Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies
Abstract
1. Introduction
2. Methods
3. Plant Sources and Chemical Properties of Curcumin
4. Absorption, Distribution, Metabolism and Exclusion of Curcumin
5. Clinical Evidence of Curcumin in Treating Metabolic Diseases
5.1. T2DM
5.1.1. Curcumin Lowers Blood Glucose Levels and Improves Insulin Resistance in Patients with T2DM
5.1.2. Curcumin Improves Blood Lipid Levels in Patients with T2DM
5.1.3. Curcumin Alleviates Inflammation and Oxidative Stress in Patients with T2DM
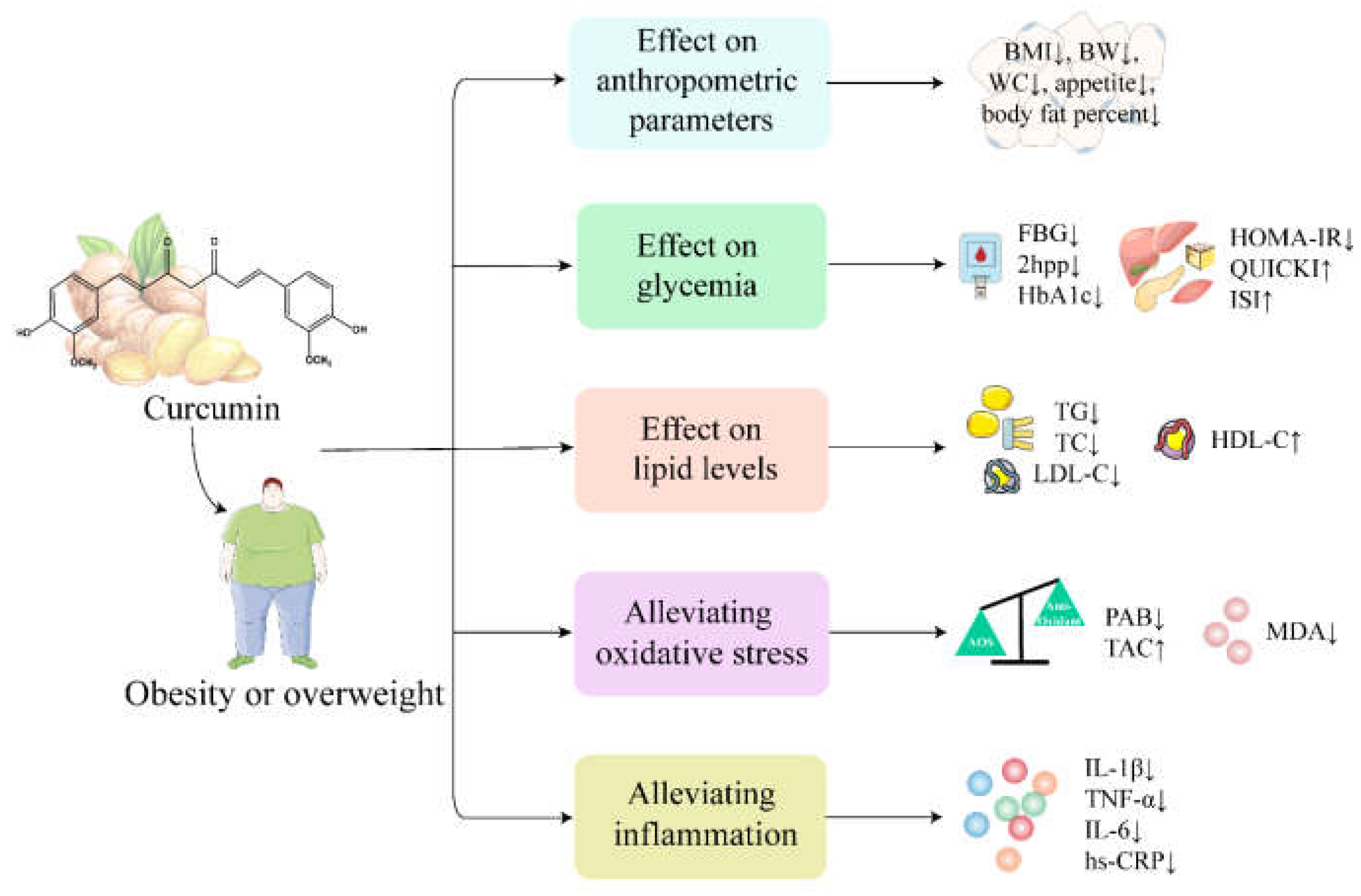
5.2. Obesity
5.2.1. Effect of Curcumin on Anthropometric Parameters in Obese or Overweight Subjects
5.2.2. Effect of Curcumin on Glycemia and Lipid Levels in Obese or Overweight Subjects
5.2.3. Curcumin Alleviates Inflammation and Oxidative Stress in Obese or Overweight Subjects
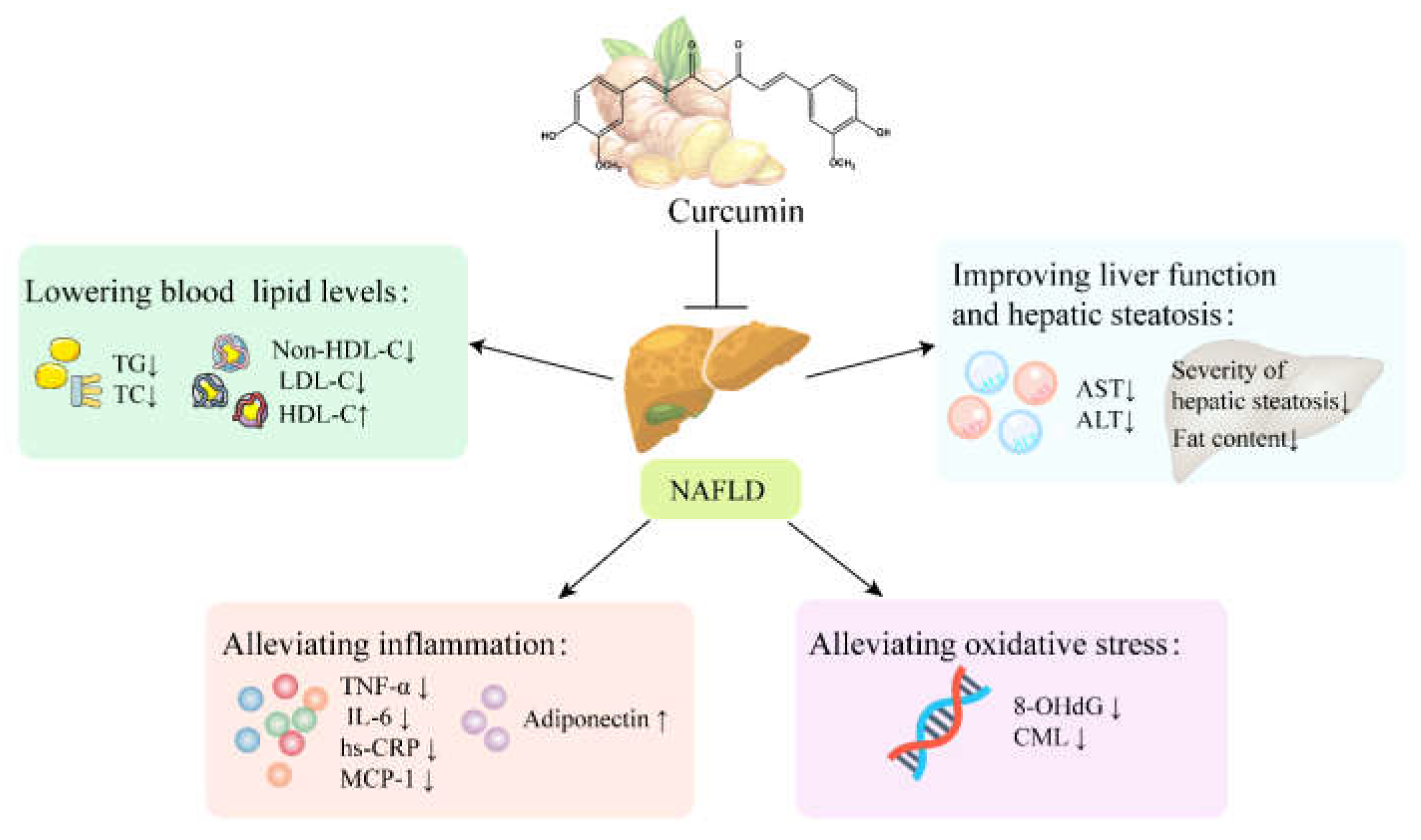
5.3. NAFLD
5.3.1. Curcumin Improves Liver Function and Hepatic Steatosis in Patients with NAFLD
5.3.2. Curcumin Lowers Blood Lipid Levels in Patients with NAFLD
5.3.3. Curcumin Alleviates Inflammation and Oxidative Stress in Patients with NAFLD
6. Side Effects of Curcumin in Human Trials
7. Conclusion and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2DM | type 2 diabetes mellitus |
| NAFLD | non-alcoholic fatty liver disease |
| CHD | coronary heart disease |
| MetS | metabolic syndrome |
| IGT | Impaired Glucose Tolerance |
| PCOS | Polycystic ovary syndrome |
| FBG | fasting blood glucose |
| HbA1c | hemoglobin A1c |
| TC | total cholesterol |
| TG | triglycerides |
| FINS | fasting insulin |
| LDL-C | low density lipoprotein cholesterol |
| HDL-C | high density lipoprotein cholesterol |
| Non-HDL-C | non-high density lipoprotein cholesterol |
| VLDL-C | very-low-density lipoprotein cholesterol |
| HDL | high density lipoprotein |
| HOMA-β | homeostasis model assessment β |
| HOMA-IR | homeostasis model assessment-insulin resistance |
| ISI | insulin sensitivity index |
| QUICKI | Quantitative Insulin Sensitivity Check Index |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| 8-OHdG | 8-Hydroxy-2′-deoxyguanosine |
| CML | carboxy methyl lysine |
| BMI | body mass index |
| BW | body weight |
| WC | Waist circumference |
| TNF-α | tumor necrosis factor-α |
| FFA | free fatty acid |
| CRP | C-reactive protein |
| hs-CRP | high sensitivity C-reactive protein |
| IL-6 | interleukin-6 |
| SOD | superoxide dismutase |
| MDA | malonaldehyde |
| LP(a) | lipoprotein(a) |
| MCP-1 | macrophage chemoattractant protein-1 |
| GSH | glutathione |
| TAC | total antioxidant capacity |
| PAB | pro-oxidant–antioxidant balance |
| PWV | pulse wave velocity |
| SUA | serum uric acid |
| γ-GTP | γ-glutamyl transpeptidase |
| 2hpp | 2-h postprandial glucose |
| IL-1β | interleukin-1β |
| IL-4 | interleukin-4 |
| VEGF | vascular endothelial growth factor |
| ox-LDL | oxidized low density lipoprotein |
| LPL | lipoprotein lipase |
| PPAR-γ | peroxisome proliferator-activated receptor |
| GOT | glutamic oxaloacetic transaminase |
| GPT | glutamate pyruvate transaminase |
| GRAS | generally recognized as safe |
| AGEs | advanced glycosylation end products |
| Cmax | maximum concentration |
References
- Wang, X.; Wang, Y.; Antony, V.; Sun, H.; Liang, G. Metabolism-Associated Molecular Patterns (MAMPs). Trends Endocrinol. Metab. TEM 2020, 31, 712–724. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Hancková, M.; Betáková, T. Pandemics of the 21st Century: The Risk Factor for Obese People. Viruses 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.M.; Price, J.F.; Glancy, S.; Perry, E.; Nee, L.D.; Hayes, P.C.; Frier, B.M.; Van Look, L.A.; Johnston, G.I.; Reynolds, R.M.; et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes Care 2011, 34, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Hoyte, C. Review of biguanide (metformin) toxicity. J. Intensive Care Med. 2019, 34, 863–876. [Google Scholar] [CrossRef]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin therapy: Review of safety and potential side effects. Acta Cardiol. Sin. 2016, 32, 631. [Google Scholar]
- Hanefeld, M.; Ganz, X.; Nolte, C. Hypoglycemia and cardiac arrhythmia in patients with diabetes mellitus type 2. Herz 2014, 39, 312–319. [Google Scholar] [CrossRef]
- Cai, L.; Liu, S.; Sun, L.; Wang, Y.; Ji, H.; Li, J. Application of tea polyphenols in combination with 6-gingerol on shrimp paste of during storage: Biogenic amines formation and quality determination. Front. Microbiol. 2015, 6, 981. [Google Scholar] [CrossRef]
- Oglah, M.K.; Mustafa, Y.F.; Bashir, M.K.; Jasim, M.H.; Mustafa, Y.F. Curcumin and its derivatives: A review of their biological activities. Syst. Rev. Pharm. 2020, 11, 472–481. [Google Scholar]
- Duan, Z.; Song, W.; Chen, K.; Qiao, X.; Ye, M. Assessment of genetic and chemical variability in curcumae longae rhizoma (Curcuma longa) based on DNA barcoding markers and HPLC fingerprints. Biol. Pharm. Bull. 2017, 40, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.F.; Xu, L.L.; Liu, H.C.; Luan, Y.F.; Xu, X.Y.; Yu, Y.M.; Lin, Y.Q. Determination of 8 Main Active Compounds in Curcumae Rhizome by HPLC Wavelength Switching Method. Chin. J. Mod. Appl. Pharm. 2021, 38, 2227–2233. [Google Scholar]
- Yu, M.T.; Tong, H.G.; Mao, C.Q.; Su, D.; Yin, F.Z.; Fei, C.H.; Wang, M.; Ji, D.; Lu, T.L. Study on quality identification of Curcumae Rhizoma from different origins based on quantitative ananlysis of appearance color and content of main components. China J. Chin. Mater. Med. 2021, 46, 1393–1400. [Google Scholar]
- Qi, A.D. Analysis of curcumin in Curcuma longa, C. wenyujin, C. kwangsiensis by HPLC. Chin. Tradit. Herb. Drugs 2002, 2002, 33–35. [Google Scholar]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Stanić, Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge—A Review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Dhakal, S.; Chao, K.; Schmidt, W.; Qin, J.; Kim, M.; Chan, D. Evaluation of Turmeric Powder Adulterated with Metanil Yellow Using FT-Raman and FT-IR Spectroscopy. Foods 2016, 5, 36. [Google Scholar] [CrossRef]
- Yin, G.P.; Zhang, Q.Z.; An, Y.W.; Zhu, J.J.; Wang, Z.M. Advance in chemical constituents and pharmacological activity of Curcuma wenyujin. China J. Chin. Mater. Med. 2012, 37, 3354–3360. [Google Scholar]
- López-Malo, D.; Villarón-Casares, C.A.; Alarcón-Jiménez, J.; Miranda, M.; Díaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a Therapeutic Option in Retinal Diseases. Antioxidants 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, purification and applications of curcumin from plant materials—A comprehensive review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Steward, W.P.; Gescher, A.J. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J. Agric. Food Chem. 2009, 57, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Jones, D.; Singh, R.; Dennison, A.; Farmer, P.; Sharma, R.; Steward, W.; Gescher, A.; Berry, D. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.-M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B 2015, 985, 38–47. [Google Scholar] [CrossRef]
- Hoehle, S.I.; Pfeiffer, E.; Sólyom, A.M.; Metzler, M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J. Agric. Food Chem. 2006, 54, 756–764. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Ghayour Mobarhan, M.; Kazemi Oskuee, R. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomedicine 2016, 6, 567–577. [Google Scholar]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Curcuminoids Plus Piperine Modulate Adipokines in Type 2 Diabetes Mellitus. Curr. Clin. Pharmacol. 2017, 12, 253–258. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: A randomized controlled trial. Inflammopharmacology 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef]
- Usharani, P.; Mateen, A.A.; Naidu, M.U.; Raju, Y.S.; Chandra, N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: A randomized, parallel-group, placebo-controlled, 8-week study. Drugs R&D 2008, 9, 243–250. [Google Scholar]
- Neerati, P.; Devde, R.; Gangi, A.K. Evaluation of the effect of curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother.Res. 2014, 28, 1796–1800. [Google Scholar] [CrossRef]
- Na, L.X.; Yan, B.L.; Jiang, S.; Cui, H.L.; Li, Y.; Sun, C.H. Curcuminoids Target Decreasing Serum Adipocyte-fatty Acid Binding Protein Levels in Their Glucose-lowering Effect in Patients with Type 2 Diabetes. Biomed. Environ.Sci. 2014, 27, 902–906. [Google Scholar]
- Na, L.X.; Li, Y.; Pan, H.Z.; Zhou, X.L.; Sun, D.J.; Meng, M.; Li, X.X.; Sun, C.H. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Mobini, M.; Raygan, F.; Aghadavod, E.; Ostadmohammadi, V.; Amirani, E.; Mansournia, M.A.; Asemi, Z. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin. Nutr. ESPEN 2020, 40, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, M.; Shimizu, K.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Wada, H.; Hasegawa, K.; et al. Effects of Highly Absorbable Curcumin in Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 8208237. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z.; Reiner, Z.; Soleimani, A.; Aghadavod, E.; Bahmani, F. The Effects of Nano-curcumin on Metabolic Status in Patients with Diabetes on Hemodialysis, a Randomized, Double Blind, Placebo-controlled Trial. Iran. J. Kidney Dis. 2020, 14, 290–299. [Google Scholar] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Sahebkar, A.; Iranshahi, M.; Amini, M.; Khojasteh, R.; Ghayour-Mobarhan, M.; Ferns, G.A. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: A randomized crossover trial. Phytother. Res. 2013, 27, 374–379. [Google Scholar] [CrossRef]
- Sahebkar, A.; Mohammadi, A.; Atabati, A.; Rahiman, S.; Tavallaie, S.; Iranshahi, M.; Akhlaghi, S.; Ferns, G.A.; Ghayour-Mobarhan, M. Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother. Res. 2013, 27, 1883–1888. [Google Scholar] [CrossRef]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Parizadeh, S.M.; Ghayour-Mobarhan, M. Investigation of the effects of curcumin on serum cytokines in obese individuals: A randomized controlled trial. Sci. World J. 2014, 2014, 898361. [Google Scholar] [CrossRef]
- Franco-Robles, E.; Campos-Cervantes, A.; Murillo-Ortiz, B.O.; Segovia, J.; López-Briones, S.; Vergara, P.; Pérez-Vázquez, V.; Solís-Ortiz, M.S.; Ramírez-Emiliano, J. Effects of curcumin on brain-derived neurotrophic factor levels and oxidative damage in obesity and diabetes. Appl. Physiol. Nutr. Metab. 2014, 39, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: A randomized placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. The nanocurcumin reduces appetite in obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nanomed. J. 2018, 5, 67–76. [Google Scholar]
- Sedighiyan, M.; Abdolahi, M.; Jafari, E.; Vahabi, Z.; Sohrabi Athar, S.; Hadavi, S.; Narimani Zamanabadi, M.; Yekaninejad, M.S.; Djalali, M. The effects of nano-curcumin supplementation on adipokines levels in obese and overweight patients with migraine: A double blind clinical trial study. BMC Res. Notes 2022, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Karandish, M.; Mozaffari-Khosravi, H.; Mohammadi, S.M.; Cheraghian, B.; Azhdari, M. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother. Res. 2021, 35, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gastrointest. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Rezai, A.; Dehabeh, M.; Nobakht, M.G.B.F.; Bidkhori, M.; Sahebkar, A.; Hariri, M. Efficacy of phytosomal curcumin among patients with non-alcoholic fatty liver disease. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J. Int. Vitaminol. Nutr. 2021, 91, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Karimian, M.; Keshvari, M.; Ghayour-Mobarhan, M.; Salehizadeh, L.; Rahmani, S.; Behnam, B.; Jamialahmadi, T.; Asgary, S.; Sahebkar, A. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102322. [Google Scholar] [CrossRef]
- Hariri, M.; Gholami, A.; Mirhafez, S.R.; Bidkhori, M.; Sahebkar, A. A pilot study of the effect of curcumin on epigenetic changes and DNA damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled, clinical trial. Complement. Ther. Med. 2020, 51, 102447. [Google Scholar] [CrossRef]
- Panahi, Y.; Valizadegan, G.; Ahamdi, N.; Ganjali, S.; Majeed, M.; Sahebkar, A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial. J. Cell. Biochem. 2019, 120, 15989–15996. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Azimi-Nezhad, M.; Dehabeh, M.; Hariri, M.; Naderan, R.D.; Movahedi, A.; Abdalla, M.; Sathyapalan, T.; Sahebkar, A. The Effect of Curcumin Phytosome on the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Adv. Exp. Med. Biol. 2021, 1308, 25–35. [Google Scholar]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Soflaei, S.S.; Sahebkar, A. Efficacy of phospholipidated curcumin in nonalcoholic fatty liver disease: A clinical study. J. Asian Nat. Prod. Res. 2019, 21, 798–805. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Khanna, V.G.; Thiruvengadam, M. Insights on the current status and advancement of diabetes mellitus type 2 and to avert complications: An overview. Biotechnol. Appl. Biochem. 2020, 67, 920–928. [Google Scholar] [CrossRef]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. (Cambridge, Mass.) 2018, 24, 59. [Google Scholar] [CrossRef]
- Kaur, P.; Kotru, S.; Singh, S.; Behera, B.S.; Munshi, A. Role of miRNAs in the pathogenesis of T2DM, insulin secretion, insulin resistance, and β cell dysfunction: The story so far. J. Physiol. Biochem. 2020, 76, 485–502. [Google Scholar] [CrossRef]
- Yuan, F.; Wu, W.; Ma, L.; Wang, D.; Hu, M.; Gong, J.; Fang, K.; Xu, L.; Dong, H.; Lu, F. Turmeric and curcuminiods ameliorate disorders of glycometabolism among subjects with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2022, 177, 106121. [Google Scholar] [CrossRef]
- Saeed, A.; Sun, W.; Agarwala, A.; Virani, S.S.; Nambi, V.; Coresh, J.; Selvin, E.; Boerwinkle, E.; Jones, P.H.; Ballantyne, C.M.; et al. Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: The Atherosclerosis Risk in Communities study. Atherosclerosis 2019, 282, 52–56. [Google Scholar] [CrossRef]
- Al-Mrabeh, A. β-Cell Dysfunction, Hepatic Lipid Metabolism, and Cardiovascular Health in Type 2 Diabetes: New Directions of Research and Novel Therapeutic Strategies. Biomedicines 2021, 9, 226. [Google Scholar] [CrossRef]
- Mihai, V.C.; Loredana, P.; Lucia, D.; Mădălina, M.; Mircea, P.; Gabriela, R.; Romulus, T.; Remus, P.A. Insulin resistance(IR) in patients with type 2 diabetes mellitus. Identifying predictors of insulin resistance and establishing a correlation between insulin resistance and cardiovascular risk. Intern. Med. 2018, 15, 7–15. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Guo, D.; Lv, Y.; Zheng, X.; Mo, Z.; Xie, W. Lipoprotein lipase transporter GPIHBP1 and triglyceride-rich lipoprotein metabolism. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 487, 33–40. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef]
- Muoio, D.M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell 2014, 159, 1253–1262. [Google Scholar] [CrossRef]
- Fernández-García, J.C.; Cardona, F.; Tinahones, F.J. Inflammation, oxidative stress and metabolic syndrome: Dietary modulation. Curr. Vasc. Pharmacol. 2013, 11, 906–919. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Milajerdi, A.; Varkaneh, H.K.; Gorjipour, M.M.; Esmaillzadeh, A. The effects of curcumin supplementation on body weight, body mass index and waist circumference: A systematic review and dose-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Haghighatdoost, F. Effect of curcumin on anthropometric measures: A systematic review on randomized clinical trials. J. Am. Coll. Nutr. 2018, 37, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, Z.; Zhao, X.; Liao, C.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X. Natural alkaloid and polyphenol compounds targeting lipid metabolism: Treatment implications in metabolic diseases. Eur. J. Pharmacol. 2020, 870, 172922. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Juanola, O.; Martínez-López, S.; Francés, R.; Gómez-Hurtado, I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int. J. Environ. Res. Public Health 2021, 18, 5227. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Chen, C.H.; Tsai, S.P.; Lu, P.J.; Wu, H.; Zeng, Y.; Ye, Y.; Tu, H.; Wen, C.; Huang, M.; et al. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Am. J. Gastroenterol. 2019, 114, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Jelinek, H.F. 8-Hydroxy-2-deoxy-guanosine identifies oxidative DNA damage in a rural prediabetes cohort. Redox Rep. Commun. Free. Radic. Res. 2010, 15, 155–160. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef]
- Mashayekhi-Sardoo, H.; Mashayekhi-Sardoo, A.; Roufogalis, B.D.; Jamialahmadi, T.; Sahebkar, A. Impact of Curcumin on Microsomal Enzyme Activities: Drug Interaction and Chemopreventive Studies. Curr. Med. Chem. 2021, 28, 7122–7140. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, X.; Bai, J.; Li, W.; Yang, J.; Deng, Z.; Wu, M.; Ying, T.; He, G. The effects of curcumin on anthropometric and cardiometabolic parameters of patients with metabolic related diseases: A systematic review and dose-effect meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Slika, L.; Patra, D. A short review on chemical properties, stability and nano-technological advances for curcumin delivery. Expert Opin. Drug Deliv. 2020, 17, 61–75. [Google Scholar] [CrossRef] [PubMed]


| Disease | Status | Phase I/II/III/IV | Estimated Enrollment | Intervention/Treatment | Dose | Duration | Start Date | Identifier |
|---|---|---|---|---|---|---|---|---|
| T2DM | - | IV | 200 | Curcumin capsules | 500 mg tid | 12 months | July 2009 | NCT01052597 |
| T2DM | - | II, III | 176 | Curcumin capsules | 400 mg bid | 26 weeks | 1 August 2018 | NCT03262363 |
| T2DM | - | II, III | 50 | Curcumin capsules | 500 mg/d | 12 weeks | July 2015 | NCT02529969 |
| T2DM | Completed | - | 44 | Curcumin capsules | 500 mg tid | 10 weeks | July 2015 | NCT02529982 |
| T2DM | Recruiting | IV | 60 | Curcumin, glimepiride and black pepper | 1100 mg/d | 3 months | 25 November 2020 | NCT04528212 |
| T2DM/NAFLD | Completed | II, III | 50 | Curcumin capsules | 1500 mg qd | 12 weeks | 10 February 2017 | NCT02908152 |
| T2DM Pre-diabetes | - | IV | 200 | Curcumin capsules | 500 mg tid | 12 months | August 2009 | NCT01052025 |
| High cholesterol obesity | Completed | - | 15 | Curcumin | 500 mg/d | 12 months | 19 September 2018 | NCT03542240 |
| Prediabetes | - | IV | 142 | Curcumin and bioperine | 500 mg qd | 3 months | 25 February 2019 | NCT03917784 |
| Obesity in childhood | - | - | 300 | Curcumin capsules and black pepper | 600 mg/d | 3 months | 8 January 2018 | NCT03670875 |
| NAFLD/insulin resistance | Completed | - | 39 | Phospholipid curcumin tablets | 500 mg bid | 6 weeks | 5 March 2019 | NCT03864783 |
| NAFLD/insulin resistance | Recruiting | - | 36 | Curcumin tablets | 100 mg bid | 11 days | 1 December 2019 | NCT04315350 |
| Disease | Sample Size (Test/Control) | Gender (Male/Female) | Curcumin Form | Purity | Dose | Duration | Outcome | Side Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| T2DM | 35/35 | 31/39 | Nano-curcumin capsules | 100% | 80 mg qd | 3 months | HbA1c↓, FBG↓, BMI↓ | No report | [31] |
| T2DM | 21/23 | 22/22 | Curcumin capsules | 88% | 500 mg tid | 10 weeks | adiponectin↑, hs-CRP↓, BW↓, FBG↓, BMI↓ | No report | [32] |
| T2DM | 50/50 | 51/49 | Curcumin C3 complex capsules | 100% | 1000 mg/d | 12 weeks | BW↓, BMI↓, TC↓, HDL-C↑, Non-HDL-C↓, LP(a)↓ | No side effects | [33] |
| T2DM | 50/50 | 51/49 | Curcumin C3 complex capsules | 100% | 500 mg qd | 3 months | BW↓, BMI↓, FBG↓, HbA1c↓, C-peptide↓, AST↓, ALT↓ | No side effects | [34] |
| T2DM | 50/50 | 51/49 | Curcumin C3 complex capsules | 100% | 1000 mg/d | 12 weeks | TNF-α↓, leptin↓, adiponectin↑, leptin: adiponectin ratio↓ | No side effects | [35] |
| T2DM | 50/50 | 51/49 | Curcumin C3 complex capsules | 100% | 1000 mg/d | 12 weeks | TAC↑, SOD↑, MDA↓ | No side effects | [36] |
| T2DM | 107/106 | 97/116 | Curcumin capsules | 100% | 750 mg bid | 6 months | adiponectin↑, leptin↓, HOMA-IR↓, TG↓, TC↓, LDL-C↓, HDL-C↑, SUA↓, PWV↓ | 2 patients had constipation and 1 patient had nausea | [37] |
| T2DM | 23/21 | 23/21 | Curcumin capsules (NCB-02) | 100% | 150 mg bid | 8 weeks | MDA↓, IL-6↓, TNF-α↓ | 2 patients had mild diarrhoea | [38] |
| T2DM | 8 | Unknown | Curcumin capsules | 100% | 475 mg qd | 10 days | LDL-C↓, VLDL-C↓, TG↓, HDL-C↑ | No side effects | [39] |
| T2DM with obesity | 50/50 | Unknown | Curcumin capsules | 100% | 150 mg bid | 3 months | A-FABP↓, TNF-α↓, CRP↓, IL-6↓, SOD↑, FFA↓ | No report | [40] |
| T2DM with obesity | 50/50 | 49/51 | Curcumin capsules | 97.49% | 150 mg bid | 3 months | FBG↓, HbA1c↓, HOMA-IR↓, FFA↓, TG↓, LPL↑ | No report | [41] |
| T2DM with obesity | 21/23 | 22/22 | Curcumin capsules | 88% | 500 mg tid | 10 weeks | FBG↓, WC↓, BW↓ | No side effects | [42] |
| T2DM with CHD | 25/24 | Unknown | Curcumin tablets | 100% | 1000 mg/d | 12 weeks | MDA↓, TAC↑, GSH↑, PPAR-γ↑ | No side effects | [43] |
| T2DM/IGT | 15/18 | 22/11 | Curcumin capsules | 100% | 90 mg bid | 6 months | TG↓, γ-GTP↓ | No report | [44] |
| T2DM on hemodialysis | 26/27 | 32/21 | Nano-curcumin capsules | 100% | 80 mg qd | 12 weeks | FBG↓, TG↓, VLDL-C↓, FINS↓, TC↓, LDL-C↓, hs-CRP↓, MDA↓, TAC↑, PPAR-γ↑ | No side effects | [45] |
| Prediabetes | 119/116 | 83/152 | Curcumin capsules | 100% | 750 mg, bid | 9 months | Adiponectin↑, HOMA-IR↓, C-peptide↓, HOMA-β↑ | 1 patient had itching, 2 patients had constipation and 1 patient had vertigo | [46] |
| Overweight | 40/40 | Unknown | Phospholipid curcumin tablets | 25% | 800 mg qd | 8 weeks | FINS↓, TG↓, GOT↓, GPT↓, cortisol↓ | 3 patients had abdominal discomfort | [47] |
| Obesity | 15/15 | 5/25 | Curcumin C3 complex capsules | 100% | 1000 mg/d | 30 days | TG↓ | 2 patients had constipation, 1 patient had diuresis and 1 patient had paramenia | [48] |
| Obesity | 15/15 | Unknown | Curcumin C3 complex capsules | 100% | 1000 mg/d | 30 days | PAB↓ | No report | [49] |
| Obesity | 15/15 | Unknown | Curcumin C3 complex capsules | 100% | 1000 mg/d | 4 weeks | IL-1β↓, IL-4↓, VEGF↓ | No report | [50] |
| Obesity | 20/20 | 40/0 | Curcumin | 70% | 500 mg 750 mg | 12 weeks | ox-LDL↓ | No report | [51] |
| Overweight/obesity | 30/30 | 0/60 | Curcumin capsules | 100% | 500 mg/d | 10weeks | IL-6↓, TAC↑, MDA↓ | No side effects | [52] |
| Obesity with NAFLD | 42/42 | 46/38 | Nano-curcumin capsules | 100% | 40 mg bid | 3 months | HDL↑, QUICKI↑, WC↓, ALT↓, AST↓, TC↓, LDL-C↓, TG↓, FBG↓, HbA1c↓, TNF-α↓, IL-6↓, hs-CRP↓, HOMA-IR↓, NAFLD severity↓ | 1 patient had nausea | [53] |
| Obesity with NAFLD | 42/42 | 46/38 | Nano-curcumin capsules | 100% | 40 mg bid | 3 months | BW↓, BMI↓, appetite↓ | No report | [54] |
| Obesity or overweight with migraine | 22/22 | 2/42 | Nano-curcumin capsules | 100% | 40 mg bid | 2 months | MCP-1↓ | No report | [55] |
| Obesity with prediabetes | 21/20 | 13/28 | Curcumin capsules | 95% | 500 mg qd | 90 days | FBG↓, 2hpp↓, HbA1c↓, FINS↓, HOMA-IR↓, ISI↑ | A few subjects had mild nausea, headaches and dizziness | [56] |
| Overweight with MetS | 22/22 | 17/27 | Curcumin tablets | 95% | 400 mg bid | 30 days | BMI↓, WC↓, BW↓, body fat percent↓ | No side effects | [57] |
| NAFLD | 37/40 | 38/39 | Curcumin (amorphous dispersion preparation) | 14% | 500 mg/d | 8 weeks | NAFLD severity↓, liver fat content↓, BW↓, BMI↓, TC↓, LDL-C↓, TG↓, HbA1c↓, AST↓, ALT↓ | 1 patient had stomachache and 2 patients had stomachache and nausea. | [58] |
| NAFLD | 44/43 | 51/36 | Phospholipid curcumin capsules | 20% | 500 mg bid | 8 weeks | TC↓, LDL-C↓, Non-HDL-C↓, TG↓, SUA↓ | No side effects | [59] |
| NAFLD | 32/29 | 37/24 | Phospholipid curcumin capsules | 20% | 250 mg qd | 8 weeks | HDL-C ↑, adiponectin↑, leptin↓ | No side effects | [60] |
| NAFLD | 22/22 | 29/15 | Phospholipid curcumin capsules | 20% | 250 mg qd | 8 weeks | 8-OHdG↓, BMI↓, CML↓, BW↓, WC↓, ALT↓, AST↓, body fat percent↓ | No side effects | [61] |
| NAFLD | 23/26 | Unknown | Curcumin C3 complex capsules | 100% | 500 mg qd | 8 weeks | NAFLD severity↓, BW↓, TNF-α↓, MCP-1↓ | 1 patient had digestive problems | [62] |
| NAFLD | 22/23 | 26/19 | Phospholipid curcumin capsules | 20% | 250 mg qd | 8 weeks | BMI↓ | No side effects | [63] |
| NAFLD | 35/35 | 31/39 | Curcumin C3 complex capsules | 100% | 500 mg qd | 12 weeks | NAFLD severity↓ | No report | [64] |
| NAFLD | 27/23 | 27/23 | Curcumin capsules | 95% | 500 mg tid | 12 weeks | - | No side effects | [65] |
| NAFLD | 35/37 | 41/31 | Phospholipid curcumin capsules | 20% | 250 mg qd | 2 months | NAFLD severity↓, AST↓ | No report | [66] |
| NAFLD | 44/43 | 36/51 | Phospholipid curcumin capsules | 20% | 500 mg bid | 8 weeks | BMI↓, WC↓, AST↓, ALT↓, NAFLD severity↓ | 5 patients had stomachache and flatulence | [5] |
| NAFLD | 36 | 19/17 | Phospholipid curcumin capsules | 20% | 1500 mg/d | 8 weeks | BMI↓, ALT↓, AST↓, LDL-C↓, TG↓, Non-HDL-C↓, SUA↓, NAFLD severity↓ | 1 patient had nausea | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Luo, Y.; Wang, L.; Zhang, K.; Peng, J.; Fan, G. Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies. Int. J. Mol. Sci. 2023, 24, 3323. https://doi.org/10.3390/ijms24043323
Zeng Y, Luo Y, Wang L, Zhang K, Peng J, Fan G. Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies. International Journal of Molecular Sciences. 2023; 24(4):3323. https://doi.org/10.3390/ijms24043323
Chicago/Turabian StyleZeng, Yujiao, Yuting Luo, Lijie Wang, Kun Zhang, Jiayan Peng, and Gang Fan. 2023. "Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies" International Journal of Molecular Sciences 24, no. 4: 3323. https://doi.org/10.3390/ijms24043323
APA StyleZeng, Y., Luo, Y., Wang, L., Zhang, K., Peng, J., & Fan, G. (2023). Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies. International Journal of Molecular Sciences, 24(4), 3323. https://doi.org/10.3390/ijms24043323





