Stress-Inducible SCAND Factors Suppress the Stress Response and Are Biomarkers for Enhanced Prognosis in Cancers
Abstract
1. Introduction
2. Results
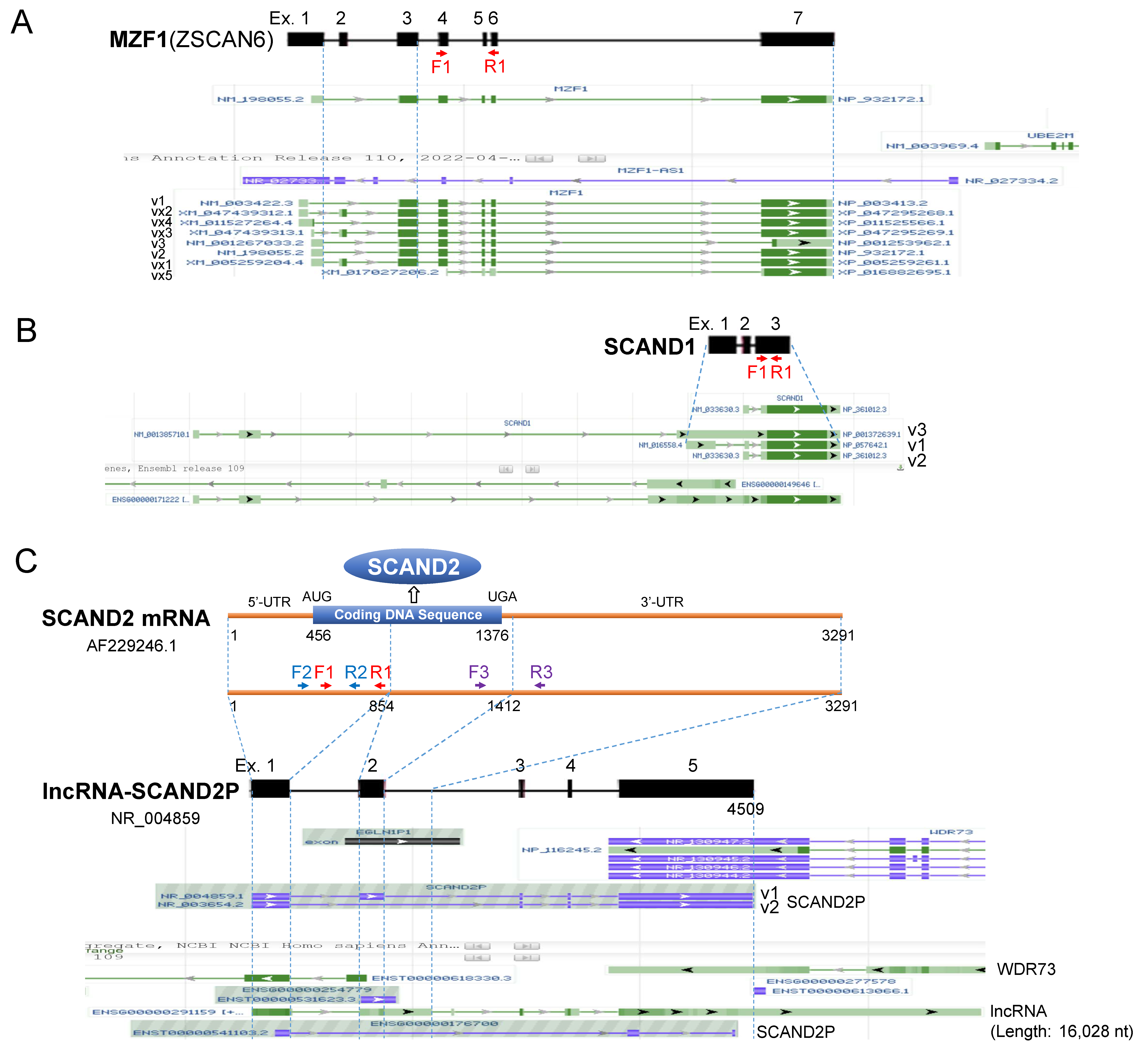
2.1. Heat Shock Elements (HSE) in the Promoter Regions of MZF1(ZSCAN6), SCAND1, and SCAND2P Genes in the Human Genome
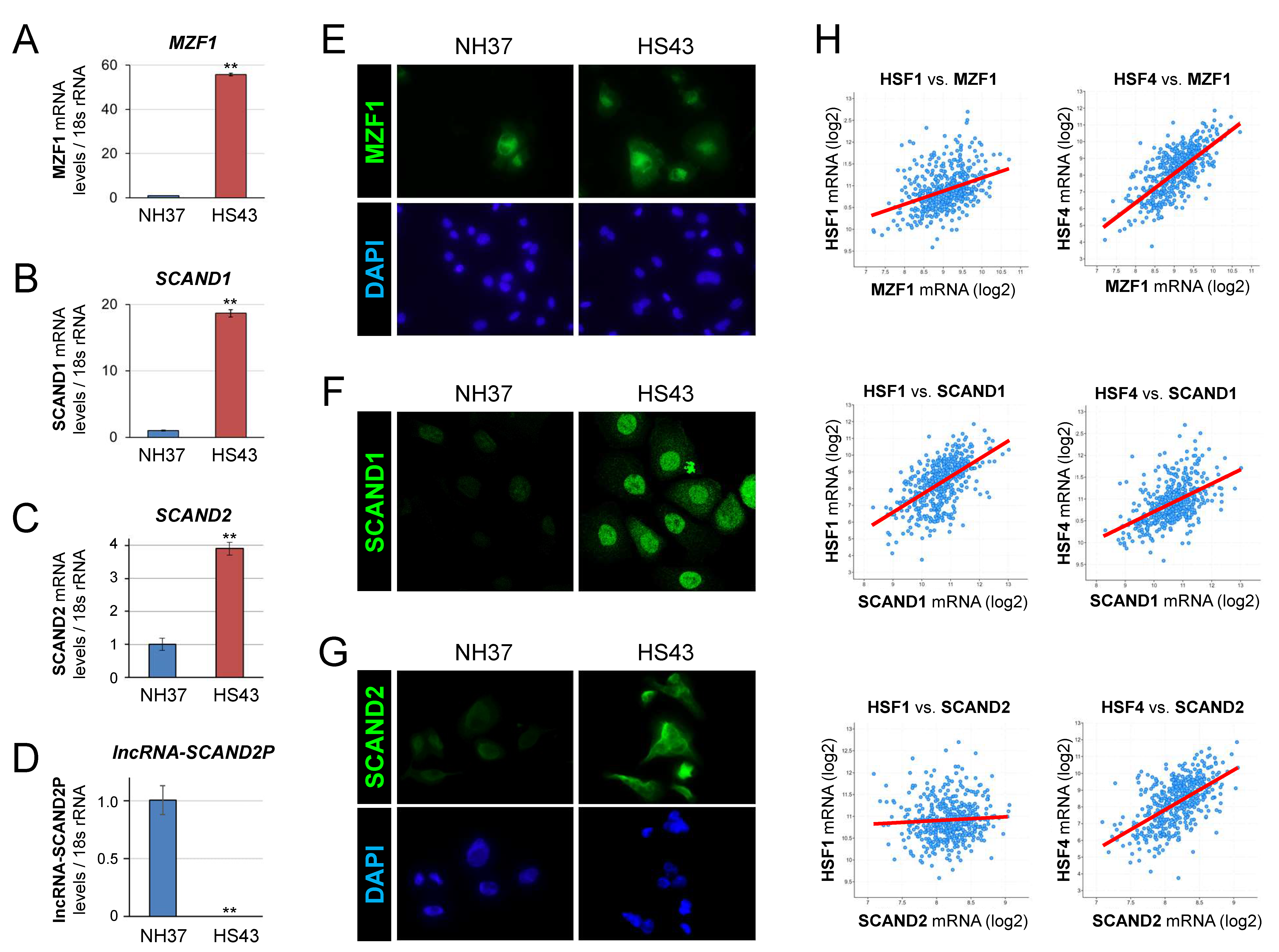
2.2. Heat Shock Stress Induces MZF1, SCAND1, and SCAND2 Gene Expression and Reduces lncRNA-SCAND2P in Prostate Cancer
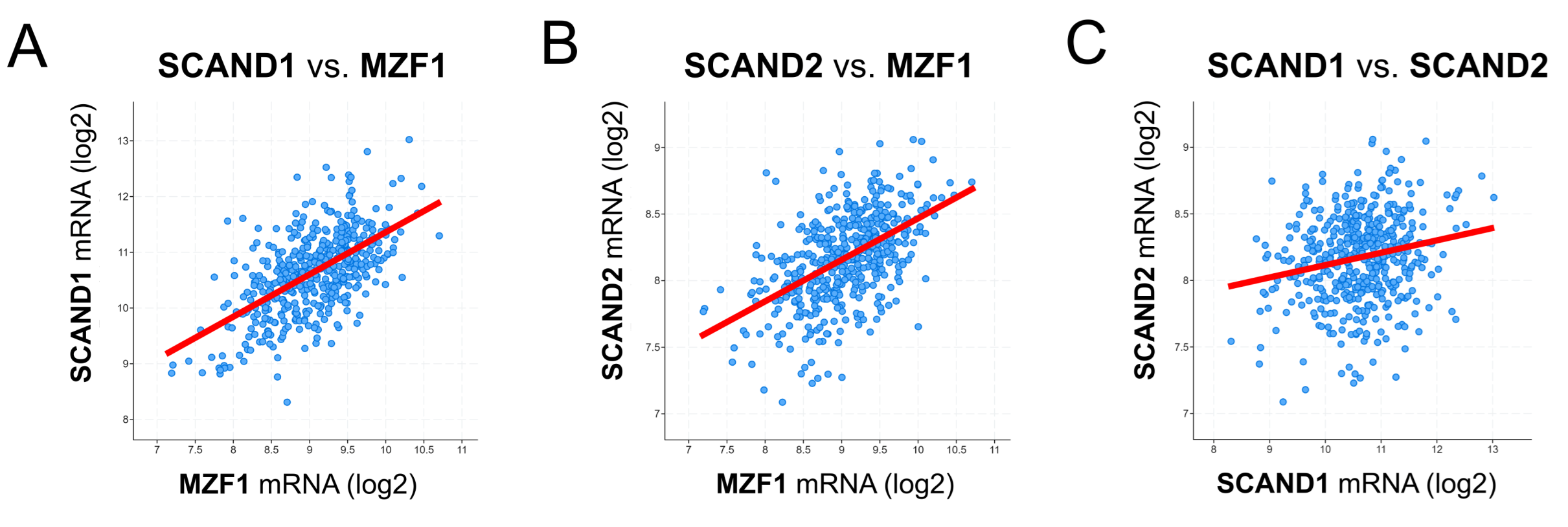
2.3. Co-Expression Correlation of SCAN-TF Genes in Prostate Cancer
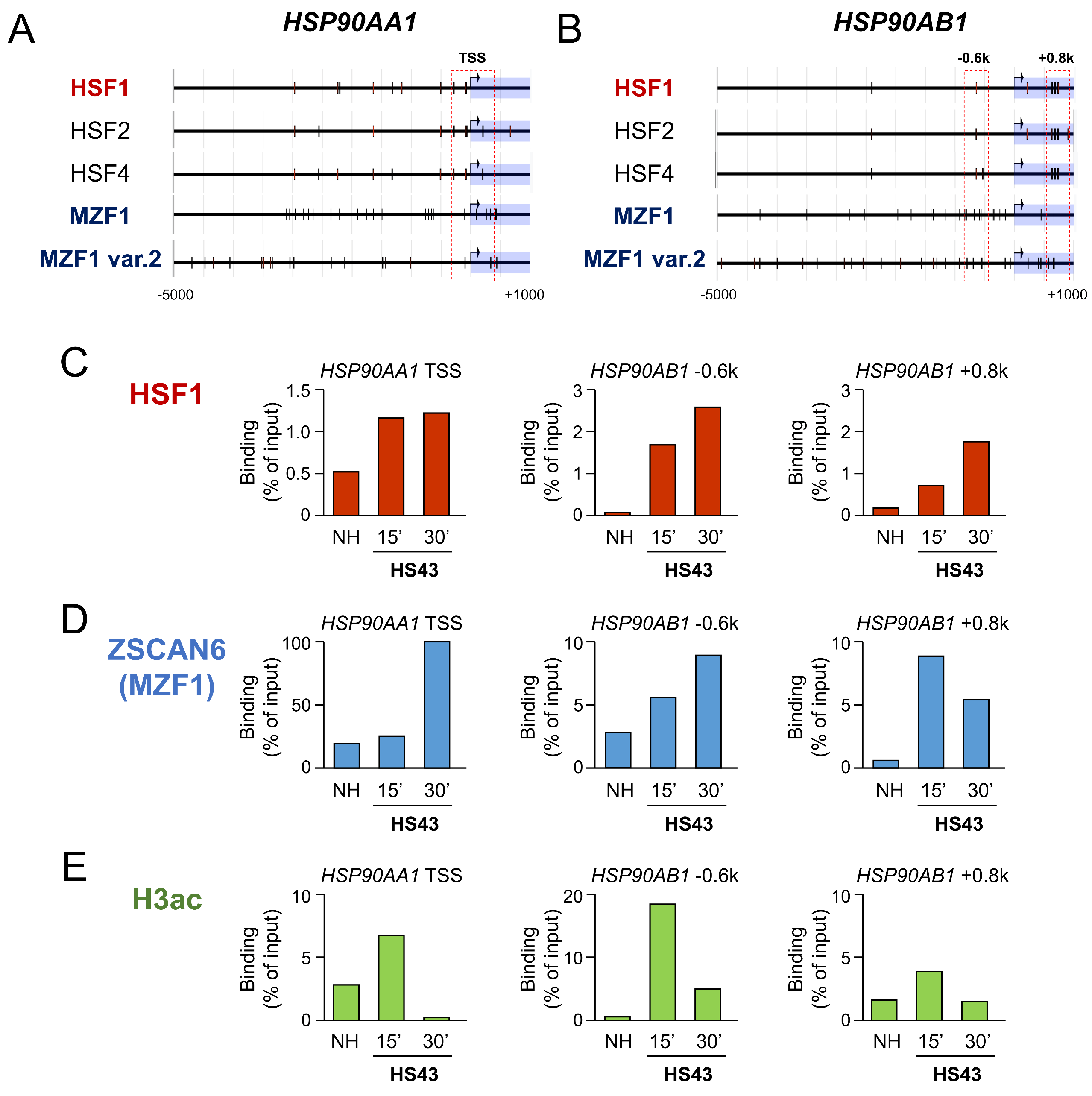
2.4. Heat Shock Stress Induces HSF1 and MZF1(ZSCAN6) Binding to HSP90 Genes
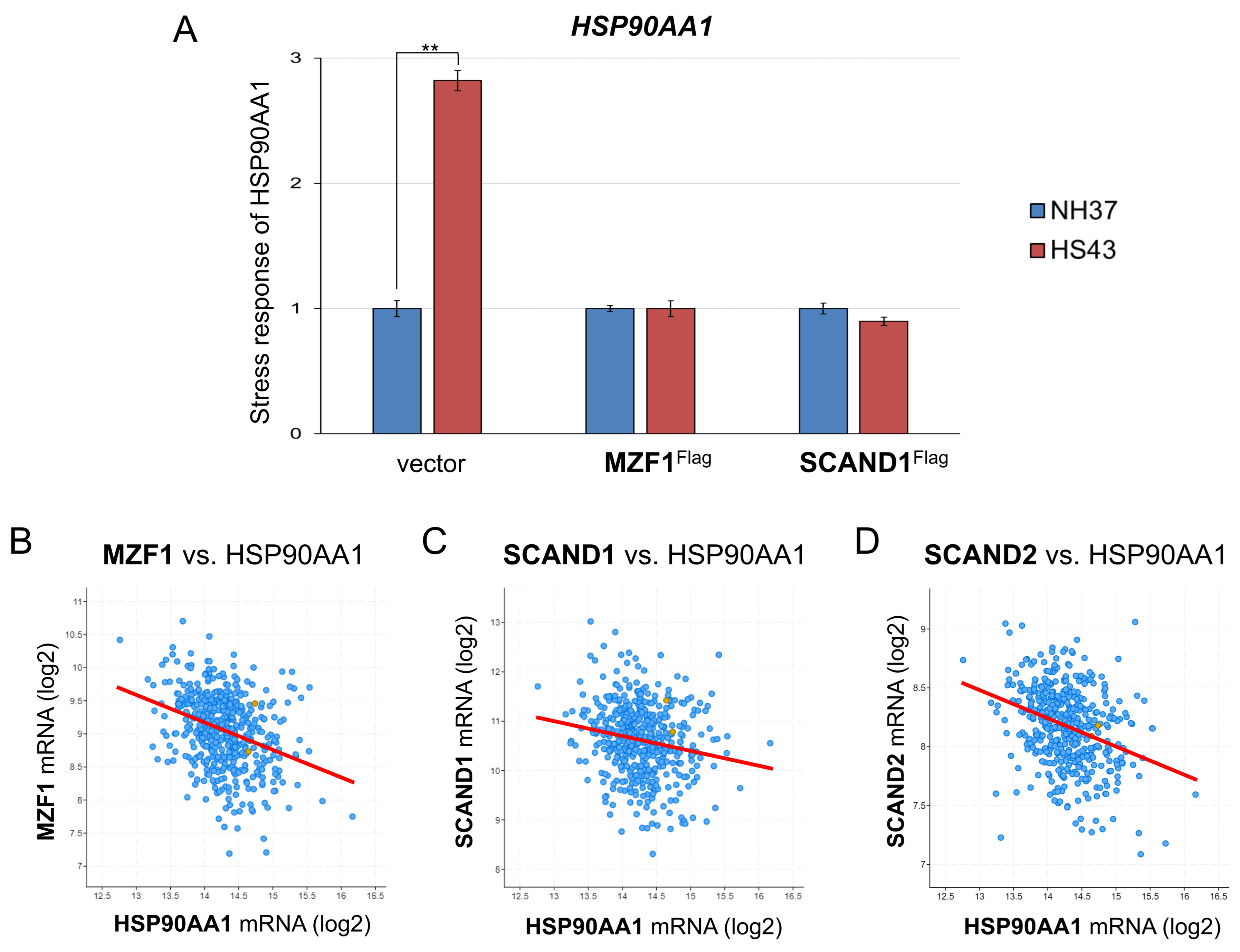
2.5. MZF1 and SCAND1 Blocks the Heat Shock Response of HSP90
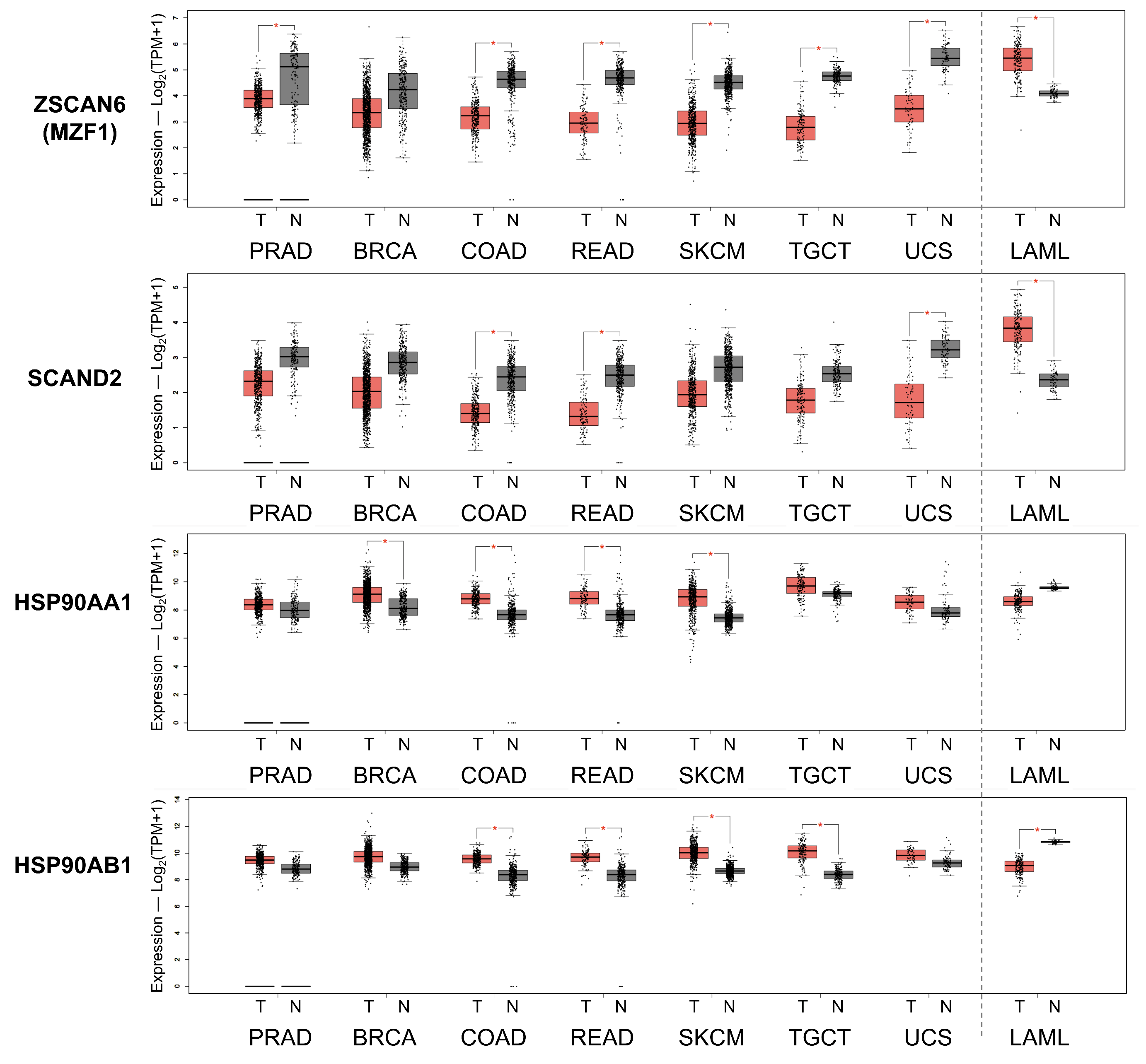
2.6. Reduced Expression of SCAND2 and MZF1 Coincide with the Increased HSP90 Expression in Tumor Tissues Compared with Normal Tissues
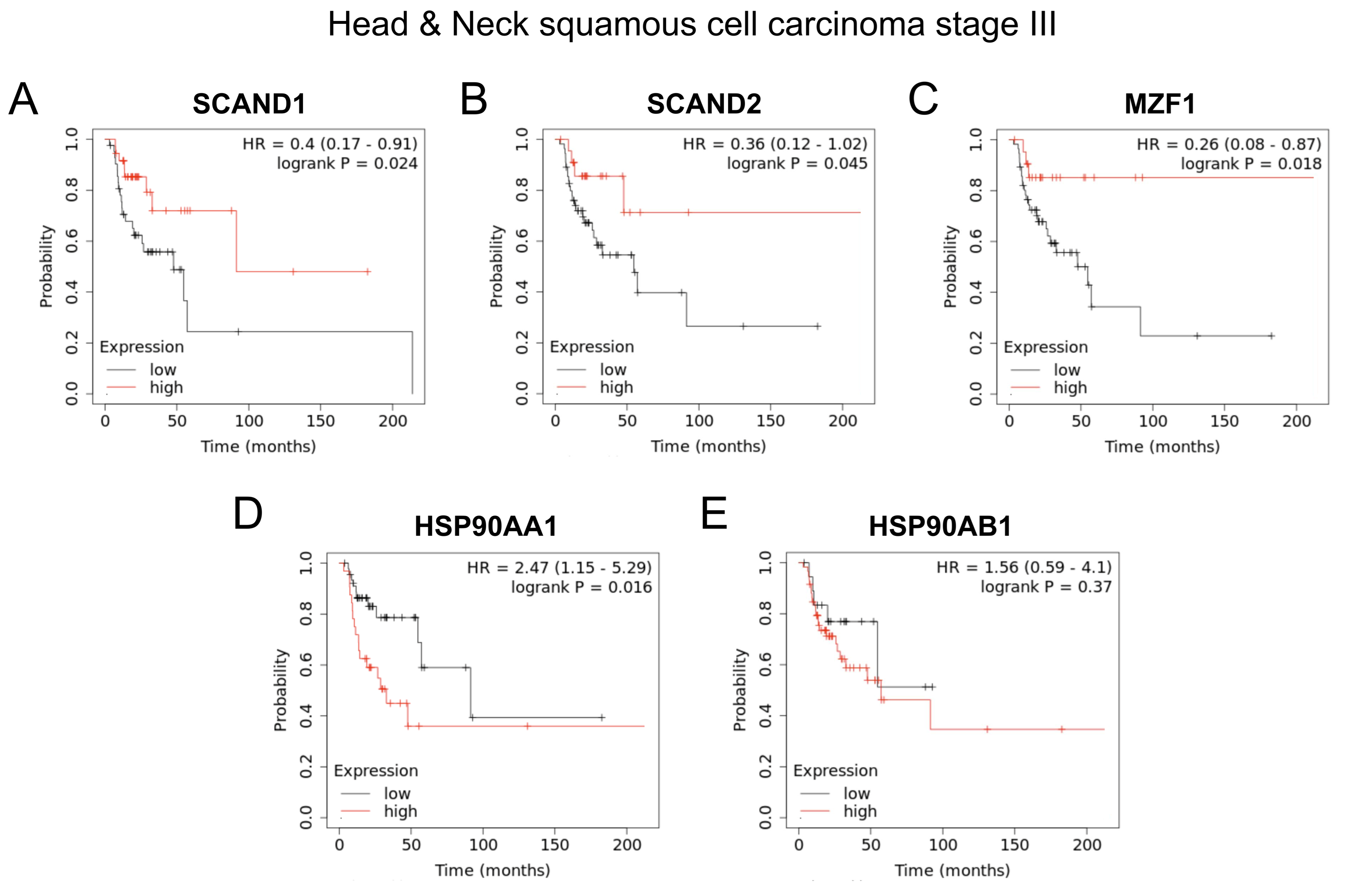
2.7. SCANDs and MZF1(ZSACAN6) Expression Correlates with Enhanced Prognoses Whereas HSP90 Expression Is Correlated with Poor Prognoses in Cancers
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Heat Shock Stress
4.2. Genome and Promoter Analysis
4.3. qRT-PCR
4.4. Plasmids and Transfection
4.5. ChIP
4.6. Immunocytochemistry and Confocal Laser Scanning Microscopy (CLSM)
4.7. Co-Expression Analysis
4.8. Gene Expression Profiling of Tumors vs. Paired Normal Tissues
4.9. Kaplan–Meier Analysis
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morimoto, R.I. The heat shock response: Systems biology of proteotoxic stress in aging and disease. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 91–99. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Murshid, A.; Eguchi, T.; Calderwood, S.K. Stress proteins in aging and life span. Int. J. Hyperth. 2013, 29, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Prince, T.L.; Lang, B.J.; Guerrero-Gimenez, M.E.; Fernandez-Munoz, J.M.; Ackerman, A.; Calderwood, S.K. HSF1: Primary Factor in Molecular Chaperone Expression and a Major Contributor to Cancer Morbidity. Cells 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Sogawa, C.; Ono, K.; Matsumoto, M.; Tran, M.T.; Okusha, Y.; Lang, B.J.; Okamoto, K.; Calderwood, S.K. Cell Stress Induced Stressome Release Including Damaged Membrane Vesicles and Extracellular HSP90 by Prostate Cancer Cells. Cells 2020, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Arrigo, A.P.; Calderwood, S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013, 87, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Lang, B.J.; Murshid, A.; Prince, T.; Gong, J.; Calderwood, S.K. Regulatory roles for Hsp70 in cancer incidence and tumor progression. In Frontiers in Structural Biology; Galigniana, M.D., Ed.; Bentham Science: Sharjah, United Arab Emirates, 2018; Volume 1, pp. 1–22. [Google Scholar]
- Chou, S.D.; Murshid, A.; Eguchi, T.; Gong, J.; Calderwood, S.K. HSF1 regulation of beta-catenin in mammary cancer cells through control of HuR/elavL1 expression. Oncogene 2015, 34, 2178–2188. [Google Scholar] [CrossRef]
- Gong, J.; Weng, D.; Eguchi, T.; Murshid, A.; Sherman, M.Y.; Song, B.; Calderwood, S.K. Targeting the hsp70 gene delays mammary tumor initiation and inhibits tumor cell metastasis. Oncogene 2015, 34, 5460–5471. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T.; Okusha, Y.; Feng, Y.; Sogawa, C.; Eguchi, T.; Kadowaki, T.; Sakai, E.; Tsukuba, T.; Okamoto, K. A novel role of HSP90 in regulating osteoclastogenesis by abrogating Rab11b-driven transport. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119096. [Google Scholar] [CrossRef] [PubMed]
- Furuta, K.; Eguchi, T. Roles of Heat Shock Proteins on Antigen Presentation. In Heat Shock Proteins in Human Diseases; Asea, A.A.A., Kaur, P., Eds.; Springer Nature: Cham, Switzerland, 2020; Volume 21, pp. 275–280. [Google Scholar]
- Lu, Y.; Eguchi, T.; Sogawa, C.; Taha, E.A.; Tran, M.T.; Nara, T.; Wei, P.; Fukuoka, S.; Miyawaki, T.; Okamoto, K. Exosome-Based Molecular Transfer Activity of Macrophage-Like Cells Involves Viability of Oral Carcinoma Cells: Size Exclusion Chromatography and Concentration Filter Method. Cells 2021, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef]
- Ono, K.; Eguchi, T.; Sogawa, C.; Calderwood, S.K.; Futagawa, J.; Kasai, T.; Seno, M.; Okamoto, K.; Sasaki, A.; Kozaki, K. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell Biochem. 2018, 119, 7350–7362. [Google Scholar] [CrossRef]
- Sasaya, T.; Kubo, T.; Murata, K.; Mizue, Y.; Sasaki, K.; Yanagawa, J.; Imagawa, M.; Kato, H.; Tsukahara, T.; Kanaseki, T.; et al. Cisplatin-induced HSF1-HSP90 axis enhances the expression of functional PD-L1 in oral squamous cell carcinoma. Cancer Med. 2022, 12, 4605–4615. [Google Scholar] [CrossRef]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Sogawa, C.; Kawai, H.; Tran, M.T.; Taha, E.A.; Lu, Y.; Oo, M.W.; Okusha, Y.; Okamura, H.; Ibaragi, S.; et al. Triple knockdown of CDC37, HSP90-alpha and HSP90-beta diminishes extracellular vesicles-driven malignancy events and macrophage M2 polarization in oral cancer. J. Extracell. Vesicles 2020, 9, 1769373. [Google Scholar] [CrossRef]
- Eguchi, T.; Ono, K.; Kawata, K.; Okamoto, K.; Calderwood, S.K. Regulatory Roles of HSP90-Rich Extracellular Vesicles. In Heat Shock Protein 90 in Human Diseases and Disorders; Asea, A.A.A., Kaur, P., Eds.; Springer Nature: Cham, Swizerland, 2019; Volume 19, pp. 3–17. [Google Scholar]
- Eguchi, T.; Sogawa, C.; Okusha, Y.; Uchibe, K.; Iinuma, R.; Ono, K.; Nakano, K.; Murakami, J.; Itoh, M.; Arai, K.; et al. Organoids with Cancer Stem Cell-like Properties Secrete Exosomes and HSP90 in a 3D NanoEnvironment. PLoS ONE 2018, 13, e0191109. [Google Scholar] [CrossRef]
- Lu, Y.; Eguchi, T. HSP Stimulation on Macrophages Activates Innate Immune System. In Heat Shock Proteins in Inflammatory Diseases; Asea, A.A.A., Punit, K., Eds.; Springer Nature: Cham, Switzerland, 2021; Volume 22, pp. 53–68. [Google Scholar]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Mendillo, M.L.; Santagata, S.; Koeva, M.; Bell, G.W.; Hu, R.; Tamimi, R.M.; Fraenkel, E.; Ince, T.A.; Whitesell, L.; Lindquist, S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 2012, 150, 549–562. [Google Scholar] [CrossRef]
- Chou, S.D.; Prince, T.; Gong, J.; Calderwood, S.K. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS ONE 2012, 7, e39679. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Calderwood, S.K.; Takigawa, M.; Kubota, S.; Kozaki, K. Intracellular MMP3 Promotes HSP Gene Expression in Collaboration With Chromobox Proteins. J. Cell Biochem. 2017, 118, 43–51. [Google Scholar] [CrossRef]
- Tadepally, H.D.; Burger, G.; Aubry, M. Evolution of C2H2-zinc finger genes and subfamilies in mammals: Species-specific duplication and loss of clusters, genes and effector domains. BMC Evol. Biol. 2008, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, L.C.; Collins, T. The SCAN domain family of zinc finger transcription factors. Gene 2005, 359, 1–17. [Google Scholar] [CrossRef]
- Sander, T.L.; Stringer, K.F.; Maki, J.L.; Szauter, P.; Stone, J.R.; Collins, T. The SCAN domain defines a large family of zinc finger transcription factors. Gene 2003, 310, 29–38. [Google Scholar] [CrossRef]
- Schumacher, C.; Wang, H.; Honer, C.; Ding, W.; Koehn, J.; Lawrence, Q.; Coulis, C.M.; Wang, L.L.; Ballinger, D.; Bowen, B.R.; et al. The SCAN domain mediates selective oligomerization. J. Biol. Chem. 2000, 275, 17173–17179. [Google Scholar] [CrossRef]
- Williams, A.J.; Blacklow, S.C.; Collins, T. The zinc finger-associated SCAN box is a conserved oligomerization domain. Mol. Cell. Biol. 1999, 19, 8526–8535. [Google Scholar] [CrossRef]
- Eguchi, T.; Prince, T.; Wegiel, B.; Calderwood, S.K. Role and Regulation of Myeloid Zinc Finger Protein 1 in Cancer. J. Cell. Biochem. 2015, 116, 2146–2154. [Google Scholar] [CrossRef]
- Sander, T.L.; Haas, A.L.; Peterson, M.J.; Morris, J.F. Identification of a novel SCAN box-related protein that interacts with MZF1B. The leucine-rich SCAN box mediates hetero- and homoprotein associations. J. Biol. Chem. 2000, 275, 12857–12867. [Google Scholar] [CrossRef]
- Perrotti, D.; Melotti, P.; Skorski, T.; Casella, I.; Peschle, C.; Calabretta, B. Overexpression of the zinc finger protein MZF1 inhibits hematopoietic development from embryonic stem cells: Correlation with negative regulation of CD34 and c-myb promoter activity. Mol. Cell. Biol. 1995, 15, 6075–6087. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Prince, T.L.; Tran, M.T.; Sogawa, C.; Lang, B.J.; Calderwood, S.K. MZF1 and SCAND1 Reciprocally Regulate CDC37 Gene Expression in Prostate Cancer. Cancers 2019, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jiao, W.; Mei, H.; Song, H.; Li, D.; Xiang, X.; Chen, Y.; Yang, F.; Li, H.; Huang, K.; et al. miRNA-337-3p inhibits gastric cancer progression through repressing myeloid zinc finger 1-facilitated expression of matrix metalloproteinase 14. Oncotarget 2016, 7, 40314–40328. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Kim, S.; Yang, K.; Kim, K. Phosphorylation-dependent stabilization of MZF1 upregulates N-cadherin expression during protein kinase CK2-mediated epithelial-mesenchymal transition. Oncogenesis 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Mohapatra, B.; Bielecki, T.A.; Mushtaq, I.; Mirza, S.; Jennings, T.A.; Clubb, R.J.; An, W.; Ahmed, D.; El-Ansari, R.; et al. Loss of the Nuclear Pool of Ubiquitin Ligase CHIP/STUB1 in Breast Cancer Unleashes the MZF1-Cathepsin Pro-oncogenic Program. Cancer Res. 2018, 78, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.K.; Gadi, A.; Maurizi, G.; Roy, U.B.; Mansukhani, A.; Basilico, C. Myeloid Zinc Finger 1 and GA Binding Protein Co-Operate with Sox2 in Regulating the Expression of Yes-Associated Protein 1 in Cancer Cells. Stem. Cells 2017, 35, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, L.; Zhou, C.; Wei, W.; Chen, X.; Yi, H.; Wu, X.; Bai, X.; Guo, S.; Yu, Y.; et al. TGF-beta1-induced CK17 enhances cancer stem cell-like properties rather than EMT in promoting cervical cancer metastasis via the ERK1/2-MZF1 signaling pathway. FEBS J. 2017, 284, 3000–3017. [Google Scholar] [CrossRef]
- Eguchi, T.; Csizmadia, E.; Kawai, H.; Sheta, M.; Yoshida, K.; Prince, T.L.; Wegiel, B.; Calderwood, S.K. SCAND1 Reverses Epithelial-to-Mesenchymal Transition (EMT) and Suppresses Prostate Cancer Growth and Migration. Cells 2022, 11, 3993. [Google Scholar] [CrossRef]
- Tsai, S.J.; Hwang, J.M.; Hsieh, S.C.; Ying, T.H.; Hsieh, Y.H. Overexpression of myeloid zinc finger 1 suppresses matrix metalloproteinase-2 expression and reduces invasiveness of SiHa human cervical cancer cells. Biochem. Biophys. Res. Commun. 2012, 425, 462–467. [Google Scholar] [CrossRef]
- Wu, D.; Tan, H.; Su, W.; Cheng, D.; Wang, G.; Wang, J.; Ma, D.A.; Dong, G.M.; Sun, P. MZF1 mediates oncogene-induced senescence by promoting the transcription of p16(INK4A). Oncogene 2022, 41, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Noll, L.; Peterson, F.C.; Hayes, P.L.; Volkman, B.F.; Sander, T. Heterodimer formation of the myeloid zinc finger 1 SCAN domain and association with promyelocytic leukemia nuclear bodies. Leuk. Res. 2008, 32, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, D.; Aubert, I.; Dupérat, V.G.; Petit, J.; Taine, L.; Stef, M.; Bloch, B.; Arveiler, B. Mapping, characterization, and expression analysis of the SM-20 human homologue, c1orf12, and identification of a novel related gene, SCAND2. Genomics 2000, 69, 348–354. [Google Scholar] [CrossRef]
- Anufrieva, K.S.; Shender, V.O.; Arapidi, G.P.; Pavlyukov, M.S.; Shakhparonov, M.I.; Shnaider, P.V.; Butenko, I.O.; Lagarkova, M.A.; Govorun, V.M. Therapy-induced stress response is associated with downregulation of pre-mRNA splicing in cancer cells. Genome Med. 2018, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Akaike, Y.; Masuda, K.; Kuwano, Y.; Nishida, K.; Kajita, K.; Kurokawa, K.; Satake, Y.; Shoda, K.; Imoto, I.; Rokutan, K. HuR regulates alternative splicing of the TRA2beta gene in human colon cancer cells under oxidative stress. Mol. Cell Biol. 2014, 34, 2857–2873. [Google Scholar] [CrossRef]
- Zhou, H.L.; Hinman, M.N.; Barron, V.A.; Geng, C.; Zhou, G.; Luo, G.; Siegel, R.E.; Lou, H. Hu proteins regulate alternative splicing by inducing localized histone hyperacetylation in an RNA-dependent manner. Proc. Natl. Acad. Sci. USA 2011, 108, E627–E635. [Google Scholar] [CrossRef]
- Lee, S.; Wei, L.; Zhang, B.; Goering, R.; Majumdar, S.; Wen, J.; Taliaferro, J.M.; Lai, E.C. ELAV/Hu RNA binding proteins determine multiple programs of neural alternative splicing. PLoS Genet. 2021, 17, e1009439. [Google Scholar] [CrossRef]
- Izquierdo, J.M. Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J. Biol. Chem. 2008, 283, 19077–19084. [Google Scholar] [CrossRef]
- Lin, J.-C.; Hsu, M.; Tarn, W.-Y. Cell stress modulates the function of splicing regulatory protein RBM4 in translation control. Proc. Natl. Acad. Sci. USA 2007, 104, 2235–2240. [Google Scholar] [CrossRef]
- Nakai, A.; Tanabe, M.; Kawazoe, Y.; Inazawa, J.; Morimoto, R.I.; Nagata, K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell. Biol. 1997, 17, 469–481. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Cheng, P.; Yue, K.; Tang, M.; Li, Y.; Guo, Q.; Zhang, Y. HSF4 promotes tumor progression of colorectal cancer by transactivating c-MET. Mol. Cell. Biochem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Tang, W.G.; Hu, J.W.; Hao, Y.; Xiong, L.K.; Wang, M.M.; Liu, H.; Bo, W.H.; Yu, K.H. HSP4 triggers epithelial-mesenchymal transition and promotes motility capacities of hepatocellular carcinoma cells via activating AKT. Liver Int. 2020, 40, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Eroglu, B.; Cho, W.; Yamaguchi, Y.; Moskophidis, D.; Mivechi, N.F. Inactivation of heat shock factor Hsf4 induces cellular senescence and suppresses tumorigenesis in vivo. Mol. Cancer Res. 2012, 10, 523–534. [Google Scholar] [CrossRef]
- Eguchi, T.; Sheta, M.; Fujii, M.; Calderwood, S.K. Cancer Extracellular Vesicles, Tumoroid Models, and Tumor Microenvironment. Semin. Cancer Biol. 2022, 86, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Namba, Y.; Sogawa, C.; Okusha, Y.; Kawai, H.; Itagaki, M.; Ono, K.; Murakami, J.; Aoyama, E.; Ohyama, K.; Asaumi, J.; et al. Depletion of Lipid Efflux Pump ABCG1 Triggers the Intracellular Accumulation of Extracellular Vesicles and Reduces Aggregation and Tumorigenesis of Metastatic Cancer Cells. Front. Oncol. 2018, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kawai, H.; Eguchi, T.; Sukegawa, S.; Oo, M.W.; Anqi, C.; Takabatake, K.; Nakano, K.; Okamoto, K.; Nagatsuka, H. Tumor Angiogenic Inhibition Triggered Necrosis (TAITN) in Oral Cancer. Cells 2019, 8, 761. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Fais, S.; Venturi, G.; Gatenby, B. Microenvironmental acidosis in carcinogenesis and metastases: New strategies in prevention and therapy. Cancer Metastasis Rev. 2014, 33, 1095–1108. [Google Scholar] [CrossRef]
- Ischia, J.; So, A.I. The role of heat shock proteins in bladder cancer. Nat. Rev. Urol. 2013, 10, 386–395. [Google Scholar] [CrossRef]
- Torigoe, T.; Tamura, Y.; Sato, N. Heat shock proteins and immunity: Application of hyperthermia for immunomodulation. Int. J. Hyperth. 2009, 25, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, V.R.; Michalak, M.; Abdullah, T.M.; Bremer, E.; Eggleton, P. Mechanisms of Translocation of ER Chaperones to the Cell Surface and Immunomodulatory Roles in Cancer and Autoimmunity. Front. Oncol. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, Y.; Saito, A.; Imaizumi, K. Impact of Nuclear Envelope Stress on Physiological and Pathological Processes in Central Nervous System. Neurochem. Res. 2022, 47, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Panagaki, D.; Croft, J.T.; Keuenhof, K.; Larsson Berglund, L.; Andersson, S.; Kohler, V.; Buttner, S.; Tamas, M.J.; Nystrom, T.; Neutze, R.; et al. Nuclear envelope budding is a response to cellular stress. Proc. Natl. Acad. Sci. USA 2021, 118, e2020997118. [Google Scholar] [CrossRef]
- Xu, B.; Sun, Z.; Liu, Z.; Guo, H.; Liu, Q.; Jiang, H.; Zou, Y.; Gong, Y.; Tischfield, J.A.; Shao, C. Replication stress induces micronuclei comprising of aggregated DNA double-strand breaks. PLoS ONE 2011, 6, e18618. [Google Scholar] [CrossRef]
- Ahn, S.G.; Thiele, D.J. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003, 17, 516–528. [Google Scholar] [CrossRef]
- Gong, J.L.; Lang, B.J.; Weng, D.S.; Eguchi, T.; Murshid, A.; Borges, T.J.; Doshi, S.; Song, B.Z.; Stevenson, M.A.; Calderwood, S.K. Genotoxic stress induces Sca-1-expressing metastatic mammary cancer cells. Mol. Oncol. 2018, 12, 1249–1263. [Google Scholar] [CrossRef]
- Hitomi, K.; Okada, R.; Loo, T.M.; Miyata, K.; Nakamura, A.J.; Takahashi, A. DNA Damage Regulates Senescence-Associated Extracellular Vesicle Release via the Ceramide Pathway to Prevent Excessive Inflammatory Responses. Int. J. Mol. Sci. 2020, 21, 3720. [Google Scholar] [CrossRef]
- Guang, M.H.Z.; Kavanagh, E.L.; Dunne, L.P.; Dowling, P.; Zhang, L.; Lindsay, S.; Bazou, D.; Goh, C.Y.; Hanley, C.; Bianchi, G.; et al. Targeting Proteotoxic Stress in Cancer: A Review of the Role that Protein Quality Control Pathways Play in Oncogenesis. Cancers 2019, 11, 66. [Google Scholar] [CrossRef]
- Okusha, Y.; Eguchi, T.; Tran, M.T.; Sogawa, C.; Yoshida, K.; Itagaki, M.; Taha, E.A.; Ono, K.; Aoyama, E.; Okamura, H.; et al. Extracellular Vesicles Enriched with Moonlighting Metalloproteinase Are Highly Transmissive, Pro-Tumorigenic, and Trans-Activates Cellular Communication Network Factor (CCN2/CTGF): CRISPR against Cancer. Cancers 2020, 12, 881. [Google Scholar] [CrossRef]
- Taha, E.A.; Sogawa, C.; Okusha, Y.; Kawai, H.; Oo, M.W.; Elseoudi, A.; Lu, Y.; Nagatsuka, H.; Kubota, S.; Satoh, A.; et al. Knockout of MMP3 Weakens Solid Tumor Organoids and Cancer Extracellular Vesicles. Cancers 2020, 12, 1260. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2022, 24, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Z.; Zhang, W.; Ye, Z.; Liu, F. GEPIA2021: Integrating multiple deconvolution-based analysis into GEPIA. Nucleic. Acids Res. 2021, 49, W242–W246. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, Q.; Shanmugam, R.; Piskol, R.; Kohler, J.; Young, A.N.; Liu, K.I.; Zhang, R.; Ramaswami, G.; Ariyoshi, K.; et al. Dynamic landscape and regulation of RNA editing in mammals. Nature 2017, 550, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Munkacsy, G.; Gyorffy, B. Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef]
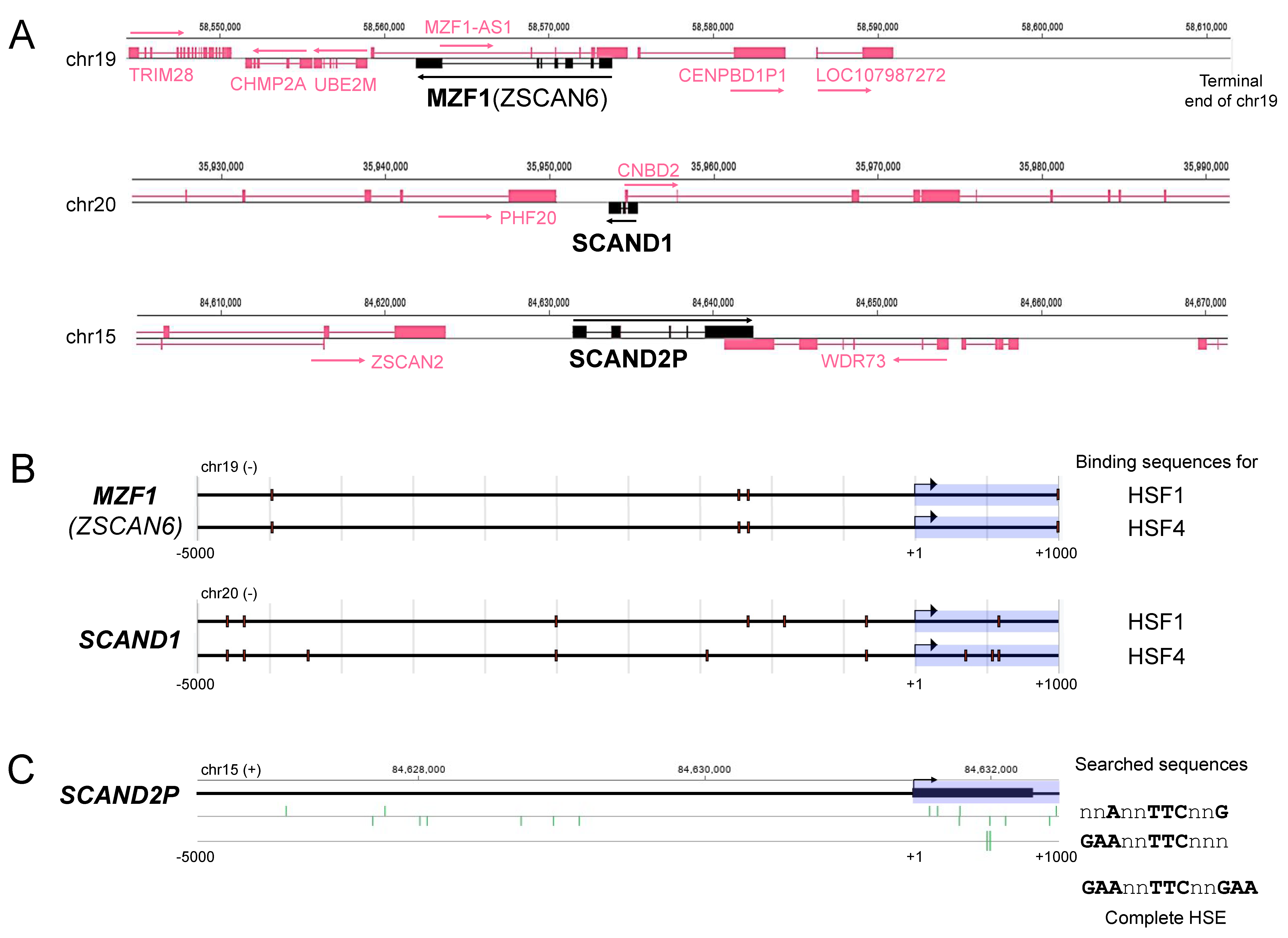


| Gene Promoter 1 | HSF1-BS | HSF4-BS | MZF1-BS | MZF1-BS var.2 |
|---|---|---|---|---|
| SCAND1 | 7 | 9 | 5 | 15 |
| MZF1 | 4 | 4 | 27 | 30 |
| Gene | Correlated Gene | Spearman’s Correlation 1 | p-Value 2 | q-Value 3 |
|---|---|---|---|---|
| HSF1 vs. | MZF1 | 0.375 | 9.52 × 10–18 | 7.37 × 10–17 |
| HSF1 vs. | SCAND1 | 0.518 | 6.73 × 10–35 | 2.92 × 10–33 |
| HSF1 vs. | SCAND2 | 0.0925 | 4.12 × 10–2 | 6.42 × 10–2 |
| HSF4 vs. | MZF1 | 0.705 | 1.97 × 10–74 | 3.03 × 10–72 |
| HSF4 vs. | SCAND1 | 0.585 | 3.99 × 10–46 | 1.86 × 10–44 |
| HSF4 vs. | SCAND2 | 0.55 | 5.55 × 10–40 | 1.89 × 10–38 |
| Gene | Correlated Gene | Spearman’s Correlation 1 | p-Value 2 | q-Value 3 |
|---|---|---|---|---|
| MZF1 vs. | SCAND1 | 0.548 | 1.27 × 10–39 | 6.16 × 10–38 |
| MZF1 vs. | SCAND2 | 0.524 | 1.01 × 10–35 | 3.95 × 10–34 |
| SCAND1 vs. | SCAND2 | 0.170 | 2.20 × 10–4 | 4.16 × 10–4 |
| Promoter | HSF1-BS | HSF4-BS | MZF1-BS | MZF1-BS var.2 |
|---|---|---|---|---|
| HSP90AA1 | 9 | 12 | 22 | 18 |
| HSP90AB1 | 8 | 8 | 22 | 28 |
| Gene | Correlated Gene | Spearman’s Correlation 1 | p-Value 2 | q-Value 3 |
|---|---|---|---|---|
| HSP90AA1 vs. | MZF1 | −0.321 | 3.63 × 10–13 | 3.35 × 10–11 |
| HSP90AA1 vs. | SCAND2 | −0.32 | 4.34 × 10–13 | 3.88 × 10–11 |
| HSP90AA1 vs. | SCAND1 | −0.188 | 2.86 × 10–5 | 1.71 × 10–4 |
| HSP90AA1 vs. | HSF4 | −0.241 | 6.97 × 10–8 | 9.73 × 10–7 |
| HSP90AA1 vs. | HSF1 | −0.045 | 3.21 × 10–1 | 4.38 × 10–1 |
| HSP90AA1 vs. | HSF2 | −0.0164 | 7.17 × 10–1 | 7.94 × 10–1 |
| HSP90AA1 vs. | HSF5 | −0.00297 | 9.48 × 10–1 | 0.963 × 10–1 |
| vs. MZF1 | vs. SCAND1 | vs. SCAND2 | ||||
|---|---|---|---|---|---|---|
| Correlated Gene | Spearman’s Correlation 1 | p-Value 2 | Spearman’s Correlation | p-Value | Spearman’s Correlation | p-Value |
| HSPA13 | −0.448 | 1.75 × 10–25 | −0.572 | 7.79 × 10–44 | −0.357 | 4.18 × 10–16 |
| HSPA4 | −0.358 | 3.41 × 10–16 | −0.303 | 7.97 × 10–12 | −0.394 | 1.52 × 10–19 |
| HSPA4L | −0.326 | 1.58 × 10–13 | −0.411 | 2.66 × 10–21 | −0.0341 | 4.52 × 10–1 |
| HSP90AA1 | −0.321 | 3.63 × 10–13 | −0.188 | 2.86 × 10–5 | −0.32 | 4.34 × 10–13 |
| HSPH1 | −0.300 | 1.42 × 10–11 | −0.191 | 2.20 × 10–5 | −0.312 | 1.67 × 10–12 |
| Cancer Type | Log-Rank P | N | ||
|---|---|---|---|---|
| SCAND2 | SCAND1 | MZF1 | ||
| Pancreatic adenocarcinoma | 0.00018 *** | 0.0041 ** | 0.0009 ** | 177 |
| Head & Neck SCC (stage III) | 0.045 * | 0.024 * | 0.018 * | 78 |
| Lung adenocarcinoma | 0.00032 *** | 0.32 | 0.21 | 504 |
| Sarcoma | 0.0096 ** | 0.11 | 0.14 | 259 |
| Cervical SCC | 0.015 * | 0.34 | 0.21 | 304 |
| Primer Name | Sequences (5′ to 3′) |
|---|---|
| HSP90AA1 h −100F | GGCTGGGGAGGGTTCTTC |
| HSP90AA1 h +200R | GAGGCCTCCGGAATAGAAAG |
| HSP90AB1 h −800F | CCTGAGGATTGGGCTGGTA |
| HSP90AB1 h −430R | CATCTGCCCTACACATCTCG |
| HSP90AB1 h +600F | GTCTCCAGCACCCGATACTC |
| HSP90AB1 h +900R | GAACAGGACCAAACCCAAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheta, M.; Yoshida, K.; Kanemoto, H.; Calderwood, S.K.; Eguchi, T. Stress-Inducible SCAND Factors Suppress the Stress Response and Are Biomarkers for Enhanced Prognosis in Cancers. Int. J. Mol. Sci. 2023, 24, 5168. https://doi.org/10.3390/ijms24065168
Sheta M, Yoshida K, Kanemoto H, Calderwood SK, Eguchi T. Stress-Inducible SCAND Factors Suppress the Stress Response and Are Biomarkers for Enhanced Prognosis in Cancers. International Journal of Molecular Sciences. 2023; 24(6):5168. https://doi.org/10.3390/ijms24065168
Chicago/Turabian StyleSheta, Mona, Kunihiro Yoshida, Hideka Kanemoto, Stuart K. Calderwood, and Takanori Eguchi. 2023. "Stress-Inducible SCAND Factors Suppress the Stress Response and Are Biomarkers for Enhanced Prognosis in Cancers" International Journal of Molecular Sciences 24, no. 6: 5168. https://doi.org/10.3390/ijms24065168
APA StyleSheta, M., Yoshida, K., Kanemoto, H., Calderwood, S. K., & Eguchi, T. (2023). Stress-Inducible SCAND Factors Suppress the Stress Response and Are Biomarkers for Enhanced Prognosis in Cancers. International Journal of Molecular Sciences, 24(6), 5168. https://doi.org/10.3390/ijms24065168









