The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy
Abstract
:1. Introduction
2. Results
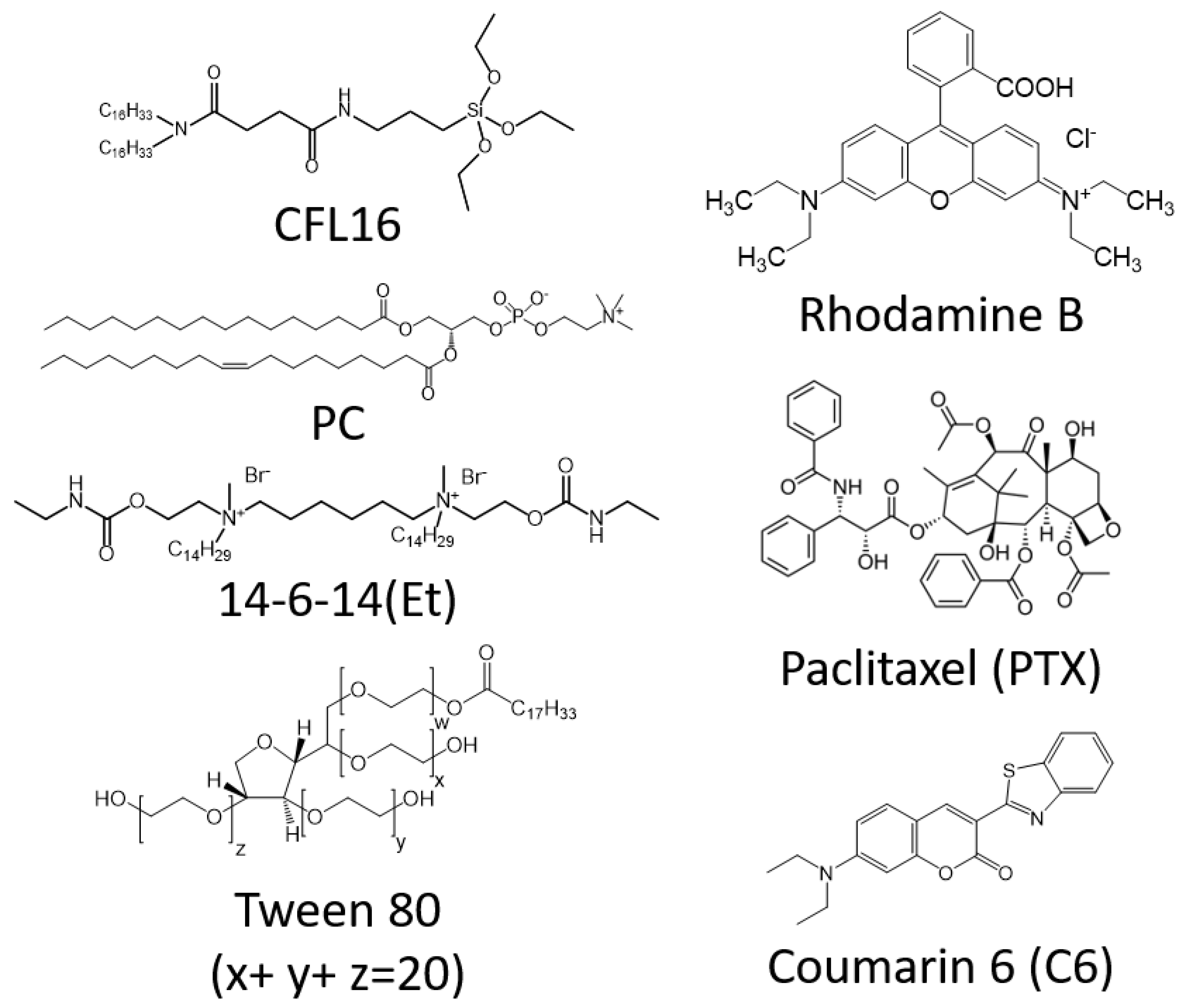
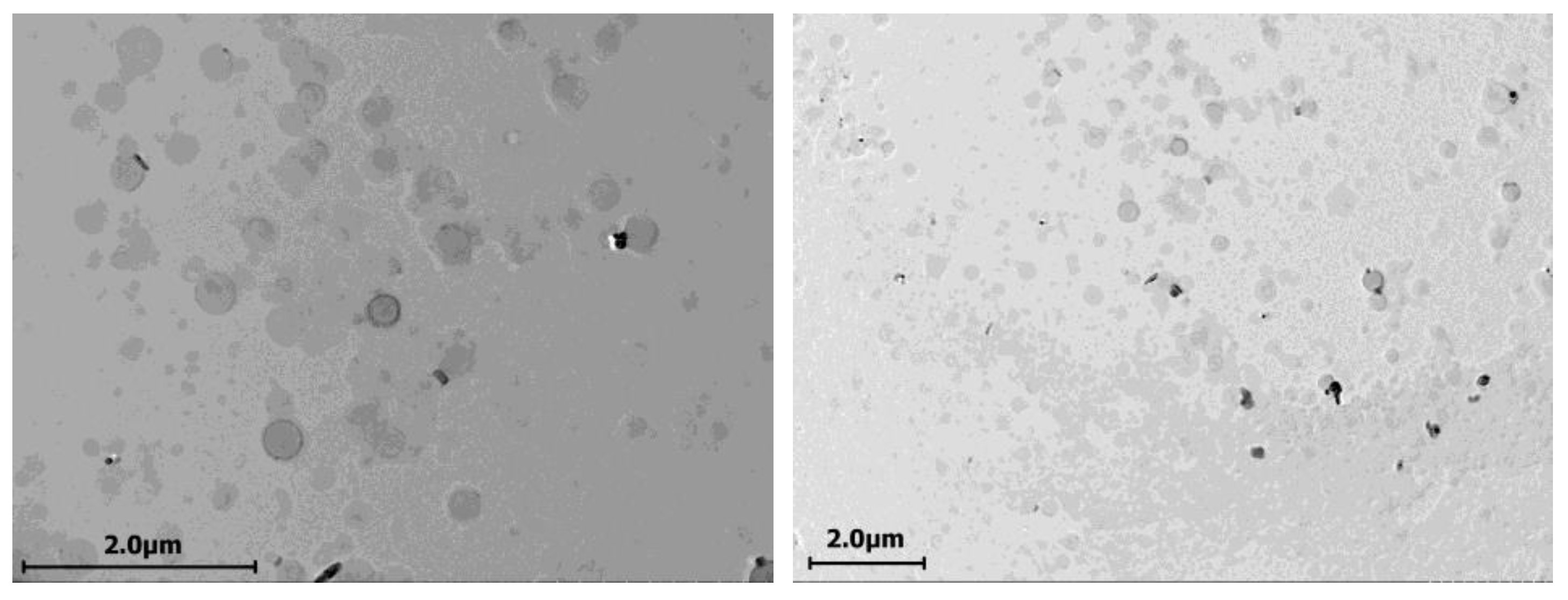
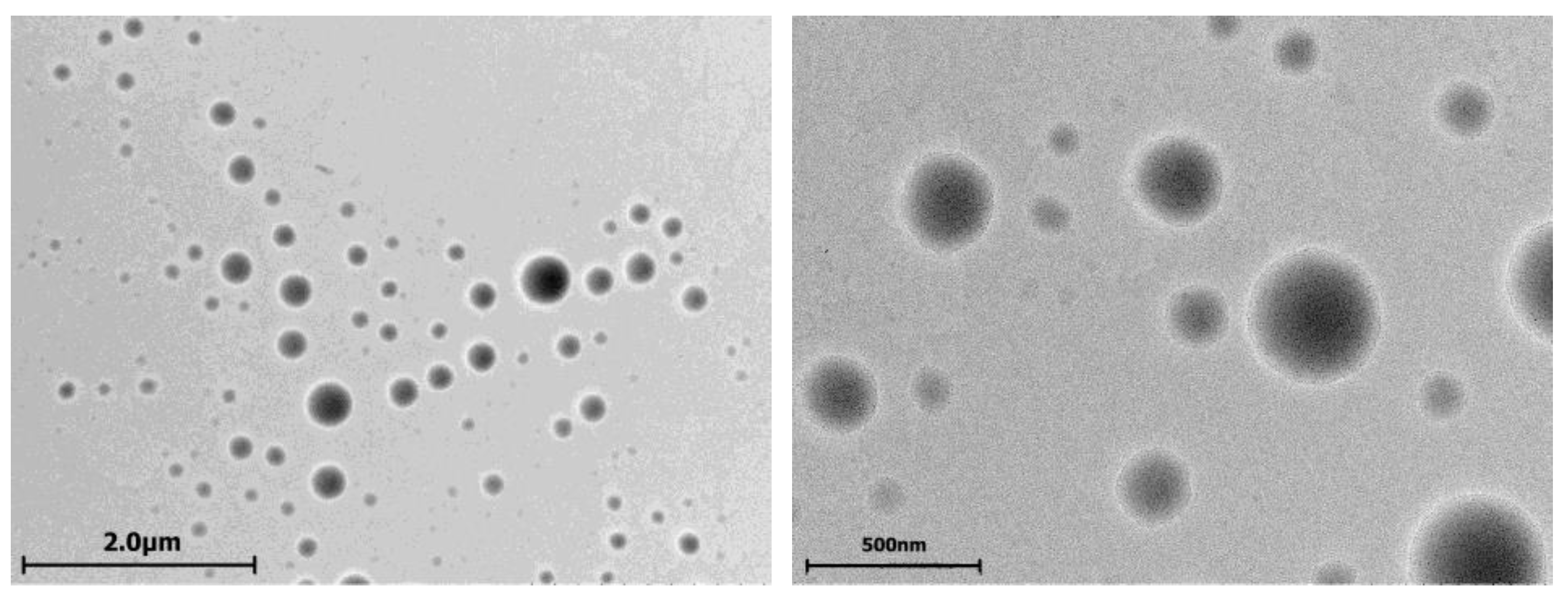
2.1. Cerasomes Obtained via Ethanol Sol Injection Method
2.2. Cerasomes Obtained via Thin Film Method
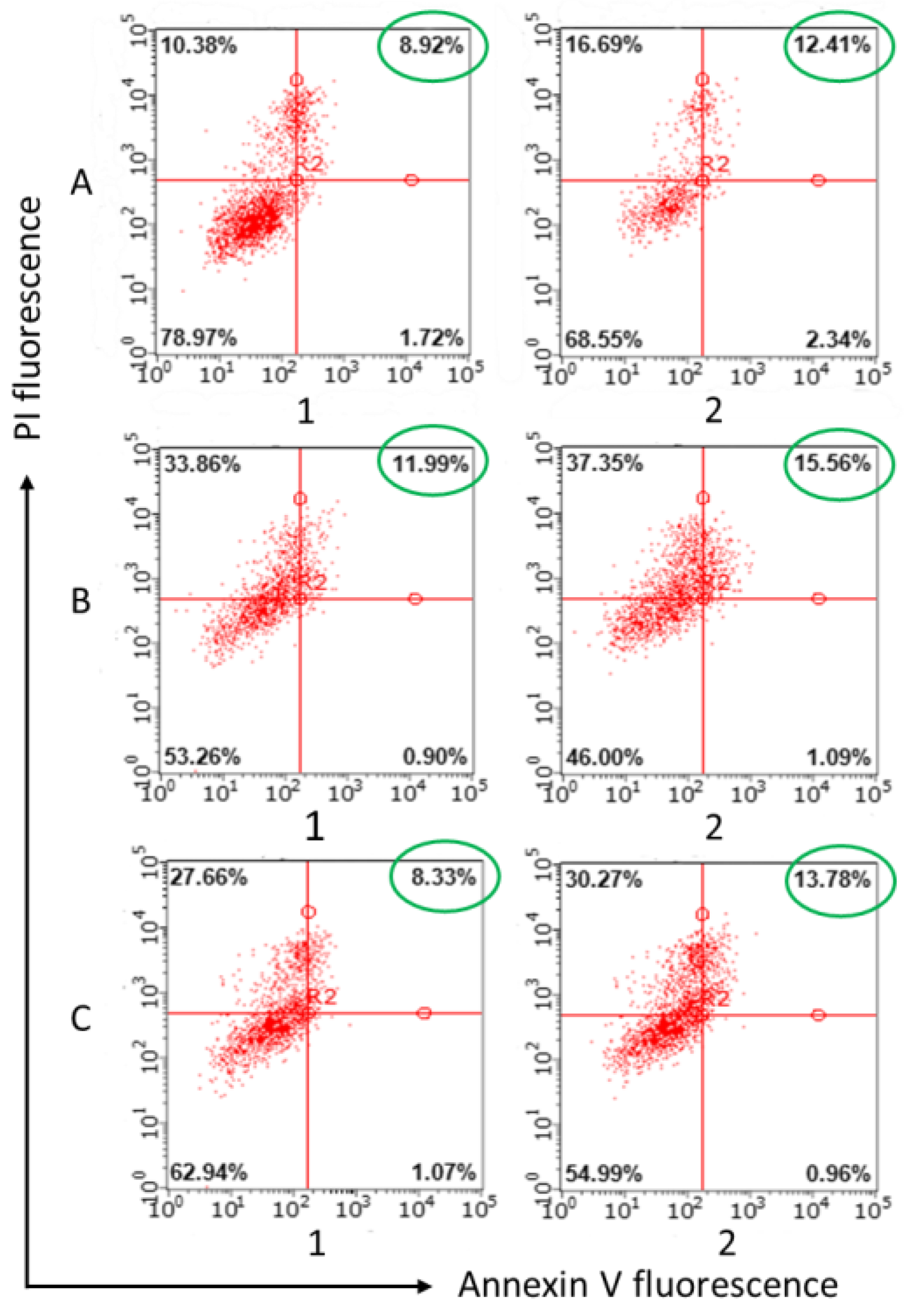
2.3. Evaluation of Cerasome Toxicity
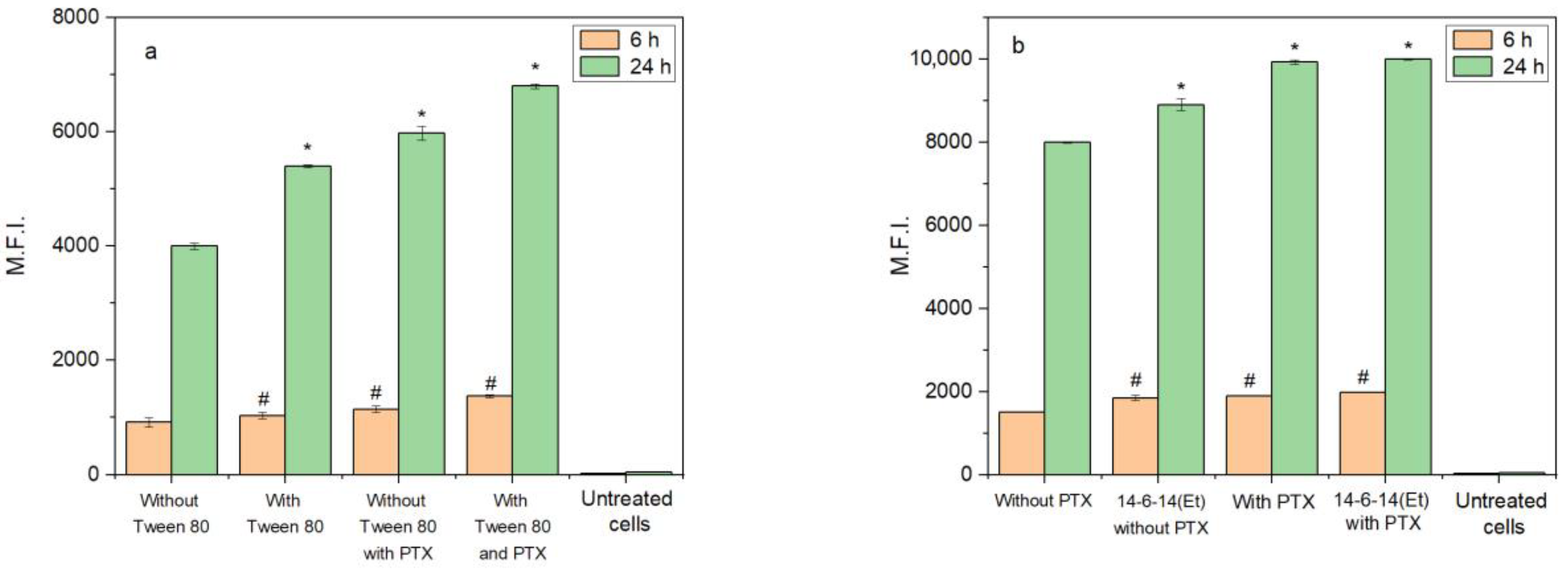
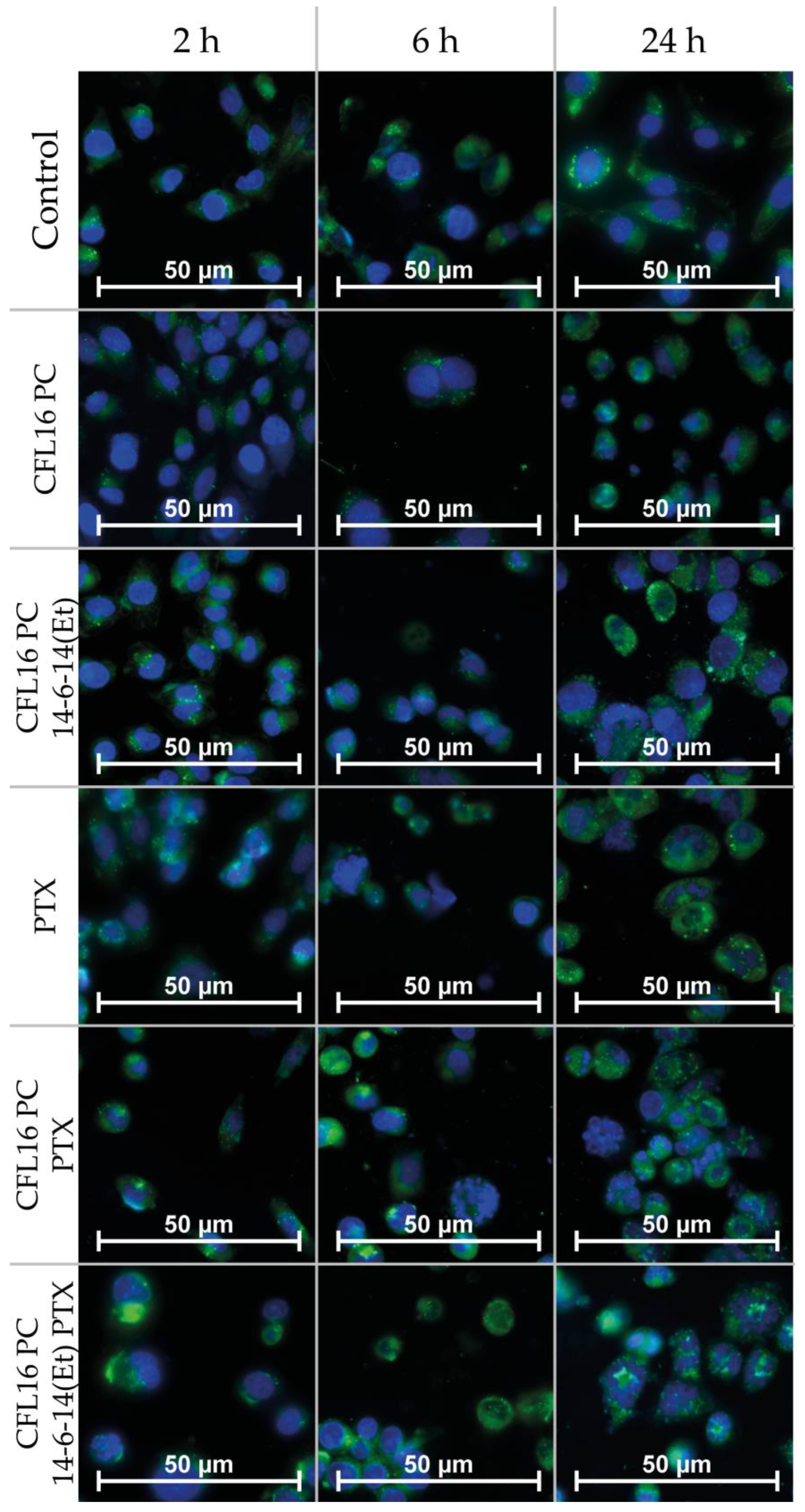
2.4. Cerasome Uptake by T98G Cells
2.5. Analysis of Cerasome Compatibility with Blood
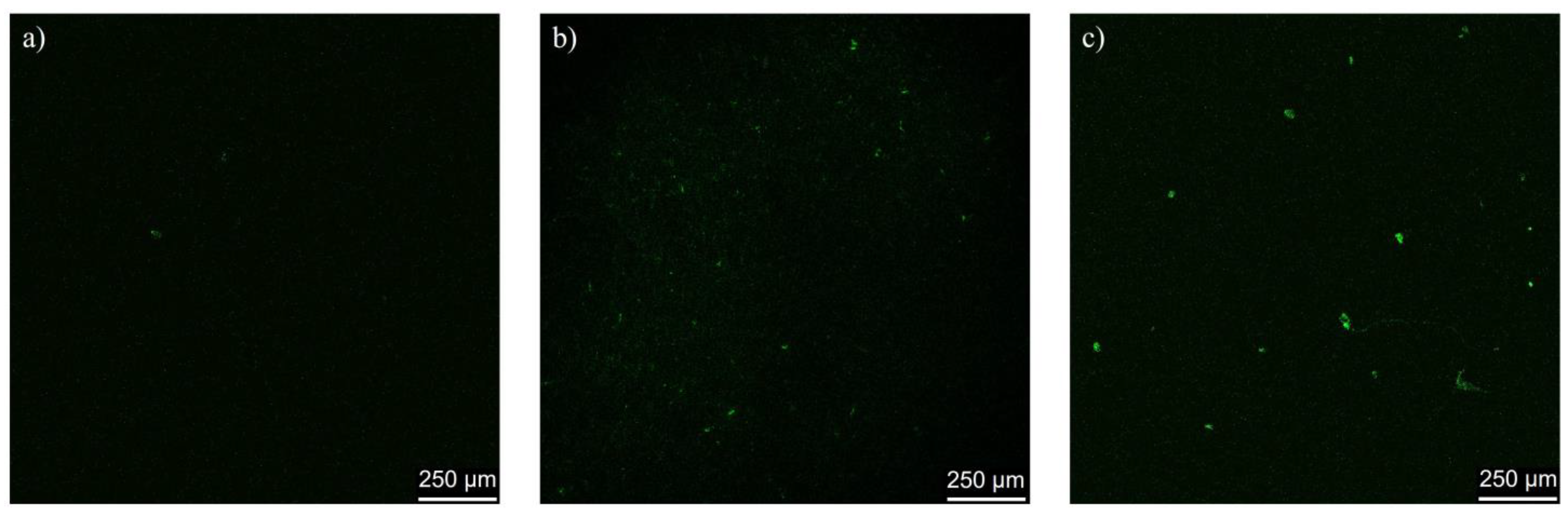
2.6. In Vivo Assessment of Cerasome Ability to Cross the BBB
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cerasome Preparation
4.3. Cerasome Characterization
4.4. Cell Culture
4.5. Cytotoxicity Assessment via MTT-Assay
4.6. Induction of Apoptotic Effects
4.7. Flow Cytometry Measurement of Cell Association
4.8. Fluorescence Microscopy
4.9. In Vivo Analysis of BBB Penetration Using Confocal Microscopy of Brain Slises
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laffleur, F.; Keckeis, V. Advances in Drug Delivery Systems: Work in Progress Still Needed? Int. J. Pharm. 2020, 590, 119912. [Google Scholar] [CrossRef] [PubMed]
- Rudokas, M.; Najlah, M.; Alhnan, M.A.; Elhissi, A. Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications. Med. Princ. Pract. 2016, 25, 60–72. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-responsive Liposomes for Drug Delivery. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Riaz, M.; Zhang, X.; Lin, C.; Wong, K.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef]
- Mojarad-Jabali, S.; Farshbaf, M.; Walker, P.R.; Hemmati, S.; Fatahi, Y.; Zakeri-Milani, P.; Sarfraz, M.; Valizadeh, H. An Update on Actively Targeted Liposomes in Advanced Drug Delivery to Glioma. Int. J. Pharm. 2021, 602, 120645. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-Based Drug Co-Delivery Systems in Cancer Cells. Mater. Sci. Eng. C 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-Niosomes as Nanoscale Drug Delivery Systems: An Illustrated Review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Benson, H.A. Transfersomes for Transdermal Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737. [Google Scholar] [CrossRef]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembling Drug Formulations with Tunable Permeability and Biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef]
- Hameed, S.; Bhattarai, P.; Dai, Z. Cerasomes and Bicelles: Hybrid Bilayered Nanostructures with Silica-like Surface in Cancer Theranostics. Front. Chem. 2018, 6, 127. [Google Scholar] [CrossRef]
- Barbosa-Barros, L.; Rodríguez, G.; Barba, C.; Cócera, M.; Rubio, L.; Estelrich, J.; López-Iglesias, C.; de la Maza, A.; López, O. Bicelles: Lipid Nanostructured Platforms with Potential Dermal Applications. Small 2012, 8, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Ibragimova, A.; Pavlov, R.; Gabdrakhmanov, D.; Kashapova, N.; Burilova, E.; Zakharova, L.; Sinyashin, O. Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles. Int. J. Mol. Sci. 2021, 22, 7055. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Dai, Z. Recent Advances in Liposomal Nanohybrid Cerasomes as Promising Drug Nanocarriers. Adv. Colloid Interface Sci. 2014, 207, 32–42. [Google Scholar] [CrossRef]
- Zhang, D.; Culver, H.R.; Bowman, C.N. Hybrid Cerasomes Composed of Phosphatidylcholines and Silica Networks for the Construction of Vesicular Materials with Functionalized Shells. ACS Appl. Nano Mater. 2019, 2, 7549–7558. [Google Scholar] [CrossRef]
- Ma, X.; Yao, M.; Shi, J.; Li, X.; Gao, Y.; Luo, Q.; Hou, R.; Liang, X.; Wang, F. High Intensity Focused Ultrasound-Responsive and Ultrastable Cerasomal Perfluorocarbon Nanodroplets for Alleviating Tumor Multidrug Resistance and Epithelial–Mesenchymal Transition. ACS Nano 2020, 14, 15904–15918. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Hashizume, M.; Ariga, K.; Terashima, T.; Kikuchi, J.I. Preparation and Characterization of a Novel Organic-Inorganic Nanohybrid “Cerasome” Formed with a Liposomal Membrane and Silicate Surface. Chem.-Eur. J. 2007, 13, 5272–5281. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Pan, H.; Dai, Z. Liposomal Nanohybrid Cerasomes for Controlled Insulin Release. RSC Adv. 2014, 4, 42808–42815. [Google Scholar] [CrossRef]
- Gileva, A.; Sarychev, G.; Kondrya, U.; Mironova, M.; Sapach, A.; Selina, O.; Budanova, U.; Burov, S.; Sebyakin, Y.; Markvicheva, E. Lipoamino Acid-Based Cerasomes for Doxorubicin Delivery: Preparation and In Vitro Evaluation. Mater. Sci. Eng. C 2019, 100, 724–734. [Google Scholar] [CrossRef]
- Kawataki, T.; Yasuhara, K.; Kikuchi, J. Remarkable Long-Term Stability of Cerasome as an Organic–Inorganic Hybrid Nanocontainer for Water-Soluble Macromolecules. Chem. Lett. 2011, 40, 461–463. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, X.; Tong, S.; Bao, G.; Ren, Q.; Dai, Z. Gold Nanoshell Nanomicelles for Potential Magnetic Resonance Imaging, Light-Triggered Drug Release, and Photothermal Therapy. Adv. Funct. Mater. 2013, 23, 815–822. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Jing, L.; Xue, P.; Jiang, L.; Ren, Q.; Dai, Z. Design and Synthesis of Lipidic Organoalkoxysilanes for the Self-Assembly of Liposomal Nanohybrid Cerasomes with Controlled Drug Release Properties. Chem.-Eur. J. 2013, 19, 16113–16121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, L.; Xing, J.; Jalde, S.; Li, Y.; Cai, J.; Chen, J.; Liu, P.; Gu, N.; Ji, M. Redox Responsive Liposomal Nanohybrid Cerasomes for Intracellular Drug Delivery. Colloids Surf. B Biointerfaces 2016, 148, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, X.; Yue, X.; Dai, Z. Conjugation of Porphyrin to Nanohybrid Cerasomes for Photodynamic Diagnosis and Therapy of Cancer. Angew. Chem.-Int. Ed. 2011, 50, 11622–11627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Liao, H.; Song, X.; Wu, H.; Wang, H.; Shen, H.; Ma, X.; Tan, M. Liposomal Nanohybrid Cerasomes for Mitochondria-Targeted Drug Delivery. J. Mater. Chem. B 2015, 3, 7291–7299. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yin, G.; Pu, X.; Huang, Z.; Liao, X.; Chen, X. A Novel Tumor-Targeted Thermosensitive Liposomal Cerasome Used for Thermally Controlled Drug Release. Int. J. Pharm. 2019, 570, 118660. [Google Scholar] [CrossRef]
- Li, Y.; Du, Y.; Liang, X.; Sun, T.; Xue, H.; Tian, J.; Jin, Z. EGFR-Targeted Liposomal Nanohybrid Cerasomes: Theranostic Function and Immune Checkpoint Inhibition in a Mouse Model of Colorectal Cancer. Nanoscale 2018, 10, 16738–16749. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, S.; Liang, X.; Jin, Y.; Wu, Y.; Bai, H.; Liu, R.; Dai, Z.; Liang, Z.; Shi, T. Doping Hydroxylated Cationic Lipid into PEGylated Cerasome Boosts In Vivo SiRNA Transfection Efficacy. Bioconjug. Chem. 2014, 25, 2055–2066. [Google Scholar] [CrossRef]
- Liang, X.; Gao, J.; Jiang, L.; Luo, J.; Jing, L.; Li, X.; Jin, Y.; Dai, Z. Nanohybrid Liposomal Cerasomes with Good Physiological Stability and Rapid Temperature Responsiveness for High Intensity Focused Ultrasound Triggered Local Chemotherapy of Cancer. ACS Nano 2015, 9, 1280–1293. [Google Scholar] [CrossRef]
- Leung, S.L.; Zha, Z.; Teng, W.; Cohn, C.; Dai, Z.; Wu, X. Organic–Inorganic Nanovesicles for Doxorubicin Storage and Release. Soft Matter 2012, 8, 5756. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Sun, Y.; Wang, P.; Zhang, J.; Du, W.; Wang, S.; Liang, X. Bubble-Manipulated Local Drug Release from a Smart Thermosensitive Cerasome for Dual-Mode Imaging Guided Tumor Chemo-Photothermal Therapy. Theranostics 2019, 9, 8138–8154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized Delivery of Curcumin into Brain with Polysorbate 80-Modified Cerasomes by Ultrasound-Targeted Microbubble Destruction for Improved Parkinson’s Disease Therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef] [PubMed]
- Parvaz, S.; Taheri-Ledari, R.; Esmaeili, M.S.; Rabbani, M.; Maleki, A. A Brief Survey on the Advanced Brain Drug Administration by Nanoscale Carriers: With a Particular Focus on AChE Reactivators. Life Sci. 2020, 240, 117099. [Google Scholar] [CrossRef]
- Kreuter, J.; Ramge, P.; Petrov, V.; Hamm, S.; Gelperina, S.E.; Engelhardt, B.; Alyautdin, R.; Von Briesen, H.; Begley, D.J. Direct Evidence That Polysorbate-80-Coated Poly(Butylcyanoacrylate) Nanoparticles Deliver Drugs to the CNS via Specific Mechanisms Requiring Prior Binding of Drug to the Nanoparticles. Pharm. Res. 2003, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.H.; Lin, X.N.; Wei, F.; Feng, W.; Huang, Z.C.; Wang, P.; Ren, L.; Diao, Y. Enhanced Brain Targeting of Temozolomide in Polysorbate-80 Coated Polybutylcyanoacrylate Nanoparticles. Int. J. Nanomed. 2011, 6, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Koffie, R.M.; Farrar, C.T.; Saidi, L.-J.; William, C.M.; Hyman, B.T.; Spires-Jones, T.L. Nanoparticles Enhance Brain Delivery of Blood–Brain Barrier-Impermeable Probes for In Vivo Optical and Magnetic Resonance Imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 18837–18842. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.H. T98G: An Anchorage-Independent Human Tumor Cell Line That Exhibits Stationary Phase G1 Arrest In Vitro. J. Cell. Physiol. 1979, 99, 43–54. [Google Scholar] [CrossRef]
- Kriel, J.; Müller-Nedebock, K.; Maarman, G.; Mbizana, S.; Ojuka, E.; Klumperman, B.; Loos, B. Coordinated Autophagy Modulation Overcomes Glioblastoma Chemoresistance through Disruption of Mitochondrial Bioenergetics. Sci. Rep. 2018, 8, 10348. [Google Scholar] [CrossRef]
- Krakstad, C.; Chekenya, M. Survival Signalling and Apoptosis Resistance in Glioblastomas: Opportunities for Targeted Therapeutics. Mol. Cancer 2010, 9, 135. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Hamasaki, R.; Ariga, K.; Kikuchi, J.I. Preparation and Surface Modification of Novel Vesicular Nano-Particle “Cerasome” with Liposomal Bilayer and Silicate Surface. J. Sol-Gel Sci. Technol. 2003, 26, 393–396. [Google Scholar] [CrossRef]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, R.V.; Gaynanova, G.A.; Kuznetsov, D.M.; Ivanov, Y.A.; Amerkhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Zakharova, L.Y. A Study Involving PC-3 Cancer Cells and Novel Carbamate Gemini Surfactants: Is Zeta Potential the Key to Control Adhesion to Cells? Smart Mater. Med. 2023, 4, 123–133. [Google Scholar] [CrossRef]
- Onishchenko, N.; Tretiakova, D.; Vodovozova, E. Spotlight on the Protein Corona of Liposomes. Acta Biomater. 2021, 134, 57–78. [Google Scholar] [CrossRef]
- Kemper, E.M.; van Zandbergen, A.E.; Cleypool, C.; Mos, H.A.; Boogerd, W.; Beijnen, J.H.; van Tellingen, O. Increased Penetration of Paclitaxel into the Brain by Inhibition of P-Glycoprotein1. Clin. Cancer Res. 2003, 9, 2849–2855. [Google Scholar] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Eytan, G.D.; Regev, R.; Oren, G.; Hurwitz, C.D.; Assaraf, Y.G. Efficiency of P-Glycoprotein-Mediated Exclusion of Rhodamine Dyes from Multidrug-Resistant Cells Is Determined by Their Passive Transmembrane Movement Rate. Eur. J. Biochem. 1997, 248, 104–112. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Eckersley, R. Statistics for Biomedical Engineers and Scientists; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-08-102939-8. [Google Scholar]
- Sharipova, R.R.; Belenok, M.G.; Garifullin, B.F.; Sapunova, A.S.; Voloshina, A.D.; Andreeva, O.V.; Strobykina, I.Y.; Skvortsova, P.V.; Zuev, Y.F.; Kataev, V.E. Synthesis and Anti-Cancer Activities of Glycosides and Glycoconjugates of Diterpenoid Isosteviol. MedChemComm 2019, 10, 1488–1498. [Google Scholar] [CrossRef]
- Banerjee, N.; Sengupta, S.; Roy, A.; Ghosh, P.; Das, K.; Das, S. Functional Alteration of a Dimeric Insecticidal Lectin to a Monomeric Antifungal Protein Correlated to Its Oligomeric Status. PLoS ONE 2011, 6, e18593. [Google Scholar] [CrossRef]
| Sample Composition | Dh, nm | PdI | Zeta Potential, mV |
|---|---|---|---|
| 100% CFL16 0% PC | 333 ± 20 | 0.300 | −20 ± 0.3 |
| 90% CFL16 10% PC | 252 ± 16 | 0.263 | +16.1 ± 0.7 * |
| 80% CFL16 20% PC | 216 ± 8 | 0.275 | −15.6 ± 0.2 |
| 70% CFL16 30% PC | 203 ± 2 | 0.259 | −14.6 ± 0.4 |
| 60% CFL16 40% PC | 191 ± 8 | 0.265 | −13.1 ± 0.2 |
| 50% CFL16 50% PC | 153 ± 1 | 0.262 | −12.2 ± 0.1 |
| 25% CFL16 75% PC | 88 ± 3 | 0.245 | −7.6 ± 0.4 |
| 0% CFL16 100% PC | 89 ± 8 | 0.268 | −7.0 ± 0.5 |
| 50% CFL16 50% PC + 1/35 14-6-14(Et) | 161 ± 8 | 0.268 | 40.7 ± 0.8 |
| Sample Composition | Dh, nm | Intercept | ||||
|---|---|---|---|---|---|---|
| Initial | 1 h | 24 h | Initial | 1 h | 24 h | |
| 80% CFL16 20% PC | 216 | 188 | 258 | 0.741 | 0.743 | 0.519 |
| 70% CFL16 30% PC | 203 | 184 | 222 | 0.750 | 0.763 | 0.370 |
| 60% CFL16 40% PC | 191 | 144 | 184 | 0.706 | 0.778 | 0.490 |
| 50% CFL16 50% PC | 153 | 117 | 129 | 0.789 | 0.793 | 0.719 |
| 25% CFL16 75% PC | 88 | 102 | 190 | 0.739 | 0.537 | 0.158 |
| 0% CFL16 100% PC | 89 | 73 | 52,212 | 0.664 | 0.492 | 0.189 |
| Sample Composition | IC50 (CFL), μM | IC50 (PTX), μM |
|---|---|---|
| CFL 1 mM PC 1 mM | 354.8 ± 32.7 | |
| CFL 1 mM PC 1 mM Tw80 0.2 mM | 320.6 ± 29.0 | |
| CFL 1 mM PC 1 mM +14-6-14(Et) | 341.2 ± 9.6 | |
| PTX | 8.8 ± 0.98 | |
| CFL 1 mM PC 1 mM + PTX | 2.9 ± 0.07 * | 0.24 ± 0.1 ** |
| CFL 1 mM PC 1 mM Tw80 0.2 mM + PTX | 3.3 ± 0.37 * | 0.26 ± 0.04 ** |
| CFL 1 mM PC 1 mM +14-6-14(Et) + PTX | 4.6 ± 0.20 * | 0.4 ± 0.04 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlov, R.; Romanova, E.; Kuznetsov, D.; Lyubina, A.; Amerhanova, S.; Voloshina, A.; Buzyurova, D.; Babaev, V.; Zueva, I.; Petrov, K.; et al. The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy. Int. J. Mol. Sci. 2023, 24, 3632. https://doi.org/10.3390/ijms24043632
Pavlov R, Romanova E, Kuznetsov D, Lyubina A, Amerhanova S, Voloshina A, Buzyurova D, Babaev V, Zueva I, Petrov K, et al. The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy. International Journal of Molecular Sciences. 2023; 24(4):3632. https://doi.org/10.3390/ijms24043632
Chicago/Turabian StylePavlov, Rais, Elvira Romanova, Denis Kuznetsov, Anna Lyubina, Syumbelya Amerhanova, Alexandra Voloshina, Daina Buzyurova, Vasily Babaev, Irina Zueva, Konstantin Petrov, and et al. 2023. "The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy" International Journal of Molecular Sciences 24, no. 4: 3632. https://doi.org/10.3390/ijms24043632
APA StylePavlov, R., Romanova, E., Kuznetsov, D., Lyubina, A., Amerhanova, S., Voloshina, A., Buzyurova, D., Babaev, V., Zueva, I., Petrov, K., Lukashenko, S., Gaynanova, G., & Zakharova, L. (2023). The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy. International Journal of Molecular Sciences, 24(4), 3632. https://doi.org/10.3390/ijms24043632







