FT-IR Analysis of P. aeruginosa Bacteria Inactivation by Femtosecond IR Laser Radiation
Abstract
1. Introduction
2. Results and Discussion
2.1. CFU Count
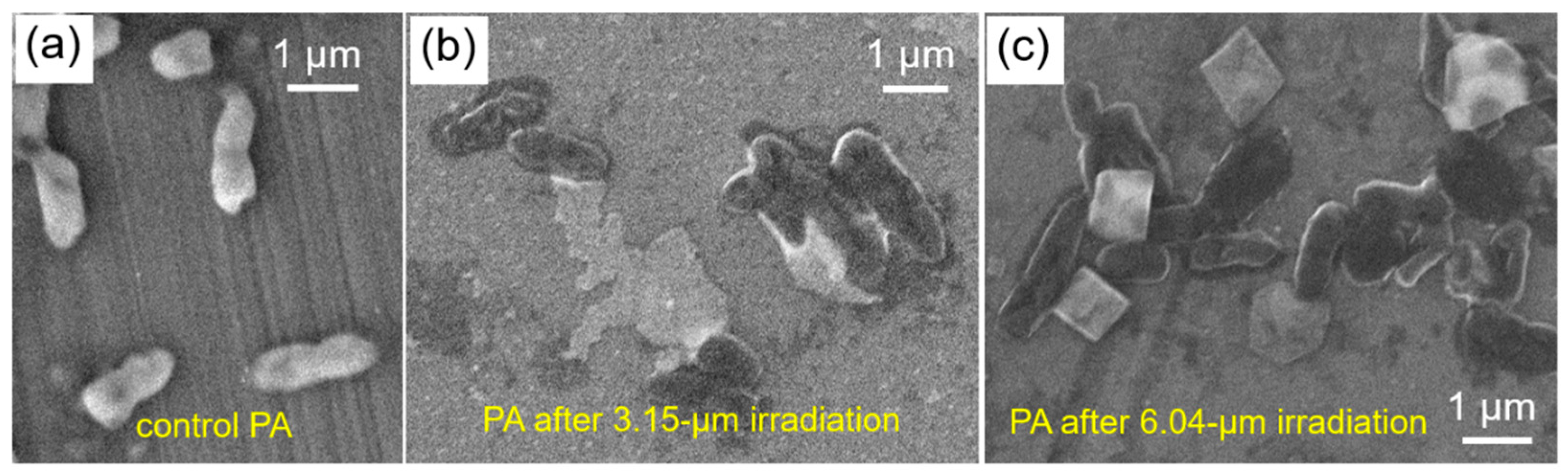
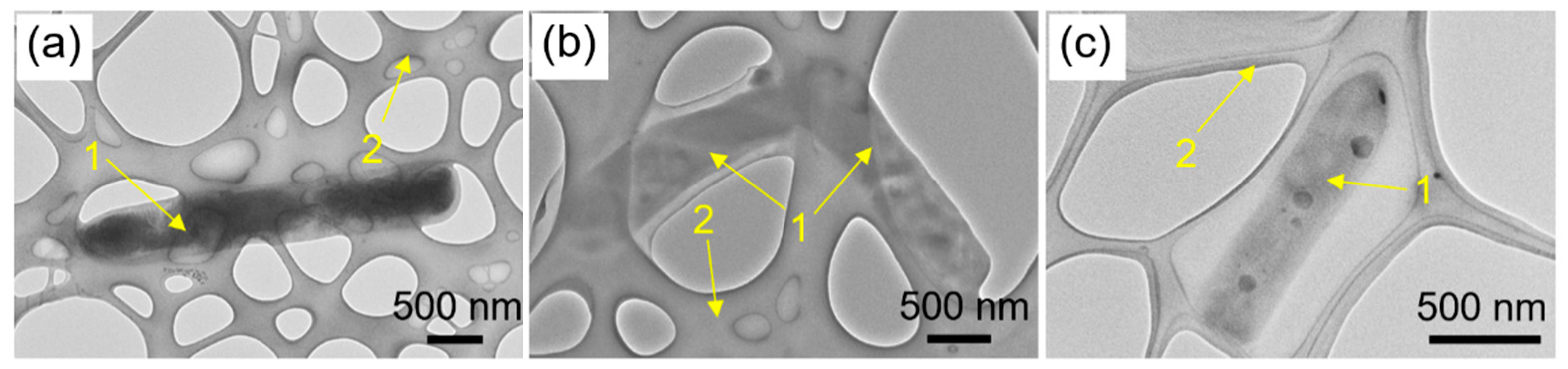
2.2. SEM and TEM Characterization
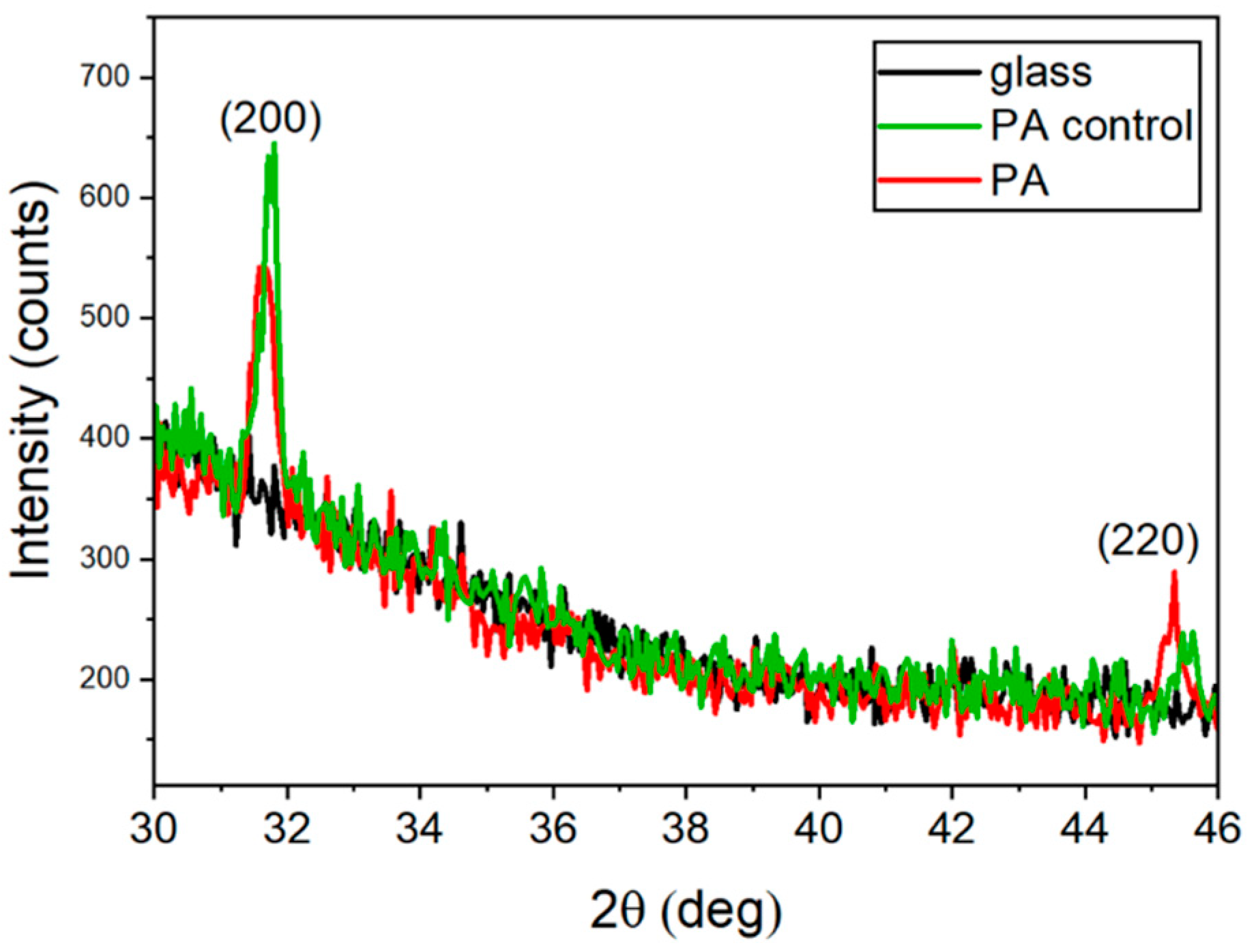
2.3. XRD Analysis
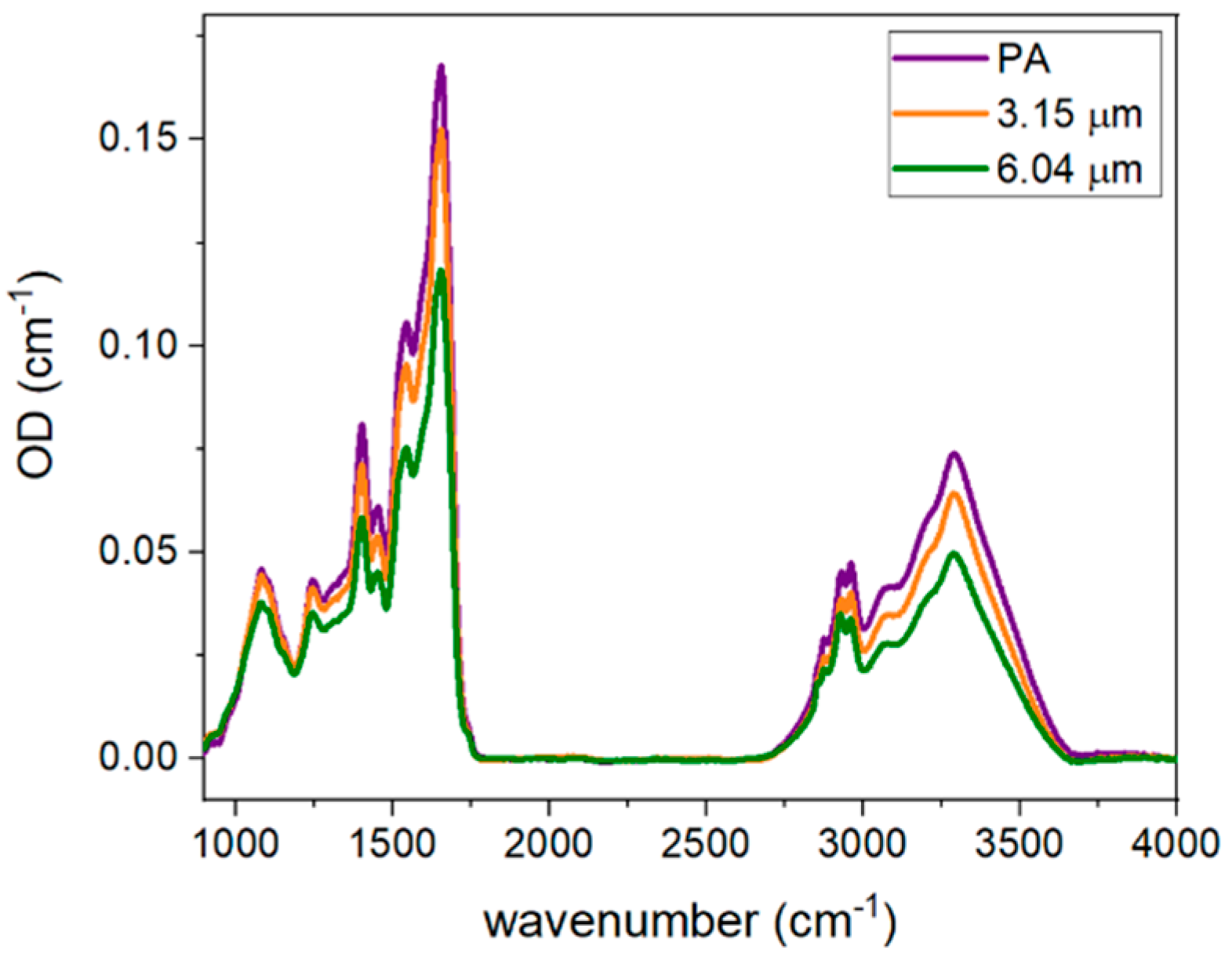
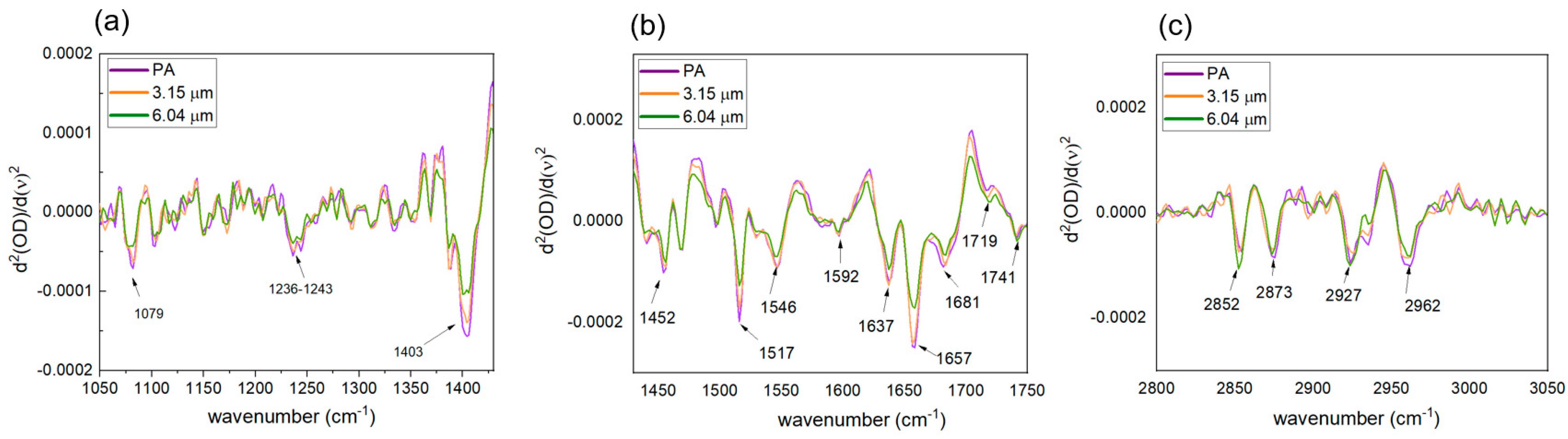
2.4. FT-IR Spectral Analysis
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyagi, L.; Sharma, G.P.; Verma, R.C.; Jain, S.K.; Murdia, L.K.; Mathur, S.M. Infrared heating in food processing: An overview. IJCS 2020, 8, 327–336. [Google Scholar] [CrossRef]
- Elliott, D.C.; Elliott, E.W. Biochemistry and Molecular Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Sawai, J.; Fujisawa, M.; Kokugan, T.; Shimizu, M.; Igarashi, H.; Hashimoto, A.; Kojima, H. Pasteurization of bacterial spores in liquid medium by far-infrared irradiation. J. Chem. Eng. Jpn. 1997, 30, 170–172. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Demirci, A.L.I.; Irudayaraj, J. Inactivation of Staphylococcus aureus by pulsed UV-light sterilization. J. Food Prot. 2004, 67, 1027–1030. [Google Scholar] [CrossRef]
- Hamanaka, D.; Uchino, T.; Furuse, N.; Han, W.; Tanaka, S.I. Effect of the wavelength of infrared heaters on the inactivation of bacterial spores at various water activities. Int. J. Food Microbiol. 2006, 108, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Oduola, A.A.; Bowie, R.; Wilson, S.A.; Mohammadi Shad, Z.; Atungulu, G.G. Impacts of broadband and selected infrared wavelength treatments on inactivation of microbes on rough rice. J. Food Saf. 2020, 40, e12764. [Google Scholar] [CrossRef]
- Schoop, U.; Moritz, A.; Kluger, W.; Patruta, S.; Goharkhay, K.; Sperr, W.; Georgopoulos, A. The Er: YAG laser in endodontics: Results of an in vitro study. Lasers Surg. Med. Off. J. Am. Soc. Lasers Surg. Med. 2002, 30, 360–364. [Google Scholar] [CrossRef]
- Folwaczny, M.; Mehl, A.; Aggstaller, H.; Hickel, R. Antimicrobial effects of 2.94 μm Er: YAG laser radiation on root surfaces: An in vitro study. J. Clin. Periodontol. 2002, 29, 73–78. [Google Scholar] [CrossRef]
- Hashimoto, A.; Yamazaki, Y.; Shimizu, M.; Oshita, S.I. Drying characteristics of gelatinous materials irradiated by infrared radiation. Dry. Technol. 1994, 12, 1029–1052. [Google Scholar] [CrossRef]
- Pawar, S.B.; Pratape, V.M. Fundamentals of infrared heating and its application in drying of food materials: A review. J. Food Proc. Eng. 2017, 40, e12308. [Google Scholar] [CrossRef]
- Kompanets, V.O.; Kudryashov, S.I.; Totordava, E.R.; Shelygina, S.N.; Sokolova, V.V.; Saraeva, I.N.; Kovalev, M.S.; Ionin, A.A.; Chekalin, S.V. Femtosecond infrared laser spectroscopy of characteristic molecular vibrations in bacteria in the 6-µm spectral range. JETP Lett. 2021, 113, 365–369. [Google Scholar] [CrossRef]
- Kompanets, V.; Shelygina, S.; Tolordava, E.; Kudryashov, S.; Saraeva, I.; Rupasov, A.; Kovalev, M. Spectrally-selective mid-IR laser-induced inactivation of pathogenic bacteria. Biomed. Opt. Express 2021, 12, 6317–6325. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Dashti, A.; Nasiri, S.; Monshi, A.; Vilkauskas, A.; Janusas, G. Measurement Modulus of elasticity related to the atomic density of planes in unit cell of crystal lattices. Materials 2020, 13, 4380. [Google Scholar] [CrossRef]
- Nikaido, H.; Nakae, T. The outer membrane of Gram-negative bacteria. Adv. Microb. Physiol. 1980, 20, 163–250. [Google Scholar] [CrossRef]
- Tang, M.; McEwen, G.D.; Wu, Y.; Miller, C.D.; Zhou, A. Characterization and analysis of mycobacteria and Gram-negative bacteria and co-culture mixtures by Raman microspectroscopy, FTIR, and atomic force microscopy. Anal. Bioanal. Chem. 2013, 405, 1577–1591. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Mauer, L.J. Fourier transform infrared FT-IR spectroscopy: A rapid tool for detection and analysis of foodborne pathogenic bacteria. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb.Biotechnol. 2010, 2, 1582–1594. [Google Scholar]
- Melin, A.M.; Perromat, A.; Déléris, G. Pharmacologic application of Fourier transform IR spectroscopy: In vivo toxicity of carbon tetrachloride on rat liver. Biopolym. Orig. Res. Biomol. 2000, 53, 160–168. [Google Scholar] [CrossRef]
- Gorgulu, S.T.; Dogan, M.; Severcan, F. The characterization and differentiation of higher plants by Fourier transform infrared spectroscopy. Appl. Spectrosc. 2007, 61, 300–308. [Google Scholar] [CrossRef]
- Naumann, D. FT-infrared and FT-Raman spectroscopy in biomedical research. Appl. Spec. Rev. 2001, 36, 239–298. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Tugarova, A.V.; Dyatlova, Y.A.; Tarantilis, P.A.; Grigoryeva, O.P.; Fainleib, A.M.; De Luca, S. Methodological effects in Fourier transform infrared FTIR spectroscopy: Implications for structural analyses of biomacromolecular samples. Spec. Act. Part A Mol. Biomol. Spec. 2018, 193, 558–564. [Google Scholar] [CrossRef]
- Quilès, F.; Humbert, F.; Delille, A. Analysis of changes in attenuated total reflection FTIR fingerprints of Pseudomonas fluorescens from planktonic state to nascent biofilm state. Spec. Act. Part A Mol. Biomol. Spec. 2010, 75, 610–616. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, H.Y.; Kong, Y.; Yu, Y.W.; Xu, X.Y. Component analysis of extracellular polymeric substances during aerobic sludge granulation using FTIR and 3D-EEM technologies. Bioresour. Technol. 2012, 124, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lam, J.S. WaaP of Pseudomonas aeruginosa is a novel eukaryotic type protein-tyrosine kinase as well as a sugar kinase essential for the biosynthesis of core lipopolysaccharide. J. Biol. Chem. 2002, 277, 4722–4730. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Sowa, M.G.; Mantsch, H.H. Infrared spectroscopy: A new frontier in medicine. Biophys. Chem. 1997, 68, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Garip, S.; Gozen, A.C.; Severcan, F. Use of Fourier transform infrared spectroscopy for rapid comparative analysis of Bacillus and Micrococcus isolates. Food Chem. 2009, 113, 1301–1307. [Google Scholar] [CrossRef]

| Exposure Time, min | P. aeruginosa, CFU/mL | |
|---|---|---|
| Control | - | 5·104 |
| After 3.15 μm | 3 5 7 | 5·103 3·103 2·103 |
| After 6.04 μm | 3 5 7 | 1·102 5·103 4·103 |
| Functional Groups | Frequency (cm−1) Bandwidth (a.u.) Area (a.u.) | ||
|---|---|---|---|
| Control | 3.15 μm Exposure | 6.04 μm Exposure | |
| PO2 str (sym) of nucleic acids and phospholipids | 1079.97 163.82 11.01 | 1079.97 123.37 6.76 | 1079.97 177.61 11.04 |
| P=O str (asym) of phosphodiesters | 1240.04 52.95 1.88 | 1240.04 53.28 1.67 | 1240.04 53.35 1.34 |
| COO¯ | 1403.97 48.13 4.13 | 1402.04 45.25 3.29 | 1402.04 47.21 2.75 |
| C-H def of CH2 | 1450.25 32.97 1.33 | 1450.25 40.19 1.61 | 1450.25 40.21 1.32 |
| Tyrosine O-H, C-C, C-H | 1517.75 38.78 2.13 | 1517.75 60.31 4.18 | 1517.75 64.62 3.28 |
| Amide II N–H bend, C–N str of proteins | 1544.75 48.19 3.95 | 1544.75 52.94 3.50 | 1542.82 58.79 3.06 |
| Amide I β-pleated sheets | 1637.32 51.26 7.21 | 1637.32 47.80 5.76 | 1637.32 48.61 4.59 |
| Amide I α-helices | 1658.53 34.06 4.43 | 1658.53 34.86 4.42 | 1658.53 34.45 3.33 |
| Amide I β-pleated sheets | 1681.67 29.08 2.84 | 1681.67 28.60 2.41 | 1681.67 27.87 1.82 |
| C-H str (sym) of CH2 in fatty acids | 2852.29 32.79 0.43 | 2852.29 22.25 0.18 | 2852.29 11.11 0.06 |
| C-H str (sym) of CH3 CH3 str (sym) of mainly proteins | 2873.51 27.65 0.51 | 2873.51 30.36 0.53 | 2875.43 69.43 1.27 |
| C-H str (asym) in CH2 of mainly lipids | 2927.50 34.59 1.19 | 2927.50 36.56 1.20 | 2925.58 42.66 1.43 |
| C-H str (asym) in CH3 of fatty acids | 2962.22 49.46 2.27 | 2962.22 46.98 1.85 | 2962.22 40.09 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraeva, I.; Tolordava, E.; Sheligyna, S.; Nastulyavichus, A.; Khmelnitskii, R.; Pokryshkin, N.; Khmelenin, D.; Kudryashov, S.; Ionin, A.; Akhmatkhanov, A. FT-IR Analysis of P. aeruginosa Bacteria Inactivation by Femtosecond IR Laser Radiation. Int. J. Mol. Sci. 2023, 24, 5119. https://doi.org/10.3390/ijms24065119
Saraeva I, Tolordava E, Sheligyna S, Nastulyavichus A, Khmelnitskii R, Pokryshkin N, Khmelenin D, Kudryashov S, Ionin A, Akhmatkhanov A. FT-IR Analysis of P. aeruginosa Bacteria Inactivation by Femtosecond IR Laser Radiation. International Journal of Molecular Sciences. 2023; 24(6):5119. https://doi.org/10.3390/ijms24065119
Chicago/Turabian StyleSaraeva, Irina, Eteri Tolordava, Svetlana Sheligyna, Alyona Nastulyavichus, Roman Khmelnitskii, Nikolay Pokryshkin, Dmitriy Khmelenin, Sergey Kudryashov, Andrey Ionin, and Andrey Akhmatkhanov. 2023. "FT-IR Analysis of P. aeruginosa Bacteria Inactivation by Femtosecond IR Laser Radiation" International Journal of Molecular Sciences 24, no. 6: 5119. https://doi.org/10.3390/ijms24065119
APA StyleSaraeva, I., Tolordava, E., Sheligyna, S., Nastulyavichus, A., Khmelnitskii, R., Pokryshkin, N., Khmelenin, D., Kudryashov, S., Ionin, A., & Akhmatkhanov, A. (2023). FT-IR Analysis of P. aeruginosa Bacteria Inactivation by Femtosecond IR Laser Radiation. International Journal of Molecular Sciences, 24(6), 5119. https://doi.org/10.3390/ijms24065119






