The Molecular Basis for Zinc Bioavailability
Abstract
1. Introduction
2. Unique Chemistry of Zinc
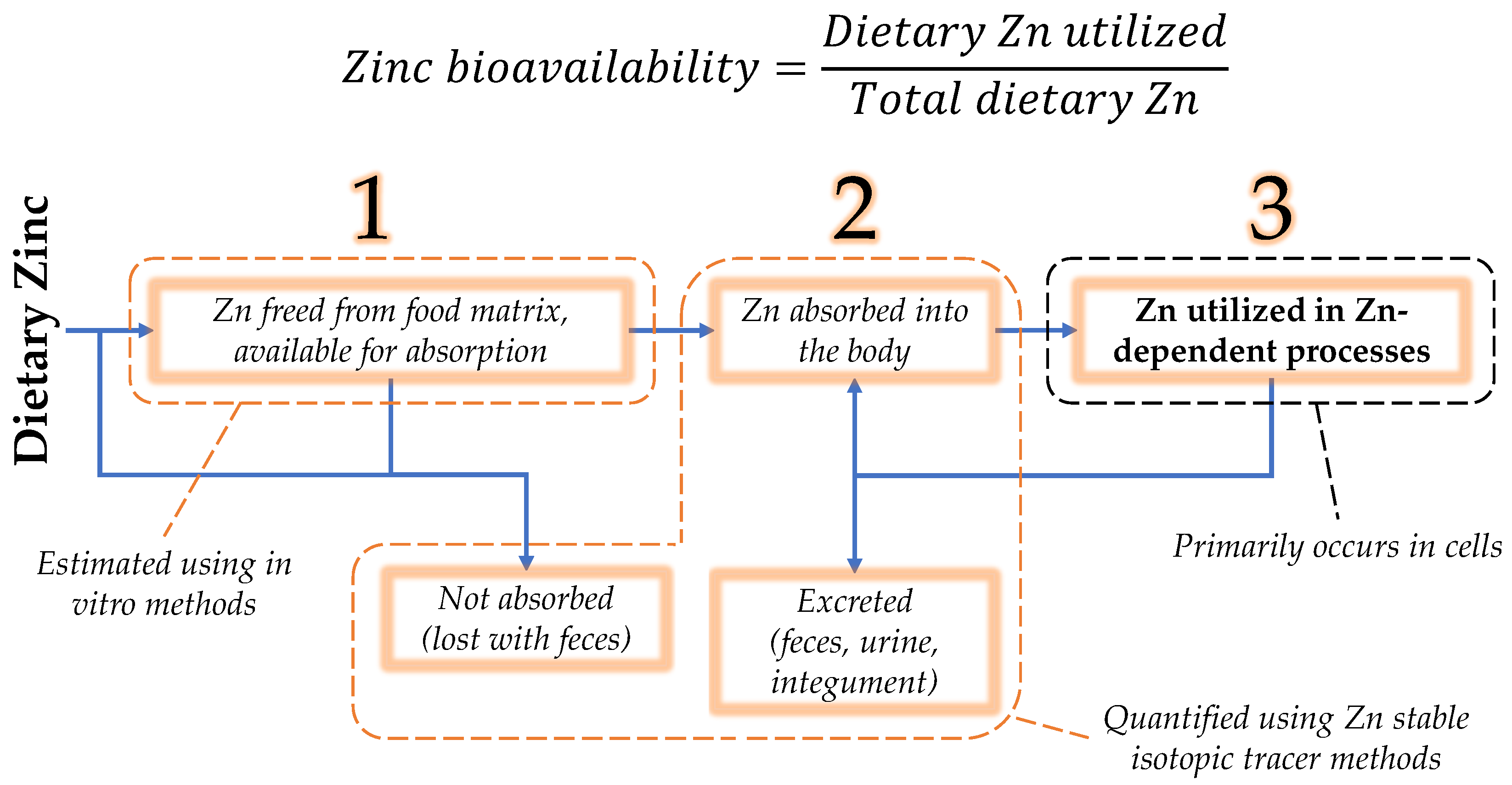
3. Zinc Bioavailability
3.1. Zinc Luminal Availability and Absorption
3.1.1. Effects of Dietary Zinc
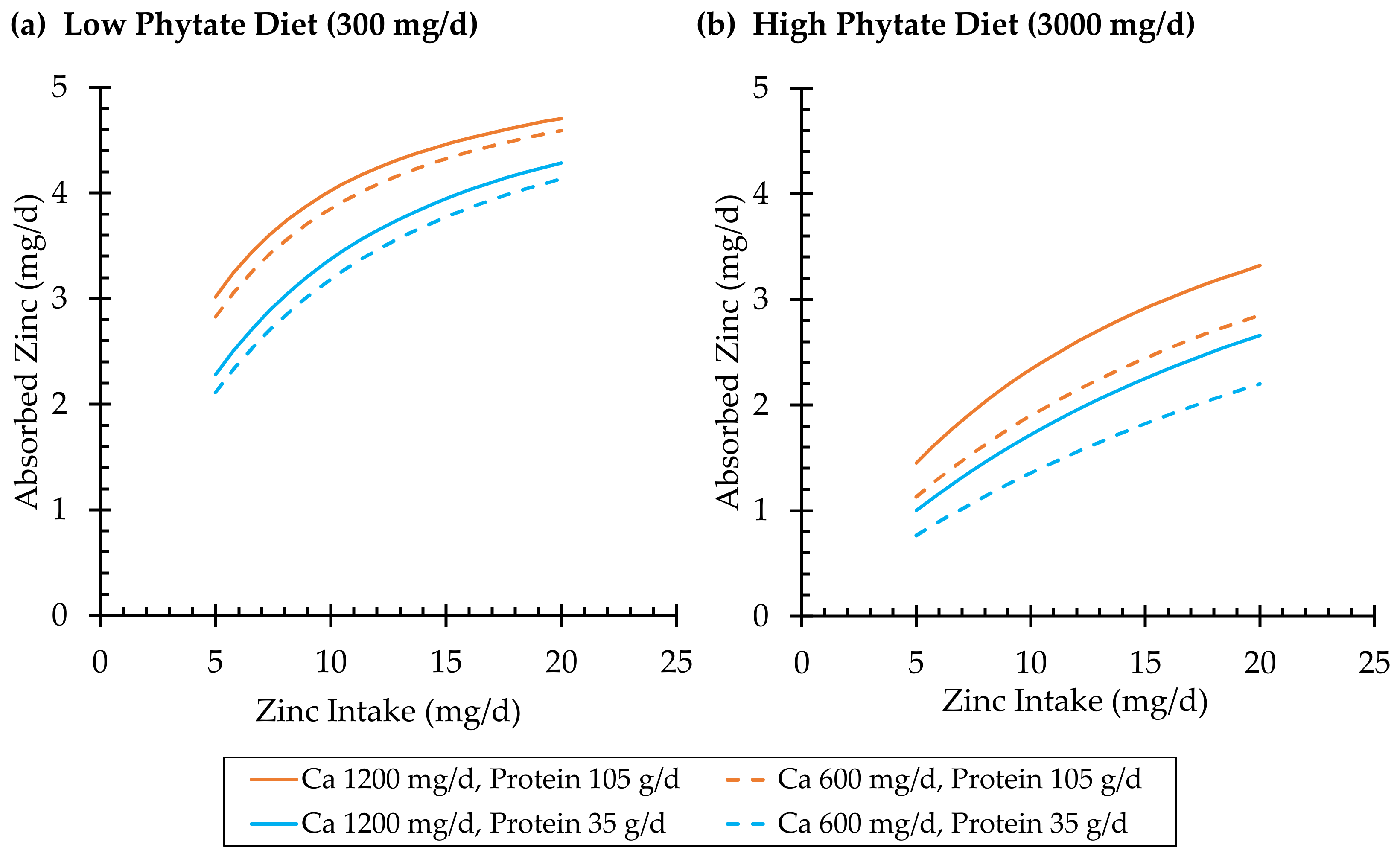
3.1.2. Effects of Dietary Phytate and Calcium
| Type | Zinc Ligand | Dissociation Constant (Kd) a | pKd (−log Kd) | Ref. |
|---|---|---|---|---|
| Protein zinc-binding domains | Zip4 M2 region | 2.9 × 10−5 M | 4.5 | [50] |
| Zip4 M1, M2 average | 6.2 × 10−7 M | 6.2 | [50] | |
| Zip4 M3M4 region | 6.0 × 10−9 M | 8.2 | [53] | |
| MT, α domain | 2.5 × 10−13 − 3.2 × 10−14 M | 12.6 − 13.5 b | [61] | |
| MT, β domain | 4.0 × 10−12 − 6.3 × 10−13 M | 11.4 − 12.2 c | [61] | |
| Albumin | 4.0 × 10−7 M | 6.4 d | [62] | |
| α-2-macroglobulin | 8 × 10−7 M | 6.1 e | [63] | |
| CA2 | 8.0 × 10−13 M | 12.1 | [64] | |
| SOD1 | 4.2 × 10−14 M | 13.4 | [65] | |
| Amino acids and peptides | Cysteine | 9.8 × 10−12 M2 | 11.0 | [14] |
| Histidine | 3.1 × 10−9 M2 | 8.5 | [14] | |
| Aspartic acid | 4.1 × 10−6 M2 | 5.4 | [14] | |
| Glycine | 1.3 × 10−5 M2 | 4.9 | [14] | |
| Glutamic acid | 2.2 × 10−5 M2 | 4.7 | [14] | |
| Cys-Gly | 1.5 × 10−6 M2 | 5.8 | [66] | |
| Gly-His | 1.4 × 10−4 M2 | 3.9 | [66] | |
| Gly-Gly-His | 1.6 × 10−4 M2 | 3.8 | [66] | |
| Gly-Cys-Glu f | 2.3 × 10−4 M | 3.6 | [66] | |
| Other | Clioquinol g | 7.9 × 10−17 M2 | 16.1 | [67] |
| EDTA | 2.3 × 10−14 M | 13.6 | [14] | |
| Citric acid | 1.2 × 10−12 M2 | 11.9 | [14] | |
| Picolinic acid | 1.3 × 10−12 M2 | 11.9 | [14] | |
| D-penicillamine | 2.8 × 10−12 M2 | 11.6 | [14] | |
| Pyrithione | 5.0 × 10−12 M2 | 11.3 | [14] | |
| Phytic acid | 3.7 × 10−11 M | 10.4 | [14] | |
| PBT2 g | 7.1 × 10−7 M2 | 6.2 | [68] | |
| Folic acid | 2.2 × 10−6 M2 | 5.7 | [14] |
3.1.3. Effects of Dietary Protein
3.1.4. Effects of Dietary Iron
3.1.5. Summary of Zinc Absorption
3.2. Zinc Utilization
3.2.1. Metallothionein
3.2.2. Albumin
3.2.3. Summary of Zinc Utilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Hall, A.G. Zinc (Submitted). In Modern Nutrition in Health and Disease, 12th ed.; Tucker, K.L., Ross, A.C., Jensen, G.L., Peterson, K.E., Touger-Decker, R., Duggan, C.P., Eds.; Jones & Bartlett Learning: Burlington, MA, USA, 2023. [Google Scholar]
- Lassi, Z.S.; Moin, A.; Bhutta, Z.A. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst. Rev. 2016, 12, CD005978. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, W.; Zheng, W.; Fang, X.; Chen, L.; Rink, L.; Min, J.; Wang, F. Zinc supplementation improves glycemic control for diabetes prevention and management: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 110, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Pompano, L.M.; Boy, E. Effects of Dose and Duration of Zinc Interventions on Risk Factors for Type 2 Diabetes and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 141–160. [Google Scholar] [CrossRef]
- Hess, S.Y. National Risk of Zinc Deficiency as Estimated by National Surveys. Food Nutr. Bull. 2017, 38, 3–17. [Google Scholar] [CrossRef]
- Wessells, K.R.; Singh, G.M.; Brown, K.H. Estimating the global prevalence of inadequate zinc intake from national food balance sheets: Effects of methodological assumptions. PLoS ONE 2012, 7, e50565. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Southon, S. Bioavailability of Nutrients. Encycl. Food Sci. Nutr. (Second Ed.) 2003, 1, 478–484. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Prasad, A.S.; Schulert, A.R.; Farid, Z.; Miale, A., Jr.; Bassilly, S.; Darby, W.J. Human zinc deficiency, endocrine manifestations and response to treatment. Am. J. Clin. Nutr. 1967, 20, 422–442. [Google Scholar] [CrossRef]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. Mathematical model of zinc absorption: Effects of dietary calcium, protein and iron on zinc absorption. Br. J. Nutr. 2013, 109, 695–700. [Google Scholar] [CrossRef]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Padjasek, M.; Kocyla, A.; Kluska, K.; Kerber, O.; Tran, J.B.; Krezel, A. Structural zinc binding sites shaped for greater works: Structure-function relations in classical zinc finger, hook and clasp domains. J. Inorg. Biochem. 2020, 204, 110955. [Google Scholar] [CrossRef]
- Xia, J.; Cai, L.H.; Wu, H.; MacKintosh, F.C.; Weitz, D.A. Anomalous mechanics of Zn(2+)-modified fibrin networks. Proc. Natl. Acad. Sci. USA 2021, 118, e2020541118. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lim, C. Physical basis of structural and catalytic Zn-binding sites in proteins. J. Mol. Biol. 2008, 379, 545–553. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. Order of Stability of Metal Complexes. Nature 1948, 162, 746–747. [Google Scholar] [CrossRef]
- Foster, A.W.; Osman, D.; Robinson, N.J. Metal preferences and metallation. J. Biol. Chem. 2014, 289, 28095–28103. [Google Scholar] [CrossRef]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics 2010, 2, 306–317. [Google Scholar] [CrossRef]
- Van Eunen, K.; Bouwman, J.; Daran-Lapujade, P.; Postmus, J.; Canelas, A.B.; Mensonides, F.I.; Orij, R.; Tuzun, I.; van den Brink, J.; Smits, G.J.; et al. Measuring enzyme activities under standardized in vivo-like conditions for systems biology. FEBS J. 2010, 277, 749–760. [Google Scholar] [CrossRef]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef] [PubMed]
- Kwapiszewska, K.; Szczepanski, K.; Kalwarczyk, T.; Michalska, B.; Patalas-Krawczyk, P.; Szymanski, J.; Andryszewski, T.; Iwan, M.; Duszynski, J.; Holyst, R. Nanoscale Viscosity of Cytoplasm Is Conserved in Human Cell Lines. J. Phys. Chem. Lett. 2020, 11, 6914–6920. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Kozyreva, T.; Zovo, K.; Palumaa, P. Affinity gradients drive copper to cellular destinations. Nature 2010, 465, 645–648. [Google Scholar] [CrossRef]
- Thompson, M.W. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals 2022, 35, 187–213. [Google Scholar] [CrossRef]
- Kocyla, A.; Tran, J.B.; Krezel, A. Galvanization of Protein-Protein Interactions in a Dynamic Zinc Interactome. Trends Biochem. Sci. 2021, 46, 64–79. [Google Scholar] [CrossRef]
- Godber, J.S. Nutrient Bioavailability in Humans and Experimental Animals. J. Food Qual. 1990, 13, 21–36. [Google Scholar] [CrossRef]
- Price, G.; Patel, D.A. Drug Bioavailability. In StatPearls; Statpearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Matseshe, J.W.; Phillips, S.F.; Malagelada, J.R.; McCall, J.T. Recovery of dietary iron and zinc from the proximal intestine of healthy man: Studies of different meals and supplements. Am. J. Clin. Nutr. 1980, 33, 1946–1953. [Google Scholar] [CrossRef]
- Dominguez-Munoz, J.E.; Martinez, S.M.; Leodolter, A.; Malfertheiner, P. Quantification of pancreatic zinc output as pancreatic function test: Making the secretin-caerulein test applicable to clinical practice. Pancreatology 2004, 4, 57–62. [Google Scholar] [CrossRef]
- Gibson, R.S.; King, J.C.; Lowe, N. A Review of Dietary Zinc Recommendations. Food Nutr. Bull. 2016, 37, 443–460. [Google Scholar] [CrossRef]
- Neve, J.; Hanocq, M.; Peretz, A.; Abi Khalil, F.; Pelen, F.; Famaey, J.P.; Fontaine, J. Pharmacokinetic study of orally administered zinc in humans: Evidence for an enteral recirculation. Eur. J. Drug Metab. Pharmacokinet. 1991, 16, 315–323. [Google Scholar] [CrossRef]
- Tran, C.D.; Miller, L.V.; Krebs, N.F.; Lei, S.; Hambidge, K.M. Zinc absorption as a function of the dose of zinc sulfate in aqueous solution. Am. J. Clin. Nutr. 2004, 80, 1570–1573. [Google Scholar] [CrossRef]
- Neve, J.; Hanocq, M.; Peretz, A.; Khalil, F.A.; Pelen, F. Absorption and metabolism of oral zinc gluconate in humans in fasting state, during, and after a meal. Biol. Trace Elem. Res. 1992, 32, 201–212. [Google Scholar] [CrossRef]
- Lowe, N.M.; Woodhouse, L.R.; King, J.C. A comparison of the short-term kinetics of zinc metabolism in women during fasting and following a breakfast meal. Br. J. Nutr. 1998, 80, 363–370. [Google Scholar] [CrossRef]
- Massih, Y.N.; Hall, A.G.; Suh, J.; King, J.C. Zinc Supplements Taken with Food Increase Essential Fatty Acid Desaturation Indices in Adult Men Compared with Zinc Taken in the Fasted State. J. Nutr. 2021, 151, 2583–2589. [Google Scholar] [CrossRef]
- Burello, E.; Worth, A.P. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology 2011, 5, 228–235. [Google Scholar] [CrossRef]
- Wegmuller, R.; Tay, F.; Zeder, C.; Brnic, M.; Hurrell, R.F. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef]
- Henderson, L.M.; Brewer, G.J.; Dressman, J.B.; Swidan, S.Z.; DuRoss, D.J.; Adair, C.H.; Barnett, J.L.; Berardi, R.R. Effect of intragastric pH on the absorption of oral zinc acetate and zinc oxide in young healthy volunteers. JPEN J. Parenter. Enter. Nutr. 1995, 19, 393–397. [Google Scholar] [CrossRef]
- Pieper, R.; Dadi, T.H.; Pieper, L.; Vahjen, W.; Franke, A.; Reinert, K.; Zentek, J. Concentration and chemical form of dietary zinc shape the porcine colon microbiome, its functional capacity and antibiotic resistance gene repertoire. ISME J. 2020, 14, 2783–2793. [Google Scholar] [CrossRef]
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J. Nutr. 2007, 137, 135–141. [Google Scholar] [CrossRef]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc absorption in human small intestine. Am. J. Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, C.; Hop, C.; Wright, M.R.; Hu, M.; Lai, Y.; Khojasteh, S.C.; Humphreys, W.G. Intestinal Excretion, Intestinal Recirculation, and Renal Tubule Reabsorption Are Underappreciated Mechanisms That Drive the Distribution and Pharmacokinetic Behavior of Small Molecule Drugs. J. Med. Chem. 2021, 64, 7045–7059. [Google Scholar] [CrossRef] [PubMed]
- Kasana, S.; Din, J.; Maret, W. Genetic causes and gene-nutrient interactions in mammalian zinc deficiencies: Acrodermatitis enteropathica and transient neonatal zinc deficiency as examples. J. Trace Elem. Med. Biol. 2015, 29, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Kuliyev, E.; Zhang, C.; Sui, D.; Hu, J. Zinc transporter mutations linked to acrodermatitis enteropathica disrupt function and cause mistrafficking. J. Biol. Chem. 2021, 296, 100269. [Google Scholar] [CrossRef]
- Moynahan, E.J. Letter: Acrodermatitis enteropathica: A lethal inherited human zinc-deficiency disorder. Lancet 1974, 2, 399–400. [Google Scholar] [CrossRef]
- Hurley, L.S.; Duncan, J.R.; Sloan, M.V.; Eckhert, C.D. Zinc-binding ligands in milk and intestine: A role in neonatal nutrition? Proc. Natl. Acad. Sci. USA 1977, 74, 3547–3549. [Google Scholar] [CrossRef]
- Wiuf, A.; Steffen, J.H.; Becares, E.R.; Gronberg, C.; Mahato, D.R.; Rasmussen, S.G.F.; Andersson, M.; Croll, T.; Gotfryd, K.; Gourdon, P. The two-domain elevator-type mechanism of zinc-transporting ZIP proteins. Sci. Adv. 2022, 8, eabn4331. [Google Scholar] [CrossRef]
- Zhang, T.; Kuliyev, E.; Sui, D.; Hu, J. The histidine-rich loop in the extracellular domain of ZIP4 binds zinc and plays a role in zinc transport. Biochem. J. 2019, 476, 1791–1803. [Google Scholar] [CrossRef]
- Zhang, T.; Sui, D.; Zhang, C.; Cole, L.; Hu, J. Asymmetric functions of a binuclear metal center within the transport pathway of a human zinc transporter ZIP4. FASEB J. 2020, 34, 237–247. [Google Scholar] [CrossRef]
- Mao, X.; Kim, B.E.; Wang, F.; Eide, D.J.; Petris, M.J. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J. Biol. Chem. 2007, 282, 6992–7000. [Google Scholar] [CrossRef]
- Bafaro, E.M.; Antala, S.; Nguyen, T.V.; Dzul, S.P.; Doyon, B.; Stemmler, T.L.; Dempski, R.E. The large intracellular loop of hZIP4 is an intrinsically disordered zinc binding domain. Metallomics 2015, 7, 1319–1330. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Guo, L.; Chang, S.M.; Cousins, R.J. Kruppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G517–G523. [Google Scholar] [CrossRef]
- Hennigar, S.R.; Olson, C.I.; Kelley, A.M.; McClung, J.P. Slc39a4 in the small intestine predicts zinc absorption and utilization: A comprehensive analysis of zinc transporter expression in response to diets of varied zinc content in young mice. J. Nutr. Biochem. 2022, 101, 108927. [Google Scholar] [CrossRef]
- Weaver, B.P.; Dufner-Beattie, J.; Kambe, T.; Andrews, G.K. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol. Chem. 2007, 388, 1301–1312. [Google Scholar] [CrossRef]
- King, J.C.; Shames, D.M.; Lowe, N.M.; Woodhouse, L.R.; Sutherland, B.; Abrams, S.A.; Turnlund, J.R.; Jackson, M.J. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am. J. Clin. Nutr. 2001, 74, 116–124. [Google Scholar] [CrossRef]
- Chung, C.S.; Stookey, J.; Dare, D.; Welch, R.; Nguyen, T.Q.; Roehl, R.; Peerson, J.M.; King, J.C.; Brown, K.H. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am. J. Clin. Nutr. 2008, 87, 1224–1229. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Miller, L.V.; Mazariegos, M.; Westcott, J.; Solomons, N.W.; Raboy, V.; Kemp, J.F.; Das, A.; Goco, N.; Hartwell, T.; et al. Upregulation of Zinc Absorption Matches Increases in Physiologic Requirements for Zinc in Women Consuming High- or Moderate-Phytate Diets during Late Pregnancy and Early Lactation. J. Nutr. 2017, 147, 1079–1085. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478S–1483S. [Google Scholar] [CrossRef]
- Pinter, T.B.; Stillman, M.J. Putting the pieces into place: Properties of intact zinc metallothionein 1A determined from interaction of its isolated domains with carbonic anhydrase. Biochem. J. 2015, 471, 347–356. [Google Scholar] [CrossRef]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. Albumin as a zinc carrier: Properties of its high-affinity zinc-binding site. Biochem. Soc. Trans. 2008, 36, 1317–1321. [Google Scholar] [CrossRef]
- Pratt, C.W.; Pizzo, S.V. The effect of zinc and other divalent cations on the structure and function of human alpha 2-macroglobulin. Biochim. Biophys. Acta 1984, 791, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.A.; Ahmed, M.; Fierke, C.A. Metal binding specificity in carbonic anhydrase is influenced by conserved hydrophobic core residues. Biochemistry 1999, 38, 9054–9062. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.P.; Sampson, J.B.; Zhuang, Y.; Thompson, J.A.; Beckman, J.S. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J. Neurochem. 1997, 69, 1936–1944. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Zinc bioavailability from whey. Enthalpy-entropy compensation in protein binding. Food Res. Int. 2016, 89, 749–755. [Google Scholar] [CrossRef]
- Budimir, A.; Humbert, N.; Elhabiri, M.; Osinska, I.; Birus, M.; Albrecht-Gary, A.M. Hydroxyquinoline based binders: Promising ligands for chelatotherapy? J. Inorg. Biochem. 2011, 105, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Compaño, R.; Bars, E. Solvent extraction of zinc with 5,7-dichloro-2-methyl-8-hydroxyquinoline into chloroform. Talanta 1986, 33, 463–466. [Google Scholar] [CrossRef]
- Ohyoshi, E.; Hamada, Y.; Nakata, K.; Kohata, S. The interaction between human and bovine serum albumin and zinc studied by a competitive spectrophotometry. J. Inorg. Biochem. 1999, 75, 213–218. [Google Scholar] [CrossRef]
- Masuoka, J.; Hegenauer, J.; Van Dyke, B.R.; Saltman, P. Intrinsic stoichiometric equilibrium constants for the binding of zinc(II) and copper(II) to the high affinity site of serum albumin. J. Biol. Chem. 1993, 268, 21533–21537. [Google Scholar] [CrossRef]
- Marolt, G.; Gricar, E.; Pihlar, B.; Kolar, M. Complex Formation of Phytic Acid With Selected Monovalent and Divalent Metals. Front. Chem. 2020, 8, 582746. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Revisiting phytate-element interactions: Implications for iron, zinc and calcium bioavailability, with emphasis on legumes. Crit. Rev. Food Sci. Nutr. 2022, 62, 1696–1712. [Google Scholar] [CrossRef]
- Forbes, R.M.; Parker, H.M.; Erdman, J.W., Jr. Effects of dietary phytate, calcium and magnesium levels on zinc bioavailability to rats. J. Nutr. 1984, 114, 1421–1425. [Google Scholar] [CrossRef]
- Fordyce, E.J.; Forbes, R.M.; Robbins, K.R.; Erdman, J.W., Jr. Phytate * calcium/zinc molar ratios: Are they predictive of zinc bioavilability? J. Food Sci. 1987, 52, 440–444. [Google Scholar] [CrossRef]
- Hunt, J.R.; Beiseigel, J.M. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am. J. Clin. Nutr. 2009, 89, 839–843. [Google Scholar] [CrossRef]
- Rafferty, K.; Walters, G.; Heaney, R.P. Calcium fortificants: Overview and strategies for improving calcium nutriture of the U.S. population. J. Food Sci. 2007, 72, R152–R158. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Cederblad, A.; Davidsson, L.; Sandstrom, B. The effect of individual components of soy formula and cows’ milk formula on zinc bioavailability. Am. J. Clin. Nutr. 1984, 40, 1064–1070. [Google Scholar] [CrossRef]
- Konietzny, U.; Greiner, R. PHYTIC ACID|Properties and Determination. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4546–4555. [Google Scholar]
- Greiner, R. (Department of Food Technology and Bioprocess Engineering, Max Rubner-Institut, Federal Research Institute of Nutrition and Food, 76131 Karlsruhe, Germany); Hall, A. (Department of Nutritional Sciences & Toxicology, University of California, Berkeley, CA 94720, USA). Personal communication, 2023.
- Lease, J.G. Availability to the chick of zinc-phytate complexes isolated from oil seed meals by an in vitro digestion method. J. Nutr. 1967, 93, 523–532. [Google Scholar] [CrossRef]
- Lease, J.G. Effect of a Soluble Fraction of Oil Seed Meals on Uptake of 65Zn from Ca·Mg·65Zn·Phytate Complexes by the Chick. J. Nutr. 1968, 96, 126–138. [Google Scholar] [CrossRef]
- Hansen, M.; Sandstrom, B.; Lonnerdal, B. The effect of casein phosphopeptides on zinc and calcium absorption from high phytate infant diets assessed in rat pups and Caco-2 cells. Pediatr. Res. 1996, 40, 547–552. [Google Scholar] [CrossRef]
- Doyle, C.J.; Yancey, K.; Pitt, H.A.; Wang, M.; Bemis, K.; Yip-Schneider, M.T.; Sherman, S.T.; Lillemoe, K.D.; Goggins, M.D.; Schmidt, C.M. The proteome of normal pancreatic juice. Pancreas 2012, 41, 186–194. [Google Scholar] [CrossRef]
- Easley, D.; Krebs, N.; Jefferson, M.; Miller, L.; Erskine, J.; Accurso, F.; Hambidge, K.M. Effect of pancreatic enzymes on zinc absorption in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 136–139. [Google Scholar] [CrossRef]
- Wapnir, R.A.; Khani, D.E.; Bayne, M.A.; Lifshitz, F. Absorption of zinc by the rat ileum: Effects of histidine and other low-molecular-weight ligands. J. Nutr. 1983, 113, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Tacnet, F.; Lauthier, F.; Ripoche, P. Mechanisms of zinc transport into pig small intestine brush-border membrane vesicles. J. Physiol. 1993, 465, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Scholmerich, J.; Freudemann, A.; Kottgen, E.; Wietholtz, H.; Steiert, B.; Lohle, E.; Haussinger, D.; Gerok, W. Bioavailability of zinc from zinc-histidine complexes. I. Comparison with zinc sulfate in healthy men. Am. J. Clin. Nutr. 1987, 45, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Pfaender, S.; Hagmeyer, S.; Tarana, L.; Mattes, A.K.; Briel, F.; Kury, S.; Boeckers, T.M.; Grabrucker, A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hiPSC. Biometals 2017, 30, 643–661. [Google Scholar] [CrossRef]
- Katimba, H.A.; Wang, R.; Cheng, C. Current findings support the potential use of bioactive peptides in enhancing zinc absorption in humans. In Critical Reviews in Food Science and Nutrition; Taylor & Francis: Abingdon, UK, 2021. [Google Scholar] [CrossRef]
- Peng, M.; Lu, D.; Yu, M.; Jiang, B.; Chen, J. Identification of zinc-chelating pumpkin seed (Cucurbita pepo L.) peptides and in vitro transport of peptide-zinc chelates. J. Food Sci. 2022, 87, 2048–2057. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Ma, X.; Liu, X.; Yin, F.; Li, D.; Nakamura, Y.; Yu, C.; Zhou, D. Characterization of a synthetic zinc-chelating peptide from sea cucumber (Stichopus japonicus) and its gastrointestinal digestion and absorption in vitro. J. Sci. Food Agric. 2022, 102, 4542–4550. [Google Scholar] [CrossRef]
- Institute of Medicine (U.S.) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: A Report of the Panel on Micronutrients; Food and Nutrition Board, Institute of Medicine; National Academy Press: Washington, DC, USA, 2001; 773p. [Google Scholar]
- Lombeck, T.; Schnippering, H.G.; Ritzl, F.; Feinendegen, L.E.; Bremer, H.J. Letter: Absorption of zinc in acrodermatitis enteropathica. Lancet 1975, 1, 855. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Stanislowski, A.G.; Hurley, L.S. Isolation of a low molecular weight zinc binding ligand from human milk. J. Inorg. Biochem. 1980, 12, 71–78. [Google Scholar] [CrossRef]
- Evans, G.W.; Johnson, P.E. Characterization and quantitation of a zinc-binding ligand in human milk. Pediatr. Res. 1980, 14, 876–880. [Google Scholar] [CrossRef]
- Krieger, I.; Cash, R.; Evans, G.W. Picolinic acid in acrodermatitis enteropathica: Evidence for a disorder of tryptophan metabolism. J. Pediatr. Gastroenterol. Nutr. 1984, 3, 62–68. [Google Scholar] [CrossRef]
- Jackson, M.J. Zinc and di-iodohydroxyquinoline therapy in acrodermatitis enteropathica. J. Clin. Pathol. 1977, 30, 284–287. [Google Scholar] [CrossRef]
- Geiser, J.; De Lisle, R.C.; Finkelstein, D.; Adlard, P.A.; Bush, A.I.; Andrews, G.K. Clioquinol synergistically augments rescue by zinc supplementation in a mouse model of acrodermatitis enteropathica. PLoS ONE 2013, 8, e72543. [Google Scholar] [CrossRef]
- Scavo, S.; Oliveri, V. Zinc ionophores: Chemistry and biological applications. J. Inorg. Biochem. 2022, 228, 111691. [Google Scholar] [CrossRef]
- Larasati, Y.A.; Savitsky, M.; Koval, A.; Solis, G.P.; Valnohova, J.; Katanaev, V.L. Restoration of the GTPase activity and cellular interactions of Galphao mutants by Zn(2+) in GNAO1 encephalopathy models. Sci. Adv. 2022, 8, eabn9350. [Google Scholar] [CrossRef]
- Bohlmann, L.; De Oliveira, D.M.P.; El-Deeb, I.M.; Brazel, E.B.; Harbison-Price, N.; Ong, C.Y.; Rivera-Hernandez, T.; Ferguson, S.A.; Cork, A.J.; Phan, M.D.; et al. Chemical Synergy between Ionophore PBT2 and Zinc Reverses Antibiotic Resistance. mBio 2018, 9, 02391-18. [Google Scholar] [CrossRef]
- Harbison-Price, N.; Ferguson, S.A.; Heikal, A.; Taiaroa, G.; Hards, K.; Nakatani, Y.; Rennison, D.; Brimble, M.A.; El-Deeb, I.M.; Bohlmann, L.; et al. Multiple Bactericidal Mechanisms of the Zinc Ionophore PBT2. mSphere 2020, 5, 00157-20. [Google Scholar] [CrossRef]
- Hempe, J.M.; Cousins, R.J. Effect of EDTA and zinc-methionine complex on zinc absorption by rat intestine. J. Nutr. 1989, 119, 1179–1187. [Google Scholar] [CrossRef]
- Brnic, M.; Wegmuller, R.; Zeder, C.; Senti, G.; Hurrell, R.F. Influence of phytase, EDTA, and polyphenols on zinc absorption in adults from porridges fortified with zinc sulfate or zinc oxide. J. Nutr. 2014, 144, 1467–1473. [Google Scholar] [CrossRef]
- Savoie, L.; Agudelo, R.A.; Gauthier, S.F.; Marin, J.; Pouliot, Y. In vitro determination of the release kinetics of peptides and free amino acids during the digestion of food proteins. J. AOAC Int. 2005, 88, 935–948. [Google Scholar] [CrossRef]
- Escudero, E.; Sentandreu, M.A.; Toldra, F. Characterization of peptides released by in vitro digestion of pork meat. J. Agric. Food Chem. 2010, 58, 5160–5165. [Google Scholar] [CrossRef]
- Marukhlenko, A.V.; Morozova, M.A.; Mbarga, A.M.J.; Antipova, N.V.; Syroeshkin, A.V.; Podoprigora, I.V.; Maksimova, T.V. Chelation of Zinc with Biogenic Amino Acids: Description of Properties Using Balaban Index, Assessment of Biological Activity on Spirostomum Ambiguum Cellular Biosensor, Influence on Biofilms and Direct Antibacterial Action. Pharmaceuticals 2022, 15, 979. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C. Maternal essential fatty acid supplementation increases zinc absorption in neonatal rats: Relevance to the defect in zinc absorption in acrodermatitis enteropathica. Pediatr. Res. 1982, 16, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Cragg, R.A.; Christie, G.R.; Phillips, S.R.; Russi, R.M.; Kury, S.; Mathers, J.C.; Taylor, P.M.; Ford, D. A novel zinc-regulated human zinc transporter, hZTL1, is localized to the enterocyte apical membrane. J. Biol. Chem. 2002, 277, 22789–22797. [Google Scholar] [CrossRef] [PubMed]
- Hardyman, J.E.; Tyson, J.; Jackson, K.A.; Aldridge, C.; Cockell, S.J.; Wakeling, L.A.; Valentine, R.A.; Ford, D. Zinc sensing by metal-responsive transcription factor 1 (MTF1) controls metallothionein and ZnT1 expression to buffer the sensitivity of the transcriptome response to zinc. Metallomics 2016, 8, 337–343. [Google Scholar] [CrossRef]
- Suzuki, E.; Ogawa, N.; Takeda, T.A.; Nishito, Y.; Tanaka, Y.K.; Fujiwara, T.; Matsunaga, M.; Ueda, S.; Kubo, N.; Tsuji, T.; et al. Detailed analyses of the crucial functions of Zn transporter proteins in alkaline phosphatase activation. J. Biol. Chem. 2020, 295, 5669–5684. [Google Scholar] [CrossRef]
- Jackson, K.A.; Helston, R.M.; McKay, J.A.; O’Neill, E.D.; Mathers, J.C.; Ford, D. Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J. Biol. Chem. 2007, 282, 10423–10431. [Google Scholar] [CrossRef]
- Thornton, J.K.; Taylor, K.M.; Ford, D.; Valentine, R.A. Differential subcellular localization of the splice variants of the zinc transporter ZnT5 is dictated by the different C-terminal regions. PLoS ONE 2011, 6, e23878. [Google Scholar] [CrossRef]
- Jou, M.Y.; Hall, A.G.; Philipps, A.F.; Kelleher, S.L.; Lonnerdal, B. Tissue-specific alterations in zinc transporter expression in intestine and liver reflect a threshold for homeostatic compensation during dietary zinc deficiency in weanling rats. J. Nutr. 2009, 139, 835–841. [Google Scholar] [CrossRef]
- Cragg, R.A.; Phillips, S.R.; Piper, J.M.; Varma, J.S.; Campbell, F.C.; Mathers, J.C.; Ford, D. Homeostatic regulation of zinc transporters in the human small intestine by dietary zinc supplementation. Gut 2005, 54, 469–478. [Google Scholar] [CrossRef]
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J. Biol. Chem. 2007, 282, 14389–14393. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef]
- Valberg, L.S.; Flanagan, P.R.; Chamberlain, M.J. Effects of iron, tin, and copper on zinc absorption in humans. Am. J. Clin. Nutr. 1984, 40, 536–541. [Google Scholar] [CrossRef]
- Sandstrom, B.; Davidsson, L.; Cederblad, A.; Lonnerdal, B. Oral iron, dietary ligands and zinc absorption. J. Nutr. 1985, 115, 411–414. [Google Scholar] [CrossRef]
- Antala, S.; Ovchinnikov, S.; Kamisetty, H.; Baker, D.; Dempski, R.E. Computation and Functional Studies Provide a Model for the Structure of the Zinc Transporter hZIP4. J. Biol. Chem. 2015, 290, 17796–17805. [Google Scholar] [CrossRef]
- Davidsson, L.; Almgren, A.; Sandstrom, B.; Hurrell, R.F. Zinc absorption in adult humans: The effect of iron fortification. Br. J. Nutr. 1995, 74, 417–425. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Wharf, S.G.; Fox, T.E. Zinc absorption in infants fed iron-fortified weaning food. Am. J. Clin. Nutr. 1995, 62, 785–789. [Google Scholar] [CrossRef]
- Domellof, M.; Hernell, O.; Abrams, S.A.; Chen, Z.; Lonnerdal, B. Iron supplementation does not affect copper and zinc absorption in breastfed infants. Am. J. Clin. Nutr. 2009, 89, 185–190. [Google Scholar] [CrossRef]
- Esamai, F.; Liechty, E.; Ikemeri, J.; Westcott, J.; Kemp, J.; Culbertson, D.; Miller, L.V.; Hambidge, K.M.; Krebs, N.F. Zinc absorption from micronutrient powder is low but is not affected by iron in Kenyan infants. Nutrients 2014, 6, 5636–5651. [Google Scholar] [CrossRef] [PubMed]
- Szymlek-Gay, E.A.; Domellof, M.; Hernell, O.; Hurrell, R.F.; Lind, T.; Lonnerdal, B.; Zeder, C.; Egli, I.M. Mode of oral iron administration and the amount of iron habitually consumed do not affect iron absorption, systemic iron utilisation or zinc absorption in iron-sufficient infants: A randomised trial. Br. J. Nutr. 2016, 116, 1046–1060. [Google Scholar] [CrossRef]
- Hettiarachchi, M.; Liyanage, C.; Hilmers, D.; Griffin, I.; Abrams, S.A. Changing the zinc:iron ratio in a cereal-based nutritional supplement has no effect on percent absorption of iron and zinc in Sri Lankan children. Br. J. Nutr. 2010, 103, 1015–1022. [Google Scholar] [CrossRef]
- Li, M.; Wu, J.; Ren, T.; Wang, R.; Li, W.; Piao, J.; Wang, J.; Yang, X. Effect of NaFeEDTA-fortified soy sauce on zinc absorption in children. Food Funct. 2015, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- FAO/IZiNCG. FAO/INFOODS/IZiNCG Global Food Composition Database for Phytate Version 1.0-PhyFoodComp 1.0. 2018. Available online: https://www.fao.org/infoods/infoods/tables-and-databases/faoinfoods-databases/en/ (accessed on 22 December 2022).
- O’Brien, K.O.; Zavaleta, N.; Caulfield, L.E.; Wen, J.; Abrams, S.A. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J. Nutr. 2000, 130, 2251–2255. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Samman, S.; Madsen, L.T.; Jensen, M.; Sorensen, S.S.; Sandstrom, B. Folic acid enrichment of bread does not appear to affect zinc absorption in young women. Am. J. Clin. Nutr. 2001, 74, 125–129. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Ziller, A.; Fraissinet-Tachet, L. Metallothionein diversity and distribution in the tree of life: A multifunctional protein. Metallomics 2018, 10, 1549–1559. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J. Am. Chem. Soc. 2007, 129, 10911–10921. [Google Scholar] [CrossRef]
- Pinter, T.B.; Stillman, M.J. The zinc balance: Competitive zinc metalation of carbonic anhydrase and metallothionein 1A. Biochemistry 2014, 53, 6276–6285. [Google Scholar] [CrossRef]
- Maret, W.; Vallee, B.L. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc. Natl. Acad. Sci. USA 1998, 95, 3478–3482. [Google Scholar] [CrossRef]
- Haase, H.; Maret, W. Partial oxidation and oxidative polymerization of metallothionein. Electrophoresis 2008, 29, 4169–4176. [Google Scholar] [CrossRef]
- Andrews, G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000, 59, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Babu, C.S.; Lee, Y.M.; Dudev, T.; Lim, C. Modeling Zn(2)(+) release from metallothionein. J. Phys. Chem. A 2014, 118, 9244–9252. [Google Scholar] [CrossRef]
- Smith, K.T.; Failla, M.L.; Cousins, R.J. Identification of albumin as the plasma carrier for zinc absorption by perfused rat intestine. Biochem. J. 1979, 184, 627–633. [Google Scholar] [CrossRef]
- Stewart, A.J.; Blindauer, C.A.; Berezenko, S.; Sleep, D.; Sadler, P.J. Interdomain zinc site on human albumin. Proc. Natl. Acad. Sci. USA 2003, 100, 3701–3706. [Google Scholar] [CrossRef]
- Chilvers, D.C.; Dawson, J.B.; Bahreyni-Toosi, M.H.; Hodgkinson, A. Identification and determination of copper--and zinc--protein complexes in blood plasma after chromatographic separation on DEAE-Sepharose CL-6B. Analyst 1984, 109, 871–876. [Google Scholar] [CrossRef]
- Michielsen, C.M.S.; van Aalen, E.A.; Merkx, M. Ratiometric Bioluminescent Zinc Sensor Proteins to Quantify Serum and Intracellular Free Zn(2). ACS Chem. Biol. 2022, 17, 1567–1576. [Google Scholar] [CrossRef]
- Bengtsson, G.; Olivecrona, T. Lipoprotein lipase. Mechanism of product inhibition. Eur. J. Biochem. 1980, 106, 557–562. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Nabben, M.; Luiken, J.J.F.P. CD36 (SR-B2) as master regulator of cellular fatty acid homeostasis. Curr. Opin. Lipidol. 2022, 33, 103–111. [Google Scholar] [CrossRef]
- Lu, J.; Stewart, A.J.; Sleep, D.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. A molecular mechanism for modulating plasma Zn speciation by fatty acids. J. Am. Chem. Soc. 2012, 134, 1454–1457. [Google Scholar] [CrossRef]
- Reinhold, J.G. High phytate content of rural Iranian bread: A possible cause of human zinc deficiency. Am. J. Clin. Nutr. 1971, 24, 1204–1206. [Google Scholar] [CrossRef]
| Pathway | Evidence of Contribution to Dietary Zinc Absorption |
|---|---|
| Zip4 | Mutations resulting in loss of Zip4 function cause severe zinc deficiency [46]. The molecular mechanism of Zip4 transport and cellular regulation [50,53] is consistent with the kinetics of zinc absorption measured in humans [34]. |
| ZnT5B | Apically localized on brush border membrane enterocytes [113,114,115], ZnT5B increases zinc absorption in cultured enterocytes [116]. In conditions of excess zinc or overexpressed MT, ZnT5 is transcriptionally downregulated [110,112]. Zinc supplementation in humans causes a reduction in Zip4 and ZnT5B protein measured in ileal biopsies [115]. |
| Co-transport with amino acids | Initially proposed based on observations that zinc-binding amino acids increase zinc absorption when in molar parity with zinc and competitively inhibit zinc absorption when in molar excess of zinc [85,86]. Zinc complexed with amino acids is absorbed by cultured enterocytes lacking Zip4 [88]. |
| Co-transport with hydrophobic peptides or zinc ionophores | Hydrophobic zinc ionophores induce zinc absorption [97] and reduce the demand for therapeutic zinc in patients lacking Zip4 [96,98]. Hydrophobic zinc-binding peptides increase zinc uptake from cell culture media [89,90,91]. Intragastric administration of essential fatty acids leads to a dose-dependent increase in zinc tracer absorption in neonatal rats [108]. |
| Passive transport | High-dose zinc supplementation induces zinc absorption in patients lacking Zip4. However, interaction with alternative zinc absorptive pathways was not ruled out. Kinetic studies find no evidence of passive absorption at zinc intakes below 30 mg Zn/d [34]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, A.G.; King, J.C. The Molecular Basis for Zinc Bioavailability. Int. J. Mol. Sci. 2023, 24, 6561. https://doi.org/10.3390/ijms24076561
Hall AG, King JC. The Molecular Basis for Zinc Bioavailability. International Journal of Molecular Sciences. 2023; 24(7):6561. https://doi.org/10.3390/ijms24076561
Chicago/Turabian StyleHall, Andrew G., and Janet C. King. 2023. "The Molecular Basis for Zinc Bioavailability" International Journal of Molecular Sciences 24, no. 7: 6561. https://doi.org/10.3390/ijms24076561
APA StyleHall, A. G., & King, J. C. (2023). The Molecular Basis for Zinc Bioavailability. International Journal of Molecular Sciences, 24(7), 6561. https://doi.org/10.3390/ijms24076561






