Bio-Guided Isolation of Compounds from Fraxinus excelsior Leaves with Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Characterisation of Ash Leaves Infusion
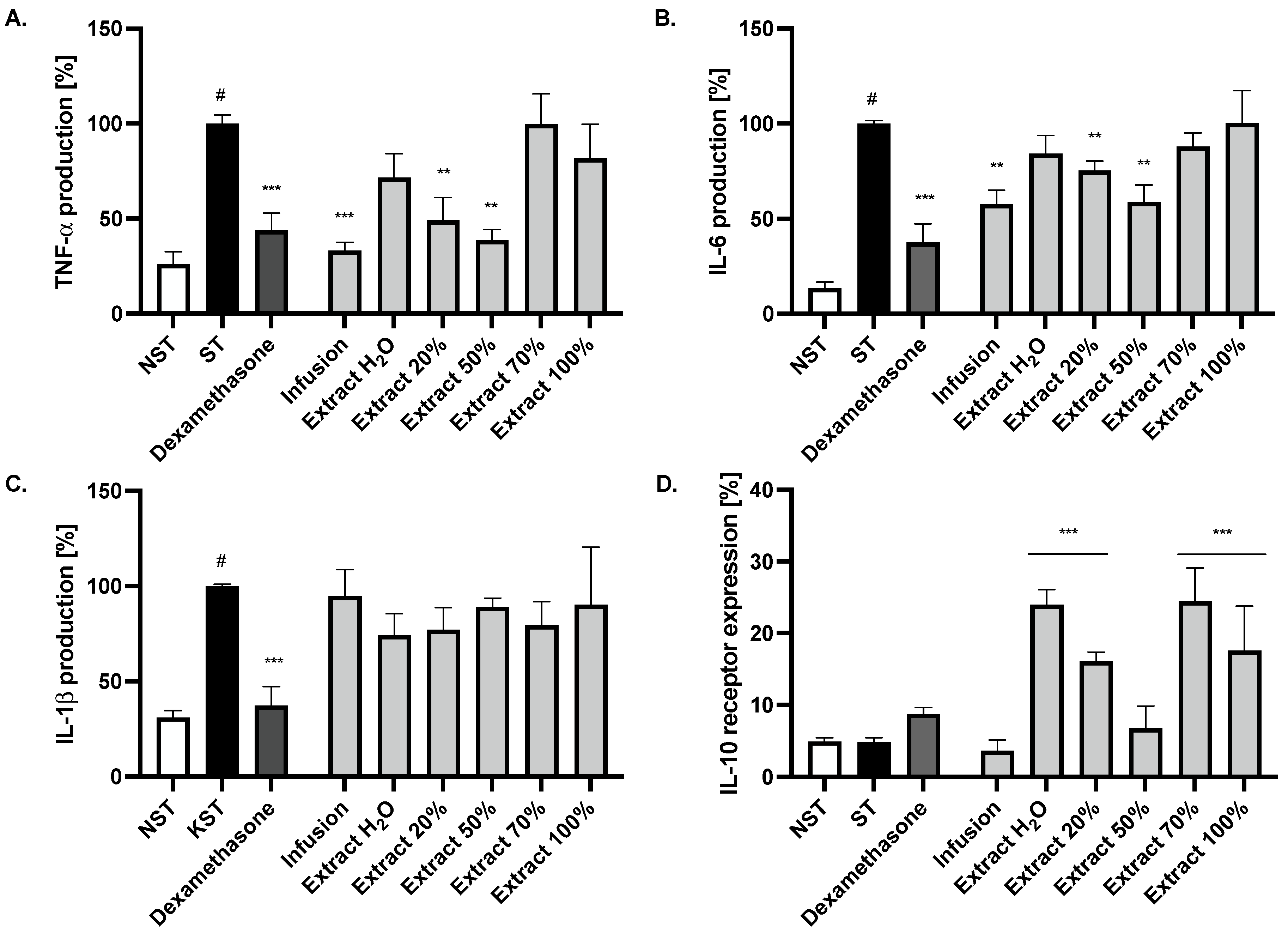
2.2. Effect of Ash Leaves Infusion and Its Fractions on the Proinflammatory Function of LPS-Stimulated Monocytes
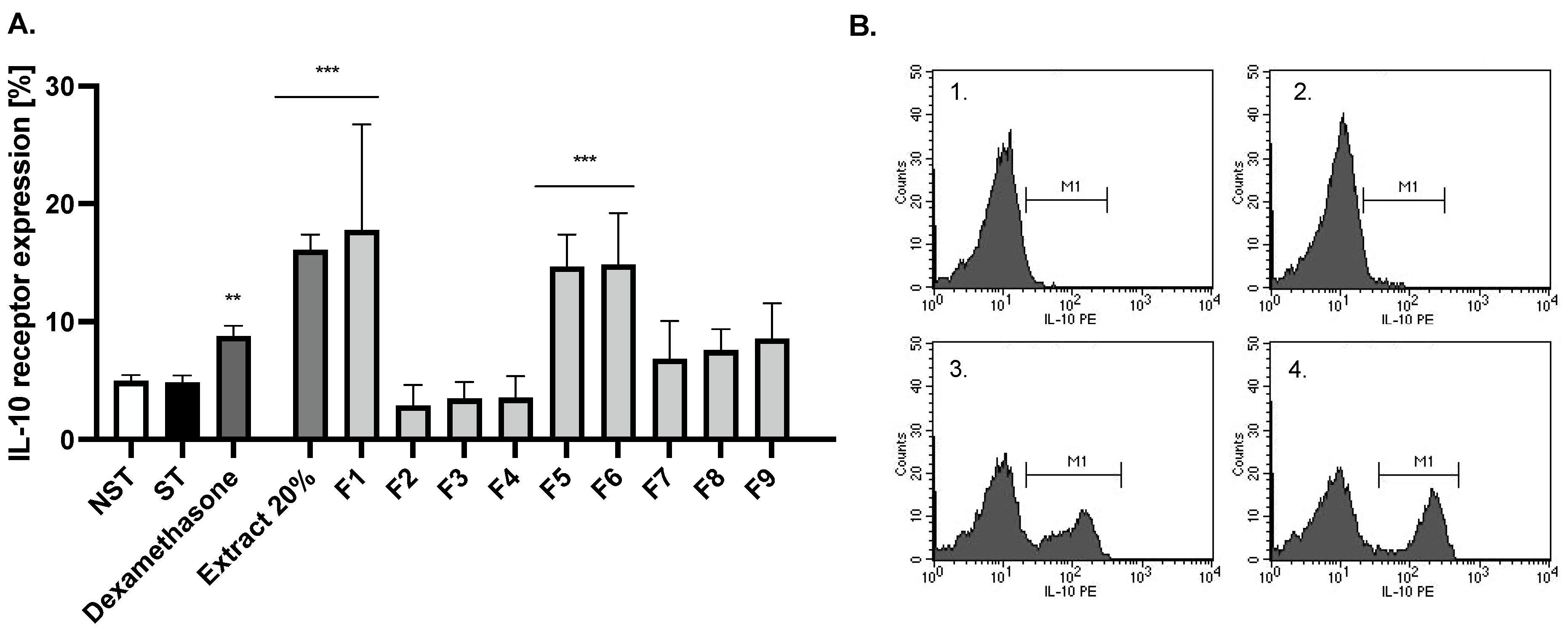
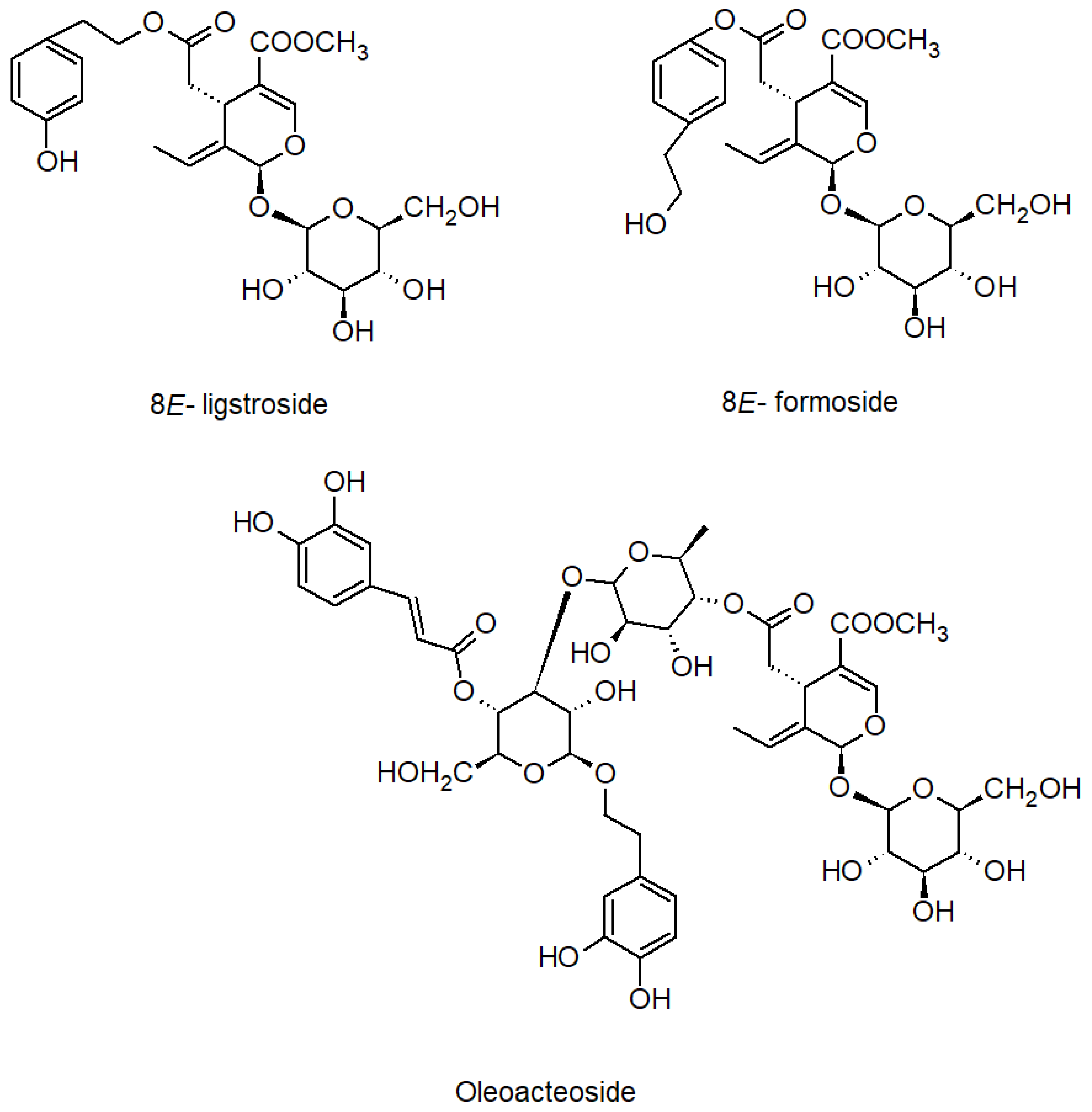
2.3. Bio-Guided Isolation of Active Compounds from 20% and 50% Extracts Obtained from Ash Leaves Infusion
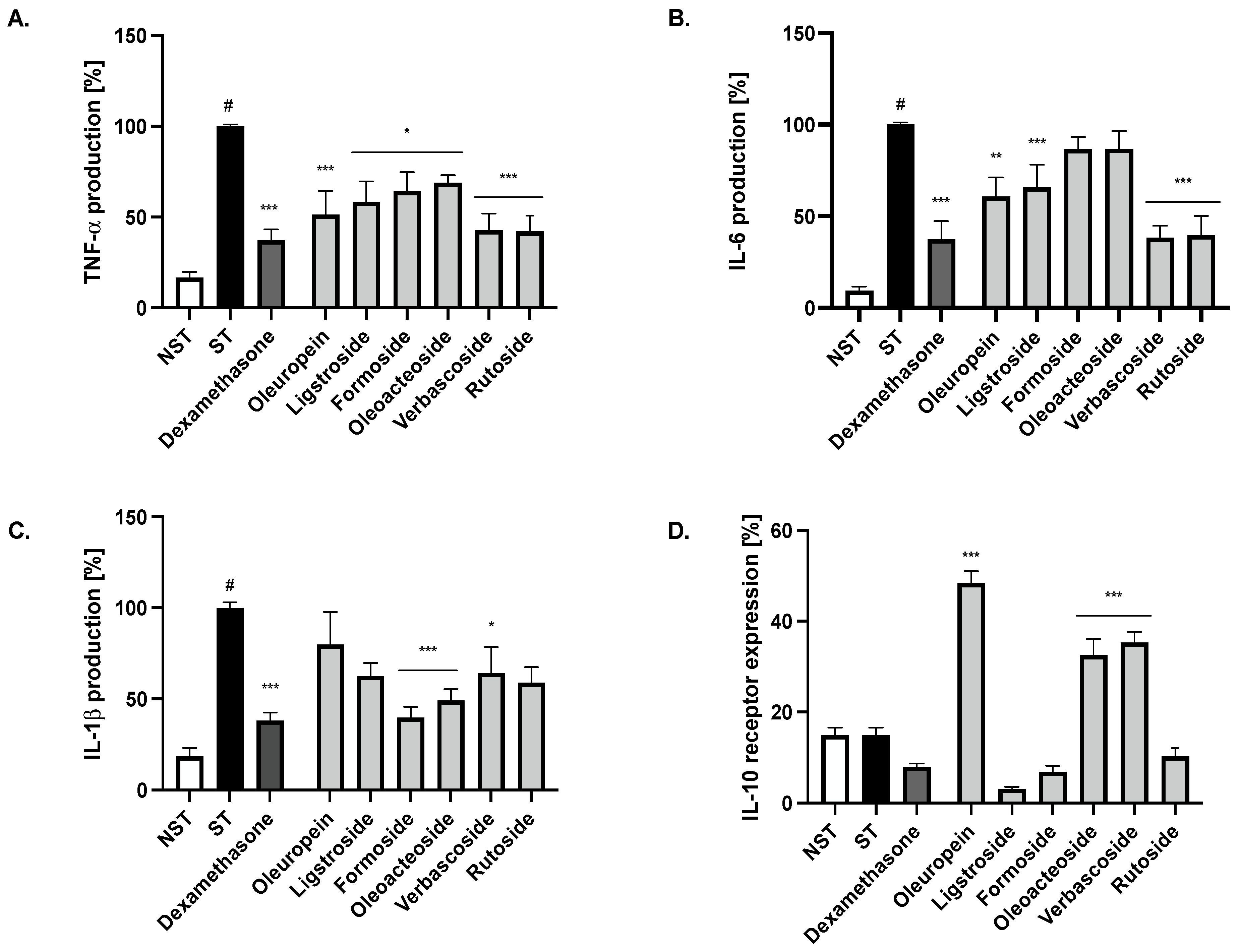
2.4. Bioactivity of Compounds Present in the Most Active Fractions
3. Discussion
4. Materials and Methods
4.1. Chemicals and General Experimental Procedures
4.2. Plant Material
4.3. Extracts Preparation, Fractionation and Isolation of Active Compounds
4.4. Preparation of Solutions of Extracts and Compounds for Bioassay
4.5. Isolation of Human Monocytes
4.6. Cytotoxicity
4.7. IL-6, IL-1β and TNF-α Secretion
4.8. Expression of IL-10 Receptor at the Surface of Monocytes/Macrophages
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caudullo, G.; Houston Durrant, T. Fraxinus angustifolia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016. [Google Scholar]
- Beck, P.; Caudullo, G.; Tinner, W.; de Rigo, D. Fraxinus excelsior in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 98–99. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europeae; Cambridge University Press: London, UK, 1972. [Google Scholar]
- Agelet, A.; Vallès, J. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part II. New or very rare uses of previously known medicinal plants. J. Ethnopharmacol. 2003, 84, 211–227. [Google Scholar] [CrossRef]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Tita, I.; Mogosanu, G.D.; Tita, M.G. Ethnobotanical inventory of medicinal plants from the South-West of Romania. Farmacia 2009, 57, 141–156. [Google Scholar]
- Menković, N.; Savikin, K.; Tasić, S.; Zdunić, G.; Stesević, D.; Milosavljević, S.; Vincek, D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 2011, 133, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Sarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical survey of traditionally used plants in human therapy of east, north and north-east Bosnia and Herzegovina. J. Ethnopharmacol. 2011, 133, 1051–1076. [Google Scholar] [CrossRef]
- Menendez-Baceta, G.; Aceituno-Mata, L.; Molina, M.; Reyes-García, V.; Tardío, J.; Pardo-de-Santayana, M. Medicinal plants traditionally used in the northwest of the Basque Country (Biscay and Alava), Iberian Peninsula. J. Ethnopharmacol. 2014, 152, 113–134. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Pranskuniene, Z.; Dauliute, R.; Pranskunas, A.; Bernatoniene, J. Ethnopharmaceutical knowledge in Samogitia region of Lithuania: Where old traditions overlap with modern medicine. J. Ethnobiol. Ethnomed. 2018, 14, 70. [Google Scholar] [CrossRef]
- Bencheikh, N.; Elbouzidi, A.; Kharchoufa, L.; Ouassou, H.; Alami Merrouni, I.; Mechchate, H.; Es-Safi, I.; Hano, C.; Addi, M.; Bouhrim, M.; et al. Inventory of Medicinal Plants Used Traditionally to Manage Kidney Diseases in North-Eastern Morocco: Ethnobotanical Fieldwork and Pharmacological Evidence. Plants 2021, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.H.; Sadeghi, Z.; Hosseini, S.V.; Bussmann, R.W. Ethnopharmacological study of medicinal plants in Sarvabad, Kurdistan province, Iran. J. Ethnopharmacol. 2022, 288, 114985. [Google Scholar] [CrossRef]
- Ayouni, K.; Berboucha-Rahmani, M.; Kim, H.K.; Atmani, D.; Verpoorte, R.; Choi, Y.H. Metabolomic tool to identify antioxidant compounds of Fraxinus angustifolia leaf and stem bark extracts. Ind. Crops Prod. 2016, 88, 65–77. [Google Scholar] [CrossRef]
- Ahmane, N.; Atmani-Kilani, D.; Chaher, N.; Ayouni, K.; Rahmani-Berboucha, M.; Da Costa, G.; Debbache-Benaida, N.; Richard, T.; Atmani, D. Identification of bioactive compounds from Fraxinus angustifolia extracts with anti-NADH oxidase activity of bovine milk xanthine oxidoreductase. Turk. J. Biol. 2019, 43, 133–147. [Google Scholar] [CrossRef]
- Kiss, A.K.; Michalak, B.; Patyra, A.; Majdan, M. UHPLC-DAD-ESI-MS/MS and HPTLC profiling of ash leaf samples from different commercial and natural sources and their in vitro effects on mediators of inflammation. Phytochem. Anal. 2020, 31, 57–67. [Google Scholar] [CrossRef]
- Kasmi, S.; Hamdi, A.; Atmani-Kilani, D.; Debbache-Benaida, N.; Jaramillo-Carmona, S.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Ayouni, K.; Atmani, D.; Guillén-Bejarano, R. Characterization of phenolic compounds isolated from the Fraxinus angustifolia plant and several associated bioactivities. J. Herb. Med. 2021, 29, 100485. [Google Scholar] [CrossRef]
- Bouguellid, G.; Russo, C.; Lavorgna, M.; Piscitelli, C.; Ayouni, K.; Wilson, E.; Kim, H.K.; Verpoorte, R.; Choi, Y.H.; Kilani-Atmani, D.; et al. Antimutagenic, antigenotoxic and antiproliferative activities of Fraxinus angustifolia Vahl. leaves and stem bark extracts and their phytochemical composition. PLoS ONE 2020, 15, e0230690. [Google Scholar] [CrossRef]
- Kostova, I.; Iossifova, T. Chemical components of Fraxinus species. Fitoterapia 2007, 78, 85–106. [Google Scholar] [CrossRef]
- Iossifova, T.; Kostova, I.; Evstatieva, L.N. Secoirydoids and hydroxycoumarins in Bulgarian Fraxinus species. Biochem. Syst. Ecol. 1997, 25, 271–274. [Google Scholar] [CrossRef]
- Damtoft, S.; Franzyk, H.; Jensen, S.R. Excelsioside, a secoiridoid glucoside from Fraxinus excelsior. Phytochemistry 1992, 31, 4197–4201. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J. Leukoc. Biol. 2007, 81, 584–592. [Google Scholar] [CrossRef]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef] [PubMed]
- Thaxton, J.E.; Sharma, S. Interleukin-10: A multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 2010, 63, 482–491. [Google Scholar] [CrossRef]
- Sun, J.B.; Li, Z.L. Relationship between gene polymorphism at rs2228055 locus in the exon region of interleukin-10 receptor A and susceptibility to food allergy in children. Zhonghua Er Ke Za Zhi 2020, 58, 559–563. [Google Scholar] [CrossRef]
- Gassas, A.; Courtney, S.; Armstrong, C.; Kapllani, E.; Muise, A.M.; Schechter, T. Unrelated donor hematopoietic stem cell transplantation for infantile enteropathy due to IL-10/IL-10 receptor defect. Pediatr. Transplant. 2015, 19, E101–E103. [Google Scholar] [CrossRef]
- Kotlarz, D.; Beier, R.; Murugan, D.; Diestelhorst, J.; Jensen, O.; Boztug, K.; Pfeifer, D.; Kreipe, H.; Pfister, E.D.; Baumann, U.; et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: Implications for diagnosis and therapy. Gastroenterology 2012, 143, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, T.; Zhong, C.; Wang, Y.; Chang, M.; Liu, X. IL-10 and IL-10 Receptor Mutations in Very Early Onset Inflammatory Bowel Disease. Gastroenterol. Res. 2017, 10, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, T.; Watanabe, H.; Itoh, A.; Nagakura, N.; Inoue, K.; Ono, M.; Fujita, T.; Morita, M.; Chen, C.-C. Five secoiridoid glucosides from Fraxinus formosana. Phytochemistry 1992, 32, 133–136. [Google Scholar] [CrossRef]
- Machida, K.; Matsuoka, E.; Kikuchi, M. Structural revision of oleoacteoside and oleoechinacoside. Nat. Prod. Res. 2010, 24, 737–742. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Dudek, M.K.; Michalak, B.; Woźniak, M.; Czerwińska, M.E.; Filipek, A.; Granica, S.; Kiss, A.K. Hydroxycinnamoyl derivatives and secoiridoid glycoside derivatives from Syringa vulgaris flowers and their effects on the pro-inflammatory responses of human neutrophils. Fitoterapia 2017, 121, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gao, H.; Han, S.; Yuan, R.; He, J.; Zhuo, Y.; Feng, Y.L.; Tang, M.; Feng, J.; Yang, S. Oleuropein Attenuates Lipopolysaccharide-Induced Acute Kidney Injury In Vitro and In Vivo by Regulating Toll-Like Receptor 4 Dimerization. Front. Pharmacol. 2021, 12, 617314. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef]
- Castejón, M.L.; Montoya, T.; Alarcón-de-la-Lastra, C.; González-Benjumea, A.; Vázquez-Román, M.V.; Sánchez-Hidalgo, M. Dietary oleuropein and its acyl derivative ameliorate inflammatory response in peritoneal macrophages from pristane-induced SLE mice via canonical and noncanonical NLRP3 inflammasomes pathway. Food Funct. 2020, 11, 6622–6631. [Google Scholar] [CrossRef]
- Glocker, E.O.; Kotlarz, D.; Klein, C.; Shah, N.; Grimbacher, B. IL-10 and IL-10 receptor defects in humans. Ann. N. Y. Acad. Sci. 2011, 1246, 102–107. [Google Scholar] [CrossRef]
- Engelhardt, K.R.; Shah, N.; Faizura-Yeop, I.; Kocacik Uygun, D.F.; Frede, N.; Muise, A.M.; Shteyer, E.; Filiz, S.; Chee, R.; Elawad, M.; et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 2013, 131, 825–830. [Google Scholar] [CrossRef]
- Zigmond, E.; Bernshtein, B.; Friedlander, G.; Walker, C.R.; Yona, S.; Kim, K.W.; Brenner, O.; Krauthgamer, R.; Varol, C.; Müller, W.; et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 2014, 40, 720–733. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Michel, G.; Mirmohammadsadegh, A.; Olasz, E.; Jarzebska-Deussen, B.; Müschen, A.; Kemény, L.; Abts, H.F.; Ruzicka, T. Demonstration and functional analysis of IL-10 receptors in human epidermal cells: Decreased expression in psoriatic skin, down-modulation by IL-8, and up-regulation by an antipsoriatic glucocorticosteroid in normal cultured keratinocytes. J. Immunol. 1997, 159, 6291–6297. [Google Scholar] [CrossRef]
- Shi, J.; Shi, S.; Xie, W.; Zhao, M.; Li, Y.; Zhang, J.; Li, N.; Bai, X.; Cai, W.; Hu, X.; et al. IL-10 alleviates lipopolysaccharide-induced skin scarring via IL-10R/STAT3 axis regulating TLR4/NF-κB pathway in dermal fibroblasts. J. Cell. Mol. Med. 2021, 25, 1554–1567. [Google Scholar] [CrossRef]
- Kaesler, S.; Volz, T.; Skabytska, Y.; Köberle, M.; Hein, U.; Chen, K.M.; Guenova, E.; Wölbing, F.; Röcken, M.; Biedermann, T. Toll-like receptor 2 ligands promote chronic atopic dermatitis through IL-4-mediated suppression of IL-10. J. Allergy Clin. Immunol. 2014, 134, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Duell, B.L.; Carey, A.J.; Tan, C.K.; Cui, X.; Webb, R.I.; Totsika, M.; Schembri, M.A.; Derrington, P.; Irving-Rodgers, H.; Brooks, A.J.; et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J. Immunol. 2012, 188, 781–792. [Google Scholar] [CrossRef]
- Jung, M.; Sola, A.; Hughes, J.; Kluth, D.C.; Vinuesa, E.; Viñas, J.L.; Pérez-Ladaga, A.; Hotter, G. Infusion of IL-10–expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int. 2012, 81, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Siennicka, A.; Chełstowski, K.; Widecka, K.; Goracy, I.; Hałasa, M.; Machaliński, B.; Naruszewicz, M. Is there an association between angiotensin-converting enzyme gene polymorphism and functional activation of monocytes and macrophage in young patients with essential hypertension? J. Hypertens. 2006, 24, 1565–1573. [Google Scholar] [CrossRef] [PubMed]


| Compounds | UV [nm] | Rt [min] | [M-H]− | Product Ions Main Peaks |
|---|---|---|---|---|
| 325 | 11.4 | 353 | 191, 179 |
| 325 | 16.6 | 353 | 191 |
| 256, 355 | 29.2 | 609 | 301 |
| 215, 330 | 31.1 | 623 | 461, 315, 161, 315 |
| 264, 343 | 32.7 | 593 | 285 |
| 222, 275 | 38.0 | 539 | 377, 307, 275 |
| 218 | 39.6 | 569 * | 523, 385, 299, 223 |
| 216, 325 | 39.9 | 1009 | 847, 623, 461 |
| 216, 280 | 43.0 | 523 | 523, 361 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołtun-Jasion, M.; Sawulska, P.; Patyra, A.; Woźniak, M.; Dudek, M.K.; Filipek, A.; Kiss, A.K. Bio-Guided Isolation of Compounds from Fraxinus excelsior Leaves with Anti-Inflammatory Activity. Int. J. Mol. Sci. 2023, 24, 3750. https://doi.org/10.3390/ijms24043750
Kołtun-Jasion M, Sawulska P, Patyra A, Woźniak M, Dudek MK, Filipek A, Kiss AK. Bio-Guided Isolation of Compounds from Fraxinus excelsior Leaves with Anti-Inflammatory Activity. International Journal of Molecular Sciences. 2023; 24(4):3750. https://doi.org/10.3390/ijms24043750
Chicago/Turabian StyleKołtun-Jasion, Małgorzata, Paulina Sawulska, Andrzej Patyra, Marta Woźniak, Marta Katarzyna Dudek, Agnieszka Filipek, and Anna Karolina Kiss. 2023. "Bio-Guided Isolation of Compounds from Fraxinus excelsior Leaves with Anti-Inflammatory Activity" International Journal of Molecular Sciences 24, no. 4: 3750. https://doi.org/10.3390/ijms24043750
APA StyleKołtun-Jasion, M., Sawulska, P., Patyra, A., Woźniak, M., Dudek, M. K., Filipek, A., & Kiss, A. K. (2023). Bio-Guided Isolation of Compounds from Fraxinus excelsior Leaves with Anti-Inflammatory Activity. International Journal of Molecular Sciences, 24(4), 3750. https://doi.org/10.3390/ijms24043750






