A Unique In Vitro Assay to Investigate ABCB4 Transport Function
Abstract
1. Introduction
2. Results
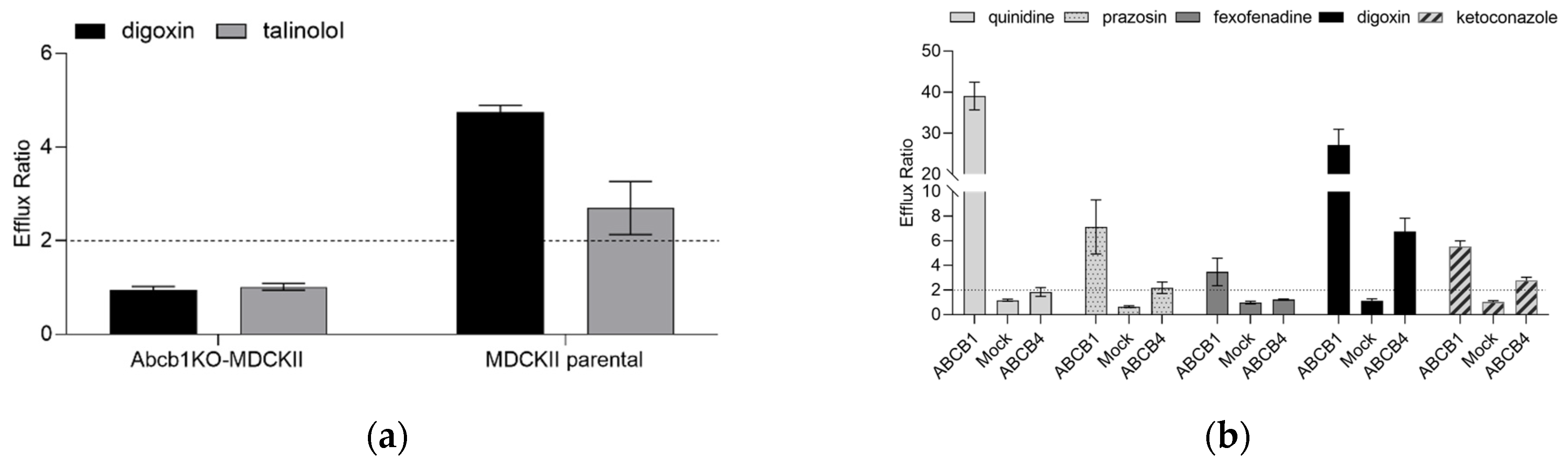
2.1. Characterization of the Abcb1 Knockout MDCKII Cell Line Engineered to Express ABCB4
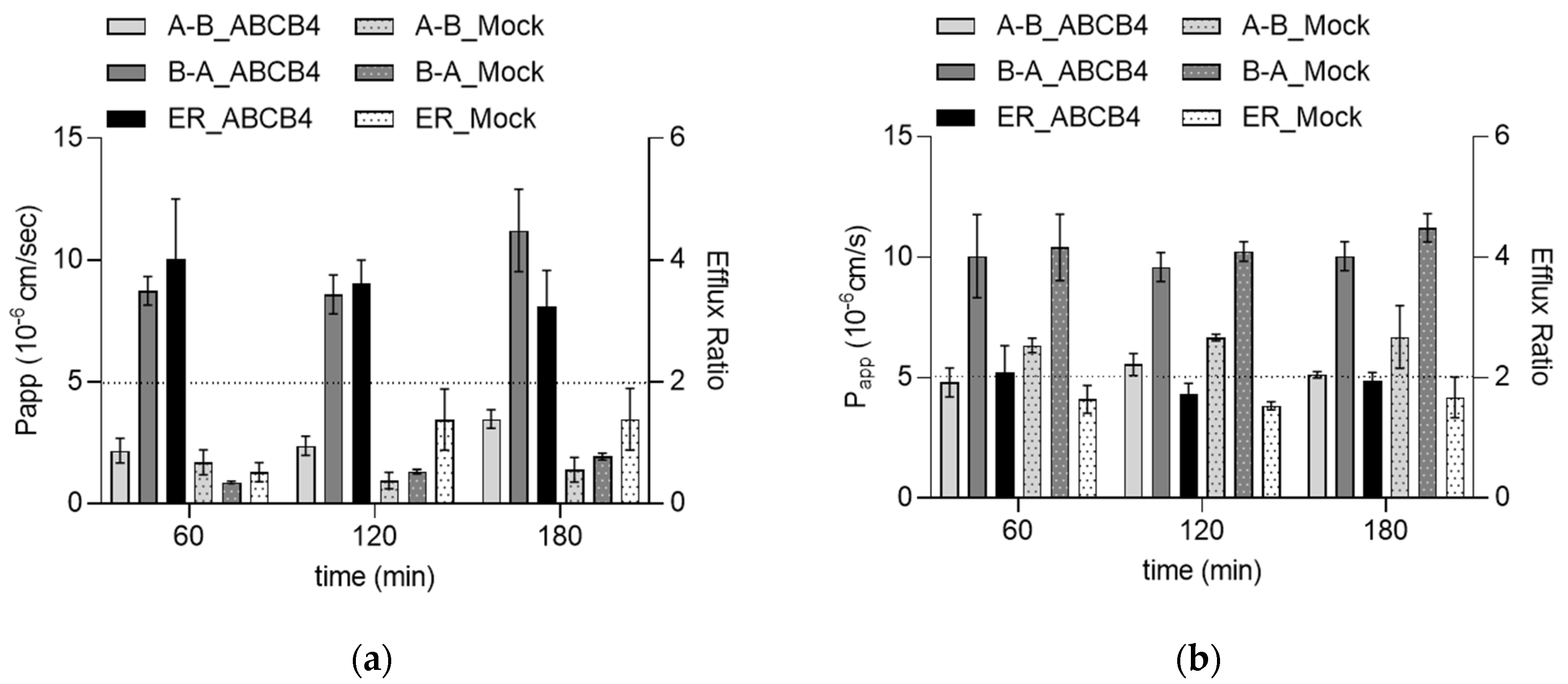
2.2. Characterization of ABCB4 Transporter Function in the Abcb1KO-MDCKII-ABCB4 Cell Line in Comparison with ABCB1 Function in Abcb1KO-MDCKII-ABCB1 Cells
2.3. Screening ABCB4 Interactors with Different DILI Concern to Test the Predictive Potential of the ABCB4 Transport Assay Using Digoxin
| DILI Concern | Compound | IC50 (µM), Based on Digoxin ER | In Vitro Literature Data (IC50, µM) | ABCB1 Interactor |
|---|---|---|---|---|
| Most DILI concern | gefitinib | 0.81 | a | Y [43] |
| imatinib | 1.24 | a | Y [44] | |
| sorafenib | 4.42 | a | Y [45] | |
| erlotinib | >5 µM, 60% inhibition at 5 µM | a | Y [46] | |
| fluconazole | >3, NI | >300 [36] | N [38] | |
| itraconazole | 0.17 | 2.1 [25], 30% inhibition at 10 µM [35], 50% inhibition at 1 µM [24], 22.5 µM [40] | Y [38] | |
| ketoconazole | 0.56 | 5.6 [25], 4.6 [40] | Y [38] | |
| lopinavir | 0.6 | a | Y [47] | |
| ritonavir | 0.73 | 9.6 [25], 11.3 [36] | Y [47] | |
| darunavir | >10 µM, 60% inhibition at 10 µM | a | Y [48] | |
| amiodarone | >10, NI | >300 [36], >100 [40] | Y [49] | |
| benzbromarone | 19.89 | 0.4 [36] | Y (in house data) | |
| carbamazepine | >80, NI | >300 [36] | Conflicting information [50] | |
| clarithromycin | >80, NI | a | Y [51] | |
| methotrexate | >10, NI | 3.1 [36] | Y [52] | |
| levofloxacin | >80, NI | a | Y [53] | |
| diltiazem | 48.61 | a | Y [54] | |
| cyclosporin A | 0.46 | >100 b [55], inhibitor d [16] | Y [56] | |
| Less DILI concern | saquinavir | 1.4 | 12.9 [25] | Y [57] |
| fenofibrate | >20, NI | a | Y [58] | |
| ivermectin | 0.53 | Substrate [16] | Y [47] | |
| amlodipine | 17.86 | a | Y [41] | |
| pantoprazole | >10, NI | a | Y [59] | |
| felodipine | >30, NI | a | Y [60] | |
| carvedilol | 0.70 | a | Y [61] | |
| quinidine | 1.09 | a | Y [62] | |
| verapamil | 0.39 | 6.3 [25], 7 [36], inhibitor d [16] | Y [63] | |
| No DILI concern | minoxidil | >20, NI | >300 [36] | N [64] |
| furosemide | >80, NI | >100 [25] | Conflicting information [65] | |
| acetylsalicylic acid | >100, NI | >300 [36] | N [66] | |
| other | idelalisib | >5, NI | a | Y [67] |
| asunaprevir c | >5, NI | a | Y [68] | |
| valspodar | 0.15 | Inhibitor d [16] | Y [69] | |
| prazosin e | 16.12 | a | Y [70] | |
| zosuquidar | 0.07 | a | Y [71] | |
| mibefradil | 0.40 | a | Y [72] | |
| elacridar | 0.15 | a | Y [73] |
2.4. Identification of a Novel ABCB4 Transporter Substrate
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines and Culture Conditions
4.3. Calcein AM-Based Fluorescence-Activated Cell Sorting (FACS)
4.4. Bidirectional Transport Assays
4.5. LC-MS Sample Preparation and Analytics
4.6. Cell Monolayer Integrity
4.7. Real-Time qPCR
4.8. Western Blot
4.9. Calculations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, A.J.; de Vree, J.M.; Ottenhoff, R.; Elferink, R.P.; Schinkel, A.H.; Borst, P. Hepatocyte-specific expression of the human MDR3 P-glycoprotein gene restores the biliary phosphati-dylcholine excretion absent in Mdr2 (-/-) mice. Hepatology 1998, 28, 530–536. [Google Scholar] [CrossRef]
- Smith, A.J.; Timmermans-Hereijgers, J.L.; Roelofsen, B.; Wirtz, K.W.; Van Blitterswijk, W.J.; Smit, J.J.; Schinkel, A.H.; Borst, P. The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett. 1994, 354, 263–266. [Google Scholar] [CrossRef]
- Prescher, M.; Smits, S.H.J.; Schmitt, L. Stimulation of ABCB4/MDR3 ATPase activity requires an intact phosphatidylcholine lipid. J. Lipid Res. 2020, 61, 1605–1616. [Google Scholar] [CrossRef]
- Linton, K.J. Lipid flopping in the liver. Biochem. Soc. Trans. 2015, 43, 1003–1010. [Google Scholar] [CrossRef]
- Smit, J.; Schinkel, A.; Elferink, R.; Groen, A.; Wagenaar, E.; van Deemter, L.; Mol, C.; Ottenhoff, R.; van der Lugt, N.; van Roon, M.; et al. Homozygous disruption of the murine MDR2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993, 75, 451–462. [Google Scholar] [CrossRef]
- Wang, H.H.; Portincasa, P.; Liu, M.; Wang, D.Q.-H. Effects of Biliary Phospholipids on Cholesterol Crystallization and Growth in Gallstone Formation. Adv. Ther. 2023, 40, 1–26. [Google Scholar] [CrossRef]
- Stättermayer, A.F.; Halilbasic, E.; Wrba, F.; Ferenci, P.; Trauner, M. Variants in ABCB4 (MDR3) across the spectrum of cholestatic liver diseases in adults. J. Hepatol. 2020, 73, 651–663. [Google Scholar] [CrossRef]
- Biyoukar, M.; Corpechot, C.; El Mouhadi, S.; Chambenois, E.; Vanderbecq, Q.; Barbu, V.; Dong, C.; Lemoinne, S.; Tordjman, M.; Jomaah, R.; et al. A BCB4 variant is associated with hepatobiliary MR abnormalities in people with low-phospholipid-associated cholelithiasis syndrome. JHEP Rep. 2022, 4, 100590. [Google Scholar] [CrossRef]
- Wang, H.H.; Portincasa, P.; Liu, M.; Wang, D.Q.-H. Genetic Analysis of ABCB4 Mutations and Variants Related to the Pathogenesis and Pathophysiology of Low Phospholipid-Associated Cholelithiasis. Genes 2022, 13, 1047. [Google Scholar] [CrossRef]
- van der Bliek, A.; Kooiman, P.; Schneider, C.; Borst, P. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene 1988, 71, 401–411. [Google Scholar] [CrossRef]
- Olsen, J.A.; Alam, A.; Kowal, J.; Stieger, B.; Locher, K.P. Structure of the human lipid exporter ABCB4 in a lipid environment. Nat. Struct. Mol. Biol. 2019, 27, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Cuperus, F.J.C.; Claudel, T.; Gautherot, J.; Halilbasic, E.; Trauner, M. The Role of Canalicular ABC Transporters in Cholestasis. Drug Metab. Dispos. 2014, 42, 546–560. [Google Scholar] [CrossRef]
- Van Helvoort, A.; Smith, A.; Sprong, H.; Fritzsche, I.; Schinkel, A.; Borst, P.; van Meer, G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specif-ically translocates phosphatidylcholine. Cell 1996, 87, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.-Y.; Kobayashi, A.; Takanezawa, Y.; Kioka, N.; Handa, T.; Arai, H.; Matsuo, M.; Ueda, K. Bile salt–dependent efflux of cellular phospholipids mediated by ATP binding cassette protein B4. Hepatology 2007, 46, 188–199. [Google Scholar] [CrossRef]
- Ruetz, S. Phosphatidylcholine translocase: A physiological role for the mdr2 gene. Cell 1994, 77, 1071–1081. [Google Scholar] [CrossRef]
- Smith, A.J.; van Helvoort, A.; van Meer, G.; Szabó, K.; Welker, E.; Szakács, G.; Váradi, A.; Sarkadi, B.; Borst, P. MDR3 P-glycoprotein, a Phosphatidylcholine Translocase, Transports Several Cytotoxic Drugs and Directly Interacts with Drugs as Judged by Interference with Nucleotide Trapping. J. Biol. Chem. 2000, 275, 23530–23539. [Google Scholar] [CrossRef]
- Ishigami, M.; Tominaga, Y.; Nagao, K.; Kimura, Y.; Matsuo, M.; Kioka, N.; Ueda, K. ATPase activity of nucleotide binding domains of human MDR3 in the context of MDR1. Biochim. Et Biophys. Acta (BBA) -Mol. Cell Biol. Lipids 2013, 1831, 683–690. [Google Scholar] [CrossRef]
- Januchowski, R.; Wojtowicz, K.; Andrzejewska, M.; Zabel, M. Expression of MDR1 and MDR3 gene products in paclitaxel-, doxorubicin- and vincristine-resistant cell lines. Biomed. Pharmacother. 2014, 68, 111–117. [Google Scholar] [CrossRef]
- Němcová-Fürstová, V.; Kopperová, D.; Balušíková, K.; Ehrlichová, M.; Brynychová, V.; Václavíková, R.; Daniel, P.; Souček, P.; Kovář, J. Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol. Appl. Pharmacol. 2016, 310, 215–228. [Google Scholar] [CrossRef]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef]
- Hontecillas-Prieto, L.; Garcia-Dominguez, D.J.; Vaca, D.P.; Garcia-Mejias, R.; Marcilla, D.; Ramirez-Villar, G.L.; Saez, C.; de Álava, E. Multidrug resistance transporter profile reveals MDR3 as a marker for stratification of blastemal Wilms tumour patients. Oncotarget 2017, 8, 11173–11186. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B. Drug safety sciences and the bottleneck in drug development. Clin. Pharmacol. Ther. 2011, 89, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Weaver, R.J.; Blomme, E.A.; Chadwick, A.; Copple, I.M.; Gerets, H.H.J.; Goldring, C.E.; Guillouzo, A.; Hewitt, P.G.; Ingelman-Sundberg, M.; Jensen, K.G.; et al. Managing the challenge of drug-induced liver injury: A roadmap for the development and deployment of preclinical predictive models. Nat. Rev. Drug Discov. 2019, 19, 131–148. [Google Scholar] [CrossRef]
- Yoshikado, T.; Takada, T.; Yamamoto, T.; Yamaji, H.; Ito, K.; Santa, T.; Yokota, H.; Yatomi, Y.; Yoshida, H.; Goto, J.; et al. Itraconazole-induced cholestasis: Involvement of the inhibition of bile canalicular phospholipid trans-locator MDR3/ABCB4. Mol. Pharmacol. 2011, 79, 241–250. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Cai, L.; Shi, Q.; Liu, H.; Woolf, T.F. Inhibition of MDR3 Activity in Human Hepatocytes by Drugs Associated with Liver Injury. Chem. Res. Toxicol. 2015, 28, 1987–1990. [Google Scholar] [CrossRef]
- Bénichou, C. Criteria of drug-induced liver disorders: Report of an International Consensus Meeting. J. Hepatol. 1990, 11, 272–276. [Google Scholar] [CrossRef]
- Yang, K.; Köck, K.; Sedykh, A.; Tropsha, A.; Brouwer, K.L. An updated review on drug-induced cholestasis: Mechanisms and investigation of physicochemical properties and pharmacokinetic parameters. J. Pharm. Sci. 2013, 102, 3037–3057. [Google Scholar] [CrossRef]
- Chatterjee, S. Drug-induced Cholestasis: Mechanisms, Models, and Markers. Curr. Drug Metab. 2018, 19, 808–818. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Stieger, B.; Meier, Y.; Kullak-Ublick, G.A.; Meier, P.J. Enterohepatic transport of bile salts and genetics of cholestasis. J. Hepatol. 2005, 43, 342–357. [Google Scholar] [CrossRef]
- Hafey, M.J.; Houle, R.; Tanis, K.Q.; Knemeyer, I.; Shang, J.; Chen, Q.; Baudy, A.; Monroe, J.; Sistare, F.D.; Evers, R. A Two-Tiered In Vitro Approach to De-Risk Drug Candidates for Potential Bile Salt Export Pump Inhibition Liabilities in Drug Discovery. Drug Metab. Dispos. 2020, 48, 1147–1160. [Google Scholar] [CrossRef]
- Schadt, S.; Simon, S.; Kustermann, S.; Boess, F.; McGinnis, C.; Brink, A.; Lieven, R.; Fowler, S.; Youdim, K.; Ullah, M.; et al. Minimizing DILI risk in drug discovery—A screening tool for drug candidates. Toxicol. Vitr. 2015, 30, 429–437. [Google Scholar] [CrossRef]
- Chan, R.; Benet, L.Z. Measures of BSEP Inhibition In Vitro Are Not Useful Predictors of DILI. Toxicol. Sci. 2017, 162, 499–508. [Google Scholar] [CrossRef]
- Morgan, R.E.; van Staden, C.J.; Chen, Y.; Kalyanaraman, N.; Kalanzi, J.; Dunn, R.T., II; Afshari, C.A.; Hamadeh, H.K. A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic com-pound development. Toxicol. Sci. 2013, 136, 216–241. [Google Scholar] [CrossRef]
- Köck, K.; Ferslew, B.C.; Netterberg, I.; Yang, K.; Urban, T.J.; Swaan, P.; Stewart, P.W.; Brouwer, K.L.R. Risk Factors for Development of Cholestatic Drug-Induced Liver Injury: Inhibition of Hepatic Basolateral Bile Acid Transporters Multidrug Resistance-Associated Proteins 3 and 4. Drug Metab. Dispos. 2013, 42, 665–674. [Google Scholar] [CrossRef]
- Mahdi, Z.M.; Synal-Hermanns, U.; Yoker, A.; Locher, K.P.; Stieger, B. Role of Multidrug Resistance Protein 3 in Antifungal-Induced Cholestasis. Mol. Pharmacol. 2016, 90, 23–34. [Google Scholar] [CrossRef]
- Aleo, M.D.; Shah, F.; He, K.; Bonin, P.D.; Rodrigues, A.D. Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem. Res. Toxicol. 2017, 30, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Tupova, L.; Ceckova, M.; Temesszentandrási-Ambrus, C.; Šorf, A.; Ptackova, Z.; Gaborik, Z.; Staud, F. Interactions between Maraviroc and the ABCB1, ABCG2, and ABCC2 Transporters: An Important Role in Transplacental Pharmacokinetics. Drug Metab. Dispos. 2019, 47, 954–960. [Google Scholar] [CrossRef]
- Wang, E.-J.; Lew, K.; Casciano, C.N.; Clement, R.P.; Johnson, W.W. Interaction of Common Azole Antifungals with P Glycoprotein. Antimicrob. Agents Chemother. 2002, 46, 160–165. [Google Scholar] [CrossRef]
- Elsby, R.; Surry, D.D.; Smith, V.N.; Gray, A.J. Validation and application of Caco-2 assays for the in vitro evaluation of development candidate drugs as substrates or inhibitors of P-glycoprotein to support regulatory submissions. Xenobiotica 2008, 38, 1140–1164. [Google Scholar] [CrossRef]
- Yucha, R.W.; He, K.; Shi, Q.; Cai, L.; Nakashita, Y.; Xia, C.Q.; Liao, M. In Vitro Drug-Induced Liver Injury Prediction: Criteria Optimization of Efflux Transporter IC50 and Physicochemical Properties. Toxicol. Sci. 2017, 157, 487–499. [Google Scholar] [CrossRef]
- Katoh, M.; Nakajima, M.; Yamazaki, H.; Yokoi, T. Inhibitory potencies of 1,4-dihydropyridine calcium antagonists to P-glycoprotein-mediated transport: Comparison with the effects on CYP3A4. Pharm. Res. 2000, 17, 1189–1197. [Google Scholar] [CrossRef]
- de Weerdt, I.; Koopmans, S.M.; Kater, A.P.; van Gelder, M. Incidence and management of toxicity associated with ibrutinib and idelalisib: A practical approach. Haematologica 2017, 102, 1629–1639. [Google Scholar] [CrossRef]
- Leggas, M.; Panetta, J.C.; Zhuang, Y.; Schuetz, J.D.; Johnston, B.; Bai, F.; Sorrentino, B.; Zhou, S.; Houghton, P.J.; Stewart, C.F. Gefitinib Modulates the Function of Multiple ATP-Binding Cassette Transporters In Vivo. Cancer Res. 2006, 66, 4802–4807. [Google Scholar] [CrossRef]
- Thomas, J.; Wang, L.; Clark, R.E.; Pirmohamed, M. Active transport of imatinib into and out of cells: Implications for drug resistance. Blood 2004, 104, 3739–3745. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Ohlfest, J.R.; Elmquist, W.F. The Role of the Breast Cancer Resistance Protein (ABCG2) in the Distribution of Sorafenib to the Brain. J. Pharmacol. Exp. Ther. 2011, 336, 223–233. [Google Scholar] [CrossRef]
- Marchetti, S.; de Vries, N.A.; Buckle, T.; Bolijn, M.J.; van Eijndhoven, M.A.J.; Beijnen, J.H.; Mazzanti, R.; van Tellingen, O.; Schellens, J.H.M. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydro-chloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol. Cancer Ther. 2008, 7, 2280–2287. [Google Scholar] [CrossRef]
- Telbisz, A.; Ambrus, C.; Mózner, O.; Szabó, E.; Várady, G.; Bakos, E.; Sarkadi, B.; Özvegy-Laczka, C. Interactions of Potential Anti-COVID-19 Compounds with Multispecific ABC and OATP Drug Transporters. Pharmaceutics 2021, 13, 81. [Google Scholar] [CrossRef]
- Fujimoto, H.; Higuchi, M.; Watanabe, H.; Koh, Y.; Ghosh, A.K.; Mitsuya, H.; Tanoue, N.; Hamada, A.; Saito, H. P-Glycoprotein Mediates Efflux Transport of Darunavir in Human Intestinal Caco-2 and ABCB1 Gene-Transfected Renal LLC-PK1 Cell Lines. Biol. Pharm. Bull. 2009, 32, 1588–1593. [Google Scholar] [CrossRef]
- Mendell, J.; Zahir, H.; Matsushima, N.; Noveck, R.; Lee, F.; Chen, S.; Zhang, G.; Shi, M. Drug-Drug Interaction Studies of Cardiovascular Drugs Involving P-Glycoprotein, an Efflux Transporter, on the Pharmacokinetics of Edoxaban, an Oral Factor Xa Inhibitor. Am. J. Cardiovasc. Drugs 2013, 13, 331–342. [Google Scholar] [CrossRef]
- Fuhr, L.M.; Marok, F.Z.; Hanke, N.; Selzer, D.; Lehr, T. Pharmacokinetics of the CYP3A4 and CYP2B6 Inducer Carbamazepine and Its Drug-Drug Interaction Potential: A Physiologically Based Pharmacokinetic Modeling Approach. Pharmaceutics 2021, 13, 270. [Google Scholar] [CrossRef]
- Lam, A.; Hoang, J.D.; Singleton, A.; Han, X.; Bleier, B.S. Itraconazole and clarithromycin inhibit P-glycoprotein activity in primary human sinonasal epithelial cells. Int. Forum Allergy Rhinol. 2015, 5, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.; Tanaka, Y. Potential of B-cell-targeting therapy in overcoming multidrug resistance and tissue invasiveness associated with P-glycoprotein expressing-B cell compartments. Immunol. Med. 2021, 44, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Sikri, V.; Pal, D.; Jain, R.; Kalyani, D.; Mitra, A.K. Cotransport of Macrolide and Fluoroquinolones, a Beneficial Interaction Reversing P-glycoprotein Efflux. Am. J. Ther. 2004, 11, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.E.; Byon, W.; Song, Y.; Wang, J.; Schuster, A.E.; Boyd, R.A.; Zhang, D.; Yu, Z.; Dias, C.; Shenker, A.; et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br. J. Clin. Pharmacol. 2015, 79, 838–846. [Google Scholar] [CrossRef]
- Abe, T.; Unno, M.; Onogawa, T.; Tokui, T.; Kondo, T.N.; Nakagomi, R.; Adachi, H.; Fujiwara, K.; Okabe, M.; Suzuki, T.; et al. LST-2, A human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 2001, 120, 1689–1699. [Google Scholar] [CrossRef]
- Anglicheau, D.; Pallet, N.; Rabant, M.; Marquet, P.; Cassinat, B.; Méria, P.; Beaune, P.; Legendre, C.; Thervet, E. Role of P-glycoprotein in cyclosporine cytotoxicity in the cyclosporine–sirolimus interaction. Kidney Int. 2006, 70, 1019–1025. [Google Scholar] [CrossRef]
- Kim, A.E.; Dintaman, J.M.; Waddell, D.S.; Silverman, J.A. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J. Pharmacol. Exp. Ther. 1998, 286, 1439–1445. [Google Scholar]
- Ehrhardt, M.; Lindenmaier, H.; Burhenne, J.; Haefeli, W.; Weiss, J. Influence of lipid lowering fibrates on P-glycoprotein activity in vitro. Biochem. Pharmacol. 2003, 67, 285–292. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Rekersbrink, S.; Klotz, U.; Fromm, M.F. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001, 364, 551–557. [Google Scholar] [CrossRef]
- Höll, V.; Kouba, M.; Dietel, M.; Vogt, G. Stereoisomers of calcium antagonists which differ markedly in their potencies as calcium blockers are equally effective in modulating drug transport by P-glycoprotein. Biochem. Pharmacol. 1992, 43, 2601–2608. [Google Scholar] [CrossRef]
- Kakumoto, M.; Sakaeda, T.; Takara, K.; Nakamura, T.; Kita, T.; Yagami, T.; Kobayashi, H.; Okamura, N.; Okumura, K. Effects of carvedilol on MDR1 -mediated multidrug resistance: Comparison with verapamil. Cancer Sci. 2003, 94, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.F.; Kim, R.B.; Stein, C.M.; Wilkinson, G.R.; Roden, D.M. Inhibition of P-glycoprotein-mediated drug transport: A unifying mechanism to explain the interaction between digoxin and quinidine. Circulation 1999, 99, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Tsume, Y.; Zur, M.; Dahan, A.; Amidon, G.L. Intestinal Permeability Study of Minoxidil: Assessment of Minoxidil as a High Permeability Reference Drug for Biopharmaceutics Classification. Mol. Pharm. 2014, 12, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Hoosain, F.G.; Choonara, Y.; Tomar, L.K.; Kumar, P.; Tyagi, C.; du Toit, L.; Pillay, V. Bypassing P-Glycoprotein Drug Efflux Mechanisms: Possible Applications in Pharmacoresistant Schizophrenia Therapy. BioMed Res. Int. 2015, 2015, 484963. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Naik, T.; Nigam, A.; Chatterjee, S.; Rajanna, P.; Shen, H.; Iyer, R. Is aspirin a substrate of MDR1/P-glycoprotein? Xenobiotica 2020, 50, 1258–1264. [Google Scholar] [CrossRef]
- Jin, F.; Nd, M.R.; Zhou, H.; Moyer, C.; Wilbert, S.; Murray, B.; Ramanathan, S. Clinical drug interaction profile of idelalisib in healthy subjects. J. Clin. Pharmacol. 2015, 55, 909–919. [Google Scholar] [CrossRef]
- Eley, T.; Garimella, T.; Li, W.; Bertz, R.J. Asunaprevir: A Review of Preclinical and Clinical Pharmacokinetics and Drug–Drug Interactions. Clin. Pharmacokinet. 2015, 54, 1205–1222. [Google Scholar] [CrossRef]
- Tai, H.L. Technology evaluation: Valspodar, Novartis AG. Curr. Opin. Mol. Ther. 2000, 2, 459–467. [Google Scholar]
- Shapiro, A.; Fox, K.; Lam, P.; Ling, V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. JBIC J. Biol. Inorg. Chem. 2001, 259, 841–850. [Google Scholar] [CrossRef]
- Rubin, E.H.; de Alwis, D.P.; Pouliquen, I.; Green, L.; Marder, P.; Lin, Y.; Musanti, R.; Grospe, S.L.; Smith, S.L.; Toppmeyer, D.L.; et al. A phase I trial of a potent P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), ad-ministered orally in combination with doxorubicin in patients with advanced malignancies. Clin. Cancer Res. 2002, 8, 3710–3717. [Google Scholar] [PubMed]
- Wandel, C.; Kim, R.B.; Guengerich, F.P.; Wood, A.J. Mibefradil is a P-glycoprotein substrate and a potent inhibitor of both P-glycoprotein and CYP3A in vitro. Drug Metab. Dispos. 2000, 28, 895–898. [Google Scholar] [PubMed]
- Dash, R.P.; Babu, R.J.; Srinivas, N.R. Therapeutic Potential and Utility of Elacridar with Respect to P-glycoprotein Inhibition: An Insight from the Published In Vitro, Preclinical and Clinical Studies. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Groen, A.; Romero, M.R.; Kunne, C.; Hoosdally, S.J.; Dixon, P.H.; Wooding, C.; Williamson, C.; Seppen, J.; Van Den Oever, K.; Mok, K.S.; et al. Complementary Functions of the Flippase ATP8B1 and the Floppase ABCB4 in Maintaining Canalicular Membrane Integrity. Gastroenterology 2011, 141, 1927–1937.e4. [Google Scholar] [CrossRef] [PubMed]
- Gordo-Gilart, R.; Andueza, S.; Hierro, L.; Martínez-Fernández, P.; D’Agostino, D.; Jara, P.; Alvarez, L. Functional analysis of ABCB4 mutations relates clinical outcomes of progressive familial intrahepatic cholestasis type 3 to the degree of MDR3 floppase activity. Gut 2014, 64, 147–155. [Google Scholar] [CrossRef]
- Evers, R.; Zaman, G.J.; Van Deemter, L.; Jansen, H.; Calafat, J.; Oomen, L.C.; Elferink, R.P.O.; Borst, P.; Schinkel, A.H. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J. Clin. Investig. 1996, 97, 1211–1218. [Google Scholar] [CrossRef]
- Goh, L.-B.; Spears, K.J.; Yao, D.; Ayrton, A.; Morgan, P.; Wolf, C.R.; Friedberg, T. Endogenous drug transporters in in vitro and in vivo models for the prediction of drug disposition in man. Biochem. Pharmacol. 2002, 64, 1569–1578. [Google Scholar] [CrossRef]
- Çakıl, Y.D.; Khunweeraphong, N.; Parveen, Z.; Schmid, D.; Artaker, M.; Ecker, G.; Sitte, H.; Pusch, O.; Stockner, T.; Chiba, P. Pore-Exposed Tyrosine Residues of P-Glycoprotein Are Important Hydrogen-Bonding Partners for Drugs. Mol. Pharmacol. 2013, 85, 420–428. [Google Scholar] [CrossRef]
- Gartzke, D.; Fricker, G. Establishment of Optimized MDCK Cell Lines for Reliable Efflux Transport Studies. J. Pharm. Sci. 2014, 103, 1298–1304. [Google Scholar] [CrossRef]
- Gartzke, D.; Delzer, J.; Laplanche, L.; Uchida, Y.; Hoshi, Y.; Tachikawa, M.; Terasaki, T.; Sydor, J.; Fricker, G. Genomic Knockout of Endogenous Canine P-Glycoprotein in Wild-Type, Human P-Glycoprotein and Human BCRP Transfected MDCKII Cell Lines by Zinc Finger Nucleases. Pharm. Res. 2014, 32, 2060–2071. [Google Scholar] [CrossRef]
- Simoff, I.; Karlgren, M.; Backlund, M.; Lindström, A.-C.; Gaugaz, F.Z.; Matsson, P.; Artursson, P. Complete Knockout of Endogenous Mdr1 (Abcb1) in MDCK Cells by CRISPR-Cas9. J. Pharm. Sci. 2016, 105, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Gong, B.; Wang, Z.; Feng, Y.; Zhang, W.; Wang, S.; Peng, Y.; Zheng, J. Glutathione Conjugation and Protein Adduction Derived from Oxidative Debromination of Benzbromarone in Mice. Drug Metab. Dispos. 2019, 47, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Zamek-Gliszczynski, M.J.; Hoffmaster, K.A.; Nezasa, K.-I.; Tallman, M.N.; Brouwer, K.L. Integration of hepatic drug transporters and phase II metabolizing enzymes: Mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites. Eur. J. Pharm. Sci. 2006, 27, 447–486. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics 2020, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Van de Steeg, E.; van Esch, A.; Wagenaar, E.; Kenworthy, K.E.; Schinkel, A.H. Influence of human OATP1B1, OATP1B3, and OATP1A2 on the pharmacokinetics of methotrexate and paclitaxel in humanized transgenic mice. Clin. Cancer Res. 2013, 19, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-X.; Tiwari, A.K.; Wu, H.-C.; Chen, Z.-S. Overexpression of P-glycoprotein induces acquired resistance to imatinib in chronic myelogenous leukemia cells. Chin. J. Cancer 2012, 31, 110–118. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Gallardo, J.L.; Ohlfest, J.R.; Elmquist, W.F. Distribution of Gefitinib to the Brain Is Limited by P-glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2)-Mediated Active Efflux. J. Pharmacol. Exp. Ther. 2010, 334, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Chen, Z.-S.; Ambudkar, S.V. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist. Updat. 2012, 15, 70–80. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Huang, X.; Li, Y.; Wu, M.; Liu, J. The drug–drug interaction of sorafenib mediated by P-glycoprotein and CYP3A4. Xenobiotica 2015, 46, 651–658. [Google Scholar] [CrossRef]
- Herweijer, H.; Sonneveld, P.; Baas, F.; Nooter, K. Expression of mdr1 and mdr3 Multidrug-resistance Genes in Human Acute and Chronic Leukemias and Association With Stimulation of Drug Accumulation by Cyclosporine. JNCI J. Natl. Cancer Inst. 1990, 82, 1133–1140. [Google Scholar] [CrossRef]
- Nooter, K.; Sonneveld, P.; Janssen, A.; Oostrum, R.; Boersma, T.; Herweijer, H.; Valerjo, D.; Hagemeijer, A.; Baas, F. Expression of the mdr3 gene in prolymphocytic leukemia: Association with cyclosporin-A-induced increase in drug accumulation. Int. J. Cancer 1990, 45, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Wen, C.-J.; Zhao, G.-Z.; Dai, Y.; Li, Y.; Wu, L.-X.; Zhou, H.-H. Overexpression of ABCB4 contributes to acquired doxorubicin resistance in breast cancer cells in vitro. Cancer Chemother. Pharmacol. 2018, 82, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, K.L.R.; Keppler, D.; Hoffmaster, K.A.; Bow, D.A.J.; Cheng, Y.; Lai, Y.; Palm, J.E.; Stieger, B.; Evers, R. In Vitro Methods to Support Transporter Evaluation in Drug Discovery and Development. Clin. Pharmacol. Ther. 2013, 94, 95–112. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temesszentandrási-Ambrus, C.; Nagy, G.; Bui, A.; Gáborik, Z. A Unique In Vitro Assay to Investigate ABCB4 Transport Function. Int. J. Mol. Sci. 2023, 24, 4459. https://doi.org/10.3390/ijms24054459
Temesszentandrási-Ambrus C, Nagy G, Bui A, Gáborik Z. A Unique In Vitro Assay to Investigate ABCB4 Transport Function. International Journal of Molecular Sciences. 2023; 24(5):4459. https://doi.org/10.3390/ijms24054459
Chicago/Turabian StyleTemesszentandrási-Ambrus, Csilla, Gábor Nagy, Annamária Bui, and Zsuzsanna Gáborik. 2023. "A Unique In Vitro Assay to Investigate ABCB4 Transport Function" International Journal of Molecular Sciences 24, no. 5: 4459. https://doi.org/10.3390/ijms24054459
APA StyleTemesszentandrási-Ambrus, C., Nagy, G., Bui, A., & Gáborik, Z. (2023). A Unique In Vitro Assay to Investigate ABCB4 Transport Function. International Journal of Molecular Sciences, 24(5), 4459. https://doi.org/10.3390/ijms24054459






