Indoxyl Sulphate Retention Is Associated with Microvascular Endothelial Dysfunction after Kidney Transplantation
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Population
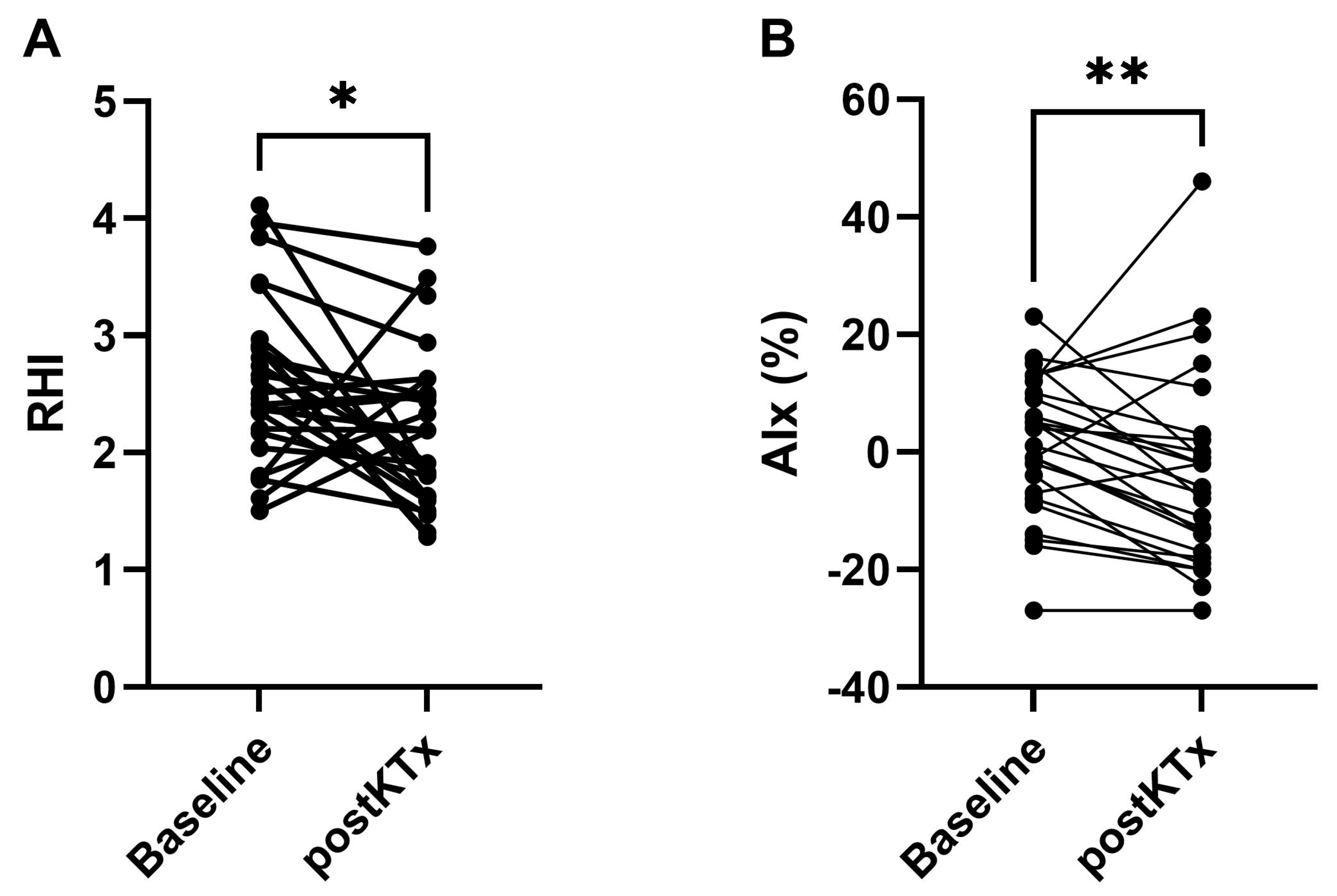
2.2. In Vivo Functional Study
2.3. Biochemical Parameters before and after KTx
2.4. Univariate Correlations and Multivariate Regression Analyses
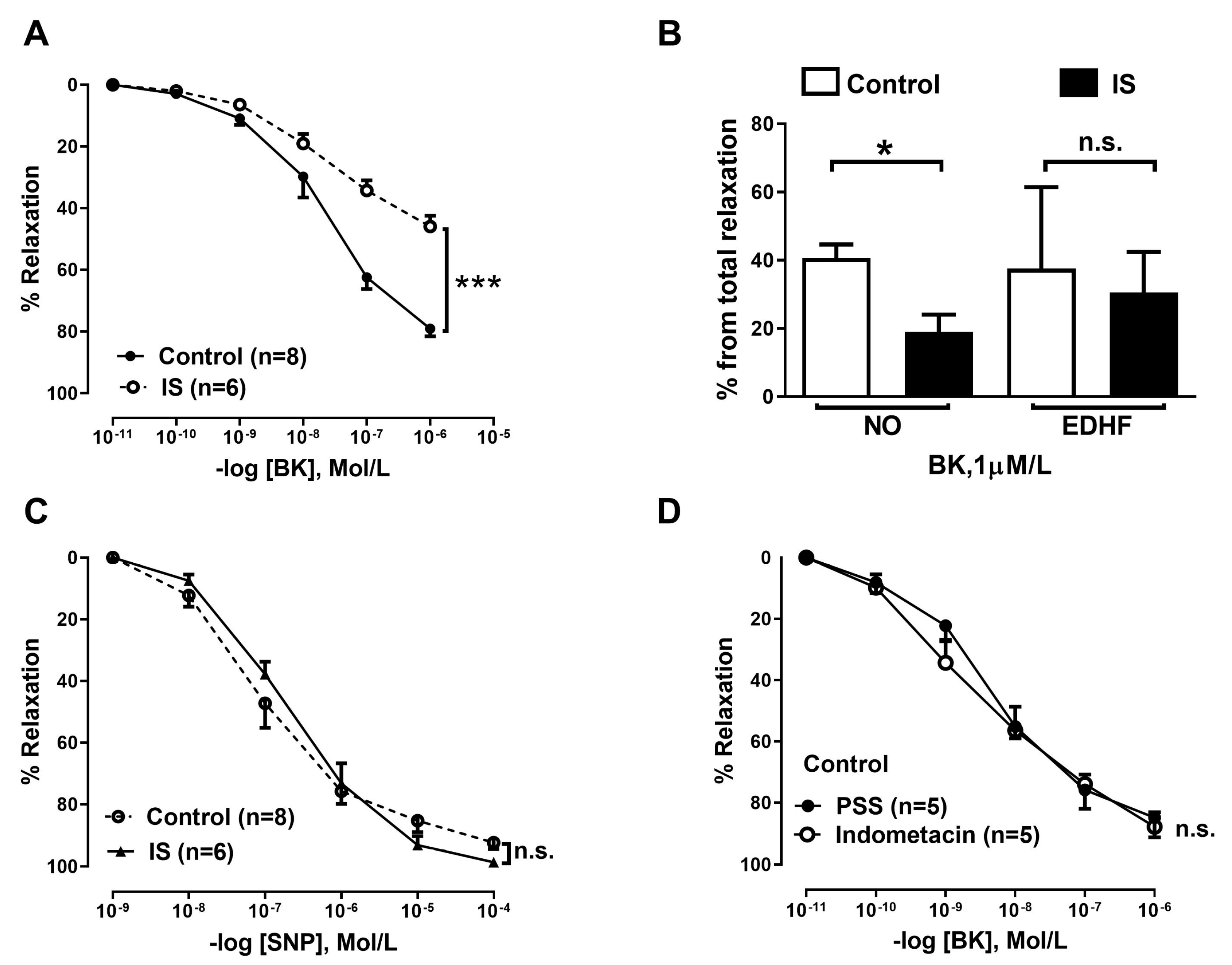
2.5. Ex Vivo Functional Study
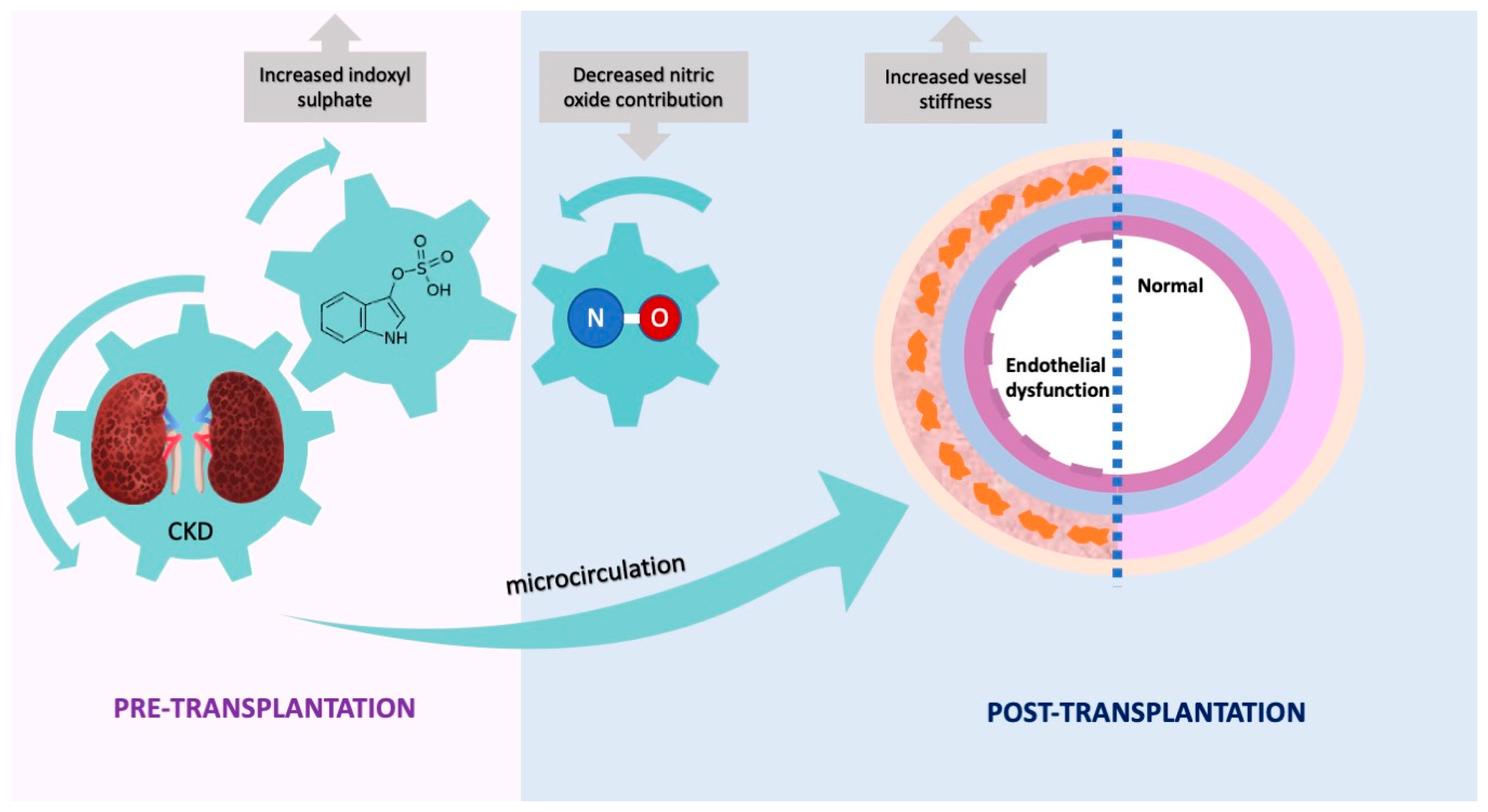
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. EndoPAT
4.3. Biochemical Analysis/Clinical Parameters
4.4. Vascular Reactivity Studies in Resistance Arteries
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. (2011) 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M.; Network, A.K.D. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef]
- Moradi, H.; Sica, D.A.; Kalantar-Zadeh, K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am. J. Nephrol. 2013, 38, 136–148. [Google Scholar] [CrossRef]
- Menon, V.; Gul, A.; Sarnak, M.J. Cardiovascular risk factors in chronic kidney disease. Kidney Int. 2005, 68, 1413–1418. [Google Scholar] [CrossRef]
- Dai, L.; Schurgers, L.J.; Shiels, P.G.; Stenvinkel, P. Early vascular ageing in chronic kidney disease: Impact of inflammation, vitamin K, senescence and genomic damage. Nephrol. Dial. Transplant. 2020, 35, ii31–ii37. [Google Scholar] [CrossRef]
- Nilsson, P.M. Early vascular aging (EVA): Consequences and prevention. Vasc. Health Risk Manag. 2008, 4, 547–552. [Google Scholar] [CrossRef]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef]
- Martens, C.R.; Kirkman, D.L.; Edwards, D.G. The Vascular Endothelium in Chronic Kidney Disease: A Novel Target for Aerobic Exercise. Exerc. Sport Sci. Rev. 2016, 44, 12–19. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Stenvinkel, P.; Sonmez, A.; Saglam, M.; Yaman, H.; Kilic, S.; Eyileten, T.; Caglar, K.; Oguz, Y.; Vural, A.; et al. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol. Dial. Transplant. 2011, 26, 3537–3543. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Park, M. Post-Transplant Cardiovascular Disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 1878–1889. [Google Scholar] [CrossRef]
- Devine, P.A.; Courtney, A.E.; Maxwell, A.P. Cardiovascular risk in renal transplant recipients. J. Nephrol. 2019, 32, 389–399. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.L.; Salimi, S.; Pierre, V.; Giffuni, J.; Katzel, L.; Parsa, A. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef]
- Stam, F.; van Guldener, C.; Becker, A.; Dekker, J.M.; Heine, R.J.; Bouter, L.M.; Stehouwer, C.D. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: The Hoorn study. J. Am. Soc. Nephrol. 2006, 17, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S. Arterial stiffness and hypertension. Clin. Hypertens. 2018, 24, 17. [Google Scholar] [CrossRef]
- Ro, H.; Kim, A.J.; Chang, J.H.; Jung, J.Y.; Chung, W.K.; Park, Y.H.; Lee, H.H. Can Kidney Transplantation Improve Arterial Stiffness in End-Stage Renal Patients? Transplant. Proc. 2016, 48, 884–886. [Google Scholar] [CrossRef]
- Kim, H.S.; Seung, J.; Lee, J.H.; Chung, B.H.; Yang, C.W. Clinical Significance of Pre-Transplant Arterial Stiffness and the Impact of Kidney Transplantation on Arterial Stiffness. PLoS ONE 2015, 10, e0139138. [Google Scholar] [CrossRef]
- Oflaz, H.; Turkmen, A.; Turgut, F.; Pamukcu, B.; Umman, S.; Ucar, A.; Akyol, Y.; Uzun, S.; Kazancioglu, R.; Kurt, R.; et al. Changes in endothelial function before and after renal transplantation. Transpl. Int. 2006, 19, 333–337. [Google Scholar] [CrossRef]
- Kaczmarska, M.; Grzelak, P.; Goździk, M.; Stefańczyk-Jakubowicz, K.; Stefańczyk, L.; Kurnatowska, I. Arterial vessel reactivity in patients in the long term after kidney transplantation—Preliminary study. Arch. Med. Sci. 2019, 15, 1240–1246. [Google Scholar] [CrossRef]
- Luksha, L.; Stenvinkel, P.; Hammarqvist, F.; Carrero, J.J.; Davidge, S.T.; Kublickiene, K. Mechanisms of endothelial dysfunction in resistance arteries from patients with end-stage renal disease. PLoS ONE 2012, 7, e36056. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Tomino, Y.; Lu, K.C. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Meijers, B.K.; Van Kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am. J. Kidney Dis. 2009, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Lano, G.; Burtey, S.; Sallée, M. Indoxyl Sulfate, a Uremic Endotheliotoxin. Toxins 2020, 12, 229. [Google Scholar] [CrossRef]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef]
- Young, A.; Garcia, M.; Sullivan, S.M.; Liu, C.; Moazzami, K.; Ko, Y.A.; Shah, A.J.; Kim, J.H.; Pearce, B.; Uphoff, I.; et al. Impaired Peripheral Microvascular Function and Risk of Major Adverse Cardiovascular Events in Patients With Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.D.; Nadar, S.K.; Lip, G.Y. The adhesion molecule P-selectin and cardiovascular disease. Eur. Heart J. 2003, 24, 2166–2179. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M.; Matetzky, S.; Prasad, M.; Goitein, O.; Goldkorn, R.; Naroditsky, M.; Koren-Morag, N.; Lerman, A. Endothelial function predicts 1-year adverse clinical outcome in patients hospitalized in the emergency department chest pain unit. Int. J. Cardiol. 2017, 240, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, U.; Mak, R.H.; Pries, A.R. Microvascular disease in chronic kidney disease: The base of the iceberg in cardiovascular comorbidity. Clin. Sci. 2020, 134, 1333–1356. [Google Scholar] [CrossRef]
- Fliser, D.; Wiecek, A.; Suleymanlar, G.; Ortiz, A.; Massy, Z.; Lindholm, B.; Martinez-Castelao, A.; Agarwal, R.; Jager, K.J.; Dekker, F.W.; et al. The dysfunctional endothelium in CKD and in cardiovascular disease: Mapping the origin(s) of cardiovascular problems in CKD and of kidney disease in cardiovascular conditions for a research agenda. Kidney Int. Suppl. (2011) 2011, 1, 6–9. [Google Scholar] [CrossRef]
- Ali, A.; Macphee, I.; Kaski, J.C.; Banerjee, D. Cardiac and vascular changes with kidney transplantation. Indian J. Nephrol. 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Kaballo, M.A.; Canney, M.; O’Kelly, P.; Williams, Y.; O’Seaghdha, C.M.; Conlon, P.J. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin. Kidney J. 2018, 11, 389–393. [Google Scholar] [CrossRef]
- Mathur, A.K.; Chang, Y.H.; Steidley, D.E.; Heilman, R.; Khurmi, N.; Wasif, N.; Etzioni, D.; Moss, A.A. Patterns of Care and Outcomes in Cardiovascular Disease After Kidney Transplantation in the United States. Transplant. Direct 2017, 3, e126. [Google Scholar] [CrossRef]
- Lentine, K.L.; Rocca-Rey, L.A.; Bacchi, G.; Wasi, N.; Schmitz, L.; Salvalaggio, P.R.; Abbott, K.C.; Schnitzler, M.A.; Neri, L.; Brennan, D.C. Obesity and cardiac risk after kidney transplantation: Experience at one center and comprehensive literature review. Transplantation 2008, 86, 303–312. [Google Scholar] [CrossRef]
- Luan, F.L.; Langewisch, E.; Ojo, A. Metabolic syndrome and new onset diabetes after transplantation in kidney transplant recipients. Clin. Transplant. 2010, 24, 778–783. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Anjum, S.; Shah, R.; Skogen, J.; Kandaswamy, C.; Danielson, B.; O’Shaughnessy, E.A.; Dahl, D.C.; Silkensen, J.R.; Sahadevan, M.; et al. Hypertension after kidney transplantation. Am. J. Kidney Dis. 2004, 43, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, M.P.; Socratous, F.; Hennebry, S.; Eikelis, N.; Lambert, E.A.; Straznicky, N.; Esler, M.D.; Lambert, G.W. Sympathetic activation in chronic renal failure. J. Am. Soc. Nephrol. 2009, 20, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, C.; Xie, J.; Chen, B.; Chang, L. Serum asymmetric dimethylarginine and endothelial function after renal transplantation. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2009, 34, 289–294. [Google Scholar]
- Caglar, K.; Yilmaz, M.I.; Saglam, M.; Cakir, E.; Kilic, S.; Eyileten, T.; Sonmez, A.; Oguz, Y.; Oner, K.; Ors, F.; et al. Endothelial dysfunction and fetuin A levels before and after kidney transplantation. Transplantation 2007, 83, 392–397. [Google Scholar] [CrossRef]
- Cottone, S.; Palermo, A.; Vaccaro, F.; Mulè, G.; Guarneri, M.; Arsena, R.; Vadalà, A.; Cerasola, G. Inflammation and endothelial activation are linked to renal function in long-term kidney transplantation. Transpl. Int. 2007, 20, 82–87. [Google Scholar] [CrossRef]
- Stadler, M.; Theuer, E.; Anderwald, C.; Hanusch-Enserer, U.; Auinger, M.; Bieglmayer, C.; Quehenberger, P.; Bischof, M.; Kästenbauer, T.; Wolzt, M.; et al. Persistent arterial stiffness and endothelial dysfunction following successful pancreas-kidney transplantation in Type 1 diabetes. Diabet. Med. 2009, 26, 1010–1018. [Google Scholar] [CrossRef]
- Junarta, J.; Hojs, N.; Ramphul, R.; Lowe-Jones, R.; Kaski, J.C.; Banerjee, D. Progression of endothelial dysfunction, atherosclerosis, and arterial stiffness in stable kidney transplant patients: A pilot study. BMC Cardiovasc. Disord. 2020, 20, 6. [Google Scholar] [CrossRef]
- Hornum, M.; Clausen, P.; Idorn, T.; Hansen, J.M.; Mathiesen, E.R.; Feldt-Rasmussen, B. Kidney transplantation improves arterial function measured by pulse wave analysis and endothelium-independent dilatation in uraemic patients despite deterioration of glucose metabolism. Nephrol. Dial. Transplant. 2011, 26, 2370–2377. [Google Scholar] [CrossRef]
- Moerland, M.; Kales, A.J.; Schrier, L.; van Dongen, M.G.; Bradnock, D.; Burggraaf, J. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. Int. J. Vasc. Med. 2012, 2012, 904141. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological Diagnostic Criteria for Vascular Failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Bammens, B.; Claes, K.; Sprangers, B.; Naesens, M.; Kuypers, D.; Augustijns, P.; Meijers, B. The influence of renal transplantation on retained microbial-human co-metabolites. Nephrol. Dial. Transplant. 2016, 31, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Kim, Y.J.; Kang, D.H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol. 2011, 6, 30–39. [Google Scholar] [CrossRef]
- Tumur, Z.; Niwa, T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. Am. J. Nephrol. 2009, 29, 551–557. [Google Scholar] [CrossRef]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Takayanagi, K.; Kojima, M.; Katome, T.; Taguchi, K.; Kobayashi, T. Direct Impairment of the Endothelial Function by Acute Indoxyl Sulfate through Declined Nitric Oxide and Not Endothelium-Derived Hyperpolarizing Factor or Vasodilator Prostaglandins in the Rat Superior Mesenteric Artery. Biol. Pharm. Bull. 2019, 42, 1236–1242. [Google Scholar] [CrossRef]
- Kovács, D.; Löcsey, L.; Szabó, L.; Fedor, R.; Laczik, R.; Asztalos, L.; Soltész, P. Noninvasive perioperative monitoring of arterial function in patients with kidney transplantation. Transplant. Proc. 2013, 45, 3682–3684. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Harada, H.; Sasaki, H.; Iwami, D.; Fukuzawa, N.; Morita, K.; Seki, T.; Togashi, M.; Nonomura, K. Successful kidney transplantation ameliorates arterial stiffness in end-stage renal disease patients. Transplant. Proc. 2012, 44, 684–686. [Google Scholar] [CrossRef]
- Zoungas, S.; Kerr, P.G.; Chadban, S.; Muske, C.; Ristevski, S.; Atkins, R.C.; McNeil, J.J.; McGrath, B.P. Arterial function after successful renal transplantation. Kidney Int. 2004, 65, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Keven, K.; Calayoglu, R.; Sengul, S.; Dincer, I.; Kutlay, S.; Erturk, S.; Erbay, B.; Nergizoglu, G. Comparative effects of renal transplantation and maintenance dialysis on arterial stiffness and left ventricular mass index. Clin. Transplant. 2008, 22, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; Denardo, S.J.; Wilkinson, I.B.; McEniery, C.M.; Cockcroft, J.; O’Rourke, M.F. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J. Clin. Hypertens. 2008, 10, 295–303. [Google Scholar] [CrossRef]
- London, G.M.; Drueke, T.B. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997, 51, 1678–1695. [Google Scholar] [CrossRef]
- Karras, A.; Boutouyrie, P.; Briet, M.; Bozec, E.; Haymann, J.P.; Legendre, C.; McMahon, L.P.; Delahousse, M. Reversal of Arterial Stiffness and Maladaptative Arterial Remodeling After Kidney Transplantation. J. Am. Heart Assoc. 2017, 6, e006078. [Google Scholar] [CrossRef]
- Evenepoel, P.; Goffin, E.; Meijers, B.; Kanaan, N.; Bammens, B.; Coche, E.; Claes, K.; Jadoul, M. Sclerostin Serum Levels and Vascular Calcification Progression in Prevalent Renal Transplant Recipients. J. Clin. Endocrinol. Metab. 2015, 100, 4669–4676. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, C.; Coche, E.; Goffin, E.; Dragean, A.; Schlieper, G.; Nguyen, P.; Floege, J.; Kanaan, N.; Devuyst, O.; Jadoul, M. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am. J. Kidney Dis. 2012, 59, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Axtell, A.L.; Gomari, F.A.; Cooke, J.P. Assessing endothelial vasodilator function with the Endo-PAT 2000. J. Vis. Exp. 2010, e2167. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef]
- de Loor, H.; Poesen, R.; De Leger, W.; Dehaen, W.; Augustijns, P.; Evenepoel, P.; Meijers, B. A liquid chromatography-tandem mass spectrometry method to measure a selected panel of uremic retention solutes derived from endogenous and colonic microbial metabolism. Anal. Chim. Acta 2016, 936, 149–156. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Halpern, W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977, 41, 19–26. [Google Scholar] [CrossRef]

| Baseline (N = 27) | postKTx (N = 27) | p-Value | |
|---|---|---|---|
| Age (years) | 45 (35–54) | 48 (31–55) | - |
| Men | 21 (78%) | 21 (78%) | - |
| BMI (kg/m2) | 24.5 (23.0–28.7) | 25.4 (24.6–29.4) | 0.019 |
| SBP (mmHg) | 138 (128–158) | 130 (119–137) | 0.005 |
| DBP (mmHg) | 90 (81–98) | 82 (78–86) | 0.032 |
| Diabetes mellitus | 1 (4%) | 2 (7%) | 1.000 |
| Lipid-lowering medication | 10 (37%) | 23 (85%) | <0.001 |
| Blood pressure-lowering medication | 27 (100%) | 25 (93%) | 0.500 |
| Dialysis treatment | 7 (26%) | - | - |
| eGFR (mL/min/1.73 m2) | - | 57 (41–69) | - |
| Albumin (g/L) | 38 (36–39) | 36 (32–38) | 0.023 |
| hsCRP (mg/L) | 1.4 (0.4–2.6) | 0.9 (0.5–3.0) | 0.976 |
| Calcium (mmol/L) | 2.3 (2.1–2.4) | 2.4 (2.3–2.5) | 0.018 |
| Phosphate (mmol/L) | 1.7 (1.5–2.0) | 0.9 (0.8–1.0) | <0.001 |
| Total chol (mmol/L) | 4.4 (3.5–5.5) | 4.1 (3.9–4.4) | 0.727 |
| HDL cholesterol (mmol/L) | 1.3 (1.0–1.5) | 1.6 (1.3–1.8) | <0.001 |
| Triglycerides (mmol/L) | 1.8 (1.0–2.0) | 1.4 (0.9–1.7) | 0.078 |
| Lipoprotein(a) (mg/L) | 18 (10–76) | 10 (10–37) | 0.019 |
| Indoxyl Sulphate (µmol/L) | 100.8 (82.8–153.5) | - | - |
| p-Cresyl Sulphate (µmol/L) | 191.6 (101.5–281.5) | - | - |
| CAC score (AU) | 3 (0–61) | 27 (0–125) | 0.001 |
| AGE score | 3.0 (2.4–3.4) | - | - |
| RHI | 2.51 (2.17–2.91) | 2.18 (1.63–2.50) | 0.019 |
| AIx (%) | 4 (−7–10) | −6 (−17–2) | 0.009 |
| P-selectin (ng/mL) | 43.0 (37.3–56.5) | 44.55 (37.3–52.3) | 0.582 |
| MMP-9 (ng/mL) | 515.1 (368.3–756.0) | 479.0 (327.9–784.3) | 0.604 |
| Indoxyl Sulphate | p-Cresyl Sulphate | ||
|---|---|---|---|
| RHI (Baseline) | ρ | −0.329 | −0.214 |
| p-value | 0.101 | 0.293 | |
| RHI (postKTx) | ρ | −0.461 | 0.053 |
| p-value | 0.018 | 0.798 | |
| AIx (Baseline) | ρ | −0.208 | 0.036 |
| p-value | 0.308 | 0.862 | |
| AIx (postKTx) | ρ | −0.176 | 0.075 |
| p-value | 0.389 | 0.717 | |
| P-selectin (Baseline) | ρ | 0.118 | −0.116 |
| p-value | 0.573 | 0.580 | |
| P-selectin (postKTx) | ρ | 0.510 | 0.122 |
| p-value | 0.009 | 0.560 | |
| MMP-9 (Baseline) | ρ | 0.017 | −0.054 |
| p-value | 0.933 | 0.792 | |
| MMP-9 (postKTx) | ρ | 0.201 | 0.236 |
| p-value | 0.326 | 0.245 |
| Independent Variables | RHI postKTx | P-Selectin postKTx (ng/mL) | |
|---|---|---|---|
| Indoxyl sulphate (µmol/L) | ꞵ | −0.506 | 0.518 |
| p-value | 0.028 | 0.036 | |
| Age (years) | ꞵ | 0.271 | −0.181 |
| p-value | 0.195 | 0.417 | |
| Sex (male) | ꞵ | −0.109 | −0.025 |
| p-value | 0.586 | 0.908 | |
| Presence of diabetes (yes/no) | ꞵ | −0.032 | −0.135 |
| p-value | 0.870 | 0.532 | |
| Dialysis treatment (yes/no) | ꞵ | 0.319 | 0.073 |
| p-value | 0.139 | 0.748 | |
| Statin usage (yes/no) | ꞵ | 0.056 | 0.243 |
| p-value | 0.804 | 0.324 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobson, S.; Arefin, S.; Rahman, A.; Hernandez, L.; Ebert, T.; de Loor, H.; Evenepoel, P.; Stenvinkel, P.; Kublickiene, K. Indoxyl Sulphate Retention Is Associated with Microvascular Endothelial Dysfunction after Kidney Transplantation. Int. J. Mol. Sci. 2023, 24, 3640. https://doi.org/10.3390/ijms24043640
Hobson S, Arefin S, Rahman A, Hernandez L, Ebert T, de Loor H, Evenepoel P, Stenvinkel P, Kublickiene K. Indoxyl Sulphate Retention Is Associated with Microvascular Endothelial Dysfunction after Kidney Transplantation. International Journal of Molecular Sciences. 2023; 24(4):3640. https://doi.org/10.3390/ijms24043640
Chicago/Turabian StyleHobson, Sam, Samsul Arefin, Awahan Rahman, Leah Hernandez, Thomas Ebert, Henriette de Loor, Pieter Evenepoel, Peter Stenvinkel, and Karolina Kublickiene. 2023. "Indoxyl Sulphate Retention Is Associated with Microvascular Endothelial Dysfunction after Kidney Transplantation" International Journal of Molecular Sciences 24, no. 4: 3640. https://doi.org/10.3390/ijms24043640
APA StyleHobson, S., Arefin, S., Rahman, A., Hernandez, L., Ebert, T., de Loor, H., Evenepoel, P., Stenvinkel, P., & Kublickiene, K. (2023). Indoxyl Sulphate Retention Is Associated with Microvascular Endothelial Dysfunction after Kidney Transplantation. International Journal of Molecular Sciences, 24(4), 3640. https://doi.org/10.3390/ijms24043640





