Long-Term Evaluation of Retinal Morphology and Function in Rosa26-Cas9 Knock-In Mice
Abstract
1. Introduction
2. Results
2.1. Cas9 Knock-In Mice Exhibit Ubiquitous Expression of SpCas9 in RPE and Retinal Layers
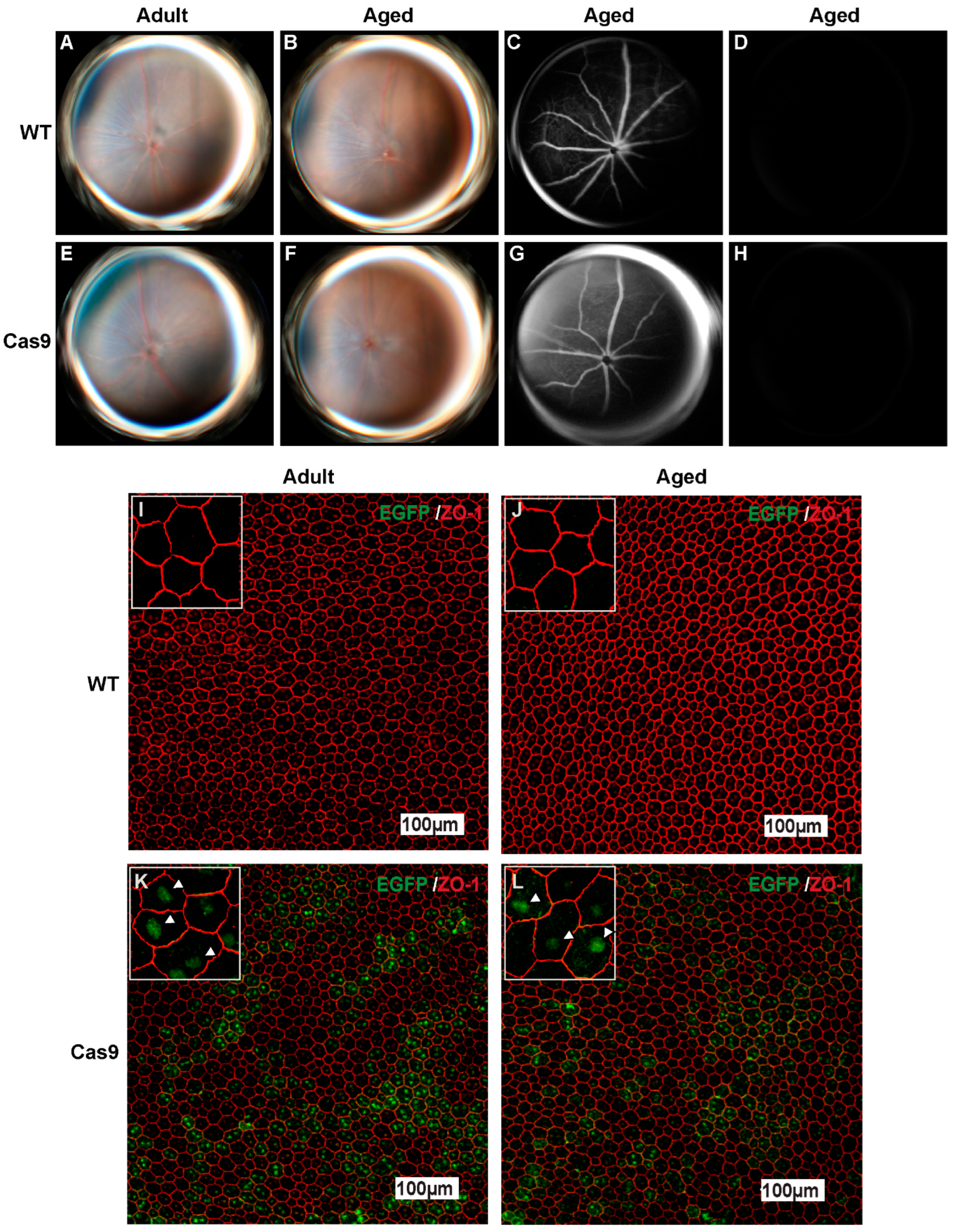
2.2. Effects of Long-Term SpCas9 Expression on Retinal Phenotype in Adult and Aged Cas9 Mice
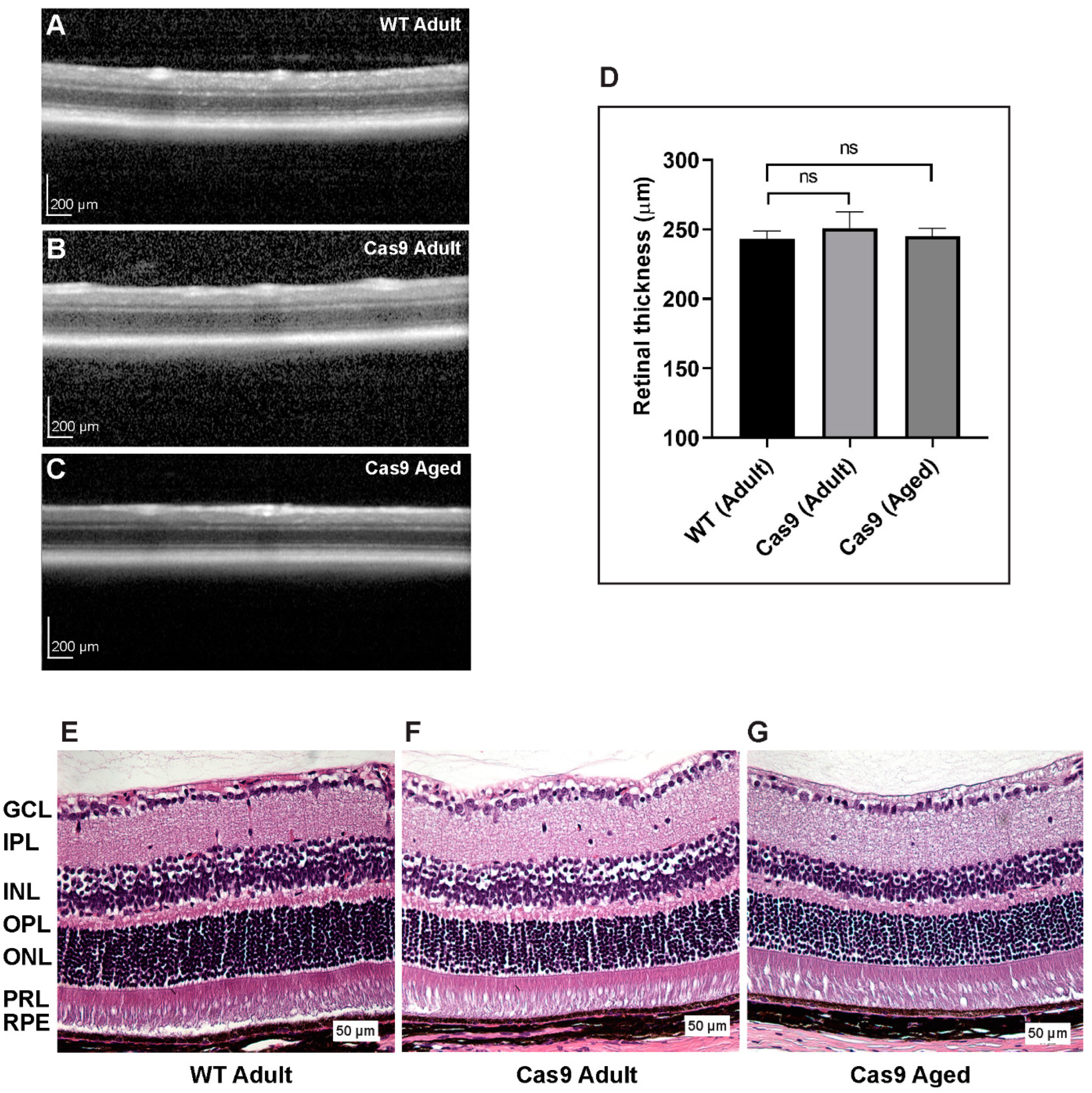
2.3. Examination of Retinal Thickness and Histologic Integrity in Mice with Endogenous Expression of SpCas9
2.4. Assessment of Retinal Function by Electroretinography
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Rd8 Sequencing in Cas9 Mice
4.3. Mice Anesthesia
4.4. Fundus Imaging
4.5. Electroretinography
4.6. Spectral-Domain Optical Coherence Tomography (SD-OCT)
4.7. Flat Mount Preparation and Immunofluorescence
4.8. H&E Staining
4.9. qPCR
4.10. Western Blot
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Mir, A.; Edraki, A.; Lee, J.; Sontheimer, E.J. Type II-C CRISPR-Cas9 Biology, Mechanism, and Application. ACS Chem. Biol. 2018, 13, 357–365. [Google Scholar] [CrossRef]
- Sapranauskas, R.; Gasiunas, G.; Fremaux, C.; Barrangou, R.; Horvath, P.; Siksnys, V. The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 2011, 39, 9275–9282. [Google Scholar] [CrossRef]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [CrossRef]
- Bibikova, M.; Beumer, K.; Trautman, J.K.; Carroll, D. Enhancing gene targeting with designed zinc finger nucleases. Science 2003, 300, 764. [Google Scholar] [CrossRef]
- Bibikova, M.; Carroll, D.; Segal, D.J.; Trautman, J.K.; Smith, J.; Kim, Y.G.; Chandrasegaran, S. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol. Cell. Biol. 2001, 21, 289–297. [Google Scholar] [CrossRef]
- Jasin, M.; de Villiers, J.; Weber, F.; Schaffner, W. High frequency of homologous recombination in mammalian cells between endogenous and introduced SV40 genomes. Cell 1985, 43, 695–703. [Google Scholar] [CrossRef]
- Rudin, N.; Sugarman, E.; Haber, J.E. Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae. Genetics 1989, 122, 519–534. [Google Scholar] [CrossRef]
- Chung, S.H.; Sin, T.N.; Dang, B.; Ngo, T.; Lo, T.; Lent-Schochet, D.; Meleppat, R.K.; Zawadzki, R.J.; Yiu, G. CRISPR-based VEGF suppression using paired guide RNAs for treatment of choroidal neovascularization. Mol. Ther. Nucleic Acids 2022, 28, 613–622. [Google Scholar] [CrossRef]
- Jo, D.H.; Song, D.W.; Cho, C.S.; Kim, U.G.; Lee, K.J.; Lee, K.; Park, S.W.; Kim, D.; Kim, J.H.; Kim, J.S.; et al. CRISPR-Cas9-mediated therapeutic editing of Rpe65 ameliorates the disease phenotypes in a mouse model of Leber congenital amaurosis. Sci. Adv. 2019, 5, eaax1210. [Google Scholar] [CrossRef]
- Kim, K.; Park, S.W.; Kim, J.H.; Lee, S.H.; Kim, D.; Koo, T.; Kim, K.E.; Kim, J.H.; Kim, J.S. Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res. 2017, 27, 419–426. [Google Scholar] [CrossRef]
- Koo, T.; Park, S.W.; Jo, D.H.; Kim, D.; Kim, J.H.; Cho, H.Y.; Kim, J.; Kim, J.H.; Kim, J.S. CRISPR-LbCpf1 prevents choroidal neovascularization in a mouse model of age-related macular degeneration. Nat. Commun. 2018, 9, 1855. [Google Scholar] [CrossRef]
- Chew, W.L.; Tabebordbar, M.; Cheng, J.K.; Mali, P.; Wu, E.Y.; Ng, A.H.; Zhu, K.; Wagers, A.J.; Church, G.M. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat. Methods 2016, 13, 868–874. [Google Scholar] [CrossRef]
- Hung, S.S.; Chrysostomou, V.; Li, F.; Lim, J.K.; Wang, J.H.; Powell, J.E.; Tu, L.; Daniszewski, M.; Lo, C.; Wong, R.C.; et al. AAV-Mediated CRISPR/Cas Gene Editing of Retinal Cells In Vivo. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3470–3476. [Google Scholar] [CrossRef]
- Jo, D.H.; Koo, T.; Cho, C.S.; Kim, J.H.; Kim, J.S.; Kim, J.H. Long-Term Effects of In Vivo Genome Editing in the Mouse Retina Using Campylobacter jejuni Cas9 Expressed via Adeno-Associated Virus. Mol. Ther. 2019, 27, 130–136. [Google Scholar] [CrossRef]
- Toral, M.A.; Charlesworth, C.T.; Ng, B.; Chemudupati, T.; Homma, S.; Nakauchi, H.; Bassuk, A.G.; Porteus, M.H.; Mahajan, V.B. Investigation of Cas9 antibodies in the human eye. Nat. Commun. 2022, 13, 1053. [Google Scholar] [CrossRef]
- Kim, E.; Koo, T.; Park, S.W.; Kim, D.; Kim, K.; Cho, H.Y.; Song, D.W.; Lee, K.J.; Jung, M.H.; Kim, S.; et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat. Commun. 2017, 8, 14500. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wing, K.; Wang, J.H.; Luu, C.D.; Bender, J.A.; Chen, J.; Wang, Q.; Lu, Q.; Nguyen Tran, M.T.; Young, K.M.; et al. Comparison of CRISPR/Cas Endonucleases for in vivo Retinal Gene Editing. Front. Cell. Neurosci. 2020, 14, 570917. [Google Scholar] [CrossRef] [PubMed]
- Platt, R.J.; Chen, S.; Zhou, Y.; Yim, M.J.; Swiech, L.; Kempton, H.R.; Dahlman, J.E.; Parnas, O.; Eisenhaure, T.M.; Jovanovic, M.; et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 2014, 159, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Burnight, E.R.; Gupta, M.; Wiley, L.A.; Anfinson, K.R.; Tran, A.; Triboulet, R.; Hoffmann, J.M.; Klaahsen, D.L.; Andorf, J.L.; Jiao, C.; et al. Using CRISPR-Cas9 to Generate Gene-Corrected Autologous iPSCs for the Treatment of Inherited Retinal Degeneration. Mol. Ther. 2017, 25, 1999–2013. [Google Scholar] [CrossRef]
- Fuster-Garcia, C.; Garcia-Garcia, G.; Gonzalez-Romero, E.; Jaijo, T.; Sequedo, M.D.; Ayuso, C.; Vazquez-Manrique, R.P.; Millan, J.M.; Aller, E. USH2A Gene Editing Using the CRISPR System. Mol. Ther. Nucleic Acids 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Hu, S.; Du, J.; Chen, N.; Jia, R.; Zhang, J.; Liu, X.; Yang, L. In Vivo CRISPR/Cas9-Mediated Genome Editing Mitigates Photoreceptor Degeneration in a Mouse Model of X-Linked Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2020, 61, 31. [Google Scholar] [CrossRef]
- Li, P.; Kleinstiver, B.P.; Leon, M.Y.; Prew, M.S.; Navarro-Gomez, D.; Greenwald, S.H.; Pierce, E.A.; Joung, J.K.; Liu, Q. Allele-Specific CRISPR-Cas9 Genome Editing of the Single-Base P23H Mutation for Rhodopsin-Associated Dominant Retinitis Pigmentosa. CRISPR J. 2018, 1, 55–64. [Google Scholar] [CrossRef]
- Ye, G.J.; Budzynski, E.; Sonnentag, P.; Nork, T.M.; Sheibani, N.; Gurel, Z.; Boye, S.L.; Peterson, J.J.; Boye, S.E.; Hauswirth, W.W.; et al. Cone-Specific Promoters for Gene Therapy of Achromatopsia and Other Retinal Diseases. Hum. Gene Ther. 2016, 27, 72–82. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Thompson, J.A.; Chen, S.C.; Huang, Z.; Jennings, L.; McLaren, T.L.; Lamey, T.M.; De Roach, J.N.; Chen, F.K.; et al. Gene correction of the CLN3 c.175G>A variant in patient-derived induced pluripotent stem cells prevents pathological changes in retinal organoids. Mol. Genet. Genom. Med. 2021, 9, e1601. [Google Scholar] [CrossRef]
- Hakim, C.H.; Kumar, S.R.P.; Perez-Lopez, D.O.; Wasala, N.B.; Zhang, D.; Yue, Y.; Teixeira, J.; Pan, X.; Zhang, K.; Million, E.D.; et al. Cas9-specific immune responses compromise local and systemic AAV CRISPR therapy in multiple dystrophic canine models. Nat. Commun. 2021, 12, 6769. [Google Scholar] [CrossRef]
- Mehta, A.; Merkel, O.M. Immunogenicity of Cas9 Protein. J. Pharm. Sci. 2020, 109, 62–67. [Google Scholar] [CrossRef]
- Wang, D.; Mou, H.; Li, S.; Li, Y.; Hough, S.; Tran, K.; Li, J.; Yin, H.; Anderson, D.G.; Sontheimer, E.J.; et al. Adenovirus-Mediated Somatic Genome Editing of Pten by CRISPR/Cas9 in Mouse Liver in Spite of Cas9-Specific Immune Responses. Hum. Gene Ther. 2015, 26, 432–442. [Google Scholar] [CrossRef]
- Sarkar, A.; Jin, Y.; DeFelice, B.C.; Logan, C.Y.; Yang, Y.; Anbarchian, T.; Wu, P.; Morri, M.; Neff, N.F.; Nguyen, H.; et al. Intermittent fasting induces rapid hepatocyte proliferation to restore the hepatostat in the mouse liver. Elife 2023, 12. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, P.; Wei, J.; Long, L.; Shi, H.; Dhungana, Y.; Chapman, N.M.; Fu, G.; Saravia, J.; Raynor, J.L.; et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8(+) T cell fate decisions. Cell 2021, 184, 1245–1261.e1221. [Google Scholar] [CrossRef]
- Laprie-Sentenac, M.; Cretet-Rodeschini, C.; Menger, L. Optimized protocol to generate genome-wide inactivated Cas9-expressing murine T cells. STAR Protoc. 2022, 4, 101922. [Google Scholar] [CrossRef]
- Burnight, E.R.; Wiley, L.A.; Drack, A.V.; Braun, T.A.; Anfinson, K.R.; Kaalberg, E.E.; Halder, J.A.; Affatigato, L.M.; Mullins, R.F.; Stone, E.M.; et al. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014, 21, 662–672. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, W.; Zhu, X.; Mao, Y.; Liu, X.; Chen, X.; Huang, J.; Tang, S.; Rizzolo, L.J. Differential expression of claudins in retinas during normal development and the angiogenesis of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7556–7564. [Google Scholar] [CrossRef]
- Olsson, J.E.; Gordon, J.W.; Pawlyk, B.S.; Roof, D.; Hayes, A.; Molday, R.S.; Mukai, S.; Cowley, G.S.; Berson, E.L.; Dryja, T.P. Transgenic mice with a rhodopsin mutation (Pro23His): A mouse model of autosomal dominant retinitis pigmentosa. Neuron 1992, 9, 815–830. [Google Scholar] [CrossRef]
- Seo, S.; Mullins, R.F.; Dumitrescu, A.V.; Bhattarai, S.; Gratie, D.; Wang, K.; Stone, E.M.; Sheffield, V.; Drack, A.V. Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6118–6132. [Google Scholar] [CrossRef]
- Tan, E.; Wang, Q.; Quiambao, A.B.; Xu, X.; Qtaishat, N.M.; Peachey, N.S.; Lem, J.; Fliesler, S.J.; Pepperberg, D.R.; Naash, M.I.; et al. The relationship between opsin overexpression and photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci. 2001, 42, 589–600. [Google Scholar]
- Schirmbeck, R.; Reimann, J.; Kochanek, S.; Kreppel, F. The immunogenicity of adenovirus vectors limits the multispecificity of CD8 T-cell responses to vector-encoded transgenic antigens. Mol. Ther. 2008, 16, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Ruan, M.Z.C.; Mahajan, V.B.; Tsang, S.H. Viral Delivery Systems for CRISPR. Viruses 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Keller, B.; Makalou, N.; Sutton, R.E. Systematic determination of the packaging limit of lentiviral vectors. Hum. Gene Ther. 2001, 12, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, J.; Ge, S.; Lai, L. CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research. Front. Med. 2021, 8, 649896. [Google Scholar] [CrossRef]
- Wang, Q.J.; Jung, K.S.; Mohan, K.; Kleinman, M.E. Imaging data on characterization of retinal autofluorescent lesions in a mouse model of juvenile neuronal ceroid lipofuscinosis (CLN3 disease). Data Brief 2020, 32, 106076. [Google Scholar] [CrossRef]

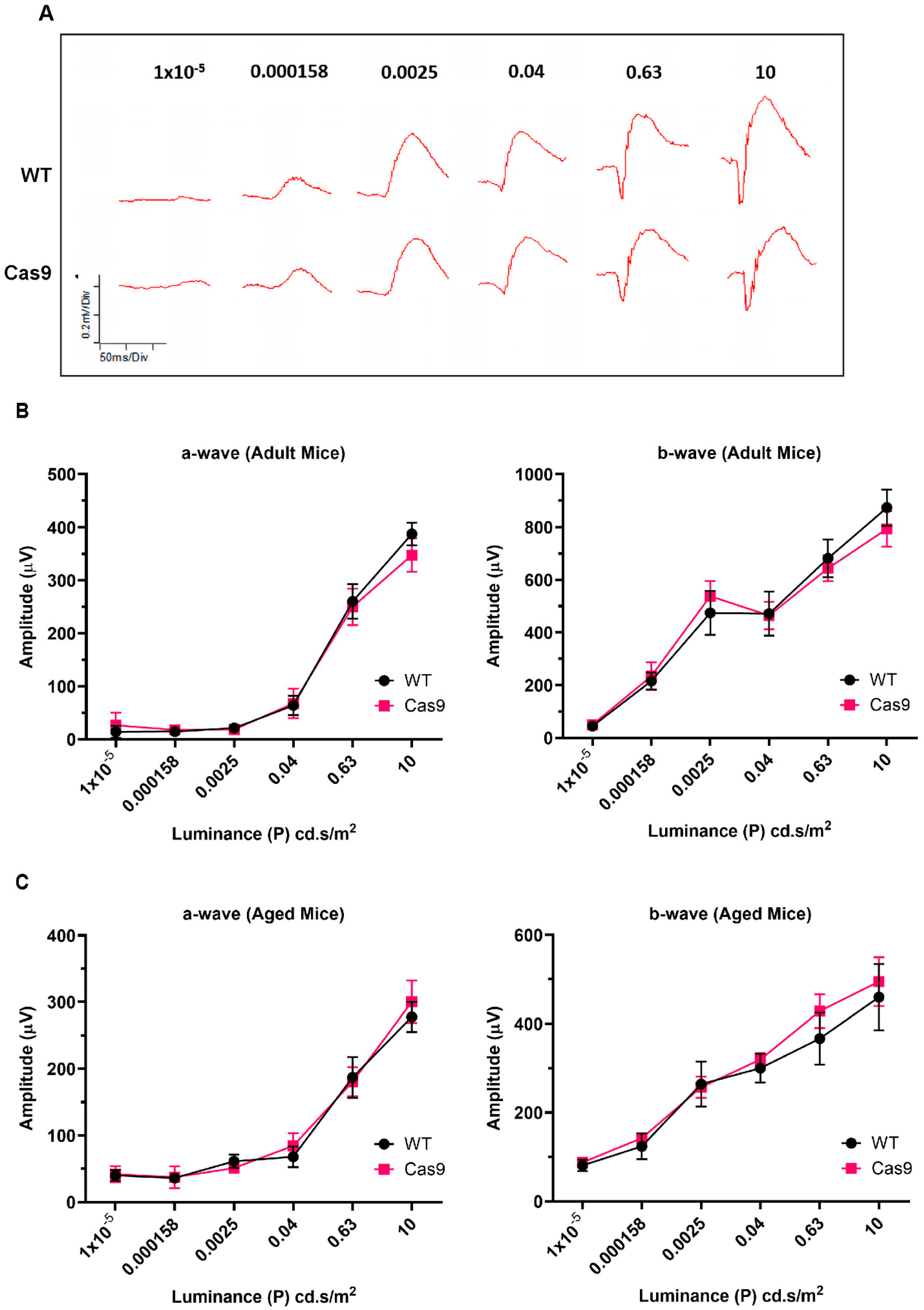
| Flash Intensity ((P) cd.s/m2) | a-Wave (4–6 Months) | b-Wave (4–6 Months) | a-Wave (10–14 Months) | b-Wave (10–14 Months) |
|---|---|---|---|---|
| 0.00001 | 0.4136 | 0.1812 | 0.6991 | 0.2403 |
| 0.000158 | 0.7546 | 0.5728 | 0.9372 | 0.132 |
| 0.0025 | 0.4908 | 0.1419 | 0.0649 | >0.9999 |
| 0.04 | 0.662 | 0.7546 | 0.132 | 0.1797 |
| 0.63 | 0.5728 | 0.345 | 0.6991 | 0.0931 |
| 10 | 0.0513 | 0.0732 | 0.3095 | 0.2403 |
| Flash Intensity ((P) cd.s/m2) | a-Wave Implicit Time (ms) | p_Value | b-Wave Implicit Time (ms) | p_Value | a-Wave Implicit Time (ms) | p_Value | b-Wave Implicit Time (ms) | p_Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult-WT | Adult-Cas9 | Adult-WT | Adult-Cas9 | Aged-WT | Aged-Cas9 | Aged-WT | Aged-Cas9 | |||||
| 0.00001 | 43.33 ± 2.84 | 43.5 ± 3.44 | 0.8168 | 111.5 ± 1.59 | 115.75 ± 1.74 | 0.0846 | 29.52 ± 1.40 | 31.34 ± 0.70 | 0.513 | 90.26 ± 3.78 | 97.73 ± 1.56 | 0.2403 |
| 0.000158 | 26 ± 4.74 | 31 ± 3.66 | 0.5851 | 110.67 ± 2.22 | 114.63 ± 1.70 | 0.0949 | 35.27 ± 1.07 | 33.81 ± 0.87 | 0.3095 | 104.05 ± 2.52 | 102.71 ± 1.51 | 0.6991 |
| 0.0025 | 26.33 ± 0.33 | 24.88 ± 2.51 | 0.1951 | 111 ± 3.48 | 112.75 ± 1.97 | 0.9191 | 34.74 ± 2.74 | 33.65 ± 1.66 | 0.5087 | 98.94 ± 2.94 | 95.57 ± 2.34 | 0.1797 |
| 0.04 | 25.67 ± 0.76 | 26.88 ± 0.91 | 0.4659 | 79.83 ± 1.76 | 86.88 ± 4.09 | 0.3097 | 29.28 ± 1.45 | 26.86 ± 0.85 | 0.0584 | 85 ± 1.79 | 81.47 ± 1.58 | 0.132 |
| 0.63 | 20.17 ± 0.65 | 22 ± 0.78 | 0.0696 | 83.67 ± 0.88 | 87.75 ± 3.48 | 0.7529 | 23.07 ± 1.42 | 21.2 ± 0.43 | 0.2359 | 70.13 ± 4.51 | 72.6 ± 1.97 | 0.9372 |
| 10 | 10.17 ± 0.17 | 10.75 ± 0.25 | 0.1189 | 75.83 ± 1.25 | 81.88 ± 3.15 | 0.2384 | 18.72 ± 0.72 | 17.17 ± 0.61 | 0.145 | 70.33 ± 8.07 | 63.17 ± 2.23 | 0.5584 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, K.; Dubey, S.K.; Jung, K.; Dubey, R.; Wang, Q.J.; Prajapati, S.; Roney, J.; Abney, J.; Kleinman, M.E. Long-Term Evaluation of Retinal Morphology and Function in Rosa26-Cas9 Knock-In Mice. Int. J. Mol. Sci. 2023, 24, 5186. https://doi.org/10.3390/ijms24065186
Mohan K, Dubey SK, Jung K, Dubey R, Wang QJ, Prajapati S, Roney J, Abney J, Kleinman ME. Long-Term Evaluation of Retinal Morphology and Function in Rosa26-Cas9 Knock-In Mice. International Journal of Molecular Sciences. 2023; 24(6):5186. https://doi.org/10.3390/ijms24065186
Chicago/Turabian StyleMohan, Kabhilan, Sushil Kumar Dubey, Kyungsik Jung, Rashmi Dubey, Qing Jun Wang, Subhash Prajapati, Jacob Roney, Jennifer Abney, and Mark Ellsworth Kleinman. 2023. "Long-Term Evaluation of Retinal Morphology and Function in Rosa26-Cas9 Knock-In Mice" International Journal of Molecular Sciences 24, no. 6: 5186. https://doi.org/10.3390/ijms24065186
APA StyleMohan, K., Dubey, S. K., Jung, K., Dubey, R., Wang, Q. J., Prajapati, S., Roney, J., Abney, J., & Kleinman, M. E. (2023). Long-Term Evaluation of Retinal Morphology and Function in Rosa26-Cas9 Knock-In Mice. International Journal of Molecular Sciences, 24(6), 5186. https://doi.org/10.3390/ijms24065186







