Novel Transcriptomic Interactomes of Noncoding RNAs in the Heart under Altered Thyroid Hormonal States
Abstract
1. Introduction
2. Results
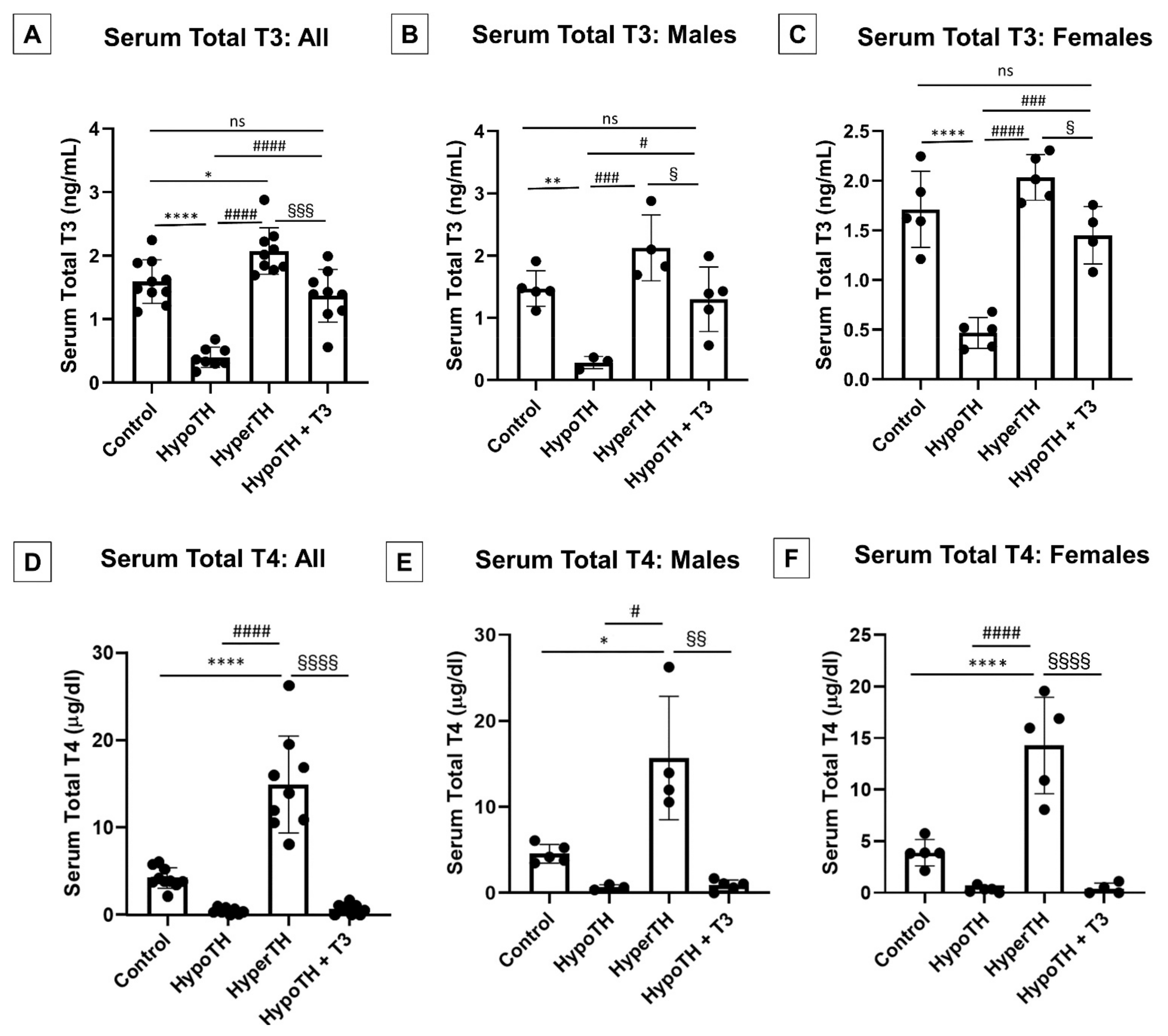
2.1. Oral T3 Supplementation Significantly Restores Serum TH Levels in HypoTH
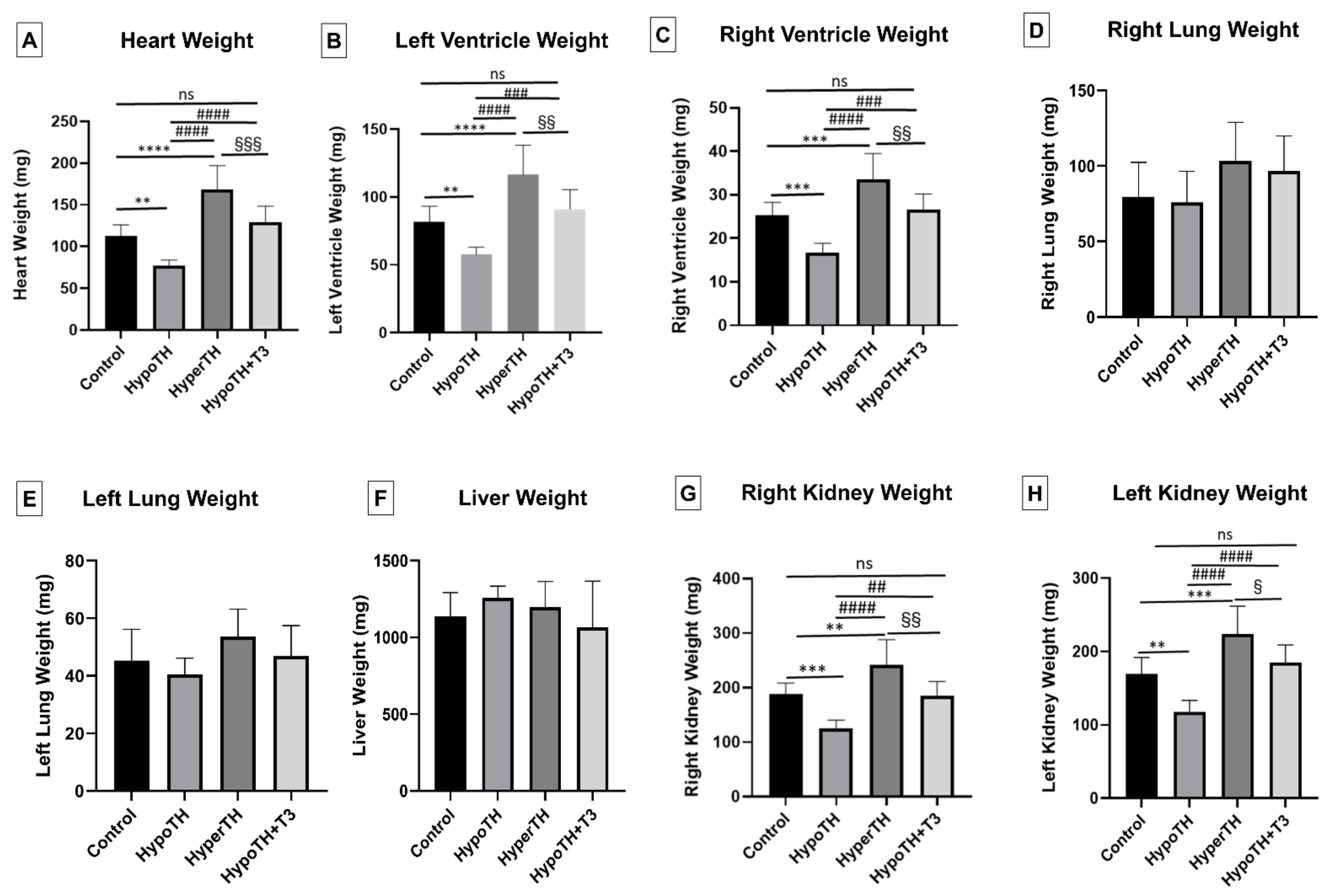
2.2. Morphometrics
2.3. Echocardiogram
2.4. Significant Impairment in Cardiac Inflammatory lncRNA Expression under Altered TH Conditions
2.5. Whole Transcriptome Analysis
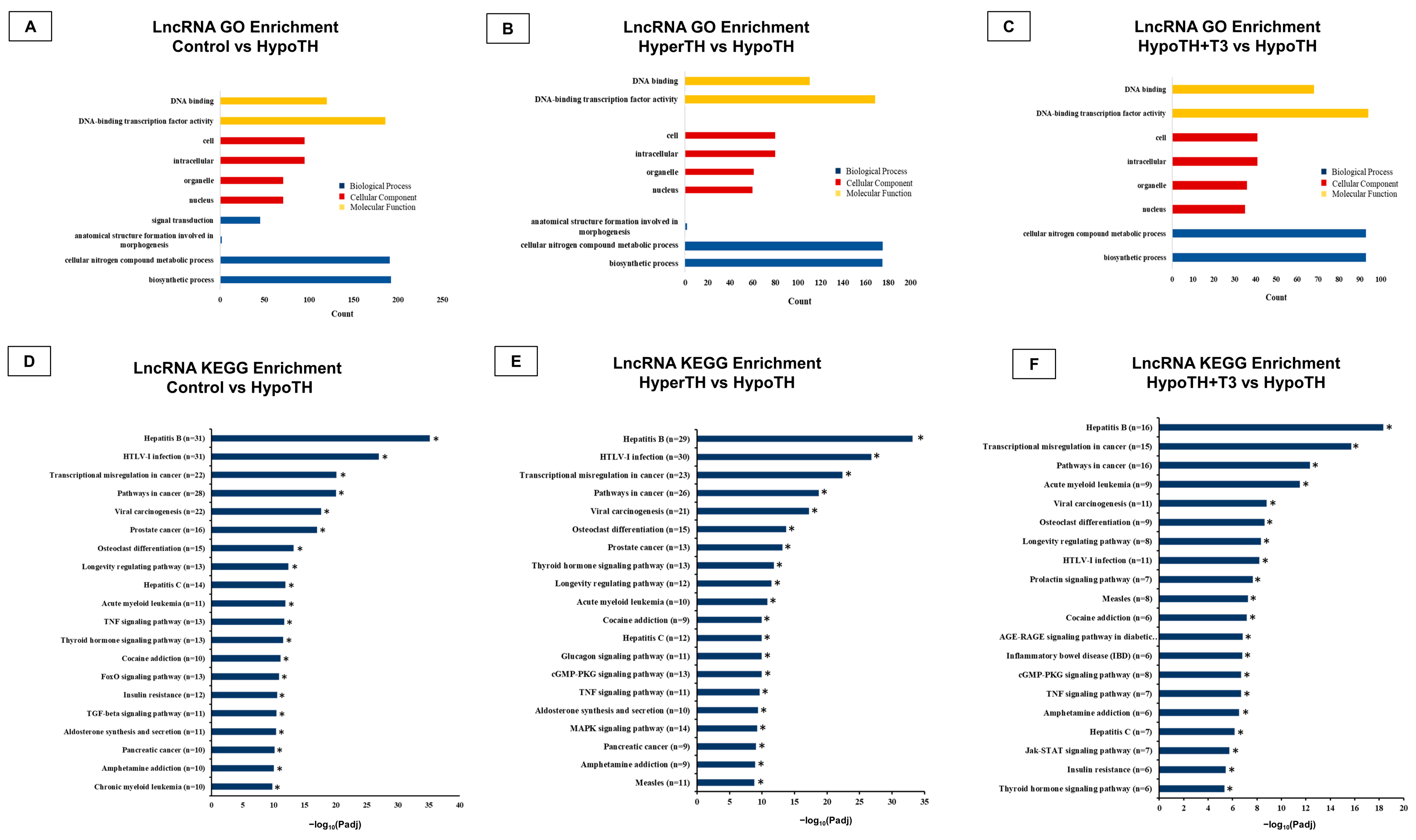
2.5.1. LncRNA Analyses
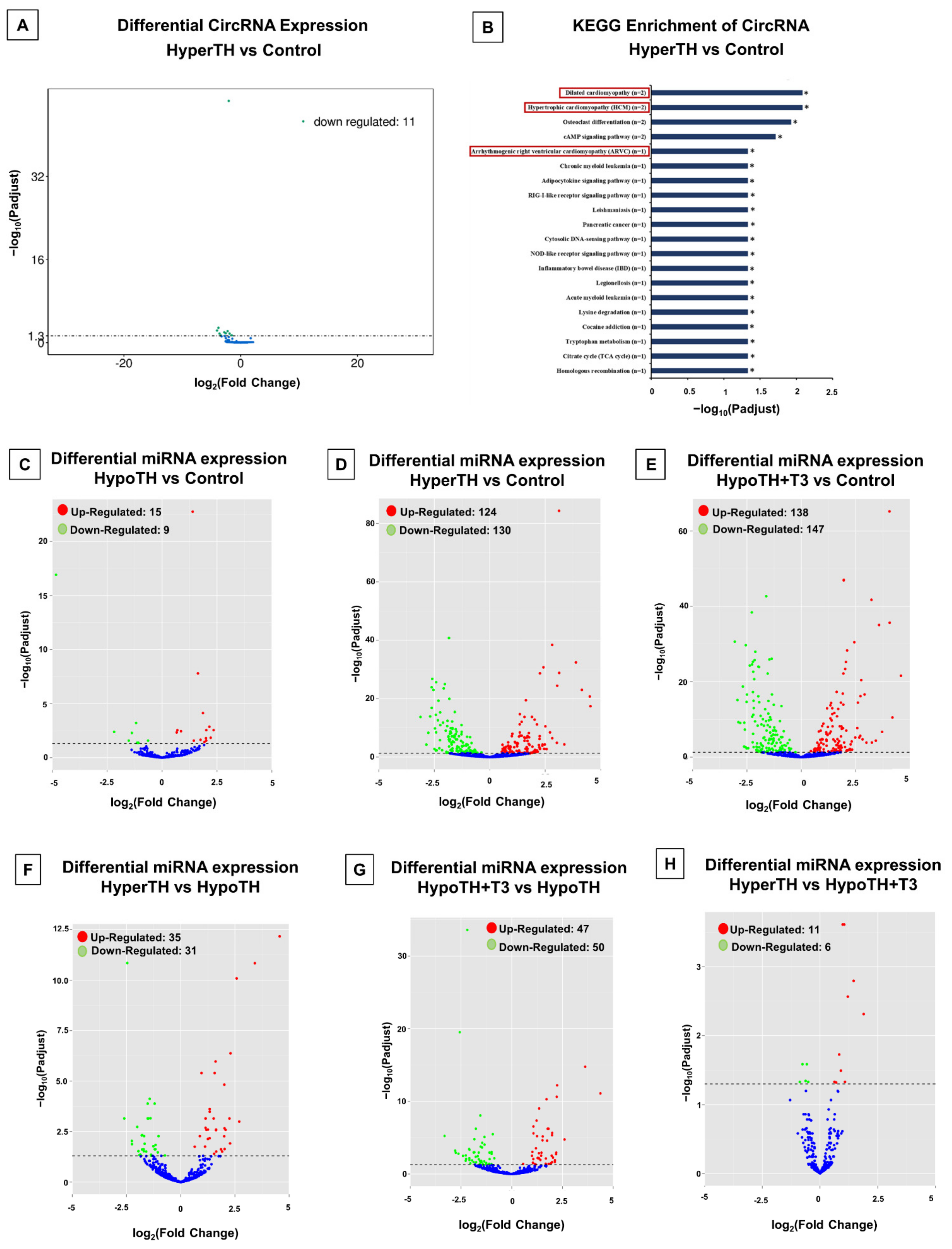
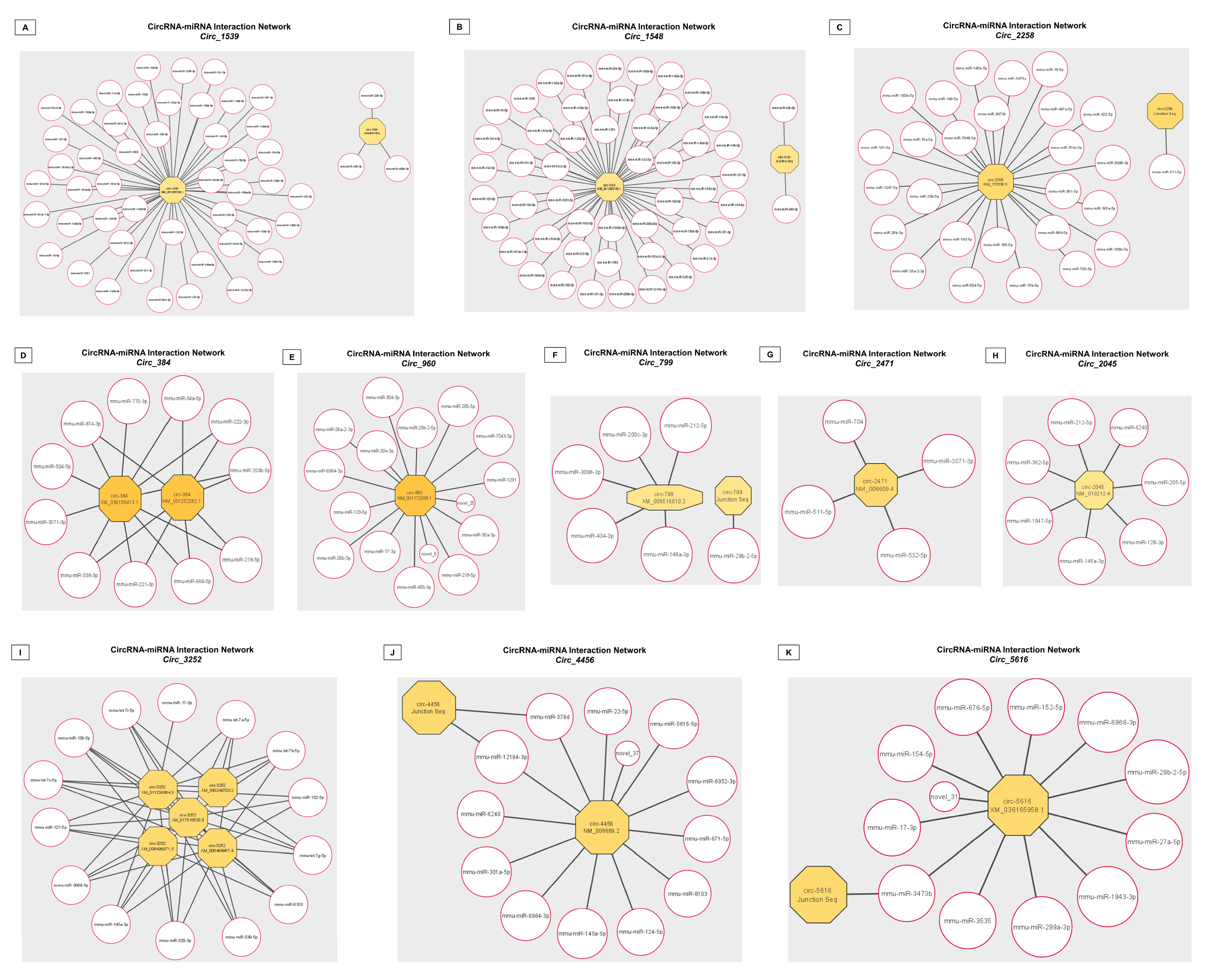
2.5.2. CircRNA Analyses
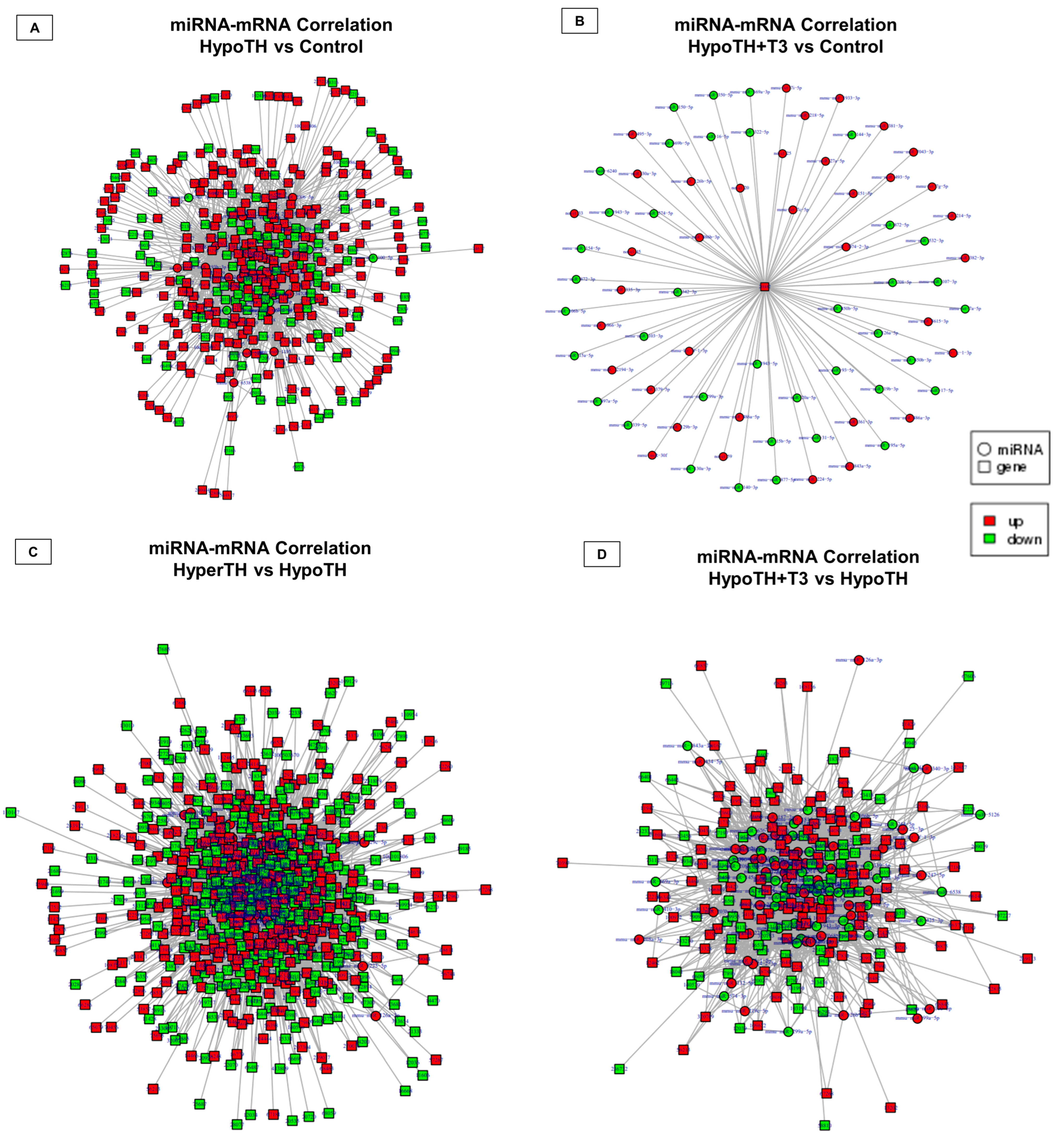
2.5.3. miRNA Analyses
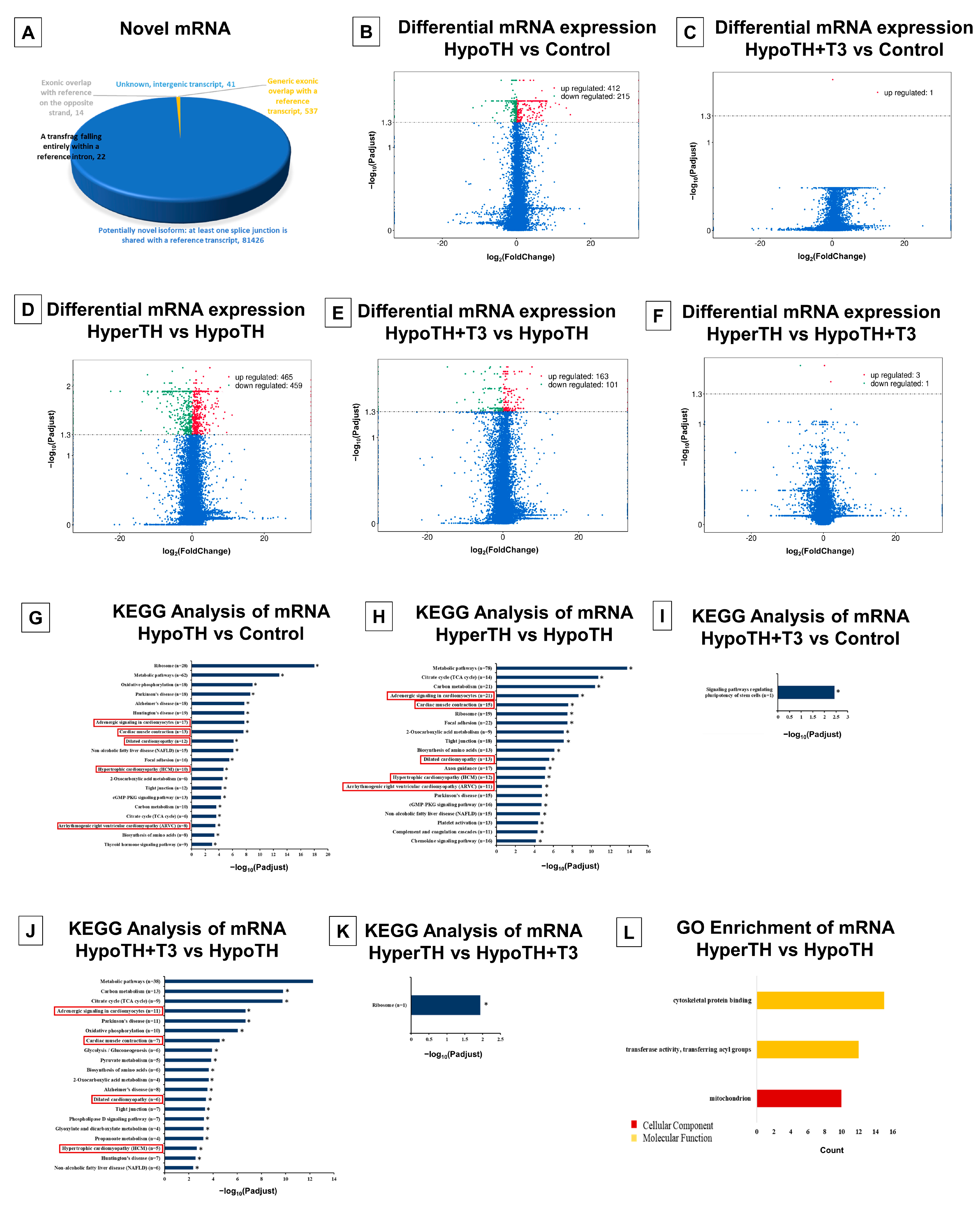
2.5.4. mRNA Analyses
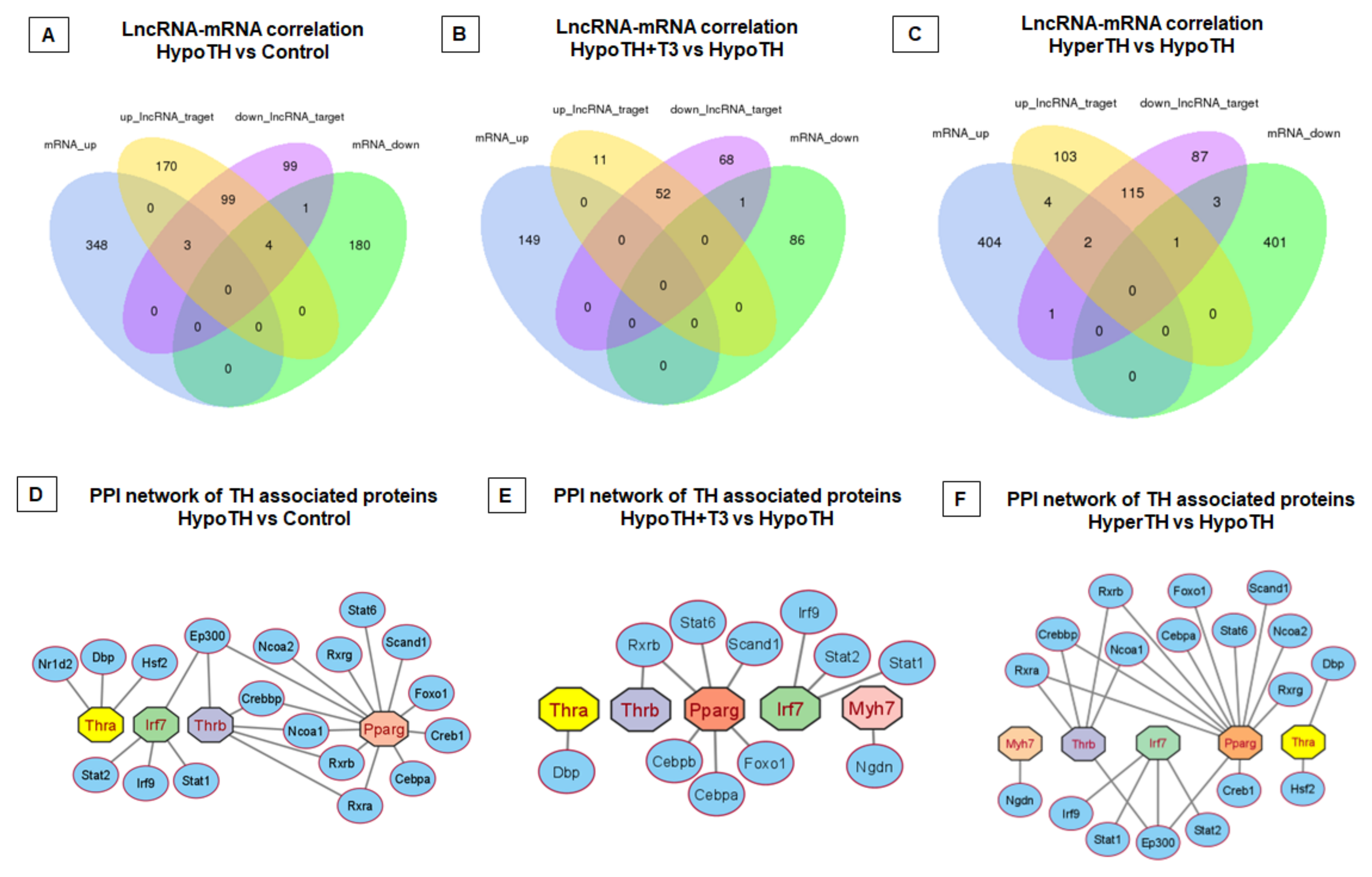
2.5.5. Interaction Networks
3. Discussion
3.1. All Major Cardiac Noncoding RNAs Are Regulated by Systemic TH Levels
3.2. Novel Interaction Networks of Cardiac Non-Coding and Coding RNAs
3.3. Non-Coding RNAs in Cardio-Thyro-Oncology
4. Materials and Methods
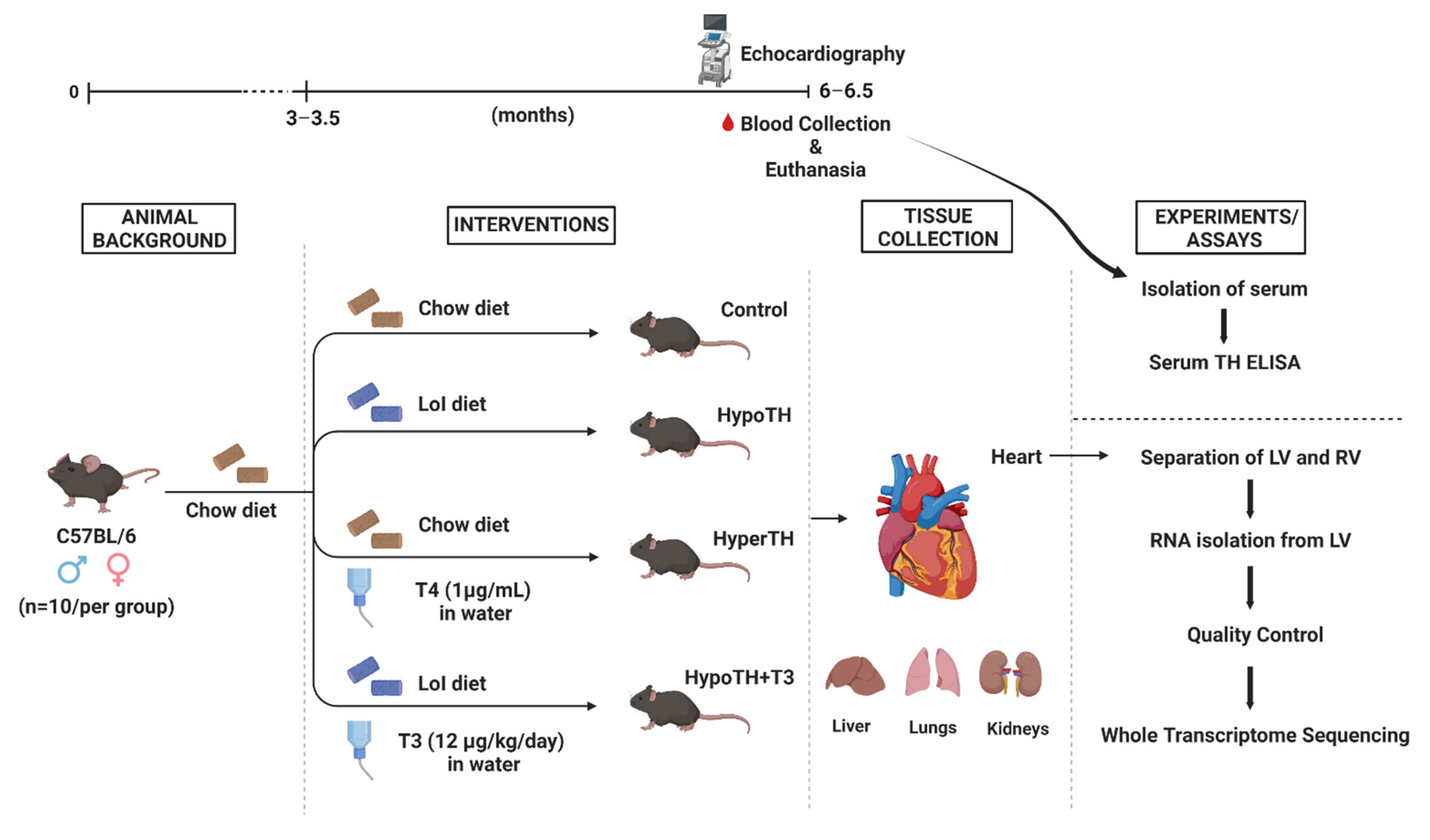
4.1. Animal Models
4.2. Echocardiography
4.3. Tissue Isolation and Sample Procurement
4.4. Serum Thyroid Hormone Levels
4.5. Tissue Processing and RNA Isolation
4.6. Quantitative Real-Time PCR
4.7. RNA Library Preparation and Whole Transcriptome Sequencing
4.8. Sample Quality Control
4.9. Data Quality Control
4.10. Statistical Analyses
4.11. LncRNA Data Analyses
4.12. CircRNA Data Analyses
4.13. Small RNA Data Analyses
4.14. Correlation and Interaction Analyses
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloyd, K.C.K.; Adams, D.J.; Baynam, G.; Beaudet, A.L.; Bosch, F.; Boycott, K.M.; Braun, R.E.; Caulfield, M.; Cohn, R.; Dickinson, M.E.; et al. The Deep Genome Project. Genome Biol. 2020, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Sallam, T.; Sandhu, J.; Tontonoz, P. Long Noncoding RNA Discovery in Cardiovascular Disease: Decoding Form to Function. Circ. Res. 2018, 122, 155–166. [Google Scholar] [CrossRef]
- McKinsey, T.A.; Vondriska, T.M.; Wang, Y. Epigenomic regulation of heart failure: Integrating histone marks, long noncoding RNAs, and chromatin architecture. F1000Research 2018, 7, 1713. [Google Scholar] [CrossRef] [PubMed]
- Bar, C.; Chatterjee, S.; Pires, I.F.; Rodrigues, P.; Sluijter, J.P.G.; Boon, R.A.; Nevado, R.M.; Andres, V.; Sansonetti, M.; de Windt, L.; et al. Non-coding RNAs: Update on mechanisms and therapeutic targets from the ESC Working Groups of Myocardial Function and Cellular Biology of the Heart. Cardiovasc. Res. 2020, 116, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.G.; Touma, M.; Wang, Y. Decoding the noncoding transcripts in human heart failure. Circulation 2014, 129, 958–960. [Google Scholar] [CrossRef]
- Robinson, E.L.; Port, J.D. Utilization and Potential of RNA-Based Therapies in Cardiovascular Disease. JACC Basic Transl. Sci. 2022, 7, 956–969. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Li, Y.; Song, T.; Wu, Y.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Pei, D.; et al. NONCODEV6: An updated database dedicated to long non-coding RNA annotation in both animals and plants. Nucleic Acids Res. 2021, 49, D165–D171. [Google Scholar] [CrossRef]
- Kazimierczyk, M.; Kasprowicz, M.K.; Kasprzyk, M.E.; Wrzesinski, J. Human Long Noncoding RNA Interactome: Detection, Characterization and Function. Int. J. Mol. Sci. 2020, 21, 1027. [Google Scholar] [CrossRef]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.-Y.; Zhou, L.-Y.; Wang, J.-X.; Wang, M.; Zhao, B.; Zhao, W.-K.; Xu, S.-J.; Fan, L.-H.; Zhang, X.-J.; et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat. Commun. 2015, 6, 6779. [Google Scholar] [CrossRef]
- Lim, T.B.; Lavenniah, A.; Foo, R.S.-Y. Circles in the heart and cardiovascular system. Cardiovasc. Res. 2020, 116, 269–278. [Google Scholar] [CrossRef]
- Aufiero, S.; Reckman, Y.J.; Pinto, Y.M.; Creemers, E.E. Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. 2019, 16, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Aufiero, S.; van den Hoogenhof, M.M.G.; Reckman, Y.J.; Beqqali, A.; van der Made, I.; Kluin, J.; Khan, M.A.F.; Pinto, Y.M.; Creemers, E.E. Cardiac circRNAs arise mainly from constitutive exons rather than alternatively spliced exons. RNA 2018, 24, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, T.; Siede, D.; Eschenbach, J.; Heumüller, A.; Busch, M.; Nietsch, R.; Meder, B.; Most, P.; Dimmeler, S.; Backs, J.; et al. Deep Characterization of Circular RNAs from Human Cardiovascular Cell Models and Cardiac Tissue. Cells 2020, 9, 1616. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zheng, Y.; Li, L.; Lu, M.; Chen, X.; Wang, Y.; Li, Y.; Liu, X.; Gao, Y.; Mao, Y.; et al. Integrated transcriptomics and epigenomics reveal chamber-specific and species-specific characteristics of human and mouse hearts. PLoS Biol. 2021, 19, e3001229. [Google Scholar] [CrossRef]
- Kukreja, R.C.; Yin, C.; Salloum, F.N. MicroRNAs: New Players in Cardiac Injury and Protection. Mol. Pharmacol. 2011, 80, 558–564. [Google Scholar] [CrossRef]
- Garikipati, V.N.S.; Krishnamurthy, P.; Verma, S.K.; Khan, M.; Abramova, T.; Mackie, A.R.; Qin, G.; Benedict, C.; Nickoloff, E.; Johnson, J.; et al. Negative Regulation of miR-375 by Interleukin-10 Enhances Bone Marrow-Derived Progenitor Cell-Mediated Myocardial Repair and Function After Myocardial Infarction. Stem Cells 2015, 33, 3519–3529. [Google Scholar] [CrossRef]
- Van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef]
- Da Costa Martins, P.A.; De Windt, L.J. MicroRNAs in control of cardiac hypertrophy. Cardiovasc. Res. 2012, 93, 563–572. [Google Scholar] [CrossRef]
- Xun, Y.; Tang, Y.; Hu, L.; Xiao, H.; Long, S.; Gong, M.; Wei, C.; Wei, K.; Xiang, S. Purification and Identification of miRNA Target Sites in Genome Using DNA Affinity Precipitation. Front. Genet. 2019, 10, 778, Corrigendum in 2020, 11, 909. [Google Scholar] [CrossRef]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from Repression to Activation: MicroRNAs Can Up-Regulate Translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- Jusic, A.; Thomas, P.B.; Wettinger, S.B.; Dogan, S.; Farrugia, R.; Gaetano, C.; Tuna, B.G.; Pinet, F.; Robinson, E.L.; Tual-Chalot, S.; et al. Noncoding RNAs in age-related cardiovascular diseases. Ageing Res. Rev. 2022, 77, 101610. [Google Scholar] [CrossRef]
- Pang, J.K.S.; Phua, Q.H.; Soh, B.-S. Applications of miRNAs in cardiac development, disease progression and regeneration. Stem Cell Res. Ther. 2019, 10, 336. [Google Scholar] [CrossRef]
- Iervasi, G.; Pingitore, A.; Landi, P.; Raciti, M.; Ripoli, A.; Scarlattini, M.; L’Abbate, A.; Donato, L. Low-T3 syndrome: A strong prognostic predictor of death in patients with heart disease. Circulation 2003, 107, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kim, M.J.; Yu, J.M.; Yoo, H.J.; Park, Y.J. Subclinical Hypothyroidism and the Risk of Cardiovascular Disease and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. Thyroid 2018, 28, 1101–1110. [Google Scholar] [CrossRef]
- Gil-Cayuela, C.; Roselló-Lletí, E.; Tarazón, E.; Ortega, A.; Sandoval, J.; Martínez-Dolz, L.; Cinca, J.; Jorge, E.; González-Juanatey, J.R.; Lago, F.; et al. Thyroid hormone biosynthesis machinery is altered in the ischemic myocardium: An epigenomic study. Int. J. Cardiol. 2017, 243, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Bental, T.; Perl, L.; Assa, H.V.; Codner, P.; Orvin, K.; Barkan, Y.T.; Levi, A.; Kornowski, R.; Perl, L. Hypothyroidism predicts worsened prognosis in patients undergoing percutaneous coronary intervention. Front. Cardiovasc. Med. 2022, 9, 984952. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshihisa, A.; Kimishima, Y.; Kiko, T.; Kanno, Y.; Yokokawa, T.; Abe, S.; Misaka, T.; Sato, T.; Oikawa, M.; et al. Low T3 Syndrome Is Associated with High Mortality in Hospitalized Patients with Heart Failure. J. Card. Fail. 2019, 25, 195–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Dedkov, E.I.; Teplitsky, D.; Weltman, N.Y.; Pol, C.J.; Rajagopalan, V.; Lee, B.; Gerdes, A.M. Both Hypothyroidism and Hyperthyroidism Increase Atrial Fibrillation Inducibility in Rats. Circ. Arrhythmia Electrophysiol. 2013, 6, 952–959. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Gerdes, A.M. Physiological and Pathological Cardiac Remodeling in Altered Thyroid Hormone States. In Thyroid and Heart: A Comprehensive Translational Essay, 2nd ed.; Iervasi, G., Pingitore, A., Gerdes, A., Razvi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 137–152. [Google Scholar]
- Rajagopalan, V.; Gerdes, A.M. Role of Thyroid Hormones in Ventricular Remodeling. Curr. Heart Fail Rep. 2014, 12, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, A.M.; Iervasi, G. Thyroid replacement therapy and heart failure. Circulation 2010, 122, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Gilani, N.; Wang, K.; Muncan, A.; Peter, J.; An, S.; Bhatti, S.; Pandya, K.; Zhang, Y.; Tang, Y.-D.; Gerdes, A.M.; et al. Triiodothyronine maintains cardiac transverse-tubule structure and function. J. Mol. Cell. Cardiol. 2021, 160, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, A.M.; Ojamaa, K. Thyroid Hormone and Cardioprotection. Compr. Physiol. 2016, 6, 1199–1219. [Google Scholar]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.S.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.P.C.; Senger, N.; Barreto-Chaves, M.L.M. The endocrinological component and signaling pathways associated to cardiac hypertrophy. Mol. Cell Endocrinol. 2020, 518, 110972. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Zhang, Y.; Ojamaa, K.; Chen, Y.-F.; Pingitore, A.; Pol, C.J.; Saunders, D.; Balasubramanian, K.; Towner, R.A.; Gerdes, A.M. Safe Oral Triiodo-L-Thyronine Therapy Protects from Post-Infarct Cardiac Dysfunction and Arrhythmias without Cardiovascular Adverse Effects. PLoS ONE 2016, 11, e0151413. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Zhang, Y.; Pol, C.; Costello, C.; Seitter, S.; Lehto, A.; Savinova, O.V.; Chen, Y.-F.; Gerdes, A.M. Modified Low-Dose Triiodo-L-thyronine Therapy Safely Improves Function Following Myocardial Ischemia-Reperfusion Injury. Front. Physiol. 2017, 8, 225. [Google Scholar] [CrossRef]
- Weltman, N.Y.; Ojamaa, K.; Schlenker, E.H.; Chen, Y.-F.; Zucchi, R.; Saba, A.; Colligiani, D.; Rajagopalan, V.; Pol, C.J.; Gerdes, A.M. Low-Dose T3 Replacement Restores Depressed Cardiac T3 Levels, Preserves Coronary Microvasculature and Attenuates Cardiac Dysfunction in Experimental Diabetes Mellitus. Mol. Med. 2014, 20, 302–312. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, Y.-D.; Zhang, Y.; Ojamaa, K.; Li, Y.; Saini, A.S.; Carrillo-Sepulveda, M.A.; Rajagopalan, V.; Gerdes, A.M. Comparison of Therapeutic Triiodothyronine Versus Metoprolol in the Treatment of Myocardial Infarction in Rats. Thyroid 2018, 28, 799–810. [Google Scholar] [CrossRef]
- Badran, H.M.; Faheem, N.; Zidan, A.; Yacoub, M.H.; Soltan, G. Effect of Short-Term L-Thyroxine Therapy on Left Ventricular Mechanics in Idiopathic Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2020, 33, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Galli, E.; Barison, A.; Iervasi, A.; Scarlattini, M.; Nucci, D.; L’Abbate, A.; Mariotti, R.; Iervasi, G. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: A randomized, placebo-controlled study. J. Clin. Endocrinol. Metab. 2008, 93, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Redetzke, R.A.; Said, S.; Pottala, J.V.; De Escobar, G.M.; Gerdes, A.M. Serum thyroid hormone levels may not accurately reflect thyroid tissue levels and cardiac function in mild hypothyroidism. Am. J. Physiol. Circ. Physiol. 2008, 294, H2137–H2143. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, A.M.; Portman, M.A.; Iervasi, G.; Pingitore, A.; Cooper, D.K.C.; Novitzky, D. Ignoring a basic pathophysiological mechanism of heart failure progression will not make it go away. Am. J. Physiol. Circ. Physiol. 2021, 320, H1919–H1922. [Google Scholar] [CrossRef]
- Falstie-Jensen, A.M.; Esen, B.; Kjærsgaard, A.; Lorenzen, E.L.; Jensen, J.D.; Reinertsen, K.V.; Dekkers, O.M.; Ewertz, M.; Cronin-Fenton, D.P. Incidence of hypothyroidism after treatment for breast cancer—A Danish matched cohort study. Breast Cancer Res. 2020, 22, 106. [Google Scholar] [CrossRef]
- Park, J.; Kim, C.; Ki, Y.; Kim, W.; Nam, J.; Kim, D.; Park, D.; Jeon, H.; Kim, D.W.; Joo, J.H. Incidence of hypothyroidism after treatment for breast cancer: A Korean population-based study. PLoS ONE 2022, 17, e0269893. [Google Scholar] [CrossRef]
- Zhang, S.; Li, R.; Ma, Y.; Yan, Y.; Ma, M.; Zhang, K.; Zhou, Y.; Li, L.; Pan, L.; Ying, H.; et al. Thyroid-stimulating hormone regulates cardiac function through modulating HCN2 via targeting microRNA-1a. FASEB J. 2022, 36, e22561. [Google Scholar] [CrossRef]
- Ejanssen, R.; Zuidwijk, M.J.; Kuster, D.; Emuller, A.; Simonides, W.S. Thyroid Hormone-Regulated Cardiac microRNAs are Predicted to Suppress Pathological Hypertrophic Signaling. Front. Endocrinol. 2014, 5, 171. [Google Scholar] [CrossRef]
- Canale, P.; Nicolini, G.; Pitto, L.; Kusmic, C.; Rizzo, M.; Balzan, S.; Iervasi, G.; Forini, F. Role of miR-133/Dio3 Axis in the T3-Dependent Modulation of Cardiac mitoK-ATP Expression. Int. J. Mol. Sci. 2022, 23, 6549. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Liu, S.; Wang, Y.-C.; Guo, F.; Zhai, Q.; Jiang, J.; Ying, H. microRNA and thyroid hormone signaling in cardiac and skeletal muscle. Cell Biosci. 2017, 7, 14. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Gorecki, M.; Costello, C.; Schultz, E.; Zhang, Y.; Gerdes, A.M. Cardioprotection by triiodothyronine following caloric restriction via long noncoding RNAs. Biomed. Pharmacother. 2020, 131, 110657. [Google Scholar] [CrossRef]
- Konishi, H.; Sato, H.; Takahashi, K.; Fujiya, M. Tumor-Progressive Mechanisms Mediating miRNA-Protein Interaction. Int. J. Mol. Sci. 2021, 22, 12303. [Google Scholar] [CrossRef]
- Moukette, B.; Barupala, N.P.; Aonuma, T.; Sepulveda, M.; Kawaguchi, S.; Kim, I.-M. Interactions between noncoding RNAs as epigenetic regulatory mechanisms in cardiovascular diseases. Methods Cell Biol. 2021, 166, 309–348. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wang, J.; Liu, Y.; Li, J.; Duan, L. Transcriptome sequencing of lncRNA, miRNA, mRNA and interaction network constructing in coronary heart disease. BMC Med. Genom. 2019, 12, 124. [Google Scholar] [CrossRef]
- López-Urrutia, E.; Montes, L.P.B.; Cervantes, D.L.D.G.; Perez-Plasencia, C.; Campos-Parra, A.D. Crosstalk Between Long Non-coding RNAs, Micro-RNAs and mRNAs: Deciphering Molecular Mechanisms of Master Regulators in Cancer. Front. Oncol. 2019, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, W.; Zheng, S.; Pyle, W.G.; Zhu, C.; Chen, H.; Kang, L.; Wu, J.; Zou, Y.; Backx, P.H.; et al. Differential mRNA Expression and Circular RNA-Based Competitive Endogenous RNA Networks in the Three Stages of Heart Failure in Transverse Aortic Constriction Mice. Front. Physiol. 2022, 13, e777284. [Google Scholar] [CrossRef] [PubMed]
- Gatsiou, A.; Stellos, K. RNA modifications in cardiovascular health and disease. Nat. Rev. Cardiol. 2022, in press. [Google Scholar] [CrossRef]
- He, C.; Hu, H.; Wilson, K.D.; Wu, H.; Feng, J.; Xia, S.; Churko, J.; Qu, K.; Chang, H.Y.; Wu, J.C. Systematic Characterization of Long Noncoding RNAs Reveals the Contrasting Coordination of Cis- and Trans- Molecular Regulation in Human Fetal and Adult Hearts. Circ. Cardiovasc. Genet. 2016, 9, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, T.; Tang, H.; Sha, Z.; Chen, R.; Chen, L.; Yu, Y.; Rowe, G.C.; Das, S.; Xiao, J. Inhibition of lncRNA MAAT Controls Multiple Types of Muscle Atrophy by cis- and trans-Regulatory Actions. Mol. Ther. 2021, 29, 1102–1119. [Google Scholar] [CrossRef]
- Tong, C.; Yin, Y. Localization of RNAs in the nucleus: Cis- and trans- regulation. RNA Biol. 2021, 18, 2073–2086. [Google Scholar] [CrossRef]
- Garcia-Padilla, C.; Lozano-Velasco, E.; Garcia-Lopez, V.; Aranega, A.; Franco, D.; Garcia-Martinez, V.; Lopez-Sanchez, C. Comparative Analysis of Non-Coding RNA Transcriptomics in Heart Failure. Biomedicines 2022, 10, 3076. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, X.; Xie, D.; Shen, M.; Lin, H.; Zhu, Y.; Ma, S.; Zheng, C.; Chen, L.; Liu, Y.; et al. RNA interactions in right ventricular dysfunction induced type II cardiorenal syndrome. Aging 2021, 13, 4215–4241. [Google Scholar] [CrossRef]
- Donzelli, R.; Colligiani, D.; Kusmic, C.; Sabatini, M.; Lorenzini, L.; Accorroni, A.; Nannipieri, M.; Saba, A.; Iervasi, G.; Zucchi, R. Effect of Hypothyroidism and Hyperthyroidism on Tissue Thyroid Hormone Concentrations in Rat. Eur. Thyroid. J. 2016, 5, 27–34. [Google Scholar] [CrossRef]
- Gil-Cayuela, C.; Ortega, A.; Tarazón, E.; Martínez-Dolz, L.; Cinca, J.; Juanatey, J.R.G.; Lago, F.; Roselló-Lletí, E.; Rivera, M.; Portolés, M. Myocardium of patients with dilated cardiomyopathy presents altered expression of genes involved in thyroid hormone biosynthesis. PLoS ONE 2018, 13, e0190987. [Google Scholar] [CrossRef]
- Weltman, N.Y.; Ojamaa, K.; Savinova, O.V.; Chen, Y.-F.; Schlenker, E.H.; Zucchi, R.; Saba, A.; Colligiani, D.; Pol, C.J.; Gerdes, A.M. Restoration of Cardiac Tissue Thyroid Hormone Status in Experimental Hypothyroidism: A Dose-Response Study in Female Rats. Endocrinology 2013, 154, 2542–2552. [Google Scholar] [CrossRef]
- Forini, F.; Nicolini, G.; Kusmic, C.; D’Aurizio, R.; Mercatanti, A.; Iervasi, G.; Pitto, L. T3 Critically Affects the Mhrt/Brg1 Axis to Regulate the Cardiac MHC Switch: Role of an Epigenetic Cross-Talk. Cells 2020, 9, 2155. [Google Scholar] [CrossRef] [PubMed]
- Reckman, Y.J.; Creemers, E.E. Circulating Circles Predict Postoperative Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7, e008261. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ojamaa, K.; Samuels, A.; Gilani, N.; Zhang, K.; An, S.; Zhang, Y.; Tang, Y.-D.; Askari, B.; Gerdes, A.M. BNP as a New Biomarker of Cardiac Thyroid Hormone Function. Front. Physiol. 2020, 11, 729. [Google Scholar] [CrossRef]
- Davydova, E.; Shimazu, T.; Schuhmacher, M.K.; Jakobsson, M.E.; Willemen, H.L.D.M.; Liu, T.; Moen, A.; Ho, A.Y.Y.; Małecki, J.; Schroer, L.; et al. The methyltransferase METTL9 mediates pervasive 1-methylhistidine modification in mammalian proteomes. Nat. Commun. 2021, 12, 891. [Google Scholar] [CrossRef]
- Tomar, D.; Elrod, J.W. Metabolite regulation of the mitochondrial calcium uniporter channel. Cell Calcium. 2020, 92, 102288. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Files, M.D.; Kajimoto, M.; O’Kelly Priddy, C.M.; Ledee, D.R.; Xu, C.; Des Rosiers, C.; Isern, N.; Portman, M.A. Triiodothyronine facilitates weaning from extracorporeal membrane oxygenation by improved mitochondrial substrate utilization. J. Am. Heart Assoc. 2014, 3, e000680. [Google Scholar] [CrossRef] [PubMed]
- Danzi, S.; Klein, S.; Klein, I. Differential regulation of the myosin heavy chain genes alpha and beta in rat atria and ventricles: Role of antisense RNA. Thyroid 2008, 18, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Pandorf, C.E.; Jiang, W.; Qin, A.X.; Bodell, P.W.; Baldwin, K.M.; Haddad, F. Regulation of an antisense RNA with the transition of neonatal to IIb myosin heavy chain during postnatal development and hypothyroidism in rat skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2012, 302, R854–R867. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I. Thyroid hormone receptor alpha1 as a novel therapeutic target for tissue repair. Ann. Transl. Med. 2018, 6, 254. [Google Scholar] [CrossRef]
- Sweeney, M.; Yiu, A.; Lyon, A.R.; Service, R.B.H.C.-O. Cardiac Atrophy and Heart Failure in Cancer. Card. Fail. Rev. 2017, 3, 62–65. [Google Scholar] [CrossRef]
- Rivas, A.M.; Larumbe-Zabala, E.; Diaz-Trastoy, O.; Schurr, R.N.; Jones, C.; Abdulrahman, R.; Dar, N.; Lado-Abeal, J. Effect of chemoradiation on the size of the thyroid gland. Bayl. Univ. Med Cent. Proc. 2020, 33, 541–545. [Google Scholar] [CrossRef]
- Huang, J.; Jin, L.; Ji, G.; Xing, L.; Xu, C.; Xiong, X.; Li, H.; Wu, K.; Ren, G.; Kong, L. Implication from thyroid function decreasing during chemotherapy in breast cancer patients: Chemosensitization role of triiodothyronine. BMC Cancer 2013, 13, 334. [Google Scholar] [CrossRef]
- Guerra, G.; Fabozzo, A.; Ragona, R.M.; Gerosa, G. Giant cardiac lesion in anaplastic thyroid cancer. JTCVS Tech. 2022, 16, 92–93. [Google Scholar] [CrossRef]
- Rakov, H.; Engels, K.; Hones, G.S.; Strucksberg, K.H.; Moeller, L.C.; Kohrle, J.; Zwanziger, D.; Fuhrer, D. Sex-specific pheno-types of hyperthyroidism and hypothyroidism in mice. Biol. Sex Differ. 2016, 7, 36. [Google Scholar] [CrossRef]
- Engels, K.; Rakov, H.; Zwanziger, D.; Hönes, G.S.; Rehders, M.; Brix, K.; Köhrle, J.; Möller, L.C.; Führer, D. Efficacy of protocols for induction of chronic hyperthyroidism in male and female mice. Endocrine 2016, 54, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Shen, S.; Park, J.W.; Lu, Z.-X.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.-Q.; Liu, X.-Q.; Zhao, S.-Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Bateman, A.; Birney, E.; Cerruti, L.; Durbin, R.; Etwiller, L.; Eddy, S.R.; Griffiths-Jones, S.; Howe, K.L.; Marshall, M.; Sonnhammer, E.L. The Pfam protein families database. Nucleic Acids Res. 2002, 30, 276–280. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, J.; Zhao, F. Circular RNA identification based on multiple seed matching. Briefings Bioinform. 2018, 19, 803–810. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Wen, M.; Shen, Y.; Shi, S.; Tang, T. miREvo: An integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinform. 2012, 13, 140. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.; Li, Z.; Li, X.; Hu, X.; Huang, Y.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; et al. Integrated profiling of microRNAs and mRNAs: MicroRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS ONE 2010, 5, e15224. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Storey, J.D. The positive false discovery rate: A bayesian interpretation and the p value. Ann. Stat. 2003, 31, 2013–2035. [Google Scholar] [CrossRef]
- Shabalina, S.A.; Spiridonov, N.A. The mammalian transcriptome and the function of non-coding DNA sequences. Genome Biol. 2004, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; van Dam, S.; Casal Novo Ribeiro, J.L.; Larbi, A.; de Magalhaes, J.P. A comparison of human and mouse gene co-expression networks reveals conservation and divergence at the tissue, pathway and disease levels. BMC Evol. Biol. 2015, 15, 259. [Google Scholar] [CrossRef] [PubMed]


| Control | HypoTH | HyperTH | HypoTH + T3 | |
|---|---|---|---|---|
| Heart rate (bpm) | 464 ± 32.7 | 337 ± 32.4 **** | 510 ± 27.6 #### | 515 ± 58.7 #### |
| FS (%) | 42.6 ± 6.6 | 28.2 ± 6 *** | 43.7 ± 5.4 #### | 48.5 ± 5.6 #### |
| IVSd (mm) | 0.82 ± 0.2 | 0.56 ± 0.1 | 0.86 ± 0.2 # | 0.99 ± 0.2 ### |
| IVSs (mm) | 1.36 ± 0.2 | 1.02 ± 0.1 ** | 1.59 ± 0.1 *,#### | 1.75 ± 0.2 ***,#### |
| LVIDd (mm) | 3.53 ± 0.2 | 3.59 ± 0.5 | 4.27 ± 0.5 *,# | 3.78 ± 0.5 |
| LVIDs (mm) | 2.02 ± 0.2 | 2.59 ± 0.5 | 2.41 ± 0.4 | 1.96 ± 0.4 # |
| LVPWd (mm) | 0.82 ± 0.3 | 0.58 ± 0.2 | 0.87 ± 0.1 | 0.87 ± 0.4 |
| LVPWs (mm) | 1.16 ± 0.5 | 0.71 ± 0.2 € | 1.28 ± 0.2 ## | 1.25 ± 0.4 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajagopalan, V.; Chakraborty, S.; Lin, R. Novel Transcriptomic Interactomes of Noncoding RNAs in the Heart under Altered Thyroid Hormonal States. Int. J. Mol. Sci. 2023, 24, 6560. https://doi.org/10.3390/ijms24076560
Rajagopalan V, Chakraborty S, Lin R. Novel Transcriptomic Interactomes of Noncoding RNAs in the Heart under Altered Thyroid Hormonal States. International Journal of Molecular Sciences. 2023; 24(7):6560. https://doi.org/10.3390/ijms24076560
Chicago/Turabian StyleRajagopalan, Viswanathan, Sankalpa Chakraborty, and Richard Lin. 2023. "Novel Transcriptomic Interactomes of Noncoding RNAs in the Heart under Altered Thyroid Hormonal States" International Journal of Molecular Sciences 24, no. 7: 6560. https://doi.org/10.3390/ijms24076560
APA StyleRajagopalan, V., Chakraborty, S., & Lin, R. (2023). Novel Transcriptomic Interactomes of Noncoding RNAs in the Heart under Altered Thyroid Hormonal States. International Journal of Molecular Sciences, 24(7), 6560. https://doi.org/10.3390/ijms24076560






