DNA Damage Response−Related Proteins Are Prognostic for Outcome in Both Adult and Pediatric Acute Myelogenous Leukemia Patients: Samples from Adults and from Children Enrolled in a Children’s Oncology Group Study
Abstract
1. Introduction
2. Results
2.1. DDR Protein Expression and Activation Status Are Abnormal in Adult AML
2.2. DDR Protein Expression Correlates with Clinical Characteristics, and Is Prognostic for Remission Duration (RD) in Adult AML
2.3. A Subgroup of DDR Proteins Is Prognostic for Overall Survival (OS)
2.4. Seven DDR Proteins Are Individually Prognostic for Either RD or OS
2.5. Novel DDR and DDR-Related Protein Groupings Are Prognostic for OS
2.6. DDR-Related Proteins Are Prognostic for OS in Patients Receiving Either Conventional Chemotherapy (CC) or Venetoclax plus Hypomethylating Agent (HMA) Therapy (VH)
2.7. DDR Protein Expression and Activation Status Are Abnormal in Pediatric AML
2.8. DDR Protein Expression Reveals Five Pediatric Clusters with Significantly Different Prognoses
3. Discussion
4. Methods
4.1. Sample Collection and Processing
4.2. RPPA Methodology
4.3. Selected DDR and DDR-Related Proteins
4.4. Proteomic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lagunas-Rangel, F.A.; Chávez-Valencia, V.; Gómez-Guijosa, M.; Cortes-Penagos, C. Acute Myeloid Leukemia-Genetic Alterations and Their Clinical Prognosis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 328–339. [Google Scholar] [PubMed]
- Yohe, S. Molecular Genetic Markers in Acute Myeloid Leukemia. J. Clin. Med. 2015, 4, 460–478. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Ganser, A. Treatment of Relapsed Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2020, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Ijsselsteijn, R.; Jansen, J.G.; de Wind, N. DNA mismatch repair-dependent DNA damage responses and cancer. DNA Repair 2020, 93, 102923. [Google Scholar] [CrossRef]
- Biechonski, S.; Yassin, M.; Milyavsky, M. DNA-damage response in hematopoietic stem cells: An evolutionary trade-off between blood regeneration and leukemia suppression. Carcinogenesis 2017, 38, 367–377. [Google Scholar] [CrossRef]
- Deriano, L.; Guipaud, O.; Merle-Béral, H.; Binet, J.L.; Ricoul, M.; Potocki-Veronese, G.; Favaudon, V.; Maciorowski, Z.; Muller, C.; Salles, B.; et al. Human chronic lymphocytic leukemia B cells can escape DNA damage-induced apoptosis through the nonhomologous end-joining DNA repair pathway. Blood 2005, 105, 4776–4783. [Google Scholar] [CrossRef]
- Stetka, J.; Gursky, J.; Liñan Velasquez, J.; Mojzikova, R.; Vyhlidalova, P.; Vrablova, L.; Bartek, J.; Divoky, V. Role of DNA Damage Response in Suppressing Malignant Progression of Chronic Myeloid Leukemia and Polycythemia Vera: Impact of Different Oncogenes. Cancers 2020, 12, 903. [Google Scholar] [CrossRef]
- Nilles, N.; Fahrenkrog, B. Taking a Bad Turn: Compromised DNA Damage Response in Leukemia. Cells 2017, 6, 11. [Google Scholar] [CrossRef]
- Hoff, F.W.; Van Dijk, A.D.; Qiu, Y.; Hu, C.W.; Ries, R.E.; Ligeralde, A.; Jenkins, G.N.; Gerbing, R.B.; Gamis, A.S.; Aplenc, R.; et al. Clinical relevance of proteomic profiling in de novo pediatric acute myeloid leukemia: A Children’s Oncology Group study. Haematologica 2022, 107, 2329–2343. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef] [PubMed]
- Kakoti, S.; Sato, H.; Laskar, S.; Yasuhara, T.; Shibata, A. DNA Repair and Signaling in Immune-Related Cancer Therapy. Front. Mol. Biosci. 2020, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Mouw, K.W.; Konstantinopoulos, P.A. From checkpoint to checkpoint: DNA damage ATR/Chk1 checkpoint signalling elicits PD-L1 immune checkpoint activation. Br. J. Cancer 2018, 118, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shih, D.J.H.; Lin, S.Y. Role of DNA repair defects in predicting immunotherapy response. Biomark. Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Yu, D.S.; Cortez, D. A role for CDK9-cyclin K in maintaining genome integrity. Cell Cycle 2011, 10, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, M.; Gutierrez-Martinez, P.; Swat, A.; Nebreda, A.R.; Fernandez-Capetillo, O. p27Kip1 stabilization is essential for the maintenance of cell cycle arrest in response to DNA damage. Cancer Res. 2009, 69, 8726–8732. [Google Scholar] [CrossRef] [PubMed]
- Liontos, M.; Velimezi, G.; Pateras, I.S.; Angelopoulou, R.; Papavassiliou, A.G.; Bartek, J.; Gorgoulis, V.G. The roles of p27(Kip1) and DNA damage signalling in the chemotherapy-induced delayed cell cycle checkpoint. J. Cell. Mol. Med. 2010, 14, 2264–2267. [Google Scholar] [CrossRef]
- Pruitt, S.C.; Freeland, A.; Rusiniak, M.E.; Kunnev, D.; Cady, G.K. Cdkn1b overexpression in adult mice alters the balance between genome and tissue ageing. Nat. Commun. 2013, 4, 2626. [Google Scholar] [CrossRef]
- Bencivenga, D.; Tramontano, A.; Borgia, A.; Negri, A.; Caldarelli, I.; Oliva, A.; Perrotta, S.; Della Ragione, F.; Borriello, A. P27Kip1 serine 10 phosphorylation determines its metabolism and interaction with cyclin-dependent kinases. Cell Cycle 2014, 13, 3768–3782. [Google Scholar] [CrossRef]
- Schiappacassi, M.; Lovisa, S.; Lovat, F.; Fabris, L.; Colombatti, A.; Belletti, B.; Baldassarre, G. Role of T198 modification in the regulation of p27(Kip1) protein stability and function. PLoS ONE 2011, 6, e17673. [Google Scholar] [CrossRef]
- Sun, C.; Wang, G.; Wrighton, K.H.; Lin, H.; Songyang, Z.; Feng, X.H.; Lin, X. Regulation of p27(Kip1) phosphorylation and G1 cell cycle progression by protein phosphatase PPM1G. Am. J. Cancer Res. 2016, 6, 2207–2220. [Google Scholar]
- Potrony, M.; Haddad, T.S.; Tell-Martí, G.; Gimenez-Xavier, P.; Leon, C.; Pevida, M.; Mateu, J.; Badenas, C.; Carrera, C.; Malvehy, J.; et al. DNA Repair and Immune Response Pathways Are Deregulated in Melanocyte-Keratinocyte Co-cultures Derived From the Healthy Skin of Familial Melanoma Patients. Front. Med. 2021, 8, 692341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sharpless, N.; Chang, S. p16(INK4a) protects against dysfunctional telomere-induced ATR-dependent DNA damage responses. J. Clin. Investig. 2013, 123, 4489–4501. [Google Scholar] [CrossRef]
- Le Gac, G.; Estève, P.O.; Ferec, C.; Pradhan, S. DNA damage-induced down-regulation of human Cdc25C and Cdc2 is mediated by cooperation between p53 and maintenance DNA (cytosine-5) methyltransferase 1. J. Biol. Chem. 2006, 281, 24161–24170. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, M.; Lu, R.; Du, J.; Zhao, Q.; Li, Z.; Li, Y.; Zhang, S. The role of CDC25C in cell cycle regulation and clinical cancer therapy: A systematic review. Cancer Cell Int. 2020, 20, 213. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Mojzych, M.; Kontek, R. Cyclin-dependent kinases in DNA damage response. Biochim. Et Biophys. Acta. Rev. Cancer 2022, 1877, 188716. [Google Scholar] [CrossRef] [PubMed]
- Mayya, V.; Rezual, K.; Wu, L.; Fong, M.B.; Han, D.K. Absolute quantification of multisite phosphorylation by selective reaction monitoring mass spectrometry: Determination of inhibitory phosphorylation status of cyclin-dependent kinases. Mol. Cell. Proteom. MCP 2006, 5, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Poon, R.Y.C. Aurora kinases and DNA damage response. Mutat. Res. 2020, 821, 111716. [Google Scholar] [CrossRef]
- Molinaro, C.; Martoriati, A.; Cailliau, K. Proteins from the DNA Damage Response: Regulation, Dysfunction, and Anticancer Strategies. Cancers 2021, 13, 3819. [Google Scholar] [CrossRef]
- Bullinger, L.; Rücker, F.G.; Kurz, S.; Du, J.; Scholl, C.; Sander, S.; Corbacioglu, A.; Lottaz, C.; Krauter, J.; Fröhling, S.; et al. Gene-expression profiling identifies distinct subclasses of core binding factor acute myeloid leukemia. Blood 2007, 110, 1291–1300. [Google Scholar] [CrossRef]
- Schoch, C.; Kern, W.; Kohlmann, A.; Hiddemann, W.; Schnittger, S.; Haferlach, T. Acute myeloid leukemia with a complex aberrant karyotype is a distinct biological entity characterized by genomic imbalances and a specific gene expression profile. Genes Chromosom. Cancer 2005, 43, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Boudny, M.; Zemanova, J.; Khirsariya, P.; Borsky, M.; Verner, J.; Cerna, J.; Oltova, A.; Seda, V.; Mraz, M.; Jaros, J.; et al. Novel CHK1 inhibitor MU380 exhibits significant single-agent activity in TP53-mutated chronic lymphocytic leukemia cells. Haematologica 2019, 104, 2443–2455. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chehab, N.H.; Pavletich, N.P. Structure and activation mechanism of the CHK2 DNA damage checkpoint kinase. Mol Cell 2009, 35, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.T.; So, C.W. DNA damage accumulation and repair defects in acute myeloid leukemia: Implications for pathogenesis, disease progression, and chemotherapy resistance. Chromosoma 2014, 123, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jia, R.; Wang, L.; Yang, Q.; Hu, X.; Fu, Q.; Zhang, X.; Li, W.; Ren, Y. The Emerging Roles of Rad51 in Cancer and Its Potential as a Therapeutic Target. Front Oncol 2022, 12, 935593. [Google Scholar] [CrossRef]
- Yang, M.; Tian, X.; Fan, Z.; Yu, W.; Li, Z.; Zhou, J.; Zhang, W.; Liang, A. Targeting RAD51 enhances chemosensitivity of adult T-cell leukemia-lymphoma cells by reducing DNA double-strand break repair. Oncol. Rep. 2019, 42, 2426–2434. [Google Scholar] [CrossRef]
- Mazzocca, A. The Systemic-Evolutionary Theory of the Origin of Cancer (SETOC): A New Interpretative Model of Cancer as a Complex Biological System. Int. J. Mol. Sci. 2019, 20, 4885. [Google Scholar] [CrossRef]
- Cavelier, C.; Didier, C.; Prade, N.; Mansat-De Mas, V.; Manenti, S.; Recher, C.; Demur, C.; Ducommun, B. Constitutive activation of the DNA damage signaling pathway in acute myeloid leukemia with complex karyotype: Potential importance for checkpoint targeting therapy. Cancer Res. 2009, 69, 8652–8661. [Google Scholar] [CrossRef]
- Lindvall, C.; Furge, K.; Björkholm, M.; Guo, X.; Haab, B.; Blennow, E.; Nordenskjöld, M.; Teh, B.T. Combined genetic and transcriptional profiling of acute myeloid leukemia with normal and complex karyotypes. Haematologica 2004, 89, 1072–1081. [Google Scholar]
- Liu, Q.; Gao, J.; Zhao, C.; Guo, Y.; Wang, S.; Shen, F.; Xing, X.; Luo, Y. To control or to be controlled? Dual roles of CDK2 in DNA damage and DNA damage response. DNA Repair 2020, 85, 102702. [Google Scholar] [CrossRef]
- Satyanarayana, A.; Kaldis, P. A dual role of Cdk2 in DNA damage response. Cell Div. 2009, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Arnold, C.; Thoma, J.; Rohde, C.; Kholmatov, M.; Garg, S.; Hsiao, C.C.; Viol, L.; Zhang, K.; Sun, R.; et al. CDK7/12/13 inhibition targets an oscillating leukemia stem cell network and synergizes with venetoclax in acute myeloid leukemia. EMBO Mol. Med. 2022, 14, e14990. [Google Scholar] [CrossRef] [PubMed]
- Moro, L.; Arbini, A.A.; Marra, E.; Greco, M. Down-regulation of BRCA2 expression by collagen type I promotes prostate cancer cell proliferation. J. Biol. Chem. 2014, 289, 17423. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Nussenzweig, A.; Lubinski, J.; Byrski, T.; Eisen, A.; Bordeleau, L.; Tung, N.M.; Manoukian, S.; Phelan, C.M.; Sun, P.; et al. The incidence of leukaemia in women with BRCA1 and BRCA2 mutations: An International Prospective Cohort Study. Br. J. Cancer 2016, 114, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Boffo, S.; Damato, A.; Alfano, L.; Giordano, A. CDK9 inhibitors in acute myeloid leukemia. J. Exp. Clin. Cancer Res. CR 2018, 37, 36. [Google Scholar] [CrossRef]
- Aplenc, R.; Meshinchi, S.; Sung, L.; Alonzo, T.; Choi, J.; Fisher, B.; Gerbing, R.; Hirsch, B.; Horton, T.; Kahwash, S.; et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: A report from the Children’s Oncology Group. Haematologica 2020, 105, 1879–1886. [Google Scholar] [CrossRef]
- Kornblau, S.M.; Coombes, K.R. Use of reverse phase protein microarrays to study protein expression in leukemia: Technical and methodological lessons learned. Methods Mol. Biol. 2011, 785, 141–155. [Google Scholar]
- Kornblau, S.M.; Womble, M.; Cade, J.S.; Lemker, E.; Qiu, Y.H. Comparative analysis of the effects of sample source and test methodology on the assessment of protein expression in acute myelogenous leukemia. Leukemia 2005, 19, 1550–1557. [Google Scholar] [CrossRef]
- Tibes, R.; Qiu, Y.; Lu, Y.; Hennessy, B.; Andreeff, M.; Mills, G.B.; Kornblau, S.M. Reverse phase protein array: Validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol. Cancer Ther. 2006, 5, 2512–2521. [Google Scholar] [CrossRef]
- Hu, C.W.; Qiu, Y.; Ligeralde, A.; Raybon, A.Y.; Yoo, S.Y.; Coombes, K.R.; Qutub, A.A.; Kornblau, S.M. A quantitative analysis of heterogeneities and hallmarks in acute myelogenous leukaemia. Nat. Biomed. Eng. 2019, 3, 889–901. [Google Scholar] [CrossRef]
- Hu, J.; He, X.; Baggerly, K.A.; Coombes, K.R.; Hennessy, B.T.; Mills, G.B. Non-parametric quantification of protein lysate arrays. Bioinformatics 2007, 23, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T. A Package for Survival Analysis in R; R Package Version 3.5-5; R Foundation: Vienna, Austria, 2023. [Google Scholar]
- Therneau, T.G.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
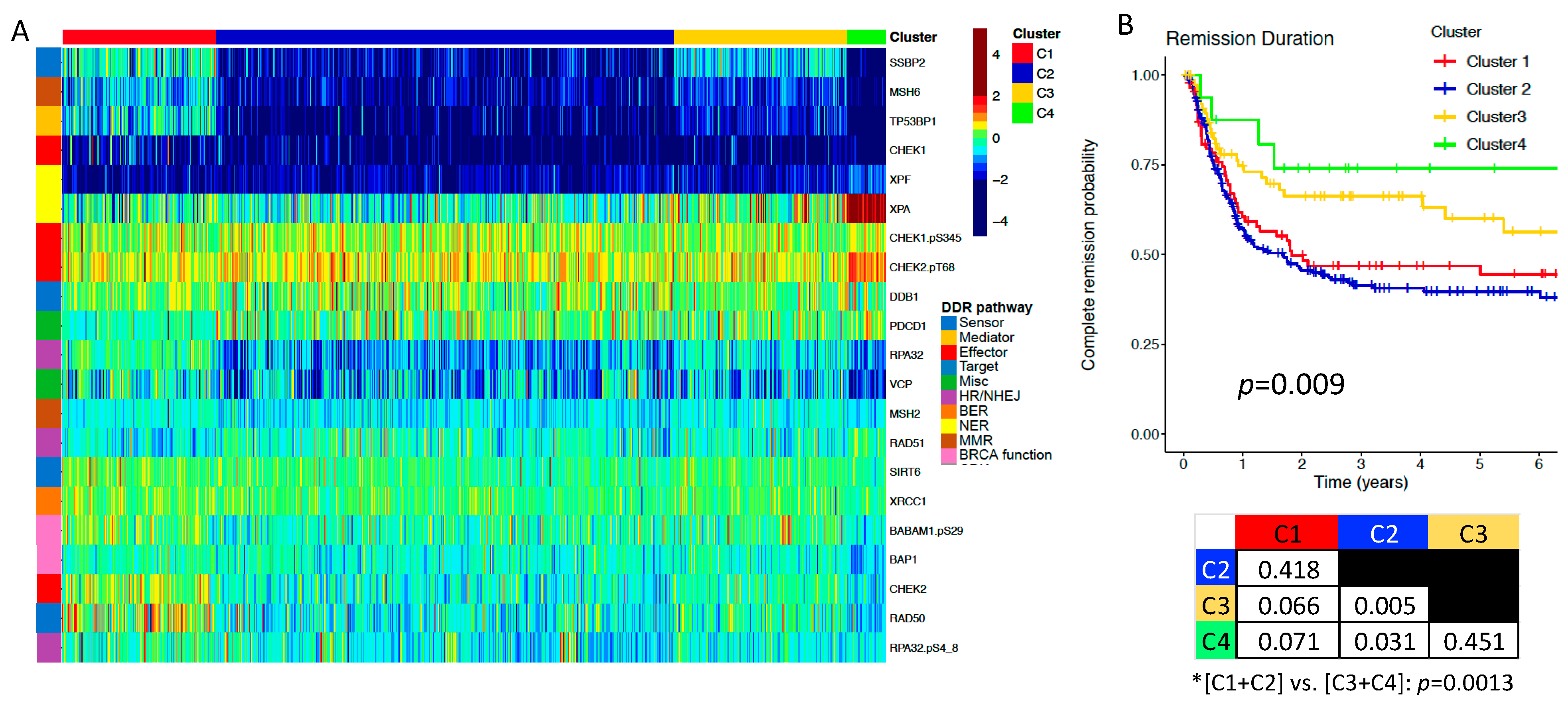
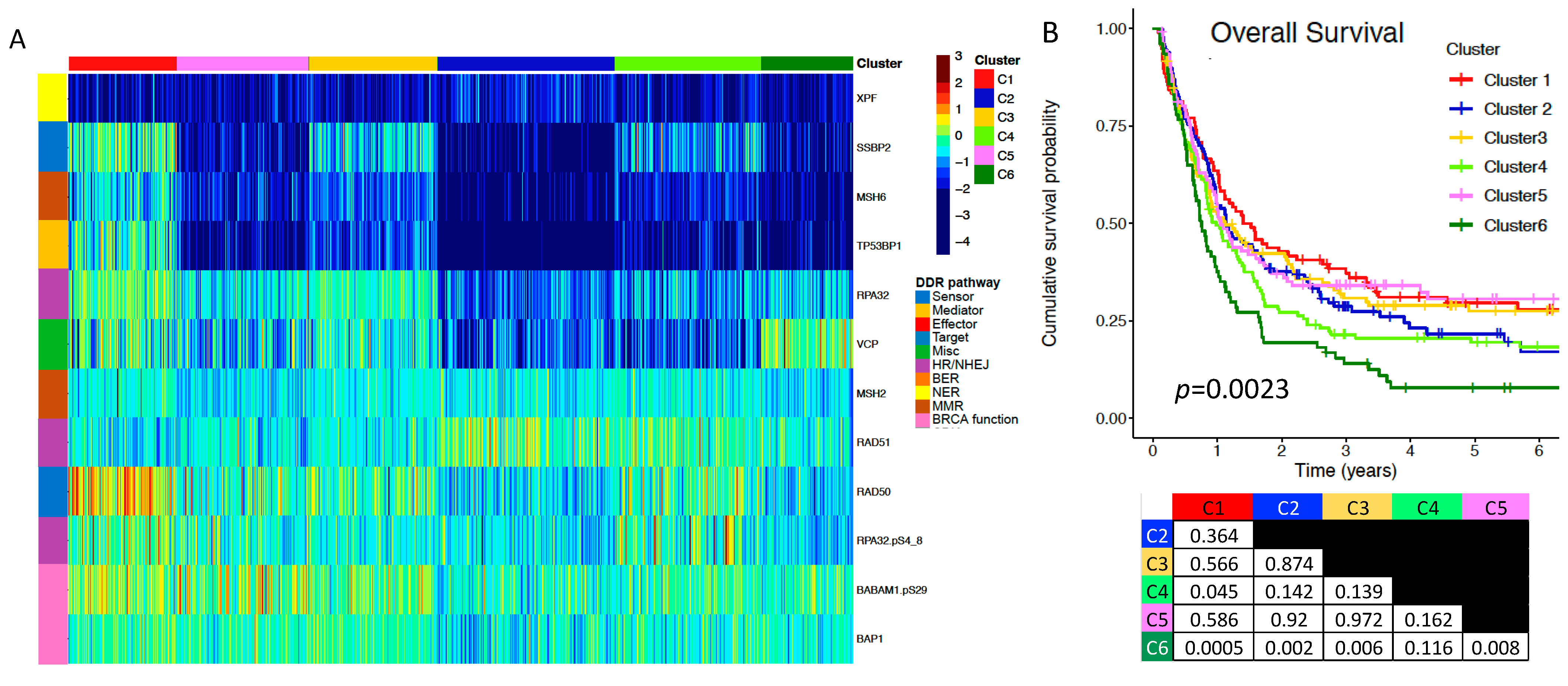
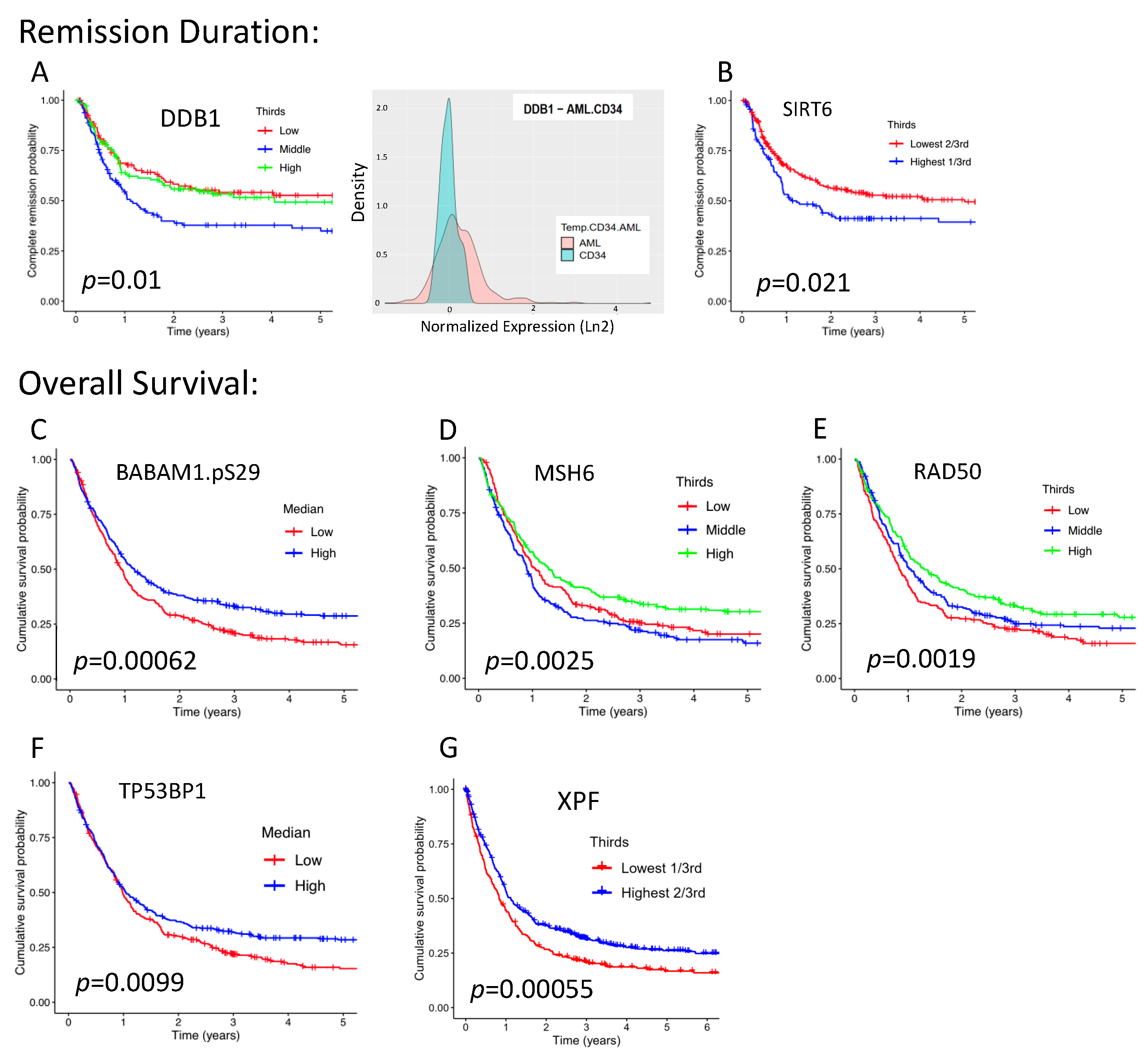
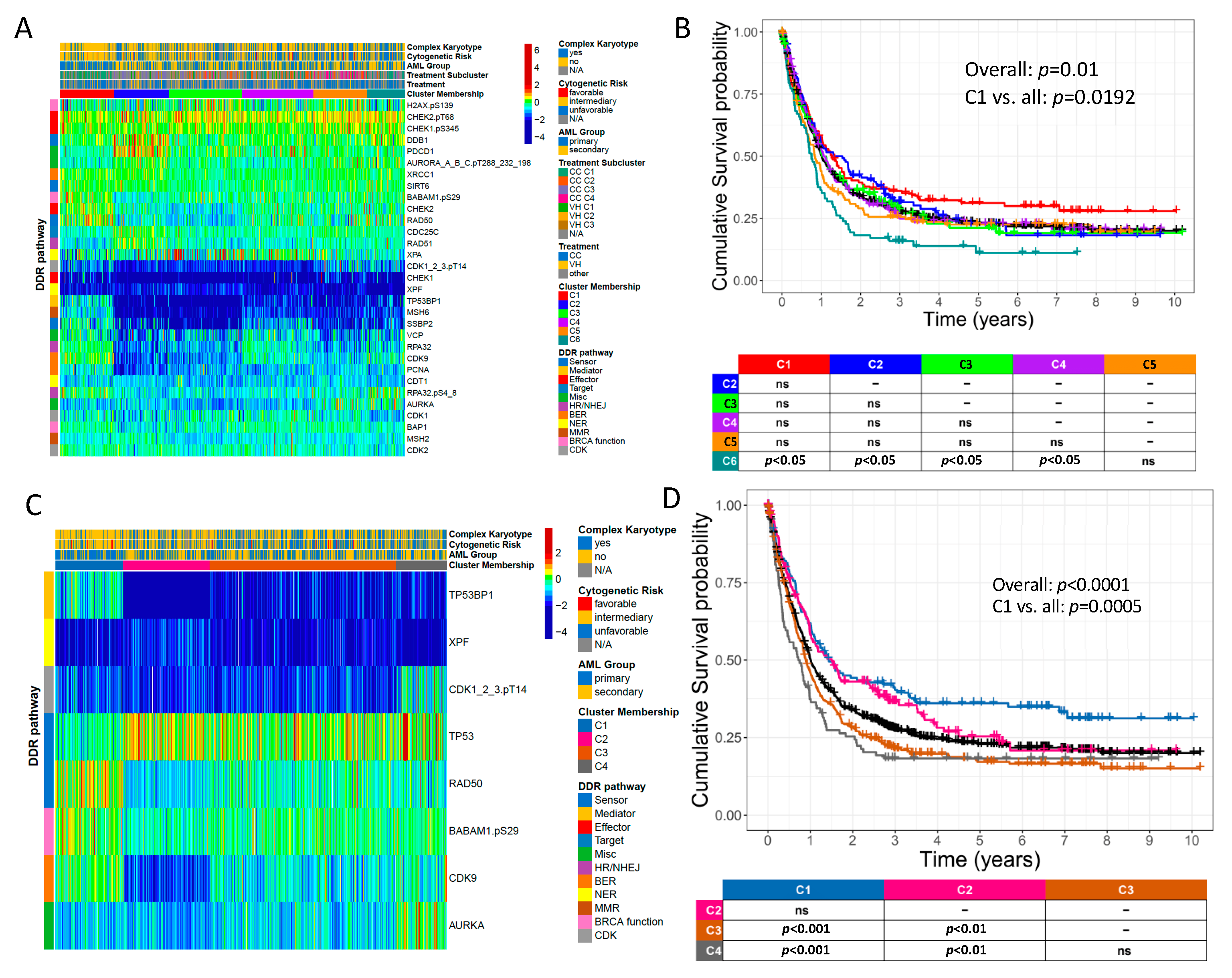

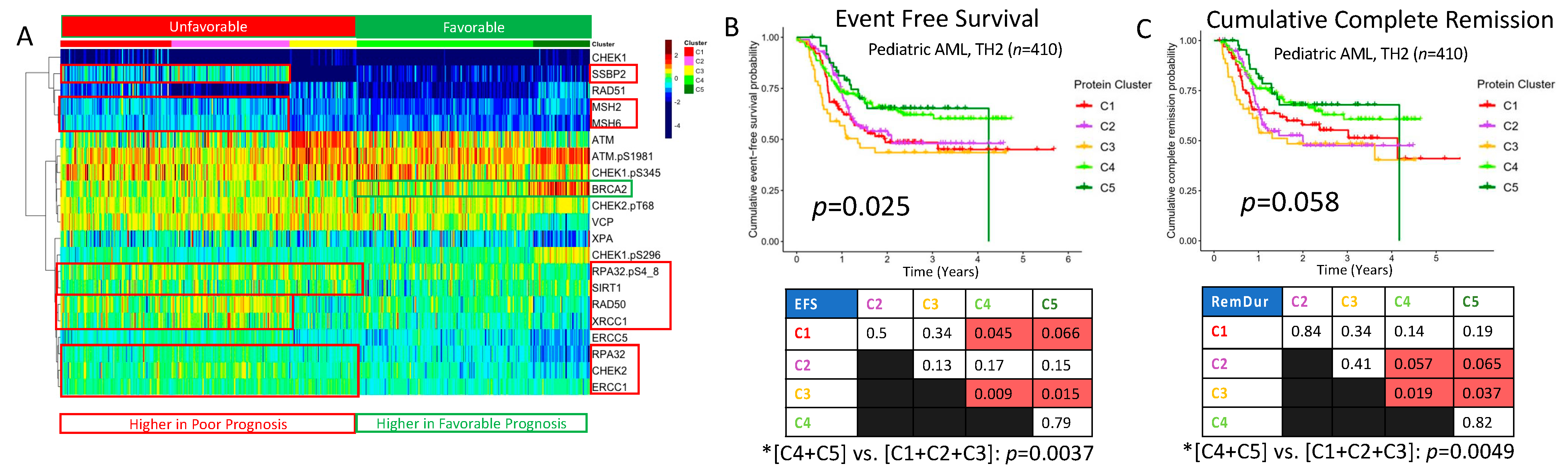
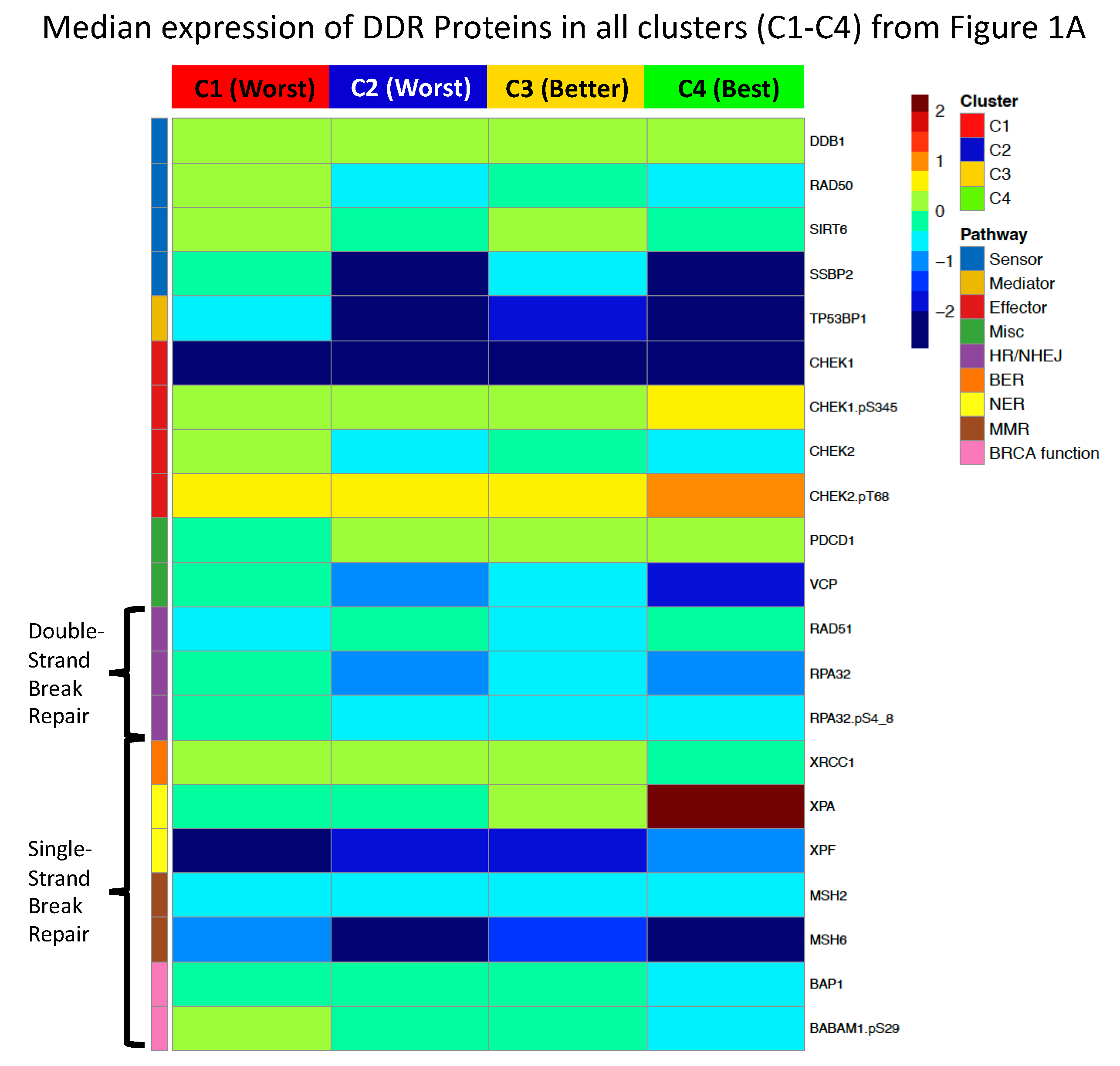
| Adult Patients | ||||||
|---|---|---|---|---|---|---|
| Expression in AML Patents Relative to Normal CD34+ Control Range | ||||||
| Protein | AML Median | Below | Within | Above | % Above or Below | CD34 Range |
| BAP1 | −0.1801 | 26% | 67% | 7% | 33% | [−0.4494, 0.6957] |
| CHEK1 | −0.3171 | 43% | 54% | 4% | 46% | [−0.3685, 0.3009] |
| CHEK1.pS345 | −2.4923 | 93% | 7% | 0% | 93% | [−1.3991, 0.9762] |
| CHEK2 | 0.3325 | 1% | 54% | 45% | 46% | [−0.2368, 0.3813] |
| CHEK2.pT68 | −0.2762 | 34% | 57% | 9% | 43% | [−0.4641, 0.4964] |
| DDB1 | 0.5638 | 1% | 72% | 27% | 28% | [−0.5936, 0.7839] |
| MSH2 | 0.1621 | 11% | 51% | 37% | 49% | [−0.3157, 0.3599] |
| MSH6 | −0.5742 | 91% | 8% | 0% | 92% | [−0.1465, 1.6748] |
| PDCD1 | −1.7839 | 90% | 10% | 0% | 90% | [−0.7149, 0.718] |
| RAD50 | −0.0009 | 5% | 91% | 4% | 9% | [−0.616, 1.3787] |
| RAD51 | −0.3321 | 36% | 55% | 9% | 45% | [−0.5228, 0.7557] |
| RPA32 | −0.3817 | 57% | 32% | 10% | 68% | [−0.2974, 0.256] |
| RPA32.pS4_8 | −0.6294 | 54% | 41% | 5% | 59% | [−0.5541, 0.2611] |
| SIRT6 | −0.4622 | 64% | 25% | 12% | 75% | [−0.3254, 0.1799] |
| SSBP2 | −0.0022 | 4% | 84% | 12% | 16% | [−0.5078, 0.3751] |
| TP53BP1 | −1.4872 | 85% | 12% | 3% | 88% | [−0.3191, 0.2933] |
| VCP | −2.0915 | 70% | 28% | 2% | 72% | [−1.5716, 0.1293] |
| XPA | −0.7430 | 17% | 80% | 2% | 20% | [−1.5616, 0.6984] |
| XPF | −0.1549 | 52% | 23% | 25% | 77% | [−0.1174, 0.3724] |
| XRCC1 | −1.9504 | 100% | 0% | 0% | 100% | [−0.5679, 0.5229] |
| Pediatric Patients | ||||||
| Expression in AML Patents Relative to Normal CD34+ Control Range | ||||||
| Protein | AML Median | Below | Within | Above | % Above or Below | CD34 Range |
| ATM | 0.4680 | 6% | 58% | 35% | 41% | [−0.6544, 0.7334] |
| ATM.pS1981 | 0.7960 | 0% | 44% | 56% | 56% | [−0.6671, 0.7286] |
| BRCA2 | 0.3560 | 1% | 60% | 38% | 39% | [−0.5431, 0.4642] |
| CHEK1 | −2.8780 | 98% | 2% | 0% | 98% | [−1.1618, 1.1895] |
| CHEK1.pS296 | −0.1890 | 0% | 97% | 3% | 3% | [−1.5256, 0.739] |
| CHEK1.pS345 | 0.6490 | 1% | 60% | 39% | 40% | [−0.4601, 0.7597] |
| CHEK2 | −0.2030 | 21% | 66% | 13% | 34% | [−0.5441, 0.2349] |
| CHEK2.pT68 | 0.4500 | 1% | 18% | 81% | 82% | [−0.4548, 0.2165] |
| ERCC1 | −0.2050 | 45.0% | 47% | 8% | 53% | [−0.2362, 0.2293] |
| ERCC5 | −0.4420 | 48.0% | 49% | 3% | 51% | [−0.4588, 0.2613] |
| MSH2 | −0.9910 | 82.0% | 17% | 0% | 82% | [−0.5737, 0.3723] |
| MSH6 | −0.9510 | 45.0% | 54% | 1% | 46% | [−0.994, 0.4958] |
| RAD50 | 0.0240 | 15% | 74% | 11% | 26% | [−0.4611, 0.646] |
| RAD51 | −1.6750 | 83.0% | 17% | 0% | 83% | [−0.9492, 0.638] |
| RPA32 | −0.3810 | 6% | 93% | 1% | 7% | [−1.0502, 0.3729] |
| RPA32.pS4_8 | 0.0950 | 9% | 67% | 24% | 33% | [−0.3958, 0.3992] |
| SIRT1 | −0.0310 | 9% | 88% | 3% | 12% | [−0.6172, 0.7012] |
| SSBP2 | −1.3520 | 78.0% | 22% | 0% | 78% | [−0.5313, 2.0961] |
| VCP | 0.4120 | 1% | 61% | 38% | 39% | [−0.9309, 0.5162] |
| XPA | −0.5240 | 36.0% | 56% | 7% | 43% | [−0.693, 0.4706] |
| XRCC1 | −0.0730 | 6% | 77% | 17% | 23% | [−0.7662, 0.3449] |
| AML Median | Below | Within | Above | % Above or Below | ||
| Color Legend | median > 0.5 | >25% Below Normal | >25% within Normal | >25% Above Normal | >75% | |
| −0.5 < median < 0.5 | >50% Below Normal | >50% within Normal | >50% | |||
| median < −0.5 | >90% Below Normal | >90% Within Normal | >25% | |||
| median < −2.0 | ||||||
| Clinical Variable | Unit of Measure | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Overall | p-Value |
|---|---|---|---|---|---|---|---|
| (N = 151) | (N = 451) | (N = 171) | (N = 37) | (N = 810) | |||
| Gender | Female | 74 (49.0%) | 171 (37.9%) | 72 (42.1%) | 15 (40.5%) | 332 (41.0%) | 0.209 |
| Age (Years) | Median | 63.9 | 67.9 | 66.4 | 65.1 | 66.6 | 0.003 |
| Min, Max | 22.8, 85.9 | 18.7, 94.0 | 19.9, 91.8 | 31.7, 85.4 | 18.7, 94.0 | ||
| Age Subgroup | 18-40 | 14.60% | 8.60% | 16.40% | 8.10% | 92 (11.4%) | 0.009 |
| 41-60 | 31.10% | 20.80% | 24.60% | 32.40% | 195 (24.1%) | ||
| >60 | 54.30% | 70.50% | 59.10% | 59.50% | 523 (64.6%) | ||
| Secondary AML | Yes | 31.10% | 55.70% | 46.20% | 54.10% | 397 (49.0%) | <0.001 |
| BM Blast Percentage | Median | 72 | 30 | 55 | 29 | 41 | <0.001 |
| Min, Max | 2.0, 96.0 | 1.0, 95.0 | 1.0, 97.0 | 10.0, 85.0 | 1.0, 97.0 | ||
| WBC | Median | 26.5 | 3.05 | 5.35 | 1.1 | 4.5 | <0.001 |
| Min, Max | 0.7, 391 | 0.3, 318 | 0.3, 363 | 0.3, 15.4 | 0.3, 391 | ||
| Hyperleukocytosis | >100,000 | 12.10% | 4.30% | 4.40% | 0.00% | 42 (5.7%) | 0.006 |
| PB Blast Percentage | Median | 66 | 5 | 31 | 2.5 | 16 | <0.001 |
| Min, Max | 0, 98.0 | 0, 92.0 | 0, 94.0 | 0, 25.0 | 0, 98.0 | ||
| PB Absolute Blast Count | Median | 16,600 | 138 | 1460 | 27 | 644 | <0.001 |
| Min, Max | 0, 379,000 | 0, 134,000 | 0, 247,000 | 0, 975 | 0, 379,000 | ||
| Platelet Count | Median | 32 | 44.5 | 41 | 37 | 40 | 0.024 |
| Min, Max | 6.0, 487 | 0, 1070 | 2.0, 1160 | 11.0, 122 | 0, 1160 | ||
| Platelets < 50,000 | Yes | 70.90% | 53.30% | 56.60% | 70.40% | 433 (58.0%) | 0.004 |
| Albumin | Median | 3.6 | 3.9 | 4 | 4.3 | 3.9 | <0.001 |
| Min, Max | 2.0, 36.0 | 2.1, 7.3 | 2.1, 5.2 | 2.9, 4.8 | 2.0, 36.0 | ||
| Total Bilirubin | Median | 0.5 | 0.7 | 0.6 | 0.6 | 0.6 | 0.009 |
| Min, Max | 0, 2.2 | 0, 8.1 | 0.2, 3.0 | 0.2, 1.1 | 0, 8.1 | ||
| LDH | Median | 1020 | 610 | 640 | 315 | 668 | <0.001 |
| Min, Max | 142, 10,600 | 117, 30,900 | 122, 16,000 | 168, 1230 | 117, 30,900 | ||
| Cytogenetics Risk Group | Favorable | 5.00% | 5.80% | 4.50% | 0.00% | 38 (5.2%) | 0.065 |
| Intermediate | 66.90% | 50.00% | 55.80% | 50.00% | 398 (54.4%) | ||
| Unfavorable | 28.10% | 44.20% | 39.60% | 50.00% | 295 (40.4%) | ||
| Complex Cytogenetics | Yes | 21.60% | 35.70% | 26.60% | 50.00% | 231 (31.6%) | 0.004 |
| ASXL1 Mutated | Yes | 10.00% | 23.10% | 18.30% | 41.70% | 101 (19.9%) | 0.02 |
| FLT3.ITD Mutated | Yes | 35.20% | 9.00% | 22.40% | 5.90% | 108 (16.8%) | <0.001 |
| IDH1/2 Mutated | Yes | 36.80% | 20.10% | 21.30% | 12.50% | 151 (23.4%) | 0.003 |
| NPM1 Mutated | Yes | 43.90% | 11.50% | 17.10% | 5.90% | 119 (18.7%) | <0.001 |
| RUNX1 Mutated | Yes | 13.30% | 16.00% | 26.20% | 36.40% | 91 (18.1%) | 0.047 |
| TP53 Mutated | Yes | 16.50% | 25.50% | 19.90% | 50.00% | 153 (23.4%) | 0.008 |
| WT1 Mutated | Yes | 13.40% | 3.90% | 7.70% | 9.10% | 33 (6.7%) | 0.027 |
| Response | Remission | 64.20% | 57.80% | 52.00% | 69.60% | 398 (58.2%) | 0.411 |
| PR/HI/SD | 5.20% | 9.30% | 8.70% | 4.50% | 56 (8.2%) | 0.841 | |
| Resistant | 25.40% | 29.40% | 33.30% | 27.30% | 201 (29.4%) | ||
| Early Death < 28 days | 5.20% | 3.40% | 6.00% | 0.00% | 29 (4.2%) | ||
| Relapse | Yes | 50.00% | 53.70% | 33.30% | 25.00% | 190 (47.7%) | 0.011 |
| % in CR at 5 years | % | 44.00% | 40.00% | 60.00% | 74.00% | 301 (46.0%) | 0.009 |
| Survival (Weeks) | Median | 72.7 | 54.4 | 47 | 122.4 | 55.6 | 0.15 |
| 95% CI | 53.1, 106.4 | 50.1, 61.4 | 42.6, 68 | 42.4, not_met | 51, 62.9 |
| Trials | Type | Agent 1 | Agent 2 | Agent 3 |
|---|---|---|---|---|
| Funded IITs | PARP inhibitor combination | PARPi (Talazoparib) | VEGFRi (Axitinib) | |
| PARPi (Talazoparib) | METi (Crizotinib) | |||
| PARPi (Talazoparib) | CDK4/6 (Palbociclib) | |||
| ATR inhibitor combination | ATRi (M6620) | PD-L1i (Avelumab) | ||
| ATRi (M6620) | DNA-Pki (M3814) | PD-L1i (Avelumab) | ||
| Upcoming studies | PARPi (Niraparib) | PD-1i (Dostarlimab) | ||
| NCI CTEP Trials | PARP inhibitor combination | PARPi (STAR: Seq. Olaparib) | WEE1i (Adavosertib) | |
| PI3Ki (COD: Copanlisib) | PARPi (Olaparib) | |||
| PI3Ki (COD: Copanlisib) | PARPi (Olaparib) | PD-L1i (Durvalumab) | ||
| PARPi (Talazoparib) | BETi (ZEN-3694) | |||
| Strategic Alliance Trials | ATR inhibitor monotherapy | ATRi (Repair) (RP-3500) | ||
| ATR inhibitor combination | ATRi (RP-3500) | PARPi (Talazoparib) | ||
| ATRi (BAY-1895344) | PARPi (Niraparib) | |||
| ATRi (RP-3500) | PARPi (Olaparib or Niraparib) | |||
| ATRi (RP-3500) | Chemo (Gemcitabine) | |||
| NEW DDR agents | PK-MYT-1i (RP-6306) | |||
| Upcoming studies | MRTX849 (KRAS G12Ci) | PARPi (Olaparib) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubner, S.E.; de Camargo Magalhães, E.S.; Hoff, F.W.; Brown, B.D.; Qiu, Y.; Horton, T.M.; Kornblau, S.M. DNA Damage Response−Related Proteins Are Prognostic for Outcome in Both Adult and Pediatric Acute Myelogenous Leukemia Patients: Samples from Adults and from Children Enrolled in a Children’s Oncology Group Study. Int. J. Mol. Sci. 2023, 24, 5898. https://doi.org/10.3390/ijms24065898
Hubner SE, de Camargo Magalhães ES, Hoff FW, Brown BD, Qiu Y, Horton TM, Kornblau SM. DNA Damage Response−Related Proteins Are Prognostic for Outcome in Both Adult and Pediatric Acute Myelogenous Leukemia Patients: Samples from Adults and from Children Enrolled in a Children’s Oncology Group Study. International Journal of Molecular Sciences. 2023; 24(6):5898. https://doi.org/10.3390/ijms24065898
Chicago/Turabian StyleHubner, Stefan E., Eduardo S. de Camargo Magalhães, Fieke W. Hoff, Brandon D. Brown, Yihua Qiu, Terzah M. Horton, and Steven M. Kornblau. 2023. "DNA Damage Response−Related Proteins Are Prognostic for Outcome in Both Adult and Pediatric Acute Myelogenous Leukemia Patients: Samples from Adults and from Children Enrolled in a Children’s Oncology Group Study" International Journal of Molecular Sciences 24, no. 6: 5898. https://doi.org/10.3390/ijms24065898
APA StyleHubner, S. E., de Camargo Magalhães, E. S., Hoff, F. W., Brown, B. D., Qiu, Y., Horton, T. M., & Kornblau, S. M. (2023). DNA Damage Response−Related Proteins Are Prognostic for Outcome in Both Adult and Pediatric Acute Myelogenous Leukemia Patients: Samples from Adults and from Children Enrolled in a Children’s Oncology Group Study. International Journal of Molecular Sciences, 24(6), 5898. https://doi.org/10.3390/ijms24065898





