Comparison between Organic and Inorganic Zinc Forms and Their Combinations with Various Dietary Fibers in Respect of the Effects on Electrolyte Concentrations and Mucosa in the Large Intestine of Pigs
Abstract
1. Introduction
2. Results
2.1. Water and Electrolyte Content
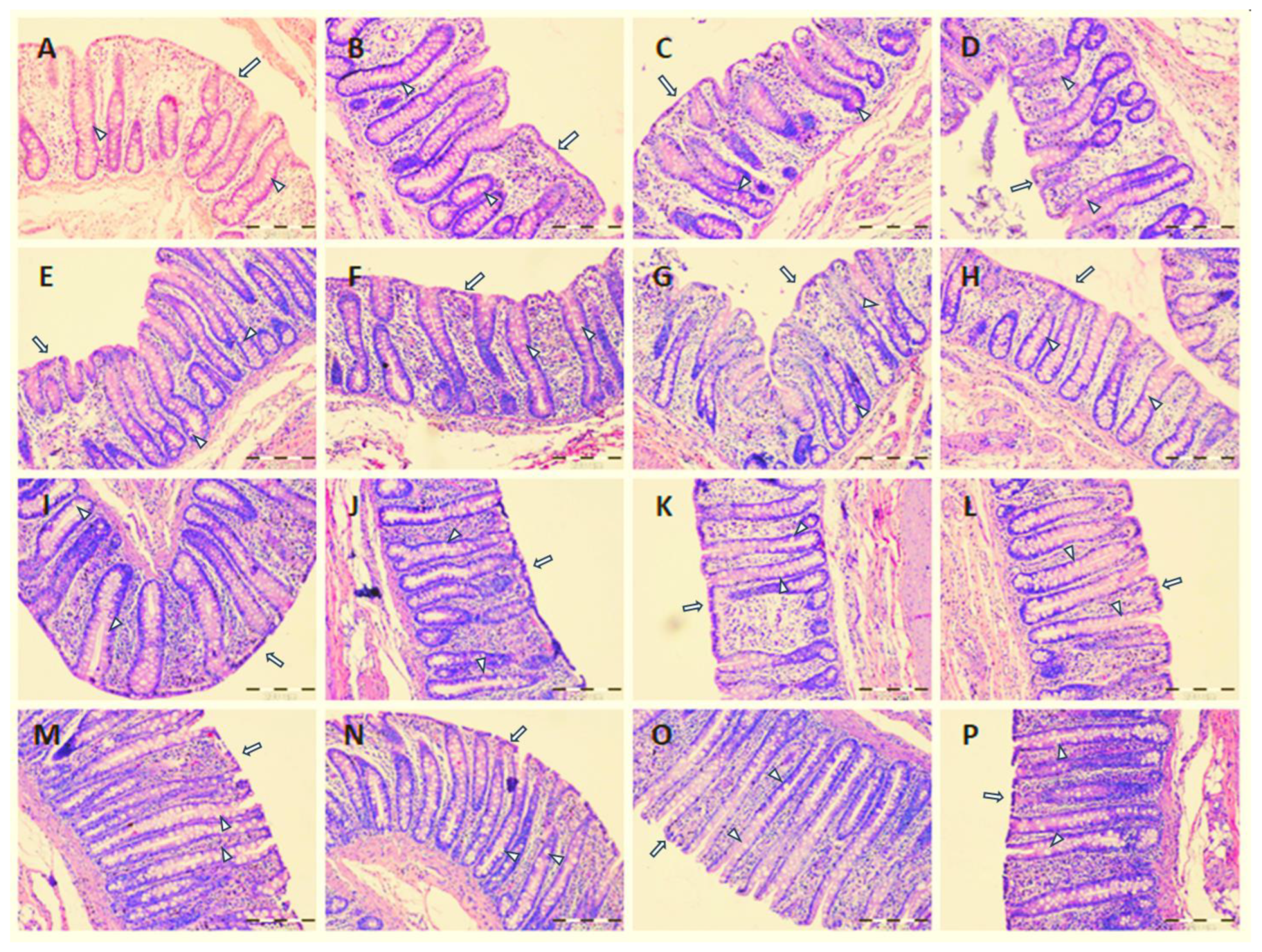
2.2. Mucus Layer Thickness and Large Intestine Morphology
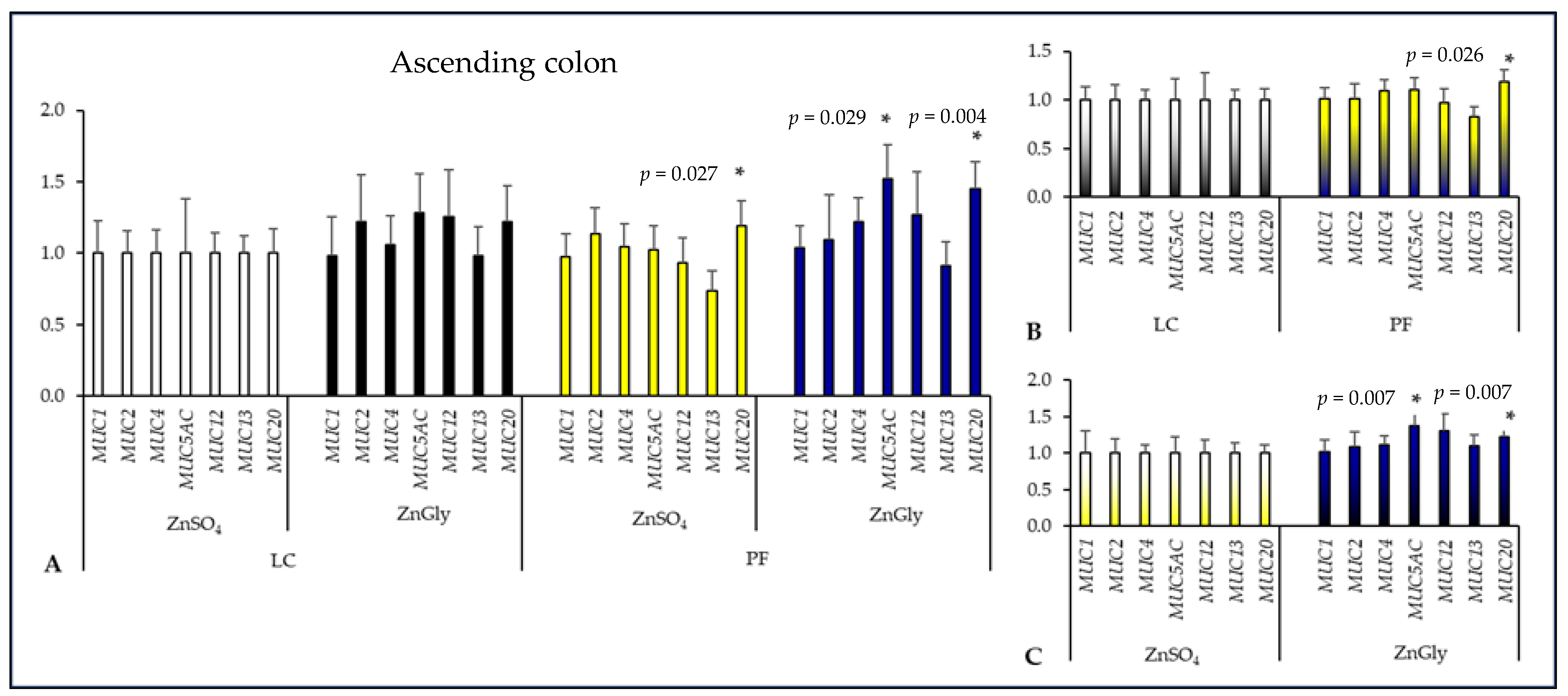
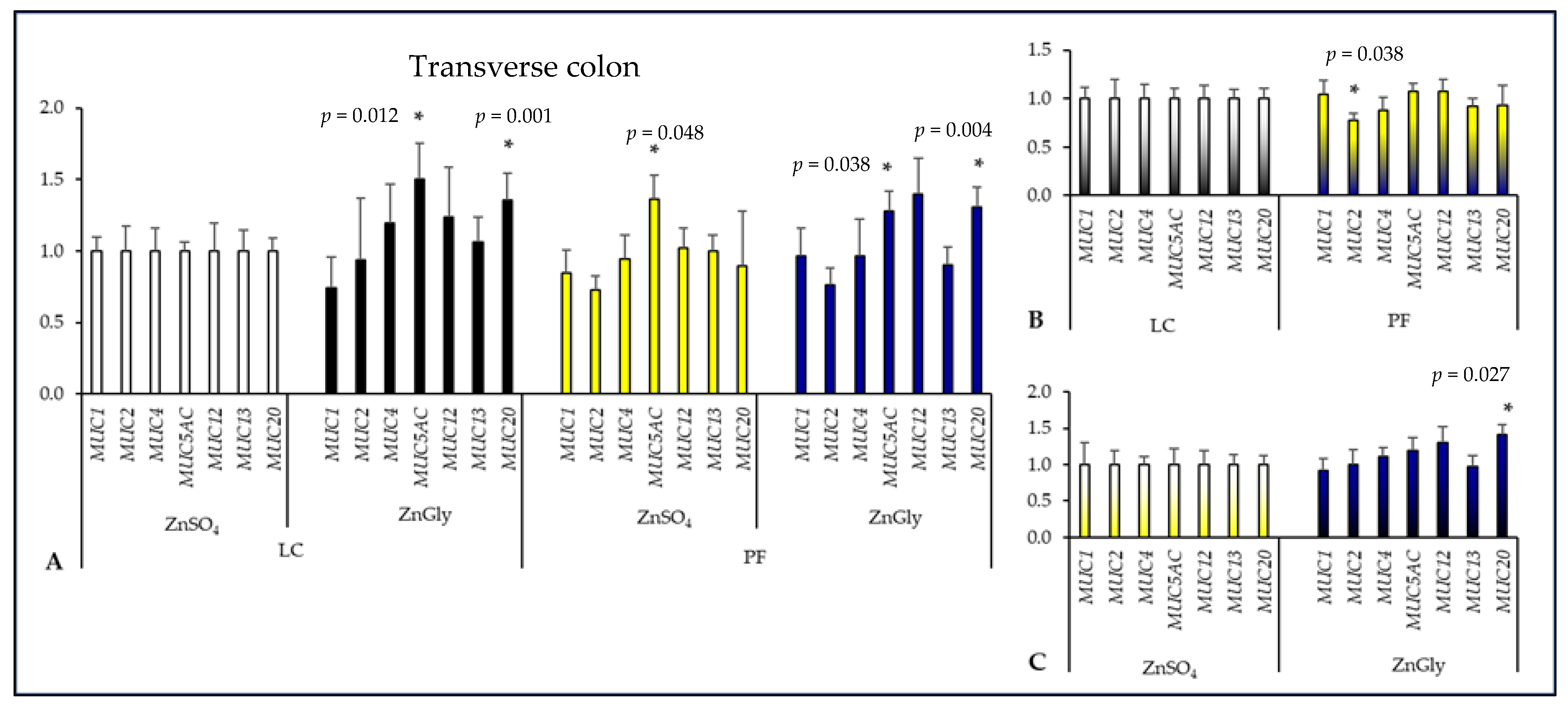
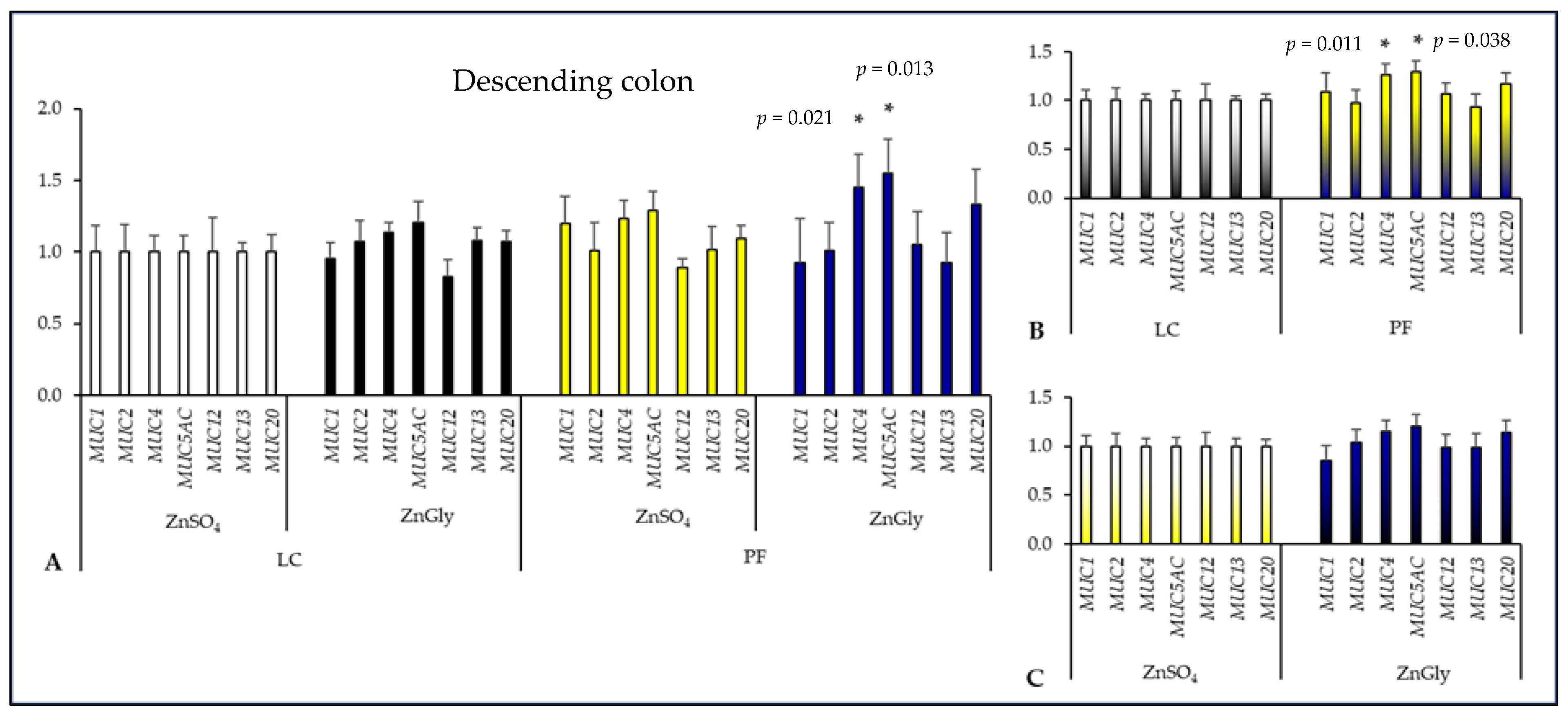
2.3. Mucin Gene Expression
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Analyses of Water Content and Electrolyte Concentrations
4.3. Measurement of Mucus Layer Thickness
4.4. Histological Examination of the Large Intestine
4.5. Total RNA Isolation
4.6. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.A.; Verstegen, M.W.A.; Tamminga, S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 2001, 14, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Bamola, V.D.; Samanta, P.; Dubey, D.; Bahadur, T.; Chandan, M.; Yiwary, S.; Gahlowt, A.; Nair, N.; Kaur, H.; et al. Immunoglobulin receptors expression in Indian colon cancer patients and healthy subjects using noninvasive approach and flow cytometry. Int. J. App. Basic Med. Res. 2020, 10, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C.; Johansson, M.E.V. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes 2010, 1, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Andriamihaja, M.; Kong, X.-F. Fate of undigested proteins in the pig large intestine: What impact on the colon epithelium? Anim. Nutr. 2022, 9, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Ambort, D.; Pelaseyed, T.; Schütte, A.; Gustafsson, J.K.; Ermund, A.; Subramani, D.B.; Holmén-Larsson, J.M.; Thomsson, K.A.; Bergström, J.H.; et al. Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 2011, 68, 3635–3641. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. 2004, 4, 45–60. [Google Scholar] [CrossRef]
- Smith, A.G.; O’Doherty, J.V.; Reilly, P.; Ryan, M.T.; Bahar, B.; Sweeney, T. The effects of laminarin derived from Laminaria digitata on measurements of gut health: Selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. Br. J. Nutr. 2011, 105, 669–677. [Google Scholar] [CrossRef]
- Pieper, R.; Kröger, S.; Richter, J.F.; Wang, J.; Martin, L.; Bindelle, J.; Htoo, J.K.; von Smolinski, D.; Vahjen, W.; Zentek, J.; et al. Fermentable fiber ameliorates fermentable protein-induced changes in microbial ecology, but not the mucosal response, in the colon of piglets. J. Nutr. 2012, 142, 661–667. [Google Scholar] [CrossRef]
- Liu, P.; Pieper, R.; Rieger, J.; Vahjen, W.; Davin, R.; Plendl, J.; Meyer, W.; Zentek, J. Effect of dietary zinc oxide on morphological characteristics, mucin composition and gene expression in the colon of weaned piglets. PLoS ONE 2014, 9, e91091. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, J.; Mu, C.; Gao, K.; Pi, Y.; Zhu, W. Low crude protein diets supplemented with casein hydrolysate enhance the intestinal barrier function and decrease the pro-inflammatory cytokine expression in the small intestine of pigs. Anim. Nutr. 2021, 7, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, Y.; Li, H.; Zhao, Y.; Wright, A.-D.G.; Cai, J.; Tian, G.; Mao, X. Differential effect of dietary fibers in intestinal health of growing pigs: Outcomes in the gut microbiota and immune-related indexes. Front. Microbiol. 2022, 13, 843045. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Xu, E.; Wang, Z.; Xiao, M.; Cao, S.; Hu, S.; Wu, Q.; Xiong, Y.; Jiang, Z.; Wang, F.; et al. Dietary Hermetia illucens larvae meal improves growth performance and intestinal barrier function of weaned pigs under the environment of enterotoxigenic Escherichia coli K88. Front. Nutr. 2022, 8, 8122011. [Google Scholar] [CrossRef] [PubMed]
- Sudan, S.; Fletcher, L.; Zhan, X.; Dingle, S.; Patterson, R.; Huber, L.-A.; Friendship, R.; Kiarie, E.G.; Li, J. Comparative efficacy of a novel Bacillus subtilis-based probiotic and pharmacological zinc oxide on growth performance and gut responses in nursery pigs. Sci. Rep. 2023, 13, 4659. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Keil, C.; Straubing, S.; Robbe-Masselot, C.; Haase, H. Zinc deficiency disturbs mucin expression, O-glycosylation and secretion by intestinal goblet cells. Int. J. Mol. Sci. 2020, 21, 6149. [Google Scholar] [CrossRef]
- Diao, H.; Yan, J.; Li, S.; Kuang, S.; Wei, X.; Zhou, M.; Zhang, J.; Huang, C.; He, P.; Tang, W. Effects of dietary zinc sources on growth performance and gut health of weaned piglets. Front. Microbiol. 2021, 12, 771617. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Kara, K.; Ozkaya, S.; Guclu, B.K.; Aktug, E.; Demir, S.; Yılmaz, S.; Pirci, G.; Yılmaz, K.; Baytok, E. In vitro ruminal fermentation and nutrient compositions of potato starch by-products. J. Anim. Feed Sci. 2023, 32, 306–315. [Google Scholar] [CrossRef]
- Metzler, B.U.; Mosenthin, R. A review of interaction between dietary fiber and the gastrointestinal microbiota and their consequences on intestinal phosphorus metabolism in growing pigs. Asian-Aust. J. Anim. Sci. 2008, 21, 603–615. [Google Scholar] [CrossRef]
- Baye, K.; Guyot, J.-P.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. [Google Scholar] [CrossRef]
- Barszcz, M.; Taciak, M.; Tuśnio, A.; Čobanová, K.; Grešáková, L. The effect of organic and inorganic zinc source, used in combination with potato fiber, on growth, nutrient digestibility and biochemical blood profile in growing pigs. Livest. Sci. 2019, 227, 34–43. [Google Scholar] [CrossRef]
- Barszcz, M.; Taciak, M.; Tuśnio, A.; Święch, E.; Skomiał, J.; Čobanová, K.; Grešáková, L. The effect of organic and inorganic zinc source, used with lignocellulose or potato fiber, on microbiota composition, fermentation, and activity of enzymes involved in dietary fiber breakdown in the large intestine of pigs. Livest. Sci. 2021, 245, 104429. [Google Scholar] [CrossRef]
- de Vogel, J.; van-Eck, W.B.; Sesink, A.L.A.; Jonker-Termont, D.S.M.L.; Kleibeuker, J.; van der Meer, R. Dietary heme injures surface epithelium resulting in hyperproliferation, inhibition of apoptosis and crypt hyperplasia in rat colon. Carcinogenesis 2008, 29, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Ncobela, C.N.; Kanengoni, A.T.; Hlatini, V.A.; Thomas, R.S.; Chimonyo, M. A review of the utility of potato by-products as a feed resource for smallholder pig production. Anim. Feed Sci. Technol. 2017, 227, 107–117. [Google Scholar] [CrossRef]
- Gralak, M.A.; Leontowicz, M.; Morawiec, M.; Bartnikowska, E.; Kulasek, G.W. Comparison of the influence of dietary fibre sources with different proportions of soluble and insoluble fibre on Ca, Mg, Fe, Zn, Mn and Cu apparent absorption in rats. Arch. Anim. Nutr. 1996, 49, 293–299. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.M.; Baird, A.W. Cytokine regulation of epithelial permeability and ion transport. Gut 1999, 44, 283–289. [Google Scholar] [CrossRef]
- Taylor, C.T.; Murphy, A.; Kelleher, D.; Baird, A.W. Changes in barrier function of a model intestinal epithelium by intraepithelial lymphocytes require new protein synthesis by epithelial cells. Gut 1997, 40, 634–640. [Google Scholar] [CrossRef][Green Version]
- Barszcz, M.; Taciak, M.; Skomiał, J. The effects of inulin, dried Jerusalem artichoke tuber and a multispecies probiotic preparation on microbiota ecology and immune status of the large intestine in young pigs. Arch. Anim. Nutr. 2016, 70, 278–292. [Google Scholar] [CrossRef]
- Barszcz, M.; Taciak, M.; Skomiał, J. Influence of different inclusion levels and chain length of inulin on microbial ecology and the state of mucosal protective barrier in the large intestine of young pigs. Anim. Prod. Sci. 2018, 58, 1109–1118. [Google Scholar] [CrossRef]
- Lapré, J.A.; De Vries, H.T.; Koeman, J.H.; Van der Meer, R. The antiproliferative effect of dietary calcium on colonic epithelium is mediated by luminal surfactants and dependent on the type of dietary fat. Cancer. Res. 1993, 53, 784–789. [Google Scholar] [PubMed]
- van Gorkom, B.A.P.; van der Meer, R.; Karrenbeld, A.; van der Sluis, T.; Zwart, N.; Termont, D.S.M.L.; Boersma-van Ek, W.; de Vries, E.G.E.; Kleibeuker, J.H. Calcium affects biomarkers of colon carcinogenesis after right hemicolectomy. Eur. J. Clin. Investig. 2002, 32, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Magee, E.A.M.; Bingham, S. Protein degradation in the large intestine: Relevance to colorectal cancer. Curr. Issues Intest. Microbiol. 2000, 1, 51–58. [Google Scholar] [PubMed]
- Tokarčiková, K.; Čobanová, K.; Takácsová, M.; Barszcz, M.; Taciak, M.; Tuśnio, A.; Grešaková, L. Trace mineral solubility and digestibility in the small intestine of piglets are affected by zinc and fibre sources. Agriculture 2022, 12, 517. [Google Scholar] [CrossRef]
- Lund, E.K.; Wharf, S.G.; Fairweather-Tait, S.J.; Johnson, I.T. Increases in the concentrations of available iron in response to dietary iron supplementation are associated with changes in crypt cell proliferation in rat large intestine. J. Nutr. 1998, 128, 175–179. [Google Scholar] [CrossRef]
- Babbs, C.F. Free radicals and the etiology of colon cancer. Free Radic. Biol. Med. 1990, 8, 191–200. [Google Scholar] [CrossRef]
- Gonciarz, R.L.; Renslo, A.R. Emerging role of ferrous iron in bacterial growth and host-pathogen interaction: New tools for chemical (micro)biology and antibacterial therapy. Curr. Opin. Chem. Biol. 2021, 61, 170–178. [Google Scholar] [CrossRef]
- Govers, M.J.; Lapré, J.A.; De Vries, H.T.; Van der Meer, R. Dietary soybean protein compared with casein damages colonic epithelium and stimulates colonic epithelial proliferation in rats. J. Nutr. 1993, 123, 1709–1713. [Google Scholar] [CrossRef]
- Martínez-Puig, D.; Castillo, M.; Nofrarias, M.; Creus, E.; Pérez, J.F. Long-term effects on the digestive tract of feeding large amounts of resistant starch: A study in pigs. J. Sci. Food Agric. 2007, 87, 1991–1999. [Google Scholar] [CrossRef]
- Nofrarías, M.; Martínez-Puig, D.; Pujols, J.; Majó, N.; Pérez, J.F. Long-term intake of resistant starch improves colonic mucosal integrity and reduces gut apoptosis and blood immune cells. Nutrition 2007, 23, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Jany, K.-D.; Berk, A.; Schulz, E.; Rechkemmer, G. Degradation of phytate in the gut of pigs—Pathway of gastrointestinal inositol phosphate hydrolysis and enzymes involved. Arch. Anim. Nutr. 2001, 55, 255–280. [Google Scholar] [CrossRef] [PubMed]
- Heyer, C.M.E.; Weiss, E.; Schmucker, S.; Rodehutscord, M.; Hoelzle, L.E.; Mosenthin, R.; Stefanski, V. The impact of phosphorus on the immune system and the intestinal microbiota with special focus on the pig. Nutr. Res. Rev. 2015, 28, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Sklan, D.; Uni, Z. Mucin dynamics in the chick small intestine are altered by starvation. J. Nutr. 2004, 134, 736–742. [Google Scholar] [CrossRef]
- Smirnov, A.; Perez, R.; Amit-Romach, E.; Sklan, D.; Uni, Z. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J. Nutr. 2005, 135, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Almagro-Moreno, S.; Pruss, K.; Taylor, R.K. Intestinal colonization dynamics of Vibrio cholerae. PLoS Pathog. 2015, 11, e1004787. [Google Scholar] [CrossRef]
- Valeri, M.; Rossi Paccani, S.; Kasendra, M.; Nesta, B.; Serino, L.; Pizza, M.; Soriani, M. Pathogenic E. coli exploits SsIE mucinase activity to translocate through the mucosal barrier and get access to host cells. PLoS ONE 2015, 10, e0117486. [Google Scholar] [CrossRef]
- Corfield, A.P. The interaction of the gut microbiota with the mucus barrier in health and disease in human. Microorganisms 2018, 6, 78. [Google Scholar] [CrossRef]
- Taciak, M.; Barszcz, M.; Święch, E.; Tuśnio, A.; Bachanek, I. Interactive effects of protein and carbohydrates on production of microbial metabolites in the large intestine of growing pigs. Arch. Anim. Nutr. 2017, 71, 192–209. [Google Scholar] [CrossRef]
- Tuśnio, A.; Barszcz, M.; Święch, E.; Skomiał, J.; Taciak, M. Large intestine morphology and microflora activity in piglets fed diets with two levels of raw or micronized blue sweet lupin seeds. Livest. Sci. 2020, 240, 104137. [Google Scholar] [CrossRef]
- Zhou, L.; Fang, L.; Sun, Y.; Su, Y.; Zhu, W. Effects of a diet high in resistant starch on fermentation end-products of protein and mucin secretion in the colons of pigs. Starke 2017, 69, 1600032. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, D.; Ha, Y.; Cho, K.-D.; Lee, B.H.; Seo, I.W.; Kim, S.-H.; Chae, C. Expression of mucins and trefoil factor family protein-1 in the colon of pigs naturally infected with Salmonella typhimurium. J. Comp. Path. 2009, 140, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The regulation of the intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.M.; Koetsier, M.A.; van Deventer, S.J.H.; van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef]
- Holodova, M.; Cobanova, K.; Sefcikova, Z.; Barszcz, M.; Tuśnio, A.; Taciak, M.; Gresakova, L. Dietary zinc and fibre source can influence the mineral and antioxidant status of piglets. Animals 2019, 9, 497. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Parameter | LC | PF | SEM | P | ||||
|---|---|---|---|---|---|---|---|---|
| ZnSO4 | ZnGly | ZnSO4 | ZnGly | Fiber | Zn | Interaction | ||
| Cecum | ||||||||
| Water, % | 86.34 | 87.50 | 88.26 | 88.23 | 0.324 | 0.039 | 0.360 | 0.334 |
| Na, mmol/g | 0.130 | 0.131 | 0.131 | 0.132 | 0.0014 | 0.769 | 0.894 | 0.979 |
| K, mmol/g | 0.015 | 0.011 | 0.014 | 0.013 | 0.0006 | 0.942 | 0.079 | 0.347 |
| Ca, μmol/g | 3.78 | 2.82 | 3.82 | 3.34 | 0.261 | 0.598 | 0.188 | 0.659 |
| Fe, μmol/g | 0.052 | 0.054 | 0.057 | 0.049 | 0.002 | 0.949 | 0.566 | 0.342 |
| Cl, μmol/g | 17.87 | 18.41 | 22.16 | 17.64 | 0.848 | 0.292 | 0.234 | 0.135 |
| Mg, μmol/g | 8.13 | 7.01 | 7.23 | 6.92 | 0.330 | 0.475 | 0.302 | 0.554 |
| P, μmol/g | 11.46 | 10.06 | 9.68 | 6.88 | 0.590 | 0.025 | 0.053 | 0.504 |
| Ascending colon | ||||||||
| Water, % | 82.62 | 83.69 | 84.10 | 84.96 | 0.339 | 0.039 | 0.139 | 0.865 |
| Na, mmol/g | 0.126 | 0.131 | 0.129 | 0.132 | 0.0014 | 0.590 | 0.209 | 0.670 |
| K, mmol/g | 0.023 | 0.019 | 0.019 | 0.020 | 0.0006 | 0.238 | 0.300 | 0.046 |
| Ca, μmol/g | 3.86 | 3.24 | 4.20 | 4.08 | 0.198 | 0.150 | 0.359 | 0.534 |
| Fe, μmol/g | 0.072 | 0.072 | 0.075 | 0.072 | 0.003 | 0.775 | 0.813 | 0.852 |
| Cl, μmol/g | 20.03 | 18.84 | 22.27 | 20.00 | 0.691 | 0.231 | 0.222 | 0.700 |
| Mg, μmol/g | 10.92 | 9.94 | 9.65 | 10.24 | 0.311 | 0.449 | 0.760 | 0.228 |
| P, μmol/g | 17.01 | 16.18 | 13.94 | 12.24 | 0.688 | 0.009 | 0.309 | 0.723 |
| Transverse colon | ||||||||
| Water, % | 77.46 | 77.75 | 80.54 | 78.76 | 0.527 | 0.053 | 0.462 | 0.312 |
| Na, mmol/g | 0.108 | 0.116 | 0.123 | 0.120 | 0.0020 | 0.020 | 0.494 | 0.123 |
| K, mmol/g | 0.037 | 0.032 | 0.031 | 0.032 | 0.0016 | 0.311 | 0.592 | 0.410 |
| Ca, μmol/g | 3.01 | 2.37 | 3.71 | 4.06 | 0.283 | 0.037 | 0.787 | 0.363 |
| Fe, μmol/g | 0.090 | 0.101 | 0.092 | 0.108 | 0.003 | 0.471 | 0.056 | 0.712 |
| Cl, μmol/g | 19.08 | 20.89 | 22.41 | 22.96 | 0.908 | 0.155 | 0.525 | 0.731 |
| Mg, μmol/g | 10.85 | 10.04 | 10.84 | 10.65 | 0.462 | 0.765 | 0.615 | 0.755 |
| P, μmol/g | 20.01 | 21.07 | 17.18 | 17.72 | 0.702 | 0.030 | 0.553 | 0.848 |
| Descending colon | ||||||||
| Water, % | 72.45 | 73.91 | 76.31 | 75.82 | 0.505 | 0.002 | 0.566 | 0.255 |
| Na, mmol/g | 0.090 | 0.101 | 0.105 | 0.104 | 0.0027 | 0.075 | 0.339 | 0.279 |
| K, mmol/g | 0.057 | 0.051 | 0.045 | 0.050 | 0.0024 | 0.202 | 0.891 | 0.239 |
| Ca, μmol/g | 2.85 | 2.83 | 4.22 | 4.24 | 0.265 | 0.009 | 0.996 | 0.960 |
| Fe, μmol/g | 0.103 | 0.117 | 0.152 | 0.154 | 0.007 | 0.001 | 0.450 | 0.590 |
| Cl, μmol/g | 19.80 | 22.94 | 25.31 | 25.71 | 1.009 | 0.041 | 0.361 | 0.477 |
| Mg, μmol/g | 12.18 | 10.78 | 9.63 | 12.22 | 0.578 | 0.632 | 0.612 | 0.096 |
| P, μmol/g | 22.64 | 24.00 | 18.44 | 20.84 | 0.819 | 0.023 | 0.222 | 0.730 |
| Parameter | LC | PF | SEM | P | ||||
|---|---|---|---|---|---|---|---|---|
| ZnSO4 | ZnGly | ZnSO4 | ZnGly | Fiber | Zn | Interaction | ||
| Cecum | ||||||||
| Crypt depth, μm | 585 | 577 | 522 | 548 | 12.1 | 0.063 | 0.700 | 0.475 |
| Muscular layer thickness, μm | 903 | 908 | 1014 | 955 | 38.1 | 0.331 | 0.736 | 0.690 |
| IEL/100 epithelial cells | 6.0 | 5.1 | 4.9 | 5.6 | 0.35 | 0.680 | 0.915 | 0.312 |
| Mucus layer thickness, μg dye/cm2 | 73.62 | 71.49 | 81.78 | 74.61 | 3.320 | 0.423 | 0.509 | 0.7191 |
| Ascending colon | ||||||||
| Crypt depth, μm | 564 | 581 | 534 | 503 | 15.5 | 0.094 | 0.821 | 0.446 |
| Muscular layer thickness, μm | 639 | 610 | 661 | 686 | 52.3 | 0.664 | 0.987 | 0.812 |
| IEL/100 epithelial cells | 9.0 | 8.2 | 8.7 | 9.3 | 0.30 | 0.552 | 0.903 | 0.282 |
| Mucus layer thickness, μg dye/cm2 | 67.71 | 70.49 | 76.52 | 78.16 | 4.062 | 0.344 | 0.798 | 0.947 |
| Transverse colon | ||||||||
| Crypt depth, μm | 610 | 566 | 605 | 561 | 13.5 | 0.849 | 0.119 | 0.999 |
| Muscular layer thickness, μm | 601 | 685 | 717 | 642 | 29.4 | 0.552 | 0.942 | 0.201 |
| IEL/100 epithelial cells | 6.6 | 6.1 | 7.3 | 5.9 | 0.43 | 0.786 | 0.290 | 0.639 |
| Mucus layer thickness, μg dye/cm2 | 60.61 | 62.58 | 75.31 | 71.96 | 3.691 | 0.120 | 0.927 | 0.724 |
| Descending colon | ||||||||
| Crypt depth, μm | 721 | 699 | 667 | 665 | 15.1 | 0.164 | 0.705 | 0.750 |
| Muscular layer thickness, μm | 688 | 675 | 742 | 697 | 26.2 | 0.497 | 0.608 | 0.771 |
| IEL/100 epithelial cells | 4.1 | 3.2 | 3.3 | 3.8 | 0.28 | 0.839 | 0.701 | 0.247 |
| Mucus layer thickness, μg dye/cm2 | 62.28 | 74.44 | 65.2 | 73.09 | 3.319 | 0.908 | 0.152 | 0.755 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barszcz, M.; Gawin, K.; Tuśnio, A.; Konopka, A.; Święch, E.; Taciak, M.; Skomiał, J.; Tokarčiková, K.; Čobanová, K.; Grešáková, Ľ. Comparison between Organic and Inorganic Zinc Forms and Their Combinations with Various Dietary Fibers in Respect of the Effects on Electrolyte Concentrations and Mucosa in the Large Intestine of Pigs. Int. J. Mol. Sci. 2023, 24, 16743. https://doi.org/10.3390/ijms242316743
Barszcz M, Gawin K, Tuśnio A, Konopka A, Święch E, Taciak M, Skomiał J, Tokarčiková K, Čobanová K, Grešáková Ľ. Comparison between Organic and Inorganic Zinc Forms and Their Combinations with Various Dietary Fibers in Respect of the Effects on Electrolyte Concentrations and Mucosa in the Large Intestine of Pigs. International Journal of Molecular Sciences. 2023; 24(23):16743. https://doi.org/10.3390/ijms242316743
Chicago/Turabian StyleBarszcz, Marcin, Kamil Gawin, Anna Tuśnio, Adrianna Konopka, Ewa Święch, Marcin Taciak, Jacek Skomiał, Katarina Tokarčiková, Klaudia Čobanová, and Ľubomira Grešáková. 2023. "Comparison between Organic and Inorganic Zinc Forms and Their Combinations with Various Dietary Fibers in Respect of the Effects on Electrolyte Concentrations and Mucosa in the Large Intestine of Pigs" International Journal of Molecular Sciences 24, no. 23: 16743. https://doi.org/10.3390/ijms242316743
APA StyleBarszcz, M., Gawin, K., Tuśnio, A., Konopka, A., Święch, E., Taciak, M., Skomiał, J., Tokarčiková, K., Čobanová, K., & Grešáková, Ľ. (2023). Comparison between Organic and Inorganic Zinc Forms and Their Combinations with Various Dietary Fibers in Respect of the Effects on Electrolyte Concentrations and Mucosa in the Large Intestine of Pigs. International Journal of Molecular Sciences, 24(23), 16743. https://doi.org/10.3390/ijms242316743








