Bioactive and Physico-Chemical Assessment of Innovative Poly(lactic acid)-Based Biocomposites Containing Sage, Coconut Oil, and Modified Nanoclay
Abstract
:1. Introduction
2. Results and Discussion
2.1. ATR-FTIR Spectra Results
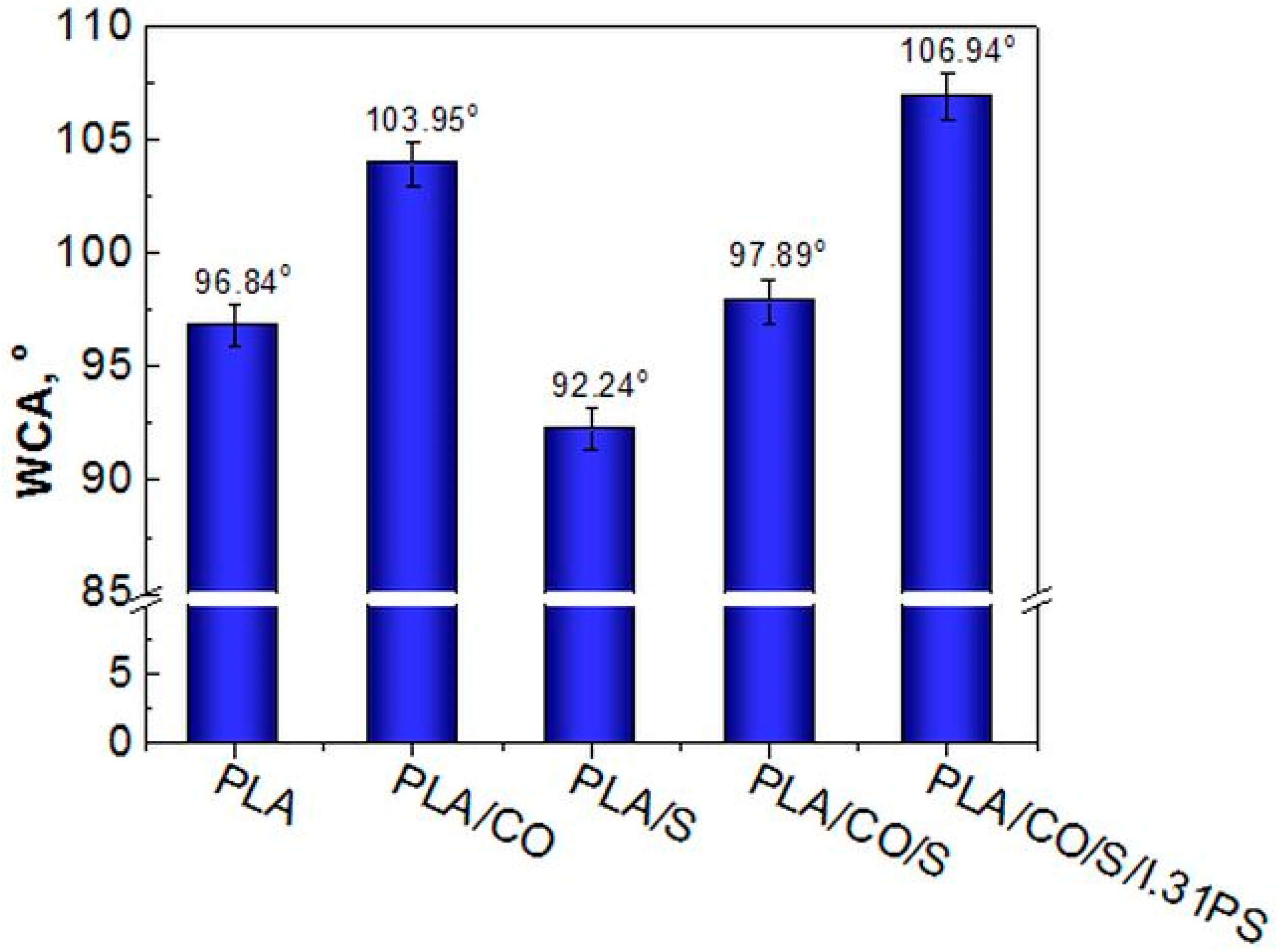
2.2. Surface Hydrophobicity Evaluation
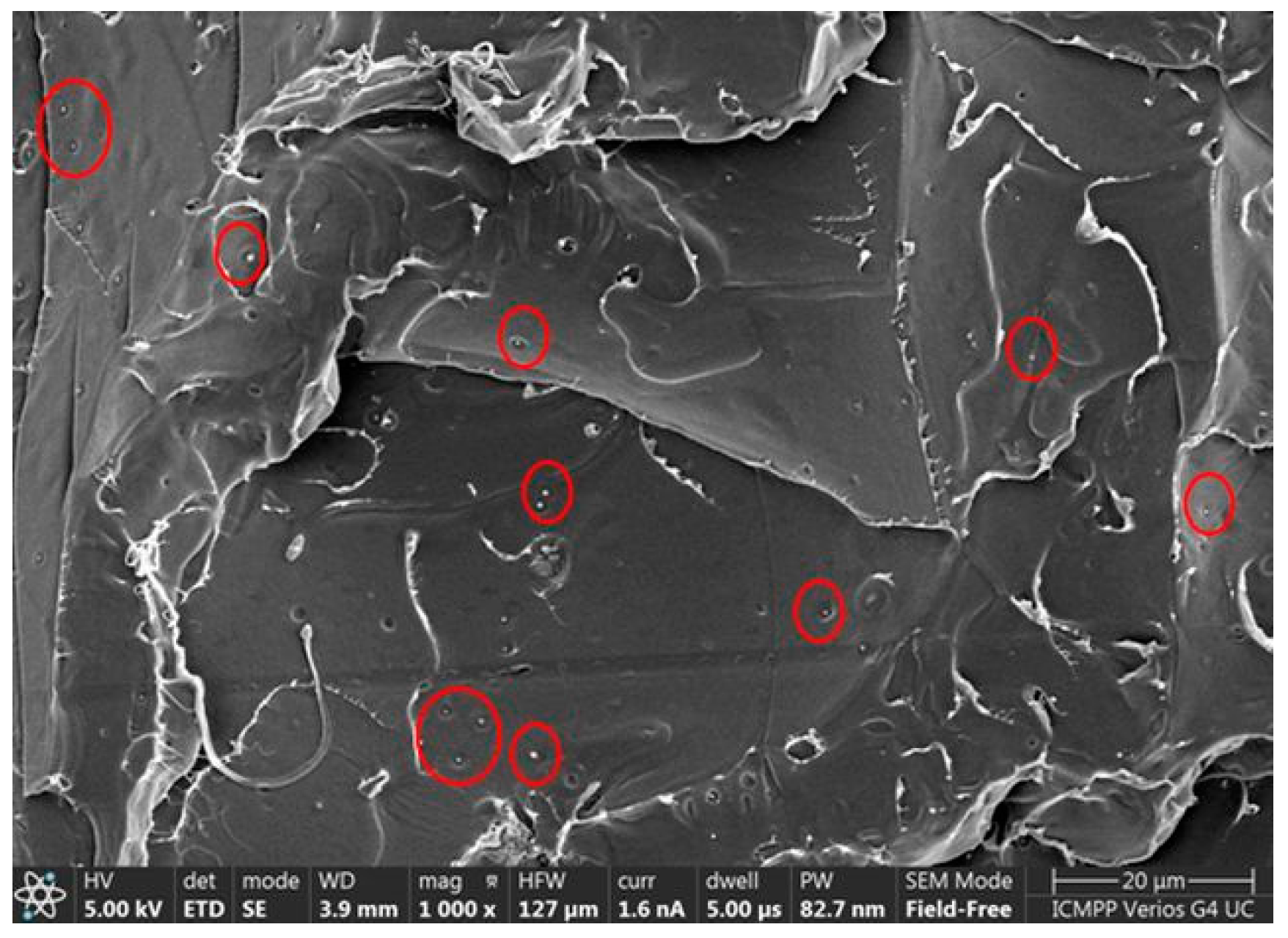
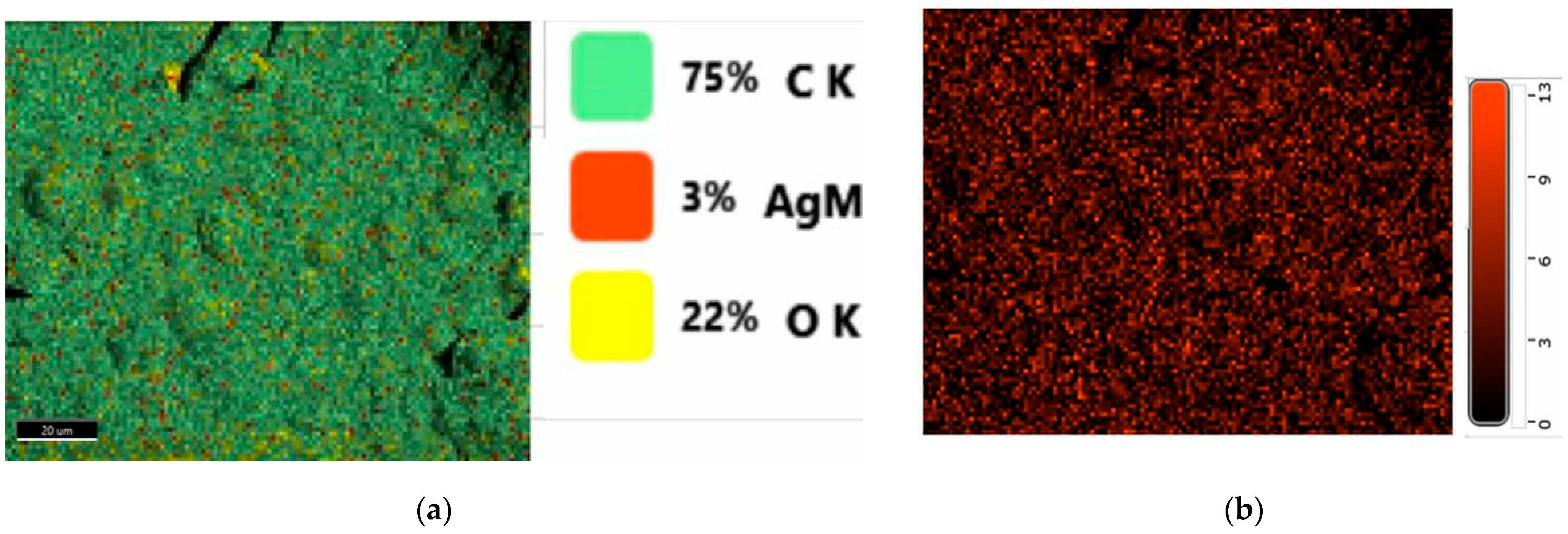
2.3. Morphology Examination
2.4. Mechanical Properties Evaluation
2.5. Antioxidant Activity
2.6. Antimicrobial Assessment
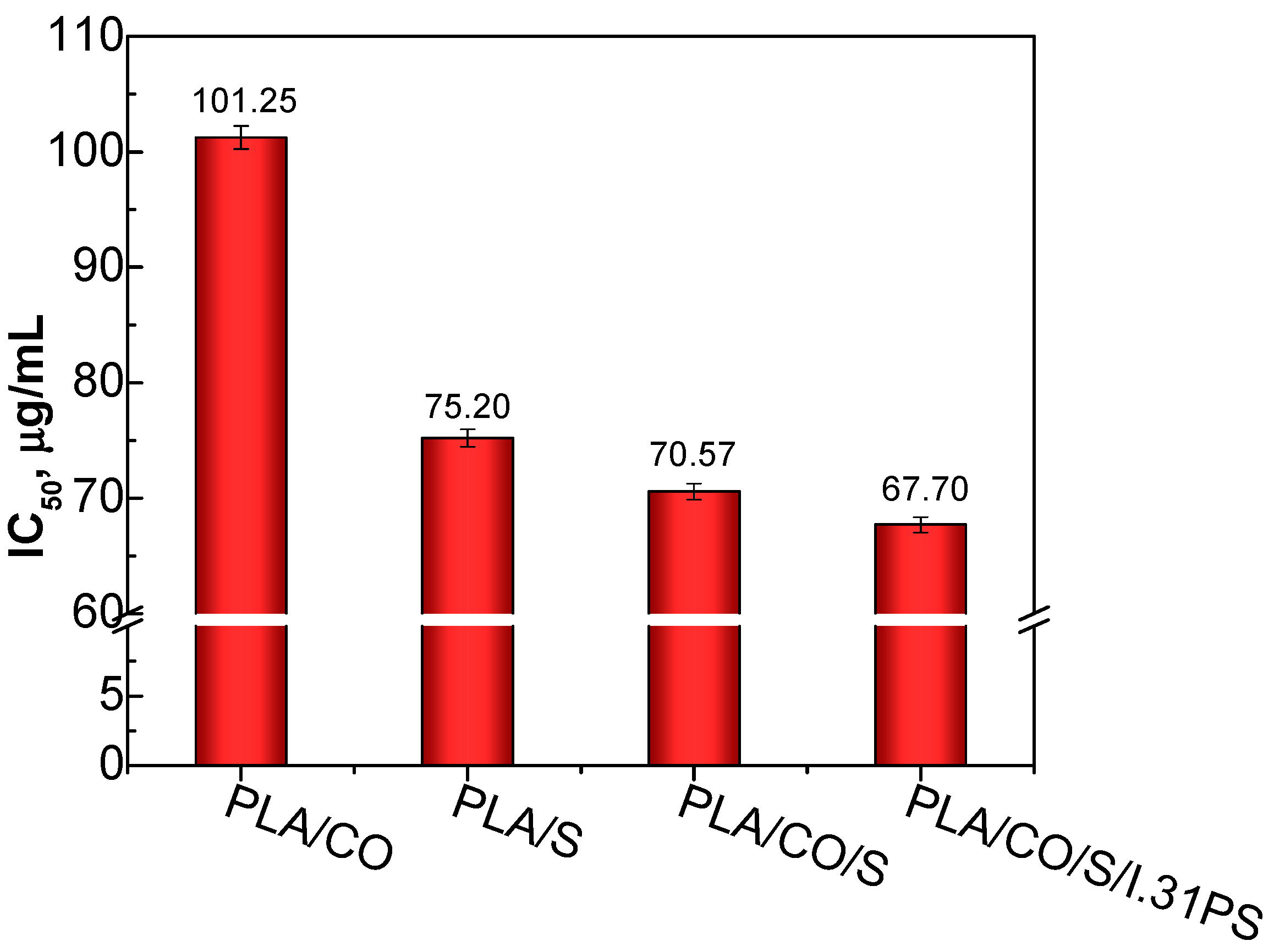
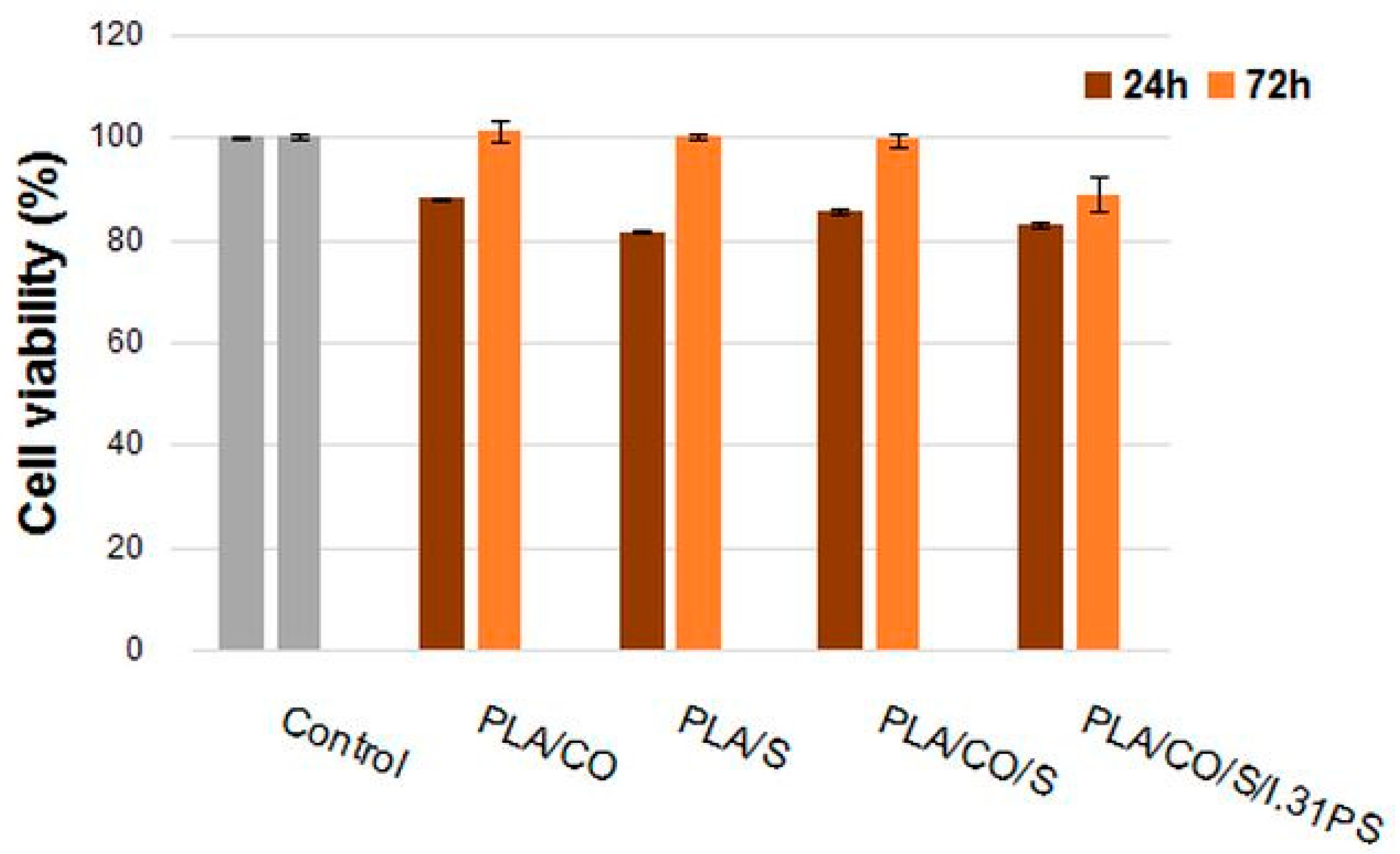
2.7. In Vitro Cytocompatibility Evaluation
3. Materials and Methods
3.1. Materials
3.2. PLA-Based Biocomposite Processing
3.3. Investigation Methods
3.3.1. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.3.2. Water Contact Angle Measurements
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. Examination of Mechanical Properties
3.3.5. DPPH Radical Scavenging Assay
3.3.6. Antimicrobial Assessment
3.3.7. In Vitro Cytocompatibility Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darie-Nita, R.N.; Rapa, M.; Frackowiak, S. Special Features of Polyester-Based Materials for Medical Applications. Polymers 2022, 14, 951. [Google Scholar] [CrossRef]
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Santos, M.; Cernadas, T.; Martins, P.; Miguel, S.P.; Correia, I.J.; Alves, P.; Ferreira, P. Polyester-based photocrosslinkable bioadhesives for wound closure and tissue regeneration support. React. Funct. Polym. 2021, 158, 104798. [Google Scholar] [CrossRef]
- Rapa, M.; Darie-Nita, R.N.; Preda, P.; Coroiu, V.; Tatia, R.; Vasile, C.; Matei, E.; Predescu, A.M.; Maxim, M.E. PLA/collagen hydrolysate/silver nanoparticles bionanocomposites for potential antimicrobial urinary drains. Polym.-Plast. Technol. Mater. 2019, 58, 2041–2055. [Google Scholar] [CrossRef]
- Salevic, A.; Prieto, C.; Cabedo, L.; Nedovic, V.; Maria Lagaron, J. Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(epsilon-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract. Nanomaterials 2019, 9, 270. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; D’Arrigo, M.; Lopresti, F.; Marino, A.; Bruno, M.; Nostro, A. Flexible mats as promising antimicrobial systems via integration of Thymus capitatus (L.) essential oil into PLA. Future Microbiol. 2020, 15, 1379–1392. [Google Scholar] [CrossRef]
- Stoleru, E.; Dumitriu, R.P.; Brebu, M.; Vasile, C.; Enache, A. Development of Bioactive Polymeric Materials by Incorporation of Essential/Vegetal Oils into Biopolymer Matrices. Proceedings 2021, 69, 25. [Google Scholar] [CrossRef]
- Darie-Nita, R.N.; Rapa, M.; Sivertsvik, M.; Rosnes, J.T.; Popa, E.E.; Dumitriu, R.P.; Marincas, O.; Matei, E.; Predescu, C.; Vasile, C. PLA-Based Materials Containing Bio-Plasticizers and Chitosan Modified with Rosehip Seed Oil for Ecological Packaging. Polymers 2021, 13, 1610. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altin, G.; Daskaya-Dikmen, C.; Martorell, M.; Ramirez-Alarcon, K.; Alarcon-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Pirbalouti, A.G.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hatami, M.; Kariman, K.; Dahaji, P.A. Phytochemical Variations and Enhanced Efficiency of Antioxidant and Antimicrobial Ingredients in Salvia officinalis as Inoculated with Different Rhizobacteria. Chem. Biodivers. 2016, 13, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Widianingrum, D.C.; Noviandi, C.T.; Salasia, S.I.O. Antibacterial and immunomodulator activities of virgin coconut oil (VCO) against Staphylococcus aureus. Heliyon 2019, 5, e02612. [Google Scholar] [CrossRef] [PubMed]
- Mercan, D.A.; Niculescu, A.G.; Grumezescu, A.M. Nanoparticles for Antimicrobial Agents Delivery-An Up-to-Date Review. Int. J. Mol. Sci. 2022, 23, 13862. [Google Scholar] [CrossRef]
- Yoon, S.; Chen, B. Modulating the Properties of Poly(glycerol sebacate)-Based Polyurethane Hydrogels Using an Organoclay. ACS Biomater. Sci. Eng. 2022, 8, 786–800. [Google Scholar] [CrossRef]
- Darie, R.N.; Paslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiata, A.; Baklavaridis, A.; Vasile, C. Effect of Nanoclay Hydrophilicity on the Poly(lactic acid)/Clay Nanocomposites Properties. Ind. Eng. Chem. Res. 2014, 53, 7877–7890. [Google Scholar] [CrossRef]
- Maiti, P.; Yamada, K.; Okamoto, M.; Ueda, K.; Okamoto, K. New polylactide/layered silicate nanocomposites: Role of organoclays. Chem. Mater. 2002, 14, 4654–4661. [Google Scholar] [CrossRef]
- Darie-Nita, R.N.; Vasile, C.; Irimia, A.; Lipsa, R.; Rapa, M. Evaluation of some eco-friendly plasticizers for PLA films processing. J. Appl. Polym. Sci. 2016, 133, 43223. [Google Scholar] [CrossRef]
- Darie-Nita, R.N.; Irimia, A.; Grigoras, V.C.; Mustata, F.; Tudorachi, N.; Rapa, M.; Ludwiczak, J.; Iwanczuk, A. Evaluation of Natural and Modified Castor Oil Incorporation on the Melt Processing and Physico-Chemical Properties of Polylactic Acid. Polymers 2022, 14, 3608. [Google Scholar] [CrossRef] [PubMed]
- Sarac, E.G.; Oner, E.; Kahraman, M.V. Microencapsulated organic coconut oil as a natural phase change material for thermo-regulating cellulosic fabrics. Cellulose 2019, 26, 8939–8950. [Google Scholar] [CrossRef]
- Mohammed, N.K. Phytochemical Screening by FTIR Spectroscopic Analysis and Anti-Bacterial Activity of Methanolic Extract of Selected Medicinal Plant of Anethum Graveolens and Plantago Major. Ann. Rom. Soc. Cell Biol. 2021, 58, 3110–3122. [Google Scholar]
- Wagner, A.; White, A.P.; Stueckle, T.A.; Banerjee, D.; Sierros, K.A.; Rojanasakul, Y.; Agarwal, S.; Gupta, R.K.; Dinu, C.Z. Early Assessment and Correlations of Nanoclay’s Toxicity to Their Physical and Chemical Properties. ACS Appl. Mater. Interfaces 2017, 9, 32323–32335. [Google Scholar] [CrossRef] [PubMed]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Khankrua, R.; Pivsa-Art, S.; Hiroyuki, H.; Suttiruengwong, S.; IOP. Grafting of poly (lactic acid) with maleic anhydride using supercritical carbon dioxide. In Proceedings of the Global Conference on Polymer and Composite Materials (PCM), Beijing, China, 16–19 May 2015. [Google Scholar]
- Tejada-Oliveros, R.; Balart, R.; Ivorra-Martinez, J.; Gomez-Caturla, J.; Montanes, N.; Quiles-Carrillo, L. Improvement of Impact Strength of Polylactide Blends with a Thermoplastic Elastomer Compatibilized with Biobased Maleinized Linseed Oil for Applications in Rigid Packaging. Molecules 2021, 26, 240. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Porte, A.; Godoy, R.L.O.; Maia-Porte, L.H. Chemical composition of sage (Salvia officinalis L.) essential oil from the Rio de Janeiro State (Brazil). Rev. Bras. Plantas Med. 2013, 15, 438–441. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Patwa, R.; Kumar, A.; Katiyar, V. Plasticizing effect of coconut oil on morphological, mechanical, thermal, rheological, barrier, and optical properties of poly(lactic acid): A promising candidate for food packaging. J. Appl. Polym. Sci. 2017, 134, 45390. [Google Scholar] [CrossRef]
- Kekevi, B.; Mert, E.H. Synthesis of beta-myrcene-based macroporous nanocomposite foams: Altering the morphological and mechanical properties by usingorgano-modifiednanoclay. J. Appl. Polym. Sci. 2021, 138, 50074. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.K.; O’Reilly, R.K. Polymers for Biomedical Applications: The Importance of Hydrophobicity in Directing Biological Interactions and Application Efficacy. Biomacromolecules 2021, 22, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Valles-Lluch, A.; Fombuena, V.; Napiwocki, B.; Lih-Sheng, T. Influence of the Hydrophobic-Hydrophilic Nature of Biomedical Polymers and Nanocomposites on In Vitro Biological Development. Macromol. Mater. Eng. 2017, 302, 1700259. [Google Scholar] [CrossRef]
- Williams, M.L.; Thomas, B.J.; Farrar, J.F.; Pollock, C.J. Visualizing the distribution of elements within barley leaves by energy-dispersive X-ray image maps. New Phytol. 1993, 125, 367–372. [Google Scholar] [CrossRef]
- Zhao, G.; Gomes, F.P.C.; Marway, H.; Thompson, M.R.; Zhu, Z. Physical Aging as the Driving Force for Brittle–Ductile Transition of Polylactic Acid. Macromol. Chem. Phys. 2020, 221, 1900475. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Stoleru, E.; Munteanu, B.S.; Darie-Niţă, R.N.; Pricope, G.M.; Lungu, M.; Irimia, A.; Râpă, M.; Lipşa, R.D.; Vasile, C. Complex poly(Lactic acid)-based biomaterial for urinary catheters: II. biocompatibility. Bioinspired Biomim. Nanobiomater. 2016, 5, 152–166. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liang, X.; Wang, S.Y.; Qin, W.; Zhang, Q. Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef]
- Stoleru, E.; Vasile, C.; Irimia, A.; Brebu, M. Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils. Molecules 2021, 26, 4500. [Google Scholar] [CrossRef]
- Zare, H. Biosynthesis of Silver Nanoparticles Using Common Sage Extract and Evaluation of Anticancer Activity. Biomed. J. Sci. Tech. Res. 2020, 28, 21179–21185. [Google Scholar] [CrossRef]
- Saud, M.A.; Saud, N.A.; Hamad, M.A.; Farhan Gar, L. Role of Salvia officinalis Silver Nanoparticles in Attenuation Renal Damage in Rabbits Exposed to Methotrexate. Arch. Razi Inst. 2022, 77, 151–162. [Google Scholar] [CrossRef]
- Marina, A.M.; Man, Y.B.C.; Nazimah, S.A.H.; Amin, I. Antioxidant capacity and phenolic acids of virgin coconut oil. Int. J. Food Sci. Nutr. 2009, 60, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.Y.; Nomanbhay, S.; Kusumo, F.; Raja Shahruzzaman, R.M.H.; Shamsuddin, A.H. Modeling and Optimization of Microwave-Based Bio-Jet Fuel from Coconut Oil: Investigation of Response Surface Methodology (RSM) and Artificial Neural Network Methodology (ANN). Energies 2021, 14, 295. [Google Scholar] [CrossRef]
- Mahfud, M.; Suryanto, A.; Qadariyah, L.; Suprapto, S.; Kusuma, H.S. Production of Methyl Ester from Coconut Oil using Microwave: Kinetic of Transesterification Reaction using Heterogeneous CaO Catalyst. Korean Chem. Eng. Res. 2018, 56, 275–280. [Google Scholar] [CrossRef]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Effects of organoclay modifiers on the flammability, thermal and mechanical properties of polycarbonate nanocomposites filled with a phosphate and organoclays. Polym. Degrad. Stab. 2012, 97, 108–117. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.-B.; Siddiqui, M.K.; Nazeer, W.; Najafi, M. A Theoretical Examination of the Antioxidant Activity of NH2, OMe, and tert-Butyl Sesamol Derivatives and Their Drug Delivery with C-60 Nanocage. Russ. J. Phys. Chem. A 2018, 92, 2757–2760. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Zhao, X.; Li, Y. Effects of nitro- and amino-group on the antioxidant activity of genistein: A theoretical study. Food Chem. 2019, 275, 339–345. [Google Scholar] [CrossRef]
- Barbalata-Mandru, M.; Serbezeanu, D.; Butnaru, M.; Rimbu, C.M.; Enache, A.A.; Aflori, M. Poly(vinyl alcohol)/Plant Extracts Films: Preparation, Surface Characterization and Antibacterial Studies against Gram Positive and Gram Negative Bacteria. Materials 2022, 15, 2493. [Google Scholar] [CrossRef]
- Zemljic, L.F.; Glaser, T.K.; Plohl, O.; Anzel, I.; Simat, V.; Cagalj, M.; Meznar, E.; Malin, V.; Sternisa, M.; Mozina, S.S. Biomass-Derived Plant Extracts in Macromolecular Chitosan Matrices as a Green Coating for PLA Films. J. Funct. Biomater. 2022, 13, 228. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Gavril, G.L.; Wrona, M.; Bertella, A.; Swieca, M.; Rapa, M.; Salafranca, J.; Nerin, C. Influence of medicinal and aromatic plants into risk assessment of a new bioactive packaging based on polylactic acid (PLA). Food Chem. Toxicol. 2019, 132, 110662. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, J.C.; Kabara, J.J. In vitro effects of monolaurin compounds on enveloped RNA and DNA viruses. J. Food Saf. 1982, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shino, B.; Peedikayil, F.C.; Jaiprakash, S.R.; Ahmed Bijapur, G.; Kottayi, S.; Jose, D. Comparison of Antimicrobial Activity of Chlorhexidine, Coconut Oil, Probiotics, and Ketoconazole on Candida albicans Isolated in Children with Early Childhood Caries: An In Vitro Study. Scientifica 2016, 2016, 7061587. [Google Scholar] [CrossRef] [PubMed]
- Verallo-Rowell, V.M.; Dillague, K.M.; Syah-Tjundawan, B.S. Novel Antibacterial and Emollient Effects of Coconut and Virgin Olive Oils in Adult Atopic Dermatitis. Dermatitis 2008, 19, 308–315. [Google Scholar] [CrossRef]
- Kamdem, S.S.; Guerzoni, M.E.; Baranyi, J.; Pin, C. Effect of capric, lauric and alpha-linolenic acids on the division time distributions of single cells of Staphylococcus aureus. Int. J. Food Microbiol. 2008, 128, 122–128. [Google Scholar] [CrossRef] [PubMed]
- DebMandal, M.; Mandal, S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac. J. Trop. Med. 2011, 4, 241–247. [Google Scholar] [CrossRef]
- Rossell, J.B. Fractionation of lauric oils. J. Am. Oil Chem. Soc. 1985, 62, 385–390. [Google Scholar] [CrossRef]
- Nair, S.S.; Manalil, J.J.; Ramavarma, S.K.; Suseela, I.M.; Thekkepatt, A.; Raghavamenon, A.C. Virgin coconut oil supplementation ameliorates cyclophosphamide-induced systemic toxicity in mice. Hum. Exp. Toxicol. 2016, 35, 205–212. [Google Scholar] [CrossRef]
- Linakurdi; Aljuhani, R. Evaluation of the cytotoxic effects of coconut juice, coconut oil and methotrexate on human breast cancer cell line MCF-7. Life Sci. J. 2020, 17, 47–57. [Google Scholar]
- Ilomuanya, M.O.; Adebona, A.C.; Wang, W.; Sowemimo, A.; Eziegbo, C.L.; Silva, B.O.; Adeosun, S.O.; Joubert, E.; De Beer, D. Development and characterization of collagen-based electrospun scaffolds containing silver sulphadiazine and Aspalathus linearis extract for potential wound healing applications. SN Appl. Sci. 2020, 2, 881. [Google Scholar] [CrossRef]
- Khakestani, M.; Jafari, S.H.; Zahedi, P.; Bagheri, R.; Hajiaghaee, R. Physical, morphological, and biological studies on PLA/nHA composite nanofibrous webs containing Equisetum arvense herbal extract for bone tissue engineering. J. Appl. Polym. Sci. 2017, 134, 45343. [Google Scholar] [CrossRef]
- Thuy Thi Thu, N.; Ghosh, C.; Hwang, S.-G.; Lam Dai, T.; Park, J.S. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Kakanuru, P.; Pochiraju, K. Moisture Ingress and Degradation of Additively Manufactured PLA, ABS and PLA/SiC Composite Parts. Addit. Manuf. 2020, 36, 101529. [Google Scholar] [CrossRef]
- van den Oever, M.J.A.; Beck, B.; Mussig, J. Agrofibre reinforced poly(lactic acid) composites: Effect of moisture on degradation and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1628–1635. [Google Scholar] [CrossRef]
- Fifere, N.; Airinei, A.; Asandulesa, M.; Rotaru, A.; Ursu, E.L.; Doroftei, F. Investigating the Vibrational, Magnetic and Dielectric Properties, and Antioxidant Activity of Cerium Oxide Nanoparticles. Int. J. Mol. Sci. 2022, 23, 13883. [Google Scholar] [CrossRef]
- Irimia, A.; Stoleru, E.; Vasile, C.; Bele, A.; Brebu, M. Application of Vegetal Oils in Developing Bioactive Paper-Based Materials for Food Packaging. Coatings 2021, 11, 1211. [Google Scholar] [CrossRef]
- Olugbami, J.O.; Gbadegesin, M.A.; Odunola, O.A. In vitro evaluation of the antioxidant potential, phenolic and flavonoid contents of the stem bark ethanol extract of Anogeissus leiocarpus. Afr. J. Med. Med. Sci. 2014, 43 (Suppl. 1), 101–110. [Google Scholar]
- Kahlmeter, G.; Brown, D.F.J.; Goldstein, F.W.; MacGowan, A.P.; Mouton, J.W.; Odenholt, I.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; Soriano, F.; et al. European Committee on Antimicrobial Susceptibility Testing (EUCAST) Technical Notes on antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2006, 12, 501–503. [Google Scholar] [CrossRef]
- Stefan, L.M.; Iosageanu, A.; Ilie, D.; Stanciuc, A.-M.; Matei, C.; Berger, D.; Craciunescu, O. Extracellular matrix biomimetic polymeric membranes enriched with silver nanoparticles for wound healing. Biomed. Mater. 2021, 16, 035010. [Google Scholar] [CrossRef] [PubMed]
- Rapa, M.; Stefan, L.M.; Seciu-Grama, A.M.; Gaspar-Pintiliescu, A.; Matei, E.; Zaharia, C.; Stanescu, P.O.; Predescu, C. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-co-3HV))/Bacterial Cellulose (BC) Biocomposites for Potential Use in Biomedical Applications. Polymers 2022, 14, 5544. [Google Scholar] [CrossRef] [PubMed]





| Sample | Mean ± SD Inhibition Zone (mm) | |
|---|---|---|
| Staphylococcus aureus | Escherichia coli | |
| S | 9.8 ± 0.2 | 8.5 ± 0.1 |
| I.31PS | 10.0 ± 0.3 | 9.2 ± 0.2 |
| PLA/S | 9.5 ± 0.3 | 8.1 ± 0.2 |
| PLA/CO | 10.4 ± 0.4 | 8.9 ± 0.6 |
| PLA/CO/S | 10.8 ± 0.3 | 9.5 ± 0.2 |
| PLA/CO/S/I.31PS | 11.6 ± 0.2 | 10.7 ± 0.3 |
| Sample Code | PLA (wt%) | S (wt%) | CO (wt%) | I.31PS (wt%) |
|---|---|---|---|---|
| PLA | 100 | - | - | - |
| PLA/S | 97 | 3 | - | - |
| PLA/CO | 85 | - | 15 | - |
| PLA/CO/S | 82.45 | 3 | 14.55 | - |
| PLA/CO/S/I.31PS | 79.90 | 3 | 14.10 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darie-Niță, R.N.; Irimia, A.; Doroftei, F.; Stefan, L.M.; Iwanczuk, A.; Trusz, A. Bioactive and Physico-Chemical Assessment of Innovative Poly(lactic acid)-Based Biocomposites Containing Sage, Coconut Oil, and Modified Nanoclay. Int. J. Mol. Sci. 2023, 24, 3646. https://doi.org/10.3390/ijms24043646
Darie-Niță RN, Irimia A, Doroftei F, Stefan LM, Iwanczuk A, Trusz A. Bioactive and Physico-Chemical Assessment of Innovative Poly(lactic acid)-Based Biocomposites Containing Sage, Coconut Oil, and Modified Nanoclay. International Journal of Molecular Sciences. 2023; 24(4):3646. https://doi.org/10.3390/ijms24043646
Chicago/Turabian StyleDarie-Niță, Raluca Nicoleta, Anamaria Irimia, Florica Doroftei, Laura Mihaela Stefan, Andrzej Iwanczuk, and Agnieszka Trusz. 2023. "Bioactive and Physico-Chemical Assessment of Innovative Poly(lactic acid)-Based Biocomposites Containing Sage, Coconut Oil, and Modified Nanoclay" International Journal of Molecular Sciences 24, no. 4: 3646. https://doi.org/10.3390/ijms24043646
APA StyleDarie-Niță, R. N., Irimia, A., Doroftei, F., Stefan, L. M., Iwanczuk, A., & Trusz, A. (2023). Bioactive and Physico-Chemical Assessment of Innovative Poly(lactic acid)-Based Biocomposites Containing Sage, Coconut Oil, and Modified Nanoclay. International Journal of Molecular Sciences, 24(4), 3646. https://doi.org/10.3390/ijms24043646









