Cocktails of NSAIDs and 17α Ethinylestradiol at Environmentally Relevant Doses in Drinking Water Alter Puberty Onset in Mice Intergenerationally
Abstract
1. Introduction
2. Results
2.1. Littermates, Body Weight of F1 and F2 Newborns and Postnatal Animals
2.2. Puberty Onset in F1 and F2 Males
2.3. Puberty Onset in F1 and F2 Females
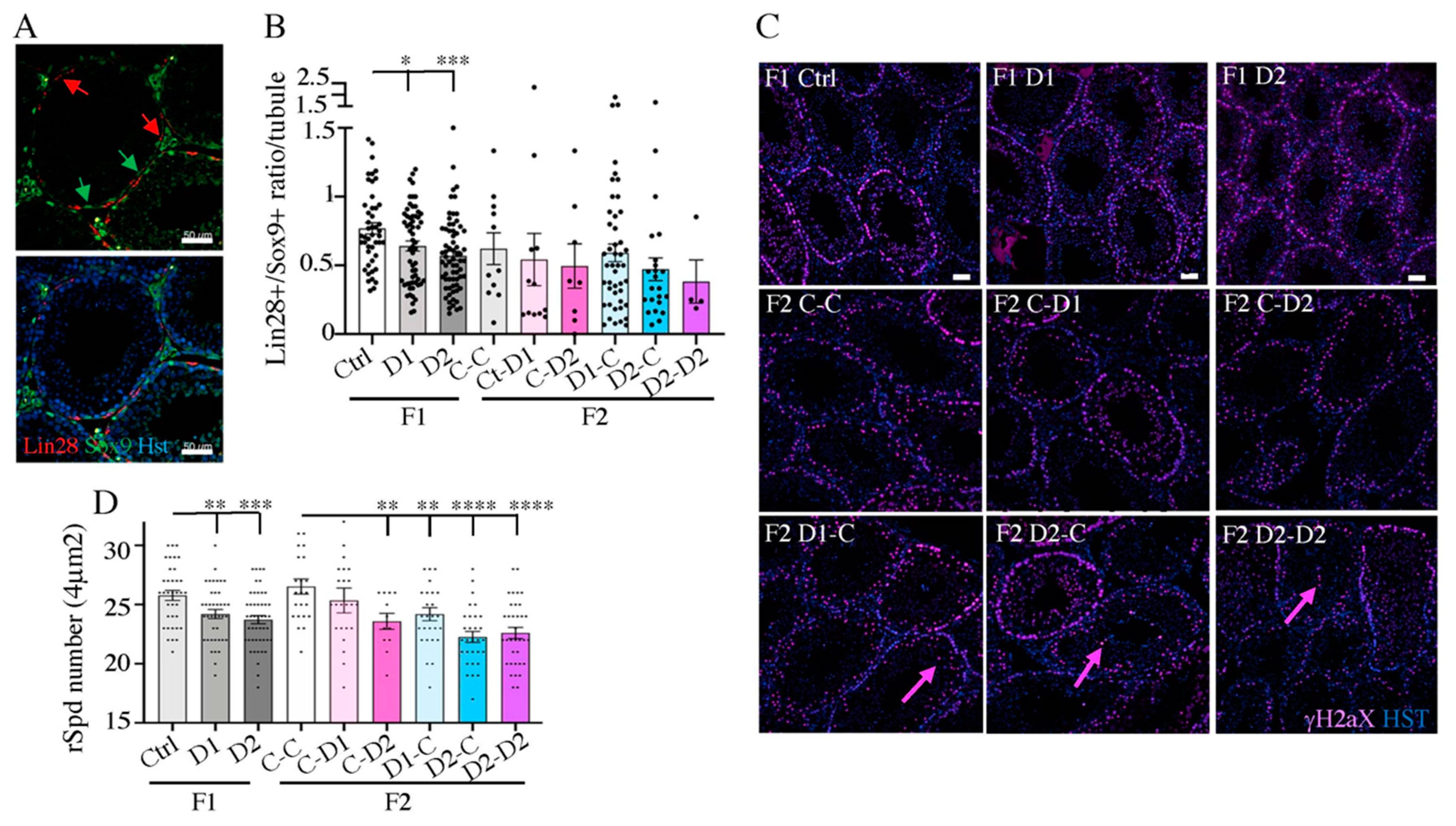
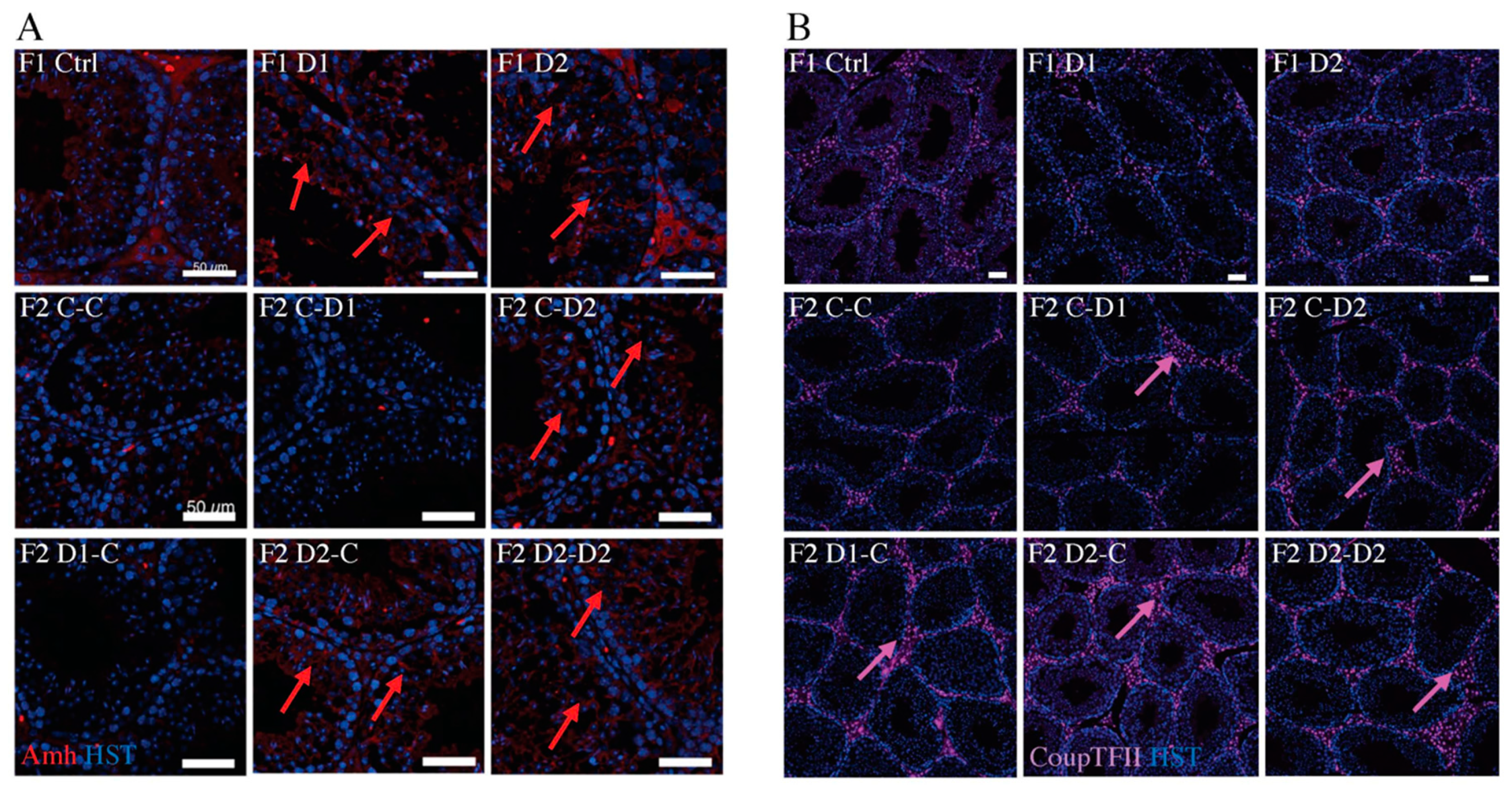
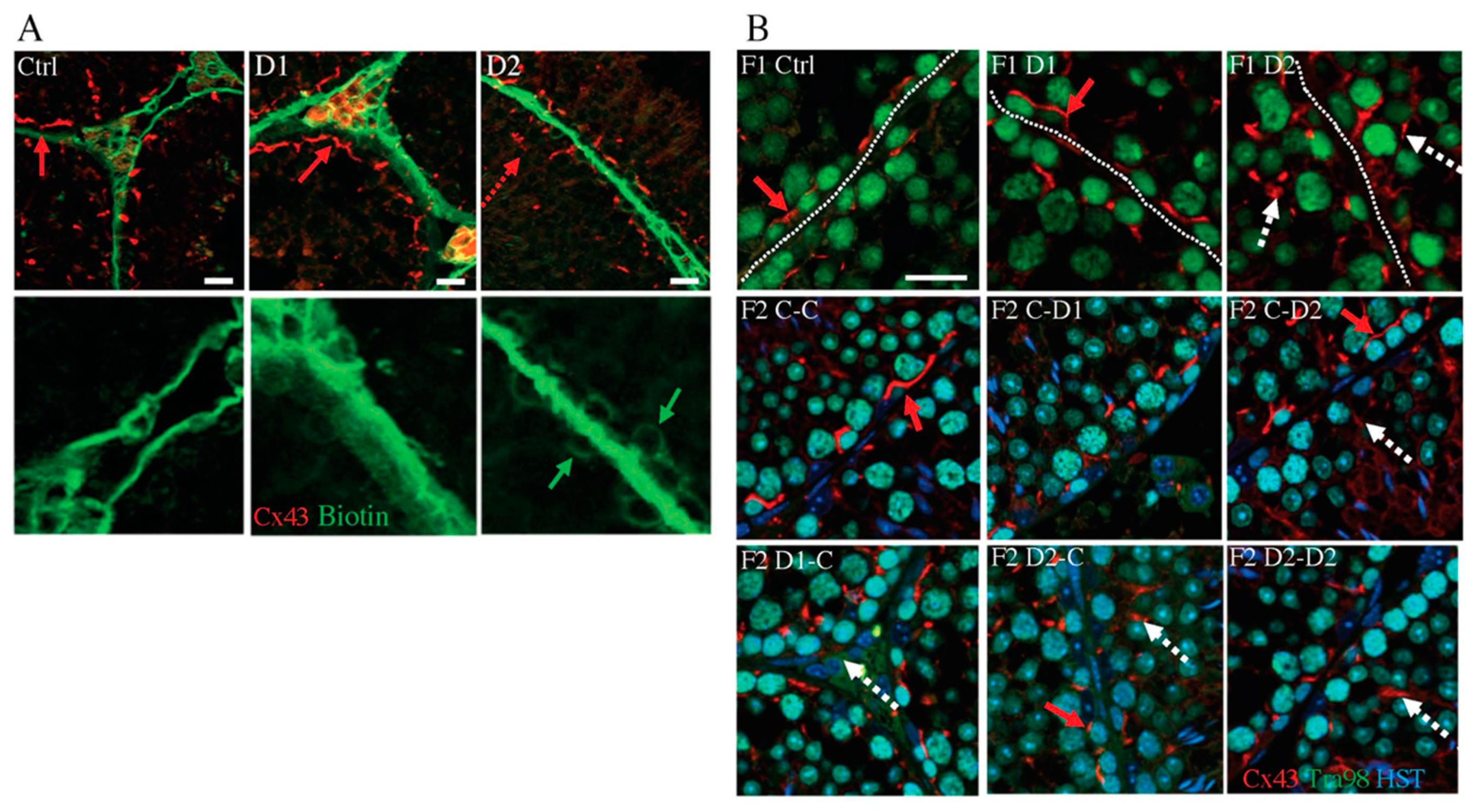
2.4. Histology (HE) and Maturation of 35 dpp F1 and F2 Testes
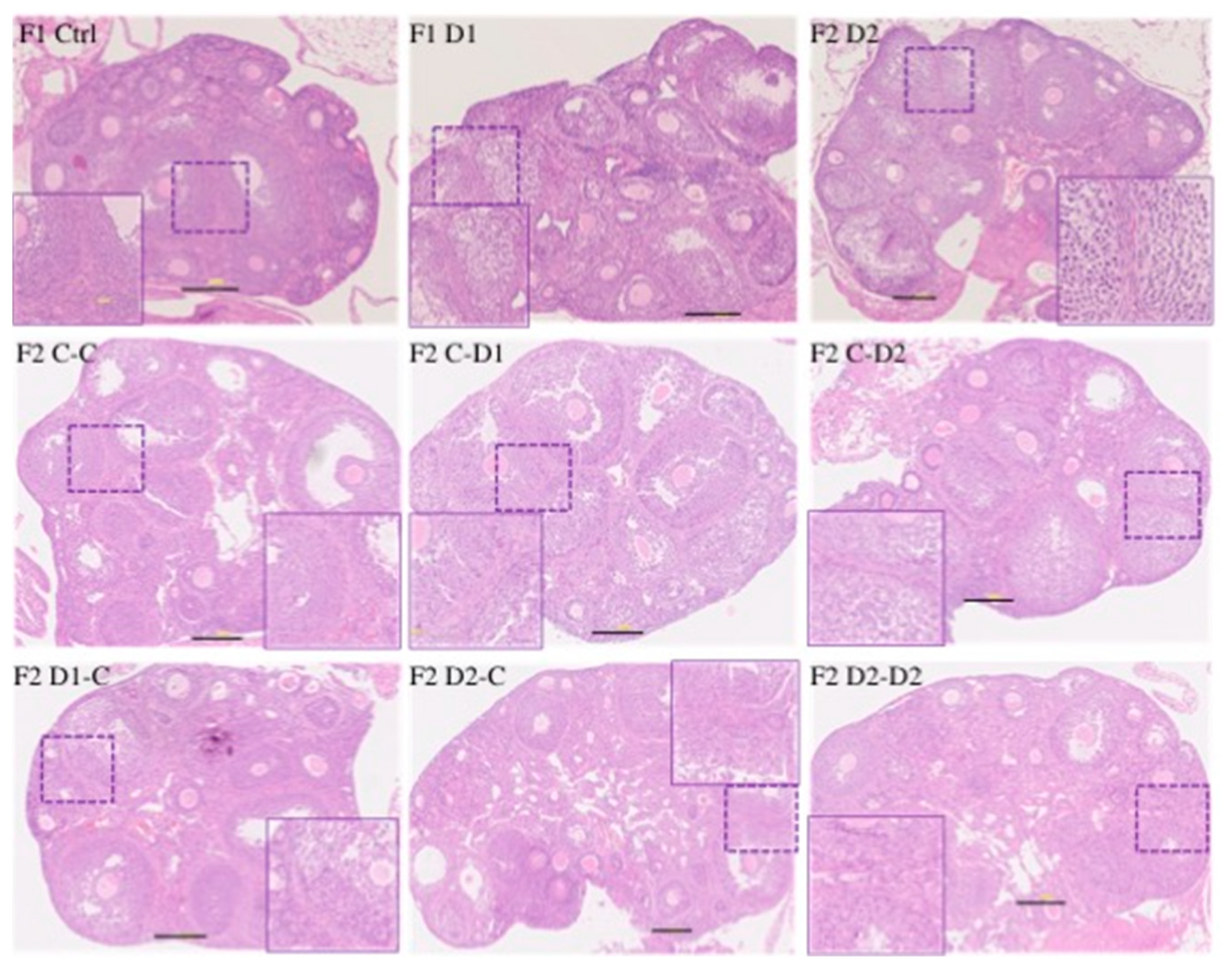
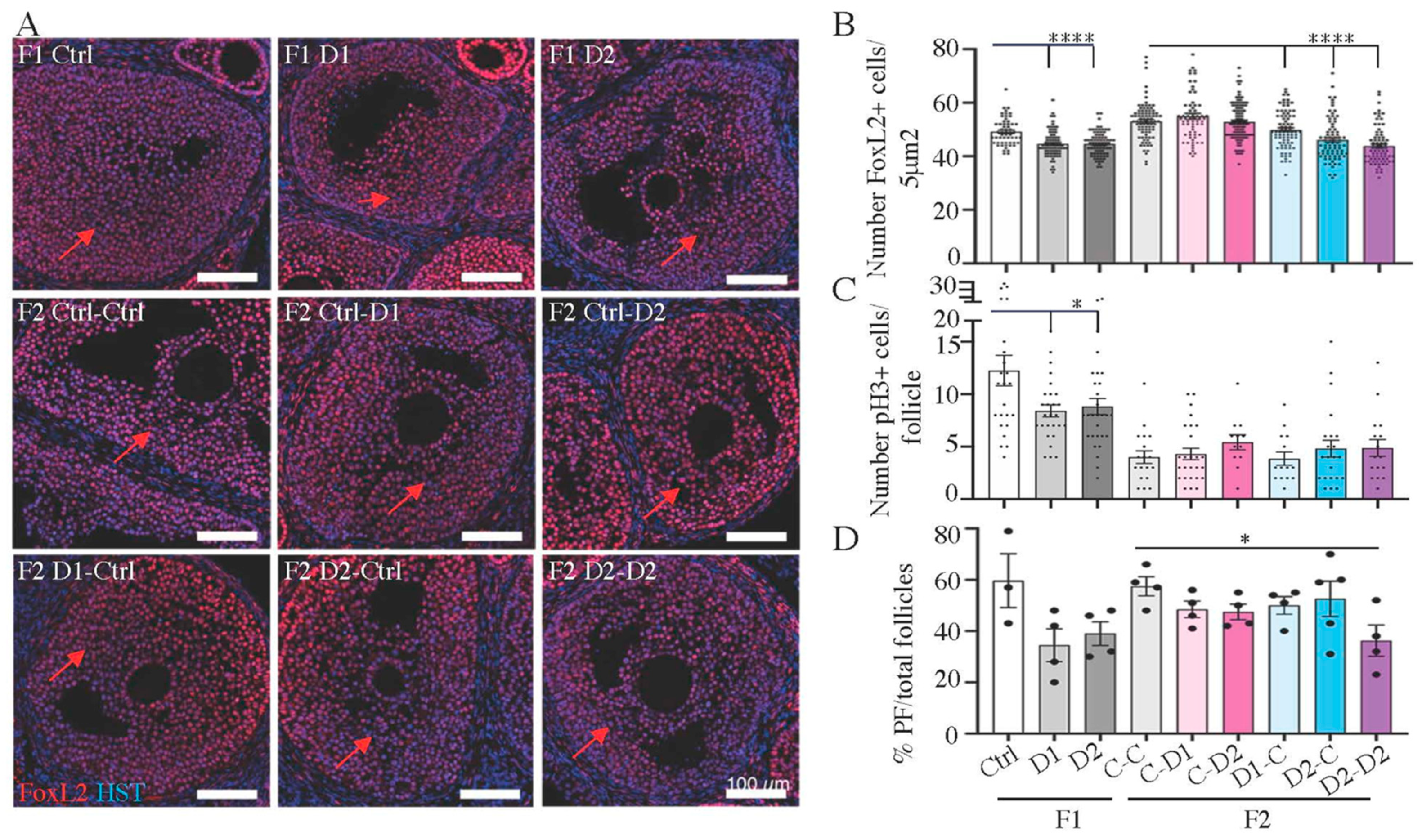
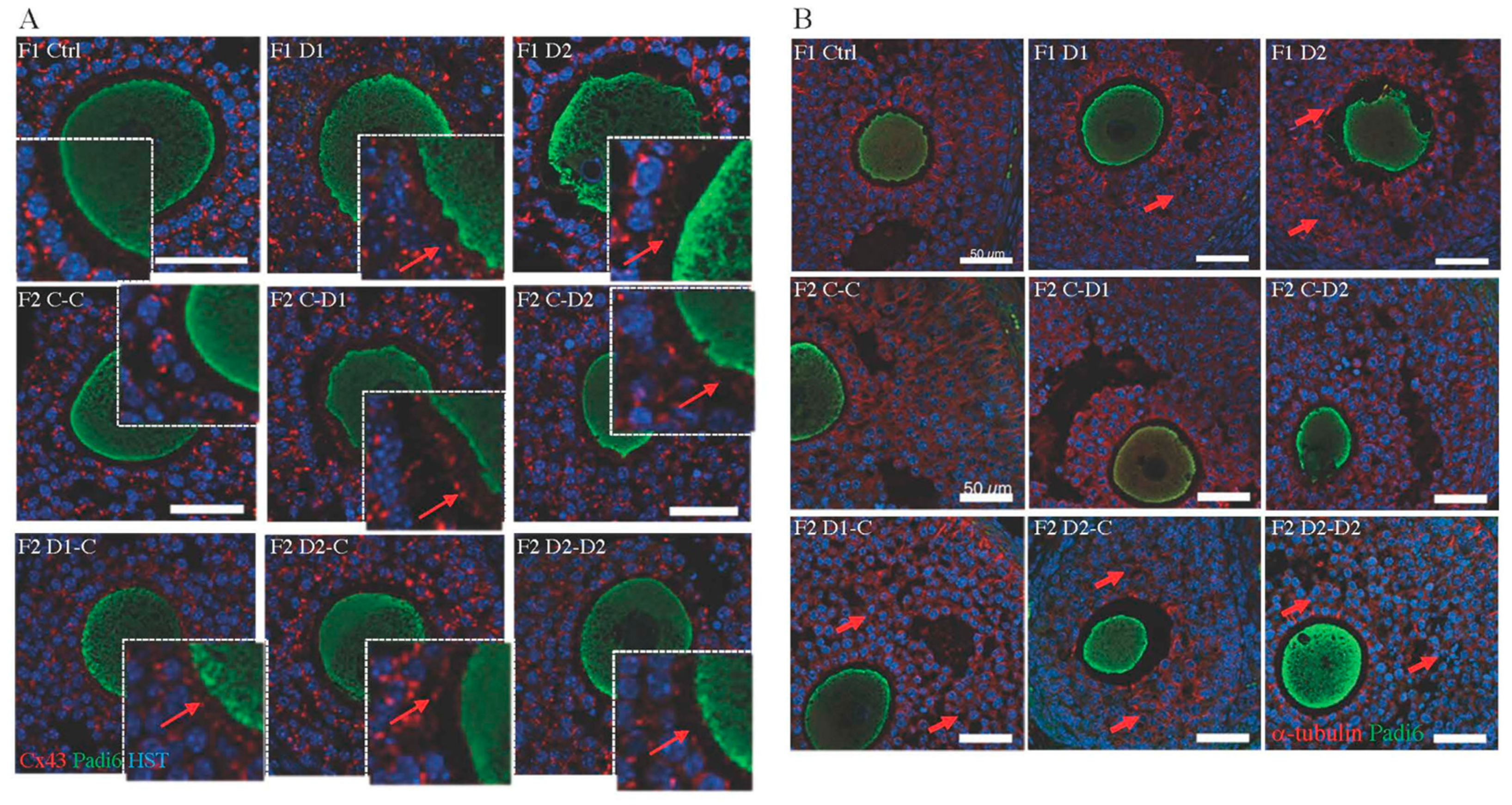
2.5. Histology and Folliculogenesis Onset in 30 dpp F1 and F2 Ovaries
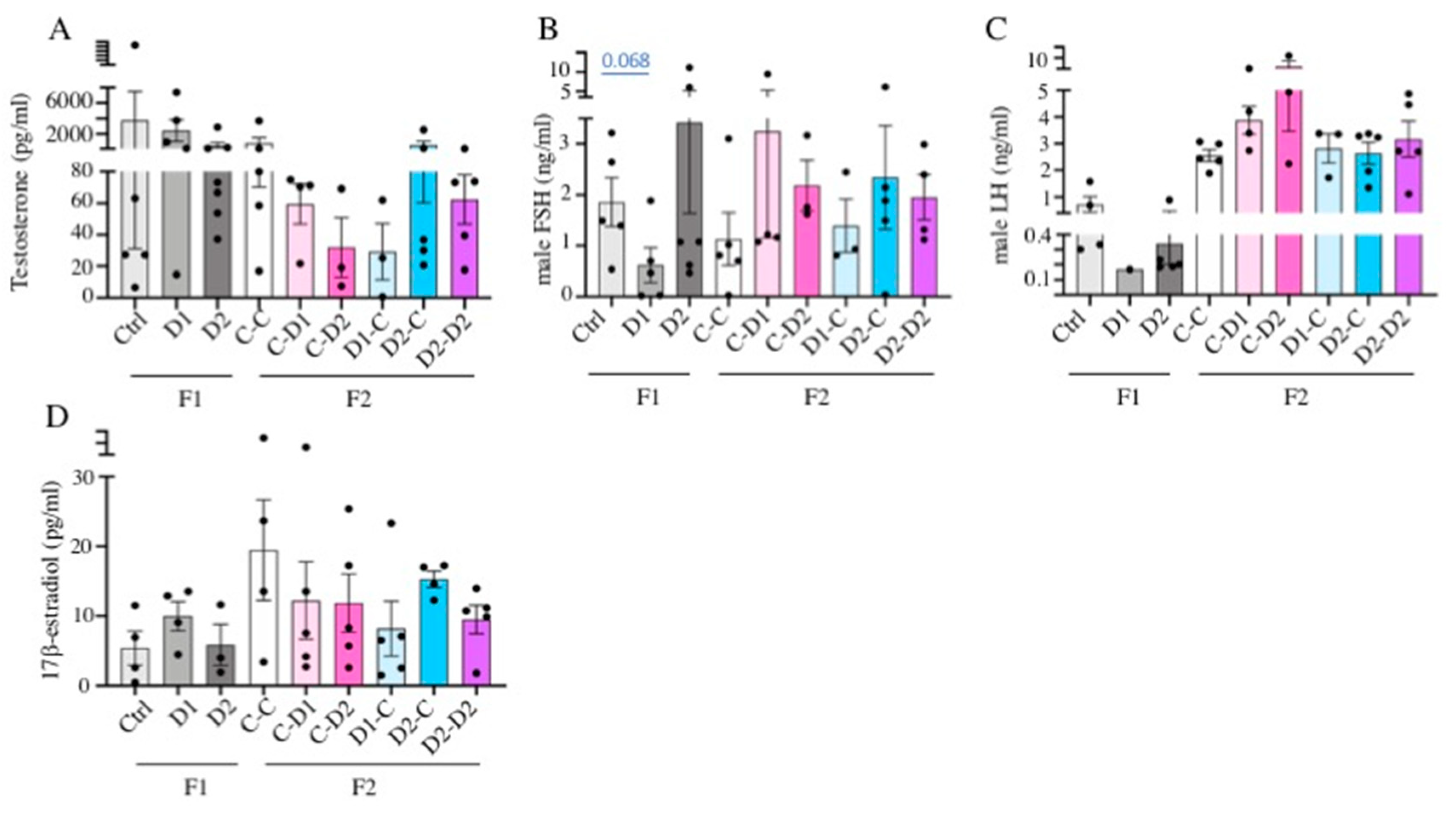
2.6. LH, FSH, Testosterone, Estradiol Quantification in Serum
2.7. Differential Gene Expression in Pubertal F1 Exposed Testis and Ovary
2.8. Biomarkers of Exposure: Genes That Are Differential Expressed Also in the F2 Generation
2.9. Putative Markers of NSAIDs and EE2 Exposure That Are Also Markers of Exposure to Other EDC
2.10. F1 DEGs Related to Human Diseases
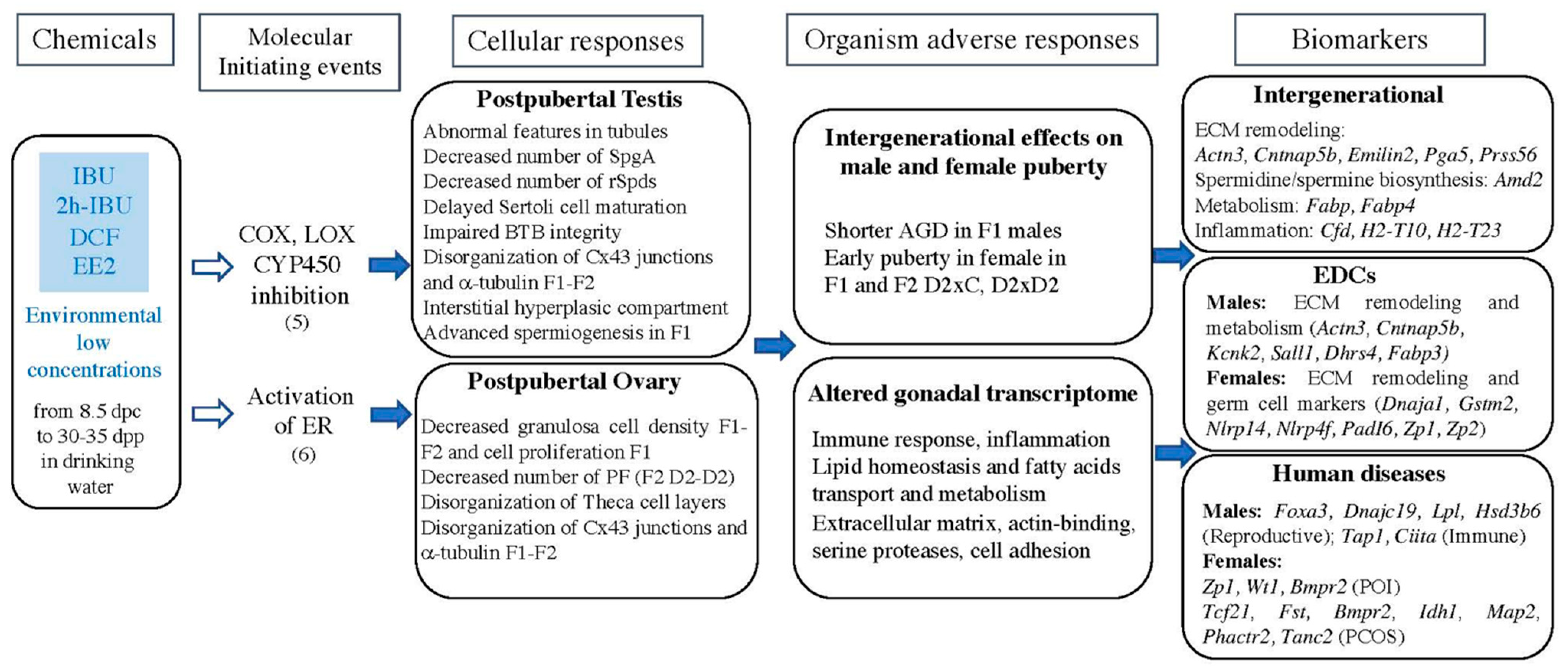
3. Discussion
4. Materials and Methods
4.1. Animal Housing, Experimental Design, and Exposures
4.2. Tissue Collection, Oocytes Isolation and Histopathological Analysis
4.3. Anogenital Distance Measurement
4.4. Hormone Concentration Quantification
4.5. RNA Extraction, RT-qPCR and Sequencing (RNA-seq)
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boxall, A.B.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Kristensen, D.M.; Mazaud-Guittot, S.; Gaudriault, P.; Lesne, L.; Serrano, T.; Main, K.M.; Jegou, B. Analgesic use-prevalence, biomonitoring and endocrine and reproductive effects. Nat. Rev. Endocrinol. 2016, 12, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, F.Z.; Archer, D.F.; Bhavnani, B.R. Ethinyl estradiol and 17beta-estradiol in combined oral contraceptives: Pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87, 706–727. [Google Scholar] [CrossRef]
- Boizet-Bonhoure, B.; Dejardin, S.; Rossitto, M.; Poulat, F.; Philibert, P. Using Experimental Models to Decipher the Effects of Acetaminophen and NSAIDs on Reproductive Development and Health. Front. Toxicol. 2022, 4, 835360. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.D. Drug Interactions Involving 17alpha-Ethinylestradiol: Considerations Beyond Cytochrome P450 3A Induction and Inhibition. Clin. Pharmacol. Ther. 2022, 111, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Naga Rani, M.A.; Joseph, T.; Narayanan, R. Placental transfer of paracetamol. J. Indian Med. Assoc. 1989, 87, 182–183. [Google Scholar] [PubMed]
- Dennis, K.K.; Carter, B.D.; Gapstur, S.M.; Stevens, V.L. Metabolomics Approach for Validation of Self-Reported Ibuprofen and Acetaminophen Use. Metabolites 2018, 8, 55. [Google Scholar] [CrossRef]
- Hutchinson, T.H.; Madden, J.C.; Naidoo, V.; Walker, C.H. Comparative metabolism as a key driver of wildlife species sensitivity to human and veterinary pharmaceuticals. Philos. Trans. R. Soc. B 2014, 369, 20130583. [Google Scholar] [CrossRef] [PubMed]
- Nuel, M.; Laurent, J.; Bois, P.; Heintz, D.; Wanko, A. Seasonal and ageing effect on the behaviour of 86 drugs in a full-scale surface treatment wetland: Removal efficiencies and distribution in plants and sediments. Sci. Total Environ. 2018, 615, 1099–1109. [Google Scholar] [CrossRef]
- Rastogi, A.; Tiwari, M.K.; Ghangrekar, M.M. A review on environmental occurrence, toxicity and microbial degradation of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). J. Environ. Manag. 2021, 300, 113694. [Google Scholar] [CrossRef]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef]
- Wolfram, J.; Stehle, S.; Bub, S.; Petschick, L.L.; Schulz, R. Water quality and ecological risks in European surface waters—Monitoring improves while water quality decreases. Environ. Int. 2021, 152, 106479. [Google Scholar] [CrossRef] [PubMed]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.Q.; Wang, R.; Feng, L.; Zhang, L.Q. Determination of selected pharmaceuticals in tap water and drinking water treatment plant by high-performance liquid chromatography-triple quadrupole mass spectrometer in Beijing, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 1854–1867. [Google Scholar] [CrossRef]
- Lopez-Pacheco, I.Y.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Silva-Nunez, A.; Arevalo-Gallegos, A.; Barcelo, D.; Afewerki, S.; Iqbal, H.M.N.; Parra-Saldivar, R. Combination of nejayote and swine wastewater as a medium for Arthrospira maxima and Chlorella vulgaris production and wastewater treatment. Sci. Total Environ. 2019, 676, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.; Andreu, V.; Pico, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Aris, A.Z.; Shamsuddin, A.S.; Praveena, S.M. Occurrence of 17alpha-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environ. Int. 2014, 69, 104–119. [Google Scholar] [CrossRef]
- Bouissou-Schurtz, C.; Houeto, P.; Guerbet, M.; Bachelot, M.; Casellas, C.; Mauclaire, A.C.; Panetier, P.; Delval, C.; Masset, D. Ecological risk assessment of the presence of pharmaceutical residues in a French national water survey. Regul. Toxicol. Pharmacol. 2014, 69, 296–303. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, D.S. Significance of metabolites in the environmental risk assessment of pharmaceuticals consumed by human. Sci. Total Environ. 2017, 592, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.M.; Eisenberg, M.L.; Jensen, T.K.; Jorgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef]
- Johansson, H.K.L.; Svingen, T.; Fowler, P.A.; Vinggaard, A.M.; Boberg, J. Environmental influences on ovarian dysgenesis—Developmental windows sensitive to chemical exposures. Nat. Rev. Endocrinol. 2017, 13, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Romano, M.E.; Karagas, M.R.; Penna, F.J. Pharmacologic and Environmental Endocrine Disruptors in the Pathogenesis of Hypospadias: A Review. Curr. Environ. Health Rep. 2018, 5, 499–511. [Google Scholar] [CrossRef]
- Vabre, P.; Gatimel, N.; Moreau, J.; Gayrard, V.; Picard-Hagen, N.; Parinaud, J.; Leandri, R.D. Environmental pollutants, a possible etiology for premature ovarian insufficiency: A narrative review of animal and human data. Environ. Health 2017, 16, 37. [Google Scholar] [CrossRef]
- Wei, Y.; Schatten, H.; Sun, Q.Y. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum. Reprod. Update 2015, 21, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Capel, B. Vertebrate sex determination: Evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 2017, 18, 675–689. [Google Scholar] [CrossRef]
- Sharpe, R.M. Androgens and the masculinization programming window: Human-rodent differences. Biochem. Soc. Trans. 2020, 48, 1725–1735. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Swan, S.H.; Kriebel, D.; Liew, Z.; Taylor, H.S.; Bornehag, C.G.; Andrade, A.M.; Olsen, J.; Jensen, R.H.; Mitchell, R.T.; et al. Paracetamol use during pregnancy—A call for precautionary action. Nat. Rev. Endocrinol. 2021, 17, 757–766. [Google Scholar] [CrossRef]
- Kristensen, D.M.; Desdoits-Lethimonier, C.; Mackey, A.L.; Dalgaard, M.D.; De Masi, F.; Munkbol, C.H.; Styrishave, B.; Antignac, J.P.; Le Bizec, B.; Platel, C.; et al. Ibuprofen alters human testicular physiology to produce a state of compensated hypogonadism. Proc. Natl. Acad. Sci. USA 2018, 115, E715–E724. [Google Scholar] [CrossRef]
- Vosges, M.; Braguer, J.C.; Combarnous, Y. Long-term exposure of male rats to low-dose ethinylestradiol (EE2) in drinking water: Effects on ponderal growth and on litter size of their progeny. Reprod. Toxicol. 2008, 25, 161–168. [Google Scholar] [CrossRef]
- Dai, R.; Edwards, M.R.; Heid, B.; Ahmed, S.A. 17beta-Estradiol and 17alpha-Ethinyl Estradiol Exhibit Immunologic and Epigenetic Regulatory Effects in NZB/WF1 Female Mice. Endocrinology 2019, 160, 101–118. [Google Scholar] [CrossRef]
- Caldwell, D.J.; Mastrocco, F.; Nowak, E.; Johnston, J.; Yekel, H.; Pfeiffer, D.; Hoyt, M.; DuPlessie, B.M.; Anderson, P.D. An assessment of potential exposure and risk from estrogens in drinking water. Environ. Health Perspect. 2010, 118, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Aydin, M.E.; Ulvi, A. Monitoring the release of anti-inflammatory and analgesic pharmaceuticals in the receiving environment. Environ. Sci. Pollut. Res. Int. 2019, 26, 36887–36902. [Google Scholar] [CrossRef]
- Carlsson, C.; Johansson, A.K.; Alvan, G.; Bergman, K.; Kuhler, T. Are pharmaceuticals potent environmental pollutants? Part I: Environmental risk assessments of selected active pharmaceutical ingredients. Sci. Total Environ. 2006, 364, 67–87. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Gaytan, F.; Morales, C.; Leon, S.; Heras, V.; Barroso, A.; Avendano, M.S.; Vazquez, M.J.; Castellano, J.M.; Roa, J.; Tena-Sempere, M. Development and validation of a method for precise dating of female puberty in laboratory rodents: The puberty ovarian maturation score (Pub-Score). Sci. Rep. 2017, 7, 46381. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Buaas, F.W.; Sharma, M.; Snyder, E.; de Rooij, D.G.; Braun, R.E. LIN28A marks the spermatogonial progenitor population and regulates its cyclic expansion. Stem Cells 2014, 32, 860–873. [Google Scholar] [CrossRef]
- Notarnicola, C.; Malki, S.; Berta, P.; Poulat, F.; Boizet-Bonhoure, B. Transient expression of SOX9 protein during follicular development in the adult mouse ovary. Gene Expr. Patterns 2006, 6, 695–702. [Google Scholar] [CrossRef]
- Franca, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- Blanco-Rodriguez, J. gammaH2AX marks the main events of the spermatogenic process. Microsc. Res. Tech. 2009, 72, 823–832. [Google Scholar] [CrossRef]
- Tran, D.; Picard, J.Y.; Campargue, J.; Josso, N. Immunocytochemical detection of anti-mullerian hormone in Sertoli cells of various mammalian species including human. J. Histochem. Cytochem. 1987, 35, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Tsai, M.J.; Tsai, S.Y. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS ONE 2008, 3, e3285. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef]
- Dunleavy, J.E.M.; O’Bryan, M.K.; Stanton, P.G.; O’Donnell, L. The cytoskeleton in spermatogenesis. Reproduction 2019, 157, R53–R72. [Google Scholar] [CrossRef]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014, 4, e979623. [Google Scholar] [CrossRef]
- Ogle, R.A.; Netherton, J.; Schneider, E.; Velkov, T.; Zhang, H.; Cole, N.; Hetherington, L.; Villaverde, A.; Baker, M.A. Nuclear heterogeneity is prevalent in high-quality fractionated human sperm cells typically used for assisted conception. Hum. Reprod. 2021, 36, 2073–2082. [Google Scholar] [CrossRef]
- De Baere, E.; Fellous, M.; Veitia, R.A. The transcription factor FOXL2 in ovarian function and dysfunction. Folia Histochem. Cytobiol. 2009, 47, S43–S49. [Google Scholar] [CrossRef]
- Ottolenghi, C.; Omari, S.; Garcia-Ortiz, J.E.; Uda, M.; Crisponi, L.; Forabosco, A.; Pilia, G.; Schlessinger, D. Foxl2 is required for commitment to ovary differentiation. Hum. Mol. Genet. 2005, 14, 2053–2062. [Google Scholar] [CrossRef]
- Fortune, J.E. The early stages of follicular development: Activation of primordial follicles and growth of preantral follicles. Anim. Reprod. Sci. 2003, 78, 135–163. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K. Cellular and molecular regulation of the activation of mammalian primordial follicles: Somatic cells initiate follicle activation in adulthood. Hum. Reprod. Update 2015, 21, 779–786. [Google Scholar] [CrossRef] [PubMed]
- di Clemente, N.; Josso, N.; Gouedard, L.; Belville, C. Components of the anti-Mullerian hormone signaling pathway in gonads. Mol. Cell. Endocrinol. 2003, 211, 9–14. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef]
- Sato, Y.; Suzuki, T.; Hidaka, K.; Sato, H.; Ito, K.; Ito, S.; Sasano, H. Immunolocalization of nuclear transcription factors, DAX-1 and COUP-TF II, in the normal human ovary: Correlation with adrenal 4 binding protein/steroidogenic factor-1 immunolocalization during the menstrual cycle. J. Clin. Endocrinol. Metab. 2003, 88, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, N.; Kurihara, I.; Lee, K.; Demayo, F.J.; Tsai, M.J.; Tsai, S.Y. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol. Endocrinol. 2005, 19, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Kidder, G.M.; Mhawi, A.A. Gap junctions and ovarian folliculogenesis. Reproduction 2002, 123, 613–620. [Google Scholar] [CrossRef]
- Teilmann, S.C. Differential expression and localisation of connexin-37 and connexin-43 in follicles of different stages in the 4-week-old mouse ovary. Mol. Cell. Endocrinol. 2005, 234, 27–35. [Google Scholar] [CrossRef]
- Gershon, E.; Plaks, V.; Dekel, N. Gap junctions in the ovary: Expression, localization and function. Mol. Cell. Endocrinol. 2008, 282, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M.; Litscher, E.S. Zona Pellucida Genes and Proteins: Essential Players in Mammalian Oogenesis and Fertility. Genes 2021, 12, 1266. [Google Scholar] [CrossRef]
- Mora, J.M.; Fenwick, M.A.; Castle, L.; Baithun, M.; Ryder, T.A.; Mobberley, M.; Carzaniga, R.; Franks, S.; Hardy, K. Characterization and significance of adhesion and junction-related proteins in mouse ovarian follicles. Biol. Reprod. 2012, 86, 153. [Google Scholar] [CrossRef] [PubMed]
- Grondahl, M.L.; Andersen, C.Y.; Bogstad, J.; Borgbo, T.; Boujida, V.H.; Borup, R. Specific genes are selectively expressed between cumulus and granulosa cells from individual human pre-ovulatory follicles. Mol. Hum. Reprod. 2012, 18, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Peluffo, M.C.; Murphy, M.J.; Baughman, S.T.; Stouffer, R.L.; Hennebold, J.D. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: Metalloproteinase involvement in follicle rupture. Endocrinology 2011, 152, 3963–3974. [Google Scholar] [CrossRef]
- Yagi, S.; Shiojiri, N. Identification of novel genetic markers for mouse yolk sac cells by using microarray analyses. Placenta 2017, 49, 68–71. [Google Scholar] [CrossRef]
- Kobayashi, K.; Endo, T.; Matsumura, T.; Lu, Y.; Yu, Z.; Matzuk, M.M.; Ikawa, M. Prss55 but not Prss51 is required for male fertility in micedagger. Biol. Reprod. 2020, 103, 223–234. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Wang, L.; Bu, T.; Liu, S.; Mao, B.; Cheng, C.Y. A local regulatory network in the testis mediated by laminin and collagen fragments that supports spermatogenesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 236–254. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, L.; Li, Y.; Zhang, F.; Xu, Y.; Pan, H.; Wan, X.; Yan, G.; Yu, F.; Li, R. EHD3 positively regulated by NR5A1 participates in testosterone synthesis via endocytosis. Life Sci. 2021, 278, 119570. [Google Scholar] [CrossRef]
- Napoli, J.L. Post-natal all-trans-retinoic acid biosynthesis. Methods Enzymol. 2020, 637, 27–54. [Google Scholar]
- Persson, K.; Heby, O.; Berger, F.G. The functional intronless S-adenosylmethionine decarboxylase gene of the mouse (Amd-2) is linked to the ornithine decarboxylase gene (Odc) on chromosome 12 and is present in distantly related species of the genus Mus. Mamm. Genome 1999, 10, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef]
- Hur, C.G.; Choe, C.; Kim, G.T.; Cho, S.K.; Park, J.Y.; Hong, S.G.; Han, J.; Kang, D. Expression and localization of two-pore domain K(+) channels in bovine germ cells. Reproduction 2009, 137, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Gewehr, C.; da Silva, M.A.; dos Santos, G.T.; Rossato, M.F.; de Oliveira, S.M.; Drewes, C.C.; Pazini, A.M.; Guerra, G.P.; Rubin, M.A.; Ferreira, J. Contribution of peripheral vanilloid receptor to the nociception induced by injection of spermine in mice. Pharmacol. Biochem. Behav. 2011, 99, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kumar, S.; Lee, K. FOXA3, a Negative Regulator of Nur77 Expression and Activity in Testicular Steroidogenesis. Int. J. Endocrinol. 2021, 2021, 6619447. [Google Scholar] [CrossRef]
- Behr, R.; Sackett, S.D.; Bochkis, I.M.; Le, P.P.; Kaestner, K.H. Impaired male fertility and atrophy of seminiferous tubules caused by haploinsufficiency for Foxa3. Dev. Biol. 2007, 306, 636–645. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, Y.; Zheng, B.; Shao, B.; Jiang, M.; Wang, G.; Zhou, T.; Wang, L.; Zhou, Z.; Guo, X.; et al. Establishment of a proteome profile and identification of molecular markers for mouse spermatogonial stem cells. J. Cell. Mol. Med. 2015, 19, 521–534. [Google Scholar] [CrossRef]
- Boyer, A.; Zhang, X.; Levasseur, A.; Abou Nader, N.; St-Jean, G.; Nagano, M.C.; Boerboom, D. Constitutive activation of CTNNB1 results in a loss of spermatogonial stem cell activity in mice. PLoS ONE 2021, 16, e0251911. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, C.; Huang, H.L.; Zeng, M.H.; Yi, D.J.; Tan, H.J.; Peng, T.L.; Yu, W.X.; Deng, H.W.; Xiao, H.M. Influence of mouse defective zona pellucida in folliculogenesis on apoptosis of granulosa cells and developmental competence of oocytesdagger. Biol. Reprod. 2019, 101, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kan, R.; Yurttas, P.; Kim, B.; Jin, M.; Wo, L.; Lee, B.; Gosden, R.; Coonrod, S.A. Regulation of mouse oocyte microtubule and organelle dynamics by PADI6 and the cytoplasmic lattices. Dev. Biol. 2011, 350, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, Y.; Fu, J.; Yu, M.; Feng, R.; Sang, Q.; Liang, B.; Chen, B.; Qu, R.; Li, B.; et al. Mutations in PADI6 Cause Female Infertility Characterized by Early Embryonic Arrest. Am. J. Hum. Genet. 2016, 99, 744–752. [Google Scholar] [CrossRef]
- Ford, E.A.; Frost, E.R.; Beckett, E.L.; Roman, S.D.; McLaughlin, E.A.; Sutherland, J.M. Transcriptomic profiling of neonatal mouse granulosa cells reveals new insights into primordial follicle activationdagger. Biol. Reprod. 2022, 106, 503–514. [Google Scholar] [CrossRef]
- Okunomiya, A.; Horie, A.; Tani, H.; Sato, Y.; Takamatsu, S.; Brown, J.B.; Sugimoto, M.; Hamanishi, J.; Kondoh, E.; Matsumura, N.; et al. Figla promotes secondary follicle growth in mature mice. Sci. Rep. 2021, 11, 9842. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yuan, D.; Rajkovic, A. Germ cell-specific transcriptional regulator sohlh2 is essential for early mouse folliculogenesis and oocyte-specific gene expression. Biol. Reprod. 2008, 79, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Ross, A.; Stallings, N.; Parker, K.L.; Capel, B.; Quaggin, S.E. Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development 2004, 131, 4095–4105. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, L.; Li, J.; Zhu, S.; Tai, M.; Mason, C.W.; Chapman, J.A.; Reynolds, E.A.; Weiner, C.P.; Zhou, H.H. MicroRNA-205 promotes cell invasion by repressing TCF21 in human ovarian cancer. J. Ovarian Res. 2017, 10, 33. [Google Scholar] [CrossRef]
- Del Collado, M.; da Silveira, J.C.; Sangalli, J.R.; Andrade, G.M.; Sousa, L.; Silva, L.A.; Meirelles, F.V.; Perecin, F. Fatty Acid Binding Protein 3 And Transzonal Projections Are Involved In Lipid Accumulation During In Vitro Maturation of Bovine Oocytes. Sci. Rep. 2017, 7, 2645. [Google Scholar] [CrossRef]
- Wieckowski, M.; Ranga, S.; Moison, D.; Messiaen, S.; Abdallah, S.; Granon, S.; Habert, R.; Rouiller-Fabre, V.; Livera, G.; Guerquin, M.J. Unexpected Interacting Effects of Physical (Radiation) and Chemical (Bisphenol A) Treatments on Male Reproductive Functions in Mice. Int. J. Mol. Sci. 2021, 22, 11808. [Google Scholar] [CrossRef]
- Nakamura, N.; Sloper, D.T.; Del Valle, P.L. Gene expression profiling of cultured mouse testis fragments treated with ethinylestradiol. J. Toxicol. Sci. 2019, 44, 667–679. [Google Scholar] [CrossRef]
- Barakat, R.; Lin, P.C.; Park, C.J.; Zeineldin, M.; Zhou, S.; Rattan, S.; Brehm, E.; Flaws, J.A.; Ko, C.J. Germline-dependent transmission of male reproductive traits induced by an endocrine disruptor, di-2-ethylhexyl phthalate, in future generations. Sci. Rep. 2020, 10, 5705. [Google Scholar] [CrossRef]
- Sadler-Riggleman, I.; Klukovich, R.; Nilsson, E.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Epigenetic transgenerational inheritance of testis pathology and Sertoli cell epimutations: Generational origins of male infertility. Environ. Epigenet. 2019, 5, dvz013. [Google Scholar] [CrossRef]
- Prokesova, S.; Ghaibour, K.; Liska, F.; Klein, P.; Fenclova, T.; Stiavnicka, M.; Hosek, P.; Zalmanova, T.; Hoskova, K.; Rimnacova, H.; et al. Acute low-dose bisphenol S exposure affects mouse oocyte quality. Reprod. Toxicol. 2020, 93, 19–27. [Google Scholar] [CrossRef]
- He, Q.L.; Wei, X.Y.; Han, X.Y.; Zhou, Q.; Wang, H.Q.; Ding, N.Z.; Meng, X.Q.; Schatten, H.; Sun, Q.Y.; Liu, S.Z. Effects of 2,3′,4,4′5-pentachlorobiphenyl exposure during pregnancy on epigenetic imprinting and maturation of offspring’s oocytes in mice. Arch. Toxicol. 2019, 93, 2575–2592. [Google Scholar] [CrossRef] [PubMed]
- Guzman, A.; Hughes, C.H.K.; Murphy, B.D. Orphan nuclear receptors in angiogenesis and follicular development. Reproduction 2021, 162, R35–R54. [Google Scholar] [CrossRef]
- Yin, Y.; Cao, S.; Fu, H.; Fan, X.; Xiong, J.; Huang, Q.; Liu, Y.; Xie, K.; Meng, T.G.; Liu, Y.; et al. A noncanonical role of NOD-like receptor NLRP14 in PGCLC differentiation and spermatogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 22237–22248. [Google Scholar] [CrossRef]
- Fan, H.; Ren, Z.; Xu, C.; Wang, H.; Wu, Z.; Rehman, Z.U.; Wu, S.; Sun, M.A.; Bao, W. Chromatin Accessibility and Transcriptomic Alterations in Murine Ovarian Granulosa Cells upon Deoxynivalenol Exposure. Cells 2021, 10, 2818. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Tian, Y.; Li, M.H.; Feng, Y.Q.; Kong, L.; Zhang, F.L.; Shen, W. Single-cell transcriptome dissection of the toxic impact of Di (2-ethylhexyl) phthalate on primordial follicle assembly. Theranostics 2021, 11, 4992–5009. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Klukovich, R.; Sadler-Riggleman, I.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Environmental toxicant induced epigenetic transgenerational inheritance of ovarian pathology and granulosa cell epigenome and transcriptome alterations: Ancestral origins of polycystic ovarian syndrome and primary ovarian insufiency. Epigenet. Off. J. DNA Methylation Soc. 2018, 13, 875–895. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Lamb, D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef]
- Walker, C.; Boisvert, A.; Malusare, P.; Culty, M. Impact of Fetal Exposure to Endocrine Disrupting Chemical Mixtures on FOXA3 Gene and Protein Expression in Adult Rat Testes. Int. J. Mol. Sci. 2023, 24, 1211. [Google Scholar] [CrossRef]
- Alvarez-Cubero, M.J.; Martinez-Gonzalez, L.J.; Saiz, M.; Carmona-Saez, P.; Alvarez, J.C.; Pascual-Geler, M.; Lorente, J.A.; Cozar, J.M. Prognostic role of genetic biomarkers in clinical progression of prostate cancer. Exp. Mol. Med. 2015, 47, e176. [Google Scholar] [CrossRef]
- Ezelle, H.J.; Malathi, K.; Hassel, B.A. The Roles of RNase-L in Antimicrobial Immunity and the Cytoskeleton-Associated Innate Response. Int. J. Mol. Sci. 2016, 17, 74. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, Y.; Gao, Y.C.; Chen, T.Y.; Li, P.C.; Xu, K.; Liu, T.; Ren, T. Targeting DNAJC19 overcomes tumor growth and lung metastasis in NSCLC by regulating PI3K/AKT signaling. Cancer Cell Int. 2021, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Chigi, Y.; Baba, T.; Ohshitanai, A.; Harada, Y.; Takahashi, F.; Morohashi, K.I. Three populations of adult Leydig cells in mouse testes revealed by a novel mouse HSD3B1-specific rat monoclonal antibody. Biochem. Biophys. Res. Commun. 2019, 511, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Olia Bagheri, F.; Alizadeh, A.; Sadighi Gilani, M.A.; Shahhoseini, M. Role of peroxisome proliferator-activated receptor gamma (PPARgamma) in the regulation of fatty acid metabolism related gene expressions in testis of men with impaired spermatogenesis. Reprod. Biol. 2021, 21, 100543. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.E.; Lindegaard, M.L.; Friis-Hansen, L.; Almstrup, K.; Leffers, H.; Nielsen, L.B.; Rajpert-De Meyts, E. Lipoprotein lipase and endothelial lipase in human testis and in germ cell neoplasms. Int. J. Androl. 2010, 33, e207–e215. [Google Scholar] [CrossRef]
- Nilsson, E.; Zhang, B.; Skinner, M.K. Gene bionetworks that regulate ovarian primordial follicle assembly. BMC Genom. 2013, 14, 496. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, X.; Pan, Y.; Shang, L.; Chen, S.; Yang, J.; Jin, L.; Zhang, F.; Wu, Y. Temporal transcriptomic landscape of postnatal mouse ovaries reveals dynamic gene signatures associated with ovarian aging. Hum. Mol. Genet. 2021, 30, 1941–1954. [Google Scholar] [CrossRef]
- Mimouni, N.E.H.; Paiva, I.; Barbotin, A.L.; Timzoura, F.E.; Plassard, D.; Le Gras, S.; Ternier, G.; Pigny, P.; Catteau-Jonard, S.; Simon, V.; et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021, 33, 513–530.e8. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Ungewitter, E.K.; Yao, H.H. How to make a gonad: Cellular mechanisms governing formation of the testes and ovaries. Sex. Dev. 2013, 7, 7–20. [Google Scholar] [CrossRef]
- Pepling, M.E. From primordial germ cell to primordial follicle: Mammalian female germ cell development. Genesis 2006, 44, 622–632. [Google Scholar] [CrossRef]
- Iguchi, T.; Sato, T.; Nakajima, T.; Miyagawa, S.; Takasugi, N. New frontiers of developmental endocrinology opened by researchers connecting irreversible effects of sex hormones on developing organs. Differentiation 2021, 118, 4–23. [Google Scholar] [CrossRef]
- Siu, M.K.; Cheng, C.Y. Extracellular matrix and its role in spermatogenesis. Adv. Exp. Med. Biol. 2008, 636, 74–91. [Google Scholar] [PubMed]
- Coticchio, G.; Dal Canto, M.; Mignini Renzini, M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef]
- Wan, H.T.; Mruk, D.D.; Wong, C.K.; Cheng, C.Y. Targeting testis-specific proteins to inhibit spermatogenesis: Lesson from endocrine disrupting chemicals. Expert Opin. Ther. Targets 2013, 17, 839–855. [Google Scholar] [CrossRef]
- Li, F.P.; Zhou, J.L.; Guo, A.W.; Liu, Y.; Zhang, F.; Xu, B.H.; Liu, R.; Wang, Y.L.; Chen, M.H.; Lin, Y.H.; et al. Di(n-butyl) phthalate exposure impairs meiotic competence and development of mouse oocyte. Environ. Pollut. 2019, 246, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.; Bhushan, S.; Fijak, M.; Meinhardt, A. Mechanism of Inflammatory Associated Impairment of Sperm Function, Spermatogenesis and Steroidogenesis. Front. Endocrinol. 2022, 13, 897029. [Google Scholar] [CrossRef]
- Gong, J.; Zeng, Q.; Yu, D.; Duan, Y.G. T Lymphocytes and Testicular Immunity: A New Insight into Immune Regulation in Testes. Int. J. Mol. Sci. 2020, 22, 57. [Google Scholar] [CrossRef]
- Kristensen, D.M.; Hass, U.; Lesne, L.; Lottrup, G.; Jacobsen, P.R.; Desdoits-Lethimonier, C.; Boberg, J.; Petersen, J.H.; Toppari, J.; Jensen, T.K.; et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum. Reprod. 2011, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.B.; Chalmey, C.; Modick, H.; Jensen, L.S.; Dierkes, G.; Weiss, T.; Jensen, B.A.; Norregard, M.M.; Borkowski, K.; Styrishave, B.; et al. Aniline Is Rapidly Converted Into Paracetamol Impairing Male Reproductive Development. Toxicol. Sci. 2015, 148, 288–298. [Google Scholar] [CrossRef]
- Ruiz-Pino, F.; Miceli, D.; Franssen, D.; Vazquez, M.J.; Farinetti, A.; Castellano, J.M.; Panzica, G.; Tena-Sempere, M. Environmentally Relevant Perinatal Exposures to Bisphenol A Disrupt Postnatal Kiss1/NKB Neuronal Maturation and Puberty Onset in Female Mice. Environ. Health Perspect. 2019, 127, 107011. [Google Scholar] [CrossRef]
- Meng, Y.; Yannan, Z.; Ren, L.; Qi, S.; Wei, W.; Lihong, J. Adverse reproductive function induced by maternal BPA exposure is associated with abnormal autophagy and activating inflamation via mTOR and TLR4/NF-kappaB signaling pathways in female offspring rats. Reprod. Toxicol. 2020, 96, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sekulovski, N.; MacLean, J.A.; Whorton, A.; Hayashi, K. Prenatal Exposure to Bisphenol A Analogues on Female Reproductive Functions in Mice. Toxicol. Sci. 2019, 168, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.C.; Hotchkiss, A.K.; Crofton, K.M.; Gray, L.E., Jr. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol. Sci. 2010, 114, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Derouiche, L.; Keller, M.; Martini, M.; Duittoz, A.H.; Pillon, D. Developmental Exposure to Ethinylestradiol Affects Reproductive Physiology, the GnRH Neuroendocrine Network and Behaviors in Female Mouse. Front. Neurosci. 2015, 9, 463. [Google Scholar] [CrossRef]
- Chakraborty, T.R.; Alicea, E.; Chakraborty, S. Relationships between urinary biomarkers of phytoestrogens, phthalates, phenols, and pubertal stages in girls. Adolesc. Health Med. Ther. 2012, 3, 17–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.X.; Wang, K.; Yan, B.Y.; Li, W. Regulation of testicular steroidogenesis by Foxa3 via transcriptional modulation of ERalpha signaling in type 2 diabetes mellitus (T2DM). Biochem. Biophys. Res. Commun. 2017, 490, 786–793. [Google Scholar] [CrossRef]
- Rossitto, M.; Marchive, C.; Pruvost, A.; Sellem, E.; Ghettas, A.; Badiou, S.; Sutra, T.; Poulat, F.; Philibert, P.; Boizet-Bonhoure, B. Intergenerational effects on mouse sperm quality after in utero exposure to acetaminophen and ibuprofen. FASEB J. 2019, 33, 339–357. [Google Scholar] [CrossRef]
- Wakui, S.; Shirai, M.; Motohashi, M.; Mutou, T.; Oyama, N.; Wempe, M.F.; Takahashi, H.; Inomata, T.; Ikegami, M.; Endou, H.; et al. Effects of in utero exposure to di(n-butyl) phthalate for estrogen receptors alpha, beta, and androgen receptor of Leydig cell on rats. Toxicol. Pathol. 2014, 42, 877–887. [Google Scholar] [CrossRef]
- Zhang, Y.; Piao, Y.; Li, Y.; Song, M.; Tang, P.; Li, C. 4-Nitrophenol induces Leydig cells hyperplasia, which may contribute to the differential modulation of the androgen receptor and estrogen receptor-alpha and -beta expression in male rat testes. Toxicol. Lett. 2013, 223, 228–235. [Google Scholar] [CrossRef]
- Guercio, G.; Saraco, N.; Costanzo, M.; Marino, R.; Ramirez, P.; Berensztein, E.; Rivarola, M.A.; Belgorosky, A. Estrogens in Human Male Gonadotropin Secretion and Testicular Physiology From Infancy to Late Puberty. Front. Endocrinol. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Furr, J.; Lambright, C.R.; Wilson, V.S.; Ryan, B.C.; Gray, L.E., Jr. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol. Sci. 2008, 102, 371–382. [Google Scholar] [CrossRef]
- Pallotti, F.; Pelloni, M.; Gianfrilli, D.; Lenzi, A.; Lombardo, F.; Paoli, D. Mechanisms of Testicular Disruption from Exposure to Bisphenol A and Phtalates. J. Clin. Med. 2020, 9, 471. [Google Scholar] [CrossRef]
- Rossitto, M.; Ollivier, M.; Dejardin, S.; Pruvost, A.; Brun, C.; Marchive, C.; Nguyen, A.L.; Ghettas, A.; Keime, C.; de Massy, B.; et al. In utero exposure to acetaminophen and ibuprofen leads to intergenerational accelerated reproductive aging in female mice. Commun. Biol. 2019, 2, 310. [Google Scholar] [CrossRef]
- Franceschi, R.; Gaudino, R.; Marcolongo, A.; Gallo, M.C.; Rossi, L.; Antoniazzi, F.; Tato, L. Prevalence of polycystic ovary syndrome in young women who had idiopathic central precocious puberty. Fertil. Steril. 2010, 93, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, B.; Meng, M.; Zhao, W.; Wang, D.; Yuan, Y.; Zheng, Y.; Qiu, J.; Li, Y.; Li, G.; et al. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Panel, V.; Ma, X.; Du, C.; Hugendubler, L.; Gavrilova, O.; Liu, A.; McLaughlin, T.; Kaestner, K.H.; Mueller, E. The winged helix transcription factor Foxa3 regulates adipocyte differentiation and depot-selective fat tissue expansion. Mol. Cell. Biol. 2013, 33, 3392–3399. [Google Scholar] [CrossRef]
- Simard, J.; Ricketts, M.L.; Moisan, A.M.; Tardy, V.; Peter, M.; Van Vliet, G.; Morel, Y. A new insight into the molecular basis of 3beta-hydroxysteroid dehydrogenase deficiency. Endocr. Res. 2000, 26, 761–770. [Google Scholar] [CrossRef]
- Hughes, L.A.; McKay-Bounford, K.; Webb, E.A.; Dasani, P.; Clokie, S.; Chandran, H.; McCarthy, L.; Mohamed, Z.; Kirk, J.M.W.; Krone, N.P.; et al. Next generation sequencing (NGS) to improve the diagnosis and management of patients with disorders of sex development (DSD). Endocr. Connect. 2019, 8, 100–110. [Google Scholar] [CrossRef]
- Ata, A.; Ozen, S.; Onay, H.; Uzun, S.; Goksen, D.; Ozkinay, F.; Ozbaran, N.B.; Ulman, I.; Darcan, S. A large cohort of disorders of sex development and their genetic characteristics: 6 novel mutations in known genes. Eur. J. Med. Genet. 2021, 64, 104154. [Google Scholar] [CrossRef]
- Reith, W.; LeibundGut-Landmann, S.; Waldburger, J.M. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 2005, 5, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, J.; Wang, X.; Yang, J.; Chen, D.; Huff, V.; Liu, Y.X. Wt1 functions in ovarian follicle development by regulating granulosa cell differentiation. Hum. Mol. Genet. 2014, 23, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, G.; Zhang, J.; Gao, F.; Li, W.; Qin, Y.; Chen, Z.J. Novel WT1 Missense Mutations in Han Chinese Women with Premature Ovarian Failure. Sci. Rep. 2015, 5, 13983. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.J.; Patino, L.C.; Suarez, Y.C.; de Jesus Rodriguez, A.; Mateus, H.E.; Jimenez, K.M.; Ortega-Recalde, O.; Diaz-Yamal, I.; Laissue, P. Next generation sequencing in women affected by nonsyndromic premature ovarian failure displays new potential causative genes and mutations. Fertil. Steril. 2015, 104, 154–162.e2. [Google Scholar] [CrossRef]
- Asghari, R.; Shokri-Asl, V.; Rezaei, H.; Tavallaie, M.; Khafaei, M.; Abdolmaleki, A.; Majdi Seghinsara, A. Alteration of TGFB1, GDF9, and BMPR2 gene expression in preantral follicles of an estradiol valerate-induced polycystic ovary mouse model can lead to anovulation, polycystic morphology, obesity, and absence of hyperandrogenism. Clin. Exp. Reprod. Med. 2021, 48, 245–254. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Reed, D.R.; Beauchamp, G.K.; Tordoff, M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef]
- Davidson, I.W.; Parker, J.C.; Beliles, R.P. Biological basis for extrapolation across mammalian species. Regul. Toxicol. Pharmacol. 1986, 6, 211–237. [Google Scholar] [CrossRef]
- Moniot, B.; Declosmenil, F.; Barrionuevo, F.; Scherer, G.; Aritake, K.; Malki, S.; Marzi, L.; Cohen-Solal, A.; Georg, I.; Klattig, J.; et al. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development 2009, 136, 1813–1821. [Google Scholar] [CrossRef]
- Rossitto, M.; Dejardin, S.; Rands, C.M.; Le Gras, S.; Migale, R.; Rafiee, M.R.; Neirijnck, Y.; Pruvost, A.; Nguyen, A.L.; Bossis, G.; et al. TRIM28-dependent SUMOylation protects the adult ovary from activation of the testicular pathway. Nat. Commun. 2022, 13, 4412. [Google Scholar] [CrossRef]
- Welsh, M.; Saunders, P.T.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Smith, L.B.; Sharpe, R.M. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Investig. 2008, 118, 1479–1490. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Mouritsen, A.; Aksglaede, L.; Sorensen, K.; Mogensen, S.S.; Leffers, H.; Main, K.M.; Frederiksen, H.; Andersson, A.M.; Skakkebaek, N.E.; Juul, A. Hypothesis: Exposure to endocrine-disrupting chemicals may interfere with timing of puberty. Int. J. Androl. 2010, 33, 346–359. [Google Scholar] [CrossRef] [PubMed]



| F1 Animals | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | D1 | D2 | Control | D1 | D2 | |||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Body weight at 21 dpp (g) (n) | 7.739 (51) | 0.229 | 9.557 a (30) | 0.263 | 8.865 c (37) | 0.299 | 7.46 (40) | 0.247 | 8.693 c (30) | 0.256 | 8.065 (43) | 0.275 |
| Body weight at 35/30 dpp (g) (n) | 27.29 (11) | 0.666 | 29.71 (8) | 0.757 | 28.37 (10) | 1.438 | 19.58 (5) | 0.685 | 21.06 (5) | 0.933 | 19.76 (5) | 0.903 |
| Gonad weight at 35/30 dpp (mg) (n) | 79.91 (11) | 4.308 | 86.21 (8) | 3.982 | 85.85 (6) | 6.009 | 2.96 (5) | 0.326 | 2.3 (3) | 0.321 | 1.94 (5) | 0.188 |
| Gonad weight/BW ratio (10−3) (n) | 2.912 (11) | 0.107 | 2.919 (8) | 0.168 | 3.072 (6) | 0.124 | 1.508 (5) | 0.156 | 1.082 (3) | 0.157 | 0.981 d (5) | 0.089 |
| AGD/BW ratio at 21 dpp (10−3) (n) | 1.017 (51) | 0.881 a (30) | 0.906 a (37) | 0.565 (40) | 0.013 | 0.541 (30) | 0.013 | 0.552 (43) | 0.012 | |||
| AGD/BW ratio at 35 dpp (10−3) (n) | 0.4802 | 0.011 | 0.472 | 0.015 | 0.466 | 0.014 | - | - | - | - | - | - |
| F2 Males | CxC | CxD1 | CxD2 | D1xC | D2xC | D2xD2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Body weight at 21 dpp (g) (n) | 12.27 (22) | 0.524 | 12.41 (22) | 0.376 | 11.97 (35) | 0.357 | 10.22 c (42) | 0.368 | 11.27 (37) | 0.30 | 12.31 (32) | 0.439 |
| AGD/BW ratio at 21 dpp (10−3) (n) | 0.7509 (22) | 0.02 | 0.7402 (28) | 0.011 | 0.762 (35) | 0.017 | 0.802 (41) | 0.0178 | 0.797 (37) | 0.014 | 0.758 (32) | 0.016 |
| Body weight at 30 dpp (g) (n) | 30.93 (6) | 1.248 | 29.20 (5) | 0.671 | 27.5 (6) | 1.018 | 25.96 (7) | 2.189 | 28.20 (6) | 1.213 | 29.93 (6) | 0.908 |
| Testis weight at 30 dpp (mg) (n) | 95.60 (6) | 4.84 | 92.06 (5) | 4.362 | 85.43 (6) | 3.927 | 73.14 d (7) | 7.608 | 83.83 (6) | 5.799 | 83.92 (6) | 2.289 |
| Testis weight/BW ratio (10−3) (n) | 3.083 (6) | 0.04 | 3.162 (5) | 0.177 | 3.113 (6) | 0.121 | 2.807 (7) | 0.198 | 2.957 (6) | 0.093 | 2.810 (6) | 0.077 |
| F2 Females | CxC | CxD1 | CxD2 | D1xC | D2xC | D2xD2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Body Weight 21 dpp (g) (n) | 12.93 (10) | 0.847 | 11.39 (15) | 0.511 | 10.92 d (34) | 0.352 | 10.92 d (25) | 0.357 | 10.53 b (30) | 0.306 | 11.48 (32) | 0.45 |
| AGD/BW ratio at 21 dpp (10−3) (n) | 0.4438 (11) | 0.024 | 0.4641 (21) | 0.016 | 0.457 (34) | 0.014 | 0.463 (25) | 0.013 | 0.495 (30) | 0.015 | 0.458 (32) | 0.013 |
| Body weight at 30 dpp (g) (n) | 21.25 (4) | 2.382 | 19.30 (5) | 1.449 | 16.87 (7) | 1.15 | 21 (6) | 0.859 | 19.67 (6) | 1.051 | 20.06 (7) | 0.598 |
| Ovary weight at 30 dpp (mg) (n) | 1.525 (4) | 0.154 | 1.38 (5) | 0.162 | 1.3 (7) | 0.198 | 1.883 (6) | 0.122 | 1.48 (5) | 0.156 | 1.617 (6) | 0.282 |
| Ovary weight/BW ratio (10−3) (n) | 0.0769 (4) | 0.016 | 0.0719 (5) | 0.0071 | 0.0743 (7) | 0.0078 | 0.0906 (6) | 0.006 | 0.0079 (5) | 0.009 | 0.0781 (6) | 0.001 |
| VO dpp | F1 Females | F2 Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| %VO | Ctrl | D1 | D2 | CxC | CxD1 | CxD2 | D1xC | D2xC | D2xD2 |
| onset | 28 | 28 | 25–26 | 28 | 28 | 28 | 28 | 27 | 26 |
| 40% | 35 | 34 | 32 | 33 | 33 | 34 | 34 | 34 | 31 |
| 60% | 36 | 35 | 33 | 34 | 35 | 36 | 36 | 35 | 32 |
| 80% | 38 | 36 | 35 | 36 | 36 | 38 | 37 | 37 | 33 |
| 90% | 39 | 37 | 36 | 38 | 38 | 38 | 38 | 37 | 34 |
| CAS Number | Concentrations in Drinking Water (ng/L) | Human Exposure (ng/kg/Day) | Mouse Exposure (ng/kg/Day) | Exposure Concentration in Mice (ng/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Maximum | Average | Maximum | Average | Maximum | Dose1 (D1) | Dose2 (D2) | ||
| IBU | 15687-27-1 | 5 | 50–100 | 0.17 | 1.7 | 0.068 | 0.68 | 11.3 | 113 |
| 2h-IBU | 51146-55-5 | 40 | 85–100 | 1.36 | 3.4 | 0.544 | 1.2 | 90 | 225 |
| DCF | 15307-86-5 | 10 | 50 | 0.34 | 1.7 | 0.136 | 0.68 | 22.5 | 112 |
| EE2 | 57-63-6 | 1–2 | 20–50 | 0.068 | 0.68 | 0.0136 | 0.27 | 2.25 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philibert, P.; Déjardin, S.; Girard, M.; Durix, Q.; Gonzalez, A.-A.; Mialhe, X.; Tardat, M.; Poulat, F.; Boizet-Bonhoure, B. Cocktails of NSAIDs and 17α Ethinylestradiol at Environmentally Relevant Doses in Drinking Water Alter Puberty Onset in Mice Intergenerationally. Int. J. Mol. Sci. 2023, 24, 5890. https://doi.org/10.3390/ijms24065890
Philibert P, Déjardin S, Girard M, Durix Q, Gonzalez A-A, Mialhe X, Tardat M, Poulat F, Boizet-Bonhoure B. Cocktails of NSAIDs and 17α Ethinylestradiol at Environmentally Relevant Doses in Drinking Water Alter Puberty Onset in Mice Intergenerationally. International Journal of Molecular Sciences. 2023; 24(6):5890. https://doi.org/10.3390/ijms24065890
Chicago/Turabian StylePhilibert, Pascal, Stéphanie Déjardin, Mélissa Girard, Quentin Durix, Anne-Alicia Gonzalez, Xavier Mialhe, Mathieu Tardat, Francis Poulat, and Brigitte Boizet-Bonhoure. 2023. "Cocktails of NSAIDs and 17α Ethinylestradiol at Environmentally Relevant Doses in Drinking Water Alter Puberty Onset in Mice Intergenerationally" International Journal of Molecular Sciences 24, no. 6: 5890. https://doi.org/10.3390/ijms24065890
APA StylePhilibert, P., Déjardin, S., Girard, M., Durix, Q., Gonzalez, A.-A., Mialhe, X., Tardat, M., Poulat, F., & Boizet-Bonhoure, B. (2023). Cocktails of NSAIDs and 17α Ethinylestradiol at Environmentally Relevant Doses in Drinking Water Alter Puberty Onset in Mice Intergenerationally. International Journal of Molecular Sciences, 24(6), 5890. https://doi.org/10.3390/ijms24065890







