Clostridia and Enteroviruses as Synergistic Triggers of Type 1 Diabetes Mellitus
Abstract
1. Introduction
2. Results
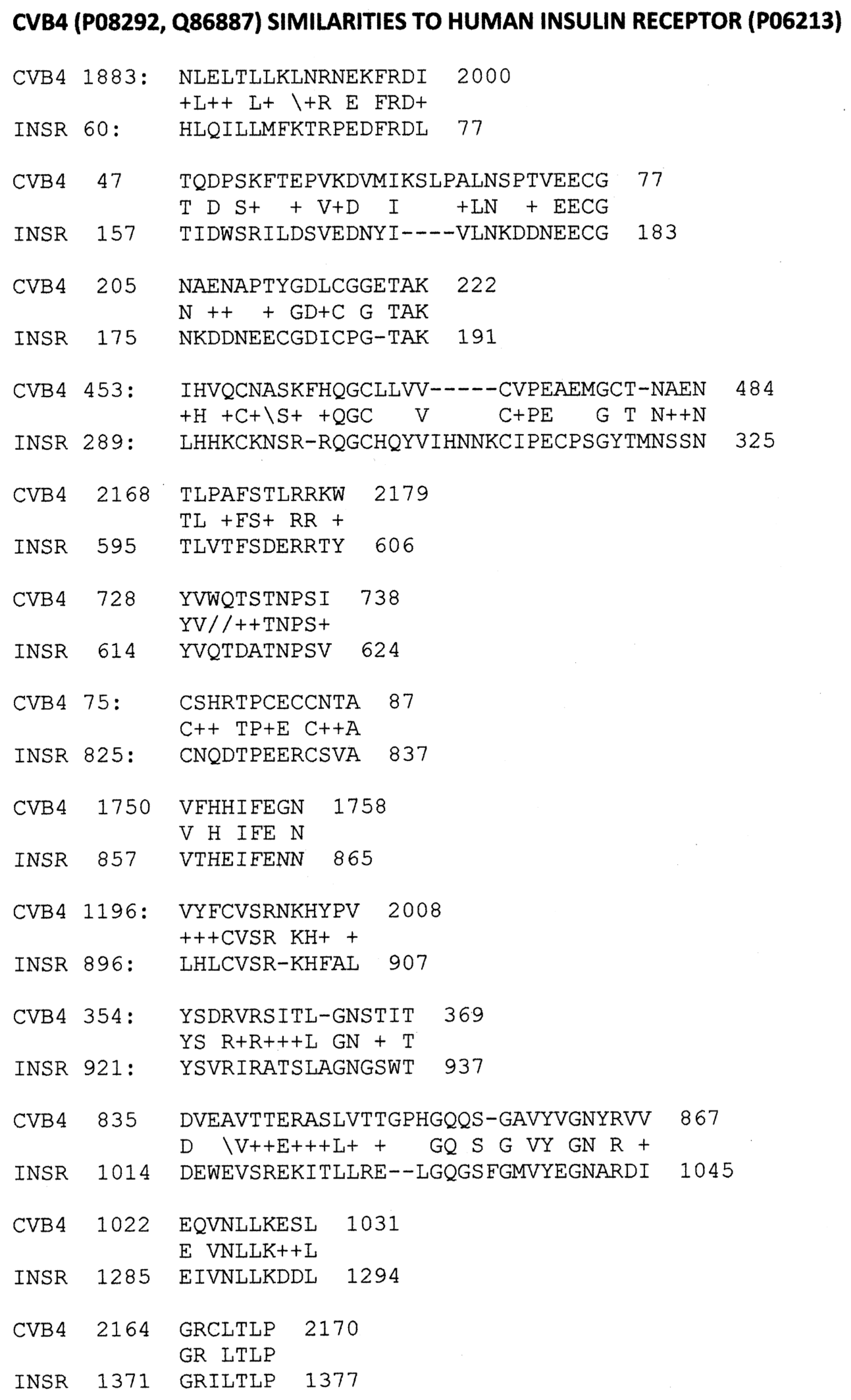
2.1. Proteomic Search for Microbial Similarities to T1DM Autoantigens
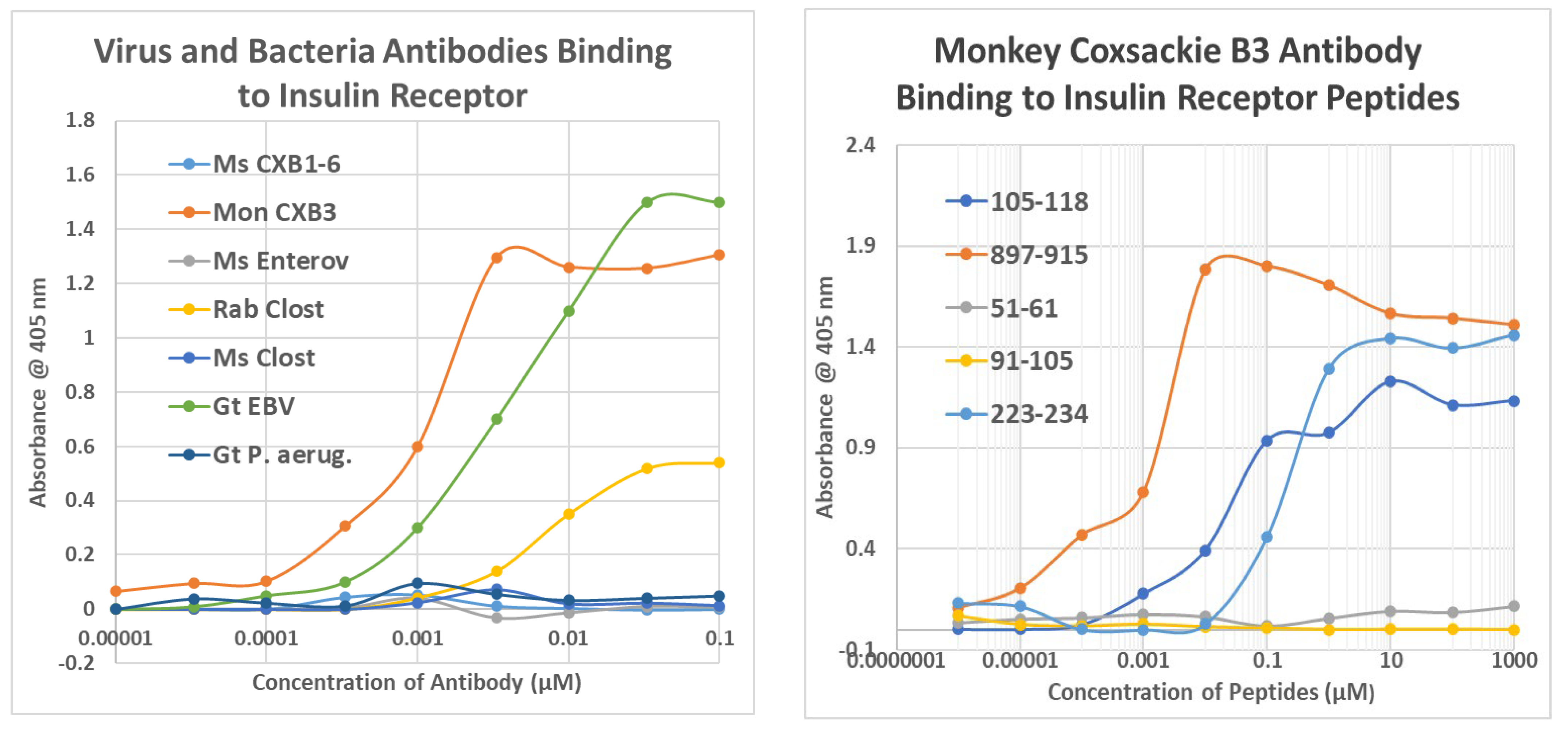
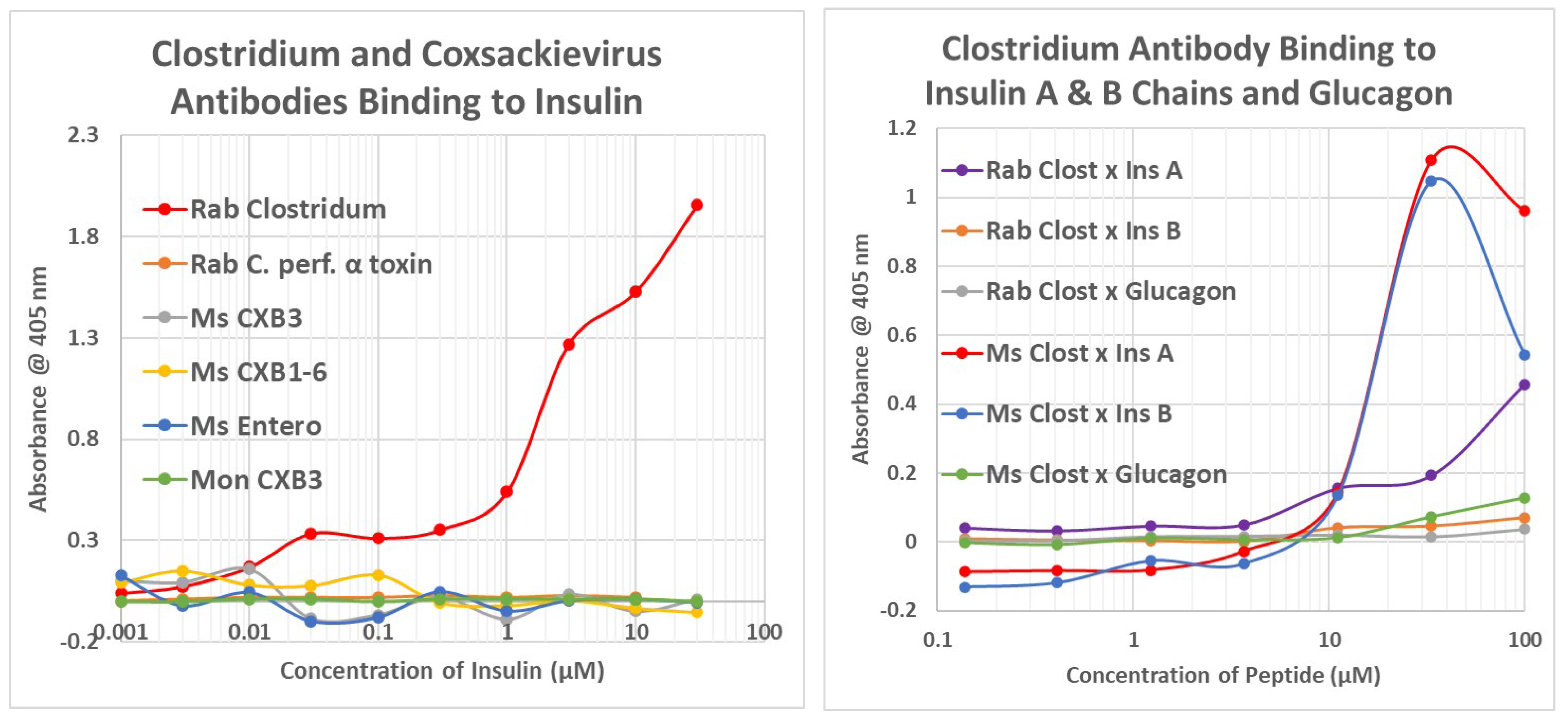
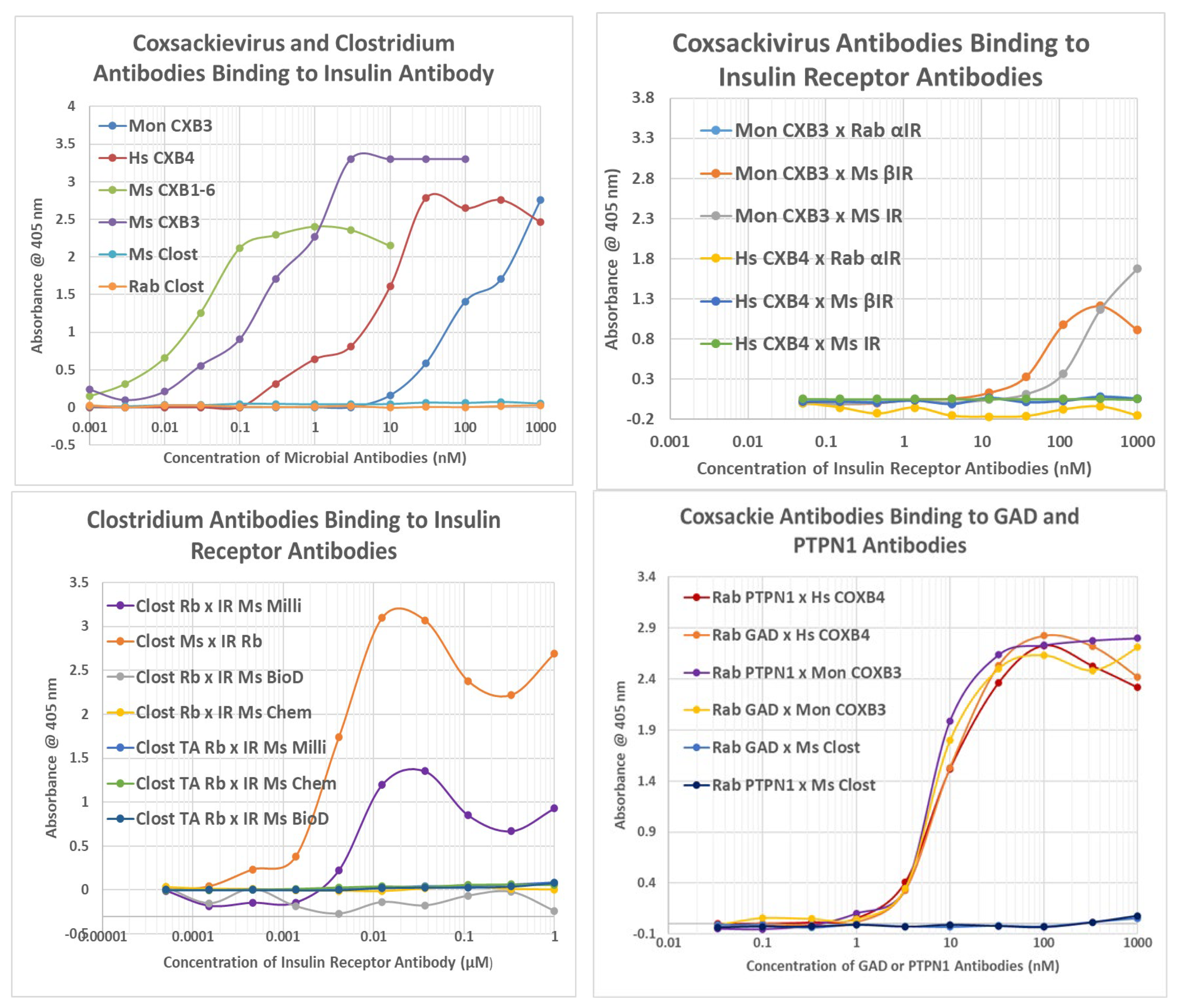
2.2. Microbial Antibody Binding to INS, INSR, and Peptides Derived from INSR
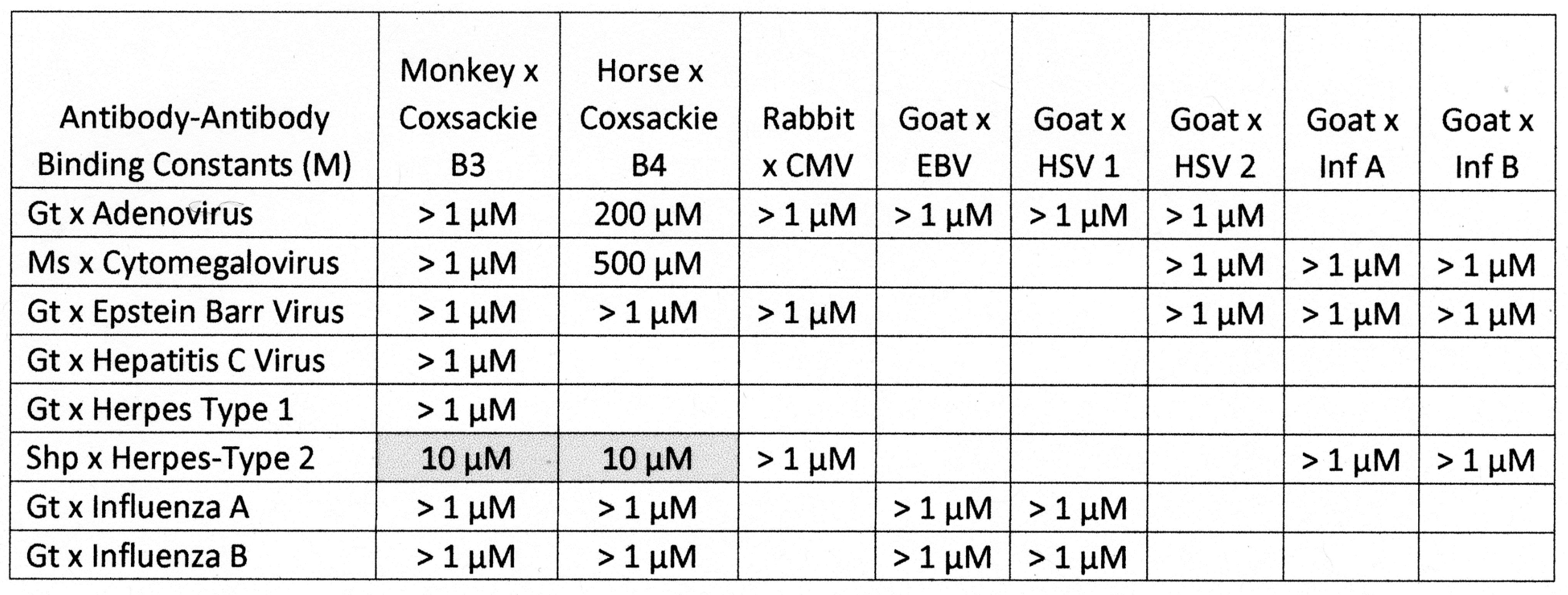
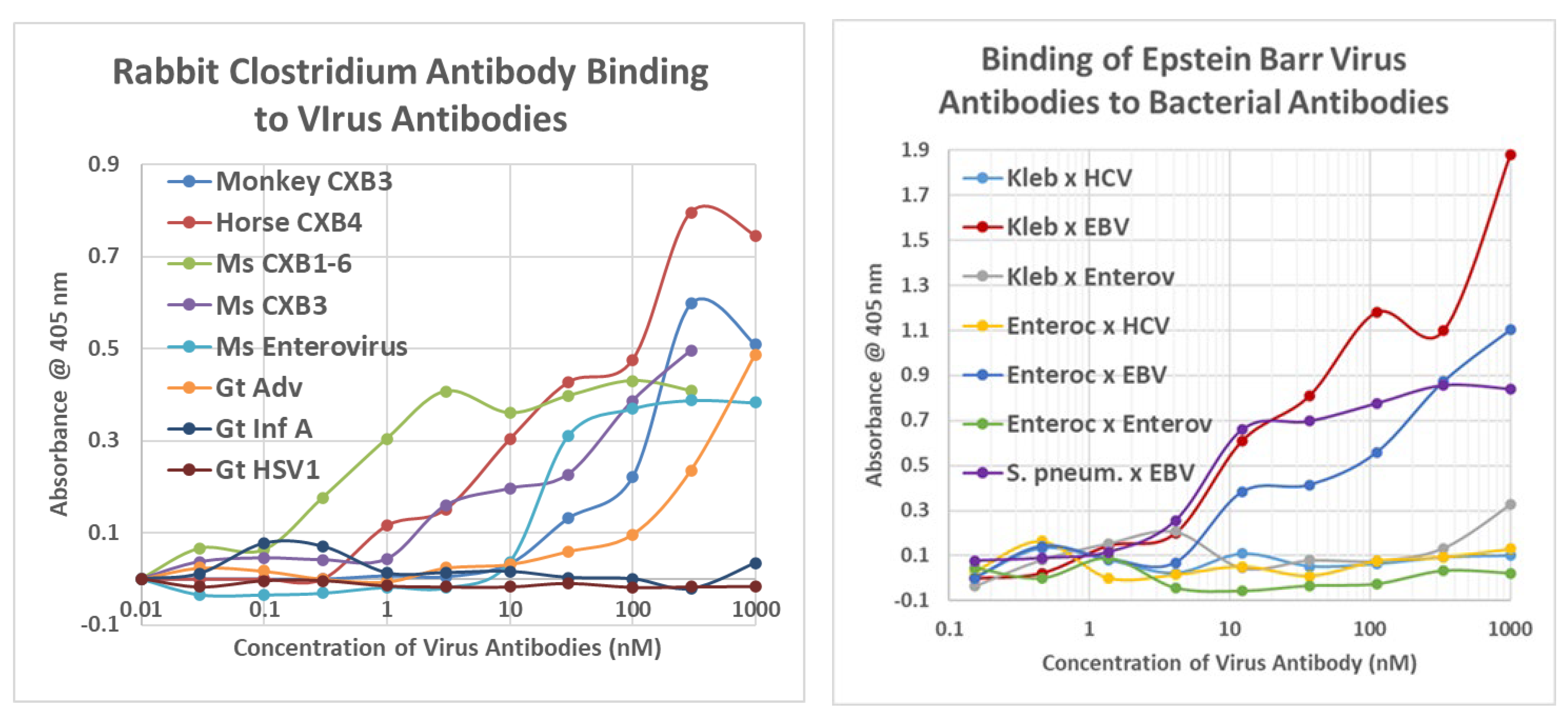
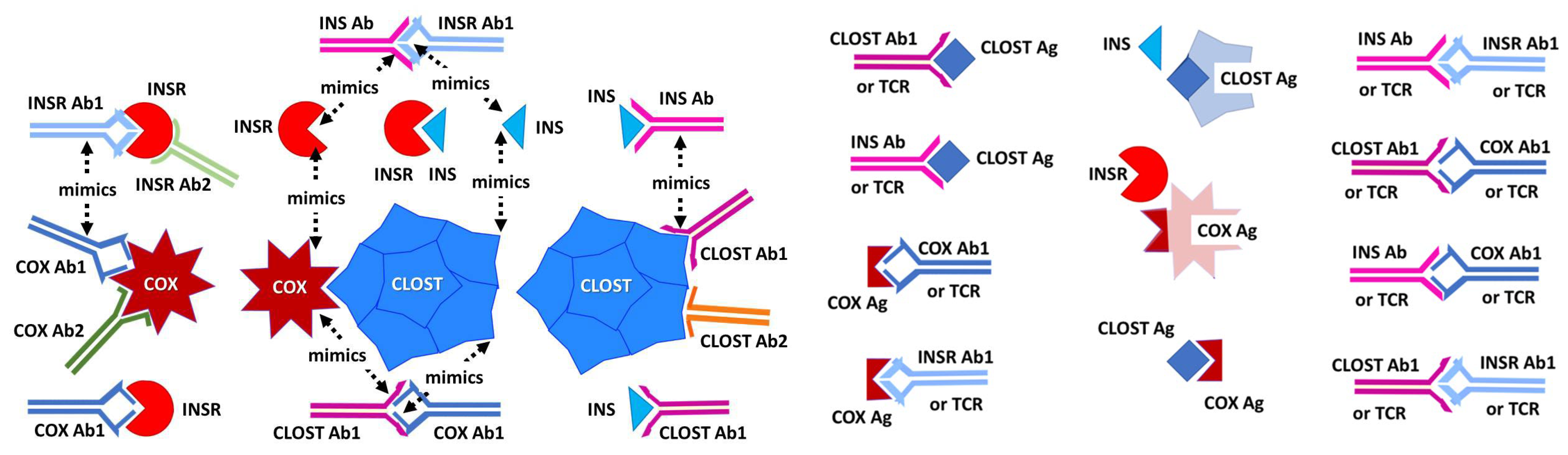
2.3. Complementarity between Virus Antibodies and Bacterial Antibodies
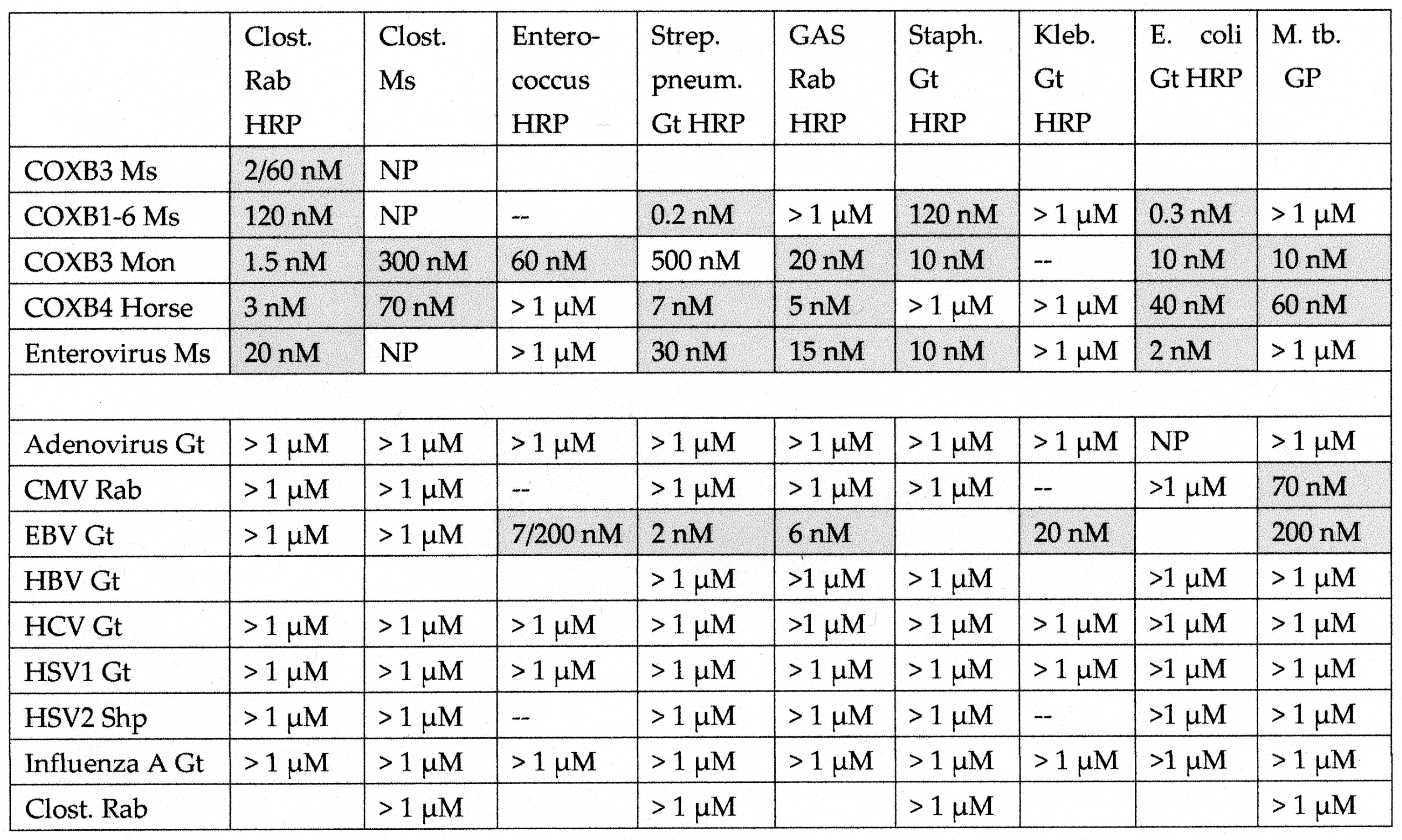
2.4. Idiotype–Anti-Idiotype Relationships between Microbial Antibodies and INS, INS Receptor, GAD-65, or PTPN(IA-2) Antibodies
2.5. Complementarity of COX and Clostridium Antigens
2.6. T1DM T Cell Receptor Recognition of COX and Clostridium Antigens
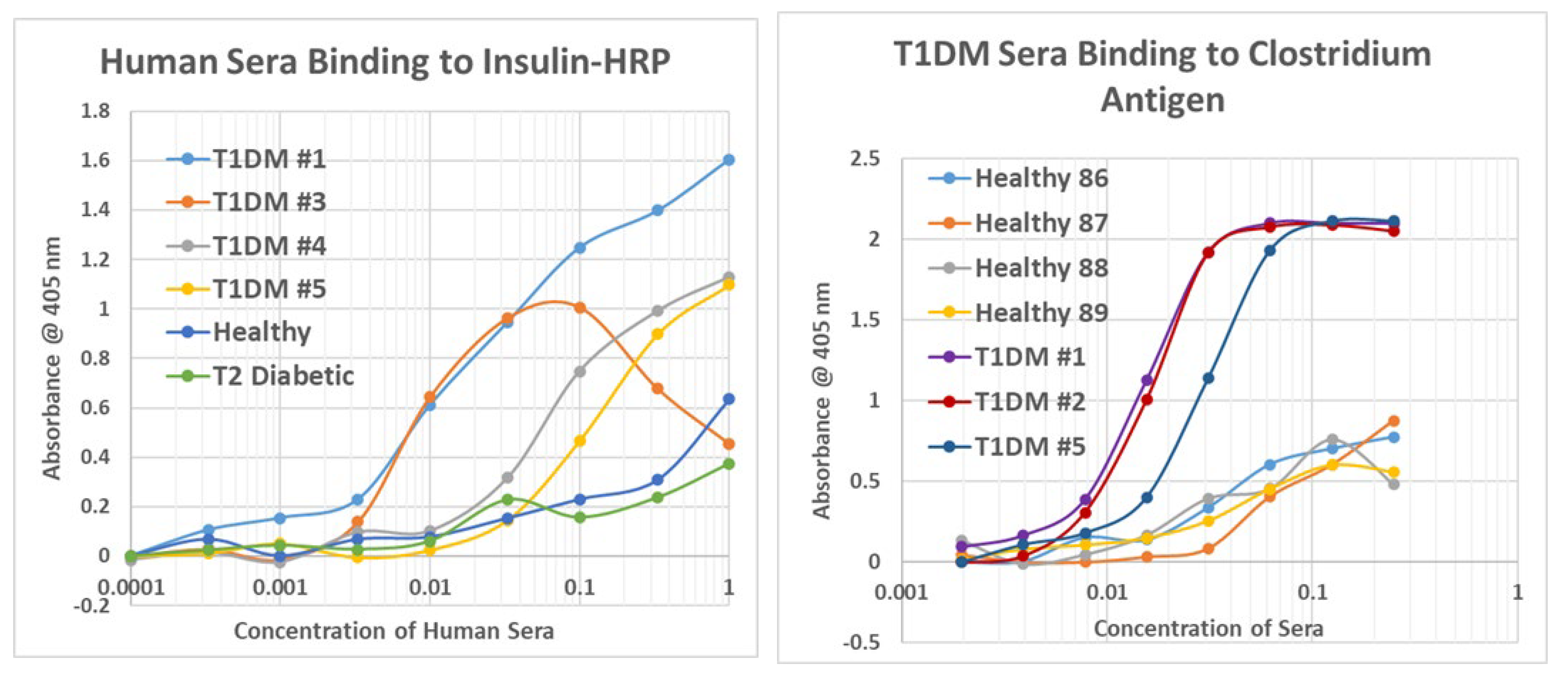
2.7. Human T1DM Sera Binding to INS and Clostridium sporogenes
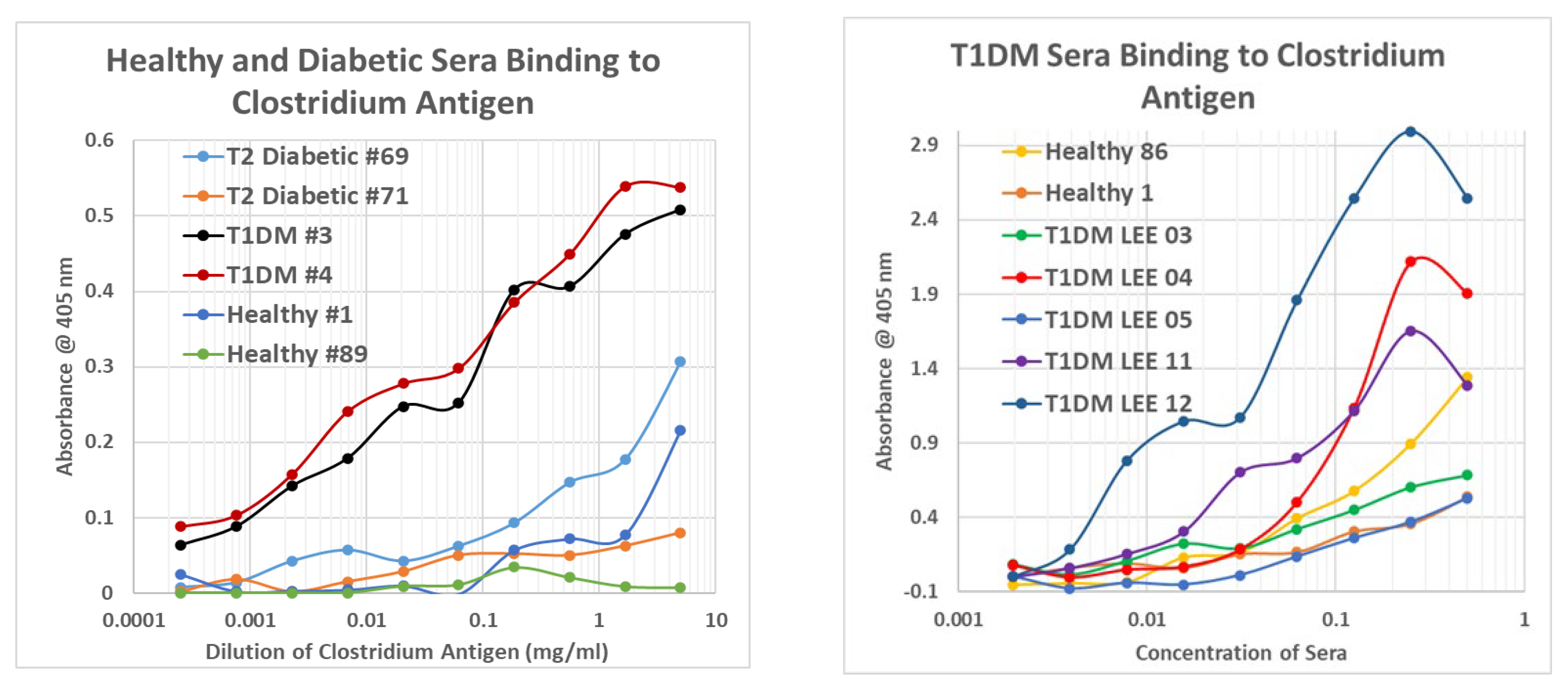
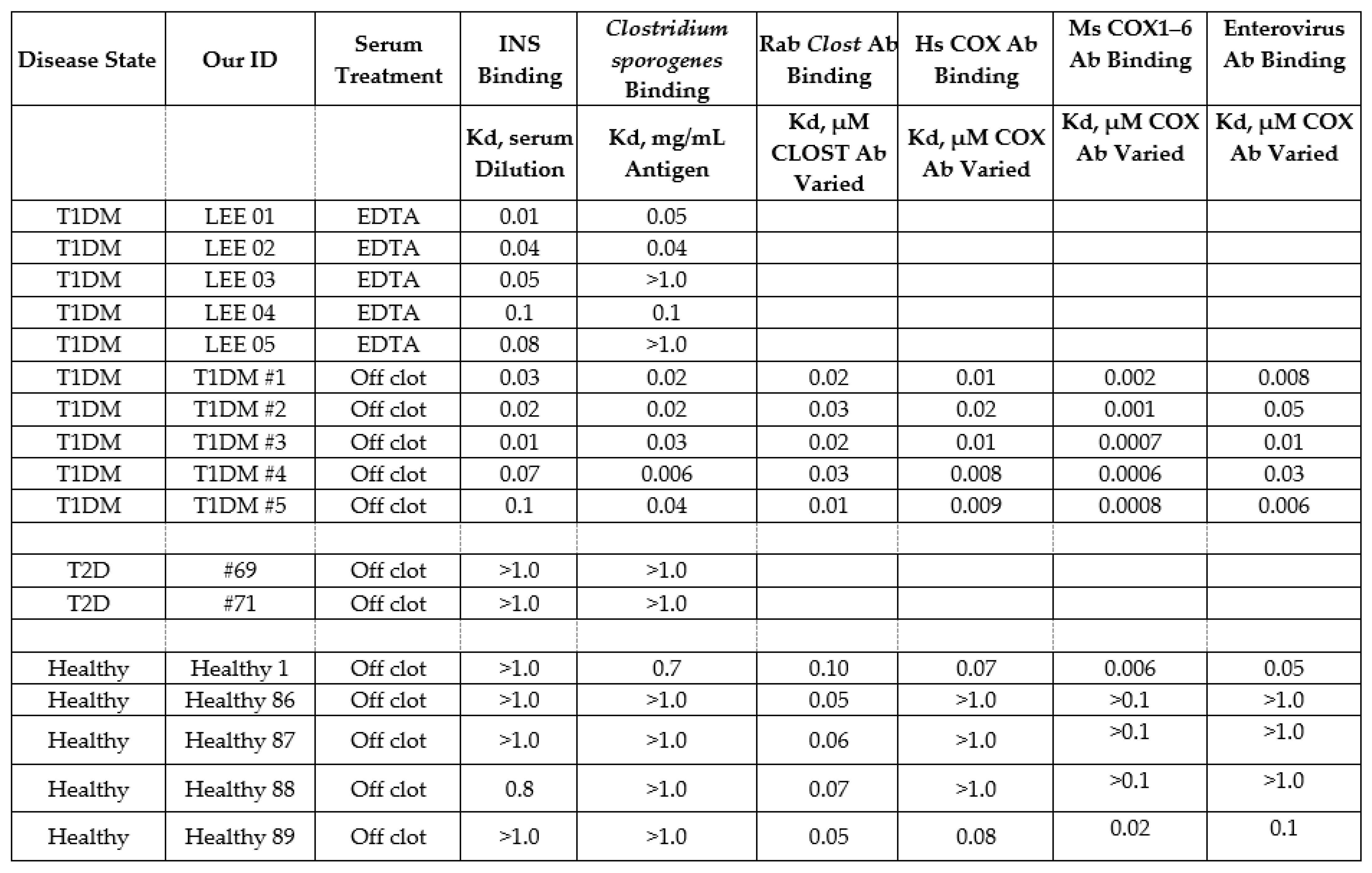
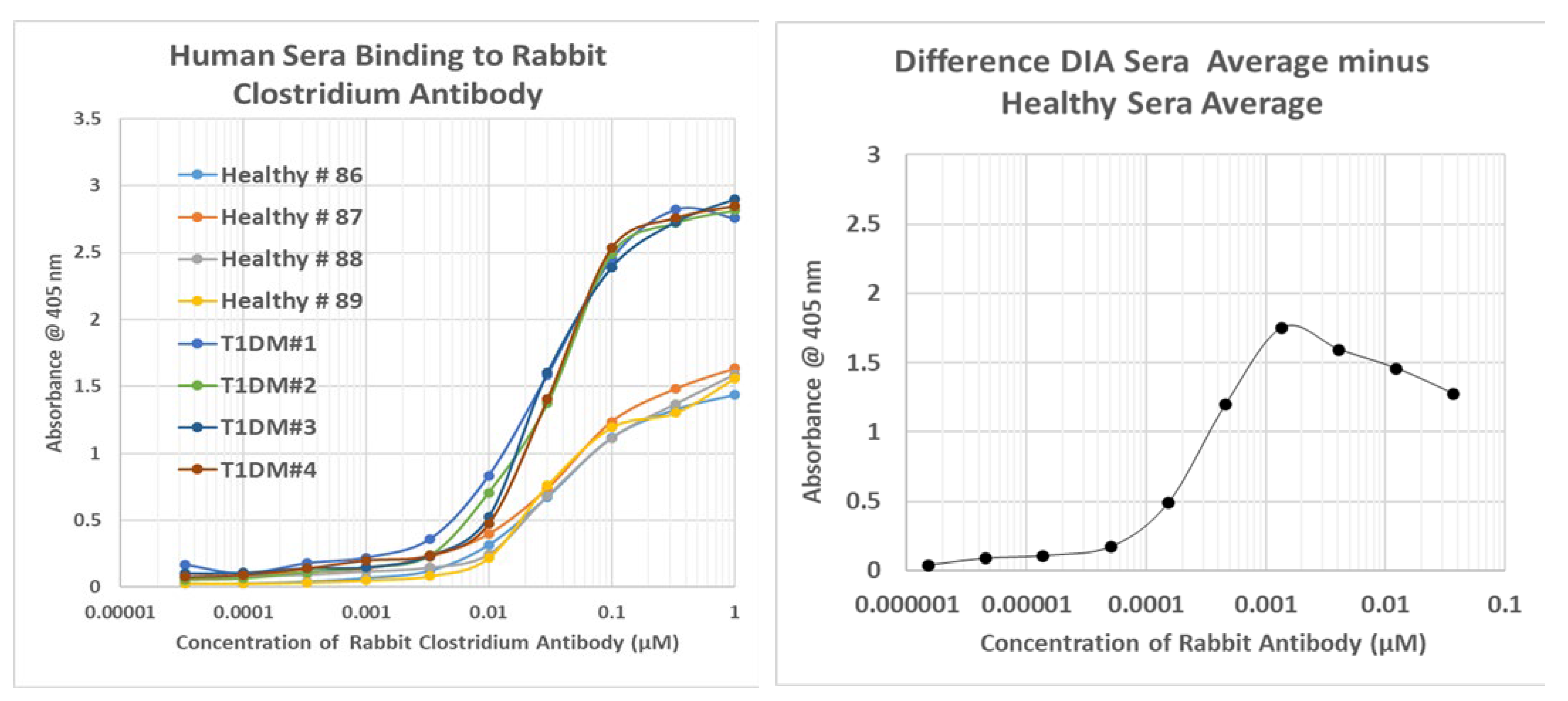
2.8. Human Sera Binding to COX and Clostridia Antibodies
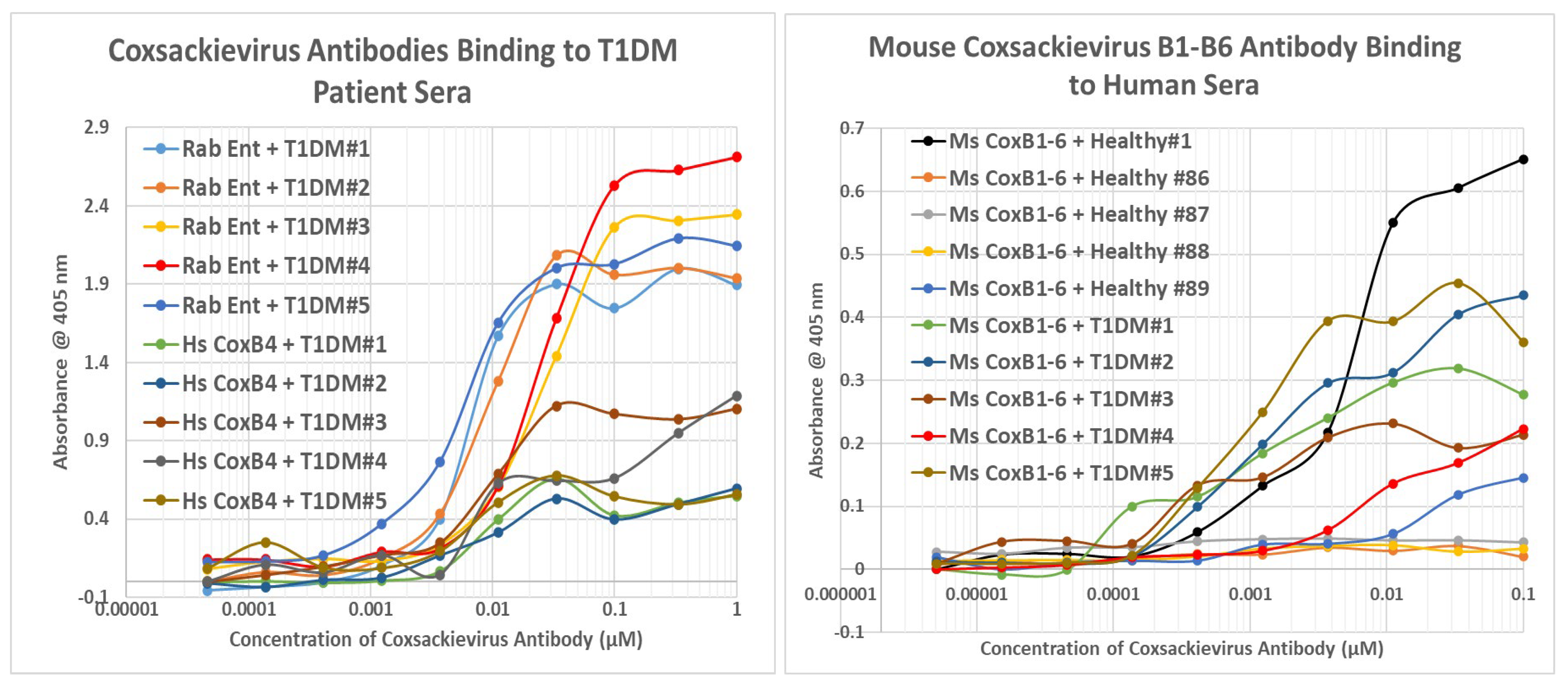
3. Discussion
3.1. Detailed Summary and Interpretation of Results
3.2. Relationship of the Experimental Findings to Previous Results
3.3. Animal Models
3.4. Epidemiology of Clostridium and Enterovirus Infections
3.5. Prevention Implications of Complementary Antigen Theory
3.6. The Need for New Animal Models of T1DM
3.7. Limitations of the Study
4. Materials and Methods
4.1. Similarity Searches
4.2. Experimental Protocols
4.3. Antigens
4.4. Antibodies
4.5. Human Sera
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
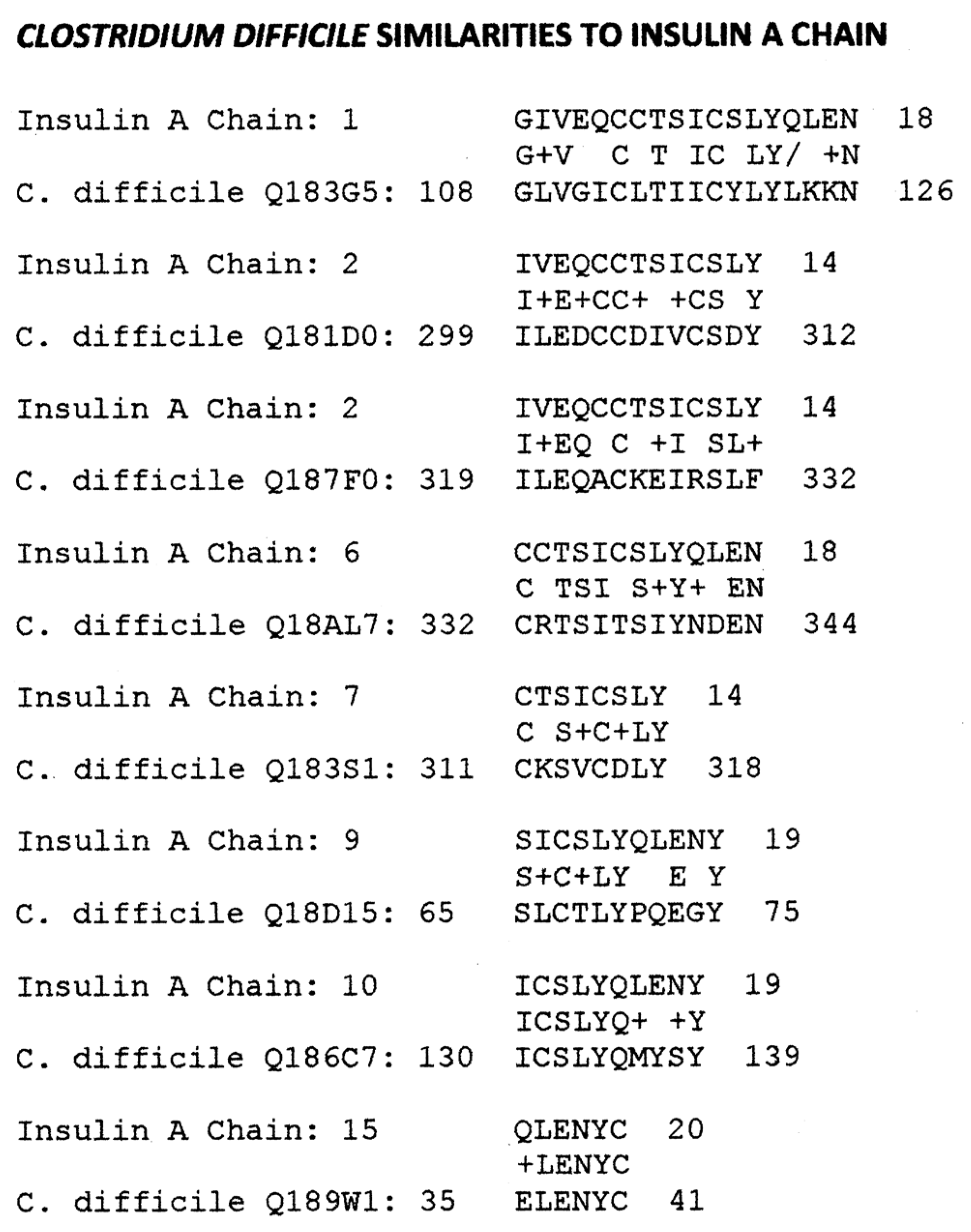
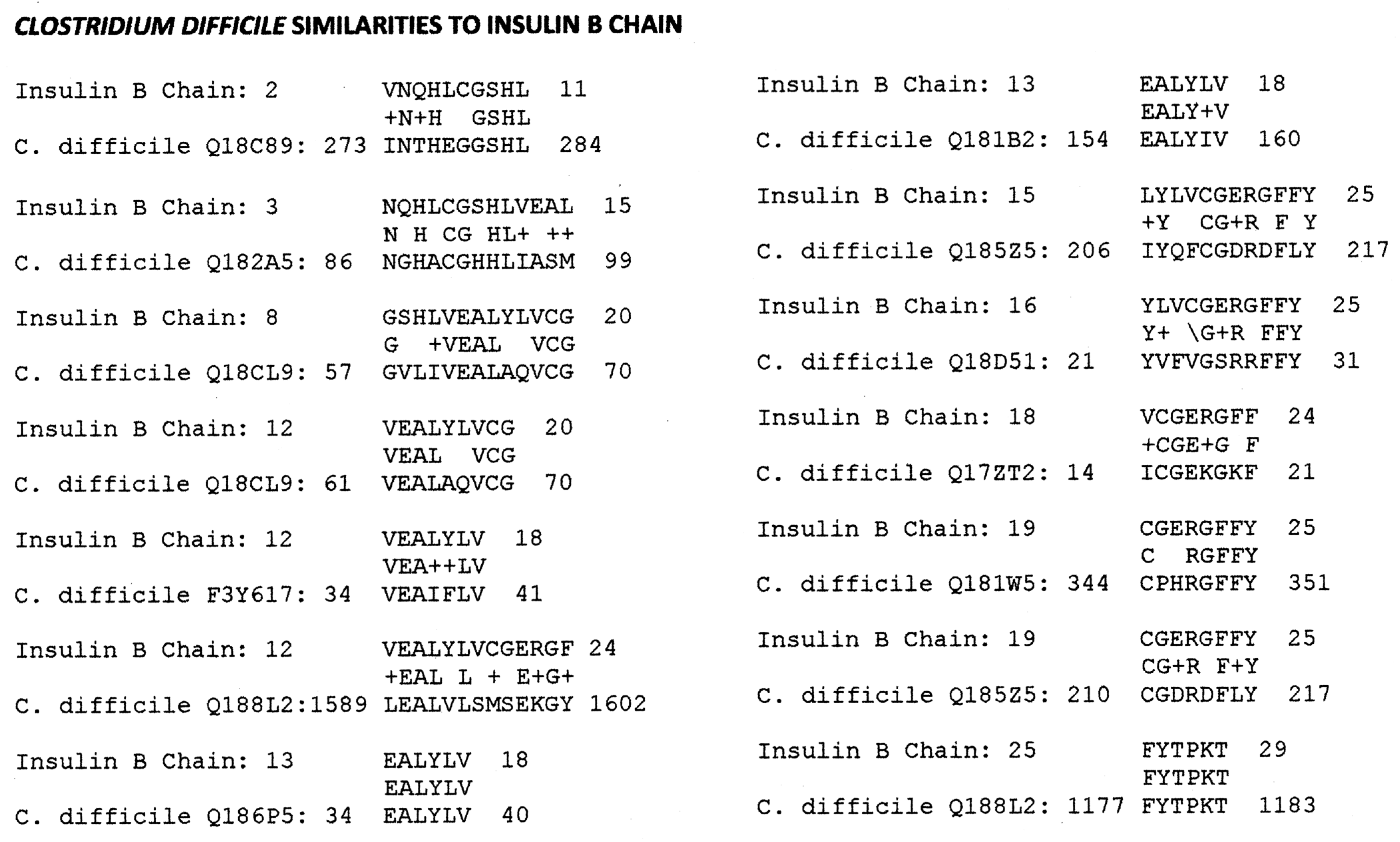
Appendix B
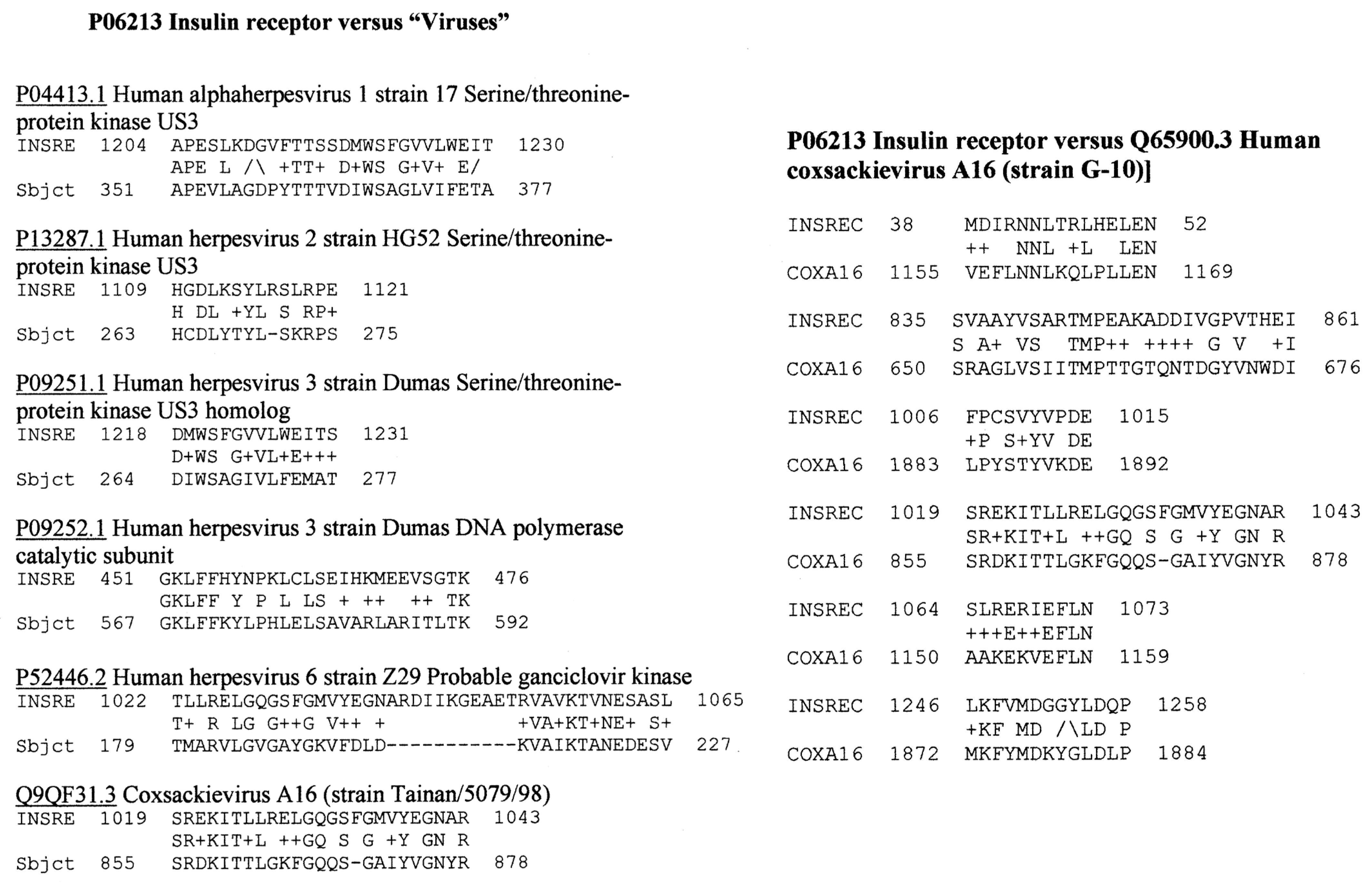
Appendix C

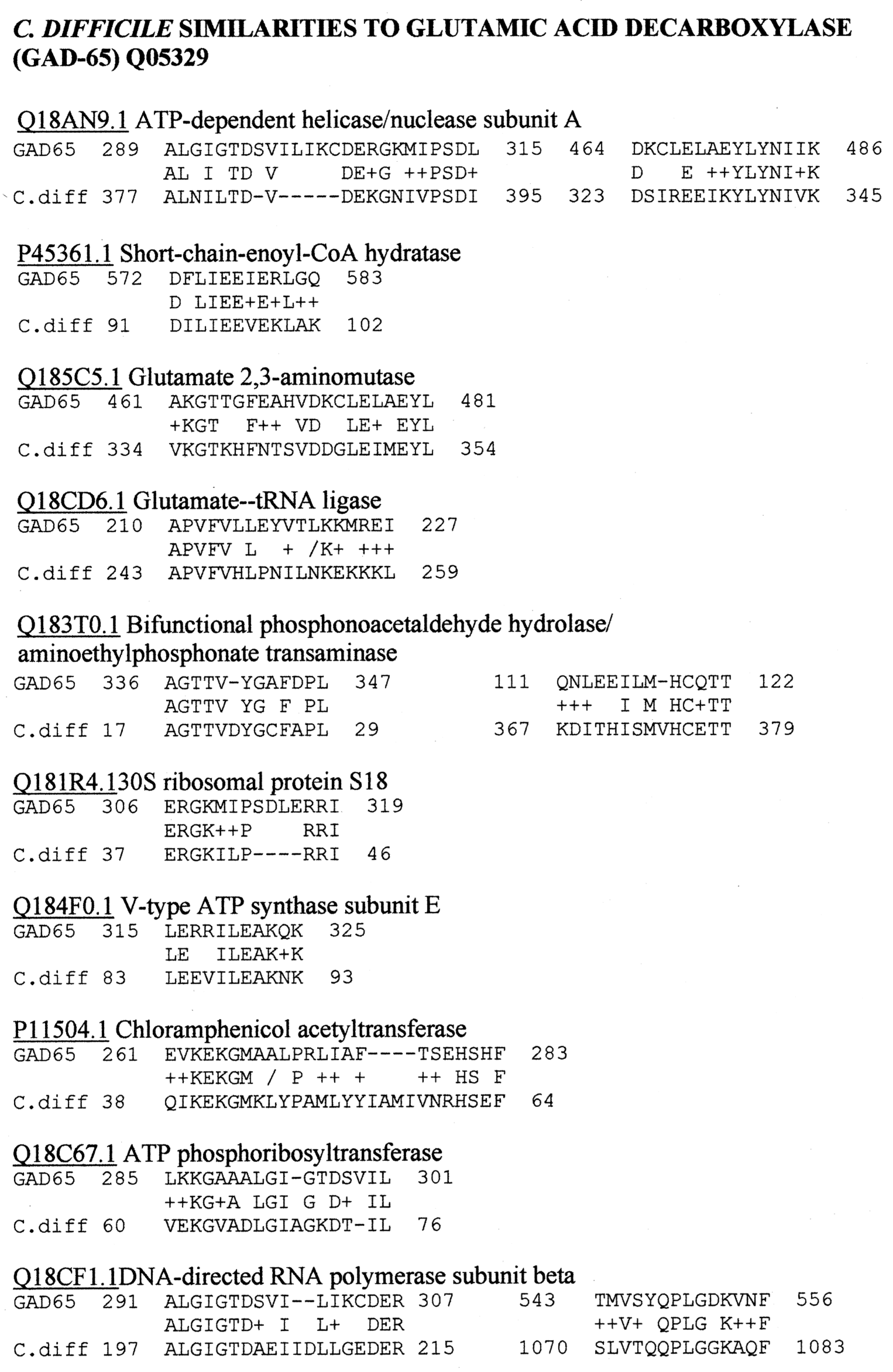

Appendix D
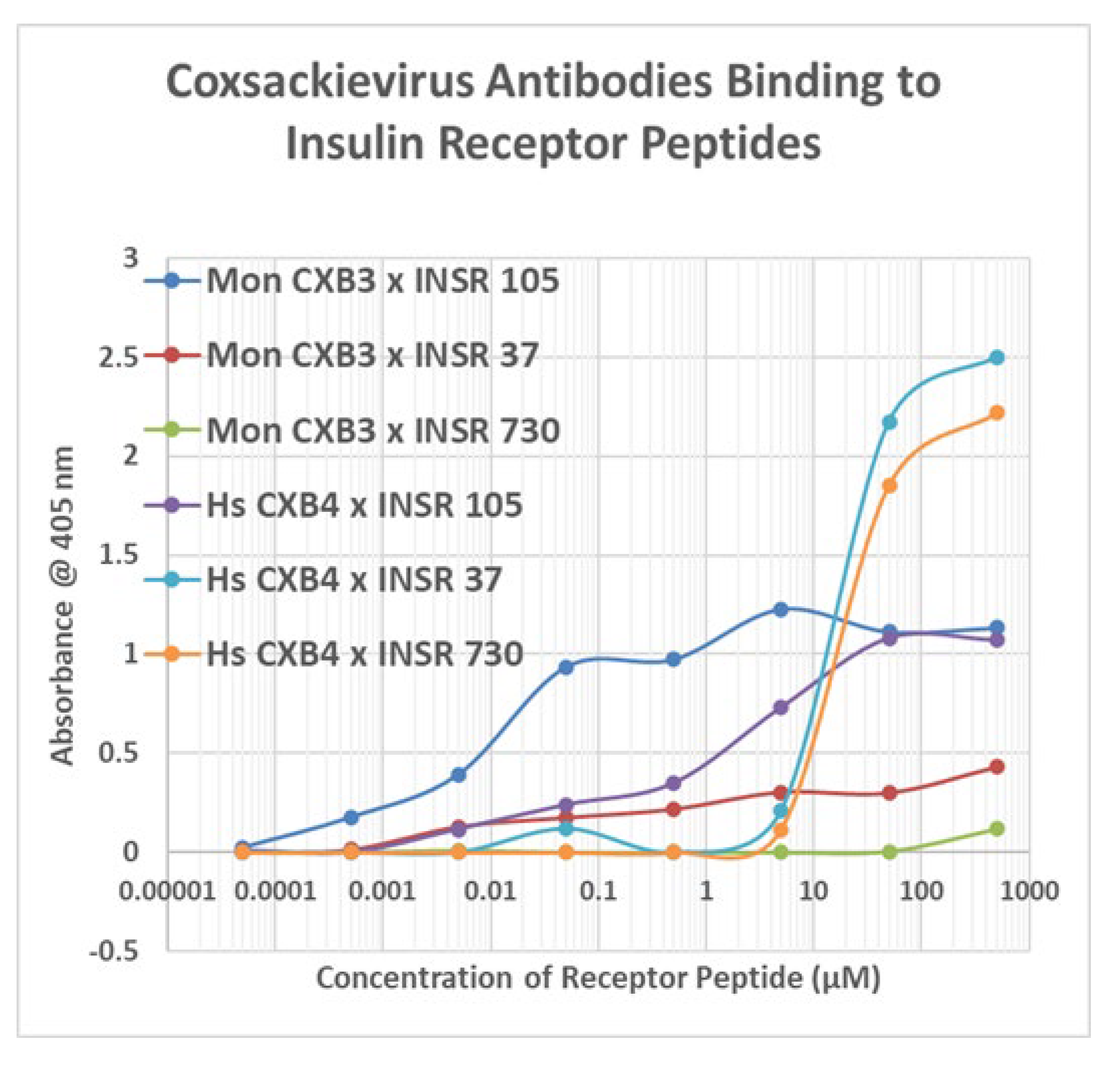
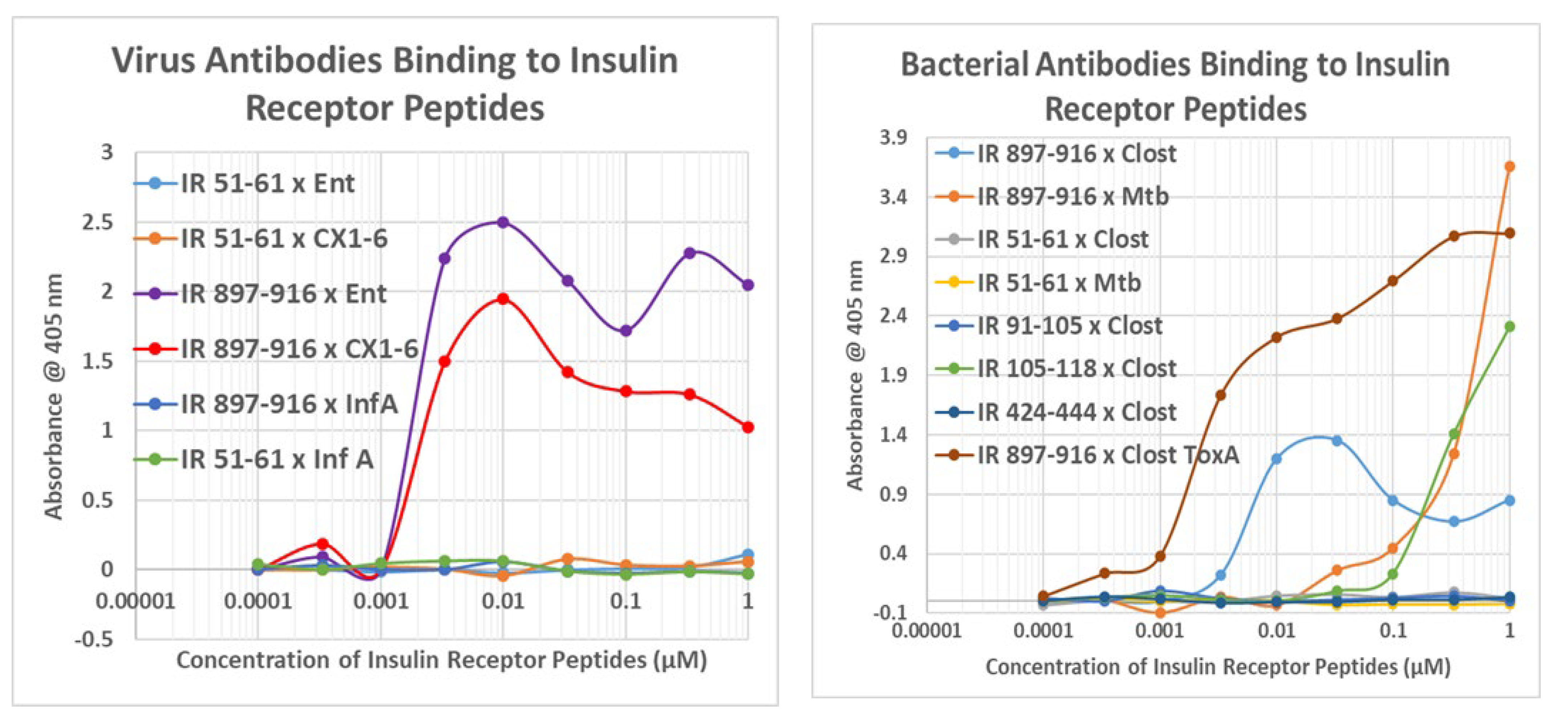
Appendix E
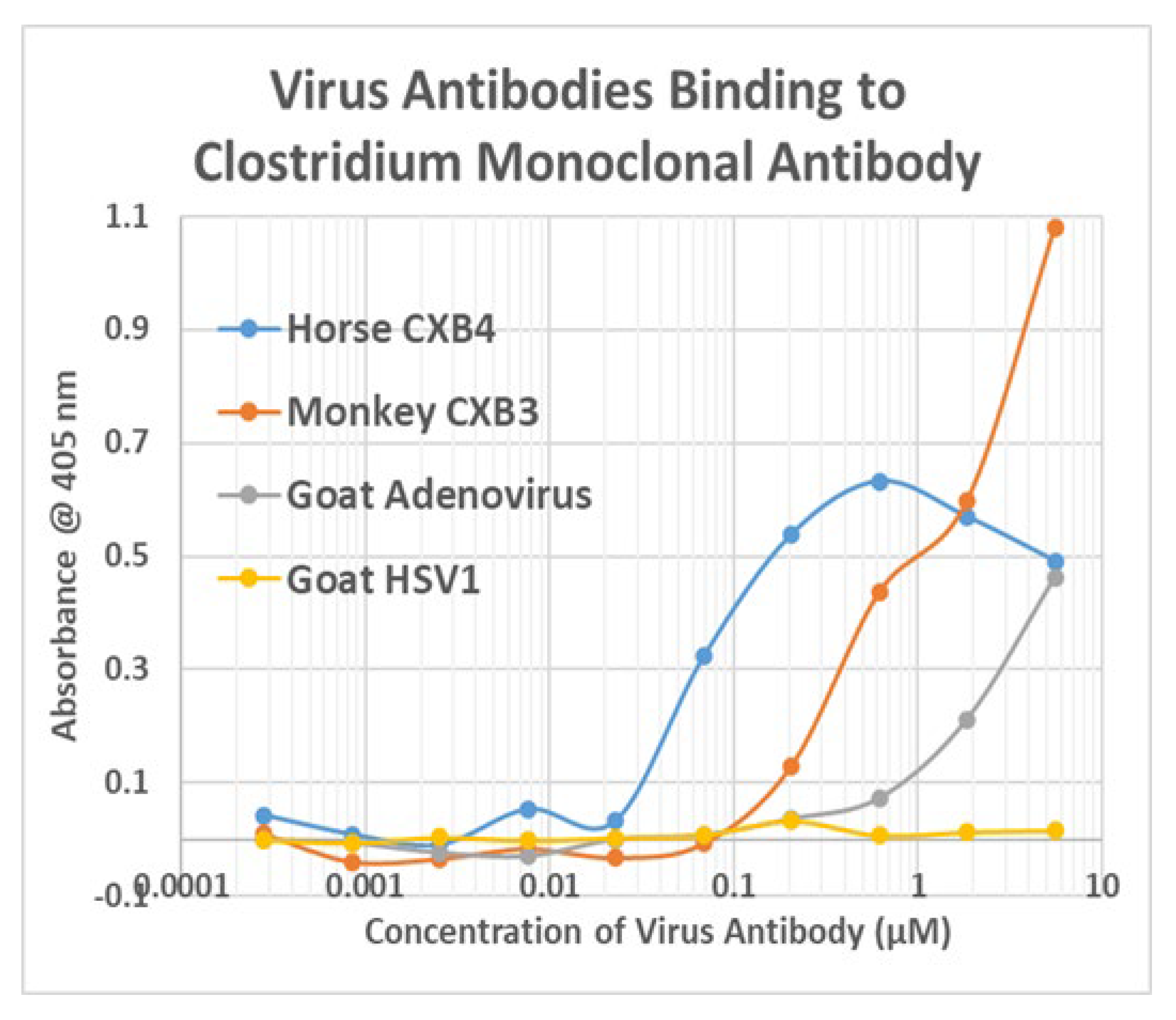

Appendix F
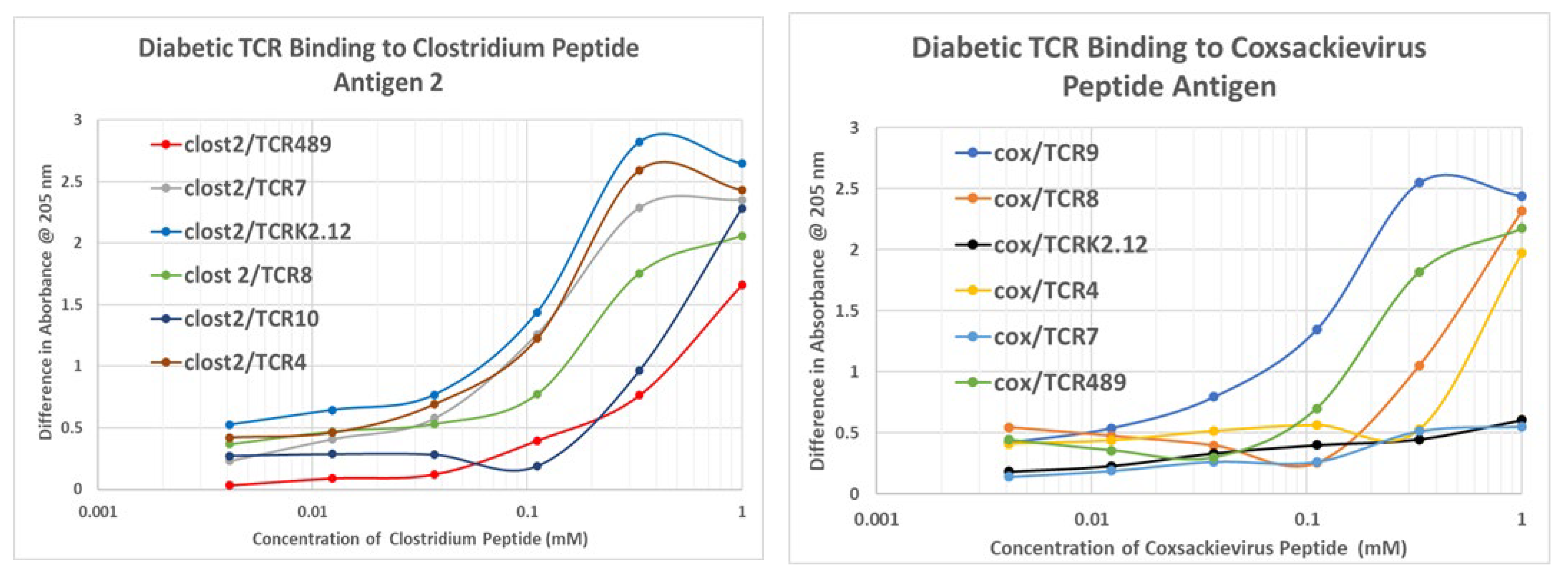
Appendix G
References
- Mallone, R.; Brezar, V.; Boitard, C. T cell recognition of autoantigens in human type 1 diabetes: Clinical perspectives. Clin. Dev. Immunol. 2011, 2011, 513210. [Google Scholar] [CrossRef] [PubMed]
- Krischer, J.P.; Liu, X.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.X.; Toppari, J.; Ziegler, A.G.; Akolkar, B.; TEDDY Study Group. Predictors of the Initiation of Islet Autoimmunity and Progression to Multiple Autoantibodies and Clinical Diabetes: The TEDDY Study. Diabetes Care 2022, 45, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Bauer, W.; Veijola, R.; Lempainen, J.; Kiviniemi, M.; Härkönen, T.; Toppari, J.; Knip, M.; Gyenesei, A.; Ilonen, J. Age at Seroconversion, HLA Genotype, and Specificity of Autoantibodies in Progression of Islet Autoimmunity in Childhood. J. Clin. Endocrinol. Metab. 2019, 104, 4521–4530. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Won, H.K.; Seok, H.; Lee, B.W.; Jung, C.H.; Lee, W.J.; Kim, J.H. High prevalence of both anti-INS and anti-INS receptor antibodies in Korean patients with INS autoimmune syndrome. Diabetes Res. Clin. Pract. 2012, 98, e12–e15. [Google Scholar] [CrossRef]
- Blackard, W.G.; Anderson, J.H.; Mullinax, F. Anti-INS receptor antibodies and diabetes. Ann. Intern. Med. 1977, 86, 584–585. [Google Scholar] [CrossRef]
- Ludwig, S.M.; Faiman, C.; Dean, H.J. INS and INS-receptor autoantibodies in children with newly diagnosed IDDM before INS therapy. Diabetes 1987, 36, 420–425. [Google Scholar] [CrossRef]
- Kahn, C.R.; Kasuga, M.; King, G.L.; Grunfeld, C. Autoantibodies to INS receptors in man: Immunological determinants and mechanism of action. In Ciba Foundation Symposium; Wiley: Hoboken, NJ, USA, 1982; pp. 91–113. [Google Scholar] [CrossRef]
- Rochet, N.; Sadoul, J.L.; Ferrua, B.; Kubar, J.; Tanti, J.F.; Bougnères, P.; Vialettes, B.; Van Obberghen, E.; Le Marchand-Brustel, Y.; Freychet, P. Autoantibodies to the INS receptor are infrequent findings in type 1 (INS-dependent) diabetes mellitus of recent onset. Diabetologia 1990, 33, 411–416. [Google Scholar] [CrossRef]
- Gergely, A.; Korányl, L.; Halmos, T.; Zsombók, M.; Péterfy, F.; Csizér, F.; Salamon, F.; Takó, J. Anti-glucagon antibodies in diabetes mellitus. Ann. Immunol. Hung. 1973, 17, 231–233. [Google Scholar]
- Asplin, C.M.; Hollander, P.; Pecoraro, R.E.; Brodsky, J.; Palmer, J.P. INS, pancreatic polypeptide, and glucagon antibodies in INS-dependent diabetes mellitus. Diabetes Care 1981, 4, 337–342. [Google Scholar] [CrossRef]
- Caforio, A.L.; Iliceto, S. Genetically determined myocarditis: Clinical presentation and immunological characteristics. Curr. Opin. Cardiol. 2008, 23, 219–226. [Google Scholar] [CrossRef]
- Li, H.S.; Ligons, D.L.; Rose, N.R. Genetic complexity of autoimmune myocarditis. Autoimmun. Rev. 2008, 7, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, T.L.; Coppieters, K.T.; von Herrath, M.G. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, L.; Köhler, K.F.; Postol, E.; Kalil, J. Genes, autoimmunity and pathogenesis of rheumatic heart disease. Ann. Pediatr. Cardiol. 2011, 4, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A. The multiple origins of Type 1 diabetes. Diabet. Med. 2013, 30, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Buschard, K. The etiology and pathogenesis of type 1 diabetes—A personal, non-systematic review of possible causes, and interventions. Front. Endocrinol. 2022, 13, 876470. [Google Scholar] [CrossRef] [PubMed]
- Mannering, S.I.; Pathiraja, V.; Kay, T.W. The case for an autoimmune aetiology of type 1 diabetes. Clin. Exp. Immunol. 2016, 183, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hober, D.; Sauter, P. Pathogenesis of type 1 diabetes mellitus: Interplay between enterovirus and host. Nat. Rev. Endocrinol. 2010, 6, 279–289. [Google Scholar] [CrossRef]
- Jaidane, H.; Hober, D. Role of COX B4 in the pathogenesis of type 1 diabetes. Diabetes Metab. 2008, 34, 537–548. [Google Scholar] [CrossRef]
- Richer, M.J.; Horwitz, M.S. COX infection as an environmental factor in the etiology of type 1 diabetes. Autoimmun. Rev. 2009, 8, 611–615. [Google Scholar] [CrossRef]
- Zipris, D. Epidemiology of type 1 diabetes and what animal models teach us about the role of viruses in disease mechanisms. Clin. Immunol. 2009, 131, 11–23. [Google Scholar] [CrossRef]
- Harms, R.Z.; Ostlund, K.R.; Cabrera, M.S.; Edwards, E.; Fisher, M.; Sarvetnick, N. Confirmation and Identification of Biomarkers Implicating Environmental Triggers in the Pathogenesis of Type 1 Diabetes. Front. Immunol. 2020, 11, 1922. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, B.; Zhang, Z.; Dai, X.; Zhang, Y.; Cui, L. Association between enterovirus infection and clinical type 1 diabetes mellitus: Systematic review and meta-analysis of observational studies. Epidemiol. Infect. 2021, 150, e23. [Google Scholar] [CrossRef] [PubMed]
- Roivainen, M.; Knip, M.; Hyöty, H.; Kulmala, P.; Hiltunen, M.; Vähäsalo, P.; Hovi, T.; Akerblom, H.K.; Childhood Diabetes in Finland (DiMe) Study Group. Several different enterovirus serotypes can be associated with prediabetic autoimmune episodes and onset of overt IDDM. J. Med. Virol. 1998, 56, 74–78. [Google Scholar] [CrossRef]
- Yeung, W.C.; Rawlinson, W.D.; Craig, M.E. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. BMJ 2011, 342, d35. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.; Krause, I. Infection and type 1 diabetes mellitus—A two edged sword? Autoimmun. Rev. 2009, 8, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Jun, H.S. Viruses in type 1 diabetes: Brief review. ILAR J. 2004, 45, 343–348. [Google Scholar] [CrossRef]
- Lammi, N.; Karvonen, M.; Tuomilehto, J. Do microbes have a causal role in type 1 diabetes? Med. Sci. Monit. 2005, 11, RA63–RA69. [Google Scholar]
- Laverdant, C.; Fromantin, M. Le diabète de l’hépatite virale. A propos de 20 observations émanant d’un groupement de 4,456 malades [Diabetes after viral hepatitis. 20 cases out of a group of 4,456 patients]. Ann. Gastroenterol. Hepatol. 1970, 6, 101–109. (In French) [Google Scholar]
- Sechi, L.A.; Rosu, V.; Pacifico, A.; Fadda, G.; Ahmed, N.; Zanetti, S. Humoral immune responses of type 1 diabetes patients to Mycobacterium avium subsp. paratuberculosis lend support to the infectious trigger hypothesis. Clin. Vaccine Immunol. 2008, 15, 320–326. [Google Scholar] [CrossRef]
- Rosu, V.; Ahmed, N.; Paccagnini, D.; Gerlach, G.; Fadda, G.; Hasnain, S.E.; Zanetti, S.; Sechi, L.A. Specific immunoassays confirm association of Mycobacterium avium Subsp. paratuberculosis with Type-1 but not Type-2 diabetes mellitus. PLoS ONE 2009, 4, e4386. [Google Scholar] [CrossRef]
- Marietti, M.; Gasbarrini, A.; Saracco, G.; Pellicano, R. Helicobacter pylori infection and diabetes mellitus: The 2013 state of art. Panminerva Med. 2013, 55, 277–281. [Google Scholar] [PubMed]
- Whalley, T.; Dolton, G.; Brown, P.E.; Wall, A.; Wooldridge, L.; van den Berg, H.; Fuller, A.; Hopkins, J.R.; Crowther, M.D.; Attaf, M.; et al. GPU-Accelerated Discovery of Pathogen-Derived Molecular Mimics of a T-Cell INS Epitope. Front. Immunol. 2020, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Senju, S.; Maenaka, K.; Iwai, L.K.; Fujii, S.; Tabata, H.; Tsukamoto, H.; Hirata, S.; Chen, Y.Z.; Nishimura, Y. Systematic analysis of the combinatorial nature of epitopes recognized by TCR leads to identification of mimicry epitopes for glutamic acid decarboxylase 65-specific TCRs. J. Immunol. 2003, 170, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 610, e25792. [Google Scholar] [CrossRef]
- De Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef]
- Vaarala, O. Human intestinal microbiota and Type 1 Diabetes. Curr. Diabetes Rep. 2013, 13, 601–607. [Google Scholar] [CrossRef]
- Murri, M.; Leiva, L.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11, 46. Available online: http://www.biomedcentral.com/1741-7015/11/46 (accessed on 28 November 2022). [CrossRef]
- Majumdar, S.; Lin, Y.; Bettini, M.L. Host-microbiota interactions shaping T-cell response and tolerance in type 1 diabetes. Front. Immunol. 2022, 13, 974178. [Google Scholar] [CrossRef]
- Girdhar, K.; Huang, Q.; Chow, I.T.; Vatanen, T.; Brady, C.; Raisingani, A.; Autissier, P.; Atkinson, M.A.; Kwok, W.W.; Kahn, C.R.; et al. A gut microbial peptide and molecular mimicry in the pathogenesis of type 1 diabetes. Proc. Natl. Acad. Sci. USA 2022, 119, e2120028119. [Google Scholar] [CrossRef]
- Cole, D.K.; Bulek, A.M.; Dolton, G.; Schauenberg, A.J.; Szomolay, B.; Rittase, W.; Trimby, A.; Jothikumar, P.; Fuller, A.; Skowera, A.; et al. Hotspot autoimmune T cell receptor binding underlies pathogen and INS peptide cross-reactivity. J. Clin. Investig. 2016, 126, 3626. [Google Scholar] [CrossRef] [PubMed]
- Von Herrath, M.G.; Holz, A.; Homann, D.; Oldstone, M.B. Role of viruses in type I diabetes. Semin. Immunol. 1998, 10, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.-G.; John, T.-J. Alteration of immune response to coxsackie B3 virus by streptozotocin in dual-aetiology diabetes mellitus in mouse. Indian J. Exp. Biol. 1987, 25, 17–21. [Google Scholar]
- Cook, S.H.; Loria, R.M.; Madge, G.E. Host factors in COX B4-induced pancreopathy. Lab Investig. 1982, 46, 377–382. [Google Scholar]
- Yoon, J.W.; London, W.T.; Curfman, B.L.; Brown, R.L.; Notkins, A.L. Coxsackie virus B4 produces transient diabetes in nonhuman primates. Diabetes 1986, 35, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Richter, W.; Mertens, T.; Schoel, B.; Muir, P.; Ritzkowsky, A.; Scherbaum, W.A.; Boehm, B.O. Sequence homology of the diabetes-associated autoantigen glutamate decarboxylase with coxsackie B4-2C protein and heat shock protein 60 mediates no molecular mimicry of autoantibodies. J. Exp. Med. 1994, 180, 721–726. [Google Scholar] [CrossRef]
- Vreugdenhil, G.R.; Batstra, M.R.; Aanstoot, H.J.; Melchers, W.J.; Galama, J.M. Analysis of antibody responses against coxsackie virus B4 protein 2C and the diabetes autoantigen GAD(65). J. Med. Virol. 1999, 59, 256–261. [Google Scholar] [CrossRef]
- Marttila, J.; Juhela, S.; Vaarala, O.; Hyöty, H.; Roivainen, M.; Hinkkanen, A.; Vilja, P.; Simell, O.; Ilonen, J. Responses of COX B4-specific T-cell lines to 2C protein-characterization of epitopes with special reference to the GAD65 homology region. Virology 2001, 284, 131–141. [Google Scholar] [CrossRef]
- Sioofy-Khojine, A.B.; Lehtonen, J.; Nurminen, N.; Laitinen, O.H.; Oikarinen, S.; Huhtala, H.; Pakkanen, O.; Ruokoranta, T.; Hankaniemi, M.M.; Toppari, J.; et al. COX B1 infections are associated with the initiation of INS-driven autoimmunity that progresses to type 1 diabetes. Diabetologia 2018, 61, 1193–1202. [Google Scholar] [CrossRef]
- Kukreja, A.; Maclaren, N.K. Current cases in which epitope mimicry is considered as a component cause of autoimmune disease: Immune-mediated (type 1) diabetes. Cell. Mol. Life Sci. 2000, 57, 534–541. [Google Scholar] [CrossRef]
- Sarugeri, E.; Dozio, N.; Meschi, F.; Pastore, M.R.; Bonifacio, E. T cell responses to type 1 diabetes related peptides sharing homologous regions. J. Mol. Med. 2001, 79, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lönnrot, M.; Hyöty, H.; Knip, M.; Roivainen, M.; Kulmala, P.; Leinikki, P.; Akerblom, H.K.; Childhood Diabetes in Finland Study Group. Antibody cross-reactivity induced by the homologous regions in glutamic acid decarboxylase (GAD65) and 2C protein of COX B4. Clin. Exp. Immunol. 1996, 104, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Onodera, T.; Jenson, A.B.; Notkins, A.L. Virus-induced diabetes mellitus. XI. Replication of coxsackie B3 virus in human pancreatic beta cell cultures. Diabetes 1978, 27, 778–781. [Google Scholar] [CrossRef]
- Honkanen, H.; Oikarinen, S.; Nurminen, N.; Laitinen, O.H.; Huhtala, H.; Lehtonen, J.; Ruokoranta, T.; Hankaniemi, M.M.; Lecouturier, V.; Almond, J.W.; et al. Detection of enteroviruses in stools precedes islet autoimmunity by several months: Possible evidence for slowly operating mechanisms in virus-induced autoimmunity. Diabetologia 2017, 60, 424–431. [Google Scholar] [CrossRef]
- Morse, Z.J.; Simister, R.L.; Crowe, S.A.; Horwitz, M.S.; Osborne, L.C. Virus induced dysbiosis promotes type 1 diabetes onset. Front. Immunol. 2023, 14, 1096323. [Google Scholar] [CrossRef] [PubMed]
- Viskari, H.; Oikarinen, S.; Hoppu, S.; Vuorinen, T.; Huhtala, H.; Toppari, J.; Veijola, R.; Ilonen, J.; Knip, M.; Hyöty, H. Live attenuated enterovirus vaccine (OPV) is not associated with islet autoimmunity in children with genetic susceptibility to type 1 diabetes: Prospective cohort study. Diabetologia 2018, 61, 203–209. [Google Scholar] [CrossRef]
- Rasquinha, M.T.; Lasrado, N.; Sur, M.; Mone, K.; Qiu, H.; Riethoven, J.J.; Sobel, R.A.; Reddy, J. A Monovalent Mt10-CVB3 Vaccine Prevents CVB4-Accelerated Type 1 Diabetes in NOD Mice. Vaccines 2022, 11, 76. [Google Scholar] [CrossRef]
- el-Reshaid, K.; al-Mofti, S.; Stepic, N.R. Induction of INS resistance by autoantibodies to INS receptors following on an acute Coxsackie B4 infection. Diabetes Res. Clin. Pract. 1994, 25, 207–210. [Google Scholar] [CrossRef]
- Boettler, T.; von Herrath, M. Protection against or triggering of Type 1 diabetes? Different roles for viral infections. Expert Rev. Clin. Immunol. 2011, 7, 45–53. [Google Scholar] [CrossRef]
- Huang, S.W.; Taylor, G.; Basid, A. The effect of pertussis vaccine on the INS-dependent diabetes induced by streptozotocin in ice. Pediatr. Res. 1984, 18, 221–226. [Google Scholar] [CrossRef]
- Nomaguchi, H.; Yogi, Y.; Kawatsu, K.; Okamura, H.; Ozawa, Y.; Kasatani, T. Prevention of diabetes in non-obese diabetic mice by a single immunization with Mycobacterium leprae. Nihon Hansenbyo Gakkai Zasshi 2002, 71, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.A.; Baxter, A.G. The NOD mouse as a model of SLE. Autoimmunity 2001, 34, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shan, K.; Pan, L.L.; Feng, N.; Lv, Z.; Sun, Y.; Li, J.; Wu, C.; Zhang, H.; Chen, W.; et al. Clostridium butyricum CGMCC0313.1 Protects against Autoimmune Diabetes by Modulating Intestinal Immune Homeostasis and Inducing Pancreatic Regulatory T Cells. Front. Immunol. 2017, 8, 1345. [Google Scholar] [CrossRef] [PubMed]
- Dovi, K.S.; Bajinka, O.; Conteh, I. Evidence and possible mechanisms of probiotics in the management of type 1 diabetes mellitus. J. Diabetes Metab. Disord. 2022, 21, 1081–1094. [Google Scholar] [CrossRef]
- Horwitz, M.S.; Bradley, L.M.; Harbertson, J.; Krahl, T.; Lee, J.; Sarvetnick, N. Diabetes induced by Coxsackie virus: Initiation by bystander damage and not molecular mimicry. Nat. Med. 1998, 4, 781–785. [Google Scholar] [CrossRef]
- Sané, F.; Moumna, I.; Hober, D. Group B COX and autoimmunity: Focus on Type 1 diabetes. Expert Rev. Clin. Immunol. 2011, 7, 357–366. [Google Scholar] [CrossRef]
- Filippi, C.; von Herrath, M. How viral infections affect the autoimmune process leading to type 1 diabetes. Cell. Immunol. 2005, 233, 125–132. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Molecular complementarity III. peptide complementarity as a basis for peptide receptor evolution: A bioinformatic case study of INS, glucagon and gastrin. J. Theor. Biol. 2002, 218, 71–84. [Google Scholar] [CrossRef]
- Rhinesmith, T.; Turkette, T.; Root-Bernstein, R. Rapid Non-Enzymatic Glycation of the INS Receptor under Hyperglycemic Conditions Inhibits INS Binding In Vitro: Implications for INS Resistance. Int. J. Mol. Sci. 2017, 18, 2602. [Google Scholar] [CrossRef]
- Kent, S.C.; Chen, Y.; Bregoll, L.; Clemmings, S.M.; Kenyon, N.S.; Ricordi, C.; Hering, B.J.; Hafler, D.A. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an INS epitope. Nature 2005, 435, 224–228. [Google Scholar] [CrossRef]
- Durinovic-Bello, I.; Steinle, A.; Ziegler, A.G.; Schendel, D.J. HLA-DQ-restricted, islet-specific T-cell clones of a type I diabetic patient. T-cell receptor sequence similarities to insulitis-inducing T-cells of nonobese diabetic mice. Diabetes 1994, 43, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R. Autoreactive T-cell receptor (Vbeta/D/Jbeta) sequences in diabetes are homologous to INS, glucagon, the INS receptor, and the glucagon receptor. J. Mol. Recognit. 2009, 22, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Podufaly, A. T Cell Receptor Variable Regions in Diabetes Bind to Each Other, to INS, Glucagon or INS Receptor, and to Their Antibodies. Open Autoimmun. J. 2012, 4, 10–22. [Google Scholar] [CrossRef]
- Bhave, M.S.; Hassanbhai, A.M.; Anand, P.; Luo, K.Q.; Teoh, S.H. Effect of Heat-Inactivated Clostridium sporogenes and Its Conditioned Media on 3-Dimensional Colorectal Cancer Cell Models. Sci. Rep. 2015, 5, 15681. [Google Scholar] [CrossRef]
- Kondrashova, A.; Hyöty, H. Role of viruses and other microbes in the pathogenesis of type 1 diabetes. Int. Rev. Immunol. 2014, 33, 284–295. [Google Scholar] [CrossRef]
- Real-Fernández, F.; Gallo, A.; Nuti, F.; Altamore, L.; Del Vescovo, G.G.; Traldi, P.; Ragazzi, E.; Rovero, P.; Lapolla, A.; Papini, A.M. Cross-reactive peptide epitopes of Enterovirus Coxsackie B4 and human glutamic acid decarboxylase detecting anti-bodies in latent autoimmune diabetes in adults versus type 1 diabetes. Clin. Chim. Acta 2021, 515, 73–79. [Google Scholar] [CrossRef]
- Alhazmi, A.; Nekoua, M.P.; Mercier, A.; Vergez, I.; Sane, F.; Alidjinou, E.K.; Hober, D. Combating coxsackievirus B infections. Rev. Med. Virol. 2023, 33, e2406. [Google Scholar] [CrossRef]
- Dotta, F.; Censini, S.; van Halteren, A.G.; Marselli, L.; Masini, M.; Dionisi, S.; Mosca, F.; Boggi, U.; Muda, A.O.; Del Prato, S.; et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA 2007, 104, 5115–5120. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, T. Enterovirus infection and type 1 diabetes: Unraveling the crime scene. Clin. Exp. Immunol. 2019, 195, 15–24. [Google Scholar] [CrossRef]
- Ylipaasto, P.; Klingel, K.; Lindberg, A.M.; Otonkoski, T.; Kandolf, R.; Hovi, T.; Roivainen, M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004, 47, 225–239. [Google Scholar] [CrossRef]
- Kim, K.W.; Horton, J.L.; Pang, C.N.I.; Jain, K.; Leung, P.; Isaacs, S.R.; Bull, R.A.; Luciani, F.; Wilkins, M.R.; Catteau, J.; et al. Higher abundance of enterovirus A species in the gut of children with islet auto-immunity. Sci. Rep. 2019, 9, 1749. [Google Scholar] [CrossRef]
- Batarseh, H.; Thompson, R.A.; Odugbesan, O.; Barnett, A.H. INS receptor antibodies in diabetes mellitus. Clin. Exp. Immunol. 1988, 71, 85–90. [Google Scholar] [PubMed]
- Fonseca, V.; Khokher, M.A.; Dandona, P. INS receptor antibodies causing steroid responsive diabetes mellitus in a patient with myositis. Br. Med. J. (Clin. Res. Ed.) 1984, 288, 1578. [Google Scholar] [CrossRef] [PubMed]
- Maron, R.; Elias, D.; de Jongh, B.M.; Bruining, G.J.; van Rood, J.J.; Schechter, Y.; Cohen, I.R. Autoantibodies to the INS receptor in juvenile onset INS-dependent diabetes. Nature 1983, 303, 817–818. [Google Scholar] [CrossRef]
- Elias, D.; Bone, A.J.; Baird, J.D.; Cooke, A.; Cohen, I.R. INS-mimicking anti-idioptypic antibodies in development of spontaneous autoimmune diabetes in BB/E rats. Diabetes 1990, 39, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Afonso, G.; Mallone, R. Infectious triggers in type 1 diabetes: Is there a case for epitope mimicry? Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 82–88. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Hiemstra, H.S.; Schloot, N.C.; De Vries, R.R.; Chaudhuri, A.; Behan, P.O.; Drijfhout, J.W. Molecular mimicry in type 1 diabetes: Immune cross-reactivity between islet autoantigen and human cytomegalovirus but not Coxsackie virus. Ann. N. Y. Acad. Sci. 2002, 958, 163–165. [Google Scholar] [CrossRef]
- Schloot, N.C.; Willemen, S.J.; Duinkerken, G.; Drijfhout, J.W.; de Vries, R.R.; Roep, B.O. Molecular mimicry in type 1 diabetes mellitus revisited: T-cell clones to GAD65 peptides with sequence homology to Coxsackie or proINS peptides do not crossreact with homologous counterpart. Hum. Immunol. 2001, 62, 299–309. [Google Scholar] [CrossRef]
- Vreugdenhil, G.R.; Geluk, A.; Ottenhoff, T.H.; Melchers, W.J.; Roep, B.O.; Galama, J.M. Molecular mimicry in diabetes mellitus: The homologous domain in coxsackie B virus protein 2C and islet autoantigen GAD65 is highly conserved in the coxsackie B-like enteroviruses and binds to the diabetes associated HLA-DR3 molecule. Diabetologia 1998, 41, 40–46. [Google Scholar] [CrossRef]
- Hou, J.; Said, C.; Franchi, D.; Dockstader, P.; Chatterjee, N.K. Antibodies to glutamic acid decarboxylase and P2-C peptides in sera from coxsackie virus B4-infected mice and IDDM patients. Diabetes 1994, 43, 1260–1266. [Google Scholar] [CrossRef]
- Horwitz, M.S.; Ilic, A.; Fine, C.; Rodriguez, E.; Sarvetnick, N. Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J. Clin. Investig. 2002, 109, 79–87. [Google Scholar] [CrossRef]
- Narendran, P.; Williams, A.J.; Elsegood, K.; Leech, N.J.; Dayan, C.M. Humoral and cellular immune responses to proINS in adults with newly diagnosed type 1 diabetes. Diabetes Metab. Res. Rev. 2003, 19, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.; Zhao, C.W.; Wegmann, K.W.; Hickley, W.F. Experimental autoimmune insulitis. Induction by T lymphocytes specific for a peptide of proINS. Am. J. Pathol. 1995, 147, 845–857. [Google Scholar] [PubMed]
- Schloot, N.C.; Willemen, S.; Duinkerken, G.; de Vries, R.R.; Roep, B.O. Cloned T cells from a recent onset IDDM patient reactive with INS B-chain. J. Autoimmun. 1998, 11, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Abiru, N.; Moriyama, H.; Babaya, N.; Liu, E.; Miao, D.; Yu, L.; Wegmann, D.R.; Hutton, J.C.; Elliott, J.F.; et al. Prime role for an INS epitope in the development of type 1 diabetes in NOD mice. Nature 2005, 435, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Pinkse, G.G.; Tysma, O.H.; Bergen, C.A.; Kester, M.G.; Ossendorp, F.; van Veelen, P.A.; Keymeulen, B.; Pipeleers, D.; Drijfhout, J.W.; Roep, B.O. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2005, 102, 18425–18430. [Google Scholar] [CrossRef]
- Pinkse, G.G.; Boitard, C.; Tree, T.I.; Peakman, M.; Roep, B.O. HLA class I epitope discovery in type 1 diabetes: Independent and reproducible identification of proINS epitopes of CD8 T cells-report of the IDS T Cell Workshop Committee. Ann. N. Y. Acad. Sci. 2006, 1079, 19–23. [Google Scholar] [CrossRef]
- Rabin, D.U.; Pleasic, S.M.; Shapiro, J.A.; Yoo-Warren, H.; Oles, J.; Hicks, J.M.; Goldstein, D.E.; Rae, P.M. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J. Immunol. 1994, 152, 3183–3188. [Google Scholar] [CrossRef]
- Jarchum, I.; Baker, J.C.; Yamada, T.; Takaki, T.; Marron, M.P.; Serreze, D.V.; DiLorenzo, T.P. In vivo cytotoxicity of INS-specific CD8+ T-cells in HLA-A*0201 transgenic NOD mice. Diabetes 2007, 56, 2551–2560. [Google Scholar] [CrossRef]
- Bonifacio, E.; Lampasona, V.; Genovese, S.; Ferrari, M.; Bosi, E. Identification of protein tyrosine phosphotase-like IA-2 (islet cell antigen 512) as the INS-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J. Immunol. 1995, 155, 5419–5426. [Google Scholar] [CrossRef]
- Solimena, M.; Dirkx RJr Hermel, J.-M.; Pleasic-Williams, S.; Shapiro, J.A.; Caron, L.; Rabin, D.U. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 1996, 15, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Honeyman, M.C.; Stone, N.L.; Harrison, L.C. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: Potential for mimicry with rotavirus and other environmental agents. Mol. Med. 1998, 4, 231–239. [Google Scholar] [CrossRef]
- Iddings, A.C.; Shenoi, A.N.; Morales Pozzo, A.; Kiessling, S.G. Hemolytic uremic syndrome complicated by Clostridium septicum bacteremia and new-onset type 1 diabetes mellitus. Report of a case. Clin. Nephrol. 2017, 87, 207–211. [Google Scholar] [CrossRef]
- Mirzai, S.; Rifai, A.O.; Webb, S.; Rifai, K.; Reiner, A. Probable Clostridium septicum pneumocephalus in a user of natural remedies with newly diagnosed diabetes mellitus type 1. IDCases 2019, 17, e00581. [Google Scholar] [CrossRef]
- Jamshidi, P.; Hasanzadeh, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Mota, J.F.; Sechi, L.A.; Nasiri, M.J. Is there any association be-tween gut microbiota and type 1 diabetes? A systematic review. Gut Pathog. 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, S.; Cavagnaro, M.J.; Zhang, W.; Shi, J. Quantitative Analysis and Visualization of the Interaction Between Intestinal Microbiota and Type 1 Diabetes in Children Based on Multi-Databases. Front. Pediatr. 2021, 9, 752250. [Google Scholar] [CrossRef] [PubMed]
- Cinek, O.; Kramna, L.; Mazankova, K.; Odeh, R.; Alassaf, A.; Ibekwe, M.U.; Ahmadov, G.; Elmahi, B.M.E.; Mekki, H.; Lebl, J.; et al. The bacteriome at the onset of type 1 diabetes: A study from four geographically distant African and Asian countries. Diabetes Res. Clin. Pract. 2018, 144, 51–62. [Google Scholar] [CrossRef]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.X.; et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018, 562, 589–594. [Google Scholar] [CrossRef]
- Endesfelder, D.; Engel, M.; Davis-Richardson, A.G.; Ardissone, A.N.; Achenbach, P.; Hummel, S.; Winkler, C.; Atkinson, M.; Schatz, D.; Triplett, E.; et al. Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome 2016, 4, 17. [Google Scholar] [CrossRef]
- Traversi, D.; Scaioli, G.; Rabbone, I.; Carletto, G.; Ferro, A.; Franchitti, E.; Carrera, D.; Savastio, S.; Cadario, F.; Siliquini, R.; et al. Gut microbiota, behavior, and nutrition after type 1 diabetes diagnosis: A longitudinal study for supporting data in the metabolic control. Front. Nutr. 2022, 9, 968068. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Autoimmunity and the microbiome: T-cell receptor mimicry of “self” and microbial antigens mediates self tolerance in holobionts: The concepts of “holoim-munity” (TcR-mediated tolerance for the holobiont) and “holoautoimmunity” (loss of tol-erance for the holobiont) are introduced. Bioessays 2016, 38, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Rudy, G.; Stone, N.; Harrison, L.C.; Colman, P.G.; McNair, P.; Brusic, V.; French, M.B.; Honeyman, M.C.; Tait, B.; Lew, A.M. Similar peptides from two beta cell autoantigens, proINS and glutamic acid decarboxylase, stimulate T cells of individuals at risk for INS-dependent diabetes. Mol. Med. 1995, 1, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Alkanani, A.K.; Ir, D.; Robertson, C.E.; Wagner, B.D.; Frank, D.N.; Zipris, D. Prevention of virus-induced type 1 diabetes with antibiotic therapy. J. Immunol. 2012, 189, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, T.P.; Peakman, M.; Roep, B.O. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin. Exp. Immunol. 2007, 148, 1–16. [Google Scholar] [CrossRef]
- Tanca, A.; Palomba, A.; Fraumene, C.; Manghina, V.; Silverman, M.; Uzzau, S. Clostridial Butyrate Biosynthesis Enzymes Are Significantly Depleted in the Gut Microbiota of Nonobese Diabetic Mice. mSphere 2018, 3, e00492-18. [Google Scholar] [CrossRef]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-diabetic Effects of Clostridium butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017, 7, 7046. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.Y.; Liu, Y. Effect of fecal microbiota transplantation on type 1 diabetes mellitus in non-obese diabetic mice and its underlying mechanism. Zhonghua Yi Xue Za Zhi 2022, 102, 1224–1231. (In Chinese) [Google Scholar] [CrossRef]
- Brown, K.; Godovannyi, A.; Ma, C.; Zhang, Y.; Ahmadi-Vand, Z.; Dai, C.; Gorzelak, M.A.; Chan, Y.; Chan, J.M.; Lochner, A.; et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016, 10, 321–332. [Google Scholar] [CrossRef]
- Depestel, D.D.; Aronoff, D.M. Epidemiology of Clostridium difficile infection. J. Pharm. Pract. 2013, 26, 464–475. [Google Scholar] [CrossRef]
- Finn, E.; Andersson, F.L.; Madin-Warburton, M. Burden of Clostridioides difficile infection (CDI)—A systematic review of the epidemiology of primary and recurrent CDI. BMC Infect. Dis. 2021, 21, 456. [Google Scholar] [CrossRef]
- Tougas, S.R.; Lodha, N.; Vandermeer, B.; Lorenzetti, D.L.; Tarr, P.I.; Tarr, G.A.M.; Chui, L.; Vanderkooi, O.G.; Freedman, S.B. Prevalence of Detection of Clostridioides difficile Among Asymptomatic Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2021, 175, e212328. [Google Scholar] [CrossRef] [PubMed]
- Brennhofer, S.A.; Rogawski McQuade, E.T.; Liu, J.; Guerrant, R.L.; Platts-Mills, J.A.; Warren, C.A. Clostridioides difficile colonization among very young children in resource-limited settings. Clin. Microbiol. Infect. 2022, 28, 996–1002. [Google Scholar] [CrossRef]
- Romero, J.R. Pediatric group B COX infections. Curr. Top. Microbiol. Immunol. 2008, 323, 223–239. [Google Scholar] [CrossRef]
- Khetsuriani, N.; Lamonte-Fowlkes, A.; Oberst, S.; Pallansch, M.A.; Centers for Disease Control and Prevention. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill. Summ. 2006, 55, 1–20. [Google Scholar] [PubMed]
- Brouwer, L.; Moreni, G.; Wolthers, K.C.; Pajkrt, D. World-Wide Prevalence and Genotype Distribution of Enteroviruses. Viruses 2021, 13, 434. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, Z.; Liu, H.; Xu, J.; Zhao, L.; Gao, L.; Zuo, Z.; Song, Y.; Han, Z.; Zhang, Y.; et al. Epidemiology of hand, foot, and mouth disease and the genetic characteristics of COX A16 in Taiyuan, Shanxi, China from 2010 to 2021. Front. Cell. Infect. Microbiol. 2022, 12, 1040414. [Google Scholar] [CrossRef] [PubMed]
- Leslie, R.D.; Evans-Molina, C.; Freund-Brown, J.; Buzzetti, R.; Dabelea, D.; Gillespie, K.M.; Goland, R.; Jones, A.G.; Kacher, M.; Phillips, L.S.; et al. Adult-Onset Type 1 Diabetes: Current Understanding and Challenges. Diabetes Care 2021, 44, 2449–2456. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef]
- Moltchanova, E.V.; Schreier, N.; Lammi, N.; Karvonen, M. Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabet. Med. 2009, 26, 673–678. [Google Scholar] [CrossRef]
- Ricci, S.; Perugia, F.; Piccini, B.; Lodi, L.; Pegoraro, F.; Giovannini, M.; Rombolà, G.; Perferi, G.; Toni, S.; Azzari, C. DR4/DQ2 haplotype confers susceptibility to T1DM with early clinical disease onset: A retrospective analysis in a tertiary-care hospital in Italy. PLoS ONE 2022, 17, e0276896. [Google Scholar] [CrossRef]
- Morran, M.P.; Vonberg, A.; Khadra, A.; Pietropaolo, M. Immunogenetics of type 1 diabetes mellitus. Mol. Asp. Med. 2015, 42, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.G.; Kick, K.; Bonifacio, E.; Haupt, F.; Hippich, M.; Dunstheimer, D.; Lang, M.; Laub, O.; Warncke, K.; Lange, K.; et al. Yield of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany. JAMA 2020, 323, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Raab, J.; Haupt, F.; Scholz, M.; Matzke, C.; Warncke, K.; Lange, K.; Assfalg, R.; Weininger, K.; Wittich, S.; Löbner, S.; et al. Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: Design and initial results of the Fr1da study. BMJ Open 2016, 6, e011144. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.J.; Steck, A.K.; Pugliese, A. Genetics of type 1 diabetes. Pediatr. Diabetes 2018, 19, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Steck, A.K.; Rewers, M.J. Genetics of type 1 diabetes. Clin. Chem. 2011, 57, 176–185. [Google Scholar] [CrossRef]
- Steck, A.K.; Armstrong, T.K.; Babu, S.R.; Eisenbarth, G.S.; Type 1 Diabetes Genetics Consortium. Stepwise or linear decrease in penetrance of type 1 diabetes with lower-risk HLA genotypes over the past 40 years. Diabetes 2011, 60, 1045–1049. [Google Scholar] [CrossRef]
- Carré, A.; Vecchio, F.; Flodstrom-Tullberg, M.; You, S.; Mallone, R. COX and type 1 diabetes: Diabetogenic mechanisms and implications for prevention. Endocr. Rev. 2023, bnad007. [Google Scholar] [CrossRef]
- Lan, H.; Hosomi, K.; Kunisawa, J. Clostridium perfringens enterotoxin-based protein engineering for the vaccine design and delivery system. Vaccine 2019, 37, 6232–6239. [Google Scholar] [CrossRef]
- Hosomi, K.; Hinenoya, A.; Suzuki, H.; Nagatake, T.; Nishino, T.; Tojima, Y.; Hirata, S.I.; Matsunaga, A.; Kondoh, M.; Yamasaki, S.; et al. Development of a bivalent food poisoning vaccine: Augmented antigenicity of the C-terminus of Clostridium perfringens enterotoxin by fusion with the B subunit of Escherichia coli Shiga toxin 2. Int. Immunol. 2019, 31, 91–100. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Ferreira, M.R.A.; Kremer, F.S.; Donassolo, R.A.; Júnior, C.M.; Alves, M.L.F.; Conceição, F.R. Recombinant Vaccine Design against Clostridium spp. Toxins Using Immunoinformatics Tools. Methods Mol. Biol. 2022, 2412, 457–470. [Google Scholar] [CrossRef]
- Saadh, M.J.; Lafi, F.F.; Dahadha, A.A.; Albannan, M.S. Immunogenicity of a newly developed vaccine against Clostridium perfringens alpha-toxin in rabbits and cattle. Vet. World 2022, 15, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Scalia, G.; Lunetta, M.; Condorelli, F.; Di Mauro, M.; Barcellini, W.; Stracuzzi, S.; Pagano, M.; Meroni, P.L. Correlation between islet cell antibodies and anti-cytomegalovirus IgM and IgG antibodies in healthy first-degree relatives of type 1 (INS-dependent) diabetic patients. Clin. Immunol. Immunopathol. 1990, 55, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Imagawa, A.; Fukui, K.; Uno, S.; Kozawa, J.; Sakai, M.; Yumioka, T.; Iwahashi, H.; Shimomura, I. A Histological Study of Fulminant Type 1 Diabetes Mellitus Related to Human Cytomegalovirus Reactivation. J. Clin. Endocrinol. Metab. 2017, 102, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Aarnisalo, J.; Veijola, R.; Vainionpää, R.; Simell, O.; Knip, M.; Ilonen, J. Cytomegalovirus infection in early infancy: Risk of induction and progression of autoimmunity associated with type 1 diabetes. Diabetologia 2008, 51, 769–772. [Google Scholar] [CrossRef]
- Ataei-Pirkooh, A.; Tehrani, M.; Keyvani, H.; Esghaei, M.; Tavakoli, A.; Nikmanesh, B.; Farahmand, M.; Ghaffari, H.; Monavari, S.H. Rotavirus Infection Enhances Levels of Autoantibodies Against Islet Cell Antigens GAD65 and IA-2 in Children with Type 1 Diabetes. Fetal Pediatr. Pathol. 2019, 38, 103–111. [Google Scholar] [CrossRef]
- Rudensky, Y.; Preston-Hurlburt, P.; Hong, S.C.; Barlow, A.; Janeway, C.A., Jr. Sequence analysis of peptides bound to MHC class II molecules. Nature 1991, 353, 622–627. [Google Scholar] [CrossRef]
- Suri, A.; Lovitch, S.B.; Unanue, E.R. The wide diversity and complexity of peptides bound to class II MHC molecules. Curr. Opin. Immunol. 2006, 18, 70–77. [Google Scholar] [CrossRef]
- Cunningham, M.W.; McCormack, J.M.; Fenderson, P.G.; Ho, M.K.; Beachey, E.H.; Dale, J.B. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J. Immunol. 1989, 143, 2677–2683. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Human Immunodeficiency Virus Proteins Mimic Human T Cell Receptors Inducing Cross-Reactive Antibodies. Int. J. Mol. Sci. 2017, 18, 2091. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Vonck, J.; Podufaly, A. Antigenic complementarity between coxsackie virus and streptococcus in the induction of rheumatic heart disease and autoimmune myocarditis. Autoimmunity 2009, 42, 1–16. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Huber, J.; Ziehl, A. Complementary Sets of Autoantibodies Induced by SARS-CoV-2, Adenovirus and Bacterial Antigens Cross-React with Human Blood Protein Antigens in COVID-19 Coagulopathies. Int. J. Mol. Sci. 2022, 23, 11500. [Google Scholar] [CrossRef] [PubMed]




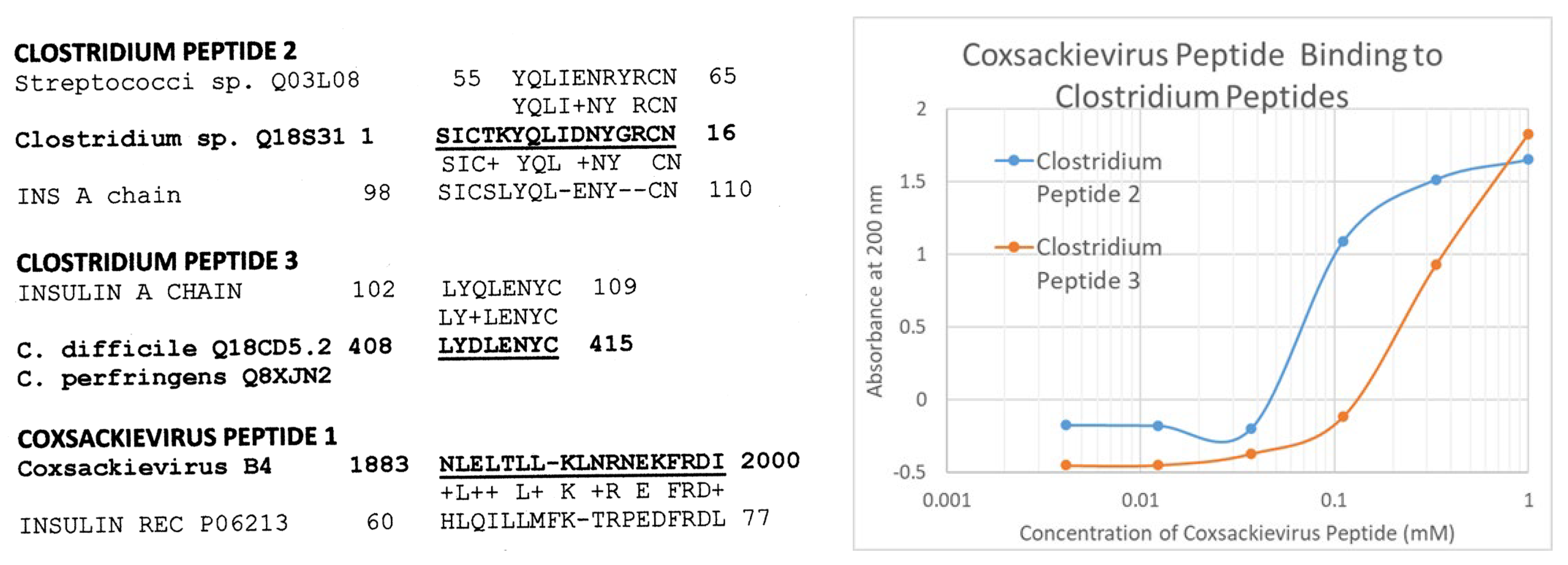
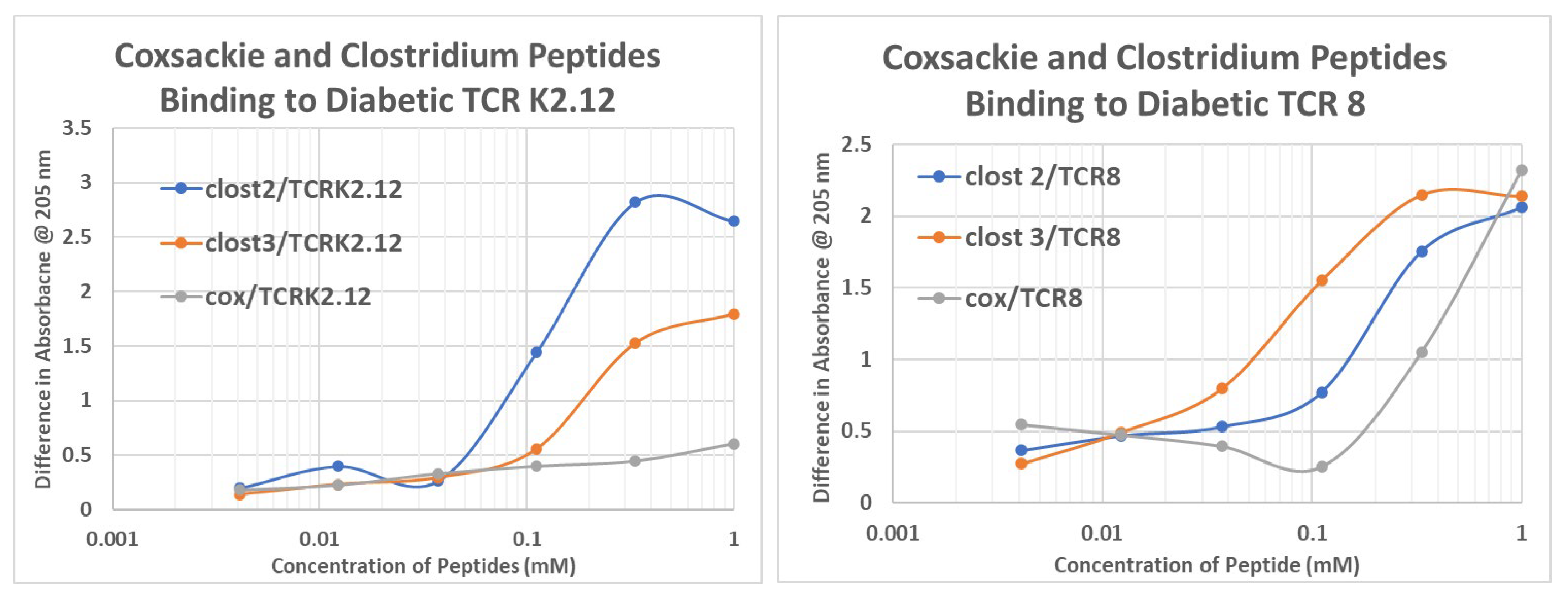
| Binding Constants (µM) | Cox Pep #1 | Ins Rec 105–118 | Ins Rec 897–915 | Clost Pep #2 | Clost Pep #3 | INS |
|---|---|---|---|---|---|---|
| TCR DIA 1 (Gluc) CASSIYLCSVEATRAD | 400 | 125 | 300 | 100 | 100 | 80 |
| TCR DIA 2, K2.16 (GR) CASSLAVIRT | 1000 | >1000 | >1000 | 110 | >10,000 | >1000 |
| TCR DIA 4, K2.4 (IR) CASSLATSGGGSDTQ | 1000 | >1000 | >1000 | 130 | 70 | 15 |
| TCR DIA 7 (IR) CASSFRRVTDTQ | >10,000 | >1000 | >1000 | 120 | 100 | 70 |
| TCR DIA 8 (Ins, GR) CASSQVRLAGGGEQ | 750 | 120 | 130 | 300 | 70 | 130 |
| TCR DIA 9 (Ins, GR) CASSLGQGETEAFF | 100 | 150 | 145 | 500 | 100 | 23 |
| TCR DIA 10 (IR) CASRNLGLNTE | 2000 | 110 | 140 | 1000 | 80 | 130 |
| TCR DIA 4,8,9 (Ins, IR, GR) DSALYLCASSLG | 200 | >1000 | >1000 | 3000 | 150 | >1000 |
| TCR DIA K2.12 (IR) CASSDRLGNQPQH | >10,000 | 120 | >1000 | 100 | 200 | 75 |
| PRODUCT NAME | SPECIES | SUPPLIER | PRODUCT # | Purity |
|---|---|---|---|---|
| Insulin (INS) | Human (recombinant) | Sigma-Aldrich | I2643 | 100% |
| INS A chain, oxidized | Bovine | Sigma-Aldrich | I1633 | >80% |
| INS B chain, oxidized | Bovine | Sigma-Aldrich | I1764 | >95% |
| INS C chain | Human (recombinant) | Sigma-Aldrich | C9781 | >95% |
| INS Receptor | Human (recombinant) | R&D Systems | 1544-IR | >95% |
| Glucagon | Human (recombinant) | Sigma-Aldrich | G2044 | 100% |
| Beta 2 Adrenergic Receptor | Rat (recombinant) | MyBioScource | MBS7111177 | >90% |
| VIRUS ANTIBODIES | SPECIES | SUPPLIER | PRODUCT # |
|---|---|---|---|
| Adenovirus | Goat | Millipore | AB1056 |
| COX B3 | Monkey | ATCC (NIH Reference Reagent) | VO31-501-563 |
| COX B4 | Horse | ATCC (NIH Reference Reagent) | VO30-501-560 |
| COX B3 | Mouse | Millipore | MAB948 |
| Coxsackie Virus B1-B6 Blend | Mouse | Millipore | MAB9410 |
| Cytomegalovirus | Goat | Biodesign International | B562756 |
| Cytomegalovirus | Mouse | Biodesign International | C65861M |
| Enterovirus pan VP3 | Mouse | MyBioSource | MBS319564 |
| Epstein-Barr Virus | Rabbit | Invitrogen | PA5-115471 |
| Hepatitis C Virus core Antigen | Rabbit | Invitrogen | PA1-4113 |
| Herpes Simplex Virus Type 1 | Goat | Invitrogen | PA1-7493 |
| Herpes Simplex Virus Type 1/2 | Rabbit | Invitrogen | PA1-7214 |
| Influenza A HRP | Goat | Biodesign International | B65243G |
| Influenza B HRP | Rabbit | Biodesign International | B653446 |
| BACTERIA ANTIBODIES | SPECIES | SUPPLIER | PRODUCT # |
|---|---|---|---|
| Clostridia | Rabbit | Invitrogen | PA1-7210 |
| Clostridium sp. HRP | Rabbit | US Biological | C5853-25C |
| Clostridium alpha toxin-HRP | Rabbit | Bioss | Bs-2273R-HRP |
| Enterococcus HRP | Rabbit | Invitrogen | PA1-73122 |
| Escherichia coli | Goat | abcam | AB13627 |
| Klebsiella pneumoniae HRP | Rabbit | Invitrogen | PA1-73176 |
| Mycobacterium tuberculosis | Rabbit | ABD Serotec | OBT0947 |
| Mycobacterium tuberculosis | Guinea Pig | MyBioSource | MBS315001 |
| Pseudomonas aeruginosa | Guinea Pig | Biodesign International | B47578P |
| Staphylococcus aureus | Rabbit | Invitrogen | PA1-7246 |
| Staphylococcus aureus HRP | Rabbit | Invitrogen | PA1-73173 |
| Streptococcus Group A | Goat | Invitrogen | PA1-7249 |
| Streptococcus Group A HRP | Rabbit | Acris Antibodies | BP2026HRP |
| Streptococcus pneumoniae | Rabbit | Biodesign International | B65831R |
| Streptococcus pneumoniae | Rabbit | Invitrogen | PA1-7259 |
| PROTEIN ANTIBODIES | SPECIES | SUPPLIER | PRODUCT # |
|---|---|---|---|
| Glutamic acid decarboxylase 65 | Rabbit | Millipore | ABN101 |
| Insulin (INS) | Rabbit | Sigma | HPA004932 |
| INS Receptor | Mouse | Chemicon | MAB105 |
| INS Receptor alpha | Rabbit | Biodesign International | K54244R |
| INS Receptor alpha | Mouse | Biodesign International | K54241M |
| INS Receptor beta | Mouse | Millipore | 05-1104 |
| PTPN(IA-2) | Rabbit | Sigma | HPA007179 |
| SECONDARY ANTIBODIES | SPECIES | SUPPLIER | PRODUCT # |
|---|---|---|---|
| Anti-Guinea Pig-HRP | Rabbit | abcam | AB6771 |
| Anti-Horse IgG-HRP | Goat | Santa Cruze Biotechnology | SC-2448 |
| Anti-Human IgG-HRP | Goat | Sigma | AO170 |
| Anti-Mouse IgG-HRP | Goat | Sigma-Aldrich | A9917 |
| Anti-Rabbit IgGHRP | Goat | Invitrogen | 65-6120 |
| Disease State | Supplier | Our ID | Sample ID | Age | Sex | Race | HbA1c % | Serum Treatment |
|---|---|---|---|---|---|---|---|---|
| T1DM | Lee Biosolutions | LEE 01 | 09E5731 A1c-50.11 | 39 | M | Caucasian | 11.5 | EDTA |
| T1DM | Lee Biosolutions | LEE 02 | 09E5731 A1c-50.12 | 36 | F | Caucasian | 14.4 | EDTA |
| T1DM | Lee Biosolutions | LEE 03 | 09E5731 A1c-03 | 29 | F | Caucasian | 10.3 | EDTA |
| T1DM | Lee Biosolutions | LEE 04 | 09E5731 A1c-04 | 30 | M | Caucasian | 10.5 | EDTA |
| T1DM | Lee Biosolutions | LEE 05 | 09E5731 A1c-05 | 24 | F | Caucasian | 11.3 | EDTA |
| T1DM | Innovative Resesarch | T1DM #1 | HMN889069 | 61 | M | Caucasian | Off the clot | |
| T1DM | Innovative Resesarch | T1DM #2 | HMN889070 | 36 | M | Asian | Off the clot | |
| T1DM | Innovative Resesarch | T1DM #3 | HMN889071 | 56 | F | Caucasian | Off the clot | |
| T1DM | Innovative Resesarch | T1DM #4 | HMN889090 | 58 | M | Hispanic | Off the clot | |
| T1DM | Innovative Resesarch | T1DM #5 | HMN889091 | 49 | F | African American | Off the clot | |
| T2D | ZenBio | #69 | SER-DPLE2ML | M | Caucasian | Off the clot | ||
| T2D | ZenBio | #71 | SER-DPLE2ML | F | African Amderican | Off the clot | ||
| Healthy | Zen-Bio | Healthy 1 | HSER-2ML | F | Caucasian | Off the clot | ||
| Healthy | Innovative Resesarch | Healthy 86 | 39521-086 | 46 | M | African American | Off the clot | |
| Healthy | Innovative Resesarch | Healthy 87 | 39521-087 | 36 | F | African American | Off the clot | |
| Healthy | Innovative Resesarch | Healthy 88 | 39521-088 | 40 | M | African American | Off the clot | |
| Healthy | Innovative Resesarch | Healthy 89 | 39521-089 | 59 | M | African American | Off the clot |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Root-Bernstein, R.; Chiles, K.; Huber, J.; Ziehl, A.; Turke, M.; Pietrowicz, M. Clostridia and Enteroviruses as Synergistic Triggers of Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 8336. https://doi.org/10.3390/ijms24098336
Root-Bernstein R, Chiles K, Huber J, Ziehl A, Turke M, Pietrowicz M. Clostridia and Enteroviruses as Synergistic Triggers of Type 1 Diabetes Mellitus. International Journal of Molecular Sciences. 2023; 24(9):8336. https://doi.org/10.3390/ijms24098336
Chicago/Turabian StyleRoot-Bernstein, Robert, Kaylie Chiles, Jack Huber, Alison Ziehl, Miah Turke, and Maja Pietrowicz. 2023. "Clostridia and Enteroviruses as Synergistic Triggers of Type 1 Diabetes Mellitus" International Journal of Molecular Sciences 24, no. 9: 8336. https://doi.org/10.3390/ijms24098336
APA StyleRoot-Bernstein, R., Chiles, K., Huber, J., Ziehl, A., Turke, M., & Pietrowicz, M. (2023). Clostridia and Enteroviruses as Synergistic Triggers of Type 1 Diabetes Mellitus. International Journal of Molecular Sciences, 24(9), 8336. https://doi.org/10.3390/ijms24098336








