetable dependencies in SE networks | [37,38] |
| Signaling pathways | PI3K, RAS | Pathway activation (mutation, amplification) | Enhanced survival and proliferation | PI3K/mTOR or RAS/MAPK inhibitors | [14,24,37,41] |
| Tumor suppressor targets | DAPK1, GREM1, HEY1 | Altered expression via PAX3-FOXO1 | Suppressed apoptosis, altered differentiation | Indirect targeting possible | [30] |
| Core regulatory TFs (CR TFs) | MYOD1, MYOG, MYCN | SE-driven overexpression | Define oncogenic transcriptional circuitry | Epigenetic therapy, CR TF network disruption | [42] |
| RTK | Role in FP-RMS | Key Inhibitors | Clinical Status | Source |
|---|---|---|---|---|
| FGFR4 | Overexpressed; transcriptional target of PAX3-FOXO1; promotes proliferation | FGF401, INCB062079 | Preclinical and early-phase trials in FGFR4-driven tumors | [127] |
| IGF1R | Activates PI3K/AKT signaling; supports cell survival and proliferation | Linsitinib (OSI-906), Xentuzumab | Phase I/II trials; limited efficacy in FP-RMS | [128] |
| PDGFRα/β | Enhances tumor growth through autocrine signaling loops | Imatinib, Sunitinib | Preclinical and early trials in RMS; not approved | [41] |
| MET | Direct PAX3-FOXO1 target; promotes cell motility and invasion | Crizotinib, Tivantinib | Phase I/II trials; limited clinical benefit in RMS | [17] |
| ALK | Aberrant expression; associated with resistance and poor prognosis | Ceritinib, Lorlatinib | Explored in pediatric tumors; limited RMS-specific data | [129] |
| EGFR | Occasionally activated; may cooperate with IGF1R signaling | Erlotinib, Gefitinib | Explored in sarcoma; limited data in RMS | [130] |
| VEGFR1/2 | Supports angiogenesis and tumor–stroma interactions | Bevacizumab, Axitinib | Studied in soft tissue sarcomas; no RMS-specific results | [131] |
| Target | Biological Role | Mechanism in ARMS | Therapeutics Investigated | Preclinical Results | Clinical Outcome |
|---|---|---|---|---|---|
| IGF-1R | RTK involved in growth, proliferation, survival | Overexpressed in ARMS; transcriptional target of PAX3-FOXO1 | Cixutumumab, R1507, AMG479 (Ganitumab) | Effective in vitro and in xenografts; synergistic with mTOR/SFK inhibitors | Limited efficacy in trials (NCT00642941, NCT00831844); combination trials ongoing (NCT03041701) |
| ALK | RTK from insulin receptor family; regulates growth signaling | Overexpressed in high-risk RMS; no gene amplification or activating mutations | Crizotinib, Ceritinib | Crizotinib effective in vitro; Ceritinib effective in Rh41 model | Crizotinib accelerated tumor growth in PDX; limited activity in clinical trial (NCT01524926) |
| EphB4 | RTK; regulates cell positioning and vasculature | Overexpressed in ARMS; dual role in apoptosis and proliferation via PDGFRβ | Dasatinib, VasG3 antibody, soluble EphB4 | Dual inhibition of EphB4 and PDGFRβ reduced growth and improved survival | Therapeutic ambiguity; direct inhibition ineffective |
| Futibatinib | Pan-FGFR irreversible inhibitor | Inhibits FGFR1–4 signaling; targets FGFR4, which is upregulated in ARMS | Futibatinib | Inhibits RMS cell proliferation in vitro; synergy with chemotherapy | Poor response in xenograft models; not effective in FP-RMS |
| EGFR | RTK involved in cell proliferation and survival | Expressed in RMS; unclear dependency | Erlotinib, Gefitinib | Some in vitro activity; enhanced by IGF-1R inhibition | No clinical success reported |
| SFK–CRKL–YES | Non-receptor tyrosine kinase cascade | YES activation bypasses IGF-1R inhibition; CRKL regulates RTK signaling | Dasatinib | Dual inhibition with IGF-1R antibody enhanced response | Phase I/II trial in progress (NCT03041701) |
| FAK–Src | Cytoplasmic kinases involved in adhesion, migration, and survival | Hyperactivated in ARMS; promotes invasiveness | FAK inhibitors + Dasatinib | Combination showed synergy in reducing motility and invasion | Clinical data not available |
| AKT | Central node in PI3K signaling; regulates survival and metabolism | Hyperactivation supports cell survival; regulated by IGF-1R, TRIB3 | AKT inhibitors (various) | Inhibition reduces proliferation; affected by multiple feedback loops | Not evaluated as monotherapy in ARMS clinical trials |
| mTOR | Downstream kinase of PI3K/AKT; regulates protein synthesis | Activated in ARMS; promotes growth and resistance | Temsirolimus + Cixutumumab | Effective in vitro and xenografts; synergistic with IGF-1R inhibitors | Failed to produce objective responses in Phase II (NCT01614795) |
| NF-Y | Transcription factor controlling cell cycle genes | Supports PAX3-FOXO1 expression; controls growth and survival | siRNA, potential future inhibitors | NF-Y inhibition reduces tumor cell proliferation and PAX3-FOXO1 expression | Not yet explored in clinical settings |
| PODXL | Anti-adhesive transmembrane protein | Direct target of PAX3-FOXO1; supports migration, invasion, drug resistance | Knockdown models | Depletion impairs ARMS cell growth and survival | No clinical inhibitors; promising target for further research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemba, B.; Lukow, K. Molecular Targets in Alveolar Rhabdomyosarcoma: A Narrative Review of Progress and Pitfalls. Int. J. Mol. Sci. 2025, 26, 5204. https://doi.org/10.3390/ijms26115204
Ziemba B, Lukow K. Molecular Targets in Alveolar Rhabdomyosarcoma: A Narrative Review of Progress and Pitfalls. International Journal of Molecular Sciences. 2025; 26(11):5204. https://doi.org/10.3390/ijms26115204
Chicago/Turabian StyleZiemba, Barbara, and Klaudia Lukow. 2025. "Molecular Targets in Alveolar Rhabdomyosarcoma: A Narrative Review of Progress and Pitfalls" International Journal of Molecular Sciences 26, no. 11: 5204. https://doi.org/10.3390/ijms26115204
APA StyleZiemba, B., & Lukow, K. (2025). Molecular Targets in Alveolar Rhabdomyosarcoma: A Narrative Review of Progress and Pitfalls. International Journal of Molecular Sciences, 26(11), 5204. https://doi.org/10.3390/ijms26115204






