Potential of uPAR, αvβ6 Integrin, and Tissue Factor as Targets for Molecular Imaging of Oral Squamous Cell Carcinoma: Evaluation of Nine Targets in Primary Tumors and Metastases by Immunohistochemistry
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
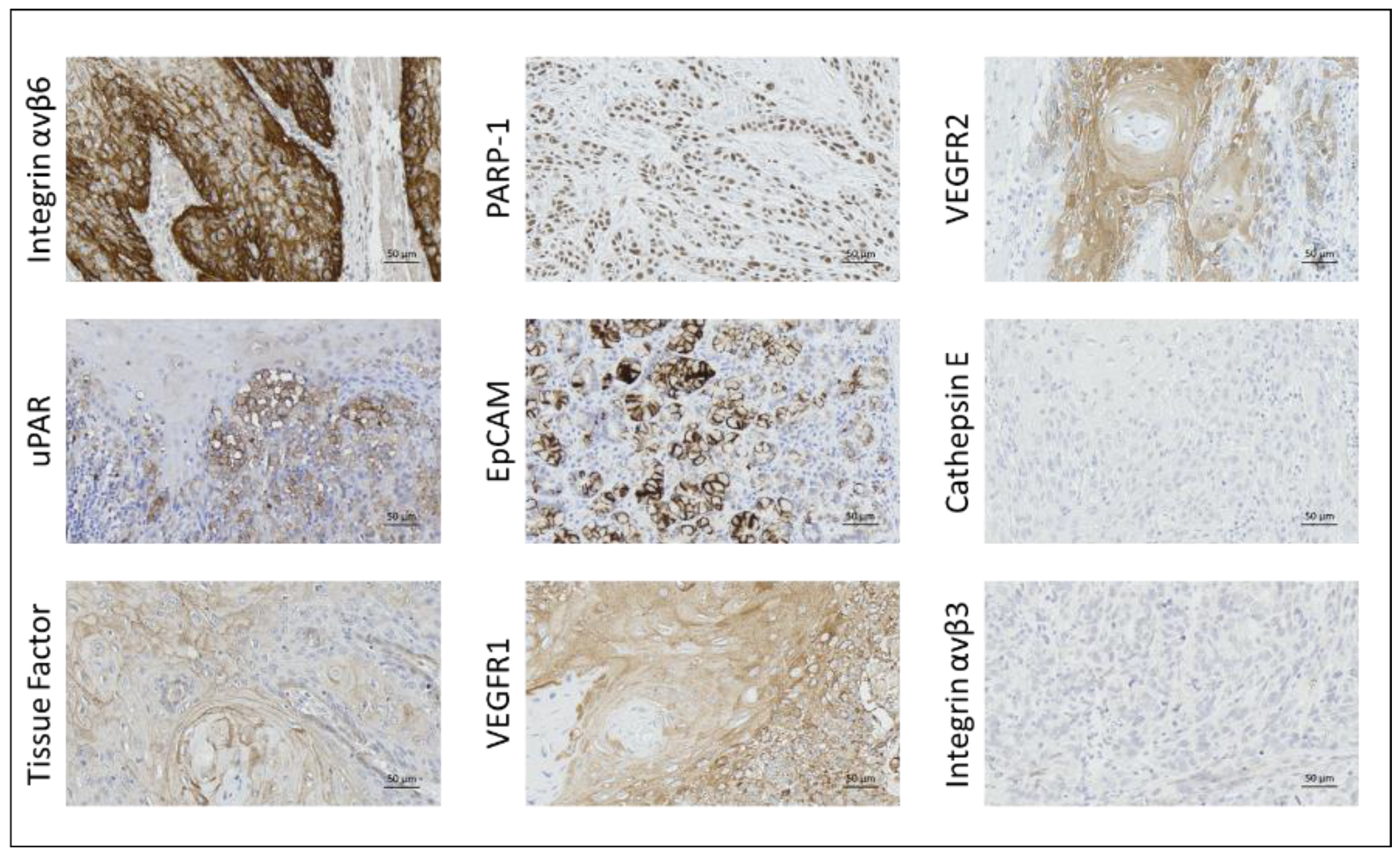
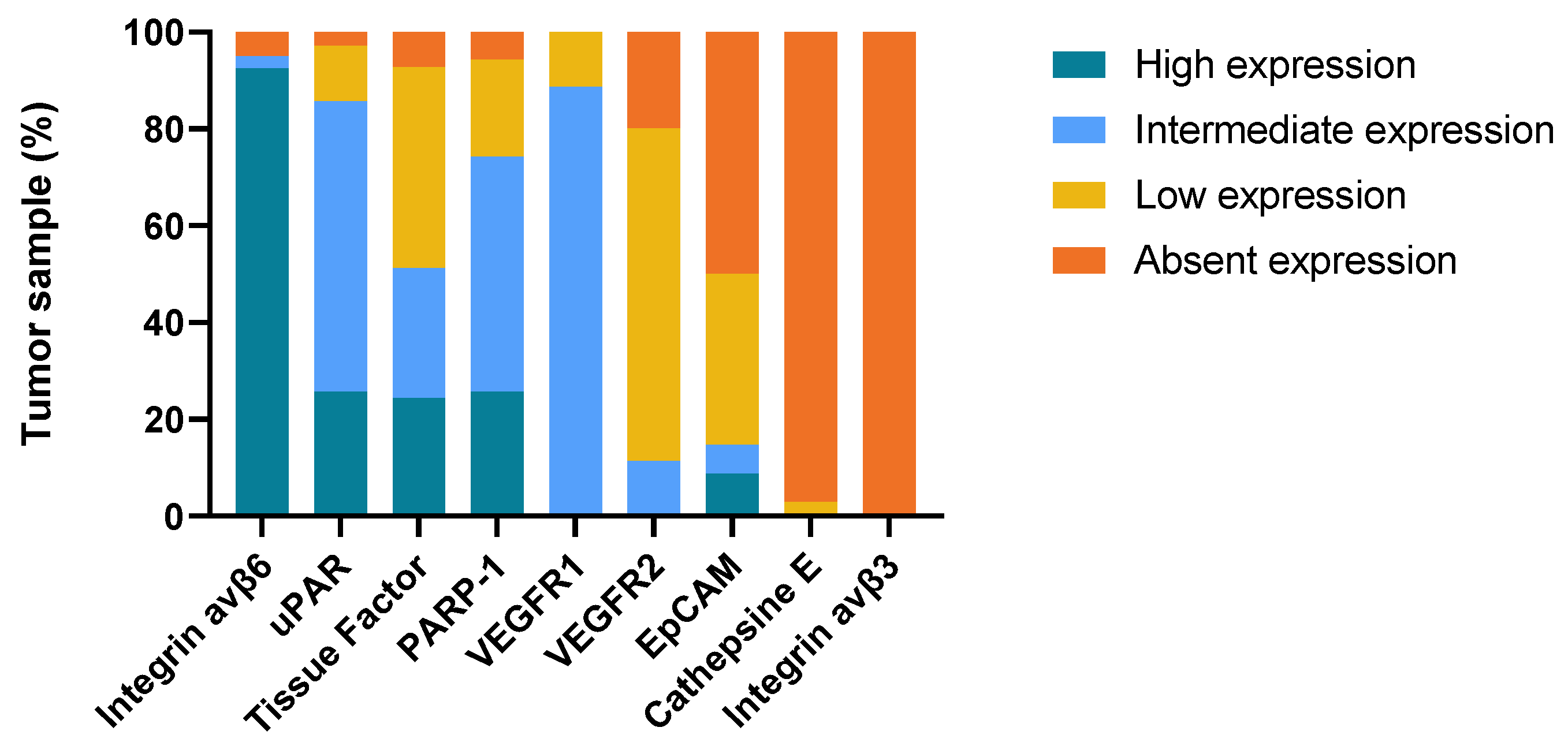
2.2. Immunohistochemical Staining
2.2.1. Integrin αvβ6
2.2.2. uPAR
2.2.3. Tissue Factor
2.2.4. PARP-1
2.2.5. VEGFR1
2.2.6. EpCAM
2.2.7. VEGFR2
2.2.8. Cathepsin E and Integrin αvβ3
2.3. Intensity of Staining in Normal Oral Mucosal Epithelium vs. Tumor Tissue
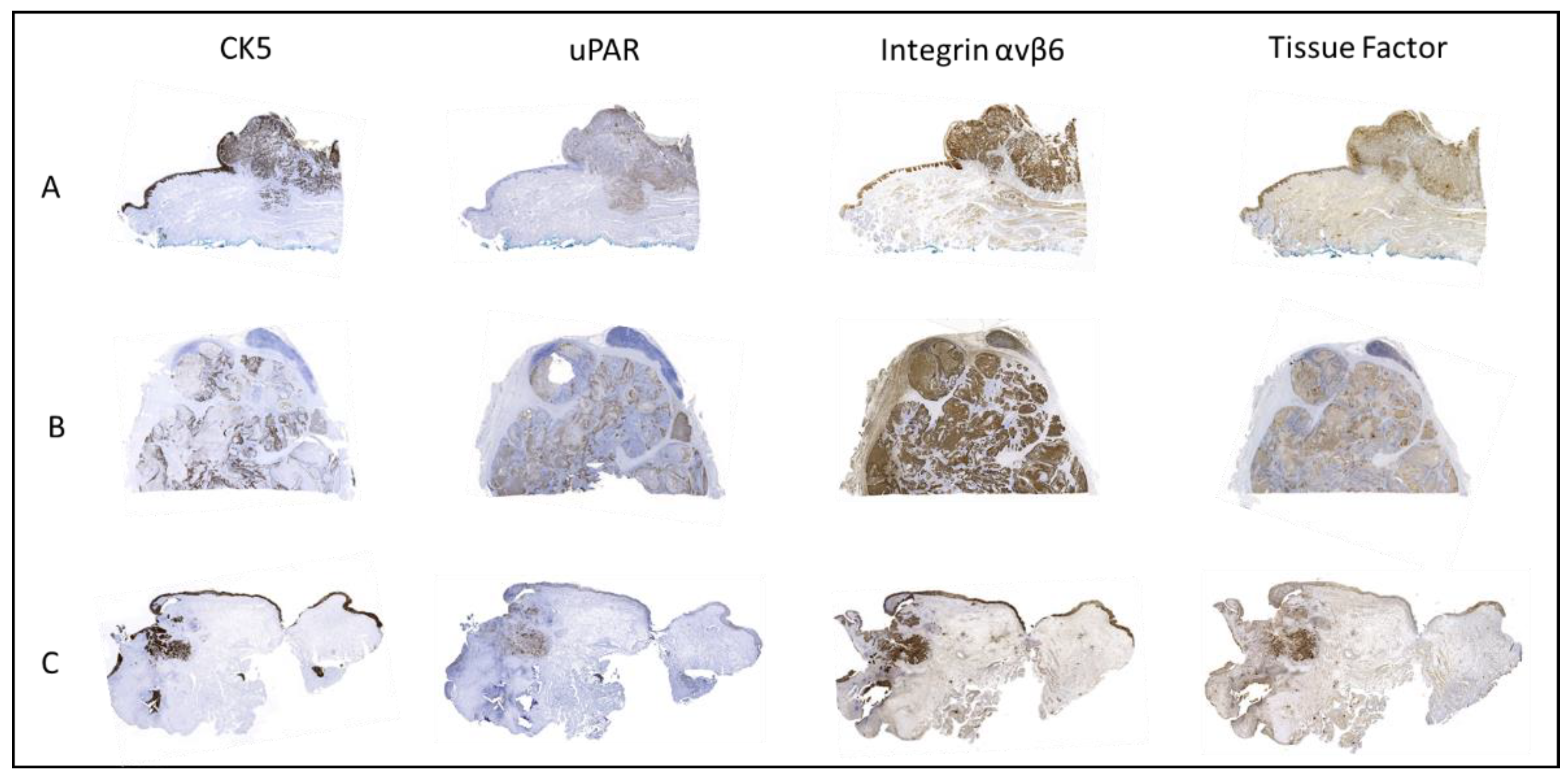
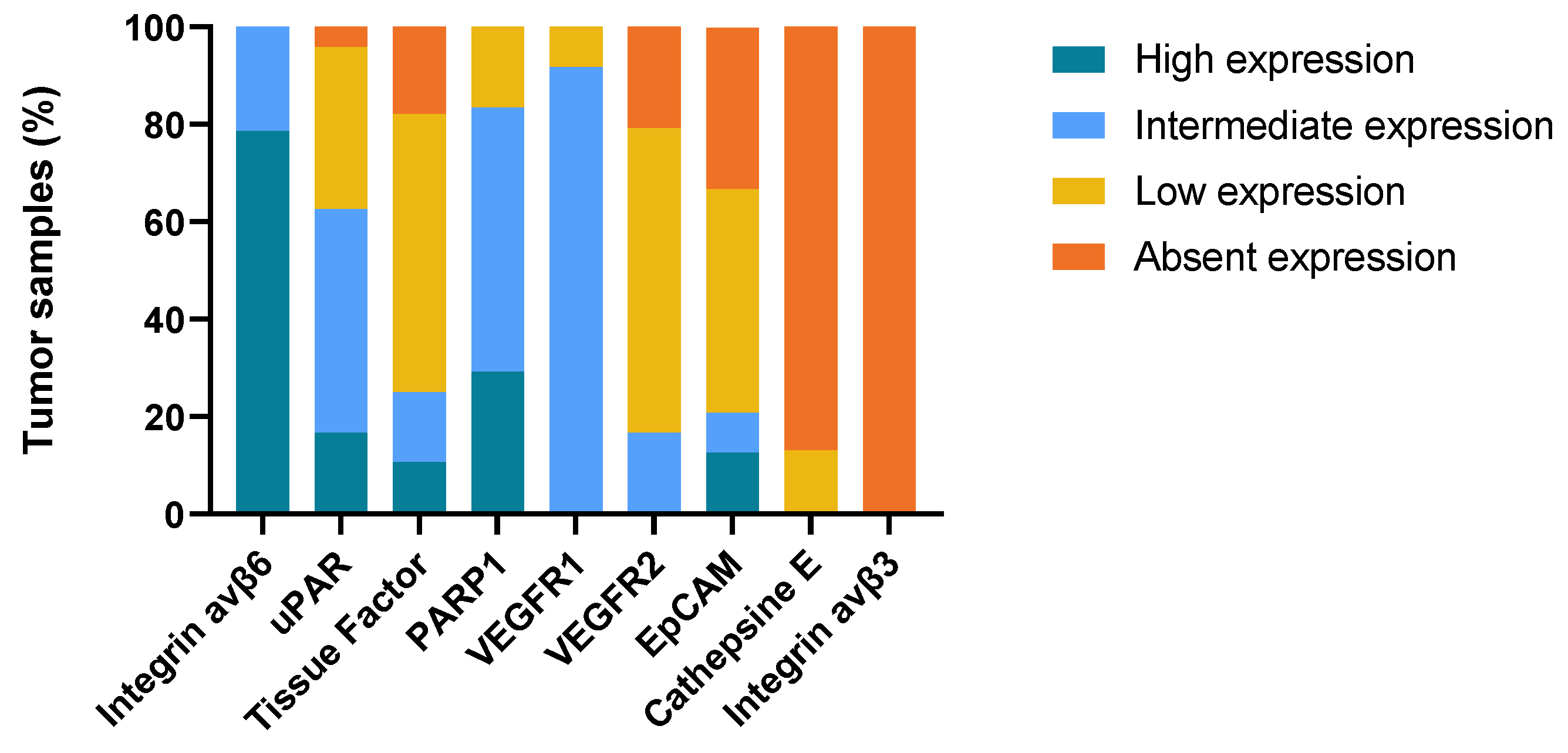
2.4. Biomarker Expression in Primary Tumor Compared to Lymph Node Metastases and Tissue from Local Recurrence (T-Site)
3. Discussion
4. Materials and Methods
4.1. Patient and Tissue Selection
4.2. Selection of Imaging Targets
4.3. Immunohistochemistry
4.4. Assessment of Immunohistochemical Staining
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Target | Source | Catalog Number | Species | Monoclonal/Polyclonal | Dilution | Staining Method |
|---|---|---|---|---|---|---|
| Cytokeratin 5 | Novocastra | CK5-L-CE | Mouse | Monoclonal | No dilution | Automatic, VENTANA BenchMark IHC |
| Integrin αvβ3 | R&D Systems | MAB3050 | Mouse | Monoclonal | 1:500 | Automatic, VENTANA BenchMark IHC |
| Integrin αvβ6 | ABCAM | ab181551 | Mouse | Monoclonal | 1:500 | Automatic, VENTANA BenchMark IHC |
| Tissue Factor | Sekisui | 4509 | Mouse | Monoclonal | 1:50 | Automatic, VENTANA BenchMark IHC |
| EPCAM | Cell Marque | 5435676001 | Mouse | Monoclonal | No dilution | Automatic, VENTANA BenchMark IHC |
| Cathepsin E | Abcam | Ab36996 | Rabbit | Polyclonal | 1:2000 | Manual |
| PARP1 | Abcam | Ab32138 | Rabbit | Monoclonal | 1:25 | Manual |
| uPAR | Genetex | GTX100467 | Mouse | Monoclonal | 1:500 | Manual |
| VEGFR1 | Abcam | Ab32152 | Rabbit | Monoclonal | 1:75 | Manual |
| VEGFR2 | Cell Signaling | 55B11 | Rabbit | Monoclonal | 1:300 | Manual |
References
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Giraldi, L.; Pastorino, R.; Arzani, D.; Petrelli, L.; Wünsch-Filho, V.; Toporcov, T.N.; Moyses, R.A.; et al. Tumour Stage and Gender Predict Recurrence and Second Primary Malignancies in Head and Neck Cancer: A Multicentre Study within the INHANCE Consortium. Eur. J. Epidemiol. 2018, 33, 1205. [Google Scholar] [CrossRef] [PubMed]
- Orosco, R.K.; Tapia, V.J.; Califano, J.A.; Clary, B.; Cohen, E.E.W.; Kane, C.; Lippman, S.M.; Messer, K.; Molinolo, A.; Murphy, J.D.; et al. Positive Surgical Margins in the 10 Most Common Solid Cancers. Sci. Rep. 2018, 8, 5686. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, H.; Macmillan, C.; Elwell, C.; Hammod, A. The Effect of the Surgical Margins on the Outcome of Patients with Head and Neck Squamous Cell Carcinoma: Single Institution Experience. Cancer Biol. Med. 2012, 9, 29–33. [Google Scholar] [CrossRef]
- Binahmed, A.; Nason, R.W.; Abdoh, A.A. The Clinical Significance of the Positive Surgical Margin in Oral Cancer. Oral Oncol. 2007, 43, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Mcmahon, J.; O’brien, C.J.; Pathak, I.; Hamill, R.; Mcneil, E.; Hammersley, N.; Gardiner, S.; Junor, E. Influence of Condition of Surgical Margins on Local Recurrence and Disease-Specific Survival in Oral and Oropharyngeal Cancer. Br. J. Oral Maxillofac. Surg. 2003, 41, 224–231. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Khawar, A.; Ahmed, J.; Ajmal, M.; Bangash, W.K.; Akhter, M.R. Occult Metastasis in Carcinoma of Oral Cavity. J. Coll. Physicians Surg. Pak. 2007, 17, 313–315. [Google Scholar]
- Du, E.; Ow, T.J.; Lo, Y.T.; Gersten, A.; Schiff, B.A.; Tassler, A.B.; Smith, R.V. Refining the Utility and Role of Frozen Section in Head and Neck Squamous Cell Carcinoma Resection. Laryngoscope 2016, 126, 1768–1775. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, Y.; Tao, X.F.; Lyu, P. Targeted Molecular Imaging of Head and Neck Squamous Cell Carcinoma: A Window into Precision Medicine. Chin. Med. J. 2020, 133, 1325. [Google Scholar] [CrossRef]
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the Margins: Real-Time Detection of Cancer Using Targeted Fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347. [Google Scholar] [CrossRef]
- Van Schaik, J.E.; Halmos, G.B.; Witjes, M.J.H.; Plaat, B.E.C. An Overview of the Current Clinical Status of Optical Imaging in Head and Neck Cancer with a Focus on Narrow Band Imaging and Fluorescence Optical Imaging. Oral Oncol. 2021, 121, 105504. [Google Scholar] [CrossRef]
- Crawford, K.L.; Pacheco, F.V.; Lee, Y.J.; Hom, M.; Rosenthal, E.L.; Nguyen, Q.T.; Orosco, R.K. A Scoping Review of Ongoing Fluorescence-Guided Surgery Clinical Trials in Otolaryngology. Laryngoscope 2022, 132, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, S.; Logan, R.M. The Role of Vascular Endothelial Growth Factor (VEGF) in Oral Dysplasia and Oral Squamous Cell Carcinoma. Oral Oncol. 2006, 42, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Stîngǎ, A.C.; Mǎrgǎritescu, O.; Stîngǎ, A.S.; Pirici, D.; Ciurea, R.; Bunget, A.; Cruce, M. VEGFR1 and VEGFR2 Immunohistochemical Expression in Oral Squamous Cell Carcinoma: A Morphometric Study. Rom. J. Morphol. Embryol. 2011, 52, 1269–1275. [Google Scholar] [PubMed]
- Masłowska, K.; Halik, P.K.; Tymecka, D.; Misicka, A.; Gniazdowska, E. The Role of VEGF Receptors as Molecular Target in Nuclear Medicine for Cancer Diagnosis and Combination Therapy. Cancers 2021, 13, 1072. [Google Scholar] [CrossRef]
- Brooks, P.C.; Clark, R.A.F.; Cheresh, D.A. Requirement of Vascular Integrin Avβ3 for Angiogenesis. Science 1994, 264, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.J.; Schwaiger, M. Imaging of Integrin Avβ3 Expression. Cancer Metastasis Rev. 2008, 27, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, Z. The Roles of Integrin Avβ6 in Cancer. Cancer Lett. 2017, 403, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Hausner, S.H.; Bold, R.J.; Cheuy, L.Y.; Chew, H.K.; Daly, M.E.; Davis, R.A.; Foster, C.C.; Kim, E.J.; Sutcliffe, J.L. Preclinical Development and First-in-Human Imaging of the Integrin a v b 6 with [18 F]a v b 6 -Binding Peptide in Metastatic Carcinoma. Clin. Cancer Res. 2019, 25, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Quigley, N.G.; Steiger, K.; Hoberück, S.; Czech, N.; Zierke, M.A.; Kossatz, S.; Pretze, M.; Richter, F.; Weichert, W.; Pox, C.; et al. PET/CT Imaging of Head-and-Neck and Pancreatic Cancer in Humans by Targeting the “Cancer Integrin” Avβ6 with Ga-68-Trivehexin. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1136–1147. [Google Scholar] [CrossRef]
- Li, H.X.; Zheng, J.H.; Fan, H.X.; Li, H.P.; Gao, Z.X.; Chen, D. Expression of Avβ6 Integrin and Collagen Fibre in Oral Squamous Cell Carcinoma: Association with Clinical Outcomes and Prognostic Implications. J. Oral Pathol. Med. 2013, 42, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.M.; Dang, D.; Sadler, S. The Role of the Integrin Avβ6 in Regulating the Epithelial to Mesenchymal Transition in Oral Cancer. Anticancer Res. 2009, 29, 125–130. [Google Scholar]
- Ramos, D.M.; But, M.; Regezi, J.; Schmidt, B.L.; Atakilit, A.; Dang, D.; Ellis, D.; Jordan, R.; Li, X. Expression of Integrin Β6 Enhances Invasive Behavior in Oral Squamous Cell Carcinoma. Matrix Biol. 2002, 21, 297–307. [Google Scholar] [CrossRef]
- Winter, M.J.; Nagtegaal, I.D.; Van Krieken, J.H.J.M.; Litvinov, S.V. The Epithelial Cell Adhesion Molecule (Ep-CAM) as a Morphoregulatory Molecule Is a Tool in Surgical Pathology. Am. J. Pathol. 2003, 163, 2139. [Google Scholar] [CrossRef] [PubMed]
- van Driel, P.B.A.A.; Boonstra, M.C.; Prevoo, H.A.J.M.; van de Giessen, M.; Snoeks, T.J.A.; Tummers, Q.R.J.G.; Keereweer, S.; Cordfunke, R.A.; Fish, A.; van Eendenburg, J.D.H.; et al. EpCAM as Multi-Tumour Target for near-Infrared Fluorescence Guided Surgery. BMC Cancer 2016, 16, 884. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, L.S.F.; Boonstra, M.C.; Prevoo, H.A.J.M.; Handgraaf, H.J.M.; Kuppen, P.J.K.; Van De Velde, J.H.; Fish, A.; Cordfunke, R.A.; Rob, A.; Valentijn, P.M.; et al. Fluorescence-Guided Tumor Detection with a Novel Anti-EpCAM Targeted Antibody Fragment: Preclinical Validation. Surg. Oncol. 2019, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pontious, C.; Kaul, S.; Hong, M.; Hart, P.A.; Krishna, S.G.; Lara, L.F.; Conwell, D.L.; Cruz-Monserrate, Z. Cathepsin E Expression and Activity: Role in the Detection and Treatment of Pancreatic Cancer. Pancreatology 2019, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, C.; Li, F.; Guan, D.; Wu, X.; Fu, X.; Lu, A.; Zhang, G. PARP1 in Carcinomas and PARP1 Inhibitors as Antineoplastic Drugs. Int. J. Mol. Sci. 2017, 18, 2111. [Google Scholar] [CrossRef]
- Kossatz, S.; Brand, C.; Gutiontov, S.; Liu, J.T.C.; Lee, N.Y.; Gonen, M.; Weber, W.A.; Reiner, T. Detection and Delineation of Oral Cancer with a PARP1 Targeted Optical Imaging Agent. Sci. Rep. 2016, 6, 21371. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.A.; Kossatz, S.; Weber, W.; Beheshti, M.; Morgenroth, A.; Mottaghy, F.M. Advancements in PARP1 Targeted Nuclear Imaging and Theranostic Probes. J. Clin. Med. 2020, 9, 2130. [Google Scholar] [CrossRef]
- Demétrio de Souza França, P.; Kossatz, S.; Brand, C.; Karassawa Zanoni, D.; Roberts, S.; Guru, N.; Adilbay, D.; Mauguen, A.; Valero Mayor, C.; Weber, W.A.; et al. A Phase I Study of a PARP1-Targeted Topical Fluorophore for the Detection of Oral Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3618–3630. [Google Scholar] [CrossRef]
- Abd-Elgaliel, W.R.; Cruz-Monserrate, Z.; Logsdon, C.D.; Tung, C.H. Molecular Imaging of Cathepsin E-Positive Tumors in Mice Using a Novel Protease-Activatable Fluorescent Probe. Mol. Biosyst. 2011, 7, 3207. [Google Scholar] [CrossRef]
- Noh, H.; Hong, S.; Huang, S. Role of Urokinase Receptor in Tumor Progression and Development. Theranostics 2013, 3, 487–495. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, Y.; Jiang, X.; Yuan, H.; Wang, H.; Cui, X.; Xu, J.; Zhao, J.; Wang, J. UPAR: An Essential Factor for Tumor Development. J. Cancer 2021, 12, 7026. [Google Scholar] [CrossRef]
- Van Den Berg, Y.W.; Osanto, S.; Reitsma, P.H.; Versteeg, H.H. The Relationship between Tissue Factor and Cancer Progression: Insights from Bench and Bedside. Blood 2012, 119, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Seto, S.; Onodera, H.; Kaido, T.; Yoshikawa, A.; Ishigami, S.; Arii, S.; Imamura, M. Tissue Factor Expression in Human Colorectal Carcinoma Correlation with Hepatic Metastasis and Impact on Prognosis. Cancer 2000, 88, 295–301. [Google Scholar] [CrossRef]
- Akashi, T.; Furuya, Y.; Ohta, S.; Fuse, H. Tissue Factor Expression and Prognosis in Patients with Metastatic Prostate Cancer. Urology 2003, 62, 1078–1082. [Google Scholar] [CrossRef]
- Christensen, A.; Grønhøj, C.; Jensen, J.S.; Lelkaitis, G.; Kiss, K.; Juhl, K.; Charabi, B.W.; Mortensen, J.; Kjær, A.; Buchwald, C. von Expression Patterns of UPAR, TF and EGFR and Their Potential as Targets for Molecular Imaging in Oropharyngeal Squamous Cell Carcinoma. Oncol. Rep. 2022, 48, 147. [Google Scholar] [CrossRef]
- Baart, V.M.; van Duijn, C.; van Egmond, S.L.; Dijckmeester, W.A.; Jansen, J.C.; Vahrmeijer, A.L.; Sier, C.F.M.; Cohen, D. Egfr and Avβ6 as Promising Targets for Molecular Imaging of Cutaneous and Mucosal Squamous Cell Carcinoma of the Head and Neck Region. Cancers 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Serpa, M.S.; Mafra, R.P.; Queiroz, S.I.M.L.; da Silva, L.P.; de Souza, L.B.; Pinto, L.P. Expression of Urokinase-Type Plasminogen Activator and Its Receptorin Squamous Cell Carcinoma of the Oral Tongue. Braz. Oral Res. 2018, 32. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Loft, M.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Knigge, U.; Kjaer, A. Prospective Phase II Trial of Prognostication by 68 Ga-NOTA-AE105 UPAR PET in Patients with Neuroendocrine Neoplasms: Implications for UPAR Targeted Therapy. J. Nucl. Med. 2022, 63, 1371–1377. [Google Scholar] [CrossRef]
- Fosbøl, M.Ø.; Kurbegovic, S.; Johannesen, H.H.; Røder, M.A.; Hansen, A.E.; Mortensen, J.; Loft, A.; Petersen, P.M.; Madsen, J.; Brasso, K.; et al. Urokinase-Type Plasminogen Activator Receptor (UPAR) PET/MRI of Prostate Cancer for Noninvasive Evaluation of Aggressiveness: Comparison with Gleason Score in a Prospective Phase 2 Clinical Trial. J. Nucl. Med. 2021, 62, 354–359. [Google Scholar] [CrossRef]
- Persson, M.; Skovgaard, D.; Brandt-Larsen, M.; Christensen, C.; Madsen, J.; Nielsen, C.H.; Thurison, T.; Klausen, T.L.; Holm, S.; Loft, A.; et al. First-in-Human UPAR PET: Imaging of Cancer Aggressiveness. Theranostics 2015, 5, 1303–1316. [Google Scholar] [CrossRef]
- Risoer, L.M.; Clausen, M.M.; Ujmajuridze, Z.; Farhadi, M.; Andersen, K.F.; Loft, A.; Kjaer, A. Prognostic Value of Urokinase-Type Plasminogen Activator Receptor (UPAR)-PET/CT in Head and Neck Squamous Cell Carcinomas and Comparison with 18 F-FDG-PET/CT: A Single-Center Prospective Study. J. Nucl. Med. 2021, 63, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.; Juhl, K.; Persson, M.; Charabi, B.W.; Mortensen, J.; Kiss, K.; Lelkaitis, G.; Rubek, N.; von Buchwald, C.; Kjær, A. UPAR-Targeted Optical near-Infrared (NIR) Fluorescence Imaging and PET for Image-Guided Surgery in Head and Neck Cancer: Proof-of-Concept in Orthotopic Xenograft Model. Oncotarget 2017, 8, 15407–15419. [Google Scholar] [CrossRef]
- Boonstra, M.C.; Van Driel, P.B.A.A.; Keereweer, S.; Prevoo, H.A.J.M.; Stammes, M.A.; Baart, V.M.; Löwik, C.W.G.M.; Mazar, A.P.; van de Velde, C.J.H.; Vahrmeijer, A.L.; et al. Preclinical UPAR-Targeted Multimodal Imaging of Locoregional Oral Cancer. Oral Oncol. 2017, 66, 1–8. [Google Scholar] [CrossRef]
- Christensen, A.; Kiss, K.; Lelkaitis, G.; Juhl, K.; Persson, M.; Charabi, B.W.; Mortensen, J.; Forman, J.L.; Sørensen, A.L.; Jensen, D.H.; et al. Urokinase-Type Plasminogen Activator Receptor (UPAR), Tissue Factor (TF) and Epidermal Growth Factor Receptor (EGFR): Tumor Expression Patterns and Prognostic Value in Oral Cancer. BMC Cancer 2017, 17, 572. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, Q.; Jiang, D.; Zhao, H.; Kutyreff, C.J.; Engle, J.W.; Liu, J.; Cai, W. Tissue Factor-Targeted ImmunoPET Imaging and Radioimmunotherapy of Anaplastic Thyroid Cancer. Adv. Sci. 2020, 7, 1903595. [Google Scholar] [CrossRef]
- Nielsen, C.H.; Erlandsson, M.; Jeppesen, T.E.; Jensen, M.M.; Kristensen, L.K.; Madsen, J.; Petersen, L.C.; Kjaer, A. Quantitative PET Imaging of Tissue Factor Expression Using 18F-Labeled Active Site–Inhibited Factor VII. J. Nucl. Med. 2016, 57, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Takashima, H.; Tsuji, A.B.; Saga, T.; Yasunaga, M.; Koga, Y.; Kuroda, J.I.; Yano, S.; Kuratsu, J.I.; Matsumura, Y. Molecular Imaging Using an Anti-Human Tissue Factor Monoclonal Antibody in an Orthotopic Glioma Xenograft Model. Sci. Rep. 2017, 71, 12341. [Google Scholar] [CrossRef] [PubMed]
- Sugyo, A.; Aung, W.; Tsuji, A.B.; Sudo, H.; Takashima, H.; Yasunaga, M.; Matsumura, Y.; Saga, T.; Higashi, T. Anti-tissue Factor Antibody-mediated Immuno-SPECT Imaging of Tissue Factor Expression in Mouse Models of Pancreatic Cancer. Oncol. Rep. 2019, 41, 2371–2378. [Google Scholar] [CrossRef]
- Food and Drug Administration FDA Grants Accelerated Approval to Tisotumab Vedotin-Tftv for Recurrent or Metastatic Cervical Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tisotumab-vedotin-tftv-recurrent-or-metastatic-cervical-cancer (accessed on 19 September 2022).
- Loft, M.; Christensen, C.; Clausen, M.M.; Carlsen, E.A.; Hansen, C.P.; Kroman, N.; Langer, S.W.; Høgdall, C.; Madsen, J.; Gillings, N.; et al. First-in-Human PET Imaging of Tissue Factor in Patients with Primary and Metastatic Cancers Using 18F-Labeled Active-Site Inhibited Factor VII (18F-ASIS): Potential as Companion Diagnostic. J. Nucl. Med. 2022, 63, 1871–1879. [Google Scholar] [CrossRef]
- Yakavets, I.; Francois, A.; Guiot, M.; Lequeux, N.; Fragola, A.; Pons, T.; Bezdetnaya, L.; Marchal, F. NIR Imaging of the Integrin-Rich Head and Neck Squamous Cell Carcinoma Using Ternary Copper Indium Selenide/Zinc Sulfide-Based Quantum Dots. Cancers 2020, 12, 3727. [Google Scholar] [CrossRef]
- de Valk, K.S.; Deken, M.M.; Handgraaf, H.J.M.; Bhairosingh, S.S.; Bijlstra, O.D.; van Esdonk, M.J.; Terwisscha van Scheltinga, A.G.T.; Valentijn, A.R.P.M.; March, T.L.; Vuijk, J.; et al. First-in-Human Assessment of CRGD-ZW800-1, a Zwitterionic, Integrin-Targeted, Near-Infrared Fluorescent Peptide in Colon Carcinoma. Clin. Cancer Res. 2020, 26, 3990–3998. [Google Scholar] [CrossRef] [PubMed]
- Hyytiäinen, A.; Wahbi, W.; Väyrynen, O.; Saarilahti, K.; Karihtala, P.; Salo, T.; Al-Samadi, A. Angiogenesis Inhibitors for Head and Neck Squamous Cell Carcinoma Treatment: Is There Still Hope? Front. Oncol. 2021, 11, 2123. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.; Crane, L.M.A.; Bart, J.; Van Leeuwen, F.W.; Van Dam, G.M. Selecting Potential Targetable Biomarkers for Imaging Purposes in Colorectal Cancer Using TArget Selection Criteria (TASC): A Novel Target Identification Tool. Transl. Oncol. 2011, 4, 71–82. [Google Scholar] [CrossRef]
- van der Fels, C.A.M.; Palthe, S.; Buikema, H.; van den Heuvel, M.C.; Leliveld, A.; de Jong, I.J. Potential Receptors for Targeted Imaging of Lymph Node Metastases in Penile Cancer. Diagnostics 2020, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- de Gooyer, J.M.; Versleijen-Jonkers, Y.M.H.; Hillebrandt-Roeffen, M.H.S.; Frielink, C.; Desar, I.M.E.; de Wilt, J.H.W.; Flucke, U.; Rijpkema, M. Immunohistochemical Selection of Biomarkers for Tumor-Targeted Image-Guided Surgery of Myxofibrosarcoma. Sci. Rep. 2020, 10, 2915. [Google Scholar] [CrossRef] [PubMed]
- Linders, D.; Deken, M.; van der Valk, M.; Tummers, W.; Bhairosingh, S.; Schaap, D.; van Lijnschoten, G.; Zonoobi, E.; Kuppen, P.; van de Velde, C.; et al. CEA, EpCAM, Avβ6 and UPAR Expression in Rectal Cancer Patients with a Pathological Complete Response after Neoadjuvant Therapy. Diagnostics 2021, 11, 516. [Google Scholar] [CrossRef]
| Characteristics | n (%) or Median (Range) |
|---|---|
| Age, years | 58 (23–81) |
| Gender | |
| Male | 26 (63%) |
| Female | 15 (37%) |
| Location | |
| Tongue | 18 (44%) |
| Floor of mouth | 23 (56%) |
| Tumor differentiation | |
| Low | 5 (12%) |
| Moderate | 30 (73%) |
| High | 6 (15%) |
| Pathologic T-stage | |
| T1 | 11(27%) |
| T2 | 17 (42%) |
| T3 | 5 (12%) |
| T4 | 5 (12%) |
| Missing | 3 (7%) |
| Pathologic N-stage | |
| N0 | 3 (7%) |
| N+ | 38 (93%) |
| Target | Primary Tumor | Lymph Node Metastases | Local Recurrence | Normal Epithelium | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Intensity score (IQR) | Proportion (IQR) | TIS-score (IQR) | n | Intensity score (IQR) | Proportion (IQR) | TIS-score (IQR) | n | Intensity score (IQR) | Proportion (IQR) | TIS-score (IQR) | n | Intensity score (IQR) | |
| Integrin αvβ6 | 40 | 3(3-3) | 4(4-4) | 12(12-12) | 28 | 3(3-3) | 4(4-4) | 12(9.75-12) | 8 | 3(3-3) | 4(4-4) | 12(12-12) | 47 | 3(3-3) |
| Tissue factor | 41 | 3(2-3) | 2(1-3) | 6(2.5-7.5) | 28 | 2(1-3) | 1(1-2) | 2(1-5.5) | 8 | 2.5(0-3) | 1.5(0-2) | 4(0-6) | 47 | 1(1-1) |
| PARP1 | 35 | 2(2-3) | 3(3-4) | 6(4-9) | 24 | 2(2-3) | 3(3-3) | 6(6-9) | 7 | 2(2-2) | 3(3-4) | 6(4-8) | 39 | 2(1-2) |
| uPAR | 34 | 3(2.75-3) | 2(2-3) | 6(6-9) | 23 | 3(2-3) | 2(2-3) | 6(4-6) | 6 | 3(2.75-3) | 2.5(2-3.25) | 6(6-9.75) | 37 | 0(0-0) |
| VEGFR1 | 35 | 2(2-2) | 4(3-4) | 8(6-8) | 24 | 2(2-2) | 4(3.25-4) | 8(6.5-8) | 7 | 2(1-2) | 4(3-4) | 8(3-8) | 40 | 1.5(1-2) |
| EpCAM | 34 | 0.5(0-2) | 0.5(0-2) | 0.5(0-2.5) | 24 | 1.5(0-3) | 1(0-1) | 1.5(0-3) | 7 | 1(0-3) | 1(0-2) | 1(0-6) | 34 | 0(0-0) |
| VEGFR2 | 35 | 1(1-2) | 1(1-2) | 2(1-4) | 24 | 1(1-2) | 2(1-2) | 2(1-4) | 7 | 1(0-1) | 2(0-2) | 2(0-2) | 39 | 0(0-1) |
| Cathepsin E | 35 | 0(0-0) | 0(0-0) | 0(0-0) | 23 | 0(0-0) | 0(0-0) | 0(0-0) | 7 | 0(0-0) | 0(0-0) | 0(0-0) | 40 | 0(0-0.5) |
| Integrin αvβ3 | 36 | 0(0-0) | 0(0-0) | 0(0-0) | 27 | 0(0-0) | 0(0-0) | 0(0-0) | 8 | 0(0-0) | 0(0-0) | 0(0-0) | 44 | 0(0-0) |
| Target | Tumor-Specific | Homogenous Expression in Tumor Compartment | Expression Rate Primary Tumor | Expression Rate Lymph Node Metastasis | Superficial Margin Contrast Tumor vs. Epithelium | Profound Margin Contrast Tumor vs. Normal Cells |
|---|---|---|---|---|---|---|
| Integrin αvβ6 | Partly | 80% | 95% | 100% | No | Yes |
| Tissue factor | Yes | 3% | 93% | 80% | Yes | Yes |
| PARP1 | No | 0% | 94% | 100% | Yes | No |
| uPAR | Yes | 51% | 97% | 96% | Yes | Yes |
| VEGFR1 | No | 5% | 100% | 100% | Yes | No |
| EpCAM | No | 3% | 50% | 66% | No | No |
| VEGFR2 | No | 0% | 80% | 81% | Yes | No |
| Cathepsin E | NA | 0% | 3% | 13% | NA | NA |
| Integrin αvβ3 | NA | NA | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawaetz, M.; Christensen, A.; Juhl, K.; Karnov, K.; Lelkaitis, G.; Kanstrup Fiehn, A.-M.; Kjaer, A.; von Buchwald, C. Potential of uPAR, αvβ6 Integrin, and Tissue Factor as Targets for Molecular Imaging of Oral Squamous Cell Carcinoma: Evaluation of Nine Targets in Primary Tumors and Metastases by Immunohistochemistry. Int. J. Mol. Sci. 2023, 24, 3853. https://doi.org/10.3390/ijms24043853
Lawaetz M, Christensen A, Juhl K, Karnov K, Lelkaitis G, Kanstrup Fiehn A-M, Kjaer A, von Buchwald C. Potential of uPAR, αvβ6 Integrin, and Tissue Factor as Targets for Molecular Imaging of Oral Squamous Cell Carcinoma: Evaluation of Nine Targets in Primary Tumors and Metastases by Immunohistochemistry. International Journal of Molecular Sciences. 2023; 24(4):3853. https://doi.org/10.3390/ijms24043853
Chicago/Turabian StyleLawaetz, Mads, Anders Christensen, Karina Juhl, Kirstine Karnov, Giedrius Lelkaitis, Anne-Marie Kanstrup Fiehn, Andreas Kjaer, and Christian von Buchwald. 2023. "Potential of uPAR, αvβ6 Integrin, and Tissue Factor as Targets for Molecular Imaging of Oral Squamous Cell Carcinoma: Evaluation of Nine Targets in Primary Tumors and Metastases by Immunohistochemistry" International Journal of Molecular Sciences 24, no. 4: 3853. https://doi.org/10.3390/ijms24043853
APA StyleLawaetz, M., Christensen, A., Juhl, K., Karnov, K., Lelkaitis, G., Kanstrup Fiehn, A.-M., Kjaer, A., & von Buchwald, C. (2023). Potential of uPAR, αvβ6 Integrin, and Tissue Factor as Targets for Molecular Imaging of Oral Squamous Cell Carcinoma: Evaluation of Nine Targets in Primary Tumors and Metastases by Immunohistochemistry. International Journal of Molecular Sciences, 24(4), 3853. https://doi.org/10.3390/ijms24043853









