Is CCL2 an Important Mediator of Mast Cell–Tumor Cell Interactions in Oral Squamous Cell Carcinoma?
Abstract
1. Introduction
2. Results
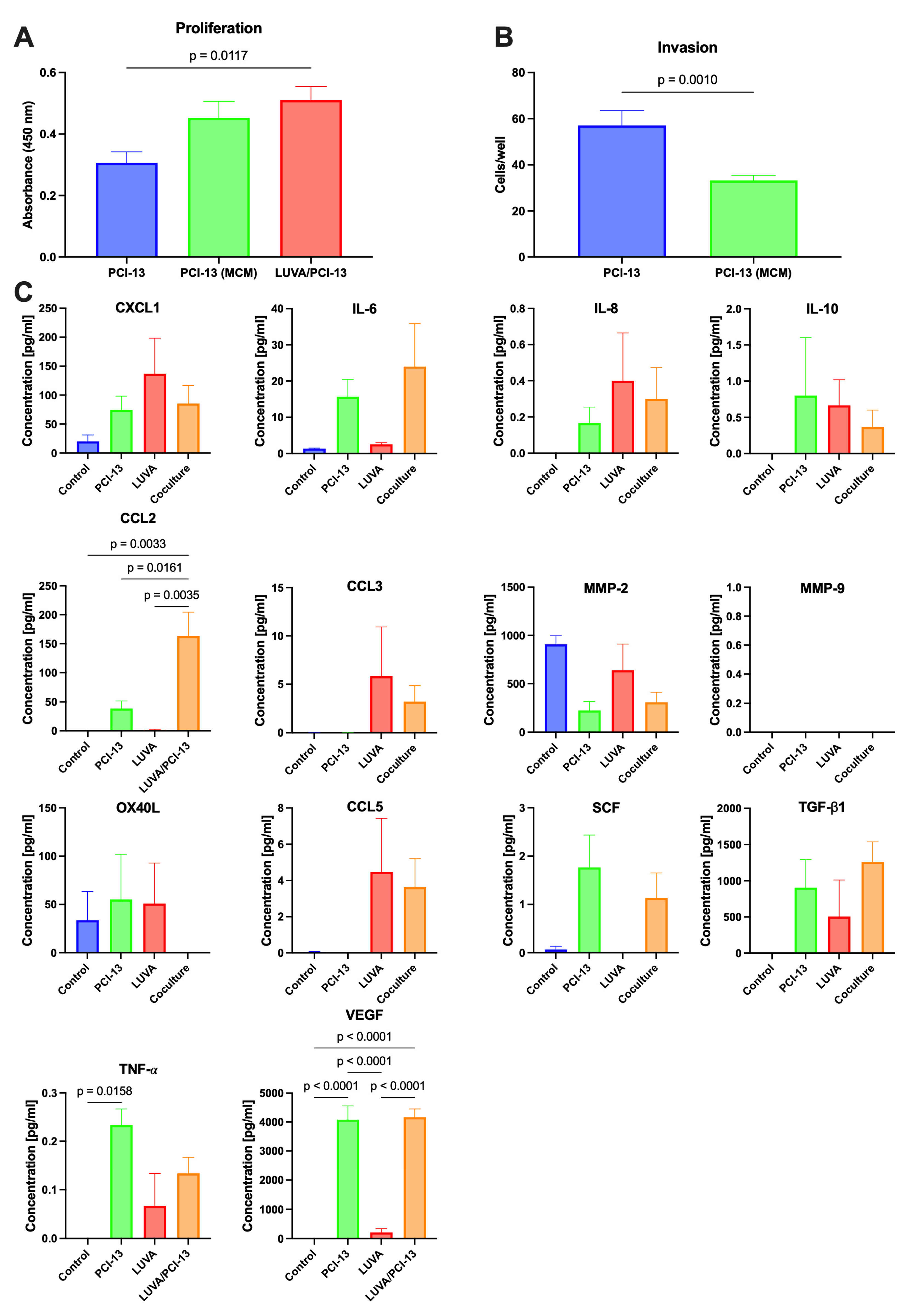
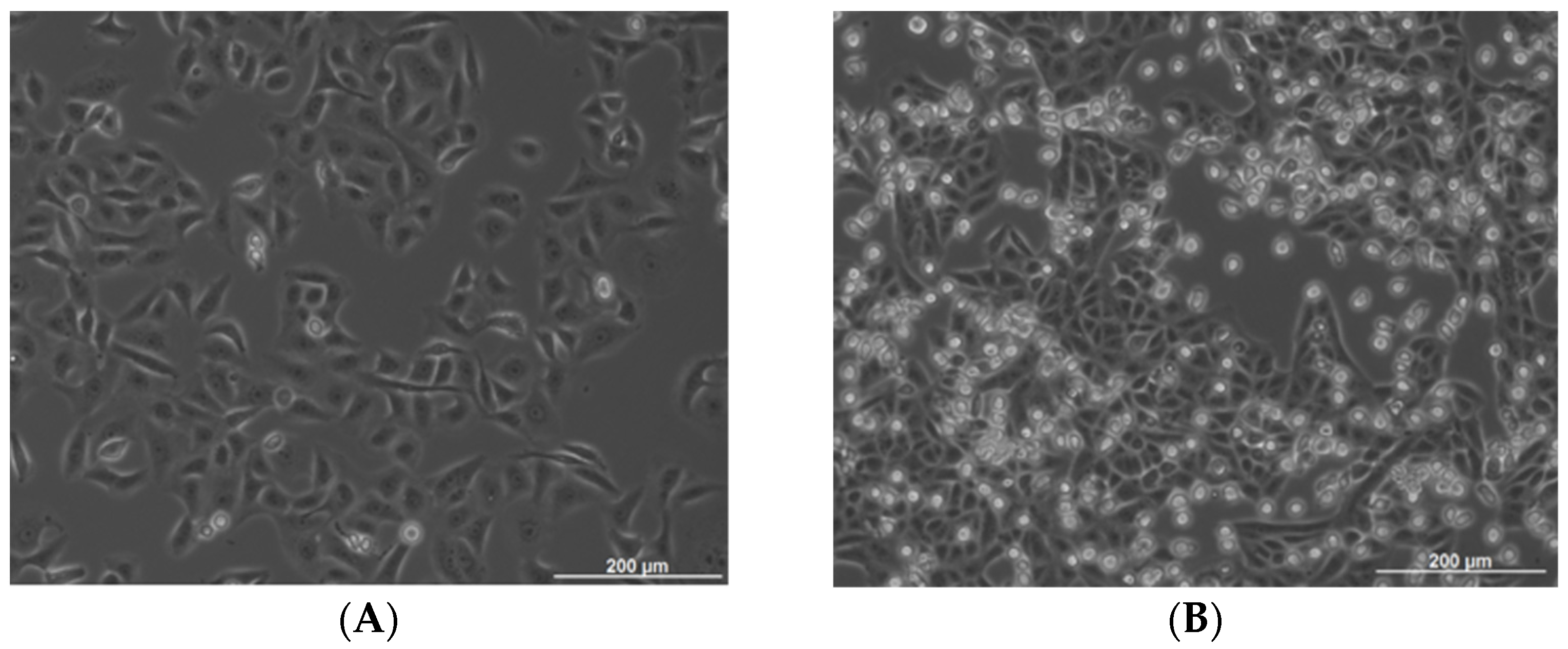
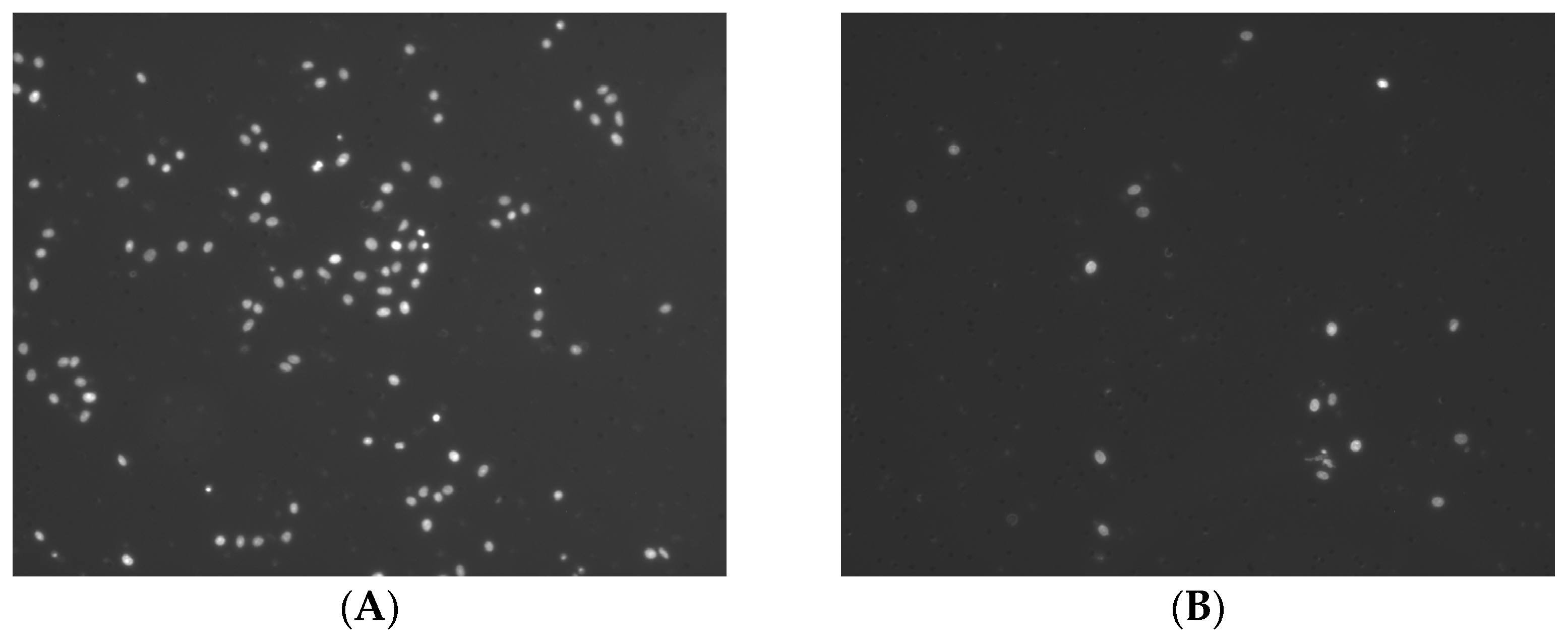
2.1. Influence of the MC/OSCC Interaction on Tumor Cell Proliferation
2.2. Influence of the MC/OSCC Interaction on Tumor Cell Invasion
2.3. Identification of Known MC/Tumor Cell Mediators
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Conditioned Cell Culture Media
4.3. Mast Cell–Tumor Cell Co-Cultures
4.4. BrdU Cell Proliferation Assay
4.5. Tumor Cell Invasion Assays
4.6. Multiplex ELISA Array
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Corsetti, P.; Moliterni, E.; Corsetti, S.; Didona, D.; Albanesi, M.; Mattozzi, C.; Lido, P.; Calvieri, S. Mast cells and cancer. Ital. J. Dermatol. Venereol. 2019, 154, 650–668. [Google Scholar] [CrossRef]
- Johansson, A.; Rudolfsson, S.; Hammarsten, P.; Halin, S.; Pietras, K.; Jones, J.; Stattin, P.; Egevad, L.; Granfors, T.; Wikstrom, P.; et al. Mast cells are novel independent prognostic markers in prostate cancer and represent a target for therapy. Am. J. Pathol. 2010, 177, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- de Souza Junior, D.A.; Santana, A.C.; da Silva, E.Z.; Oliver, C.; Jamur, M.C. The Role of Mast Cell Specific Chymases and Tryptases in Tumor Angiogenesis. Biomed. Res. Int. 2015, 2015, 142359. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Govender, D. Gene of the month: KIT. J. Clin. Pathol. 2015, 68, 671–674. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Donato, G.; Zuccala, V.; Russo, E.; Luposella, M.; Vescio, G.; Rizzuto, A.; Patruno, R.; De Sarro, G.; et al. Mast cell positivity to tryptase correlates with metastatic lymph nodes in gastrointestinal cancer patients treated surgically. Oncology 2013, 85, 111–116. [Google Scholar] [CrossRef]
- Somasundaram, R.; Connelly, T.; Choi, R.; Choi, H.; Samarkina, A.; Li, L.; Gregorio, E.; Chen, Y.; Thakur, R.; Abdel-Mohsen, M.; et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat. Commun. 2021, 12, 346. [Google Scholar] [CrossRef]
- Reddy, S.M.; Reuben, A.; Barua, S.; Jiang, H.; Zhang, S.; Wang, L.; Gopalakrishnan, V.; Hudgens, C.W.; Tetzlaff, M.T.; Reuben, J.M.; et al. Poor Response to Neoadjuvant Chemotherapy Correlates with Mast Cell Infiltration in Inflammatory Breast Cancer. Cancer Immunol. Res. 2019, 7, 1025–1035. [Google Scholar] [CrossRef]
- Gaje, P.N.; Amalia Ceausu, R.; Jitariu, A.; Stratul, S.I.; Rusu, L.C.; Popovici, R.A.; Raica, M. Mast Cells: Key Players in the Shadow in Oral Inflammation and in Squamous Cell Carcinoma of the Oral Cavity. Biomed. Res. Int. 2016, 2016, 9235080. [Google Scholar] [CrossRef]
- Mohtasham, N.; Babakoohi, S.; Salehinejad, J.; Montaser-Kouhsari, L.; Shakeri, M.T.; Shojaee, S.; Sistani, N.S.; Firooz, A. Mast cell density and angiogenesis in oral dysplastic epithelium and low- and high-grade oral squamous cell carcinoma. Acta Odontol. Scand 2010, 68, 300–304. [Google Scholar] [CrossRef]
- Shrestha, A.; Keshwar, S.; Raut, T. Evaluation of Mast Cells in Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Int. J. Dent. 2021, 2021, 5609563. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Asif, M.; Kiani, M.N.; Zareef, A.; Rashid, F.; ud Din, H.; Ansar, F. Relationship between Mast Cell Density and Microvessel Density in Oral Squamous Cell Carcinoma and Normal Oral Mucosa: Immunohistochemical Analysis using CD117 and CD34 Antibodies. Int. J. Pathol. 2022, 114–120. [Google Scholar]
- Teofilo, C.R.; Ferreira Junior, A.E.C.; Batista, A.C.; Fechini Jamacaru, F.V.; Sousa, F.B.; Lima Mota, M.R.; Silva, M.F.E.; Barros Silva, P.G.; Alves, A. Mast Cells and Blood Vessels Profile in Oral Carcinogenesis: An Immunohistochemistry Study. Asian Pac. J. Cancer Prev. 2020, 21, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, E.Z.; Markopoulos, A.K.; Antoniades, D.Z. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent. J. 2008, 2, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rojas, I.G.; Spencer, M.L.; Martinez, A.; Maurelia, M.A.; Rudolph, M.I. Characterization of mast cell subpopulations in lip cancer. J. Oral. Pathol. Med. 2005, 34, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Yagi-Nakanishi, S.; Nakanishi, Y.; Kondo, S.; Tsuji, A.; Endo, K.; Wakisaka, N.; Murono, S.; Yoshizaki, T. Expression of interleukin-33 is correlated with poor prognosis of patients with squamous cell carcinoma of the tongue. Auris Nasus Larynx 2014, 41, 552–557. [Google Scholar] [CrossRef]
- Attramadal, C.G.; Kumar, S.; Gao, J.; Boysen, M.E.; Halstensen, T.S.; Bryne, M. Low Mast Cell Density Predicts Poor Prognosis in Oral Squamous Cell Carcinoma and Reduces Survival in Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2016, 36, 5499–5506. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, P.; Kling, A.; Schulz, X.; Perske, C.; Schliephake, H.; Hemmerlein, B. High mast cell density indicates a longer overall survival in oral squamous cell carcinoma. Sci. Rep. 2017, 7, 14677. [Google Scholar] [CrossRef]
- Lichterman, J.N.; Reddy, S.M. Mast Cells: A New Frontier for Cancer Immunotherapy. Cells 2021, 10, 1270. [Google Scholar] [CrossRef] [PubMed]
- Vossen, D.M.; Verhagen, C.V.M.; Verheij, M.; Wessels, L.F.A.; Vens, C.; van den Brekel, M.W.M. Comparative genomic analysis of oral versus laryngeal and pharyngeal cancer. Oral Oncol. 2018, 81, 35–44. [Google Scholar] [CrossRef]
- Jin, J.; Lin, J.; Xu, A.; Lou, J.; Qian, C.; Li, X.; Wang, Y.; Yu, W.; Tao, H. CCL2: An Important Mediator Between Tumor Cells and Host Cells in Tumor Microenvironment. Front. Oncol. 2021, 11, 722916. [Google Scholar] [CrossRef] [PubMed]
- Gorzalczany, Y.; Merimsky, O.; Sagi-Eisenberg, R. Mast Cells Are Directly Activated by Cancer Cell-Derived Extracellular Vesicles by a CD73- and Adenosine-Dependent Mechanism. Transl. Oncol. 2019, 12, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Iamaroon, A.; Pongsiriwet, S.; Jittidecharaks, S.; Pattanaporn, K.; Prapayasatok, S.; Wanachantararak, S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2003, 32, 195–199. [Google Scholar] [CrossRef]
- Khazaie, K.; Blatner, N.R.; Khan, M.W.; Gounari, F.; Gounaris, E.; Dennis, K.; Bonertz, A.; Tsai, F.-N.; Strouch, M.J.; Cheon, E. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011, 30, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, J.; Zhang, Y.; Liao, Y.; Zhu, Y.; Wang, C.; Hou, J. LDHA Promotes Oral Squamous Cell Carcinoma Progression Through Facilitating Glycolysis and Epithelial-Mesenchymal Transition. Front. Oncol. 2019, 9, 1446. [Google Scholar] [CrossRef]
- Theoharides, T.C. Mast cells and pancreatic cancer. N. Engl. J. Med. 2008, 358, 1860. [Google Scholar] [CrossRef]
- Heo, D.S.; Snyderman, C.; Gollin, S.M.; Pan, S.; Walker, E.; Deka, R.; Barnes, E.L.; Johnson, J.T.; Herberman, R.B.; Whiteside, T.L. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 1989, 49, 5167–5175. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmerlein, B.; Reinhardt, L.; Wiechens, B.; Khromov, T.; Schliephake, H.; Brockmeyer, P. Is CCL2 an Important Mediator of Mast Cell–Tumor Cell Interactions in Oral Squamous Cell Carcinoma? Int. J. Mol. Sci. 2023, 24, 3641. https://doi.org/10.3390/ijms24043641
Hemmerlein B, Reinhardt L, Wiechens B, Khromov T, Schliephake H, Brockmeyer P. Is CCL2 an Important Mediator of Mast Cell–Tumor Cell Interactions in Oral Squamous Cell Carcinoma? International Journal of Molecular Sciences. 2023; 24(4):3641. https://doi.org/10.3390/ijms24043641
Chicago/Turabian StyleHemmerlein, Bernhard, Luisa Reinhardt, Bernhard Wiechens, Tatjana Khromov, Henning Schliephake, and Phillipp Brockmeyer. 2023. "Is CCL2 an Important Mediator of Mast Cell–Tumor Cell Interactions in Oral Squamous Cell Carcinoma?" International Journal of Molecular Sciences 24, no. 4: 3641. https://doi.org/10.3390/ijms24043641
APA StyleHemmerlein, B., Reinhardt, L., Wiechens, B., Khromov, T., Schliephake, H., & Brockmeyer, P. (2023). Is CCL2 an Important Mediator of Mast Cell–Tumor Cell Interactions in Oral Squamous Cell Carcinoma? International Journal of Molecular Sciences, 24(4), 3641. https://doi.org/10.3390/ijms24043641







