Abstract
During their life cycle, apicomplexan parasites pass through different microenvironments and encounter a range of ion concentrations. The discovery that the GPCR-like SR25 in Plasmodium falciparum is activated by a shift in potassium concentration indicates that the parasite can take advantage of its development by sensing different ionic concentrations in the external milieu. This pathway involves the activation of phospholipase C and an increase in cytosolic calcium. In the present report, we summarize the information available in the literature regarding the role of potassium ions during parasite development. A deeper understanding of the mechanisms that allow the parasite to cope with ionic potassium changes contributes to our knowledge about the cell cycle of Plasmodium spp.
Keywords:
calcium; GPCR; malaria; PfSR25; Plasmodium berghei; Plasmodium falciparum; potassium; Toxoplasma gondii 1. Introduction
The parasite that causes malaria (Plasmodium genus) is a unicellular eukaryote belonging to the phylum Apicomplexa and the family Plasmodiidae. It has a complex biological cycle consisting of different development stages comprising two phases: (i) asexual, where the parasites are found in the vertebrate host; and (ii) sexual, which occurs in the anopheline invertebrate host. During its biological cycle, the parasite undergoes morphological and biochemical changes, which make it possible to distinguish between the different stages [1,2].
Transmission occurs as a by-product of feeding, with infected female anopheline mosquitoes injecting sporozoites into the dermis of the vertebrate host, from where they eventually enter the bloodstream. Sporozoites in the bloodstream migrate to the liver and invade liver cells (hepatocytes), where they multiply asexually; this asymptomatic period lasts, on average, between six and fifteen days. At the end of the hepatic phase, the merozoites are released by rupture of the hepatocytes and subsequent invasion of erythrocytes. The erythrocytic cycle consists of parasites passing through the ring, trophozoite, and schizont stages [3,4,5,6,7].
Throughout its life cycle in the vertebrate host, the malaria parasite is exposed to extreme environmental changes, including the host’s immunological defenses, temperature, and ion concentrations, as it moves between microenvironments. Ions were shown to play an essential role in several biological processes of parasite development [8]. Regarding the concentration of potassium ions (K+), the parasite is exposed to a variation of 5 mM when outside the blood cells to 140 mM inside the parasitized cell [9]. Several studies have demonstrated the importance of the K+ ion as a regulator of events during the development of various Apicomplexan parasites, including the malaria parasite. In this present contribution, we summarize the primary information available in the literature about the essential role of K+ in the infectivity, development, and survival of Plasmodium spp.
2. Differences in Ion Concentrations in the Microenvironment Activate Signaling Pathways Required for Infection
After the mosquito injects sporozoites into the vertebrate host, infection of the hepatocytes is the next step in the parasite’s development. When in contact with the intracellular environment of these cells, the parasite initially migrates without forming a parasitophorous vacuole until it eventually reaches a cell in which it develops [5]. During this process, the parasite moves from the intracellular to the extracellular environments, and it is exposed to different [K+], from [5 mM] in the extracellular environment to [140 mM] when the parasite resides inside the host cells [10]. By studying the process of sporozoite motility to and through liver cells, Mota and coworkers suggested that this process of motility and cell-crossing is essential for the parasite to activate the signaling pathways necessary for its subsequent development in the hepatocytes. Incubation of sporozoites with host cell extracts increases the secretion of proteins involved in the invasion. Thus, parasite migration through cells induces exocytosis of apical organelles required for infection and represents an essential step in the parasite lifecycle [5,11].
Using murine malaria parasites (Plasmodium berghei), Kumar et al. (2007) observed that sporozoites incubated in a high [K+] buffer (142 mM) and subsequently placed in liver cell culture (HepG2) showed an increase in infectivity and reduced cell passage activity compared to sporozoites incubated in Na+-rich buffer [10]. When similar experiments were carried out in the presence of K+ channel blockers (20 mM tetraethylammonium chloride and 0.5 mM quinine), there was a decrease in infectivity compared to sporozoites treated with K+-rich buffer [10]. We interpret these data to suggest that: (i) the detection of K+ ions by the parasite inside cells and in the extracellular environment regulates cell infectivity, and thus, it is necessary for the progression of the parasite life cycle; (ii) the passage of sporozoites through hepatocytes may trigger the activation of signaling pathways induced by migration through host cells [5,10,11,12,13].
In T. gondii, Moudy et al. (2001) evaluated the ionic environment in the parasite egress process by investigating the influence of [Ca2+], [Na+], [Cl−], and [K+] from intracellular to extracellular levels in HFF cells. The authors showed that the only ion that plays a role in this process is K+; when [K+] is similar to cytoplasmic levels, the egress does not occur, but under low [K+] or total absence of this ion, the exit is more efficient [14]. Secretion of the microneme is critical for the successful infection of new cells and the progression of the life cycle. The environment in which the parasites find themselves has been shown to correlate directly with the activation of this process, which requires Ca2+ as an intracellular second messenger [15,16,17]. An increase in Ca2+ concentration is associated with significant changes in parasite morphology, in addition to promoting the secretion of proteins involved in invasion and egress from the host cell [18]. An increase in Ca2+cyt initiates a Ca2+ signaling cascade that promotes parasite motility and egress from the cell. Recent findings have shown that intracellular T. gondii tachyzoites are able to capture Ca2+ from the host cytoplasm to reach a cytosolic Ca2+ threshold required for successful egress; furthermore, this exit process is favored by decreasing [K+], indicating that the reduction of [K+] around the parasite is one of the factors involved in the egress rate [19].
Undoubtedly, the activation of the parasite’s motility machinery is a crucial point for the successful infection of new cells; behind this process, Ca2+ acts as an irreplaceable messenger transmitting to the intracellular medium the external stimuli from the environment [20,21]. Similar to Plasmodium, Toxoplasma also benefits from information coming from the extracellular environment to complete its life cycle, and the detection of [K+] is one of these mechanisms. One of the pieces in the K+ signaling in T. gondii is the apical protein Guanylate Cyclase (TgGC). Conditional knockdown of TgGC presents parasites defective in motility, adhesion, invasion, and egress of the host cell and is a critical protein for capturing changes in external pH and extracellular [K+] to activate the Ca2+cyt increase [22]. From this point, we draw attention to K+, an ion that is present in different concentrations in the microenvironments that parasites pass through during their development.
3. K+ in the Invasion of Erythrocytes and Progression of the Erythrocytic Cycle
After the hepatocyte cycle, the parasite reaches the bloodstream, beginning its intraerythrocytic cycle, where they reproduce asexually within the erythrocytes of its vertebrate host. Studies from the Kirk group indicate that considerable changes in cell membrane permeability occur in erythrocytes infected by P. falciparum ±15 h after the invasion and that these are detrimental to the normal ion balance. The loss of K+ and Na+ across the membrane increases, and the activity of the Na+/K+ pump decreases; these processes result in the replacement of K+ ions by Na+ ions in the erythrocyte cytosol [23,24,25,26,27]. The Na+ concentration in the cytosol of the infected cell is high, while the parasite maintains a low cytosolic Na+ concentration [27,28].
In 2016, Spillman reported a mechanism by which, via a Na+-ATPase from P. falciparum, PfATP4 expels Na+ from its cytosol into that of the host cell. In addition, the authors described PfATP4 as a target for spiroindolones, as spiroindolones disrupt the Na+ homeostasis of the parasite. Furthermore, it has been shown that mutations in PfATP4 confer resistance to antimalarial spiroindolones [29]. In another study by the group, Winterberg and Kirk (2016) reported a correlation between the intracellular concentrations of Na+ and K+ in red blood cells infected with P. falciparum. Using a high-sensitivity HPLC assay to measure intracellular Na+ and K+, the authors showed that a new spiroindolone antimalarial candidate, KAE609, can promote the disruption of parasite ion homeostasis by increasing [Na+] and decreasing [K+] after 30 min of 50 nM treatment [30].
Proteins on the surface of merozoites and proteins secreted by apical membrane-associated organelles, rhoptries, and micronemes actively participate in the erythrocyte invasion process. In 2010, Singh and colleagues found that exposure of merozoites to low [K+] (5 mM), as seen in blood plasma, promotes a PLC-dependent increase in cytosolic calcium levels (Ca2+cyt) [31]. This increase in Ca2+cyt would be associated with the release of microneme proteins, such as erythrocyte-binding antigen (EBA175) and apical membrane antigen-1 (AMA-1), to the merozoite surface [26]. The interaction of EBA175 with its receptor on erythrocytes, glycophorin A (glyA), restores basal Ca2+cyt levels and triggers the release of rhoptry proteins [31]. The authors identify for the first time an external signal responsible for the sequential release of micronemes and rhoptries proteins during erythrocyte invasion, this signal being the changes in [K+] in the parasite’s microenvironment.
In a very elegant study from the Chitins’ lab, the authors treated P. falciparum merozoites, isolated in [140 mM K+], with the calcineurin inhibitors FK506 and cyclosporin A, before transferring the parasites to an environment with low K+ concentration (5 mM). The shift of exposure of merozoites to [K+] from 140 to 5 mM triggered the release of microneme proteins, mainly the apical membrane antigen 1 (AMA1) protein. The use of calcineurin inhibitors resulted in the inhibition of AMA1 secretion to the merozoite membrane, preventing the process of erythrocytes invasion by the parasite [32,33]. This seminal work allows the authors to propose the involvement of a [K+]-dependent and calcium pathway in the erythrocyte invasion process.
4. Plasmodium falciparum Serpentine Receptor 25 (PfSR25), a GPCR-like Receptor Responsible for the Transmission of the K+ Stimulus to the Interior of the Parasite
The complex signaling pathways present in P. falciparum led Madeira et al. (2008) to search for G protein-coupled receptors (GPCRs) in the P. falciparum genome. Through a robust bioinformatic analysis of the parasite genome, the authors identified four candidates for GPCR receptors, namely, PfSR1, PfSR10, PfSR12, and PfSR25 [34]. In 2017, the P. falciparum serpentine receptor 25 (PfSR25) was identified as responsible for sensing the [K+] shift stimulus [35] (Figure 1).
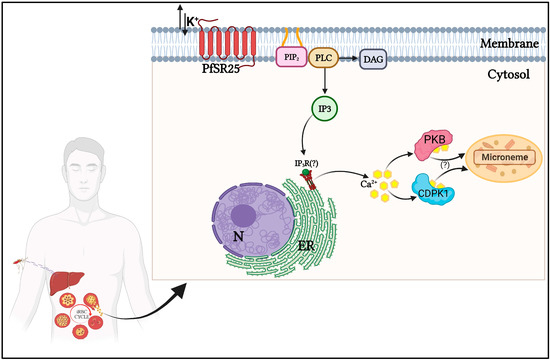
Figure 1.
Schematic representation of the signaling pathway involving K+ in Plasmodium spp. during its asexual developmental phases. In the erythrocytic stage, the exposure of P. falciparum to variations in [K+] is perceived by the GPCR-like PfSR25, which transmits the signal through the activation of phospholipase C (PLC). After PLC activation, inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) are formed. IP3 activates an IP3 receptor (hypothetical IP3R) to release calcium (Ca2+) from the endoplasmic reticulum (ER). The increase in cytosolic levels of Ca2+ activates the Ca2+-dependent protein kinases CDPK1 and PKB, leading to the release of microneme proteins essential for the infection of new erythrocytes. Created with BioRender.com.
In the work of Moraes and colleagues (2017), the authors generated a knockout of the GPCR candidate PfSR25 (PfSR25−). This tool showed that this serpentine receptor acts to sense the stimuli resulting from [K+] changes in the microenvironment, in which the parasite travels during its erythrocytic cycle. The authors investigated whether SR25 could modulate the calcium signaling in parasites at mature trophozoite stages isolated from red blood cells and incubated with high K+/low Na+ (140 mM KCl, 5.4 mM NaCl) and low K+/high Na+ (5.4 mM KCl, 140 mM NaCl) buffer supplemented with 2 mM CaCl2. They observed an increase in the cytosolic calcium concentration when parasites were transferred from high K+ to low K+ buffer, but no difference in the cytosolic calcium concentration was found in the opposite condition, i.e., low K+ to high K+ for wild-type parasites [35].
The activation of PfSR25 by shifts in [K+] increases cytosolic calcium in P. falciparum. The increase in Ca2+ is blocked by the inhibition of phospholipase C (PLC) or depletion of internal Ca2+ pools of the parasite by thapsigargin. However, PfSR25 knockout parasites did not show an increase in cytoplasmic Ca2+ when subjected to changes in [K+] from 140 mM (intracellular) to 5.4 mM (extracellular) [35]. The authors concluded that PfSR25 is a K+ sensor capable of modulating Ca2+ signaling in P. falciparum, resulting in consequent PLC activation and increased [Ca2+] cyt. This response persists in the absence of free extracellular Ca2+ and cannot be elicited by other ions such as Na+, Mg2+, or Ca2+ [35].
Interestingly, the authors found that the knockout for the PfSR25 protein is more sensitive to oxidative stress promoted by sodium nitroprusside (SNP), a nitric oxide donor. When parasites were treated with SNP, a significant decrease in parasitemia was observed, and the knockout (PfSR25-) parasites showed a 72% increase in metacaspase gene expression after exposure to SNP 0.5 mM for 3 h compared to wild-type (3D7) parasites. The wild-type parasites also showed an improved ability to survive under amino acid deprivation conditions with the lack of Albumax as supplementation to media compared to the knockout parasites [35]. This data set highlights the involvement of PfSR25 in the other mechanisms during the erythrocyte cycle.
In a recent paper, Santos and colleagues reported that PfSR25 knockout parasites show increased susceptibility to synthetic compounds derived from 1H- and 2H-1,2,3-triazole [36]. The authors identified 31 compounds with potential antimalarial activity at concentrations in the micromolar (µM) order; then, using the PfSR25− strain showed that compounds with antimalarial activity against the 3D7 strain (wild-type) showed reduced IC50 values when tested against the knockout strain [36]. In a further recent study, Santos and colleagues reported that PfSR25− showed increased susceptibility to the antimalarial drugs lumefantrine and piperaquine, which target hemozoin metabolism [37]. The authors tested different antimalarial drugs used for treating malaria with varying mechanisms of action on the 3D7 and PfSR25− strains. Among them are atovaquone (mitochondria), dihydroartemisinin and pyrimethamine (cytosol), chloroquine, mefloquine, lumefantrine, and piperaquine (digestive vacuole). As a result, they observed that the knockout parasites were more sensitive to the antimalarial activity of piperaquine and lumefantrine, presenting 44.38% and 58.12% lower IC50 values in the PfSR25− strain when compared to the 3D7 strain [37].
In the erythrocytic cycle, malaria parasites digest hemoglobin within the digestive vacuole to obtain amino acids, releasing the toxic product heme from hemoglobin. During detoxification, the heme is converted into an insoluble crystal called hemozoin by the heme detoxification protein [38]. This detoxification process is essential for the parasite’s survival and is, thus, the target of some antimalarial drugs. Interestingly, the knockout strain (PfSR25−) shows greater susceptibility to some of these compounds, such as chloroquine, piperaquine, and lumefantrine [35,37]. These compounds can reduce PfSR25 expression in the ring phase [37,39]. In an assay, Moraes and colleagues treated PfSR25− and wild-type 3D7 trophozoites (32–34 h post-infection) with piperaquine (10 μM) for 2 h and measured hemozoin formation, noting that PfSR25− parasites were more susceptible to treatment. After 2 h, the knockout inhibited hemozoin size formation by approximately 69.9 ± 2.1% compared to the wild-type parasites [35]. Thus, besides acting in detecting the K+ shift, PfSR25 can play other functions related to parasite survival.
Signaling triggered by K+ shift is one of the mechanisms involved in the invasion of new cells. Moreover, other molecular factors act in this complex event, such as the activation of signaling pathways and specific proteins during the mechanical steps of invasion [40,41,42,43,44]. In addition to the involvement of PfSR25 in the detection of K+ shift, the data found with the study of antimalarial compounds in this strain demonstrate a relationship between the action of antimalarial drugs and this GPCR-like receptor. Therefore, PfSR25 is a promising candidate for studies of the development of new antimalarial.
5. Other Apicomplexans Encode Proteins Homologous to GPCR-like PfSR25
In Toxoplasma gondii, a similar mechanism for sensing [K+] has also been described, where the decrease of [K+] from intracellular to extracellular levels in the parasitized host cell results in a PLC-dependent increase of cytoplasmic Ca2+ [14]. According to the study, this increase in Ca2+ triggers a signal to activate the parasite egress machinery [14]. It is important to note that PfSR25, as well as having a conserved structure in Plasmodium spp., also appears to have homologs in other parasites. A BLASTP (protein–protein BLAST) search using PF3D7_0713400 as an input demonstrates that other species of apicomplexan parasites, such as Hepatocystis sp., Besnoitia besnoiti, Toxoplasma gondii, Neospora caninum Liverpool, Cardiosporidium cinoae, Babesia sp., and Theileria sp., as well as the unicellular algae Vitrella brassicaformis (belonging to the eukaryotic supergroup alveolata), showed sequences that produced significant alignments with PfSR25 and may represent true functional homologs in these other organisms (Figure 2).
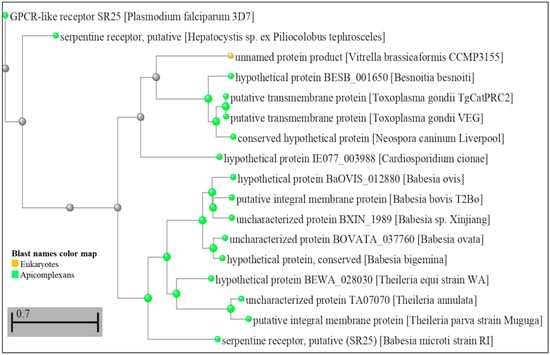
Figure 2.
Dendrogram showing amino acid sequence comparison of PfSR25 (PF3D7_0713400) with other sequences from the US National Library of Medicine database—National Center for Biotechnology Information (NCBI—https://blast.ncbi.nlm.nih.gov/Blast.cgi accessed on 6 February 2023). A search was performed on the BLAST platform that resulted in significant alignment sequences; these data were later used to build a distance tree from the results performed on the platform itself [45,46]. (Scale bar 0.7 amino acid substitutions).
These findings lead us to propose the hypothesis that parasite detection of [K+] shifts in the ionic environment may be conserved amongst different species of apicomplexan parasites. Active invasion of host cells is a characteristic of the apicomplexan, and it is tempting to suggest that this process may be regulated by sensing K+ shifts. In P. falciparum, this perception of K+ shift may be one of the mechanisms that ensure that the parasite remains in the host cell during intracellular replication and later becomes active; the increase in Ca2+cyt may be associated with the requirement for parasite activation of CDPKs (calcium-dependent kinases) in the release of microneme proteins essential for infection of new erythrocytes. At this time of egress/invasion, K+ displacement occurs, which promotes an intracellular Ca2+ increase by PLC action, which is sensed by the GPCR-like PfSR25.
6. Calcium Acts as the Second Messenger Resulting from the Signaling Triggered by [K+] Shift
Calcium is an essential ion for apicomplexan parasites, as it is involved in critical biological processes during the parasite life cycle. Classical mechanisms have been described for the uptake and release of calcium, which acts as a second messenger in signaling pathways. Some of these mechanisms are essential at different stages of the parasite life cycle [38,47,48]. Several processes, such as egress, invasion, motility, protein secretion, and cell cycle regulation, are controlled by Ca2+ signaling in the parasites [49,50]. The invasion of new red blood cells by the parasite is influenced by the extracellular ATP levels, which trigger an increase of Ca2+cyt in the parasite. Levano-Garcia and collaborators reported that the addition of ATP leads to an increase in Ca2+ in trophozoites and segmented schizonts. The presence of purinergic inhibitors KN62 and Ip5I was able to block the release of Ca2+ when ATP was added. In addition, it promoted a drastic reduction in the infection of new red blood cells [51].
It is worth emphasizing that Plasmodium can create a high Ca2+ microenvironment during its intraerythrocytic cycle [52]. Efforts by our group and others reported the host hormone, melatonin, as active in the synchronization of P. falciparum (in vitro) and P. chabaudi (in vivo) [52]. We also showed that melatonin and its precursors could mobilize Ca2+ from intracellular storage [52,53]. The signaling acting in the melatonin pathway involves the activation of the PLC-IP3 pathway that leads to the cytosolic increase of calcium and cAMP. Recently, Alves et al., 2022, identified PfMDR1 as a promising IP3 receptor candidate in P. falciparum, combining an IP3 affinity column chromatography with further bioinformatics meta-analysis. The analyses show that PfMDR1 is a potential target for binding IP3 [54]. Thus, we show that Ca2+ acts as a second messenger in several signaling pathways activated at different stages of development, essential for cycle progression and parasite survival [55].
Calcium-dependent protein kinases (CDPKs) play significant roles in the Ca2+ signaling pathways in parasites. Through crystallography, it was shown that CDPKS from T. gondii and Cryptosporidium parvum, in their self-inhibited forms (not bound to Ca2+), resembles a calmodulin protein with a long helix at the N-terminus that inhibits the protein’s kinase domain [56]. Lourido et al. (2010) used a knockdown of T. gondii, CDPK1, to demonstrate the essentiality of this kinase in the signaling that leads to the secretion of micronemes [57]. CDPK1 is a Ca2+-dependent kinase that is conserved among apicomplexan parasites. Its conditional deletion showed that CDPK1 controls Ca2+-dependent secretion from organelles called micronemes. As a result, the knockdown parasite was impaired in terms of motility, invasion, and egress from host cells. That is, in the stages where the release of the microneme is essential for the continuity of the parasite’s life cycle [57]. TgCDPK1 inhibitors (1NM-PP1) block microneme secretion and motility, promoting a blockade in invasion and parasitic development [58].
As it occurs in T. gondii, CDPK1 is also involved in the motility and invasion of new cells in P. falciparum. Green and collaborators showed that this Ca2+-dependent kinase is responsible for the phosphorylation of proteins MTIP (myosin A tail domain interaction protein) and GAP45 (glideosome-associated protein 45), both components of the motor complex that generates the necessary force to invade host cells [59]. Another relevant kinase in P. falciparum is PKB. This is an enzyme similar to protein kinase B, whose regulation is controlled by calmodulin, which in a Ca2+-dependent manner, associates with the N-terminal region of PfPKB and regulates activity [60]. The study of the Ca2+/Calmodulin-PfPKB signaling pathway showed that it might be necessary for erythrocyte invasion. Through the development of an inhibitory peptide for PfPKB, a drastic reduction in the ability of the parasite to invade new erythrocytes has been shown. This same result was also obtained when inhibitors of the activators of this pathway, calmodulin, and PLC, were used [61]. Thus, Ca2+-dependent kinases CDPK1 and PKB are highlighted as critical mediators of the signaling that leads to the secretion of micronemes, motility, and invasion in the parasite. Therefore, this set of data points to a likely conserved mechanism among parasites.
Exposure of P. falciparum merozoites to a low [K+] (5 mM), similar to that present in the bloodstream, induces the activation of phospholipase C, resulting in increased intracellular Ca2+ and activation of erythrocyte invasion mechanisms. Moraes et al. (2017) demonstrated that changing the KCl concentration from 140 mM to 5.4 mM induces an increase in cytosolic Ca2+ concentration in P. falciparum trophozoites, even in the absence of extracellular Ca2+ [35]. In T. gondii, a mechanism similar to the one mentioned above has also been described [14]. However, the GPCR-like 25 of P. falciparum presenting a representative homologous protein in the genome of T. gondii, the receptor responsible for the uptake of K+ levels and subsequent release of Ca2+cyt, was not characterized. In any case, it is vital to highlight the fundamental role of Ca2+ at the time of the invasion of new host cells, not only in P. falciparum and T. gondii, since studies show that in Trypanosoma cruzi elevations in Ca2+cyt levels are also present at the time of the invasion of new cells [62]. Another study showed that Ca2+ signaling during the invasion, as well as an increase in intracellular calcium levels, are linked to the virulence of Leishmania mexicana amazonensis [63].
There are still significant gaps to be filled in the events behind the parasite–host cell interaction processes (egress and invasion). However, Ca2+ is shown to be a ubiquitous intracellular messenger in different parasites. Although there are still many unanswered questions on this subject, identifying the SR25 protein in P. falciparum sheds light on new mechanisms by which parasites can capture information from the surrounding microenvironment and prepare themselves to initiate further steps in their life cycle.
7. Conclusions
In this present contribution, we review the importance of K+ ions in intracellular parasites, focusing on the human malaria parasite P. falciparum and the related parasite Toxoplasma gondii. Alterations in the concentration of this ion can be sensed by the GPCR-like PfSR25 and transduced to the parasite cytosol through Ca2+, a ubiquitous second messenger. This signaling performs different functions depending on the stage of the parasite’s development. However, it proves to be extremely important for the hepatic and intraerythrocytic stages of Plasmodium and other parasites. The [K+] detection mechanism is slowly unveiled and still has gaps to explore. Complete knowledge of this mechanism will contribute to our understanding of parasite cell biology and may also lead to new targets for the development of antimalarial drugs.
Author Contributions
B.M.D.S.—database analysis, writing the review; J.M.P. and C.R.S.G.—writing the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant number 2017/08684-7 and The B.M.S. was funded by FAPESP, grant number 2020/08988-9.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nadjm, B.; Behrens, R.H. Malaria: An Update for Physicians. Infect. Dis. Clin. N. Am. 2012, 26, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.H.; Duraisingh, M.T. The Molecular Basis of Erythrocyte Invasion by Malaria Parasites. Cell Host Microbe 2017, 22, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Crabb, B.S. Invasion of red blood cells by malaria parasites. Cell 2006, 124, 755–766. [Google Scholar] [CrossRef]
- Dvorak, J.A.; Miller, L.H.; Whitehouse, W.C.; Shiroishi, T. Invasion of erythrocytes by malaria merozoites. Science 1975, 187, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.M.; Pradel, G.; Vanderberg, J.P.; Hafalla, J.C.R.; Frevert, U.; Nussenzweig, R.S.; Nussenzweig, V.; Rodriguez, A. Migration of Plasmodium sporozoites through cells before infection. Science 2001, 291, 141–144. [Google Scholar] [CrossRef]
- Cowman, A.F.; Berry, D.; Baum, J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J. Cell Biol. 2012, 198, 961–971. [Google Scholar] [CrossRef]
- Amino, R.; Thiberge, S.; Martin, B.; Celli, S.; Shorte, S.; Frischknecht, F.; Ménard, R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 2006, 12, 220–224. [Google Scholar] [CrossRef]
- Rohrbach, P. Imaging ion flux and ion homeostasis in blood-stage malaria parasites. Biotechnol. J. 2009, 4, 812–825. [Google Scholar] [CrossRef]
- Pillai, A.D.; Addo, R.; Sharma, P.; Nguitragool, W.; Srinivasan, P.; Desai, S.A. Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodeling. Mol. Microbiol. 2013, 88, 20–34. [Google Scholar] [CrossRef]
- Kumar, K.A.; Garcia, C.R.S.; Chandran, V.R.; Van Rooijen, N.; Zhou, Y.; Winzeler, E.; Nussenzweig, V. Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity. Mol. Biochem. Parasitol. 2007, 156, 32–40. [Google Scholar] [CrossRef]
- Mota, M.M.; Hafalla, J.C.R.; Rodriguez, A. Migration through host cells activates Plasmodium sporozoites for infection. Nat. Med. 2002, 8, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.M.; Rodriguez, A. Migration through host cells: The first steps of Plasmodium sporozoites in the mammalian host. Cell. Microbiol. 2004, 6, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Bannister, L.H.; Mitchell, G.H. The Fine Structure of Secretion by Plasmodium knowlesi Merozoites during Red Cell Invasion. J. Protozool. 1989, 36, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Moudy, R.; Manning, T.J.; Beckers, C.J. The Loss of Cytoplasmic Potassium upon Host Cell Breakdown Triggers Egress of Toxoplasma gondii. J. Biol. Chem. 2001, 276, 41492–41501. [Google Scholar] [CrossRef]
- Lovett, J.L.; Marchesini, N.; Moreno, S.N.J.; David Sibley, L. Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from inositol 1,4,5-triphosphate(IP3)/ryanodine-sensitive stores. J. Biol. Chem. 2002, 277, 25870–25876. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Sibley, L.D. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 1999, 31, 421–428. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Giddings, O.K.; Sibley, L.D. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1999, 1, 225–235. [Google Scholar] [CrossRef]
- Arrizabalaga, G.; Boothroyd, J.C. Role of calcium during Toxoplasma gondii invasion and egress. Int. J. Parasitol. 2004, 34, 361–368. [Google Scholar] [CrossRef]
- Vella, S.A.; Moore, C.A.; Li, Z.H.; Triana, M.A.H.; Potapenko, E.; Moreno, S.N. The role of potassium and host calcium signaling in Toxoplasma gondii egress. Cell Calcium 2021, 94, 102337. [Google Scholar] [CrossRef]
- Bonhomme, A.; Bouchot, A.; Pezzella, N.; Gomez, J.; Le Moal, H.; Pinon, J.M. Signaling during the invasion of host cells by Toxoplasma gondii. FEMS Microbiol. Rev. 1999, 23, 551–561. [Google Scholar] [CrossRef]
- Roiko, M.S.; Svezhova, N.; Carruthers, V.B. Acidification activates Toxoplasma gondii motility and egress by enhancing protein secretion and cytolytic activity. PLoS Pathog. 2014, 10, e1004488. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Uboldi, A.D.; Seizova, S.; Wilde, M.L.; Coffey, M.J.; Katris, N.J.; Tonkin, C.J. An apically located hybrid guanylate cyclase–ATPase is critical for the initiation of Ca2+ signaling and motility in Toxoplasma gondii. J. Biol. Chem. 2019, 294, 8959–8972. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.; Horner, H.A.; Elford, B.C.; Ellory, J.C.; Newbold, C.I. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J. Biol. Chem. 1994, 269, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Kutner, S.; Ginsburg, H.; Cabantchik, Z.I. Permselectivity changes in malaria (Plasmodium falciparum) infected human red blood cell membranes. J. Cell. Physiol. 1983, 114, 245–251. [Google Scholar] [CrossRef]
- Ginsburg, H.; Krugliak, M.; Eidelman, O.; Ioav Cabantchik, Z. New permeability pathways induced in membranes of Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 1983, 8, 177–190. [Google Scholar] [CrossRef]
- Staines, H.M.; Ellory, J.C.; Kirk, K. Perturbation of the pump-leak balance for Na+ and K+ in malaria-infected erythrocytes. Am. J. Physiol.-Cell Physiol. 2001, 280, 1576–1587. [Google Scholar] [CrossRef]
- Mauritz, J.M.; Seear, R.; Esposito, A.; Kaminski, C.F.; Skepper, J.N.; Warley, A.; Lew, V.L.; Tiffert, T. X-ray microanalysis investigation of the changes in Na, K, and hemoglobin concentration in Plasmodium falciparum-infected red blood cells. Biophys. J. 2011, 100, 1438–1445. [Google Scholar] [CrossRef]
- Martin, R.E.; Kirk, K. Transport of the essential nutrient isoleucine in human erythrocytes infected with the malaria parasite Plasmodium falciparum. Blood 2007, 109, 2217–2224. [Google Scholar] [CrossRef]
- Spillman, N.J.; Allen, R.J.; McNamara, C.W.; Yeung, B.K.; Winzeler, E.A.; Diagana, T.T.; Kirk, K. Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 2013, 13, 227–237. [Google Scholar] [CrossRef]
- Winterberg, M.; Kiaran, K. A high-sensitivity HPLC assay for measuring intracellular Na+ and K+ and its application to Plasmodium falciparum infected erythrocytes. Sci. Rep. 2016, 6, 29241. [Google Scholar] [CrossRef]
- Singh, S.; Alam, M.M.; Pal-Bhowmick, I.; Brzostowski, J.A.; Chitnis, C.E. Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites. PLoS Pathog. 2010, 6, e1000746. [Google Scholar] [CrossRef]
- Singh, S.; More, K.R.; Chitnis, C.E. Role of calcineurin and actin dynamics in regulated secretion of microneme proteins in Plasmodium falciparum merozoites during erythrocyte invasion. Cell. Microbiol. 2014, 16, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chitnis, C.E. Signalling mechanisms involved in apical organelle discharge during host cell invasion by apicomplexan parasites. Microbes Infect. 2012, 14, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Madeira, L.; Galante, P.A.F.; Budu, A.; Azevedo, M.F.; Malnic, B.; Garcia, C.R.S. Genome-Wide Detection of Serpentine Receptor-Like Proteins in Malaria Parasites. PLoS ONE 2008, 3, e1889. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.S.; Budu, A.; Singh, M.K.; Borges-Pereira, L.; Levano-Garcia, J.; Currà, C.; Picci, L.; Pace, T.; Ponzi, M.; Pozzan, T.; et al. Plasmodium falciparum GPCR-like receptor SR25 mediates extracellular K+ sensing coupled to Ca2+ signaling and stress survival. Sci. Rep. 2017, 7, 9545. [Google Scholar] [CrossRef]
- Santos, B.M.D.; Gonzaga, D.T.G.; da Silva, F.C.; Ferreira, V.F.; Garcia, C.R.S. Plasmodium falciparum Knockout for the GPCR-Like PfSR25 Receptor Displays Greater Susceptibility to 1,2,3-Triazole Compounds That Block Malaria Parasite Development. Biomolecules 2020, 10, 1197. [Google Scholar] [CrossRef]
- Santos, B.M.; Dias, B.K.M.; Nakabashi, M.; Garcia, C.R.S. The Knockout for G Protein-Coupled Receptor-Like PfSR25 Increases the Susceptibility of Malaria Parasites to the Antimalarials Lumefantrine and Piperaquine but Not to Medicine for Malaria Venture Compounds. Front. Microbiol. 2021, 12, 638869. [Google Scholar] [CrossRef]
- Lee, A.H.; Dhingra, S.K.; Lewis, I.A.; Singh, M.K.; Siriwardana, A.; Dalal, S.; Rubiano, K.; Klein, M.S.; Baska, K.S.; Krishna, S.; et al. Evidence for regulation of hemoglobin metabolism and intracellular ionic flux by the Plasmodium falciparum chloroquine resistance transporter. Sci. Rep. 2018, 8, 13578. [Google Scholar] [CrossRef]
- Mwai, L.; Diriye, A.; Masseno, V.; Muriithi, S.; Feltwell, T.; Musyoki, J.; Lemieux, J.; Feller, A.; Mair, G.R.; Marsh, K.; et al. Genome-wide adaptations of Plasmodium falciparum in response to Lumefantrine selective drug pressure. PLoS ONE 2012, 7, e31623. [Google Scholar] [CrossRef]
- Alaganan, A.; Singh, P.; Chitnis, C.E. Molecular mechanisms that mediate invasion and egress of malaria parasites from red blood cells. Curr. Opin. Hematol. 2017, 24, 208–214. [Google Scholar] [CrossRef]
- Bannister, L.H.; Dluzewski, A.R. The ultrastructure of red cell invasion in malaria infections: A review. Blood Cells 1990, 16, 257–292. [Google Scholar] [PubMed]
- Blake, T.C.; Haase, S.; Baum, J. Actomyosin forces and the energetics of red blood cell invasion by the malaria parasite Plasmodium falciparum. PLoS Pathog. 2020, 16, e1009007. [Google Scholar] [CrossRef]
- Patel, A.; Perrin, A.; Flynn, H.R.; Bisson, C.; Withers-Martinez, C.; Treeck, M.; Flueck, C.; Nicastro, G.; Martin, S.R.; Ramos, A.; et al. Cyclic AMP signalling controls key components of malaria parasite host cell invasion machinery. PLoS Biol. 2019, 17, e3000264. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.S.; Egan, E.S.; Duraisingh, M.T. Host-parasite interactions that guide red blood cell invasion by malaria parasites. Curr. Opin. Hematol. 2015, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Wootton, J.C.; Gertz, E.M.; Agarwala, R.; Morgulis, A.; Schäffer, A.A.; Yu, Y.K. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 2005, 272, 5101–5109. [Google Scholar] [CrossRef]
- Nagamune, K.; Moreno, S.N.; Chini, E.N.; Sibley, L.D. Calcium regulation and signaling in apicomplexan parasites. Subcell. Biochem. 2008, 47, 70–81. [Google Scholar]
- Borges-Pereira, L.; Thomas, S.J.; e Silva, A.L.D.A.; Bartlett, P.J.; Thomas, A.P.; Garcia, C.R. The genetic Ca2+ sensor GCaMP3 reveals multiple Ca2+ stores differentially coupled to Ca2+ entry in the human malaria parasite Plasmodium falciparum. J. Biol. Chem. 2020, 295, 14998–15012. [Google Scholar] [CrossRef]
- Pecenin, M.F.; Borges-Pereira, L.; Levano-Garcia, J.; Budu, A.; Alves, E.; Mikoshiba, K.; Thomas, A.; Garcia, C.R. Blocking IP3 signal transduction pathways inhibits melatonin-induced Ca2+ signals and impairs P. falciparum development and proliferation in erythrocytes. Cell Calcium 2018, 72, 81–90. [Google Scholar] [CrossRef]
- Koyama, F.C.; Chakrabarti, D.; Garcia, C.R.S. Molecular machinery of signal transduction and cell cycle regulation in Plasmodium. Mol. Biochem. Parasitol. 2009, 165, 1–7. [Google Scholar] [CrossRef]
- Levano-Garcia, J.; Dluzewski, A.R.; Markus, R.P.; Garcia, C.R.S. Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic Signal. 2010, 6, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Gazarini, M.L.; Thomas, A.P.; Pozzan, T.; Garcia, C.R. Calcium signaling in a low calcium environment: How the intracellular malaria parasite solves the problem. J. Cell Biol. 2003, 161, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hotta, C.T.; Gazarini, M.L.; Beraldo, F.H.; Varotti, F.P.; Lopes, C.; Markus, R.P.; Pozzan, T.; Garcia, C.R.S. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat. Cell Biol. 2000, 2, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Nakaya, H.; Guimarães, E.; Garcia, C.R. Combining IP3 affinity chromatography and bioinformatics reveals a novel protein-IP3 binding site on Plasmodium falciparum MDR1 transporter. Curr. Res. Microb. Sci. 2022, 4, 100179. [Google Scholar] [CrossRef]
- Dos Santos, B.M.; Pereira, P.H.; Garcia, C.R. Molecular basis of synchronous replication of malaria parasites in the blood stage. Curr. Opin. Microbiol. 2021, 63, 210–215. [Google Scholar] [CrossRef]
- Wernimont, A.K.; Artz, J.D.; Finerty, P., Jr.; Lin, Y.-H.; Amani, M.; Allali-Hassani, A.; Senisterra, G.; Vedadi, M.; Tempel, W.; Mackenzie, F.; et al. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat. Publ. Gr. 2010, 17, 596–601. [Google Scholar] [CrossRef]
- Lourido, S.; Shuman, J.; Zhang, C.; Shokat, K.M.; Hui, R.; Sibley, L.D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 2010, 465, 359–362. [Google Scholar] [CrossRef]
- Sugi, T.; Kato, K.; Kobayashi, K.; Watanabe, S.; Kurokawa, H.; Gong, H.; Pandey, K.; Takemae, H.; Akashi, H. Use of the kinase inhibitor analog 1NM-PP1 reveals a role for Toxoplasma gondii CDPK1 in the invasion step. Eukaryot. Cell 2010, 9, 667. [Google Scholar] [CrossRef]
- Green, J.L.; Rees-Channer, R.R.; Howell, S.A.; Martin, S.R.; Knuepfer, E.; Taylor, H.M.; Grainger, M.; Holder, A.A. The motor complex of Plasmodium falciparum: Phosphorylation by a calcium-dependent protein kinase. J. Biol. Chem. 2008, 283, 30980–30989. [Google Scholar] [CrossRef]
- Vaid, A.; Sharma, P. PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum: II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. J. Biol. Chem. 2006, 281, 27126–27133. [Google Scholar] [CrossRef]
- Vaid, A.; Thomas, D.C.; Sharma, P. Role of Ca2+/calmodulin-PfPKB signaling pathway in erythrocyte invasion by Plasmodium falciparum. J. Biol. Chem. 2008, 283, 5589–5597. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.N.; Silva, J.; Vercesi, A.E.; Docampo, R. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J. Exp. Med. 1994, 180, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.G.; Zhong, L.; Chang, K.P.; Docampo, R. Intracellular Ca2+ pool content and signaling and expression of a calcium pump are linked to virulence in Leishmania mexicana amazonesis amastigotes. J. Biol. Chem. 1997, 272, 9464–9473. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).