Optimization of Isolation and Transformation of Protoplasts from Uncaria rhynchophylla and Its Application to Transient Gene Expression Analysis
Abstract
1. Introduction
2. Results
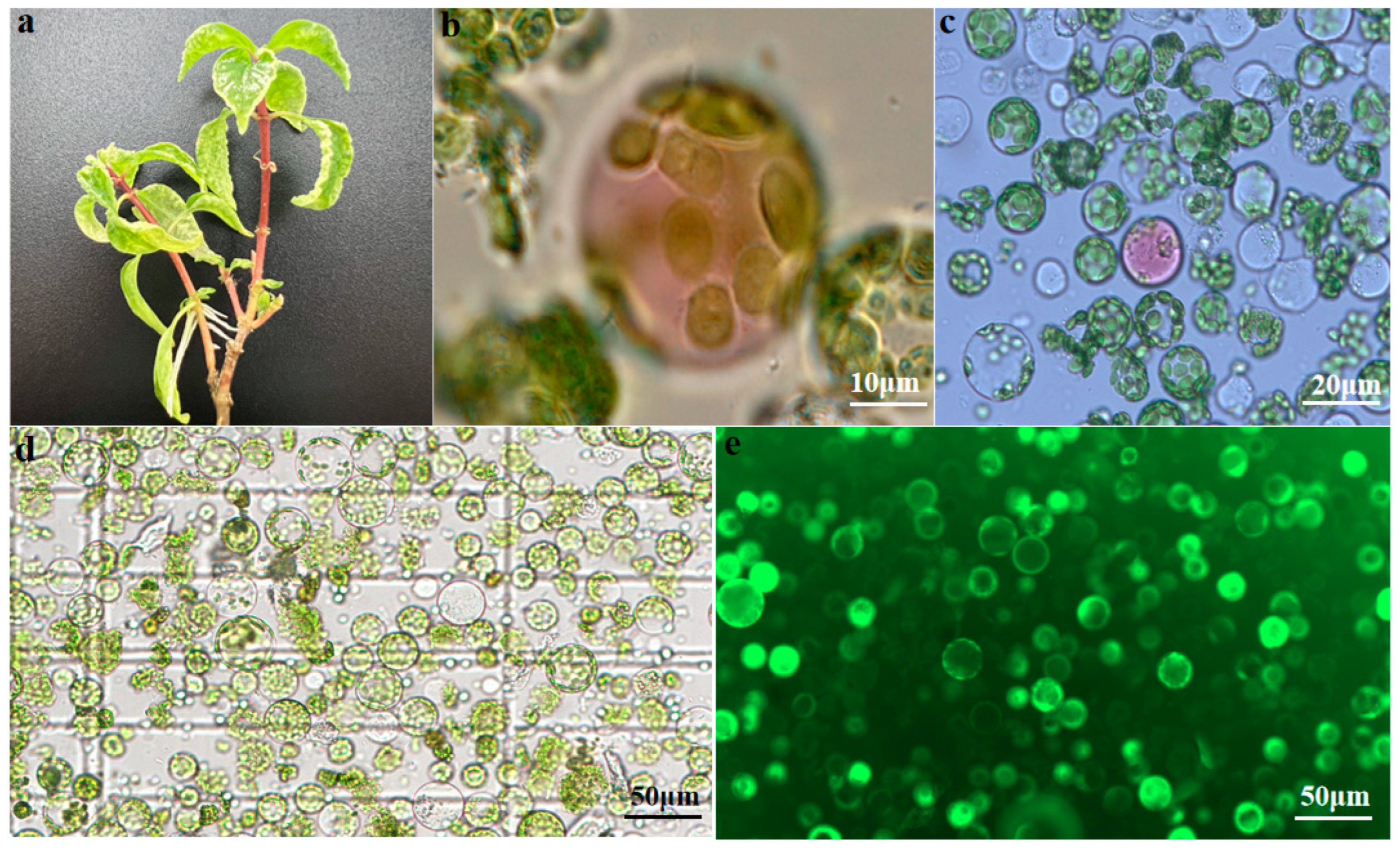
2.1. Protoplast Isolation from U. rhynchophylla Leaves
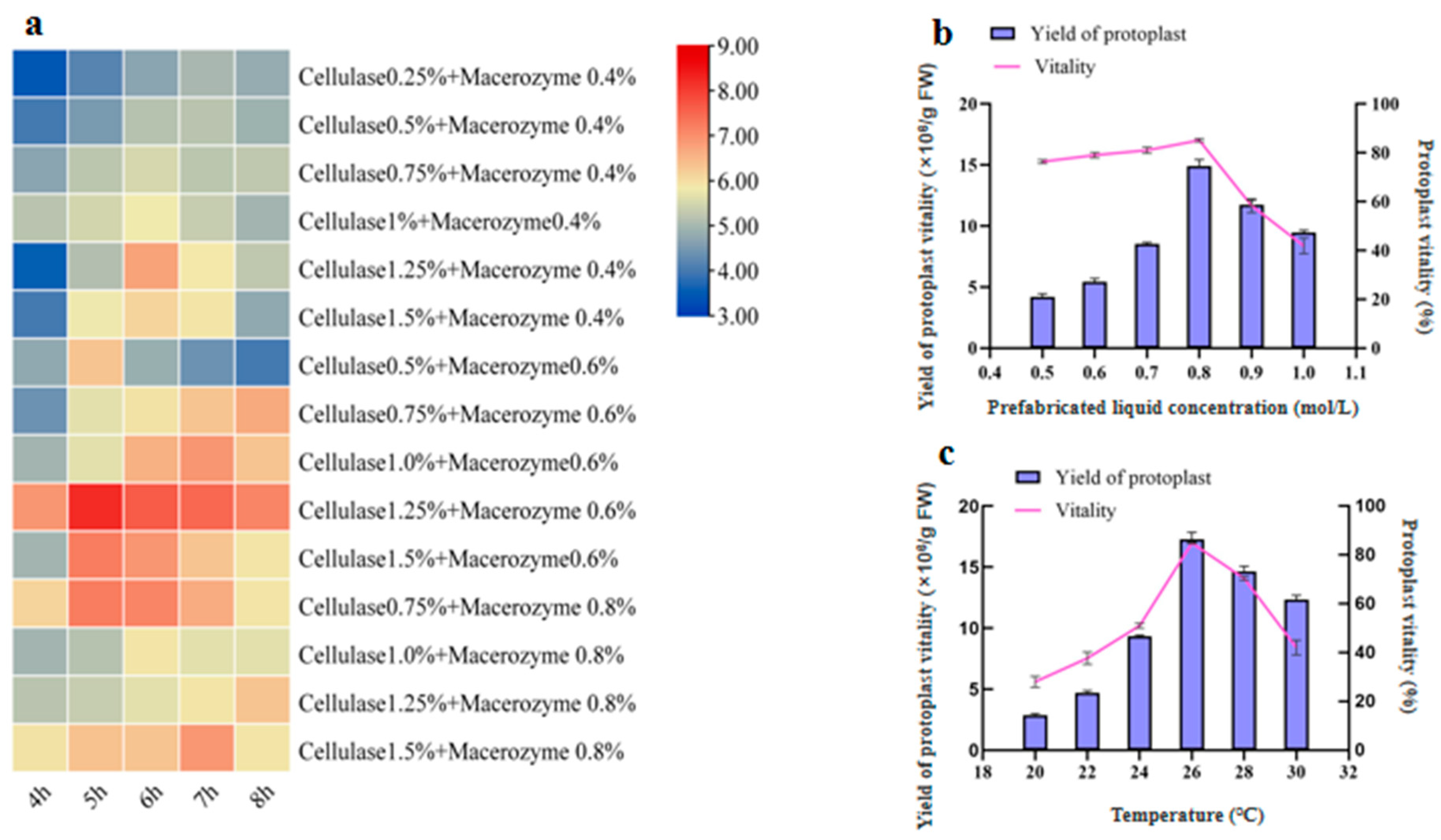
2.1.1. Effects of Enzymolysis Concentration and Duration on Protoplast Isolation
2.1.2. Effects of Prefabricated Liquid Concentration on Protoplast Isolation
2.1.3. Effects of Temperature on Protoplast Isolation
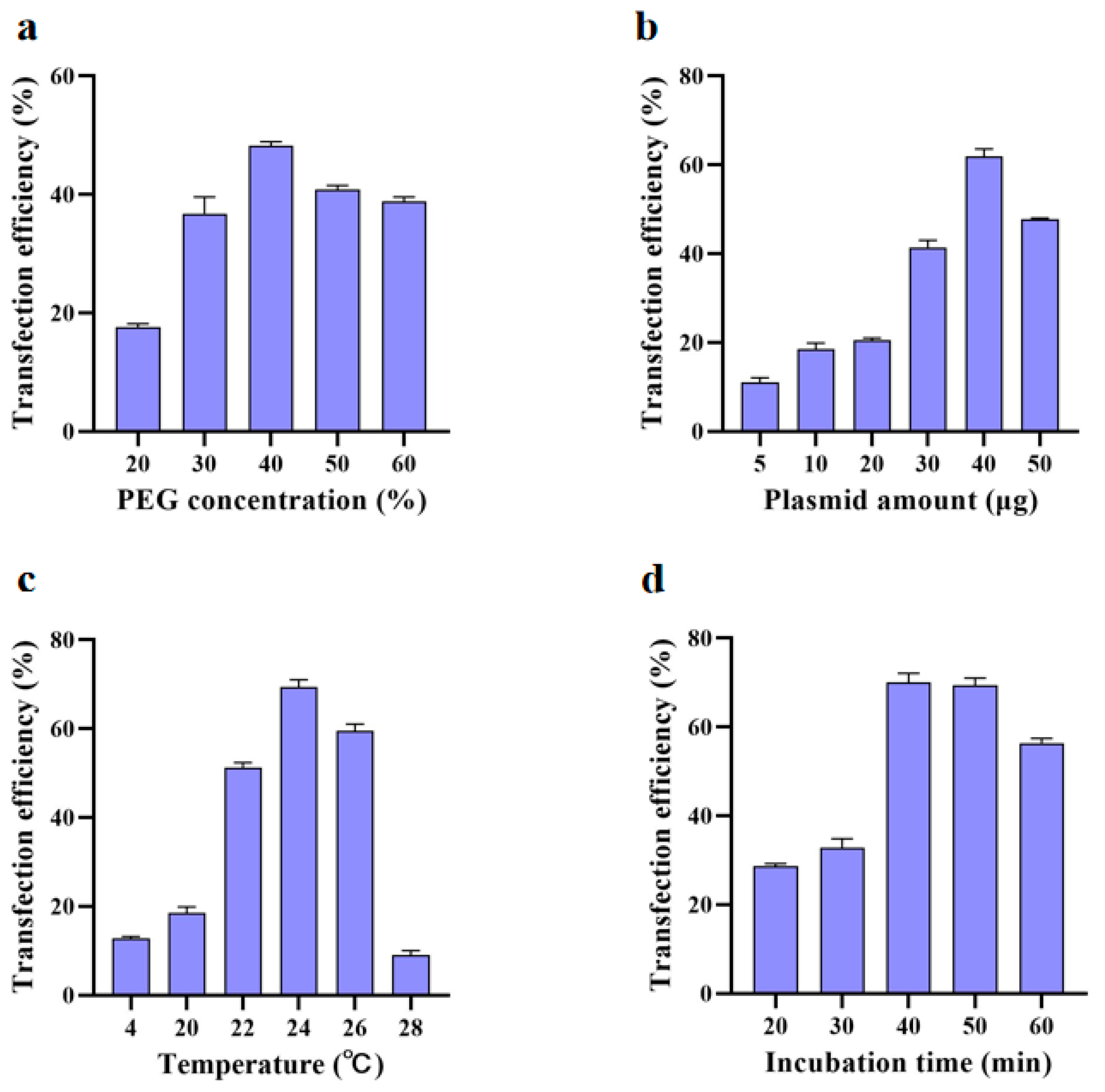
2.2. Protoplast Transformation
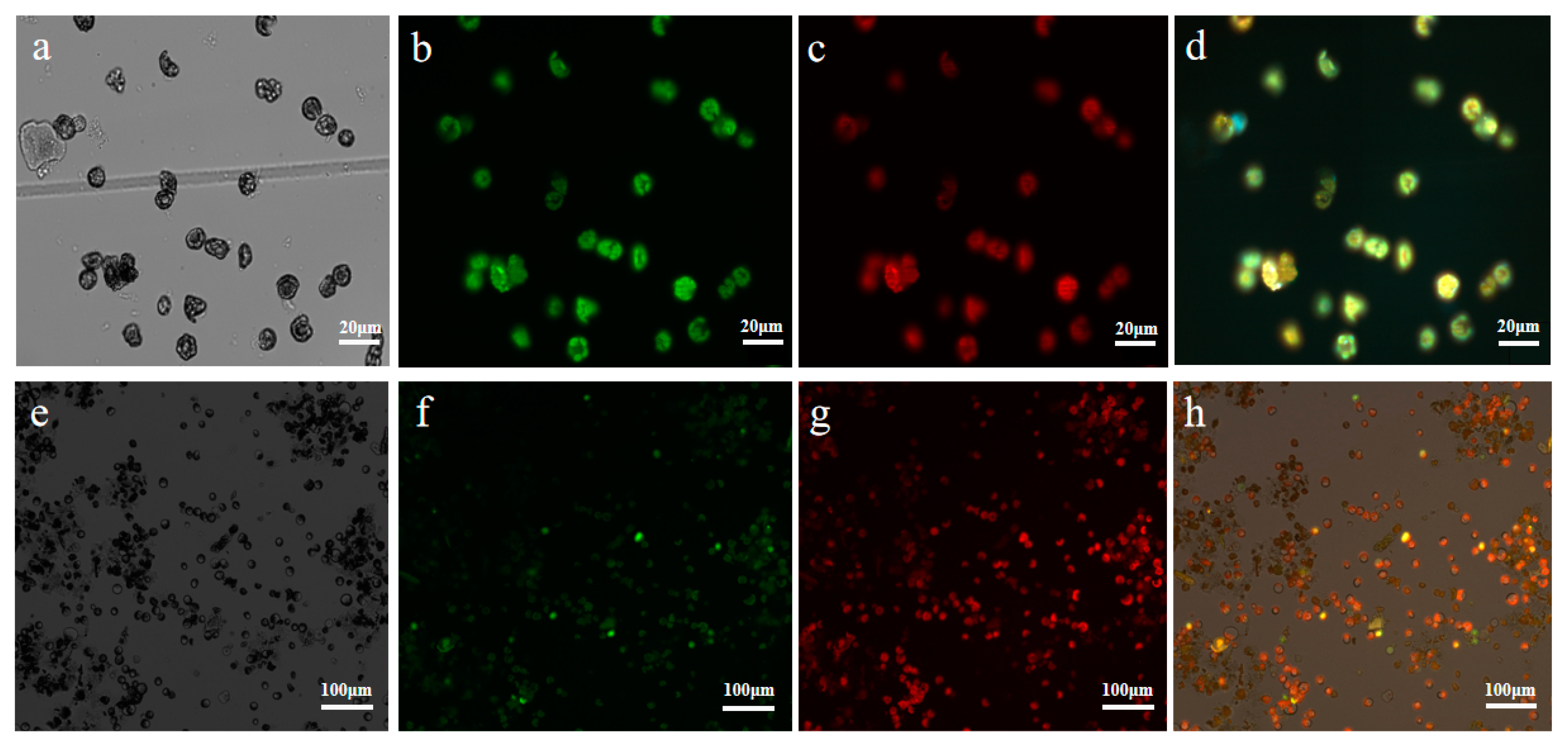
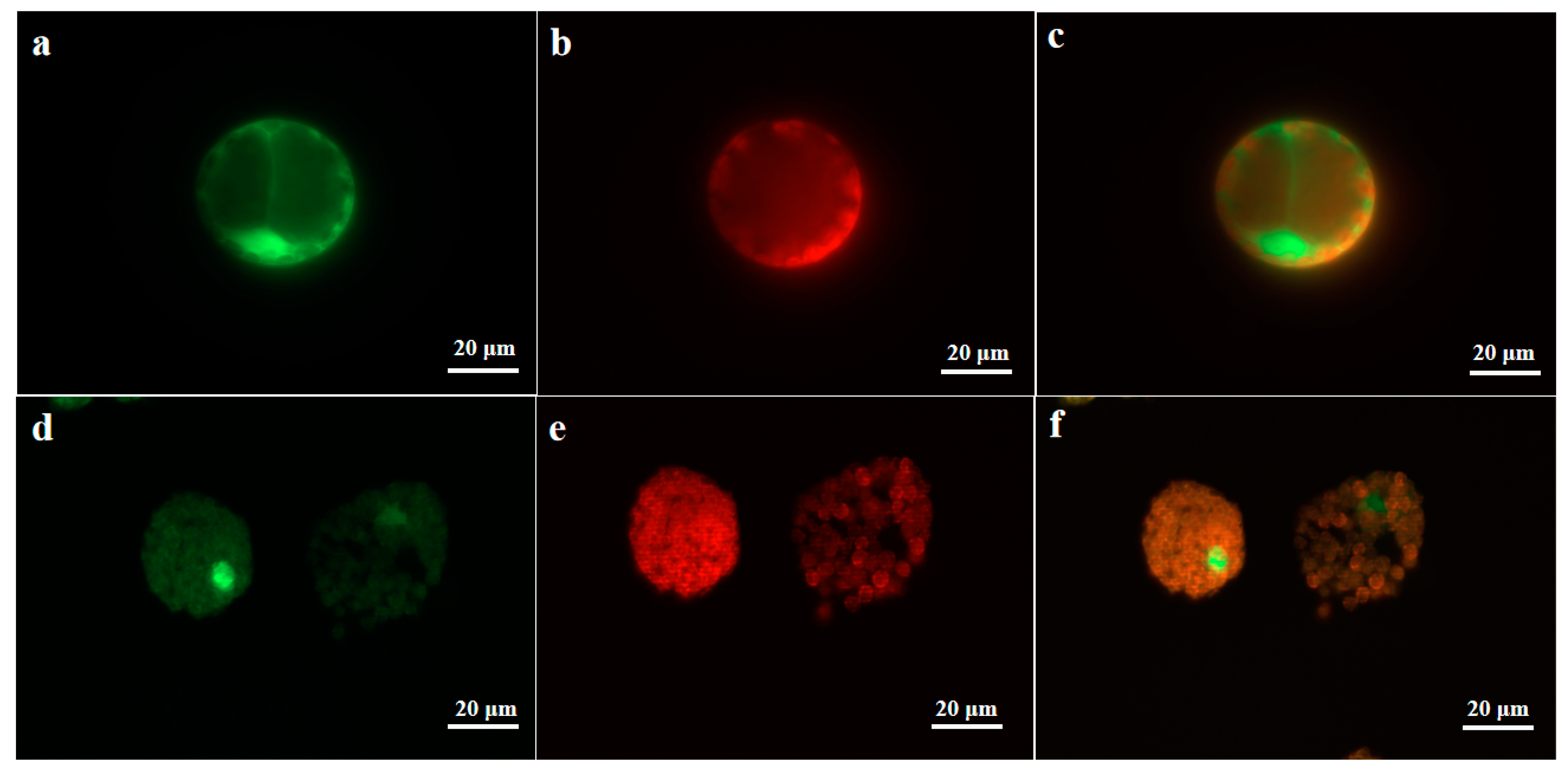
2.3. Subcellular Localization Analysis of UrWRKY37 Using U. rhynchophylla Protoplasts
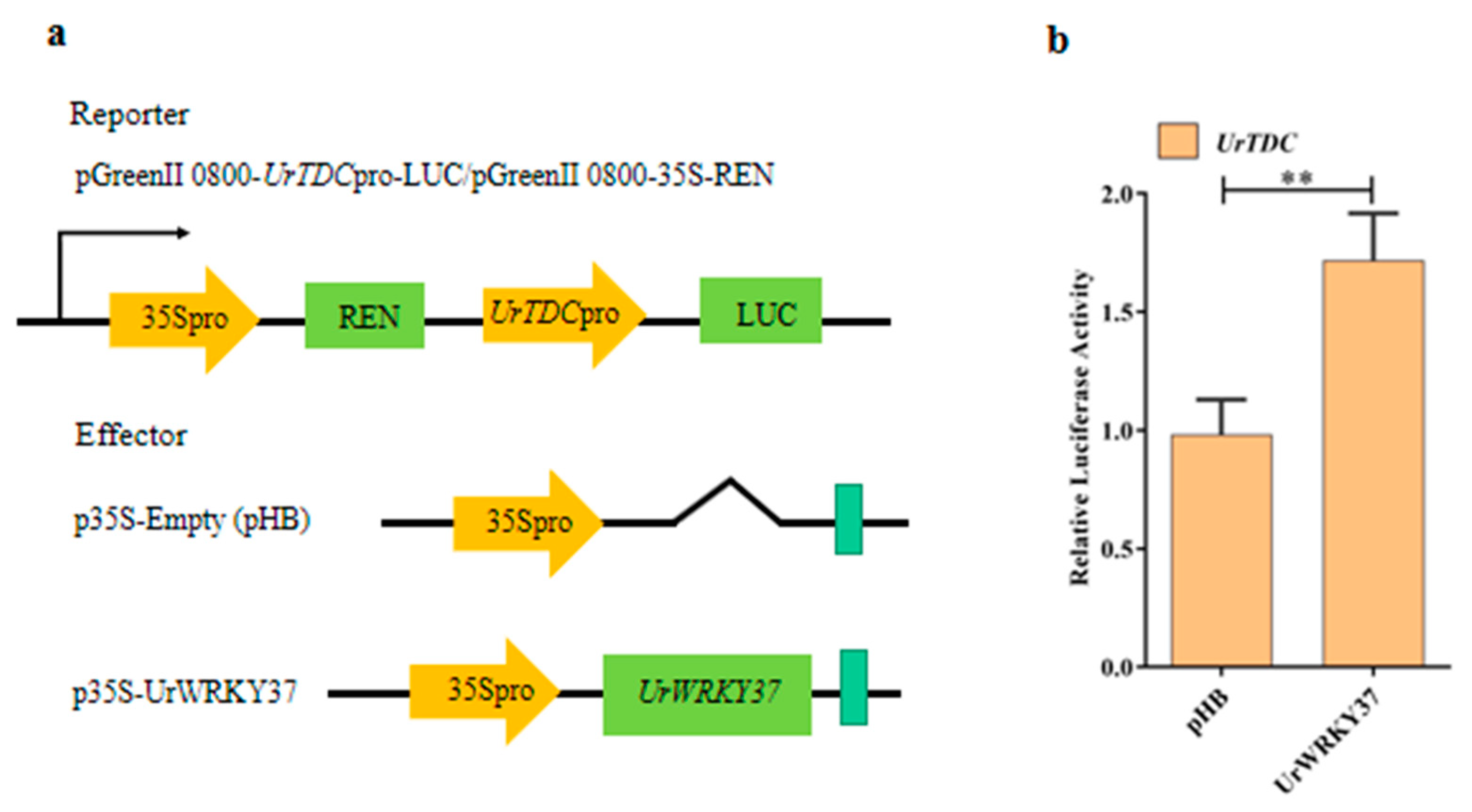
2.4. Dual-Luciferase (Dual-LUC) Assays
3. Discussion
3.1. Factors Influencing the Isolation of Protoplasts in U. rhynchophylla
3.2. Factors Influencing the Transformation Efficiency of Protoplasts in U. rhynchophylla
3.3. Utilization and Validation of the Viability of the Protoplast System of U. rhynchophylla
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Plasmid Construction
4.3. Preparation of Enzyme Solution and Prefabricated Solution
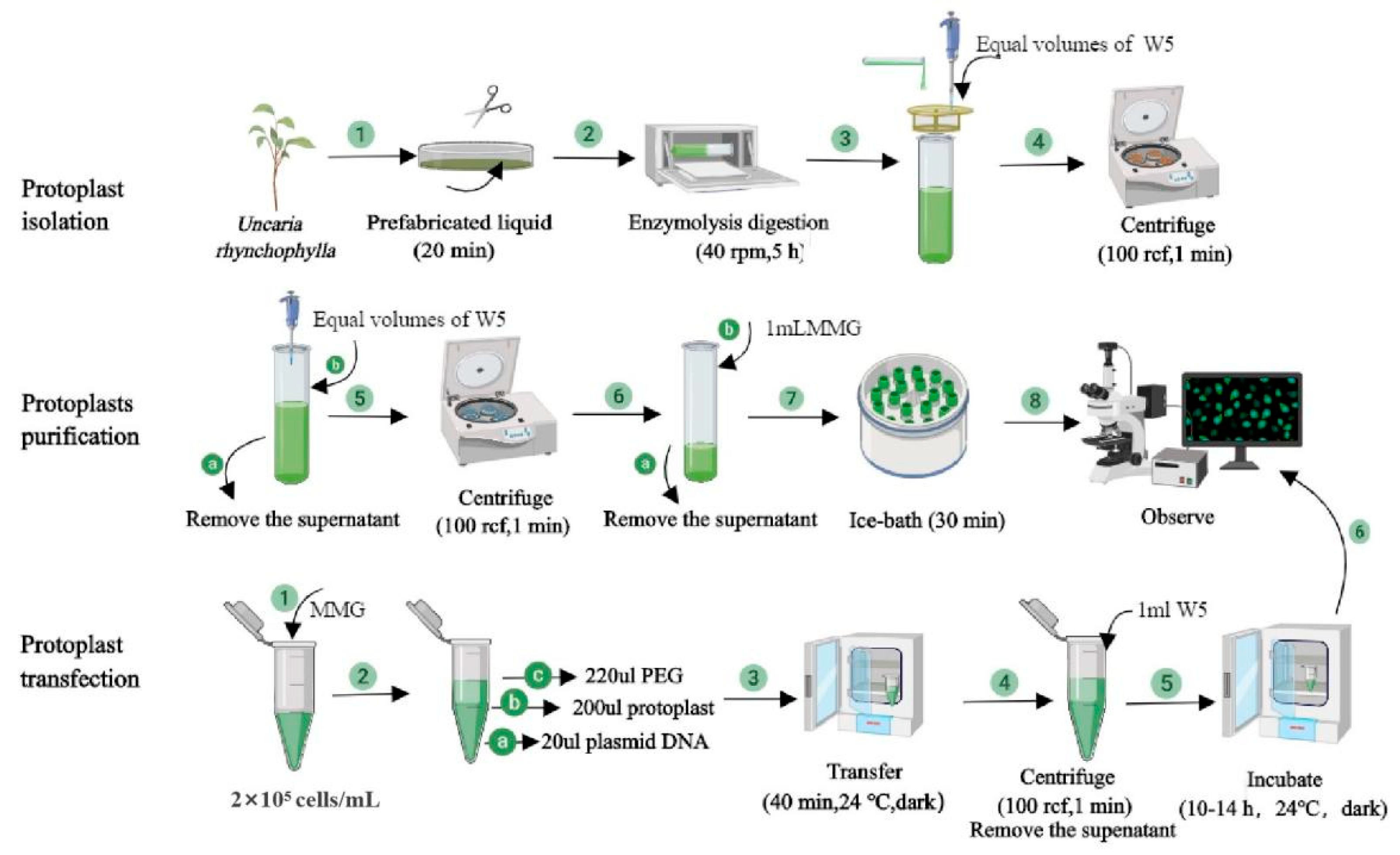
4.4. Protoplast Isolation
4.5. Protoplast Transformation
4.6. Protoplast Yield and Viability
4.7. Subcellular Localization
4.8. Dual-Luciferase Assays
4.9. Microscopy and Bioimage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kang, H.; Naing, A.H.; Park, S.K.; Chung, M.Y.; Kim, C.K. Protoplast isolation and transient gene expression in different petunia cultivars. Protoplasma 2022, 260, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Gao, J.; Lu, C.; Wei, Y.; Jin, J.; Wong, S.-M.; Zhu, G.; Yang, F. Highly efficient protoplast isolation and transient expression system for functional characterization of flowering related genes in Cymbidium Orchids. Int. J. Mol. Sci. 2020, 21, 2264. [Google Scholar] [CrossRef]
- Bai, L.; Cheng, Y.; She, J.; He, Z.; Liu, H.; Zhang, G.; Cao, R.; Chen, Y. Development of an efficient protoplast isolation and transfection system for castor bean (Ricinus communis L.). Plant Cell Tissue Organ Cult. (PCTOC) 2020, 143, 457–464. [Google Scholar] [CrossRef]
- Jo, L.; Pelletier, J.M.; Hsu, S.W.; Baden, R.; Goldberg, R.B.; Harada, J.J. Combinatorial interactions of the LEC1 transcription factor specify diverse developmental programs during soybean seed development. Proc. Natl. Acad. Sci. USA 2020, 117, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Kohlenbach, H. Differentiation of alkaloid cells in cultures of Macleaya mesophyll protoplasts. Planta Med. 1982, 46, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.T.; Wink, M. Uptake of alkaloids by latex vesicles and isolated mesophyll vacuoles of Chelidonium ntajus (Papaveraceae). Z. Naturforsch. C 1990, 45, 949–957. [Google Scholar] [CrossRef]
- Xu, Y.; Li, R.; Luo, H.; Wang, Z.; Li, M.W.; Lam, H.M.; Huang, C. Protoplasts: Small cells with big roles in plant biology. Trends Plant Sci. 2022, 27, 828–829. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, J.C.; Fang, S.C. A protoplast transient expression system to enable molecular, cellular, and functional studies in Phalaenopsis orchids. Front. Plant Sci. 2018, 9, 843. [Google Scholar] [CrossRef]
- Roest, S.; Gilissen, L.J.W. Plant regeneration from protoplasts: A literature review. Acta Bot. Neerl. 1989, 38, 1–23. [Google Scholar] [CrossRef]
- Charlot, F.; Goudounet, G.; Nogué, F.; Perroud, P.F. Physcomitrium patens protoplasting and protoplast transfection. Methods Mol. Biol. 2022, 2464, 3–19. [Google Scholar] [CrossRef]
- Chen, W.H.; Davey, M.R.; Power, J.B.; Cocking, E.C. Sugarcane protoplasts: Factors affecting division and plant regeneration. Plant Cell Rep. 1988, 7, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Lü, T.; Yang, X.; Liu, J.; Dong, Y.; Wang, Y. An efficient and universal protoplast isolation protocol suitable for transient gene expression analysis and single-cell RNA sequencing. Int. J. Mol. Sci. 2022, 23, 3419. [Google Scholar] [CrossRef]
- Berthold, F.; Roujol, D.; Hemmer, C.; Jamet, E.; Ritzenthaler, C.; Hoffmann, L.; Schmitt-Keichinger, C. Inside or outside? A new collection of Gateway vectors allowing plant protein subcellular localization or over-expression. Plasmid 2019, 105, 102436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.a.; Dong, Y.; Li, D.; Shi, S.; Li, S.; Li, L.; He, Y.; Li, J.; Chen, H.; et al. A highly efficient mesophyll protoplast isolation and PEG-mediated transient expression system in eggplant. Sci. Hortic. 2022, 304, 111303. [Google Scholar] [CrossRef]
- Adedeji, O.S.; Naing, A.H.; Kang, H.; Chung, M.Y.; Lim, K.B.; Kim, C.K. Optimization of protocol for efficient protoplast isolation and transient gene expression in carnation. Sci. Hortic. 2022, 299, 111057. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Petersen, W.L.; Buchholz, W.G.; Bowen, B.A.; Sulc, S.L. Factors affecting PEG-mediated stable transformation of maize protoplasts. Plant Cell Rep. 1990, 9, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, X.; Zhou, S.; Liu, S.; Jiang, L.; Wang, G. Efficient protoplast isolation and transient gene expression system for Phalaenopsis hybrid cultivar ‘Ruili Beauty’. In Vitro Cell. Dev. Biol. Plant 2018, 54, 87–93. [Google Scholar] [CrossRef]
- Li, R.; Cheng, J.; Jiao, M.; Li, L.; Guo, C.; Chen, S.; Liu, A. New phenylpropanoid-substituted flavan-3-ols and flavonols from the leaves of Uncaria rhynchophylla. Fitoterapia 2017, 116, 17–23. [Google Scholar] [CrossRef]
- Fujiwara, H.; Iwasaki, K.; Furukawa, K.; Seki, T.; He, M.; Maruyama, M.; Tomita, N.; Kudo, Y.; Higuchi, M.; Saido, T.C.; et al. Uncaria rhynchophylla, a Chinese medicinal herb, has potent antiaggregation effects on Alzheimer′s β-amyloid proteins. J. Neurosci. Res. 2006, 84, 427–433. [Google Scholar] [CrossRef]
- Ndagijimana, A.; Wang, X.; Pan, G.; Zhang, F.; Feng, H.; Olaleye, O. A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia 2013, 86, 35–47. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S. Antihypertensive and neuroprotective activities of rhynchophylline: The role of rhynchophylline in neurotransmission and ion channel activity. J. Ethnopharmacol. 2010, 132, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Godbole, R.C.; Pable, A.A.; Singh, S.; Barvkar, V.T. Interplay of transcription factors orchestrating the biosynthesis of plant alkaloids. 3 Biotech 2022, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Broun, P. Transcription factors as tools for metabolic engineering in plants. Curr. Opin. Plant Biol. 2004, 7, 202–209. [Google Scholar] [CrossRef]
- Jiang, W.; Fu, X.; Pan, Q.; Tang, Y.; Shen, Q.; Lv, Z.; Yan, T.; Shi, P.; Li, L.; Zhang, L.; et al. Overexpression of AaWRKY1 leads to an enhanced content of artemisinin in Artemisia annua. BioMed Res. Int. 2016, 2016, 7314971. [Google Scholar] [CrossRef]
- Wang, C.; Hao, X.; Wang, Y.; Maoz, I.; Zhou, W.; Zhou, Z.; Kai, G. Identification of WRKY transcription factors involved in regulating the biosynthesis of the anti-cancer drug camptothecin in Ophiorrhiza pumila. Hortic. Res. 2022, 9, uhac099. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Cheng, J.; Zhao, W.; Li, X.; Wang, H.; Zhang, Z.; Sui, X. An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Sci. Hortic. 2013, 150, 206–212. [Google Scholar] [CrossRef]
- Maddumage, R.; Fung, R.M.W.; Weir, I.; Ding, H.; Simons, J.L.; Allan, A.C. Efficient transient transformation of suspension culture-derived apple protoplasts. Plant Cell Tissue Organ Cult. 2002, 70, 77–82. [Google Scholar] [CrossRef]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef]
- Wu, J.Z.; Liu, Q.; Geng, X.S.; Li, K.M.; Luo, L.J.; Liu, J.P. Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz). BMC Biotechnol. 2017, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Huo, A.; Chen, Z.; Wang, P.; Yang, L.; Wang, G.; Wang, D.; Liao, S.; Cheng, T.; Chen, J.; Shi, J. Establishment of transient gene expression systems in protoplasts from Liriodendron hybrid mesophyll cells. PLoS ONE 2017, 12, e0172475. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tao, L.; Zeng, L.; Vega-Sanchez, M.E.; Umemura, K.; Wang, G.L. A highly efficient transient protoplast system for analyzing defence gene expression and protein?protein interactions in rice. Mol. Plant Pathol. 2006, 7, 417–427. [Google Scholar] [CrossRef]
- Ma, X.; Kexin, Z.; Yonggang, W.; Ebadi, A.G.; Toughani, M. Optimization of low-temperature lipase production conditions and study on enzymatic properties of Aspergillus Niger. Iran. J. Chem. Chem. Eng. 2021, 40, 1364–1374. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, Y.; Zhu, H.; Wang, Z.; Lin, J.; Liu, L.; Tang, K. Protoplast formation, regeneration and transformation from the taxol-producing fungus Ozonium sp. Afr. J. Biotechnol. 2008, 7, 2017–2024. [Google Scholar] [CrossRef]
- Cannon, A.E.; Sabharwal, T.; Roux, S.J. Spore preparation and protoplast isolation to study gravity perception and response in Ceratopteris richardii. Methods Mol. Biol. 2022, 2368, 53–60. [Google Scholar] [CrossRef]
- Klimek-Chodacka, M.; Kadluczka, D.; Lukasiewicz, A.; Malec-Pala, A.; Baranski, R.; Grzebelus, E. Effective callus induction and plant regeneration in callus and protoplast cultures of Nigella damascena L. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 143, 693–707. [Google Scholar] [CrossRef]
- Pitzschke, A.; Persak, H. Poinsettia protoplasts—a simple, robust and efficient system for transient gene expression studies. Plant Methods 2012, 8, 14. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Bae, S.; Sung, C.; Choi, S.R.; Lee, G.J.; Lim, Y.P. Optimization of protoplast isolation from leaf mesophylls of Chinese cabbage (Brassica rapa ssp. pekinensis) and subsequent transfection with a binary vector. Plants 2021, 10, 2636. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Hanzawa, Y. A simple method for isolation of soybean protoplasts and application to transient gene expression analyses. J. Vis. Exp. 2018, 131, e57258. [Google Scholar] [CrossRef]
- Page, M.T.; Parry, M.A.J.; Carmo-Silva, E. A high-throughput transient expression system for rice. Plant Cell Environ. 2019, 42, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Berges, T.; Barreau, C. Heat shock at an elevated temperature improves transformation efficiency of protoplasts from Podospora anserina. J. Gen. Microbiol. 1989, 135, 601–604. [Google Scholar] [CrossRef] [PubMed]
| Reagent | Amount |
|---|---|
| KH2PO4 | 54.4 mg |
| KNO3 | 202.0 mg |
| CaCl22H2O | 2960.0 mg |
| MgSO4 | 492 mg |
| KI | 0.32 mg |
| CuSO4 | 0.05 mg |
| ddH2O | Up to 1 L |
| Reagent | Final Concentration | Amount |
|---|---|---|
| Cellulase R-10 | 1.25% | 0.125 g |
| Macerozyme R-10 | 0.60% | 0.6 g |
| D-mannitol | 0.6 M | 1.092 g |
| 2-CPW | - | 500 μL |
| 5 mM MES | 5 mM | 1 mL |
| DEPC-treated water | 5 mL | |
| 55 °C water bath pot | Heating the solution at 55 °C for 10 min and then cool it to room temperature | |
| 5 M CaCl2 | 2.5 M | 500 μL |
| 1% BSA | 0.10% | 1 mL |
| DEPC treated water | Up to 10 mL and the PH of the enzyme solution was 5.8 | |
| Reagent | Final Concentration | Amount |
|---|---|---|
| 2 M KCl | 5 mM | 0.125 mL |
| 1 M CaCl2 | 125 mM | 6.25 mL |
| 0.5 M NaCl | 154 mM | 15.4 mL |
| 0.2 M MES | 2 mM | 0.5 mL |
| ddH2O | up to 50 mL |
| Reagent | Final Concentration | Amount |
|---|---|---|
| 0.8 M D-mannitol | 0.6 M | 3.75 mL |
| 2 M MgCl2 | 15 mM | 37.5 mL |
| 0.2 M MES | 4 mM | 0.1 mL |
| ddH2O | - | up to 5 mL |
| Reagent | Final Concentration | Amount |
|---|---|---|
| PEG4000 | 40% (m/v) | 0.4 g |
| 0.8 M D-mannitol | 0.2 M | 250 μL |
| 1 M CaCl2 | 0.2 M | 200 μL |
| ddH2O | - | up to 1 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Mu, D.; Pan, L.; Wilson, I.W.; Zheng, Y.; Zhu, L.; Lu, Z.; Wan, L.; Fu, J.; Wei, S.; et al. Optimization of Isolation and Transformation of Protoplasts from Uncaria rhynchophylla and Its Application to Transient Gene Expression Analysis. Int. J. Mol. Sci. 2023, 24, 3633. https://doi.org/10.3390/ijms24043633
Shao Y, Mu D, Pan L, Wilson IW, Zheng Y, Zhu L, Lu Z, Wan L, Fu J, Wei S, et al. Optimization of Isolation and Transformation of Protoplasts from Uncaria rhynchophylla and Its Application to Transient Gene Expression Analysis. International Journal of Molecular Sciences. 2023; 24(4):3633. https://doi.org/10.3390/ijms24043633
Chicago/Turabian StyleShao, Yingying, Detian Mu, Limei Pan, Iain W. Wilson, Yajie Zheng, Lina Zhu, Zhiguo Lu, Lingyun Wan, Jine Fu, Shugen Wei, and et al. 2023. "Optimization of Isolation and Transformation of Protoplasts from Uncaria rhynchophylla and Its Application to Transient Gene Expression Analysis" International Journal of Molecular Sciences 24, no. 4: 3633. https://doi.org/10.3390/ijms24043633
APA StyleShao, Y., Mu, D., Pan, L., Wilson, I. W., Zheng, Y., Zhu, L., Lu, Z., Wan, L., Fu, J., Wei, S., Song, L., Qiu, D., & Tang, Q. (2023). Optimization of Isolation and Transformation of Protoplasts from Uncaria rhynchophylla and Its Application to Transient Gene Expression Analysis. International Journal of Molecular Sciences, 24(4), 3633. https://doi.org/10.3390/ijms24043633






