Synthesis of Manganese Zinc Ferrite Nanoparticles in Medical-Grade Silicone for MRI Applications
Abstract
1. Introduction
2. Results
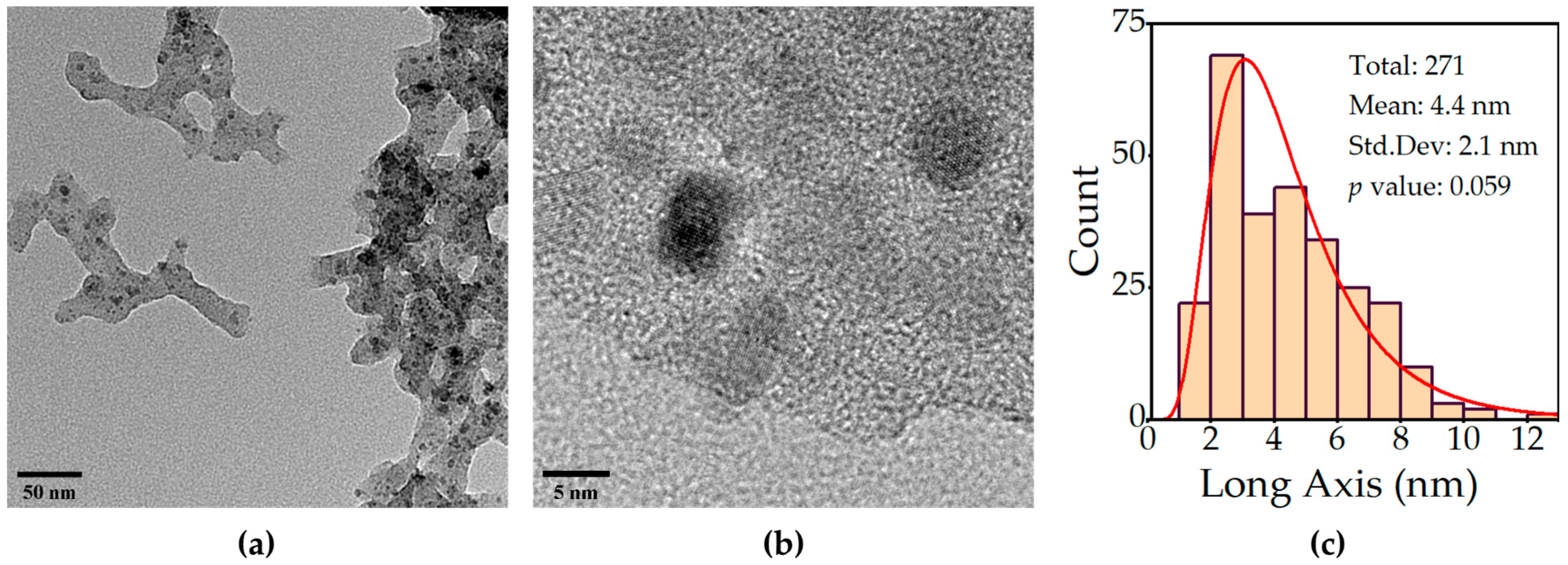
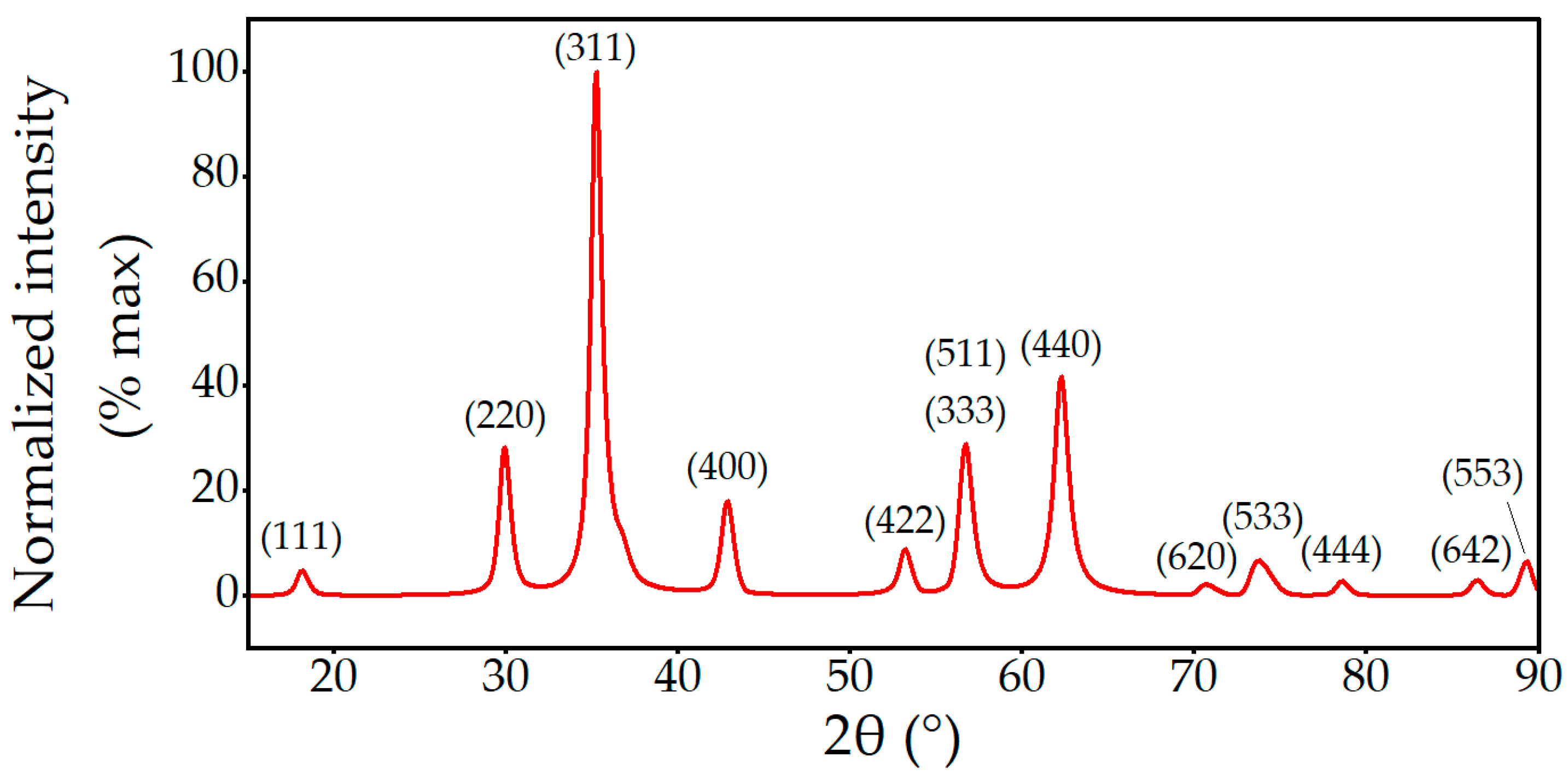
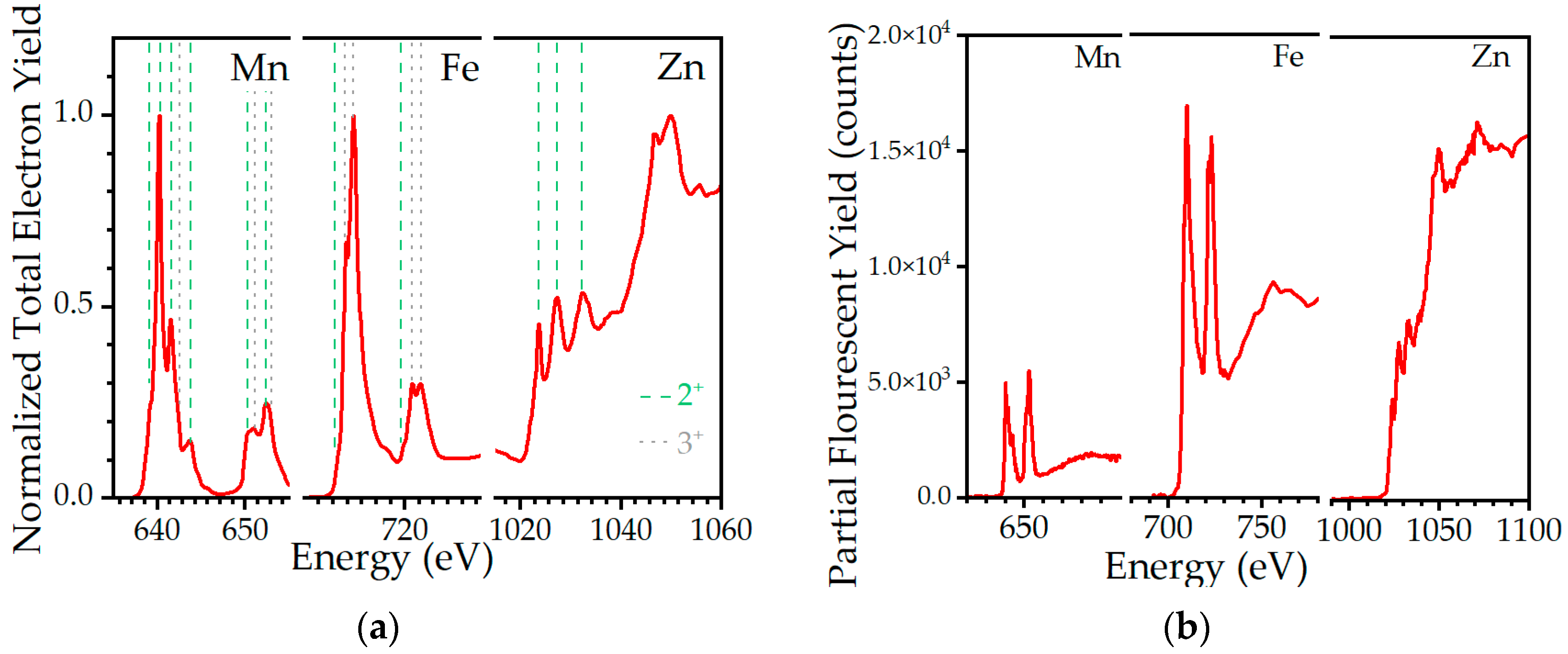
2.1. Morphological, Structural, and Chemical Characterization
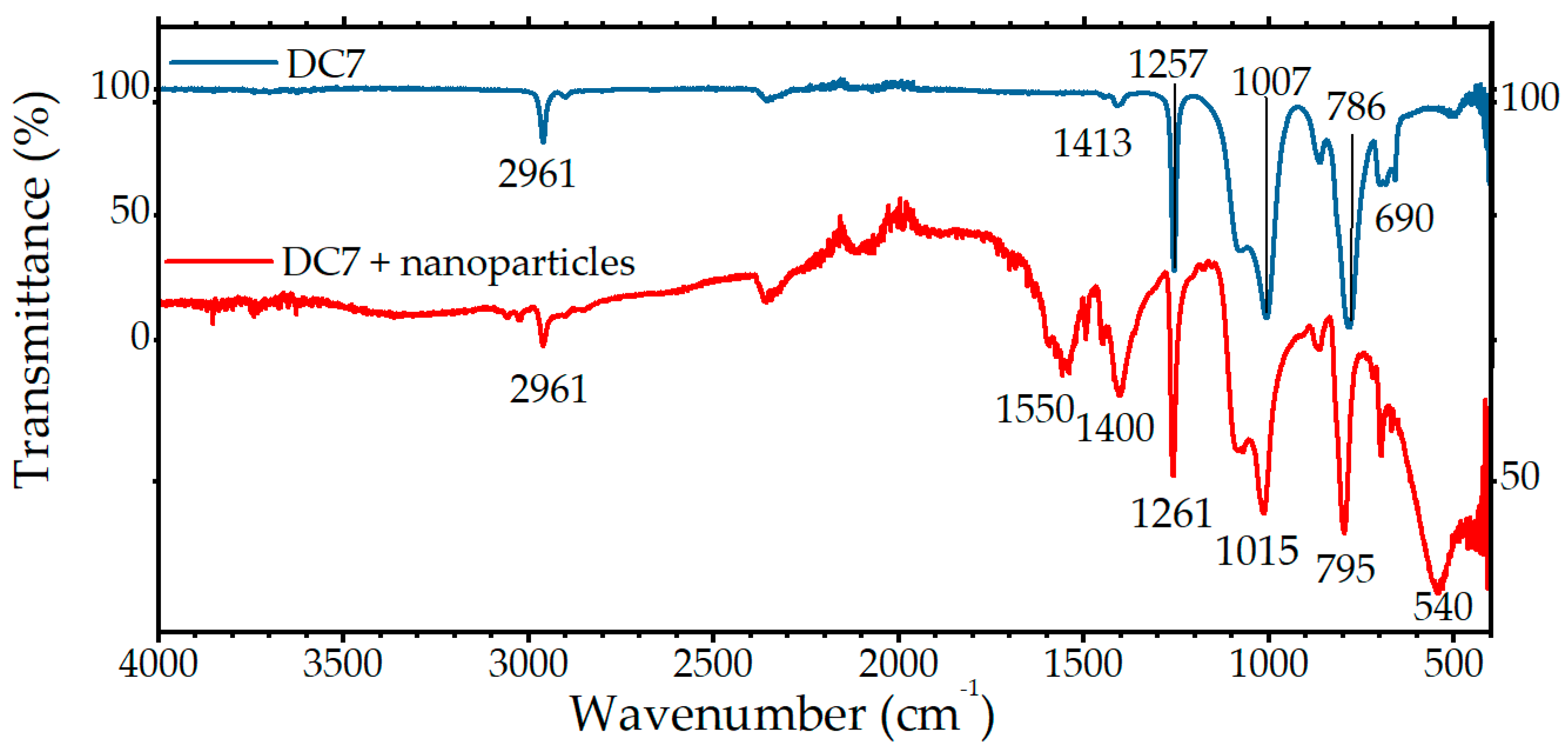
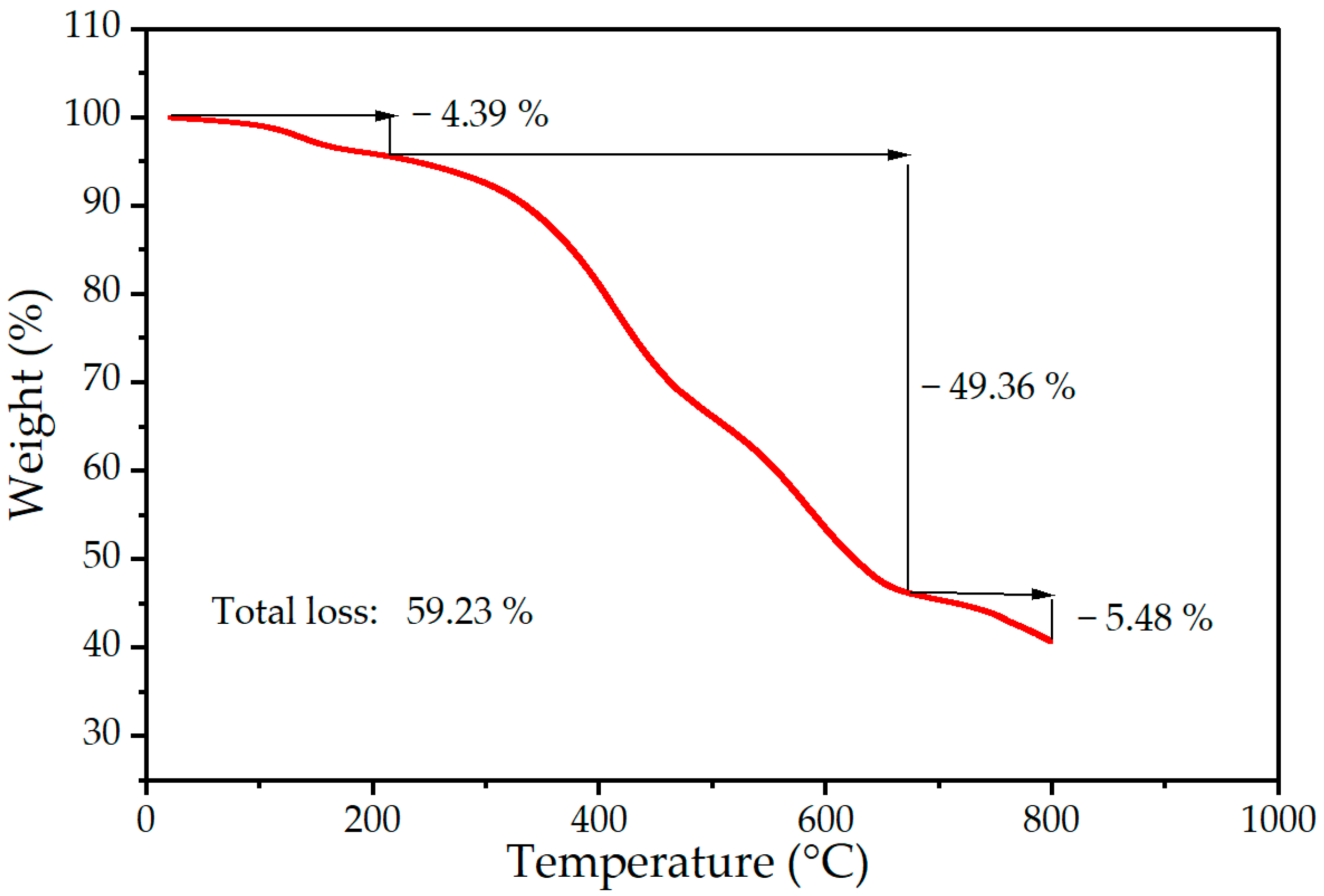
2.2. Silicone Characterization
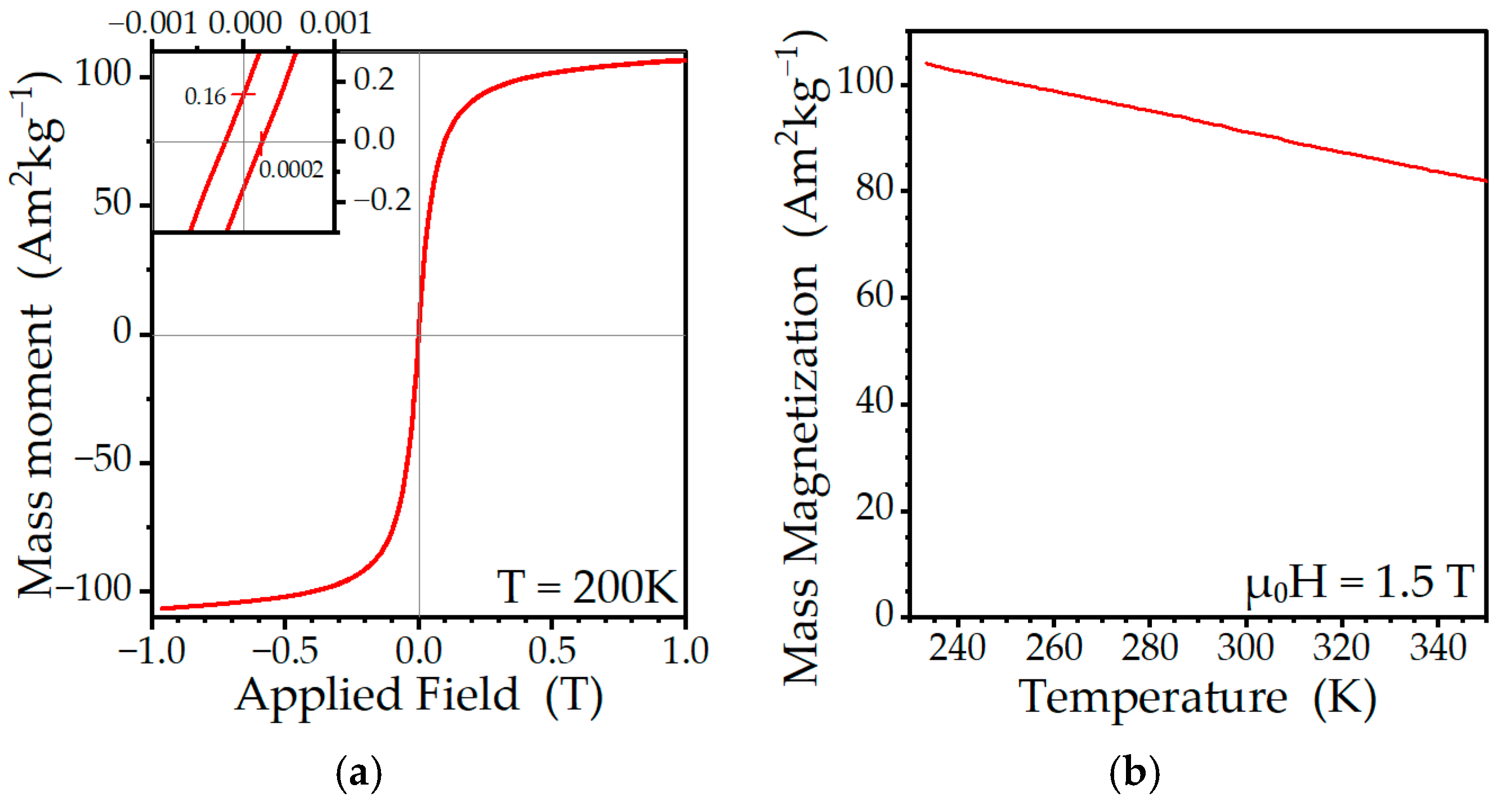
2.3. Magnetometry
2.4. Nuclear Magnetic Resonance (NMR) Measurements
2.5. Magnetic Resonance Imaging
3. Discussion
4. Materials and Methods
4.1. Synthesis of Nanoparticles in Silicone
4.2. Microscopy Methods
4.3. X-ray Diffractometry
4.4. Spectroscopy
4.5. Magnetometry
4.6. Nuclear Relaxation Time Measurements
4.7. Magnetic Resonance Imaging
4.8. Inductively Coupled Plasma-Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelesidis, G.A.; Kholghy, M.R. A Monodisperse Population Balance Model for Nanoparticle Agglomeration in the Transition Regime. Materials 2021, 14, 3882. [Google Scholar] [CrossRef] [PubMed]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M.R. Ultrasonic Dispersion of Nanoparticles for Environmental, Health and Safety Assessment—Issues and Recommendations. Nanotoxicology 2011, 5, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Özcan-Taşkin, N.G.; Padron, G.; Voelkel, A. Effect of Particle Type on the Mechanisms of Break up of Nanoscale Particle Clusters. Chem. Eng. Res. Des. 2009, 87, 468–473. [Google Scholar] [CrossRef]
- Krzosa, R.; Wojtas, K.; Golec, J.; Makowski, Ł.; Orciuch, W.; Adamek, R. Modelling Particle Deagglomeration in a Batch Homogenizer Using Full CFD and Mechanistic Models. Chem. Process Eng.-Inz. Chem. Proces. 2021, 42, 105–118. [Google Scholar] [CrossRef]
- Cohen, J.M.; Beltran-Huarac, J.; Pyrgiotakis, G.; Demokritou, P. Effective Delivery of Sonication Energy to Fast Settling and Agglomerating Nanomaterial Suspensions for Cellular Studies: Implications for Stability, Particle Kinetics, Dosimetry and Toxicity. NanoImpact 2018, 10, 81–86. [Google Scholar] [CrossRef]
- Bałdyga, J.; Makowski, Ł.; Orciuch, W.; Sauter, C.; Schuchmann, H.P. Deagglomeration Processes in High-Shear Devices. Chem. Eng. Res. Des. 2008, 86, 1369–1381. [Google Scholar] [CrossRef]
- Kim, S.C.; Chen, D.-R.; Qi, C.; Gelein, R.M.; Finkelstein, J.N.; Elder, A.; Bentley, K.; Oberdörster, G.; Pui, D.Y.H. A Nanoparticle Dispersion Method for in Vitro and in Vivo Nanotoxicity Study. Nanotoxicology 2010, 4, 42–51. [Google Scholar] [CrossRef]
- Bałdyga, J. Mixing and Fluid Dynamics Effects in Particle Precipitation Processes. KONA Powder Part. J. 2016, 33, 127–149. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle Processing: Understanding and Controlling Aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Jo, Y.-S.; Lee, H.-J.; Park, H.-M.; Na, T.-W.; Jung, J.-S.; Min, S.-H.; Kim, Y.K.; Yang, S.-M. Chemical Vapor Synthesis of Nonagglomerated Nickel Nanoparticles by In-Flight Coating. ACS Omega 2021, 6, 27842–27850. [Google Scholar] [CrossRef]
- Zheng, R.; Guo, J.; Cai, X.; Bin, L.; Lu, C.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Manganese Complexes and Manganese-Based Metal-Organic Frameworks as Contrast Agents in MRI and Chemotherapeutics Agents: Applications and Prospects. Colloids Surf. B Biointerfaces 2022, 213, 112432. [Google Scholar] [CrossRef]
- Yan, X.; Chen, J.-Q.; Hu, M.-L.; Sakiyama, H.; Muddassir, M.; Liu, J.-Q. Syntheses, Structures and Mechanisms of Interactions with DNA of Two New 20-Core Silver(I) Complexes with Different Ligands. Inorg. Chim. Acta 2023, 546, 121297. [Google Scholar] [CrossRef]
- Rösch, L.; Weidner, R. Polymerization Chemistry of Silicones. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 7635–7638. ISBN 978-0-08-043152-9. [Google Scholar]
- Wu, C.; Li, W.; Gao, D.; Jia, M. Study of Resistance of Silicone Resin to Heat and Irradiation. Polym.-Plast. Technol. Eng. 2009, 48, 1094–1100. [Google Scholar] [CrossRef]
- Marmo, A.C.; Grunlan, M.A. Biomedical Silicones: Leveraging Additive Strategies to Propel Modern Utility. ACS Macro Lett. 2023, 12, 172–182. [Google Scholar] [CrossRef]
- Chou, F.-Y.; Hara, S.; Uchida, K.; Matsuo, Y.; Masuda, T.; Yokoi, R.; Ono, T.; Anraku, M.; Isoyama, T.; Takai, M. Functionalized Silicone Elastomer via Alkaline Solution to Coat Phosphorylcholine-Based Copolymer Containing Organosilane to Improve Hemocompatibility for Medical Devices. Front. Mater. 2022, 9, 877755. [Google Scholar] [CrossRef]
- Sołoducho, J.; Zając, D.; Spychalska, K.; Baluta, S.; Cabaj, J. Conducting Silicone-Based Polymers and Their Application. Molecules 2021, 26, 2012. [Google Scholar] [CrossRef]
- Curtis, J.; Colas, A. Medical Applications of Silicones. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1106–1116. ISBN 978-0-12-374626-9. [Google Scholar]
- Evans, B.A.; Fiser, B.L.; Prins, W.J.; Rapp, D.J.; Shields, A.R.; Glass, D.R.; Superfine, R. A Highly Tunable Silicone-Based Magnetic Elastomer with Nanoscale Homogeneity. J. Magn. Magn. Mater. 2012, 324, 501–507. [Google Scholar] [CrossRef]
- Oberdick, S.D.; Russek, S.E.; Poorman, M.E.; Zabow, G. Observation of Iron Oxide Nanoparticle Synthesis in Magnetogels Using Magnetic Resonance Imaging. Soft Matter 2020, 16, 10244–10251. [Google Scholar] [CrossRef]
- Mues, B.; Buhl, E.M.; Schmitz-Rode, T.; Slabu, I. Towards Optimized MRI Contrast Agents for Implant Engineering: Clustering and Immobilization Effects of Magnetic Nanoparticles. J. Magn. Magn. Mater. 2019, 471, 432–438. [Google Scholar] [CrossRef]
- Slabu, I.; Guntherodt, G.; Schmitz-Rode, T.; Hodenius, M.; Kramer, N.; Donker, H.; Krombach, G.A.; Otto, J.; Klinge, U.; Baumann, M. Investigation of Superparamagnetic Iron Oxide Nanoparticles for MRVisualization of Surgical Implants. Curr. Pharm. Biotechnol. 2012, 13, 545–551. [Google Scholar] [CrossRef]
- Bulanov, P.A.; Nazarova, E.E.; Menshchikov, P.E.; Kupriyanov, D.A.; Tereshchenko, G.V.; Novichkova, G.A. Creation of an MRI Phantom Based on Super-Paramagnetic Nanoparticles of Iron Oxide (SPIO) for Standardization of the Conversion of T2 * Values in the Concentration of Iron in the Liver. J. Phys. Conf. Ser. 2021, 2015, 012025. [Google Scholar] [CrossRef]
- McDannold, N.J.; Jolesz, F.A. Magnetic Resonance Image-Guided Thermal Ablations. Top. Magn. Reson. Imaging 2000, 11, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Sarig, O.; Kimel, S.; Orenstein, A. Laser Treatment of Venous Malformations. Ann. Plast. Surg. 2006, 57, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Spinoza, Z.; Carter, D.; Ferrone, D.; Eksioglu, Y.; Huckins, S. The Use of MRI-Guided Laser-Induced Thermal Ablation for Epilepsy. Childs Nerv. Syst. 2013, 29, 2089–2094. [Google Scholar] [CrossRef]
- Salem, U.; Kumar, V.A.; Madewell, J.E.; Schomer, D.F.; de Almeida Bastos, D.C.; Zinn, P.O.; Weinberg, J.S.; Rao, G.; Prabhu, S.S.; Colen, R.R. Neurosurgical Applications of MRI Guided Laser Interstitial Thermal Therapy (LITT). Cancer Imaging 2019, 19, 65. [Google Scholar] [CrossRef]
- Atienza, F.; Almendral, J.; Sanchez-Quintana, D.; Zaballos, M.; Murillo, M.; Jimeno, C.; Parra, V.; Fernandez-Aviles, F. Cryoablation Time-Dependent Dose-Response Effect at Minimal Temperatures (-80 C): An Experimental Study. Europace 2009, 11, 1538–1545. [Google Scholar] [CrossRef]
- Klaver, M.N.; De Potter, T.J.R.; Iliodromitis, K.; Babkin, A.; Cabrita, D.; Fabbricatore, D.; Boersma, L.V.A. Ultralow Temperature Cryoablation Using Near-critical Nitrogen for Cavotricuspid Isthmus-ablation, First-in-human Results. J. Cardiovasc. Electrophysiol. 2021, 32, 2025–2032. [Google Scholar] [CrossRef]
- Hankiewicz, J.H.; Celinski, Z.; Camley, R.E. Measurement of Sub-zero Temperatures in MRI Using T1 Temperature Sensitive Soft Silicone Materials: Applications for MRI-guided Cryosurgery. Med. Phys. 2021, 48, 6844–6858. [Google Scholar] [CrossRef]
- Lezzoni, L.I.; Grad, O.; Moskowitz, M.A. Magnetic Resonance Imaging: Overview of the Technology and Medical Applications. Int. J. Technol. Assess. Health Care 1985, 1, 481–498. [Google Scholar] [CrossRef]
- LiveoTM 7-9600 Soft Filling Elastomer Parts A & B. Available online: https://dupont.materialdatacenter.com/products/datasheet/SI/LiveoTM%207-9600%20Soft%20Filling%20Elastomer%20Parts%20A%20%20B (accessed on 5 December 2022).
- Hankiewicz, J.H.; Stoll, J.A.; Stroud, J.; Davidson, J.; Livesey, K.L.; Tvrdy, K.; Roshko, A.; Russek, S.E.; Stupic, K.; Bilski, P.; et al. Nano-Sized Ferrite Particles for Magnetic Resonance Imaging Thermometry. J. Magn. Magn. Mater. 2018, 469, 550–557. [Google Scholar] [CrossRef]
- Guillaud, C. Propriétés Magnétiques des Ferrites. J. Phys. Radium 1951, 12, 239–248. [Google Scholar] [CrossRef]
- Aghrich, K.; Mtougui, S.; Goumrhar, F.; Abdellaoui, M.; Mamouni, N.; Fekhaoui, M.; El Moutaouakil, A.; Mounkachi, O. Experimental and Theoretical Investigation of the Synthesis, Electronic and Magnetic Properties of MnFe2O4 Spinel Ferrite. Energies 2022, 15, 8386. [Google Scholar] [CrossRef]
- Pardo, A.; Gómez-Florit, M.; Barbosa, S.; Taboada, P.; Domingues, R.M.A.; Gomes, M.E. Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano 2021, 15, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, D.; Stroud, J.; Hankiewicz, J.H.; Gassen, R.; Kmita, A.; Stepień, J.; Celinski, Z.; Sikora, M.; Zukrowski, J.; Gajewska, M.; et al. One-Step Preparation of Highly Stable Copper–Zinc Ferrite Nanoparticles in Water Suitable for MRI Thermometry. Chem. Mater. 2022, 34, 4001–4018. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.B.; Riley, D.P. An Experimental Investigation of Extrapolation Methods in the Derivation of Accurate Unit-Cell Dimensions of Crystals. Proc. Phys. Soc. 1945, 57, 160–177. [Google Scholar] [CrossRef]
- Praveena, K.; Katlakunta, S.; Virk, H.S. Structural and Magnetic Properties of Mn-Zn Ferrites Synthesized by Microwave-Hydrothermal Process. Solid State Phenom. 2015, 232, 45–64. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Orooji, Y. Sonocatalytic Activity of Biochar-Supported ZnO Nanorods in Degradation of Gemifloxacin: Synergy Study, Effect of Parameters and Phytotoxicity Evaluation. Ultrason. Sonochem. 2019, 55, 44–56. [Google Scholar] [CrossRef]
- Ghasemi, M.; Khataee, A.; Gholami, P.; Soltani, R.D.C.; Hassani, A.; Orooji, Y. In-Situ Electro-Generation and Activation of Hydrogen Peroxide Using a CuFeNLDH-CNTs Modified Graphite Cathode for Degradation of Cefazolin. J. Environ. Manag. 2020, 267, 110629. [Google Scholar] [CrossRef]
- Zając, M.; Giela, T.; Freindl, K.; Kollbek, K.; Korecki, J.; Madej, E.; Pitala, K.; Kozioł-Rachwał, A.; Sikora, M.; Spiridis, N.; et al. The First Experimental Results from the 04BM (PEEM/XAS) Beamline at Solaris. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2021, 492, 43–48. [Google Scholar] [CrossRef]
- Srinivasan, T.T.; Srivastava, C.M.; Venkataramani, N.; Patni, M.J. Infrared Absorption in Spinel Ferrites. Bull. Mater. Sci. 1984, 6, 1063–1067. [Google Scholar] [CrossRef]
- Katsnelson, E.Z.; Karoza, A.G.; Meleshchenko, L.A.; Pankov, V.V.; Khavkin, B.G. Detailed Study of IR Absorption Spectra of Manganese–Zinc Ferrites. Phys. Status Solidi B 1987, 141, 599–609. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Al-Harbi, L.M. The Influence of Self-Heating Iron on the Thermal, Mechanical, and Swelling Properties of PDMS Composites for Organic Solvents Removal. Polymers 2021, 13, 4231. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, H.; Guo, X.; Lu, J. Thermal Degradation Behaviors of Some Branched and Linear Polysiloxanes. Polym. Degrad. Stab. 2006, 91, 1471–1475. [Google Scholar] [CrossRef]
- Van Bronswijk, W.; Kirwan, L.J.; Fawell, P.D. In Situ Adsorption Densities of Polyacrylates on Hematite Nano-Particle Films as Determined by ATR-FTIR Spectroscopy. Vib. Spectrosc. 2006, 41, 176–181. [Google Scholar] [CrossRef]
- Dvornic, P. High Temperature Stability of Polysiloxanes. In Silicon Compounds: Silanes & Silicones; Arkles, B., Larson, G., Eds.; Gelest Inc.: Morrisville, PA, USA, 2013; pp. 419–431. ISBN 978-0-578-12235-9. [Google Scholar]
- Bi, C.; Fishbein, K.; Bouhrara, M.; Spencer, R.G. Stabilization of Parameter Estimates from Multiexponential Decay through Extension into Higher Dimensions. Sci. Rep. 2022, 12, 5773. [Google Scholar] [CrossRef]
- Reiter, D.A.; Lin, P.-C.; Fishbein, K.W.; Spencer, R.G. Multicomponent T2 Relaxation Analysis in Cartilage. Magn. Reson. Med. 2009, 61, 803–809. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Berret, J.-F.; Fresnais, J.; Gossuin, Y.; Sandre, O. A Universal Scaling Law to Predict the Efficiency of Magnetic Nanoparticles as MRI T2-Contrast Agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef]
- Nobles, J.E.; Hao, Y.; Goldman, S.; Stroud, J.; Stupic, K.; Hankiewicz, J.H.; Celinski, Z. Employing Gadolinium Micro-Disks as Temperature Probes for Magnetic Resonance Imaging. J. Magn. Magn. Mater. 2022, 562, 169849. [Google Scholar] [CrossRef]
- Hankiewicz, J.H.; Celinski, Z.; Stupic, K.F.; Anderson, N.R.; Camley, R.E. Ferromagnetic Particles as Magnetic Resonance Imaging Temperature Sensors. Nat. Commun. 2016, 7, 12415. [Google Scholar] [CrossRef]
- Abragam, A. The Principles of Nuclear Magnetism; The international series of monographs on physics; Reprint; Clarendon Press: Oxford, UK, 1986; ISBN 978-0-19-851236-3. [Google Scholar]
- Cohen-Addad, J.P.; Domard, M.; Boileau, S. Slow Dynamics of Entangled Polydimethylsiloxane Chains Observed by Proton Transverse Magnetic Relaxation. J. Chem. Phys. 1981, 75, 4107–4114. [Google Scholar] [CrossRef]
- Saalwachter, K. Proton Multiple-Quantum NMR for the Study of Chain Dynamics and Structural Constraints in Polymeric Soft Materials. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 51, 1–35. [Google Scholar] [CrossRef]
- Ishay, R.B.; Israel, L.L.; Eitan, E.L.; Partouche, D.M.; Lellouche, J.-P. Maghemite-Human Serum Albumin Hybrid Nanoparticles: Towards a Theranostic System with High MRI r2* Relaxivity. J. Mater. Chem. B 2016, 4, 3801–3814. [Google Scholar] [CrossRef] [PubMed]
- Yablonskiy, D.A.; Haacke, E.M. Theory of NMR Signal Behavior in Magnetically Inhomogeneous Tissues: The Static Dephasing Regime. Magn. Reson. Med. 1994, 32, 749–763. [Google Scholar] [CrossRef]
- Stroud, J.; Stupic, K.; Walsh, T.; Celinski, Z.; Hankiewicz, J. Local Heating of Metallic Objects from Switching Magnetic Gradients in MRI. In Proceedings of the Medical Imaging 2019: Imaging Informatics for Healthcare, Research, and Applications; Bak, P.R., Chen, P.-H., Eds.; SPIE: San Diego, CA, USA, 2019; p. 23. [Google Scholar]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. Physics Responsible for Heating Efficiency and Self-Controlled Temperature Rise of Magnetic Nanoparticles in Magnetic Hyperthermia Therapy. Prog. Biophys. Mol. Biol. 2018, 133, 9–19. [Google Scholar] [CrossRef]
- Pohanish, R.P.; Greene, S.A. Wiley Guide to Chemical Incompatibilities; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0-470-52330-8. [Google Scholar]
- Boss, M.A.; Dienstfrey, A.M.; Gimbutas, Z.; Keenan, K.E.; Kos, A.B.; Splett, J.D.; Stupic, K.F.; Russek, S.E. Magnetic Resonance Imaging Biomarker Calibration Service: Proton Spin Relaxation Times; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018; p. NIST SP 250-97. [Google Scholar]
- Farrar, T.C.; Becker, E.D. 4.1 Frequency Distribution of Molecular Motions. In Pulse and Fourier Transform NMR: Introduction to Theory and Methods; Academic Press: New York, NY, USA, 1971; p. 48. ISBN 978-0-12-249650-9. [Google Scholar]
- Russek, S.E.; Stupic, K.F.; Biller, J.R.; Boss, M.A.; Keenan, K.E.; Mirowski, E. Electromagnetics for Quantitative Magnetic Resonance Imaging. In Compendium on Electromagnetic Analysis; World Scientific: Singapore, 2020; pp. 95–147. [Google Scholar]
- Dietrich, O.; Biffar, A.; Baur-Melnyk, A.; Reiser, M.F. Technical Aspects of MR Diffusion Imaging of the Body. Eur. J. Radiol. 2010, 76, 314–322. [Google Scholar] [CrossRef]
- Martin, T.D.; Brockhoff, C.A.; Creed, J.T. Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; EPA: Washington, DC, USA, 1994. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoll, J.A.; Lachowicz, D.; Kmita, A.; Gajewska, M.; Sikora, M.; Berent, K.; Przybylski, M.; Russek, S.E.; Celinski, Z.J.; Hankiewicz, J.H. Synthesis of Manganese Zinc Ferrite Nanoparticles in Medical-Grade Silicone for MRI Applications. Int. J. Mol. Sci. 2023, 24, 5685. https://doi.org/10.3390/ijms24065685
Stoll JA, Lachowicz D, Kmita A, Gajewska M, Sikora M, Berent K, Przybylski M, Russek SE, Celinski ZJ, Hankiewicz JH. Synthesis of Manganese Zinc Ferrite Nanoparticles in Medical-Grade Silicone for MRI Applications. International Journal of Molecular Sciences. 2023; 24(6):5685. https://doi.org/10.3390/ijms24065685
Chicago/Turabian StyleStoll, Joshua A., Dorota Lachowicz, Angelika Kmita, Marta Gajewska, Marcin Sikora, Katarzyna Berent, Marek Przybylski, Stephen E. Russek, Zbigniew J. Celinski, and Janusz H. Hankiewicz. 2023. "Synthesis of Manganese Zinc Ferrite Nanoparticles in Medical-Grade Silicone for MRI Applications" International Journal of Molecular Sciences 24, no. 6: 5685. https://doi.org/10.3390/ijms24065685
APA StyleStoll, J. A., Lachowicz, D., Kmita, A., Gajewska, M., Sikora, M., Berent, K., Przybylski, M., Russek, S. E., Celinski, Z. J., & Hankiewicz, J. H. (2023). Synthesis of Manganese Zinc Ferrite Nanoparticles in Medical-Grade Silicone for MRI Applications. International Journal of Molecular Sciences, 24(6), 5685. https://doi.org/10.3390/ijms24065685






