Evaluation of New Potential Inflammatory Markers in Patients with Nonvalvular Atrial Fibrillation
Abstract
1. Introduction
2. Results
2.1. Demographic, Clinical, and Laboratory Characteristics
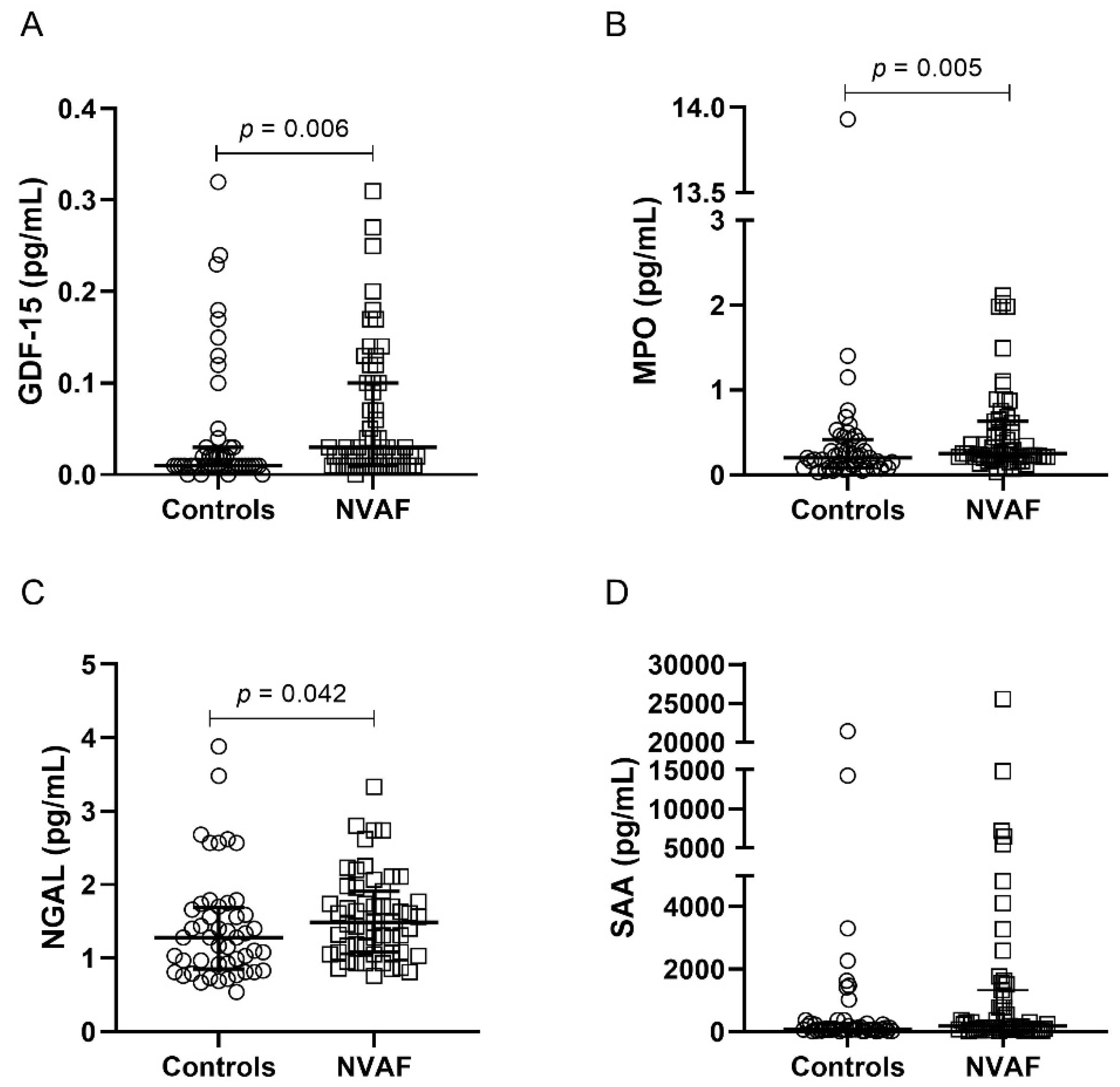
2.2. Comparison of Inflammatory Mediators between the Groups
2.3. Logistic Regression Models
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Biological Samples
4.3. Laboratory Characterization
4.4. Analysis of Inflammatory Mediators
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS13 | disintegrin and metalloproteinase with thrombospondin type 1 motif: 13 |
| AF | atrial fibrillation |
| ALT | alanine aminotransferase |
| AST | aspartate transaminase |
| CABG | coronary artery bypass grafting |
| CBA | Cytometric Bead Array |
| CHA2DS2-VASC | Congestive heart failure, Hypertension, Age ≥ 75 (doubled), Diabetes mellitus, prior stroke/transient ischemic attack/thromboembolism (doubled), Vascular disease, Age 65–74, e Sex category (female) |
| CRP | C-reactive protein |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| GDF | growth differentiation factor |
| GGT | gamma-glutamyl transferase |
| HDL | high-density lipoprotein |
| IFN-γ | interferon-gamma |
| IL | interleukin |
| IP-10 | interferon-gamma-induced protein |
| LDL | low-density lipoprotein |
| MCP | monocyte chemoattractant protein |
| MIG | monokine induced by interferon-gamma |
| MPO | myeloperoxidase |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NVAF | nonvalvular atrial fibrillation |
| RANTES | regulated on activation, normal T cells expressed and secreted |
| SAA | serum amyloid A |
| sICAM | soluble intercellular adhesion molecule |
| sVCAM | soluble vascular cell adhesion protein |
| TGF-β | human transforming growth factor-beta |
| TNF | tumor necrosis factor |
| WBC | white blood cell |
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstro, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Bassand, J.-P.; Virdone, S.; Goldhaber, S.Z.; Camm, A.J.; Fitzmaurice, D.A.; Fox, K.A.; Goto, S.; Haas, S.; Hacke, W.; Kayani, G.; et al. Early Risks of Death, Stroke/Systemic Embolism, and Major Bleeding in Patients With Newly Diagnosed Atrial Fibrillation: Results From the GARFIELD-AF Registry. Circulation 2019, 139, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.A.; Yin, X.; Fontes, J.D.; Wallace, E.R.; Skinner, A.; Wang, N.; Hammill, B.G.; Benjamin, E.J.; Curtis, L.H.; Heckbert, S.R. Hospital and clinical care costs associated with atrial fibrillation for Medicare beneficiaries in the Cardiovascular Health Study and the Framingham Heart Study. SAGE Open Med. 2018, 6, 2050312118759444. [Google Scholar] [CrossRef]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, immunity, and hypertension. Hypertension 2011, 57, 132–140. [Google Scholar] [CrossRef]
- Marzilli, M.; Merz, C.N.; Boden, W.E.; Bonow, R.O.; Capozza, P.G.; Chilian, W.M.; DeMaria, A.N.; Guarini, G.; Huqi, A.; Morrone, D.; et al. Obstructive coronary atherosclerosis and ischemic heart disease: An elusive link! J. Am. Coll. Cardiol. 2012, 60, 951–956. [Google Scholar] [CrossRef]
- Aune, D.; Feng, T.; Schlesinger, S.; Janszky, I.; Norat, T.; Riboli, E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. J. Diabet Complicat. 2018, 32, 501–511. [Google Scholar] [CrossRef]
- Lee, S.-R.; Choi, E.-K.; Ahn, H.-J.; Han, K.-D.; Oh, S.; Lip, G.Y. Association between clustering of unhealthy lifestyle factors and risk of new-onset atrial fibrillation: A nationwide population-based study. Sci. Rep. 2020, 10, 19224. [Google Scholar] [CrossRef]
- Bruins, P.; te Velthuis, H.; Yazdanbakhsh, A.P.; Jansen, P.G.; van Hardevelt, F.W.; de Beaumont, E.M.; Wildevuur, C.R.; Eijsman, L.; Trouwborst, A.; Hack, C.E. Activation of the complement system during and after cardiopulmonary bypass surgery: Postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 1997, 96, 3542–3548. [Google Scholar] [CrossRef]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef]
- Rizos, I.; Rigopoulos, A.G.; Kalogeropoulos, A.S.; Tsiodras, S.; Dragomanovits, S.; Sakadakis, E.A.; Faviou, E.; Kremastinos, D.T. Hypertension and paroxysmal atrial fibrillation: A novel predictive role of high sensitivity C-reactive protein in cardioversion and long-term recurrence. J. Hum. Hypertens. 2010, 24, 447–457. [Google Scholar] [CrossRef]
- Meyre, P.B.; Sticherling, C.; Spies, F.; Aeschbacher, S.; Blum, S.; Voellmin, G.; Madaffari, A.; Conen, D.; Osswald, S.; Kühne, M.; et al. C-reactive protein for prediction of atrial fibrillation recurrence after catheter ablation. BMC Cardiovasc. Disord. 2020, 20, 427. [Google Scholar] [CrossRef]
- Li, J.; Solus, J.; Chen, Q.; Rho, Y.H.; Milne, G.; Stein, C.M.; Darbar, D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm Off. J. Heart Rhythm Soc. 2010, 7, 438–444. [Google Scholar] [CrossRef]
- Marcus, G.M.; Smith, L.M.; Ordovas, K.; Scheinman, M.M.; Kim, A.M.; Badhwar, N.; Lee, R.J.; Tseng, Z.H.; Lee, B.K.; Olgin, J.E. Intracardiac and extracardiac markers of inflammation during atrial fibrillation. Heart Rhythm Off. J. Heart Rhythm Soc. 2010, 7, 149–154. [Google Scholar] [CrossRef]
- Kaireviciute, D.; Blann, A.D.; Balakrishnan, B.; Lane, D.A.; Patel, J.V.; Uzdavinys, G.; Norkunas, G.; Kalinauskas, G.; Sirvydis, V.; Aidietis, A.; et al. Characterisation and validity of inflammatory biomarkers in the prediction of post-operative atrial fibrillation in coronary artery disease patients. Thromb. Haemost. 2010, 104, 122–127. [Google Scholar] [CrossRef]
- Henningsen, K.M.; Therkelsen, S.K.; Bruunsgaard, H.; Krabbe, K.S.; Pedersen, B.K.; Svendsen, J.H. Prognostic impact of hs-CRP and IL-6 in patients with persistent atrial fibrillation treated with electrical cardioversion. Scand. J. Clin. Lab. Investig. 2009, 69, 425–432. [Google Scholar] [CrossRef]
- Conway, D.S.; Buggins, P.; Hughes, E.; Lip, G.Y. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J. Am. Coll. Cardiol. 2004, 43, 2075–2082. [Google Scholar] [CrossRef]
- Roldan, V.; Marin, F.; Diaz, J.; Gallego, P.; Jover, E.; Romera, M.; Manzano-Fernandez, S.; Casas, T.; Valdes, M.; Vicente, V.; et al. High sensitivity cardiac troponin T and interleukin-6 predict adverse cardiovascular events and mortality in anticoagulated patients with atrial fibrillation. J. Thromb. Haemost. JTH 2012, 10, 1500–1507. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Acampa, M.; Srivastava, U.; Bertolozzi, I.; Giabbani, B.; Finizola, F.; Vanni, F.; Dokollari, A.; Natale, M.; et al. Systemic Inflammation Rapidly Induces Reversible Atrial Electrical Remodeling: The Role of Interleukin-6—Mediated Changes in Connexin Expression. J. Am. Heart Assoc. 2019, 8, e011006. [Google Scholar] [CrossRef] [PubMed]
- Rizos, I.; Tsiodras, S.; Rigopoulos, A.G.; Dragomanovits, S.; Kalogeropoulos, A.S.; Papathanasiou, S.; Sakadakis, E.A.; Kremastinos, D.T. Interleukin-2 serum levels variations in recent onset atrial fibrillation are related with cardioversion outcome. Cytokine 2007, 40, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hak, L.; Mysliwska, J.; Wieckiewicz, J.; Szyndler, K.; Siebert, J.; Rogowski, J. Interleukin-2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG). J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2009, 29, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Bueno, F.; Medina-Palomo, C.; Ruiz-Salas, A.; Flores, A.; Rodriguez-Losada, N.; Barrera, A.; Jimenez-Navarro, M.; Alzueta, J. Serum levels of interleukin-2 predict the recurrence of atrial fibrillation after pulmonary vein ablation. Cytokine 2015, 73, 74–78. [Google Scholar] [CrossRef]
- Pinto, A.; Tuttolomondo, A.; Casuccio, A.; Di Raimondo, D.; Di Sciacca, R.; Arnao, V.; Licata, G. Immuno-inflammatory predictors of stroke at follow-up in patients with chronic non-valvular atrial fibrillation (NVAF). Clin. Sci. 2009, 116, 781–789. [Google Scholar] [CrossRef]
- Ren, M.; Li, X.; Hao, L.; Zhong, J. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: A novel potential therapeutic target? Ann. Med. 2015, 47, 316–324. [Google Scholar] [CrossRef]
- Huang, J.; Xiang, Y.; Zhang, H.; Wu, N.; Chen, X.; Wu, L.; Xu, B.; Li, C.; Zhang, Z.; Tong, S. Plasma level of interferon-γ predicts the prognosis in patients with new-onset atrial fibrillation. Heart Lung Circ. 2020, 29, e168–e176. [Google Scholar] [CrossRef]
- Pan, J.; Wang, W.; Wu, X.; Kong, F.; Lin, J.; Zhang, M. Inflammatory cytokines in cardiac pacing patients with atrial fibrillation and asymptomatic atrial fibrillation. Panminerva Med. 2018, 60, 86–91. [Google Scholar] [CrossRef]
- Rienstra, M.; Yin, X.; Larson, M.G.; Fontes, J.D.; Magnani, J.W.; McManus, D.D.; McCabe, E.L.; Coglianese, E.E.; Amponsah, M.; Ho, J.E.; et al. Relation between soluble ST2, growth differentiation factor–15, and high-sensitivity troponin I and incident atrial fibrillation. Am. Heart J. 2014, 167, 109–115.e102. [Google Scholar] [CrossRef]
- Wallentin, L.; Hijazi, Z.; Andersson, U.; Alexander, J.H.; De Caterina, R.; Hanna, M.; Horowitz, J.D.; Hylek, E.M.; Lopes, R.D.; Åsberg, S.; et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Circulation 2014, 130, 1847–1858. [Google Scholar] [CrossRef]
- Sharma, A.; Hijazi, Z.; Andersson, U.; Al-Khatib, S.M.; Lopes, R.D.; Alexander, J.H.; Held, C.; Hylek, E.M.; Leonardi, S.; Hanna, M.; et al. Use of biomarkers to predict specific causes of death in patients with Atrial fibrillation: Insights from the Aristotle Trial. Circulation 2018, 138, 1666–1676. [Google Scholar] [CrossRef]
- Hu, X.F.; Zhan, R.; Xu, S.; Wang, J.; Wu, J.; Liu, X.; Li, Y.; Chen, L. Growth differentiation factor 15 is associated with left atrial/left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Clin. Cardiol. 2018, 41, 34–38. [Google Scholar] [CrossRef]
- Gue, Y.X.; Lip, G.Y.H. Hypertension and atrial fibrillation: Closing a virtuous circle. PLoS Med. 2021, 18, e1003598. [Google Scholar] [CrossRef]
- Kreutz, R.; Camm, A.J.; Rossing, P. Concomitant diabetes with atrial fibrillation and anticoagulation management considerations. Eur. Heart J. Suppl. 2020, 22, O78–O86. [Google Scholar] [CrossRef]
- Sinner, M.F.; Wang, N.; Fox, C.S.; Fontes, J.D.; Rienstra, M.; Magnani, J.W.; Vasan, R.S.; Calderwood, A.H.; Pencina, M.; Sullivan, L.M.; et al. Relation of circulating liver transaminase concentrations to risk of new-onset atrial fibrillation. Am. J. Cardiol. 2013, 111, 219–224. [Google Scholar] [CrossRef]
- Alonso, A.; Misialek, J.R.; Amiin, M.A.; Hoogeveen, R.C.; Chen, L.Y.; Agarwal, S.K.; Loehr, L.R.; Soliman, E.Z.; Selvin, E. Circulating levels of liver enzymes and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities cohort. Heart 2014, 100, 1511–1516. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Xhepa, E.; Colleran, R.; Braun, S.; Cassese, S.; Fusaro, M.; Laugwitz, K.-L.; Kastrati, A. Gamma-glutamyl transferase and atrial fibrillation in patients with coronary artery disease. Clin. Chim. Acta 2017, 465, 17–21. [Google Scholar] [CrossRef]
- Uçar, F.M.; Ýpek, E.G.; Burak, A.; Murat, G.; Abdullah, T.; Özeke, Ö.; Geyýk, B.; Topaloglu, S.; Aras, D. Gamma-glutamyl transferase predicts recurrences of atrial fibrillation after catheter ablation. Acta Cardiol. 2016, 71, 205–210. [Google Scholar] [CrossRef]
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s Diagnostic and Laboratory Test References; Elsevier: St. Louis, Mo, USA, 2019. [Google Scholar]
- Hijazi, Z.; Wallentin, L.J.C. Renal function in atrial fibrillation: A multifaceted dilemma. Am. Heart Assoc. 2016, 134, 48–51. [Google Scholar] [CrossRef]
- Kim, S.C.; Liu, J.; Solomon, D.H. Risk of incident atrial fibrillation in gout: A cohort study. Ann. Rheum. Dis. 2016, 75, 1473–1478. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, Y.; Han, X.; Yang, Y.; Yin, X.; Qiu, J.; Liu, H.; Zhou, Y.; Liu, Y. Association between serum uric acid and atrial fibrillation: A cross-sectional community-based study in China. BMJ Open 2017, 7, e019037. [Google Scholar] [CrossRef] [PubMed]
- Gotsch, F.; Romero, R.; Friel, L.; Kusanovic, J.P.; Espinoza, J.; Erez, O.; Than, N.G.; Mittal, P.; Edwin, S.; Yoon, B.H.; et al. CXCL10/IP-10: A missing link between inflammation and anti-angiogenesis in preeclampsia? J. Matern. Fetal. Neonatal. Med. 2007, 20, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Rothenbacher, D.; Müller-Scholze, S.; Herder, C.; Koenig, W.; Kolb, H. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arter. Thromb Vasc. Biol. 2006, 26, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, C.; Zakai, N.A.; Auer, P.; Cushman, M.; Lange, E.M.; Levitan, E.B.; Olson, N.; Thornton, T.A.; Tracy, R.P.; Wilson, J.G.; et al. Interferon gamma-induced protein 10 (IP-10) and cardiovascular disease in African Americans. PLoS ONE 2020, 15, e0231013. [Google Scholar] [CrossRef]
- van den Borne, P.; Quax, P.H.; Hoefer, I.E.; Pasterkamp, G. The multifaceted functions of CXCL10 in cardiovascular disease. BioMed Res. Int. 2014, 2014, 893106. [Google Scholar] [CrossRef]
- Martins, G.L.; Duarte, R.C.F.; Vieira, É.L.M.; Rocha, N.P.; Figueiredo, E.L.; Silveira, F.R.; Caiaffa, J.R.S.; Lanna, R.P.; das Graças Carvalho, M.; Palotás, A.; et al. Comparison of Inflammatory Mediators in Patients With Atrial Fibrillation Using Warfarin or Rivaroxaban. Front. Cardiovasc. Med. 2020, 7, 114. [Google Scholar] [CrossRef]

| Parameters | Controls (N = 50) | NVAF (N = 55) | p-Value |
|---|---|---|---|
| Age, mean ± SD | 71 ± 8 | 72 ± 8 | 0.822 |
| Sex | |||
| Female, N (%) | 26 (52%) | 28 (50.9%) | 0.911 |
| Male, N (%) | 24 (48%) | 27 (49.1%) | |
| Hypertension, N (%) | 29 (58%) | 53 (96.4%) | <0.001 |
| Diabetes mellitus 2, N (%) | 5 (10%) | 20 (36.4%) | 0.002 |
| Statins, N (%) | 21 (42%) | 31 (58.5%) | 0.094 |
| CHA2DS2-VASc, median (IQR) | - | 4 (1) | - |
| Total cholesterol (mg/dL), mean ± SD | 181 ± 36 | 173 ± 39 | 0.227 |
| LDL (mg/dL), mean ± SD | 98 ± 29 | 87 ± 34 | 0.080 |
| HDL (mg/dL), mean ± SD | 54 ± 14 | 55 ± 15 | 0.682 |
| Triglycerides (mg/dL), median (IQR) | 130.5 (72.5) | 122.5 (117.5) | 0.992 |
| ALT (U/L), median (IQR) | 24 (10) | 26.5 (15.5) | 0.030 |
| AST (U/L), median (IQR) | 26 (7.25) | 30 (12.75) | 0.023 |
| GGT (U/L), median (IQR) | 23.5 (11.5) | 41.5 (33.5) | <0.001 |
| Creatinine (mg/dL), median (IQR) | 1 (0.3) | 1.1 (0.3) | 0.031 |
| Uric acid (mg/dL), median (IQR) | 5.45 (1.75) | 6.2 (2.65) | 0.002 |
| WBC, median (IQR) | 5200 (2520) | 5750 (2040) | 0.438 |
| CRP ≥ 10 mg/L, N (%) | 8 (16.3%) | 12 (21.8%) | 0.478 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Inflammatory Mediators (pg/mL) | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| IL-2 | 2.54 × 1012 (0.49–1.32 × 1025) | 0.056 | 9.58 × 1088 (0.00–) | 0.981 |
| IL-4 | 2.80 × 104 (38.90–2.01 × 107) | 0.002 | 3.61 × 10174 (0.00–) | 0.959 |
| IL-6 | 1.61 (1.26–2.05) | <0.001 | 2.85 (1.56–5.23) | 0.001 |
| IL-10 | 8.99 (3.33–24.31) | <0.001 | 6.17 (2.33–16.33) | <0.001 |
| TNF | 8.33 (3.32–20.87) | <0.001 | 12.24 (3.42–43.76) | <0.001 |
| IFN-γ | 4.91 × 1010 (2.01–1.20 × 1021) | 0.044 | 3.42 × 1042 (0.00–) | 0.984 |
| IP-10 | 1.01 (1.00–1.02) | 0.007 | 1.01 (1.00–1.02) | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, G.L.; Duarte, R.C.F.; Vieira, É.L.M.; Rocha, N.P.; Figueiredo, E.L.; Silveira, F.R.; Caiaffa, J.R.S.; Lanna, R.P.; Carvalho, M.d.G.; Palotás, A.; et al. Evaluation of New Potential Inflammatory Markers in Patients with Nonvalvular Atrial Fibrillation. Int. J. Mol. Sci. 2023, 24, 3326. https://doi.org/10.3390/ijms24043326
Martins GL, Duarte RCF, Vieira ÉLM, Rocha NP, Figueiredo EL, Silveira FR, Caiaffa JRS, Lanna RP, Carvalho MdG, Palotás A, et al. Evaluation of New Potential Inflammatory Markers in Patients with Nonvalvular Atrial Fibrillation. International Journal of Molecular Sciences. 2023; 24(4):3326. https://doi.org/10.3390/ijms24043326
Chicago/Turabian StyleMartins, Gabriela Lopes, Rita Carolina Figueiredo Duarte, Érica Leandro Marciano Vieira, Natália Pessoa Rocha, Estêvão Lanna Figueiredo, Francisco Rezende Silveira, José Raymundo Sollero Caiaffa, Rodrigo Pinheiro Lanna, Maria das Graças Carvalho, András Palotás, and et al. 2023. "Evaluation of New Potential Inflammatory Markers in Patients with Nonvalvular Atrial Fibrillation" International Journal of Molecular Sciences 24, no. 4: 3326. https://doi.org/10.3390/ijms24043326
APA StyleMartins, G. L., Duarte, R. C. F., Vieira, É. L. M., Rocha, N. P., Figueiredo, E. L., Silveira, F. R., Caiaffa, J. R. S., Lanna, R. P., Carvalho, M. d. G., Palotás, A., Ferreira, C. N., & Reis, H. J. (2023). Evaluation of New Potential Inflammatory Markers in Patients with Nonvalvular Atrial Fibrillation. International Journal of Molecular Sciences, 24(4), 3326. https://doi.org/10.3390/ijms24043326






