Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review
Abstract
1. Introduction
2. Types of Wound Dressing Materials
3. Properties of an Ideal Wound Dressings
4. Bioactive Polysaccharide-Based Hydrogels
4.1. Chitosan
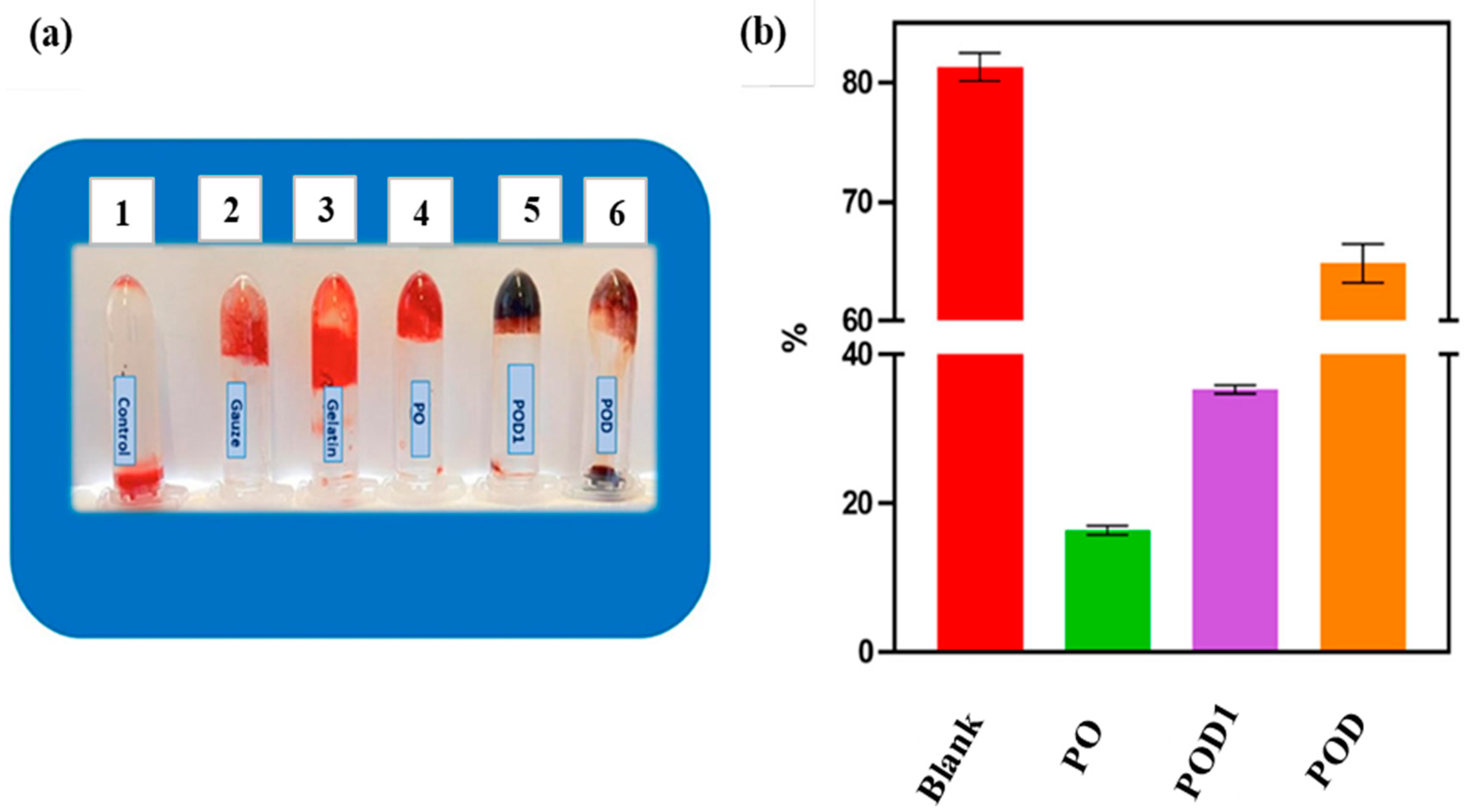
4.2. Pullulan
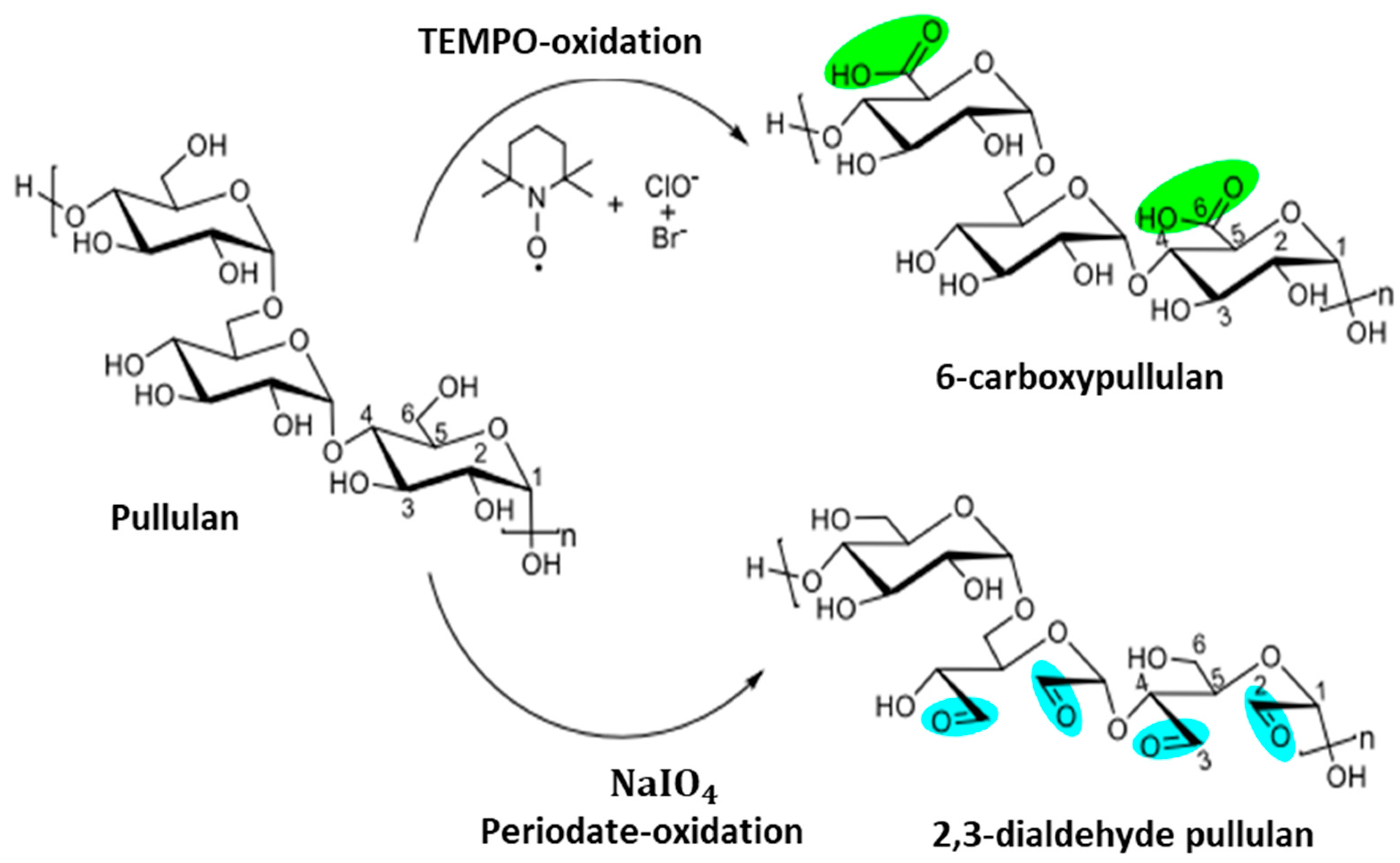
5. Oxidative Modifications of Pullulan
5.1. Pullulan Oxidation Using TEMPO
5.2. Aldehyde Modification of Pullulan
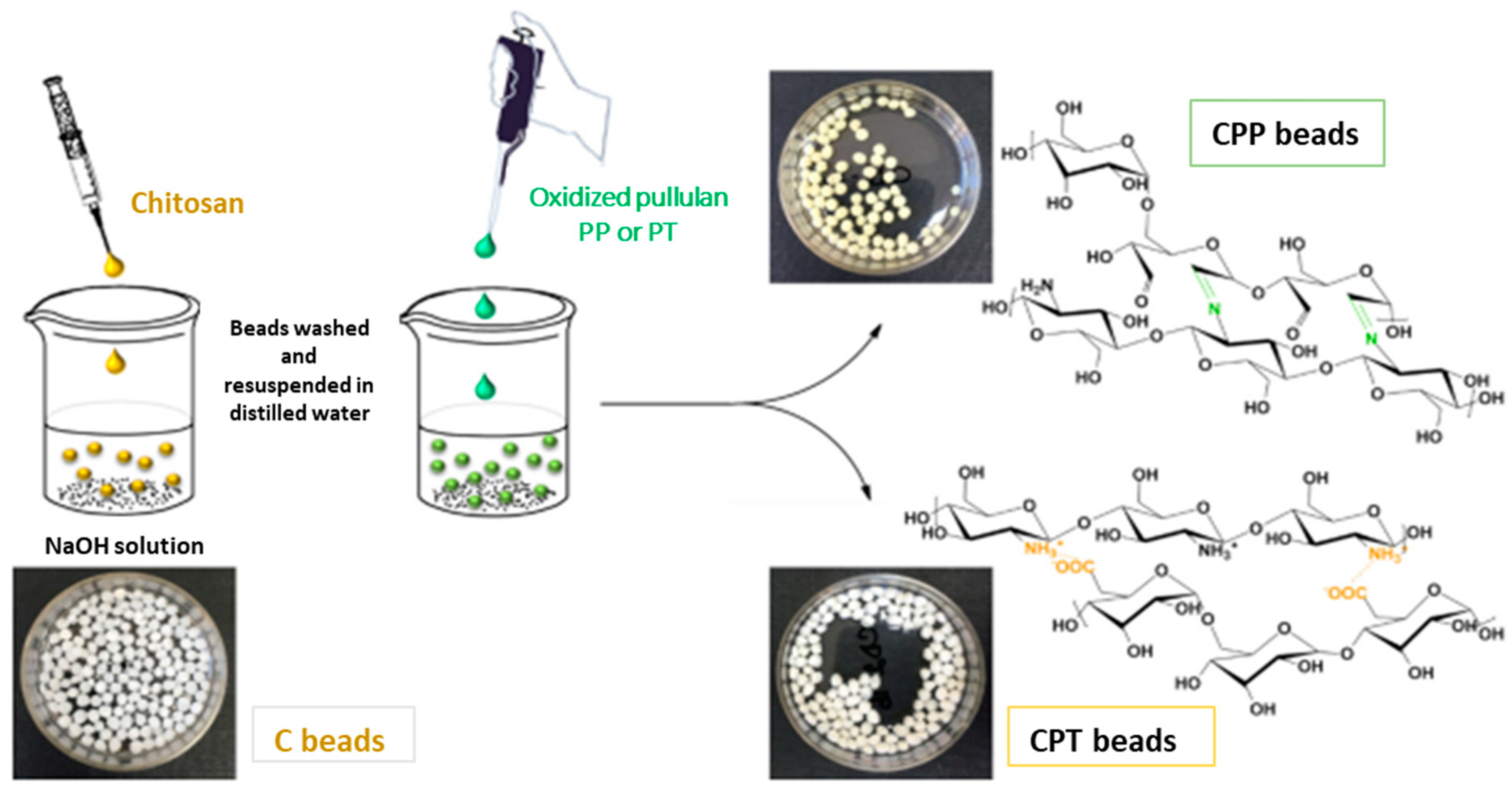
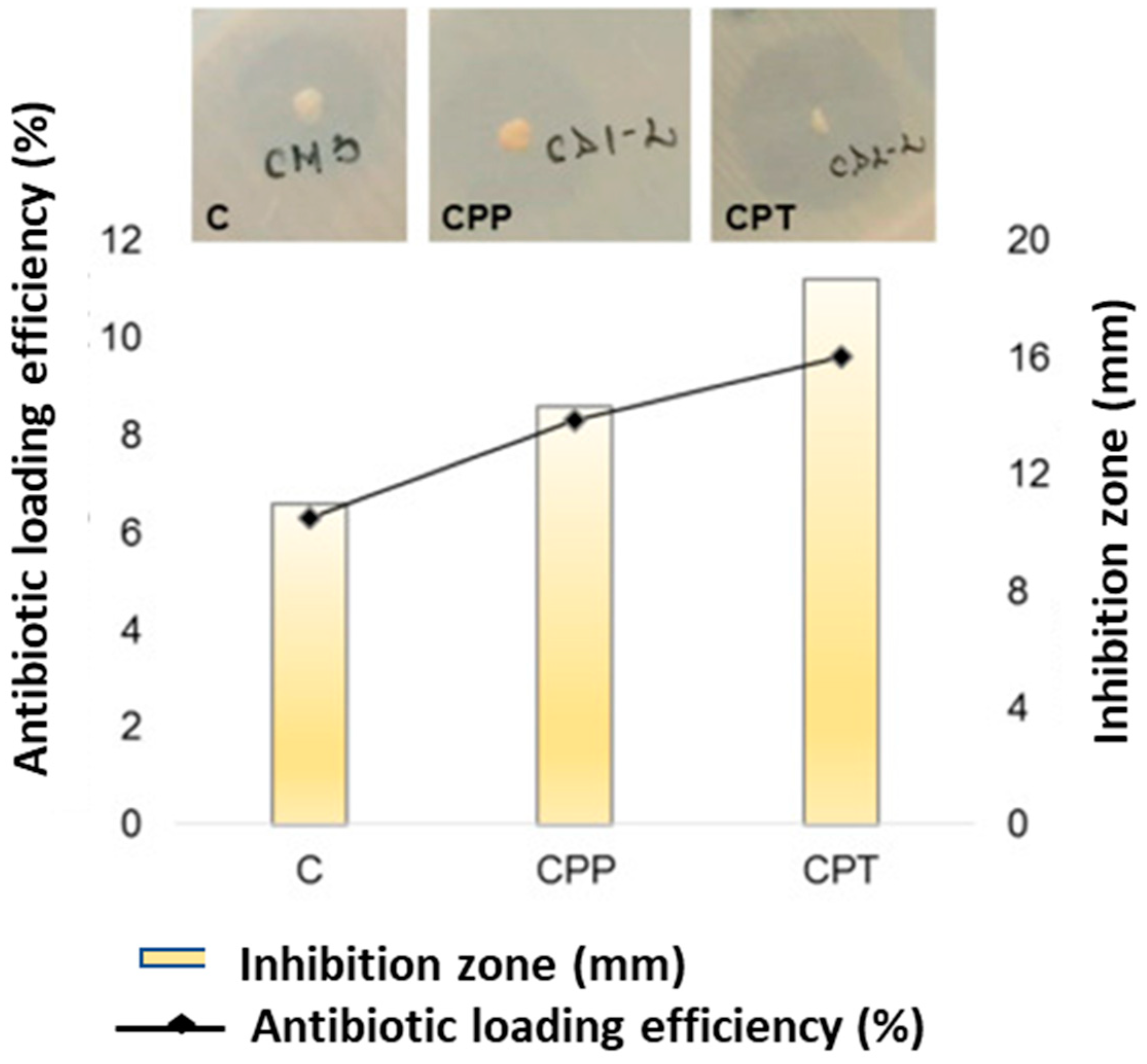
6. Applications of Pullulan-Based Biomaterials as Wound Dressings and Skin Tissue Engineering Scaffolds
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Badylak, S.F. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv. Wound Care 2015, 4, 490–500. [Google Scholar] [CrossRef]
- Sivaraj, D.; Chen, K.; Chattopadhyay, A.; Henn, D.; Wu, W.; Noishiki, C.; Magbual, N.J.; Mittal, S.; Mermin-Bunnell, A.M.; Bonham, C.A.; et al. Hydrogel scaffolds to deliver cell therapies for wound healing. Front. Bioeng. Biotechnol. 2021, 9, 660145. [Google Scholar] [CrossRef] [PubMed]
- Naseri-Nosar, M.; Ziora, Z.M. Wound Dressings from Naturally occurring Polymers: A Review on Homopolysac charide-based Composites. Carbohydr. Polym. 2018, 89, 379–398. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Samadian, H.; Mahakizadeh, S.; Tajerian, R.; Rahmati, M.; Vaez, A.; Salehi, M. Taurine-loaded poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. J. Bioact. Compat. Polym. 2018, 33, 282–294. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuța, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Bai, M.Y.; Chen, M.C.; Yu, W.C.; Lin, J.Y. Foam dressing incorporating herbal extract: An all-natural dressing for potential use in wound healing. J. Bioact. Compat. Polym. 2017, 32, 293–308. [Google Scholar] [CrossRef]
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, Y.; Jain, S.K. Perspectives of biodegradable natural polysaccharides for site specific drug delivery to the colon. J. Pharm. Pharm. Sci. 2007, 10, 86–128. [Google Scholar]
- d’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Cui, R.; Zhang, L.; Ou, R.; Xu, Y.; Xu, L.; Zhan, X.-Y.; Li, D. Polysaccharide-Based Hydrogels for Wound Dressing: Design Considerations and Clinical Applications. Front. Bioeng. Biotechnol. 2022, 10, 845735. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, R.; Zheng, Y.; Wang, J.; Khatib, M.; Jiang, X.; Zhou, C.; Omar, R.; Saliba, W.; Wu, W.; et al. Highly Efficient Self-Healing Multifunctional Dressing with Antibacterial Activity for Sutureless Wound Closure and Infected Wound Monitoring. Adv. Mater. 2022, 34, 2106842. [Google Scholar] [CrossRef]
- Yu, H.C.; Zhang, H.; Ren, K.; Ying, Z.; Zhu, F.; Qian, J.; Ji, J.; Wu, Z.L.; Zheng, Q. Ultrathin κ-Carrageenan/Chitosan Hydrogel Films with High Toughness and Antiadhesion Property. ACS Appl. Mater. Inter. 2018, 10, 9002–9009. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.G.; Jones, V.; Price, P. Topical Treatment: Which Dressing to Choose. Diabetes Metab. Res. Rev. 2000, 16, 47–50. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; de Sousa, H.C. Recent Advances on the Development of Wound Dressings for Diabetic Foot Ulcer Treatment-A Review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Kibungu, C.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. This Review Recent Advances in Chitosan and Alginate-Based Hydrogels for Wound Healing Application. Front. Mater. 2021, 8, 681960. [Google Scholar] [CrossRef]
- Line, A.S.; Morykwas, M.J.; Line, S.W. Use of Cultured Human Epidermal Xenografts for Wound Treatment in Nonhuman Primates. J. Zoo Wildl. Med. 1995, 26, 517–524. Available online: https://www.jstor.org/stable/20095517 (accessed on 7 February 2023).
- Greenwood, J.E.; Dearman, B.L. Split Skin Graft Application over an Integrating, Biodegradable Temporizing Polymer Matrix. J. Burn Care Res. 2012, 33, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D. Wounds—What Should a Dressing Formulary Include? Hosp. Pharm. 2002, 9, 261–266. [Google Scholar]
- Dhivya, S.; Padmab, V.V.; Santhini, E. Wound dressings–a review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar Ricardo, A. Poly (vinyl alcohol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Membrane Sci. 2014, 469, 262–271. [Google Scholar] [CrossRef]
- Heilmann, S.; Kuchler, S.; Wischke, C.; Lendlein, A.; Stein, C.; Schafer-Korting, M. A thermosensitive morphine-containing hydrogel for the treatment of large-scale skin wounds. Int. J. Pharm. 2013, 444, 96–102. [Google Scholar] [CrossRef]
- Kong, M.S.; Koh, W.-G.; Lee, H.J. Controlled Release of Epidermal Growth Factor from Furfuryl-Gelatin Hydrogel Using in Situ Visible Light-Induced Crosslinking and Its Effects on Fibroblasts Proliferation and Migration. Gels 2022, 8, 214. [Google Scholar] [CrossRef]
- Thönes, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gómez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of Heparin-Binding EGF-like growth factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.; Freeman, R.; Percival, S.L. In Vitro study of Sustained Antimicrobial Activity of a New Silver Alginate Dressing. J. Am. Coll. Certif. Wound Spéc. 2009, 1, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.F.; Ferreira, P.; Lopes, A.; Gil, M.H. Photocrosslinkable Biodegradable Responsive Hydrogels as Drug Delivery Systems. Int. J. Biol. Macromol. 2011, 49, 948–954. [Google Scholar] [CrossRef]
- Liang, M.; Chen, Z.; Wang, F.; Liu, L.; Wei, R.; Zhang, M. Preparation of Self-regulating/anti-adhesive Hydrogels and Their Ability to Promote Healing in Burn Wounds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz-Bortuzzo, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Hydrogel Matrices Based on Elastin and Alginate for Tissue Engineering Applications. Int. J. Biol. Macromol. 2018, 114, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Inter. 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Klapczynski, A.; Kuch, N.; Arpino, F.; Simon-Keller, K.; De la Torre, C.; Sticht, C.; van Abeelen, F.A.; Oversluizen, G.; Gretz, N. Gene Expression Profiling Reveals Aryl Hydrocarbon Receptor as a Possible Target for Photobiomodulation when Using Blue Light. Sci. Rep. 2016, 6, 33847. [Google Scholar] [CrossRef]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-ard, P.; Charumanee, S. Injectable Thermosensitive Chitosan/Pullulan-Based Hydrogels with Improved Mechanical Properties and Swelling Capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, L.; Yang, L.Y.; Huang, F.; Ouyang, X. PEI-modified core-shell/bead-like amino silica enhanced poly (vinyl alcohol)/chitosan for diclofenac sodium efficient adsorption. Carbohydr. Polym. 2020, 229, 115459. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wu, H.; Xie, T.; Chen, L.; Hu, S.; Tian, H.; Wang, Y.; Wang, J. Characterization and antibacterial effect of quaternized chitosan anchored cellulose beads. Int. J. Biol. Macromol. 2020, 155, 1325–1332. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The Production and Application of Hydrogels for Wound Management: A Review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764. [Google Scholar] [CrossRef]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and appli cations in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Lee, Y.R.; Kang, T.H.; Choi, J.W.; Kimura, S.; Wada, M. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohydr. Polym. 2017, 163, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Liu, W.; Xiao, J.; Zhao, X.; Zhu, Y.; Wu, Y. Preparation and characterization of dialdehyde starch by one-step acid hydrolysis and oxidation. Int. J. Biol. Macromol. 2017, 103, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Bai, R.; Bi, F.; Liu, J.; Qin, Y.; Liu, J. Synthesis, characterization, antioxidant and antimicrobial activities of starch aldehyde-quercetin conjugate. Int. J. Biol. Macromol. 2020, 156, 462–470. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate /gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef]
- Flynn, J.; Durack, E.; Collins, M.N.; Hudson, S.P. Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J. Mater. Chem. B 2020, 8, 4029–4038. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate oxidation of xanthan gum and its crosslinking effects on gelatin-based edible films. Food Hydrocoll. 2014, 39, 243–250. [Google Scholar] [CrossRef]
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869. [Google Scholar] [CrossRef]
- Valachova, K.; Svik, K.; Biro, C.; Collins, M.N.; Jurcik, R.; Ondruska, L.; Soltes, L. Impact of ergothioneine, hercynine, and histidine on oxidative degradation of hyaluronan and wound healing. Polymers 2020, 13, 95. [Google Scholar] [CrossRef]
- Duceac, A.I.; Vereștiuc, L.; Coroaba, A.; Arotăriței, D.; Coseri, S. All-polysaccharide hydrogels for drug delivery applications: Tunable chitosan beads surfaces via physical or chemical interactions, using oxidized pullulan. Int. J. Biol. Macromol. 2021, 181, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.K. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Chen, L.; Zhou, Q.; Qiu, J.; Xin, X.; Zhang, Y.; Yuan, W.; Tian, C.; Yu, X.; et al. High-level production of pullulan from high concentration of glucose by mutagenesis and adaptive laboratory evolution of Aureobasidium pullulans. Carbohydr. Polym. 2023, 302, 120426. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, S.; Ahmad, A.; Chen, L.; Bai, W. Ultra-high molecular weight pullulan-based material with high deformability and shape-memory properties. Carbohydr. Polym. 2022, 295, 119836. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.K. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Wypij, M.; Ingle, A.P.; Trzcinska-Wencel, J.; Golinska, P. Emerging Trends in Pullulan-Based Antimicrobial Systems for Various Applications. Int. J. Mol. Sci. 2021, 22, 13596. [Google Scholar] [CrossRef]
- Luís, A.; Ramos, A.; Domingues, F. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Krasniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan—Biopolymer with Potential for Use as Food Packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for Advanced Sustainable Body- and Skin-Contact Applications. J. Funct. Biomater. 2020, 11, 20. [Google Scholar] [CrossRef]
- Luís, A.; Ramos, A.; Domingues, F. Pullulan–Apple Fiber Biocomposite Films: Optical, Mechanical, Barrier, Antioxidant and Antibacterial Properties. Polymers 2021, 13, 870. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B. Antitumor/antiviral carbon quantum dots based on carrageenan and pullulan. Int. J. Biol. Macromol. 2020, 170, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yi, J.; Yu, X.; Wang, Z.; Wang, L. Preparation and characterization of pullulan derivative/chitosan composite film for potential antimicrobial applications. Int. J. Biol. Macromol. 2020, 148, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.; Hernández-Iturriaga, M.; Loarca-Piña, G.; Luna-Barcenas, G.; Mendoza, S. Antimicrobial effect of nisin electrospun amaranth: Pullulan nanofibers in apple juice and fresh cheese. Int. J. Food Microbiol. 2019, 295, 25–32. [Google Scholar] [CrossRef]
- Hamidi, M.; Okoro, V.O.; Milan, B.P.; Khalili, R.M.; Samadian, H.; Nie, L.; Shavandi, A. Fungal exopolysaccharides: Properties, sources, modifications, and biomedical applications. Carbohydr. Polym. 2022, 284, 119152. [Google Scholar] [CrossRef]
- Tabasum, S.; Noreen, A.; Maqsood, M.F.; Umar, H.; Akram, N.; Nazli, Z.-i-H.; Chatha, S.A.S.; Zia, K.M. Review on versatile applications of blends and composites of pullulan with natural and synthetic polymers. Int. J. Biol. Macromol. 2018, 120, 603–632. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef]
- Gniewosz, M.; Krasniewska, K.; Woreta, M.; Kosakowska, O. Antimicrobial Activity of a Pullulan-Caraway Essential Oil Coating on Reduction of Food Microorganisms and Quality in Fresh Baby Carrot. J. Food Sci. 2013, 78, 1242–1248. [Google Scholar] [CrossRef]
- Pinto, R.; Almeida, A.; Fernandes, S.C.; Freire, C.; Silvestre, A.; Neto, C.P.; Trindade, T. Antifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus niger. Colloids Surf. B Biointerfaces 2013, 103, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.H.; Cutter, C.N. Development and evaluation of pullulan-based composite antimicrobial films (CAF) incorporated with nisin, thymol and lauric arginate to reduce foodborne pathogens associated with muscle foods. Int. J. Food Microbiol. 2020, 320, 108519. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Tenorio, F.; Giraldo-Estrada, C. Characterization and chemical modification of pullulan produced from a submerged culture of Aureobasidium pullulans ATCC 15233. Polym. Test. 2022, 114, 107686. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Zheng, X.; Zhang, A.; Zhang, X.; Tang, K. Pullulan dialdehyde crosslinked gelatin hydrogels with high strength for biomedical applications. Carbohydr. Polym. 2019, 216, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S.; Bercea, M.; Harabagiu, V.; Budtova, T. Oxidation vs. degradation in polysaccharides: Pullulan—A case study. Eur. Polym. J. 2016, 85, 82–91. [Google Scholar] [CrossRef]
- Bruneel, D.; Schacht, E. Chemical modification of pullulan: Periodate oxidation. Polymer 1993, 34, 2628–2632. [Google Scholar] [CrossRef]
- De Nooy, A.E.J.; Besemer, A.C.; Van Bekkum, H.; Van Dijk, J.A.P.P.; Smit, J.A.M. TEMPO-mediated oxidation of pullulan and influence of ionic strength and linear charge density on the dimensions of the obtained polyelectrolyte chains. Macromolecules 1996, 29, 6541–6547. [Google Scholar] [CrossRef]
- Spatareanu, A.; Bercea, M.; Budtova, T.; Harabagiu, V.; Sacarescu, L.; Coseri, S. Synthesis, characterization and solution behaviour of oxidized pullulan. Carbohydr. Polym. 2014, 111, 63–71. [Google Scholar] [CrossRef]
- Coseri, S.; Spatareanu, A.; Sacarescu, L.; Rimbu, C.; Suteu, D.; Spirk, S.; Harabagiu, V. Green synthesis of the silver nanoparticles mediated by pullulan and 6-carboxypullulan. Carbohydr. Polym. 2015, 116, 9–17. [Google Scholar] [CrossRef]
- Baron, R.I.; Bercea, M.; Avadanei, M.; Lisa, G.; Biliuta, G.; Coseri, S. Green route for the fabrication of self-healable hydrogels based on tricarboxy cellulose and poly(vinyl alcohol). Int. J. Biol. Macromol. 2019, 123, 744–751. [Google Scholar] [CrossRef]
- Agrawal, S.; Budhwani, D.; Gurjar, P.; Telange, D.; Lambole, V. Pullulan based derivatives: Synthesis, enhanced physicochemical properties, and applications. Drug Deliv. 2022, 29, 3328–3339. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Qian, Y.; Zhang, Z.; Lyu, L.; Wang, Y.; Ye, F. Study on the electrospinning of gelatin/pullulan composite nanofibers. Polymers 2019, 11, 1424. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Wang, J.; Tang, R.; Wei, B.; Jiang, Q. Succinyl Pullulan-Crosslinked Carboxymethyl Chitosan Sponges for Potential Wound Dressing. Int. J. Polym. Mater. Polym. 2017, 66, 61–70. [Google Scholar] [CrossRef]
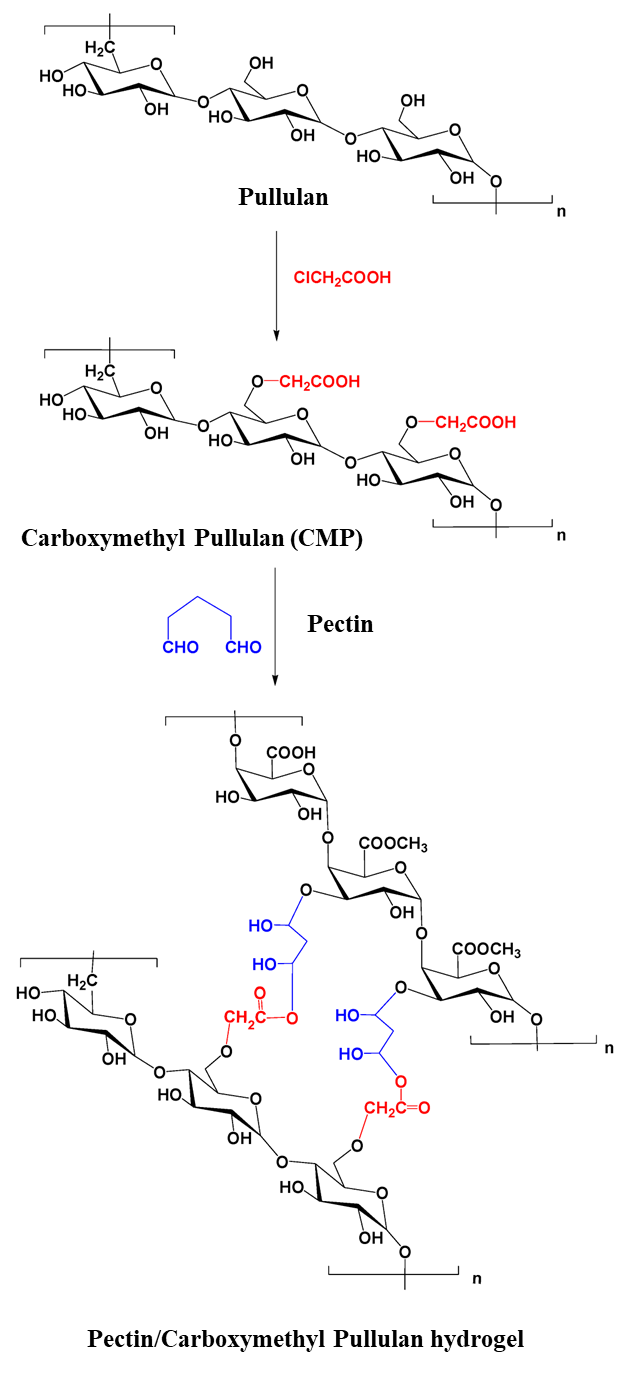
- Emam, H.E.; Mohamed, A.L. Controllable Release of Povidone-Iodine from Networked Pectin@Carboxymethyl Pullulan Hydrogel. Polymers 2021, 13, 3118. [Google Scholar] [CrossRef] [PubMed]
- Priya, V.S.; Iyappan, K.; Gayathri, V.S.; William, S.; Suguna, L. Influence of pullulan hydrogel on sutureless wound healing in rats. Wound Med. 2016, 14, 1–5. [Google Scholar] [CrossRef]
- Chen, K.; Sivaraj, D.; Davitt, M.F.; Leeolou, M.C.; Henn, D.; Steele, S.R.; Huskins, S.L.; Trotsyuk, A.A.; Kussie, H.C.; Greco, A.H.; et al. Pullulan-Collagen hydrogel wound dressing promotes dermal remodelling and wound healing compared to commercially available collagen dressings. Wound Rep. Reg. 2022, 30, 397–408. [Google Scholar] [CrossRef]
- Barrera, J.A.; Trotsyuk, A.A.; Maan, Z.N.; Bonham, C.A.; Larson, M.M.R.; Mittermiller, P.A.; Henn, D.; Chen, K.; Mays, M.C.J.; Mittal, M.S.; et al. Adipose-derived stromal cells seeded in pullulan-collagen hydrogels improve healing in murine burns. Tissue Eng. Part A 2021, 27, 844–856. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Cellularized bilayer pullulan-gelatin hydrogel for skin regeneration. Tissue Eng. Part A 2016, 9, 754–764. [Google Scholar] [CrossRef]
- Suguna, L. Wound healing potential of a biodegradable film from pullulan in rats. Clin. Exp. Dermatol. 2014, 5, 63. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Shah, S.A.; Mahmood, A.; Kousar, M.; Jabeen, N. Bioactive and multifunctional keratin-pullulan based hydrogel membranes facilitate re-epithelization in diabetic model. Int. J. Biol. Macromol. Part B 2022, 209, 1826–1836. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. J. Biomed. Mater. Res. 2011, 98A, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Thu, H.E.; Abbasi, M.; Kashif, M.U.R. Curcumin-laden hyaluronic acid-co-Pullulan-based biomaterials as a potential platform to synergistically enhance the diabetic wound repair. Int. J. Biol. Macromol. 2021, 185, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Rustad, K.C.; Glotzbach, J.P.; Sorkin, M.; Inayathullah, M.; Major, M.R.; Longaker, M.T.; Rajadas, J.; Gurtner, G.C. Pullulan hydrogels improve mesenchymal stem cell delivery into high-oxidative-stress wounds. Macromol. Biosci. 2011, 11, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.I.; Duceac, I.A.; Morariu, S.; Bostanaru-Iliescu, A.-C.; Coseri, S. Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings. Gels 2022, 8, 726. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of tannic acid/chitosan/pullulan composite nanofibers from aqueous solution for potential applications as wound dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef]
- Atila, D.; Karataş, A.; Keskin, D.; Tezcaner, A. Pullulan hydrogel-immobilized bacterial cellulose membranes with dual-release of vitamin C and E for wound dressing applications. Int. J. Biol. Macromol. 2022, 218, 760–774. [Google Scholar] [CrossRef]
- Wong, V.W.; Rustad, K.C.; Galvez, M.G.; Neofytou, E.; Glotzbach, J.P.; Januszyk, M.; Major, M.R.; Sorkin, M.; Longaker, M.T.; Rajadas, J.; et al. Engineered pullulan-collagen composite dermal hydrogels improve early cutaneous wound healing. Tissue Eng. Part A 2011, 17, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Lonchin, S. Bioactive functional collagen-oxidized pullulan scaffold loaded with polydatin for treating chronic wounds. Biomater. Adv. 2022, 140, 213078. [Google Scholar] [CrossRef]
- Kobayashi, H.; Katakura, O.; Morimoto, N.; Akiyoshi, K.; Kasugai, S. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. J. 2009, 91, 55–60. [Google Scholar] [CrossRef]
- Mert, H.; Ozkahraman, B.; Damar, H. A novel wound dressing material: Pullulan grafted copolymer hydrogel via UV copolymerization and crosslinking. J. Drug Deliv. Sci. Technol. 2020, 60, 101962. [Google Scholar] [CrossRef]
- Omar, N.A.; Amédée, J.; Letourneur, D.; Fricain, J.-C.; Fenelon, M. Recent Advances of Pullulan and/or Dextran-Based Materials for Bone Tissue Engineering Strategies in Preclinical Studies: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 889481. [Google Scholar] [CrossRef]
- Bae, H.; Ahari, A.F.; Shin, H.; Nichol, J.W.; Hutson, C.B.; Masaeli, M.; Kim, S.H.; Aubin, H.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter 2011, 7, 1903–1911. [Google Scholar] [CrossRef]
- Fricain, J.C.; Schlaubitz, S.; Le Visage, C.; Arnault, I.; Derkaoui, S.M.; Siadous, R.; Catros, S.; Lalande, C.; Bareille, R.; Renard, M.; et al. A nano-hydroxyapatite-pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013, 34, 2947–2959. [Google Scholar] [CrossRef]
- Galvez, M.G.; Wong, V.W.; Chang, E.I.; Major, M.; Carre, L.; Kandimalla, R.; Bhatt, K.A.; Rajadas, J.; Longaker, M.T.; Gurtner, G.C. Pullulan-collagen hydrogel scaffold as a dermal substitute. J. Am. Coll. Surg. 2009, 209, 78. [Google Scholar] [CrossRef]
- Iswariya, S.; Bhanu Keerthi, A.V.; Velswamy, P.; Uma, T.S.; Perumal, P.T. Design and development of a piscine collagen blended pullulan hydrogel for skin tissue engineering. RSC Adv. 2016, 6, 57863–57871. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr. Polym. 2015, 133, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Younas, A.; Dong, Z.; Hou, Z.; Asad, M.; Li, M.; Zhang, N. A chitosan/fucoidan nanoparticle-loaded pullulan microneedle patch for differential drug release to promote wound healing. Carbohydr. Polym. 2023, 306, 120593. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Kaur, N.; Singh, D.; Purewal, S.S.; Kennedy, F.J. Pullulan in pharmaceutical and cosmeceutical formulations: A review. Int. J. Biol. Macromol. 2023, 231, 123353. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Z.; Li, Y.; Wang, L.; Fu, F.; Diao, H.; Liu, X. A novel pullulan oxidation approach to preparing a shape memory sponge with rapid reaction capability for massive hemorrhage. Chem. Eng. J. 2022, 447, 137482. [Google Scholar] [CrossRef]

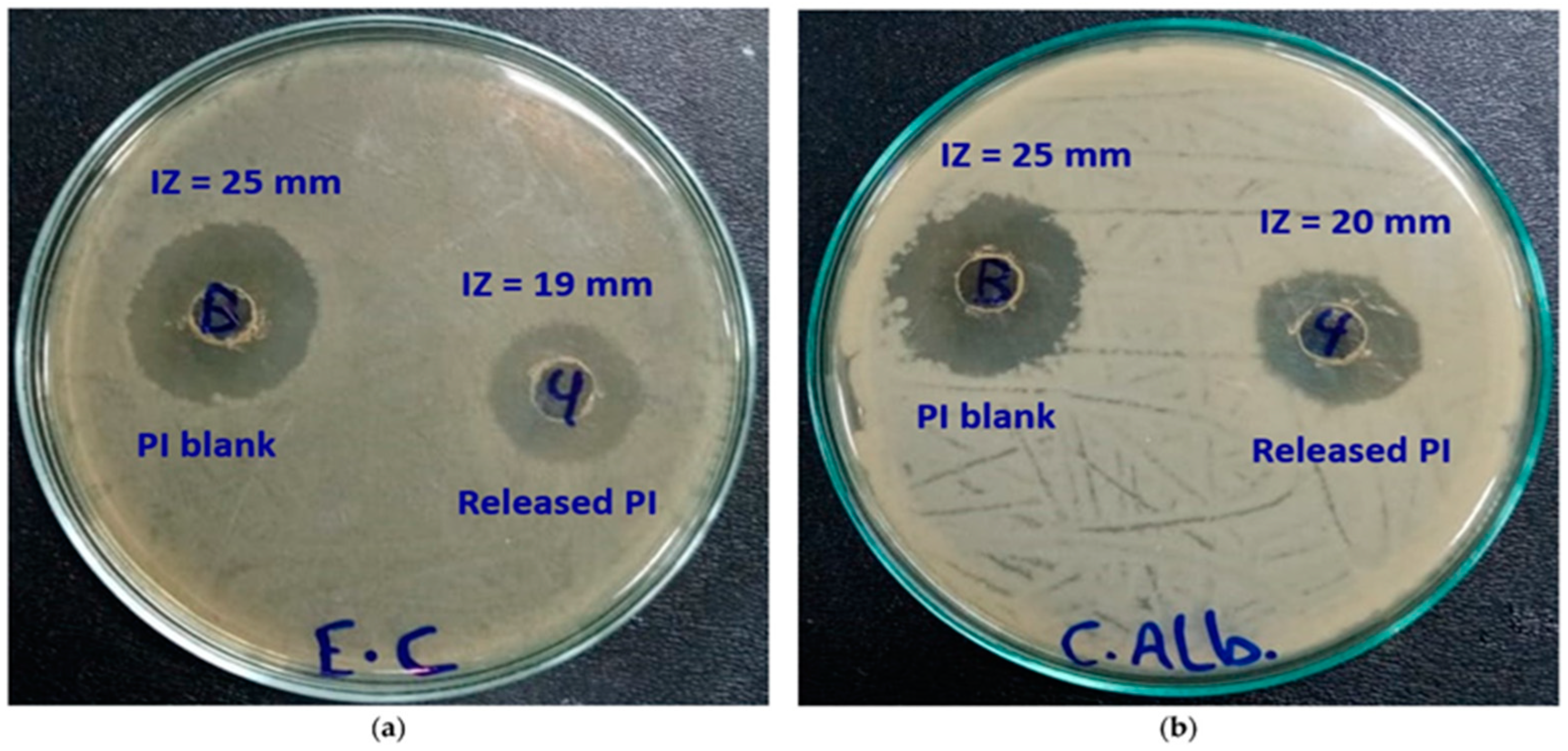
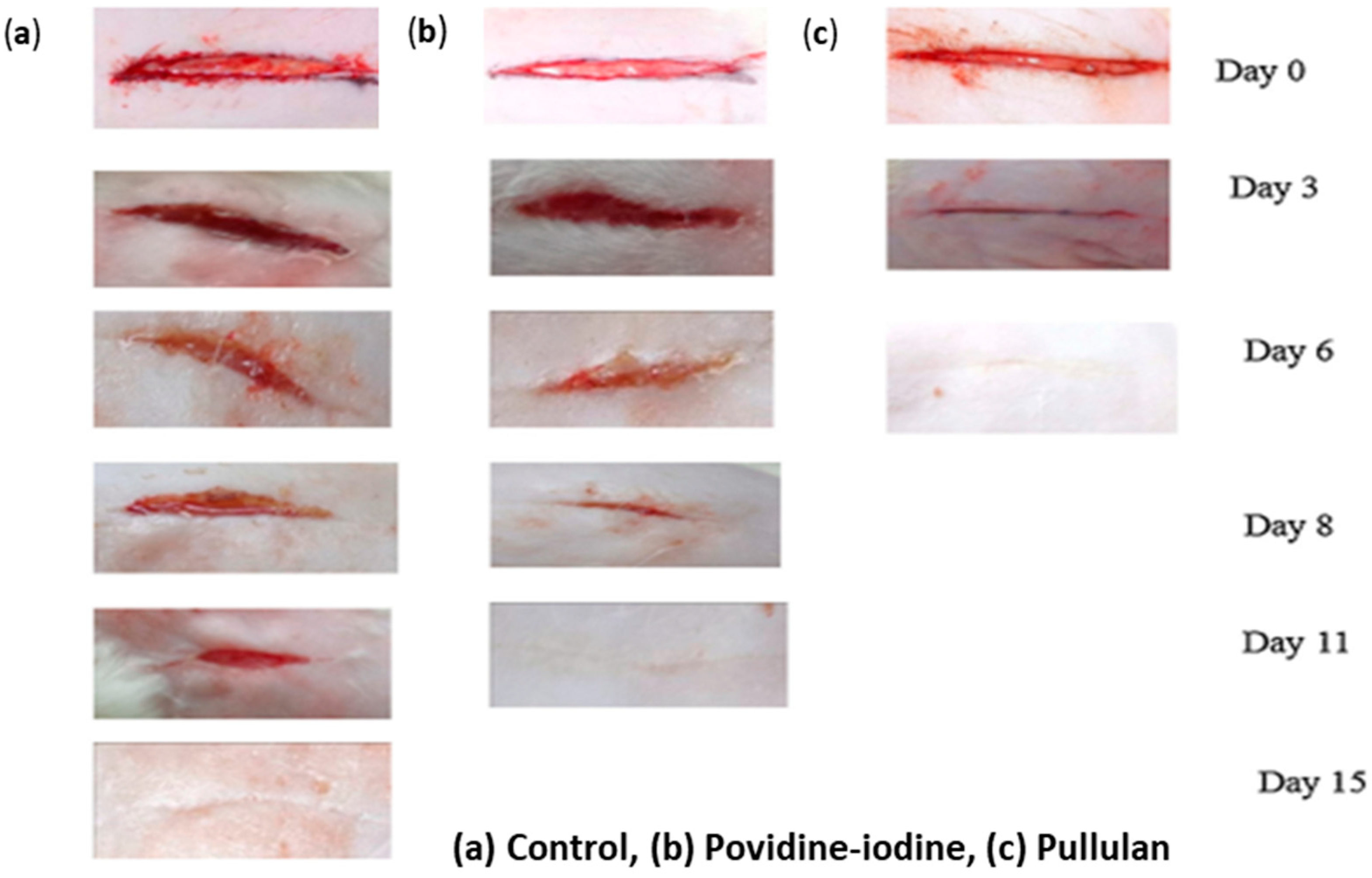
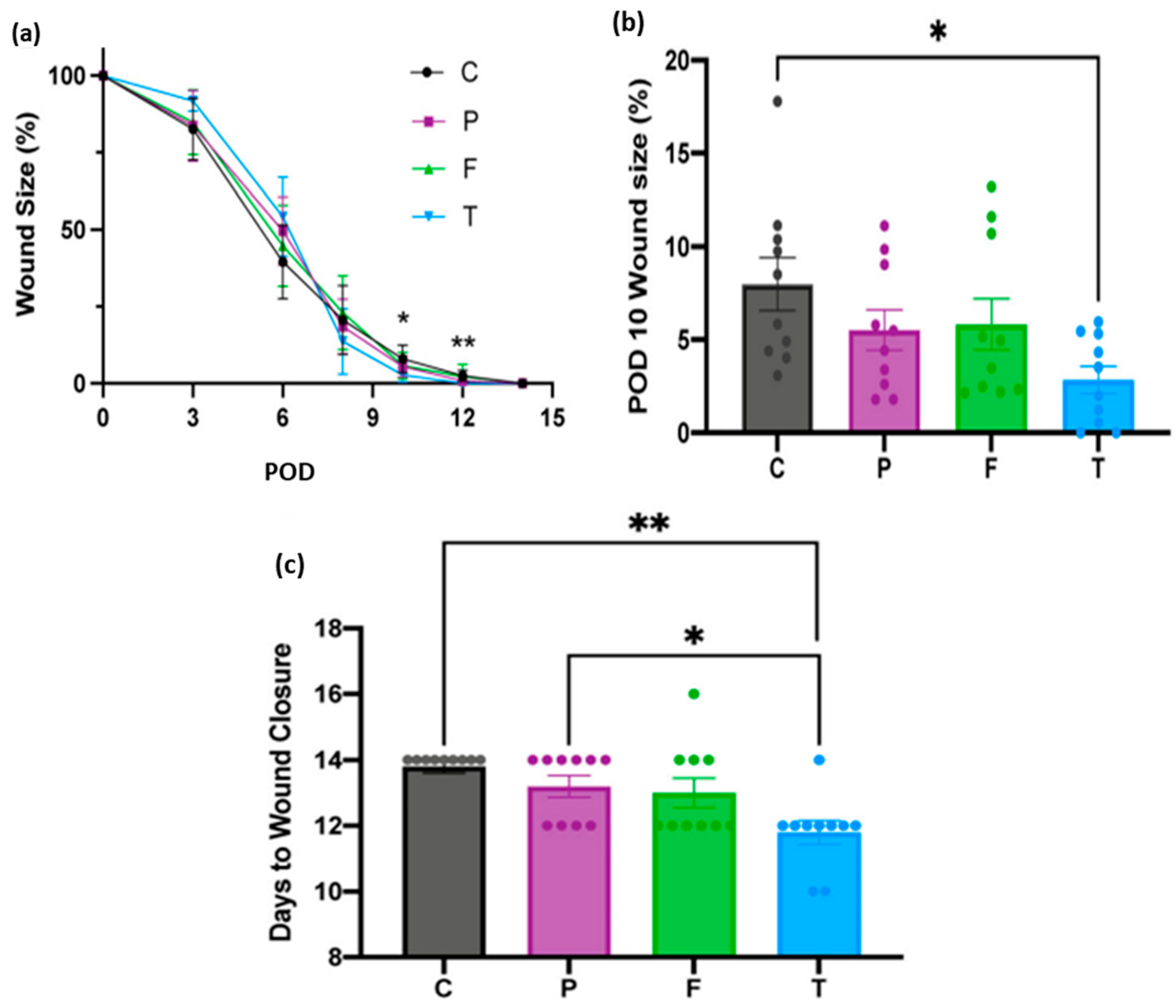
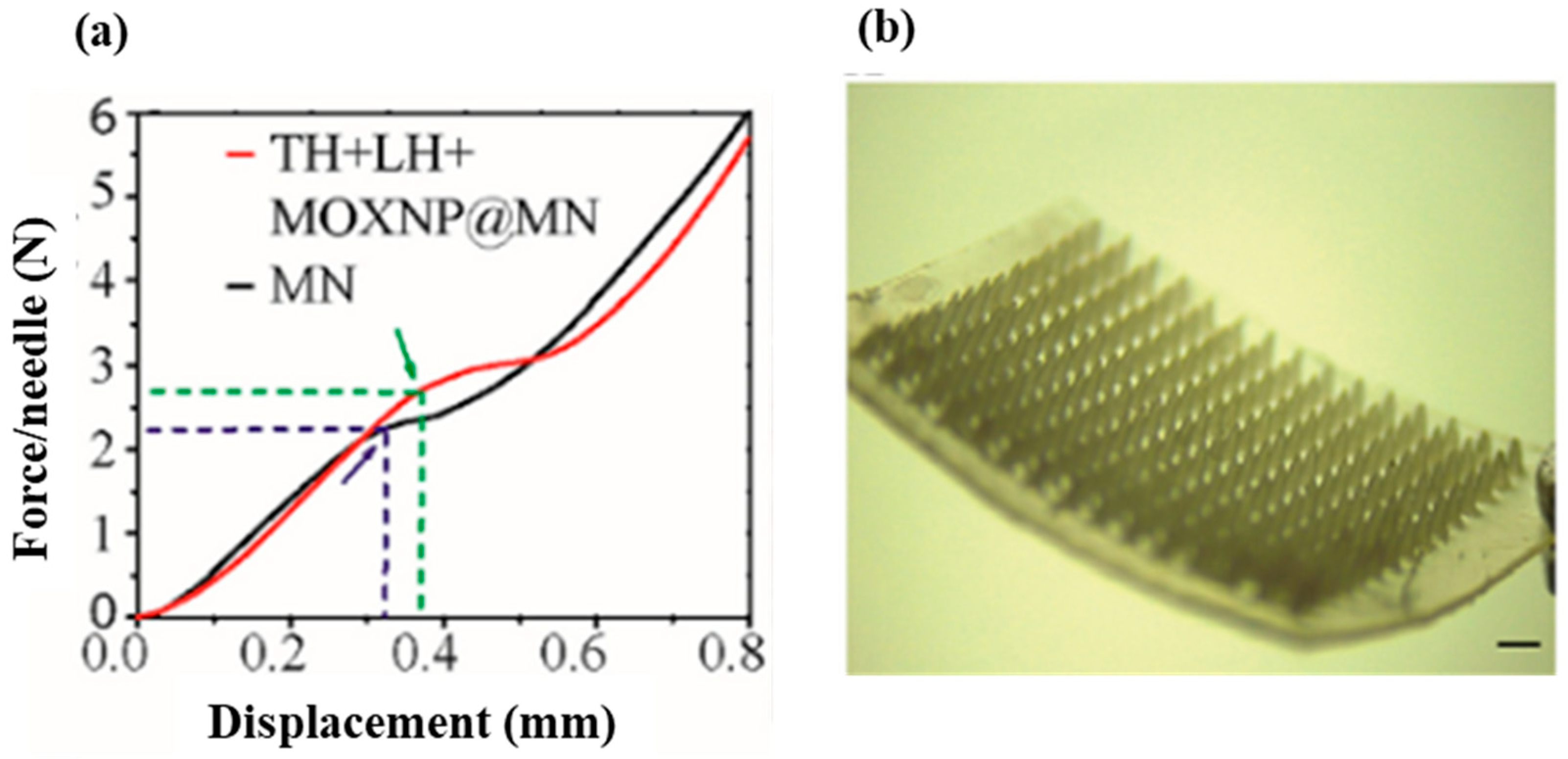
| Category | Benefit | Examples | Reference |
|---|---|---|---|
| Passive wound dressing | Mainly protect the wound from the external surroundings | Gauze, bandages and plasters | [28] |
| Allografts (skin substitutes) | Accelerate wound closure and replace the skin function, thereby boosting healing | Allografts, tissue-engineered derivatives, autografts | [29] |
| Bioactive natural dressing | Have antimicrobial, antioxidant, bio-adhesive, and proliferative properties | Collagen, chitosan, alginate, chitin | [30] |
| Artificial materials | Enhance wound healing and offer barriers against bacterial infections | Sprays, hydrocolloids, films, foams and gels | [31] |
| Property | Description |
|---|---|
| Maintain wound moisture | Prevents wound from drying |
| Excellent gas transmission | Allows the exchange of oxygen between the wound and the environment |
| Superabsorbent capabilities | Removes excess exudates |
| Protect against microbial contaminations | Possesses antimicrobial properties |
| Eco-friendly | Biodegradable |
| Excellent wound healing regulator | Reduces inflammation, stimulates release of growth factors, tissue regeneration, and prevents scaring |
| Provide mechanical protection | Acts as a physical barrier to prevent further damage to the wound |
| Stop bleeding | Possesses excellent homeostatic properties to prevent further blood loss |
| Adhesiveness | Possesses easy and comfortable removal properties |
| Nonimmunogenic and biocompatible | Is non-allergic and non-toxic to the body |
| Cost-effective and commercially available | Improves patients’ compliance through accessibility and affordability |
| Application | System Used | Drug/Growth Enhancing Factor(s) | Cell Types Treated | Reference |
|---|---|---|---|---|
| Wound healing | Pullulan film | - | Rat skin cells | [88] |
| Wound dressing | Keratin/pullulan/PVA hydrogel membrane | Cefotaxime | Incision on male SD rat skin cells | [89] |
| Wound dressing and antibacterial effect | Carboxymethyl pullulan hydrogels | Gentamicin | In vitro drug release studies in phosphate buffer saline solution | [90] |
| Wound healing | Hyaluronic/pullulan/ grafted-Pluronic F127 hydrogel | Curcumin | Female rat skin cells | [91] |
| High oxidative stress wound dressing | Pullulan hydrogels | Mesenchymal stromal cell | Anterior and posterior full thickness transverse incisions on skin cells | [92] |
| Wound dressing | Pullulan/dopamine hydrogel | - | In vitro studies of sheep blood | [93] |
| Wound healing | Pullulan/chitosan composite nanofibers | Tannic acid | NIH 3T3 mouse embryonic fibroblast cells | [94] |
| Wound dressing and antioxidant effect | Pullulan/bacterial cellulose hydrogel | Vitamin C and E | In vitro studies in phosphate saline buffer | [95] |
| Early cutaneous wound healing | Pullulan/collagen composite hydrogels | - | Full thickness skin cells incisions | [96] |
| Wound dressing | Collagen/pullulan hydrogel | Polydatin | Wistar rat skin cells | [97] |
| Wound healing | Cholesterol bearing pullulan nanogels | Prostaglandin E1 | Full thickness defect on rat dermal cells | [98] |
| Wound dressing and drug delivery system | Pu-g-p(AA-co-IA) hydrogel film | Ampicillin | In vitro drug release study in phosphate buffer saline solution | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M.V. Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. Int. J. Mol. Sci. 2023, 24, 4962. https://doi.org/10.3390/ijms24054962
Elangwe CN, Morozkina SN, Olekhnovich RO, Polyakova VO, Krasichkov A, Yablonskiy PK, Uspenskaya MV. Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. International Journal of Molecular Sciences. 2023; 24(5):4962. https://doi.org/10.3390/ijms24054962
Chicago/Turabian StyleElangwe, Collins N., Svetlana N. Morozkina, Roman O. Olekhnovich, Victoria O. Polyakova, Alexander Krasichkov, Piotr K. Yablonskiy, and Mayya V. Uspenskaya. 2023. "Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review" International Journal of Molecular Sciences 24, no. 5: 4962. https://doi.org/10.3390/ijms24054962
APA StyleElangwe, C. N., Morozkina, S. N., Olekhnovich, R. O., Polyakova, V. O., Krasichkov, A., Yablonskiy, P. K., & Uspenskaya, M. V. (2023). Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. International Journal of Molecular Sciences, 24(5), 4962. https://doi.org/10.3390/ijms24054962







