Towards Controlling the Local Bone Tissue Remodeling—Multifunctional Injectable Composites for Osteoporosis Treatment
Abstract
1. Introduction
2. Results and Discussion
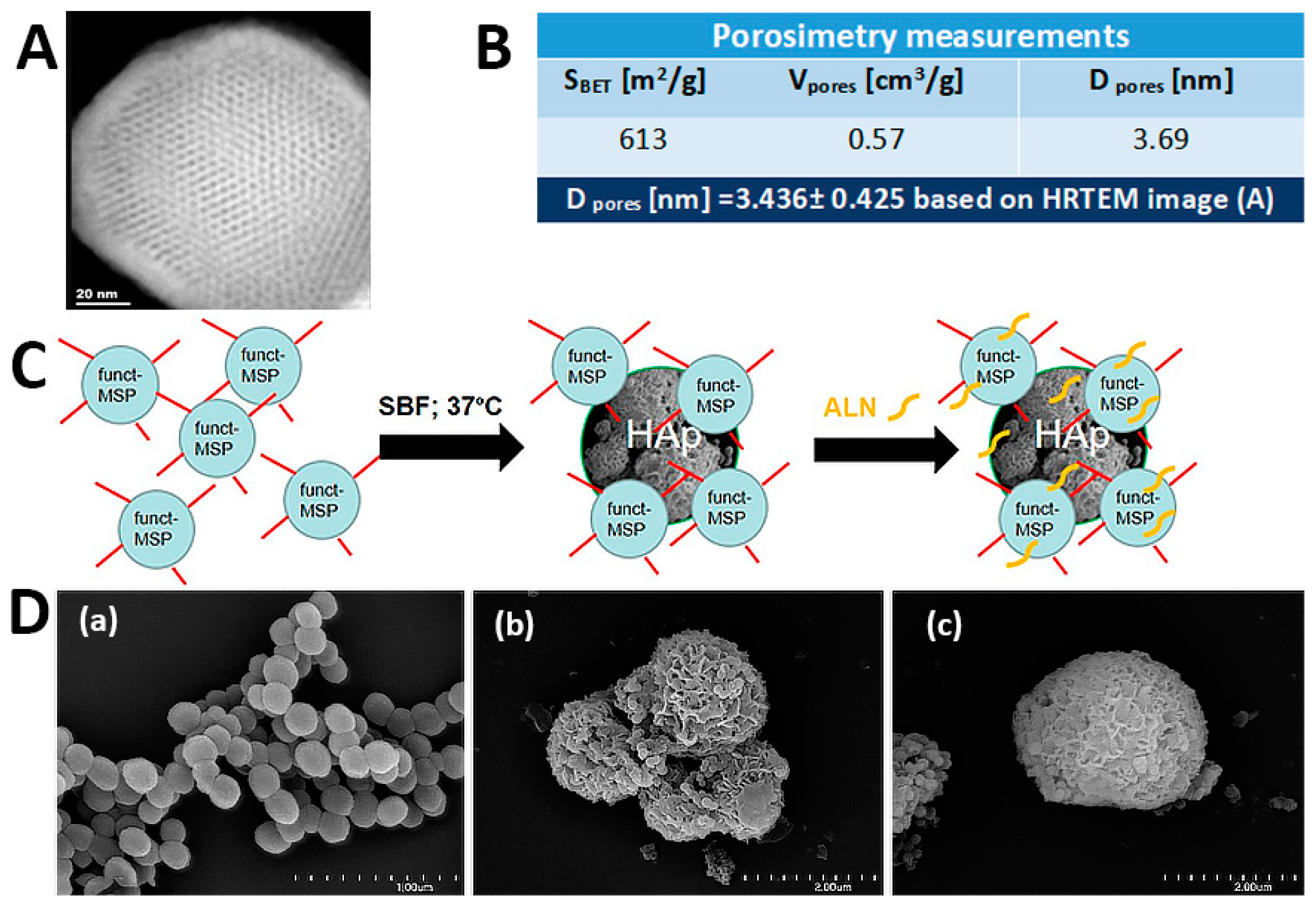
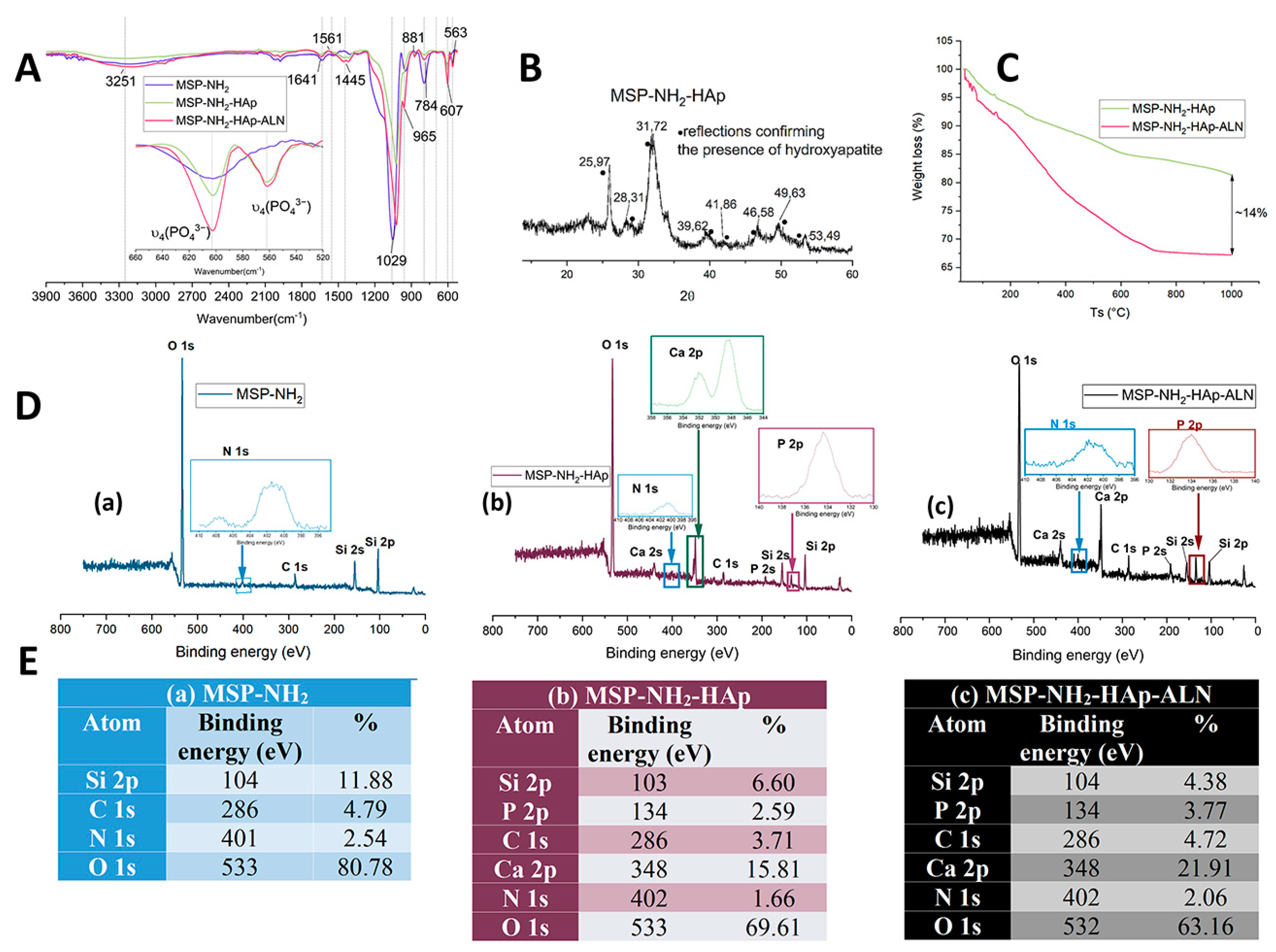
2.1. The Functionalized Mesoporous Silica-Based Particles Decorated with Hydroxyapatite and Loaded with Alendronate (MSP-NH2-HAp-ALN)—Synthesis and Characterization
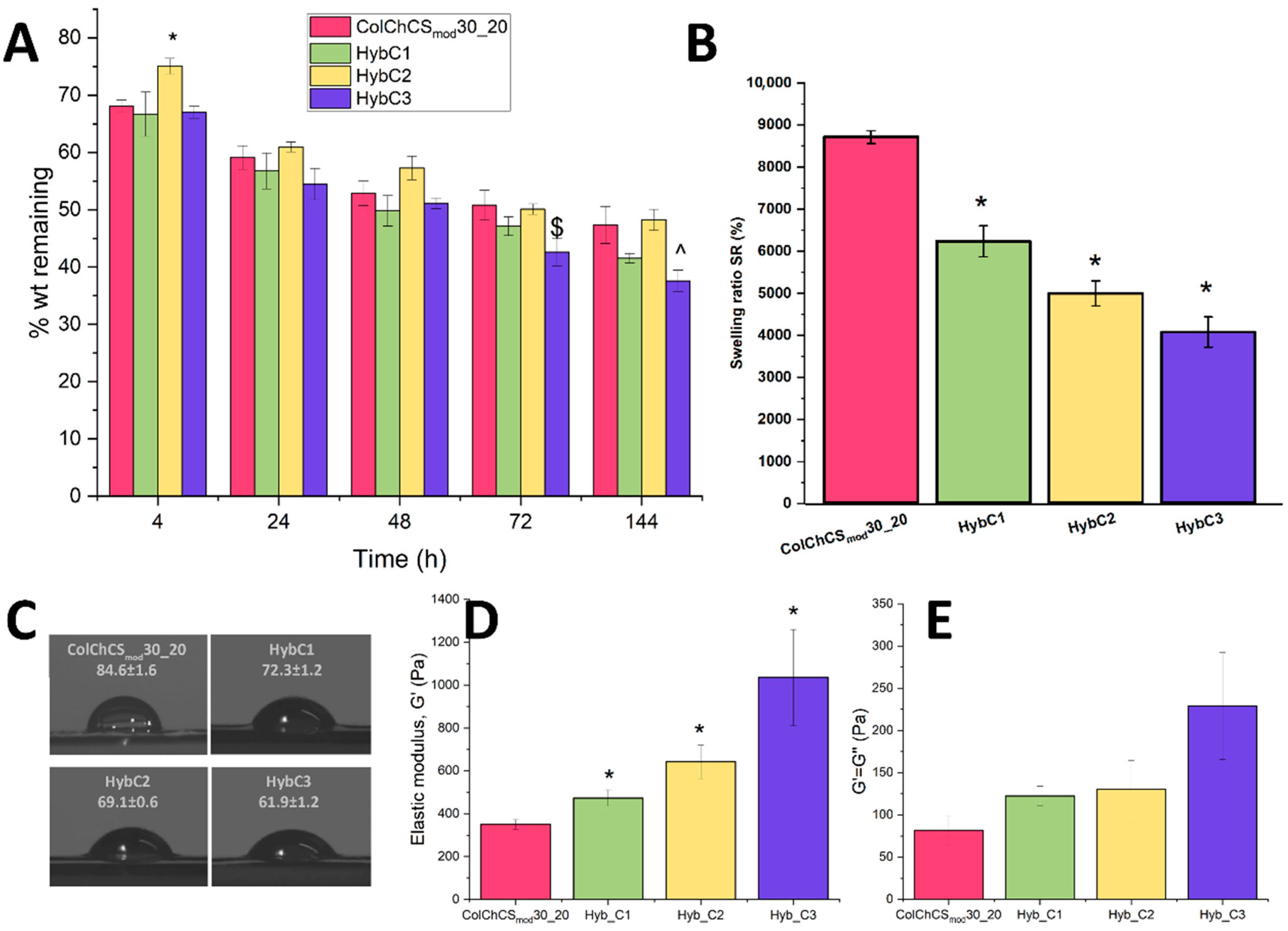
2.2. Hybrid Systems—Physicochemical Characterization
2.3. Injectability and Rheological Evaluation
2.4. Biomineralization
2.5. Model In Vitro Release Study
2.6. Biological Evaluation of Composites in Osteoblast-like and Osteoclast-like Cell Culture In Vitro
2.7. Antibacterial Activity of Materials Developed
3. Materials and Methods
3.1. Synthesis of Functionalized Mesoporous Silica Particles with Amino Groups Decorated with Hydroxyapatite and Loaded/Attached with Alendronate (MSP-NH2-HAp-ALN)
3.2. Model In Vitro Release of ALN from MSP-NH2-HAp-ALN
3.3. Characterization of MSP-NH2, MSP-NH2-HAp, and MSP-NH2-HAp-ALN Particles
3.4. Hybrid Preparation
3.5. Physicochemical Characterization of Hybrids
3.6. Drug Release Studies
3.7. In Vitro Biomineralization
3.8. Biological Experiments In Vitro Employing Osteoblast-like (MG-63) and Osteoclast-like (J774A.1) Cells Culture
3.9. In Vitro Antibacterial Activity Studies
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carlson, B.M. The Skeleton. Hum. Body 2019, 2019, 87–110. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for the Treatment of Complex Bone Diseases: Bone Cancer, Bone Infection and Osteoporosis. Pharmaceutics 2020, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, J.; Zhang, M.; Ma, Y.; Yang, Y.; Han, X.; Wang, X. Long-Term Delivery of Alendronate through an Injectable Tetra-PEG Hydrogel to Promote Osteoporosis Therapy. Biomater. Sci. 2020, 8, 3138–3146. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A New Scorecard for Osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef]
- Furman, B.L. Alendronate. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Khan, M.; Cheung, A.M.; Khan, A.A. Drug-Related Adverse Events of Osteoporosis Therapy. Endocrinol. Metab. Clin. N. Am. 2017, 46, 181–192. [Google Scholar] [CrossRef]
- Kuźnik, A.; Październiok-Holewa, A.; Jewula, P.; Kuźnik, N. Bisphosphonates—Much More than Only Drugs for Bone Diseases. Eur. J. Pharmacol. 2020, 866, 172773. [Google Scholar] [CrossRef]
- Eastell, R. Osteoporosis. Medicine 2013, 41, 586–591. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Nafee, N.; Zewail, M.; Boraie, N. Alendronate-Loaded, Biodegradable Smart Hydrogel: A Promising Injectable Depot Formulation for Osteoporosis. J. Drug Target. 2018, 26, 563–575. [Google Scholar] [CrossRef]
- Horikawa, A.; Miyakoshi, N.; Shimada, Y.; Sugimura, Y.; Kodama, H. A Comparative Study between Intravenous and Oral Alendronate Administration for the Treatment of Osteoporosis. SpringerPlus 2015, 4, 675. [Google Scholar] [CrossRef]
- Klara, J.; Lewandowska-Łańcucka, J. How Efficient Are Alendronate-Nano/Biomaterial Combinations for Anti-Osteoporosis Therapy? An Evidence-Based Review of the Literature. Int. J. Nanomed. 2022, 17, 6065–6094. [Google Scholar] [CrossRef]
- Dong, J.; Tao, L.; Abourehab, M.A.S.; Hussain, Z. Design and Development of Novel Hyaluronate-Modified Nanoparticles for Combo-Delivery of Curcumin and Alendronate: Fabrication, Characterization, and Cellular and Molecular Evidences of Enhanced Bone Regeneration. Int. J. Biol. Macromol. 2018, 116, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Dolci, L.S.; Panzavolta, S.; Albertini, B.; Campisi, B.; Gandolfi, M.; Bigi, A.; Passerini, N. Spray-Congealed Solid Lipid Microparticles as a New Tool for the Controlled Release of Bisphosphonates from a Calcium Phosphate Bone Cement. Eur. J. Pharm. Biopharm. 2018, 122, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lei, P.; Liu, G.; Shrike Zhang, Y.; Yang, J.; Zhang, L.; Xie, J.; Niu, W.; Liu, H.; Ruan, J.; et al. Reconstruction of Large-Scale Defects with a Novel Hybrid Scaffold Made from Poly(L-lactic acid)/nanohydroxyapatite/Alendronate-Loaded Chitosan Microsphere: In Vitro and in Vivo Studies. Sci. Rep. 2017, 7, 359. [Google Scholar] [CrossRef]
- Yuan, W.; Li, Z.; Xie, X.; Zhang, Z.-Y.; Bian, L. Bisphosphonate-Based Nanocomposite Hydrogels for Biomedical Applications. Bioact. Mater. 2020, 5, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Posadowska, U.; Parizek, M.; Filova, E.; Wlodarczyk-Biegun, M.; Kamperman, M.; Bacakova, L.; Pamula, E. Injectable Nanoparticle-Loaded Hydrogel System for Local Delivery of Sodium Alendronate. Int. J. Pharm. 2015, 485, 31–40. [Google Scholar] [CrossRef]
- Gilarska, A.; Hinz, A.; Bzowska, M.; Dyduch, G.; Kamiński, K.; Nowakowska, M.; Lewandowska-Łańcucka, J. Addressing the Osteoporosis Problem—Multifunctional Injectable Hybrid Materials for Controlling Local Bone Tissue Remodeling. ACS Appl. Mater. Interfaces 2021, 13, 49762–49779. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.; Raichur, A.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Klara, J.; Marczak, A.; Łatkiewicz, A.; Horak, W.; Lewandowska-Łańcucka, J. Lysine-Functionalized Chondroitin Sulfate Improves the Biological Properties of Collagen/Chitosan-Based Injectable Hydrogels. Int. J. Biol. Macromol. 2022, 202, 318–331. [Google Scholar] [CrossRef]
- Shirazi, S.; Ravindran, S.; Cooper, L.F. Topography-Mediated Immunomodulation in Osseointegration; Ally or Enemy. Biomaterials 2022, 291, 121903. [Google Scholar] [CrossRef]
- Chen, R.; Hao, Z.; Wang, Y.; Zhu, H.; Hu, Y.; Chen, T.; Zhang, P.; Li, J. Mesenchymal Stem Cell–Immune Cell Interaction and Related Modulations for Bone Tissue Engineering. Stem Cells Int. 2022, 2022, 7153584. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Colilla, M.; Vallet-Reg, M. Drug Delivery from Ordered Mesoporous Matrices. Expert Opin. Drug Deliv. 2009, 6, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Newham, G.; Evans, S.D.; Ong, Z.Y. Mechanically Tuneable Physical Nanocomposite Hydrogels from Polyelectrolyte Complex Templated Silica Nanoparticles for Anionic Therapeutic Delivery. J. Colloid Interface Sci. 2022, 617, 224–235. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of Mesoporous Silica Nanoparticles. Chem. Soc. Rev. 2013, 42, 3862. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Cho, S.B.; Miyaji, F.; Kokubo, T.; Nakanishi, K.; Soga, N.; Nakamura, T. Apatite Formation on Various Silica Gels in a Simulated Body Fluid Containing Excessive Calcium Ion. J. Ceram. Soc. Japan 1996, 104, 399–404. [Google Scholar] [CrossRef]
- Hamai, R.; Shirosaki, Y.; Miyazaki, T. Structural Effects of Sulfur-Containing Functional Groups on Apatite Formation on Ca2+-Modified Copolymers in a Simulated Body Environment. ACS Omega 2018, 3, 5627–5633. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ohtsuki, C. Design of Bioactive Bone Cement Based on Organic–Inorganic Hybrids. In Orthopaedic Bone Cements; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2008; pp. 358–376. [Google Scholar] [CrossRef]
- Alves, N.M.; Leonor, I.B.; Azevedo, H.S.; Reis, R.L.; Mano, J.F. Designing Biomaterials Based on Biomineralization of Bone. J. Mater. Chem. 2010, 20, 2911–2921. [Google Scholar] [CrossRef]
- Albayati, T.M.; Salih, I.K.; Alazzawi, H.F. Synthesis and Characterization of a Modified Surface of SBA-15 Mesoporous Silica for a Chloramphenicol Drug Delivery System. Heliyon 2019, 5, e02539. [Google Scholar] [CrossRef]
- Kotian, R.; Rao, P.P.; Madhyastha, P. X-Ray Diffraction Analysis of Hydroxyapatite-Coated in Different Plasma Gas Atmosphere on Ti and Ti-6Al-4V. Eur. J. Dent. 2017, 11, 438–446. [Google Scholar] [CrossRef]
- Rogina, A.; Šandrk, N.; Teruel-Biosca, L.; Antunović, M.; Ivanković, M.; Ferrer, G.G. Bone-Mimicking Injectable Gelatine/Hydroxyapatite Hydrogels. Chem. Biochem. Eng. Q. 2020, 33, 325–335. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. BioMed Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef] [PubMed]
- MacAya, D.; Ng, K.K.; Spector, M. Injectable Collagen–Genipin Gel for the Treatment of Spinal Cord Injury: In Vitro Studies. Adv. Funct. Mater. 2011, 21, 4788–4797. [Google Scholar] [CrossRef]
- Guo, X.; Park, H.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Mikos, A.G. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells in Vitro. AIChE Annu. Meet. Conf. Proc. 2008, 10, 541–546. [Google Scholar]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. Biomed Res. Int. 2014, 2014, 786892. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen-Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Patel, A.; Mequanint, K. Hydrogel Biomaterials. In Biomedical Engineering—Frontiers and Challenges; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Lewandowska-Łańcucka, J.; Gilarska, A.; Buła, A.; Horak, W.; Łatkiewicz, A.; Nowakowska, M. Genipin Crosslinked Bioactive Collagen/Chitosan/Hyaluronic Acid Injectable Hydrogels Structurally Amended via Covalent Attachment of Surface-Modified Silica Particles. Int. J. Biol. Macromol. 2019, 136, 1196–1208. [Google Scholar] [CrossRef]
- Zengin, A.; Castro, J.P.O.; Habibovic, P.; Van Rijt, S.H. Injectable, Self-Healing Mesoporous Silica Nanocomposite Hydrogels with Improved Mechanical Properties. Nanoscale 2021, 13, 1144–1154. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Rivera, C.; Wu, C.J.; Chan, B.K.; Schmidt, G. Photocrosslinked Nanocomposite Hydrogels from PEG and Silica Nanospheres: Structural, Mechanical and Cell Adhesion Characteristics. Mater. Sci. Eng. C 2013, 33, 1800–1807. [Google Scholar] [CrossRef]
- Arcos, D.; Boccaccini, A.R.; Bohner, M.; Díez-Pérez, A.; Epple, M.; Gómez-Barrena, E.; Herrera, A.; Planell, J.A.; Rodríguez-Mañas, L.; Vallet-Regí, M. The Relevance of Biomaterials to the Prevention and Treatment of Osteoporosis. Acta Biomater. 2014, 10, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.; Ferreira, J.A.; Rizzante, F.A.P.; Moura, G.F.; Mendonça, D.B.S.; de Magalhães, D.; Cimões, R.; Mendonça, G. Hydrophilic Titanium Surface Modulates Early Stages of Osseointegration in Osteoporosis. J. Periodontal Res. 2021, 56, 351–362. [Google Scholar] [CrossRef]
- Calciolari, E.; Hamlet, S.; Ivanovski, S.; Donos, N. Pro-Osteogenic Properties of Hydrophilic and Hydrophobic Titanium Surfaces: Crosstalk between Signalling Pathways in in Vivo Models. J. Periodontal Res. 2018, 53, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Lotz, E.M.; Schwartz, Z. Roughness and Hydrophilicity as Osteogenic Biomimetic Surface Properties. Tissue Eng.—Part A 2017, 23, 1479–1489. [Google Scholar] [CrossRef]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Shi, X.; Wang, Y. Injectable Alendronate-Functionalized GelMA Hydrogels for Mineralization and Osteogenesis. RSC Adv. 2018, 8, 22764–22776. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological Properties of Peptide-Based Hydrogels for Biomedical and Other Applications. Chem. Soc. Rev. 2010, 39, 3528. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Drabczyk, A.; Florkiewicz, W.; Głąb, M.; Kudłacik-Kramarczyk, S.; Słota, D.; Tomala, A.; Tyliszczak, B. Review of the Applications of Biomedical Compositions Containing Hydroxyapatite and Collagen Modified by Bioactive Components. Materials 2021, 14, 2096. [Google Scholar] [CrossRef]
- Kuljanin, J.; Jankovi, I.; Nedeljkovi, J.; Prstojevi, D.; Marinkovi, V. Spectrophotometric Determination of Alendronate in Pharmaceutical Formulations via Complex Formation with Fe(III) Ions. J. Pharm. Biomed. Anal. 2002, 28, 1215–1220. [Google Scholar] [CrossRef]
- Yewle, J.N.; Puleo, D.A.; Bachas, L.G. Enhanced Affinity Bifunctional Bisphosphonates for Targeted Delivery of Therapeutic Agents to Bone. Bioconjug. Chem. 2011, 22, 2496. [Google Scholar] [CrossRef]
- Capra, P.; Dorati, R.; Colonna, C.; Bruni, G.; Pavanetto, F.; Genta, I.; Conti, B. A Preliminary Study on the Morphological and Release Properties of Hydroxyapatite-Alendronate Composite Materials. J. Microencapsul. 2011, 28, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan Hydrogels for Sustained Drug Delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Qu, L.; Wu, H.; Kong, H.; Yang, Z.; Chen, D.; Mäkilä, E.; Salonen, J.; Santos, H.A.; et al. Gold Nanorods Conjugated Porous Silicon Nanoparticles Encapsulated in Calcium Alginate Nano Hydrogels Using Microemulsion Templates. Nano Lett. 2018, 18, 1448–1453. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Baishya, H. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 1000171. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm.—Drug Res. 2010, 67, 217–223. [Google Scholar]
- Paarakh, M.P.; Jose, P.A.N.I.; Setty, C.M.; Peter, G. V Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2019, 8, 12–20. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the Use of the Weibull Function for the Discernment of Drug Release Mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Filipowska, J.; Lewandowska-Łańcucka, J.; Gilarska, A.; Niedźwiedzki, Ł.; Nowakowska, M. In Vitro Osteogenic Potential of Collagen/Chitosan-Based Hydrogels-Silica Particles Hybrids in Human Bone Marrow-Derived Mesenchymal Stromal Cell Cultures. Int. J. Biol. Macromol. 2018, 113, 692–700. [Google Scholar] [CrossRef]
- Nasello, G.; Alamán-Díez, P.; Schiavi, J.; Pérez, M.Á.; McNamara, L.; García-Aznar, J.M. Primary Human Osteoblasts Cultured in a 3D Microenvironment Create a Unique Representative Model of Their Differentiation Into Osteocytes. Front. Bioeng. Biotechnol. 2020, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Liou, H.M.; Lin, C.J.; Kuo, K.L.; Hung, Y.S.; Weng, R.C.; Hsu, F.Y. MG63 Osteoblast-like Cells Exhibit Different Behavior When Grown on Electrospun Collagen Matrix versus Electrospun Gelatin Matrix. PLoS ONE 2012, 7, e31200. [Google Scholar] [CrossRef] [PubMed]
- Krajcer, A.; Klara, J.; Horak, W.; Lewandowska-Łańcucka, J. Bioactive Injectable Composites Based on Insulin-Functionalized Silica Particles Reinforced Polymeric Hydrogels for Potential Applications in Bone Tissue Engineering. J. Mater. Sci. Technol. 2022, 105, 153–163. [Google Scholar] [CrossRef]
- Moreau, M.F.; Guillet, C.; Massin, P.; Chevalier, S.; Gascan, H.; Baslé, M.F.; Chappard, D. Comparative Effects of Five Bisphosphonates on Apoptosis of Macrophage Cells in Vitro. Biochem. Pharmacol. 2007, 73, 718–723. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial Activity of New Materials Based on the Blends of Collagen/Chitosan/Hyaluronic Acid with Gentamicin Sulfate Addition. Mater. Sci. Eng. C 2018, 86, 103–108. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Guzdek-Zając, K.; Karewicz, A.; Horak, W.; Lach, R.; Wójcik, K.; Nowakowska, M. Bioactive yet Antimicrobial Structurally Stable Collagen/Chitosan/Lysine Functionalized Hyaluronic Acid-Based Injectable Hydrogels for Potential Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2020, 155, 938–950. [Google Scholar] [CrossRef]
- Son, M.J.; Lee, S.-W. Antibacterial Toxicity of Mesoporous Silica Nanoparticles with Functional Decoration of Specific Organic Moieties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127612. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/Chitosan/Hyaluronic Acid-Based Injectable Hydrogels for Tissue Engineering Applications—Design, Physicochemical and Biological Characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klara, J.; Onak, S.; Kowalczyk, A.; Horak, W.; Wójcik, K.; Lewandowska-Łańcucka, J. Towards Controlling the Local Bone Tissue Remodeling—Multifunctional Injectable Composites for Osteoporosis Treatment. Int. J. Mol. Sci. 2023, 24, 4959. https://doi.org/10.3390/ijms24054959
Klara J, Onak S, Kowalczyk A, Horak W, Wójcik K, Lewandowska-Łańcucka J. Towards Controlling the Local Bone Tissue Remodeling—Multifunctional Injectable Composites for Osteoporosis Treatment. International Journal of Molecular Sciences. 2023; 24(5):4959. https://doi.org/10.3390/ijms24054959
Chicago/Turabian StyleKlara, Joanna, Sylwia Onak, Andrzej Kowalczyk, Wojciech Horak, Kinga Wójcik, and Joanna Lewandowska-Łańcucka. 2023. "Towards Controlling the Local Bone Tissue Remodeling—Multifunctional Injectable Composites for Osteoporosis Treatment" International Journal of Molecular Sciences 24, no. 5: 4959. https://doi.org/10.3390/ijms24054959
APA StyleKlara, J., Onak, S., Kowalczyk, A., Horak, W., Wójcik, K., & Lewandowska-Łańcucka, J. (2023). Towards Controlling the Local Bone Tissue Remodeling—Multifunctional Injectable Composites for Osteoporosis Treatment. International Journal of Molecular Sciences, 24(5), 4959. https://doi.org/10.3390/ijms24054959





