Effective Pre-Clinical Treatment of Fish Envenoming with Polyclonal Antiserum
Abstract
1. Introduction
2. Results and Discussion
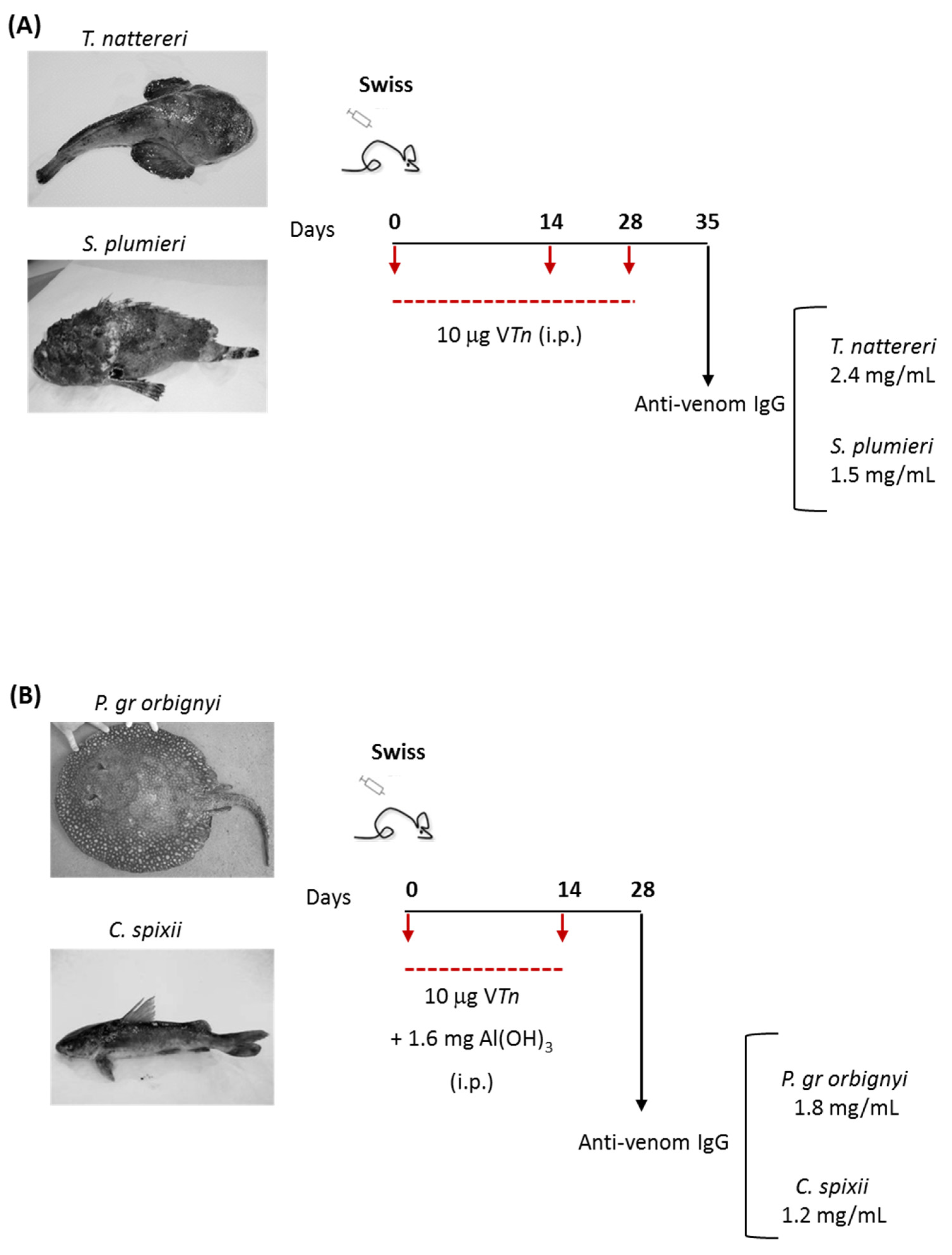
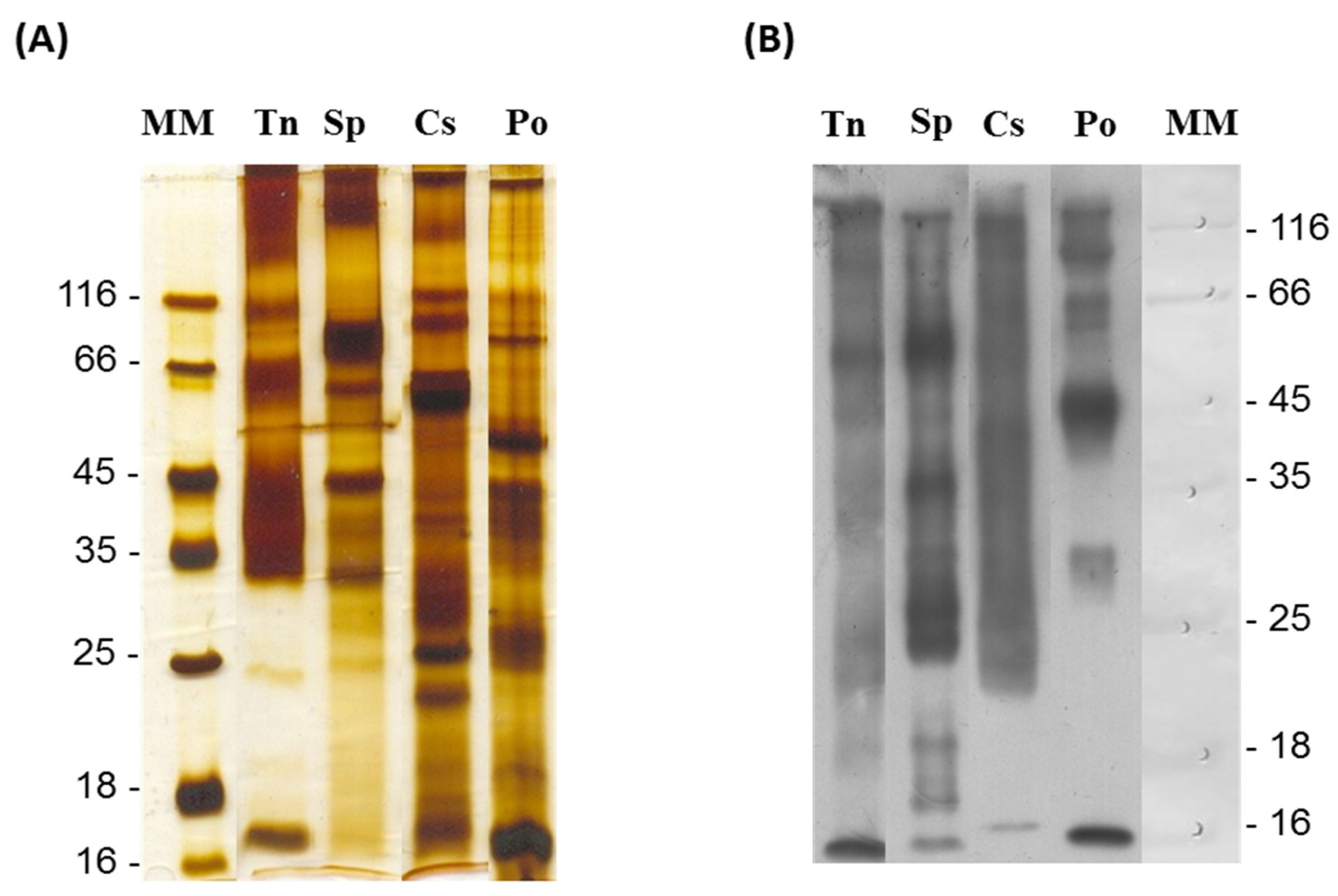
2.1. Immunogenicity Capacity of Fish Venoms to Produce Neutralizing IgG Antibodies
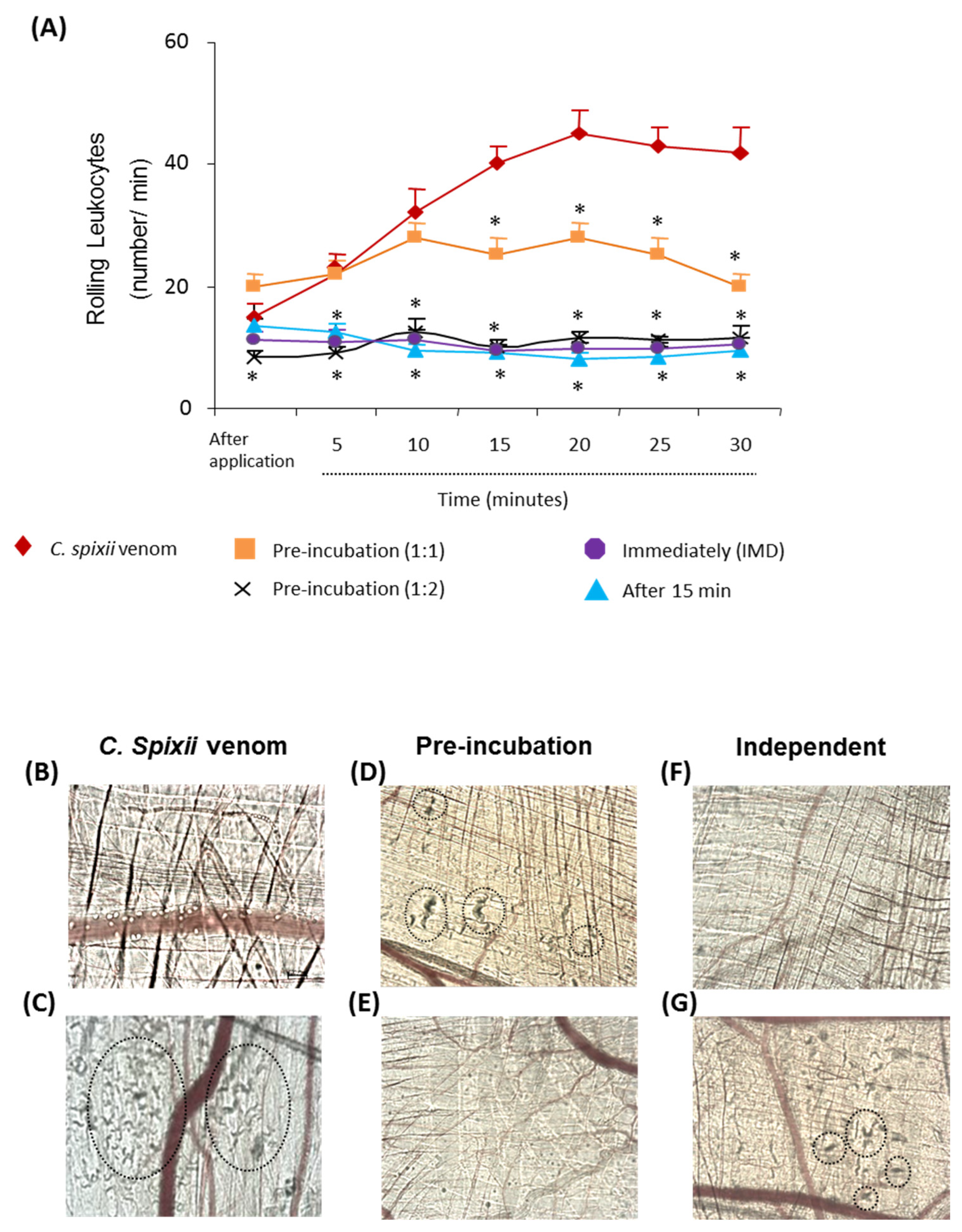
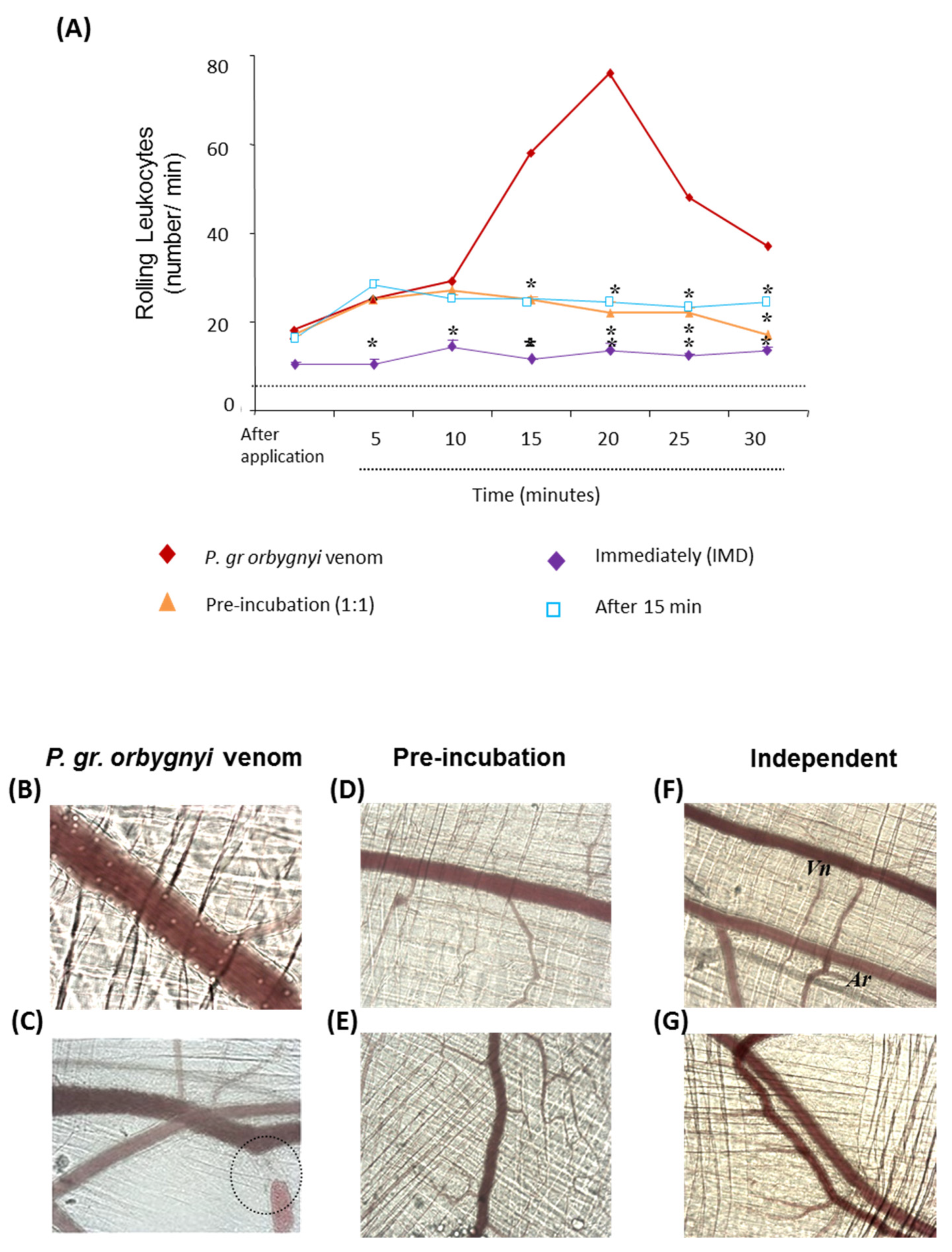
2.2. The Antiserum Attenuated Changes in the Microvasculature
2.3. The Antiserum Prevented Edema and Nociception
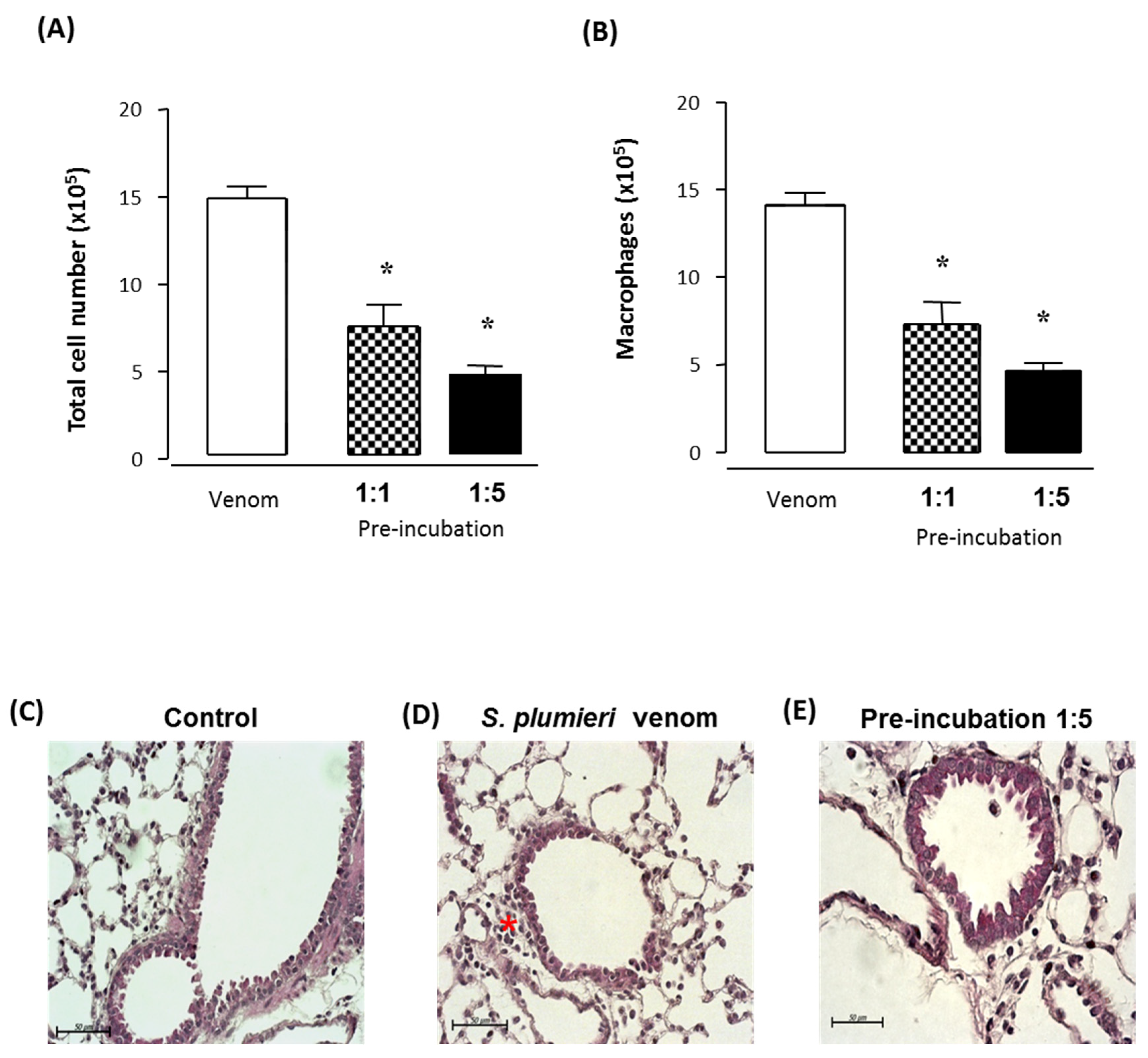
2.4. The Antiserum Neutralizes Acute Lung Inflammation
3. Materials and Methods
3.1. Mice
3.2. Fish and Venoms
3.3. Immunization Protocols for Obtaining Venom Monospecific Serum
3.4. Purification of Antiserum IgG Fraction
3.5. Methods for Studying the Neutralization Capacity of Antiserum
3.5.1. Evaluation of Microcirculation Alterations in Cremaster Muscle
3.5.2. Nociceptive Activity
3.5.3. Edema-Induced Activity
3.5.4. Acute Lung Inflammation Induced by Scorpaena plumieri Venom
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Ziegman, R.; Alewood, P. Bioactive Components in Fish Venoms. Toxins 2015, 7, 1497–1531. [Google Scholar] [CrossRef]
- Facó, P.E.; Bezerra, G.P.; Barbosa, P.S.F.; Martins, A.M.C.; Guimarães, J.A.; Ferreira, M.L.; Monteiro, H.S.A. Epidemiologia dos acidentes por Thalassophryne nattereri (niquim) no Estado do Ceará (1992–2002). Rev. Soc. Bras. Med. Trop. 2005, 38, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Haddad, V., Jr.; Pardal, P.P.O.; Cardoso, J.L.C.; Martins, I. The venomous toadfish Thalassophryne nattereri (niquim or miquim): Report of 43 injuries provoked in fishermen of Salinópolis (Pará State) and Aracaju (Sergipe State), Brazil. Rev. Do Inst. Med. Trop. São Paulo 2003, 45, 221–223. [Google Scholar] [CrossRef]
- Haddad, V., Jr.; Martins, I.A. Frequency and gravity of human envenomations caused by marine catfish (suborder siluroidei): A clinical and epidemiological study. Toxicon 2006, 47, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Haddad, V., Jr.; Martins, I.; Makyama, H.M. Injuries caused by scorpionfishes (Scorpaena plumieri Bloch, 1789 and Scorpaena brasiliensis Cuvier, 1829) in the Southwestern Atlantic Ocean (Brazilian coast): Epidemiologic, clinic and therapeutic aspects of 23 stings in humans. Toxicon 2003, 42, 79–83. [Google Scholar] [CrossRef]
- Haddad, V., Jr.; Neto, D.G.; Neto, J.B.D.P.; Marques, F.P.D.L.; Barbaro, K.C. Freshwater stingrays: Study of epidemiologic, clinic and therapeutic aspects based on 84 envenomings in humans and some enzymatic activities of the venom. Toxicon 2004, 43, 287–294. [Google Scholar] [CrossRef]
- Ministério da Saúde. SINAN SISTEMA DE INFORMAÇÃO DE AGRAVOS DE NOTIFICAÇÃO. Available online: http://www.portalsinan.saude.gov.br/ (accessed on 28 January 2023).
- Lopes-Ferreira, M.; Emim, J.A.S.; Souccar, C.; Lapa, A.J.; Cezari, M.H.S.; Juliano, L.; Moura-Da-Silva, A.M.; Mota, I. Characterization of the Nociceptive and Edematogenic Activities of the Thalassophryne Nattereri Fish Venom. Toxicon 1998, 36, 1304. [Google Scholar] [CrossRef]
- Fonseca, L.A.; Lopes-Ferreira, M. Clinical and Experimental Studies Regarding Poisoning Caused by a Fish Thalassophryne Nattereri (Niquim). An. Bras. Derm. 2000, 75, 435–443. [Google Scholar]
- Junqueira, M.E.P.; Grund, L.Z.; Orii, N.M.; Saraiva, T.C.; Lopes, C.A.D.M.; Lima, C.; Lopes-Ferreira, M. Analysis of the inflammatory reaction induced by the catfish (Cathorops spixii) venoms. Toxicon 2007, 49, 909–919. [Google Scholar] [CrossRef]
- Magalhães, K.W.; Lima, C.; Piran-Soares, A.A.; Marques, E.E.; Hiruma-Lima, C.A.; Lopes-Ferreira, M. Biological and biochemical properties of the Brazilian Potamotrygon stingrays: Potamotrygon cf. scobina and Potamotrygon gr. orbignyi. Toxicon 2006, 47, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.F.; Hardy, J.C. Stonefish envenomation. N. Engl. J. Med. 1993, 329, 510–511. [Google Scholar] [CrossRef]
- Bruni, F.M.; Coutinho, E.M.M.; Andrade-Barros, A.I.; Grund, L.Z.; Lopes-Ferreira, M.; Lima, C. Anaphylaxis induced by Thalassophryne nattereri venom in mice is an IgE/IgG1-mediated, IL-4-dependent phenomenon. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.N.J.; Ferreira, K.R.C.; Pinto, R.N.L.; Aird, S.D. A Severe Accident Caused by an Ocellate River Stingray (Potamotrygon motoro) in Central Brazil: How Well Do We Really Understand Stingray Venom Chemistry, Envenomation, and Therapeutics? Toxins 2015, 7, 2272–2288. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Emim, J.A.D.S.; Oliveira, V.; Puzer, L.; Cezari, M.H.; Araújo, M.D.S.; Juliano, L.; Lapa, A.J.; Souccar, C.; Moura-Da-Silva, A.M. Kininogenase activity of Thalassophryne nattereri fish venom. Biochem. Pharmacol. 2004, 68, 2151–2157. [Google Scholar] [CrossRef]
- da Silva, W.D.; De Andrade, S.A.; Megale, A.A.; De Souza, D.A.; Sant’Anna, O.A.; Magnoli, F.C.; Guidolin, F.R.; Godoi, K.S.; Saladini, L.Y.; Spencer, P.J.; et al. Antibodies as Snakebite Antivenoms: Past and Future. Toxins 2022, 14, 606. [Google Scholar] [CrossRef]
- Saggiomo, S.; Firth, C.; Wilson, D.; Seymour, J.; Miles, J.; Wong, Y. The Geographic Distribution, Venom Components, Pathology and Treatments of Stonefish (Synanceia spp.) Venom. Mar. Drugs 2021, 19, 302. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Moura-Da-Silva, A.; Mota, I.; Takehara, H. Neutralization of Thalassophryne nattereri (niquim) fish venom by an experimental antivenom. Toxicon 2000, 38, 1149–1156. [Google Scholar] [CrossRef]
- Piran-Soares, A.A.; Komegae, E.N.; Souza, V.M.O.; Fonseca, L.A.; Lima, C.; Lopes-Ferreira, M. Neutralizing antibodies obtained in a persistent immune response are effective against deleterious effects induced by the Thalassophryne nattereri fish venom. Toxicon 2007, 49, 920–930. [Google Scholar] [CrossRef]
- Gomes, H.L.; Menezes, T.N.; Carnielli, J.B.; Andrich, F.; Evangelista, K.S.; Chávez-Olórtegui, C.; Vassallo, D.V.; Figueiredo, S.G. Stonefish antivenom neutralises the inflammatory and cardiovascular effects induced by scorpionfish Scorpaena plumieri venom. Toxicon 2011, 57, 992–999. [Google Scholar] [CrossRef]
- Martins, A.; Barbosa, P.; Sousa, D.; Alves, C.; Menezes, D.; Lima, C.; Lopes-Ferreira, M.; Fonteles, M.; Monteiro, H. Antivenom action on renal effects induced by Thalassophryne nattereri venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 125–135. [Google Scholar] [CrossRef]
- Dunning, A.J. A model for immunological correlates of protection. Stat. Med. 2005, 25, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, A.; Zahaf, T.; Tibaldi, F. Assurance in vaccine efficacy clinical trial design based on immunological responses. Biom. J. 2021, 63, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lindgren, G.; Sandgren, K.J.; Thompson, E.A.; Francica, J.R.; Seubert, A.; De Gregorio, E.; Barnett, S.; O’Hagan, D.T.; Sullivan, N.J.; et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 2017, 9, eaal2094. [Google Scholar] [CrossRef] [PubMed]
- Kallolimath, S.; Sun, L.; Palt, R.; Stiasny, K.; Mayrhofer, P.; Gruber, C.; Kogelmann, B.; Chen, Q.; Steinkellner, H. Highly active engineered IgG3 antibodies against SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2107249118. [Google Scholar] [CrossRef]
- Kringelum, J.V.; Lundegaard, C.; Lund, O.; Nielsen, M. Reliable B Cell Epitope Predictions: Impacts of Method Development and Improved Benchmarking. PLoS Comput. Biol. 2012, 8, e1002829. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Magalhães, G.S.; Fernandez, J.H.; Junqueira-De-Azevedo, I.D.L.M.; Le Ho, P.; Lima, C.; Valente, R.H.; Moura-Da-Silva, A.M. Structural and biological characterization of Nattectin, a new C-type lectin from the venomous fish Thalassophryne nattereri. Biochimie 2011, 93, 971–980. [Google Scholar] [CrossRef]
- Magalhaes, G.; Lopesferreira, M.; Junqueiradeazevedo, I.; Spencer, P.; Araujo, M.; Portaro, F.; Ma, L.; Valente, R.; Juliano, L.; Fox, J. Natterins, a new class of proteins with kininogenase activity characterized from Thalassophryne nattereri fish venom. Biochimie 2005, 87, 687–699. [Google Scholar] [CrossRef]
- Boletini-Santos, D.; Komegae, E.N.; Figueiredo, S.G.; Haddad, V.; Lopes-Ferreira, M.; Lima, C. Systemic response induced by Scorpaena plumieri fish venom initiates acute lung injury in mice. Toxicon 2008, 51, 585–596. [Google Scholar] [CrossRef]
- Ramos, A.D.; Conceição, K.; Silva, P.I., Jr.; Richardson, M.; Lima, C.; Lopes-Ferreira, M. Specialization of the sting venom and skin mucus of Cathorops spixii reveals functional diversification of the toxins. Toxicon 2012, 59, 651–665. [Google Scholar] [CrossRef]
- Lima, C.; Disner, G.R.; Alice, M.; Falc, P.; Seni-silva, A.C.; Luis, A.; Maleski, A.; Souza, M.M.; Cristina, M.; Tonello, R.; et al. The Natterin Proteins Diversity: A Review on Phylogeny, Structure, and Immune Function. Toxins 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Liu, N.; Cheng, W.; Jiang, Y.; Sun, H.; Chen, L.; Peng, J.; Zhang, Y.; Ding, Y.; Zhang, Z.; et al. Structural basis for receptor recognition and pore formation of a zebrafish aerolysin-like protein. EMBO Rep. 2015, 17, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Falcao, M.A.P.; Andrade-Barros, A.I.; Seni-Silva, A.C.; Grund, L.Z.; Balogh, E.; Conceiçao, K.; Queniaux, V.F.; Ryffel, B.; Lopes-Ferreira, M. Natterin an aerolysin-like fish toxin drives IL-1β-dependent neutrophilic inflammation mediated by caspase-1 and caspase-11 activated by the inflammasome sensor NLRP6. Int. Immunopharmacol. 2020, 91, 107287. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Andrade-Barros, A.I.; Bernardo, J.T.G.; Balogh, E.; Quesniaux, V.F.; Ryffel, B.; Lopes-Ferreira, M. Natterin-Induced Neutrophilia Is Dependent on cGAS/STING Activation via Type I IFN Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 3600. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Moura-Da-Silva, A.M.; Piran-Soares, A.A.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Farsky, S.H. Hemostatic effects induced by Thalassophryne nattereri fish venom: A model of endothelium-mediated blood flow impairment. Toxicon 2002, 40, 1141–1147. [Google Scholar] [CrossRef]
- Rosa, R.; Charvet-Almeida, P.; Quijada, C.D.; Carrier, J.; Musick, J.; Heithaus, M. Biology of the South American Potamotrygonid Stingrays. In Sharks and Their Relatives II; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 241–281. [Google Scholar] [CrossRef]
- Lent-Schochet, D.; Jialal, I. Physiology, Edema; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lopes-Ferreira, M.; Gomes, E.M.; Bruni, F.M.; Ferreira, M.J.; Charvet, P.; Lima, C. First report of interruption of mast cell degranulation and endothelial cells activation by anti-inflammatory drugs controlling the acute response provoked by Pseudoplatystoma fasciatum fish venom. Toxicon 2014, 90, 237–248. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Carrijo, L.C.; Andrich, F.; de Lima, M.E.; Cordeiro, M.N.; Richardson, M.; Figueiredo, S.G. Biological properties of the venom from the scorpionfish (Scorpaena plumieri) and purification of a gelatinolytic protease. Toxicon 2005, 45, 843–850. [Google Scholar] [CrossRef]
- dos Santos, J.C.; Grund, L.Z.; Seibert, C.S.; Marques, E.E.; Soares, A.B.; Quesniaux, V.F.; Ryffel, B.; Lopes-Ferreira, M.; Lima, C. Stingray venom activates IL-33 producing cardiomyocytes, but not mast cell, to promote acute neutrophil-mediated injury. Sci. Rep. 2017, 7, 7912. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kurtović, T.; Balija, M.L.; Brgles, M.; Sviben, D.; Tunjić, M.; Cajner, H.; Marchetti-Deschmann, M.; Allmaier, G.; Halassy, B. Refinement strategy for antivenom preparation of high yield and quality. PLoS Negl. Trop. Dis. 2019, 13, e0007431. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes Ferreira, M.; Falcão, M.A.P.; Bruni, F.M.; Haddad, V., Jr.; Marques, E.E.; Seibert, C.S.; Lima, C. Effective Pre-Clinical Treatment of Fish Envenoming with Polyclonal Antiserum. Int. J. Mol. Sci. 2023, 24, 8338. https://doi.org/10.3390/ijms24098338
Lopes Ferreira M, Falcão MAP, Bruni FM, Haddad V Jr., Marques EE, Seibert CS, Lima C. Effective Pre-Clinical Treatment of Fish Envenoming with Polyclonal Antiserum. International Journal of Molecular Sciences. 2023; 24(9):8338. https://doi.org/10.3390/ijms24098338
Chicago/Turabian StyleLopes Ferreira, Monica, Maria Alice Pimentel Falcão, Fernanda Miriane Bruni, Vidal Haddad, Jr., Elineide Eugênio Marques, Carla Simone Seibert, and Carla Lima. 2023. "Effective Pre-Clinical Treatment of Fish Envenoming with Polyclonal Antiserum" International Journal of Molecular Sciences 24, no. 9: 8338. https://doi.org/10.3390/ijms24098338
APA StyleLopes Ferreira, M., Falcão, M. A. P., Bruni, F. M., Haddad, V., Jr., Marques, E. E., Seibert, C. S., & Lima, C. (2023). Effective Pre-Clinical Treatment of Fish Envenoming with Polyclonal Antiserum. International Journal of Molecular Sciences, 24(9), 8338. https://doi.org/10.3390/ijms24098338







