Soft X-ray Fluorescence and Near-Edge Absorption Microscopy for Investigating Metabolic Features in Biological Systems: A Review
Abstract
1. Introduction
2. STXM, μ-XRF and μ-XANES
3. Experimental Studies
3.1. Life Science and Medicine
3.2. Microbiology and Bacteriology
3.3. Environment and Agriculture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeBerardinis, R.J.; Thompson, C.B. Cellular Metabolism and Disease: What Do Metabolic Outliers Teach Us? Cell 2012, 148, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Ghesquière, B.; Wong, B.W.; Kuchnio, A.; Carmeliet, P. Metabolism of stromal and immune cells in health and disease. Nature 2014, 511, 167–176. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth–metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Scafoglio, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Ghezzi, C.; Satyamurthy, N.; Moatamed, N.A.; Huang, J.; Koepsell, H.; Barrio, J.R.; et al. Functional expression of sodium-glucose transporters in cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4111–E4119. [Google Scholar] [CrossRef] [PubMed]
- Killilea, D.W.; Maier, J.A.M. A connection between magnesium deficiency and aging: New insights from cellular studies. Magnes. Res. 2008, 21, 77–82. [Google Scholar]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Lermyte, F.; Everett, J.; Brooks, J.; Bellingeri, F.; Billimoria, K.; Sadler, P.J.; O’Connor, P.B.; Telling, N.D.; Collingwood, J.F. Emerging Approaches to Investigate the Influence of Transition Metals in the Proteinopathies. Cells 2019, 8, 1231. [Google Scholar] [CrossRef]
- Everett, J.; Brooks, J.; Lermyte, F.; O’Connor, P.B.; Sadler, P.J.; Dobson, J.; Collingwood, J.F.; Telling, N.D. Iron stored in ferritin is chemically reduced in the presence of aggregating Aβ(1-42). Sci. Rep. 2020, 10, 10332. [Google Scholar] [CrossRef]
- Everett, J.; Lermyte, F.; Brooks, J.; Tjendana-Tjhin, V.; Plascencia-Villa, G.; Hands-Portman, I.; Donnelly, J.M.; Billimoria, K.; Perry, G.; Zhu, X.; et al. Biogenic metallic elements in the human brain? Sci. Adv. 2021, 7, eabf6707. [Google Scholar] [CrossRef]
- Zhang, F.; Tao, Y.; Zhang, Z.; Guo, X.; An, P.; Shen, Y.; Wu, Q.; Yu, Y.; Wang, F. Metalloreductase Steap3 coordinates the regulation of iron homeostasis and inflammatory responses. Haematologica 2012, 97, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Zündorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Salata, C.; deAlmeida, C.E.; Ferreira-Machado, S.C.; Barroso, R.C.; Nogueira, L.P.; Mantuano, A.; Pickler, A.; Mota, C.L.; de Andrade, C.B.V. Preliminary pre-clinical studies on the side effects of breast cancer treatment. Int. J. Radiat. Biol. 2021, 97, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Pickler, A.; Mantuano, A.; Mota, C.L.; Ferreira-Machado, S.; Lau, C.C.; de Almeida, C.E.; Salata, C.; Nascimento, A.; Tanure, T.; Serqueira, L.; et al. Elemental distribution in the aortic arch using LEXRF: Side effects of angiotensin receptor blockers as antihypertensive treatment. Microchem. J. 2019, 148, 467–474. [Google Scholar] [CrossRef]
- Schiroli, D.; Marraccini, C.; Zanetti, E.; Ragazzi, M.; Gianoncelli, A.; Quartieri, E.; Gasparini, E.; Iotti, S.; Baricchi, R.; Merolle, L. Imbalance of Mg Homeostasis as a Potential Biomarker in Colon Cancer. Diagnostics 2021, 11, 727. [Google Scholar] [CrossRef]
- Vidavsky, N.; Kunitake, J.A.M.R.; Chiou, A.E.; Northrup, P.A.; Porri, T.J.; Ling, L.; Fischbach, C.; Estroff, L.A. Studying biomineralization pathways in a 3D culture model of breast cancer microcalcifications. Biomaterials 2018, 179, 71–82. [Google Scholar] [CrossRef]
- Czapla-Masztafiak, J.; Okoń, K.; Gałka, M.; Huthwelker, T.; Kwiatek, W.M. Investigating the Distribution of Chemical Forms of Sulfur in Prostate Cancer Tissue Using X-ray Absorption Spectroscopy. Appl. Spectrosc. 2015, 70, 264–271. [Google Scholar] [CrossRef]
- Xiu, J.; Liu, Y.; Wang, B.; Xue, Y.; Chen, M.; Ji, T.; Liu, H. Quantitative toxicological study of dose-dependent arsenic-induced cells via synchrotron-based STXM and FTIR measurement. Analyst 2020, 145, 4560–4568. [Google Scholar] [CrossRef]
- Doelle, H.W. Bacterial Metabolism; Elsevier Science: Amsterdam, The Netherlands, 2014; ISBN 9781483272375. [Google Scholar]
- Bergsveinson, J.; Roy, J.; Maynard, C.; Sanschagrin, S.; Freeman, C.N.; Swerhone, G.D.W.; Dynes, J.J.; Tremblay, J.; Greer, C.W.; Korber, D.R.; et al. Metatranscriptomic Insights Into the Response of River Biofilm Communities to Ionic and Nano-Zinc Oxide Exposures. Front. Microbiol. 2020, 11, 267. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Bothfeld, F.; Vargas, R.; Stuckey, J.W.; Wang, J.; Kearns, K.; Michael, H.A.; Guimond, J.; Yu, X.; Sparks, D.L. Spatial and temporal heterogeneity of geochemical controls on carbon cycling in a tidal salt marsh. Geochim. Cosmochim. Acta 2020, 282, 1–18. [Google Scholar] [CrossRef]
- Cron, B.; Macalady, J.L.; Cosmidis, J. Organic Stabilization of Extracellular Elemental Sulfur in a Sulfurovum-Rich Biofilm: A New Role for Extracellular Polymeric Substances? Front. Microbiol. 2021, 12, 720101. [Google Scholar] [CrossRef] [PubMed]
- Alleon, J.; Flannery, D.T.; Ferralis, N.; Williford, K.H.; Zhang, Y.; Schuessler, J.A.; Summons, R.E. Organo-mineral associations in chert of the 3.5 Ga Mount Ada Basalt raise questions about the origin of organic matter in Paleoarchean hydrothermally influenced sediments. Sci. Rep. 2019, 9, 16712. [Google Scholar] [CrossRef] [PubMed]
- Picard, A.; Gartman, A.; Cosmidis, J.; Obst, M.; Vidoudez, C.; Clarke, D.R.; Girguis, P.R. Authigenic metastable iron sulfide minerals preserve microbial organic carbon in anoxic environments. Chem. Geol. 2019, 530, 119343. [Google Scholar] [CrossRef]
- Li, J.; Liu, P.; Wang, J.; Roberts, A.P.; Pan, Y. Magnetotaxis as an Adaptation to Enable Bacterial Shuttling of Microbial Sulfur and Sulfur Cycling Across Aquatic Oxic-Anoxic Interfaces. J. Geophys. Res. Biogeosciences 2020, 125, e2020JG006012. [Google Scholar] [CrossRef]
- Boschker, H.T.S.; Cook, P.L.M.; Polerecky, L.; Eachambadi, R.T.; Lozano, H.; Hidalgo-Martinez, S.; Khalenkow, D.; Spampinato, V.; Claes, N.; Kundu, P.; et al. Efficient long-range conduction in cable bacteria through nickel protein wires. Nat. Commun. 2021, 12, 3996. [Google Scholar] [CrossRef]
- Monteil, C.L.; Benzerara, K.; Menguy, N.; Bidaud, C.C.; Michot-Achdjian, E.; Bolzoni, R.; Mathon, F.P.; Coutaud, M.; Alonso, B.; Garau, C.; et al. Intracellular amorphous Ca-carbonate and magnetite biomineralization by a magnetotactic bacterium affiliated to the Alphaproteobacteria. ISME J. 2021, 15, 1–18. [Google Scholar] [CrossRef]
- Duverger, A.; Berg, J.S.; Busigny, V.; Guyot, F.; Bernard, S.; Miot, J. Mechanisms of Pyrite Formation Promoted by Sulfate-Reducing Bacteria in Pure Culture. Front. Earth Sci. 2020, 8, 588310. [Google Scholar] [CrossRef]
- Berg, J.S.; Duverger, A.; Cordier, L.; Laberty-Robert, C.; Guyot, F.; Miot, J. Rapid pyritization in the presence of a sulfur/sulfate-reducing bacterial consortium. Sci. Rep. 2020, 10, 8264. [Google Scholar] [CrossRef]
- Hitchcock, A.P.; Obst, M.; Wang, J.; Lu, Y.S.; Tyliszczak, T. Advances in the Detection of As in Environmental Samples Using Low Energy X-ray Fluorescence in a Scanning Transmission X-ray Microscope: Arsenic Immobilization by an Fe(II)-Oxidizing Freshwater Bacteria. Environ. Sci. Technol. 2012, 46, 2821–2829. [Google Scholar] [CrossRef]
- Chi, Z.-L.; Yu, G.-H.; Teng, H.H.; Liu, H.-G.; Wang, J.; Liu, C.-Q.; Shen, Q.-R.; Gadd, G.M. Molecular Trade-Offs between Lattice Oxygen and Oxygen Vacancy Drive Organic Pollutant Degradation in Fungal Biomineralized Exoskeletons. Environ. Sci. Technol. 2022, 56, 8132–8141. [Google Scholar] [CrossRef]
- Žižić, M.; Dučić, T.; Grolimund, D.; Bajuk-Bogdanović, D.; Nikolic, M.; Stanić, M.; Križak, S.; Zakrzewska, J. X-ray absorption near-edge structure micro-spectroscopy study of vanadium speciation in Phycomyces blakesleeanus mycelium. Anal. Bioanal. Chem. 2015, 407, 7487–7496. [Google Scholar] [CrossRef]
- Vogel, C.; Sekine, R.; Huang, J.; Steckenmesser, D.; Steffens, D.; Huthwelker, T.; Borca, C.N.; Pradas del Real, A.E.; Castillo-Michel, H.; Adam, C. Effects of a nitrification inhibitor on nitrogen species in the soil and the yield and phosphorus uptake of maize. Sci. Total Environ. 2020, 715, 136895. [Google Scholar] [CrossRef] [PubMed]
- van Veelen, A.; Koebernick, N.; Scotson, C.S.; McKay-Fletcher, D.; Huthwelker, T.; Borca, C.N.; Mosselmans, J.F.W.; Roose, T. Root-induced soil deformation influences Fe, S and P: Rhizosphere chemistry investigated using synchrotron XRF and XANES. New Phytol. 2020, 225, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Ohkura, T.; Hikono, A.; Hashimoto, Y.; Suda, A.; Yamamoto, T.; Ando, K.; Kasuya, M.; Northrup, P.; Wang, S.-L.; et al. Microscale Heterogeneous Distribution and Speciation of Phosphorus in Soils Amended with Mineral Fertilizer and Cattle Manure Compost. Minerals 2021, 11, 121. [Google Scholar] [CrossRef]
- Tefera, W.; Liu, T.; Lu, L.; Ge, J.; Webb, S.M.; Seifu, W.; Tian, S. Micro-XRF mapping and quantitative assessment of Cd in rice (Oryza sativa L.) roots. Ecotoxicol. Environ. Saf. 2020, 193, 110245. [Google Scholar] [CrossRef]
- Limmer, M.A.; Seyfferth, A.L. Altering the localization and toxicity of arsenic in rice grain. Sci. Rep. 2022, 12, 5210. [Google Scholar] [CrossRef]
- Alves, E.E.N.; Ortega Rodriguez, D.R.; de Azevedo Rocha, P.; Vergütz, L.; Santini Junior, L.; Hesterberg, D.; Pessenda, L.C.R.; Tomazello-Filho, M.; da Costa, L.M. Synchrotron-based X-ray microscopy for assessing elements distribution and speciation in mangrove tree-rings. Results Chem. 2021, 3, 100121. [Google Scholar] [CrossRef]
- Gianoncelli, A.; Morrison, G.R.; Kaulich, B.; Bacescu, D.; Kovac, J. Scanning transmission x-ray microscopy with a configurable detector. Appl. Phys. Lett. 2006, 89, 251117. [Google Scholar] [CrossRef]
- Hornberger, B.; de Jonge, M.D.; Feser, M.; Holl, P.; Holzner, C.; Jacobsen, C.; Legnini, D.; Paterson, D.; Rehak, P.; Strüder, L.; et al. Differential phase contrast with a segmented detector in a scanning X-ray microprobe. J. Synchrotron Radiat. 2008, 15, 355–362. [Google Scholar] [CrossRef]
- Morrison, G.R.; Gianoncelli, A.; Kaulich, B. Image reconstruction using a configurable detector in STXM. J. Phys. Conf. Ser. 2009, 186, 12011. [Google Scholar] [CrossRef]
- Kaulich, B.; Gianoncelli, A.; Beran, A.; Eichert, D.; Kreft, I.; Pongrac, P.; Regvar, M.; Vogel-Mikus, K.; Kiskinova, M. Low-energy X-ray fluorescence microscopy opening new opportunities for bio-related research. J. R. Soc. Interface 2009, 6 (Suppl. 5), S641–S647. [Google Scholar] [CrossRef] [PubMed]
- Using XrayDB from Python—X-ray DB: X-ray Reference Data in SQLite. Available online: https://github.com/xraypy/XrayDB (accessed on 6 December 2022).
- Ortega, R. Direct speciation analysis of inorganic elements in single cells using X-ray absorption spectroscopy. J. Anal. At. Spectrom. 2011, 26, 23–29. [Google Scholar] [CrossRef]
- Bluhm, H.; Andersson, K.; Araki, T.; Benzerara, K.; Brown, G.E.; Dynes, J.J.; Ghosal, S.; Gilles, M.K.; Hansen, H.-C.; Hemminger, J.C.; et al. Soft X-ray microscopy and spectroscopy at the molecular environmental science beamline at the Advanced Light Source. J. Electron Spectros. Relat. Phenomena 2006, 150, 86–104. [Google Scholar] [CrossRef]
- Pascolo, L.; Gianoncelli, A.; Schneider, G.; Salomé, M.; Schneider, M.; Calligaro, C.; Kiskinova, M.; Melato, M.; Rizzardi, C. The interaction of asbestos and iron in lung tissue revealed by synchrotron-based scanning X-ray microscopy. Sci. Rep. 2013, 3, 1123. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, L.; Gianoncelli, A.; Rizzardi, C.; de Jonge, M.; Howard, D.; Paterson, D.; Cammisuli, F.; Salomé, M.; De Paoli, P.; Melato, M.; et al. Focused X-Ray Histological Analyses to Reveal Asbestos Fibers and Bodies in Lungs and Pleura of Asbestos-Exposed Subjects. Microsc. Microanal. 2016, 22, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Gianoncelli, A.; Bonanni, V.; Gariani, G.; Guzzi, F.; Pascolo, L.; Borghes, R.; Billè, F.; Kourousias, G. Soft X-ray Microscopy Techniques for Medical and Biological Imaging at TwinMic—Elettra. Appl. Sci. 2021, 11, 7216. [Google Scholar] [CrossRef]
- Salomé, M.; Cotte, M.; Baker, R.; Barrett, R.; Benseny-Cases, N.; Berruyer, G.; Bugnazet, D.; Castillo-Michel, H.; Cornu, C.; Fayard, B.; et al. The ID21 Scanning X-ray Microscope at ESRF. J. Phys. Conf. Ser. 2013, 425, 182004. [Google Scholar] [CrossRef]
- Howard, D.L.; de Jonge, M.D.; Afshar, N.; Ryan, C.G.; Kirkham, R.; Reinhardt, J.; Kewish, C.M.; McKinlay, J.; Walsh, A.; Divitcos, J.; et al. The XFM beamline at the Australian Synchrotron. J. Synchrotron Radiat. 2020, 27, 1447–1458. [Google Scholar] [CrossRef]
- Yu, S.U.; Lee, H.; Cho, W.J.; Kim, C.; Kang, M.C.; Shin, H.-J.; Kim, N.; Hahn, S.K.; Kim, K.S. Spectromicroscopic observation of a live single cell in a biocompatible liquid-enclosing graphene system. Nanoscale 2018, 10, 150–157. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, N.; Kim, H.-S.; Lee, W.-W.; Lee, C.-S.; Kim, B. A scanning transmission X-ray microscope at the Pohang Light Source. J. Synchrotron Radiat. 2018, 25, 878–884. [Google Scholar] [CrossRef]
- Arble, C.; Guo, H.; Matruglio, A.; Gianoncelli, A.; Vaccari, L.; Birarda, G.; Kolmakov, A. Addressable graphene encapsulation of wet specimens on a chip for optical, electron, infrared and X-ray based spectromicroscopy studies. Lab Chip 2021, 21, 4618–4628. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Klossek, A.; Fuchs, K.; Watts, B.; Raabe, J.; Flesch, R.; Rancan, F.; Pischon, H.; Radbruch, M.; Gruber, A.D.; et al. Soft X-ray microscopy for probing of topical tacrolimus delivery via micelles. Eur. J. Pharm. Biopharm. 2019, 139, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Raabe, J.; Tzvetkov, G.; Flechsig, U.; Böge, M.; Jaggi, A.; Sarafimov, B.; Vernooij, M.G.C.; Huthwelker, T.; Ade, H.; Kilcoyne, D.; et al. PolLux: A new facility for soft x-ray spectromicroscopy at the Swiss Light Source. Rev. Sci. Instrum. 2008, 79, 113704. [Google Scholar] [CrossRef]
- Ohigashi, T.; Arai, H.; Araki, T.; Kondo, N.; Shigemasa, E.; Ito, A.; Kosugi, N.; Katoh, M. Construction of the Scanning Transmission X-ray Microscope Beamline at UVSOR. J. Phys. Conf. Ser. 2013, 463, 012006. [Google Scholar] [CrossRef]
- Yamamoto, K.; Klossek, A.; Flesch, R.; Ohigashi, T.; Fleige, E.; Rancan, F.; Frombach, J.; Vogt, A.; Blume-Peytavi, U.; Schrade, P.; et al. Core-multishell nanocarriers: Transport and release of dexamethasone probed by soft X-ray spectromicroscopy. J. Control. Release 2016, 242, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Belkhou, R.; Stanescu, S.; Swaraj, S.; Besson, A.; Ledoux, M.; Hajlaoui, M.; Dalle, D. HERMES: A soft X-ray beamline dedicated to X-ray microscopy. J. Synchrotron Radiat. 2015, 22, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Kaznatcheev, K.V.; Karunakaran, C.; Lanke, U.D.; Urquhart, S.G.; Obst, M.; Hitchcock, A.P. Soft X-ray spectromicroscopy beamline at the CLS: Commissioning results. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 582, 96–99. [Google Scholar] [CrossRef]
- Fichtner, V.; Lange, S.M.; Krause, S.; Huthwelker, T.; Borca, C.N.; Schurr, S.L.; Immenhauser, A.; Pederson, C.L.; Treude, T.; Erhardt, A.M.; et al. Microbial activity affects sulphur in biogenic aragonite. Depos. Rec. 2021, 7, 500–519. [Google Scholar] [CrossRef]
- Farfan, G.A.; Apprill, A.; Webb, S.M.; Hansel, C.M. Coupled X-ray Fluorescence and X-ray Absorption Spectroscopy for Microscale Imaging and Identification of Sulfur Species within Tissues and Skeletons of Scleractinian Corals. Anal. Chem. 2018, 90, 12559–12566. [Google Scholar] [CrossRef]
- Mayhew, L.E.; Webb, S.M.; Templeton, A.S. Microscale imaging and identification of Fe speciation and distribution during fluid-mineral reactions under highly reducing conditions. Environ. Sci. Technol. 2011, 45, 4468–4474. [Google Scholar] [CrossRef]
- Yan, B.; Isaure, M.-P.; Mounicou, S.; Castillo-Michel, H.; De Nolf, W.; Nguyen, C.; Cornu, J.-Y. Cadmium distribution in mature durum wheat grains using dissection, laser ablation-ICP-MS and synchrotron techniques. Environ. Pollut. 2020, 260, 113987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.; Zhang, Z.; He, X.; Zhang, J.; Guo, Z.; Tai, R.; Zhao, Y.; Chai, Z. Biotransformation of Ceria Nanoparticles in Cucumber Plants. ACS Nano 2012, 6, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Medas, D.; Carlomagno, I.; Meneghini, C.; Aquilanti, G.; Araki, T.; Bedolla, D.E.; Buosi, C.; Casu, M.A.; Gianoncelli, A.; Kuncser, A.C.; et al. Zinc incorporation in marine bivalve shells grown in mine-polluted seabed sediments: A case study in the Malfidano mining area (SW Sardinia, Italy). Environ. Sci. Pollut. Res. 2018, 25, 36645–36660. [Google Scholar] [CrossRef] [PubMed]
- Northrup, P.; Leri, A.; Tappero, R. Applications of “Tender” Energy (1-5 keV) X-ray Absorption Spectroscopy in Life Sciences. Protein Pept. Lett. 2016, 23, 300–308. [Google Scholar] [CrossRef]
- Tamenori, Y.; Morita, M.; Nakamura, T. Two-dimensional approach to fluorescence yield XANES measurement using a silicon drift detector. J. Synchrotron Radiat. 2011, 18, 747–752. [Google Scholar] [CrossRef]
- Li, P.; Allain, M.; Grünewald, T.A.; Rommel, M.; Campos, A.; Carbone, D.; Chamard, V. 4th generation synchrotron source boosts crystalline imaging at the nanoscale. Light Sci. Appl. 2022, 11, 73. [Google Scholar] [CrossRef]
- Correa, J.; Mehrjoo, M.; Battistelli, R.; Lehmkühler, F.; Marras, A.; Wunderer, C.B.; Hirono, T.; Felk, V.; Krivan, F.; Lange, S.; et al. The PERCIVAL detector: First user experiments. J. Synchrotron Radiat. 2023, 30, 242–250. [Google Scholar] [CrossRef]
- Hatsui, T.; Graafsma, H. X-ray imaging detectors for synchrotron and XFEL sources. IUCrJ 2015, 2, 371–383. [Google Scholar] [CrossRef]
- Desjardins, K.; Medjoubi, K.; Sacchi, M.; Popescu, H.; Gaudemer, R.; Belkhou, R.; Stanescu, S.; Swaraj, S.; Besson, A.; Vijayakumar, J.; et al. Backside-illuminated scientific CMOS detector for soft X-ray resonant scattering and ptychography. J. Synchrotron Radiat. 2020, 27, 1577–1589. [Google Scholar] [CrossRef]
- Menk, R.H.; Arfelli, F.; Cautero, M.; Cautero, G.; Fraia, M.D.; Coreno, M.; Galdenzi, F.; Tutsch, W. On the possibility to utilize a PCO Edge 4.2 bi scientific CMOS imager for extended ultra violet and soft X-ray photon detection. J. Instrum. 2022, 17, C01058. [Google Scholar] [CrossRef]
- Cartier, S.; Kagias, M.; Bergamaschi, A.; Wang, Z.; Dinapoli, R.; Mozzanica, A.; Ramilli, M.; Schmitt, B.; Brückner, M.; Fröjdh, E.; et al. Micrometer-resolution imaging using MÖNCH: Towards G(2)-less grating interferometry. J. Synchrotron Radiat. 2016, 23, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kaneko, F.; Ishiguro, N.; Kudo, T.; Matsumoto, T.; Hatsui, T.; Tamenori, Y.; Kishimoto, H.; Takahashi, Y. Development and application of a tender X-ray ptychographic coherent diffraction imaging system on BL27SU at SPring-8. J. Synchrotron Radiat. 2021, 28, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Kourousias, G.; Billè, F.; Borghes, R.; Alborini, A.; Sala, S.; Alberti, R.; Gianoncelli, A. Compressive Sensing for Dynamic XRF Scanning. Sci. Rep. 2020, 10, 9990. [Google Scholar] [CrossRef] [PubMed]
- Kourousias, G.; Billè, F.; Borghes, R.; Pascolo, L.; Gianoncelli, A. Megapixel scanning transmission soft X-ray microscopy imaging coupled with compressive sensing X-ray fluorescence for fast investigation of large biological tissues. Analyst 2021, 146, 5836–5842. [Google Scholar] [CrossRef]
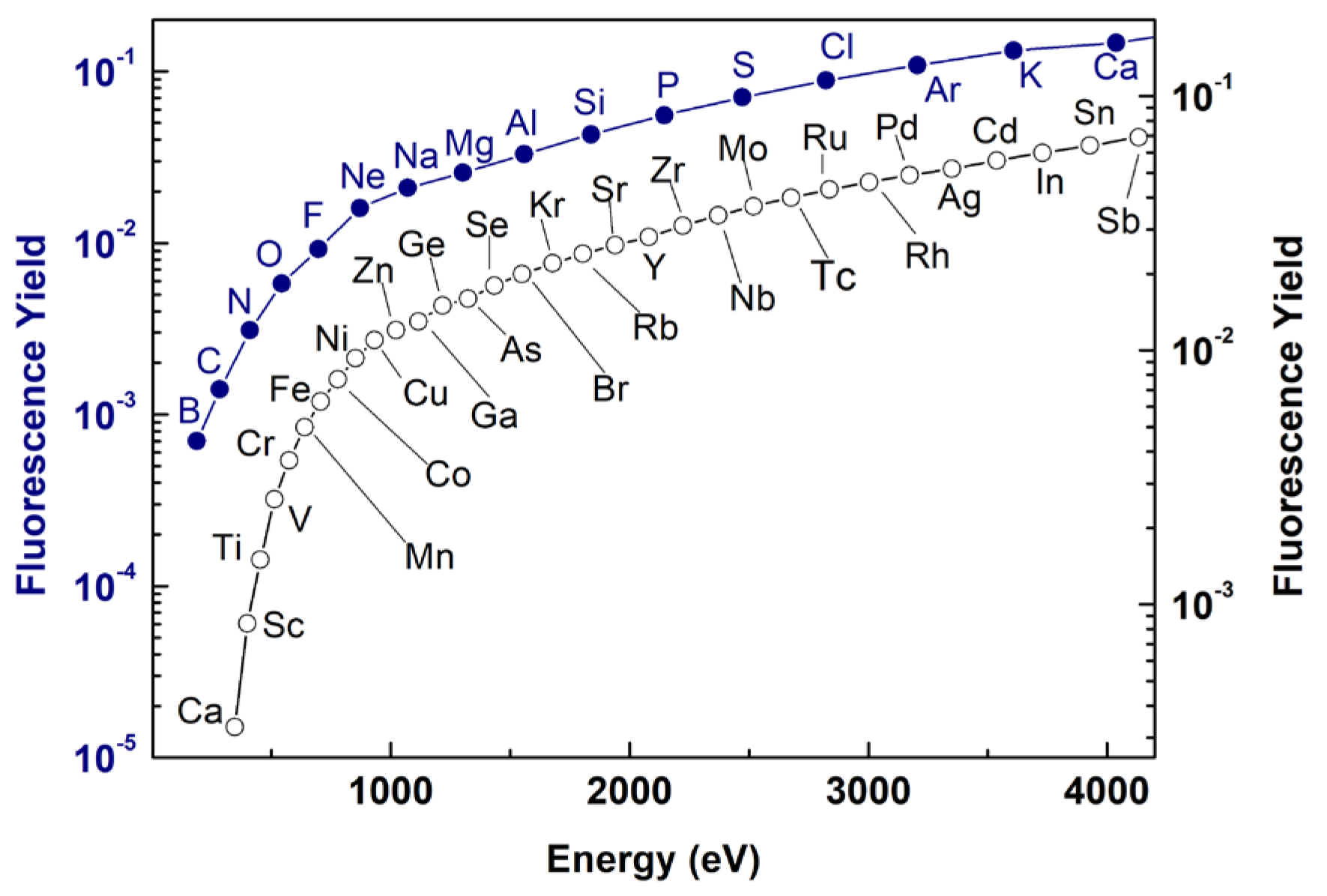

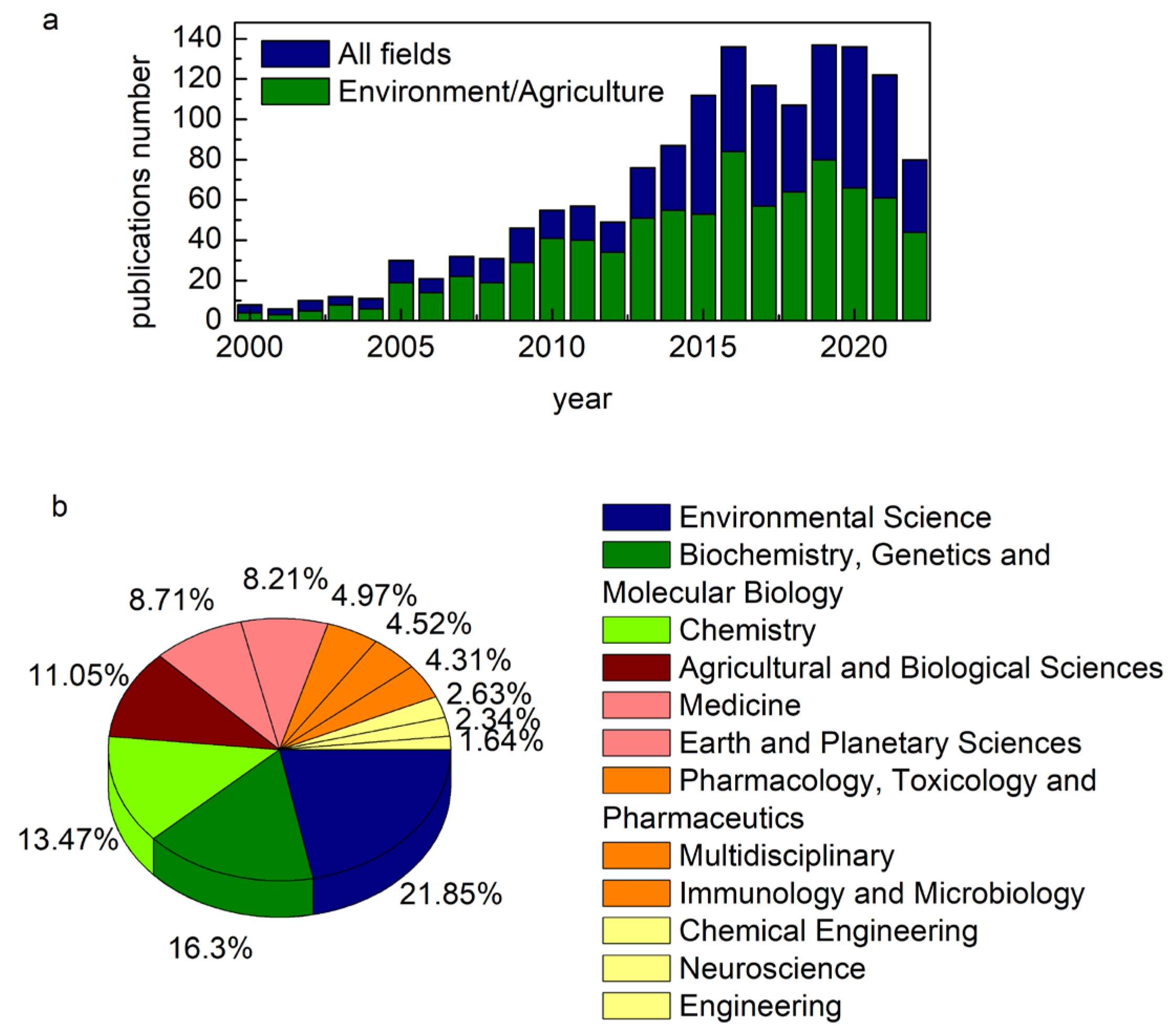
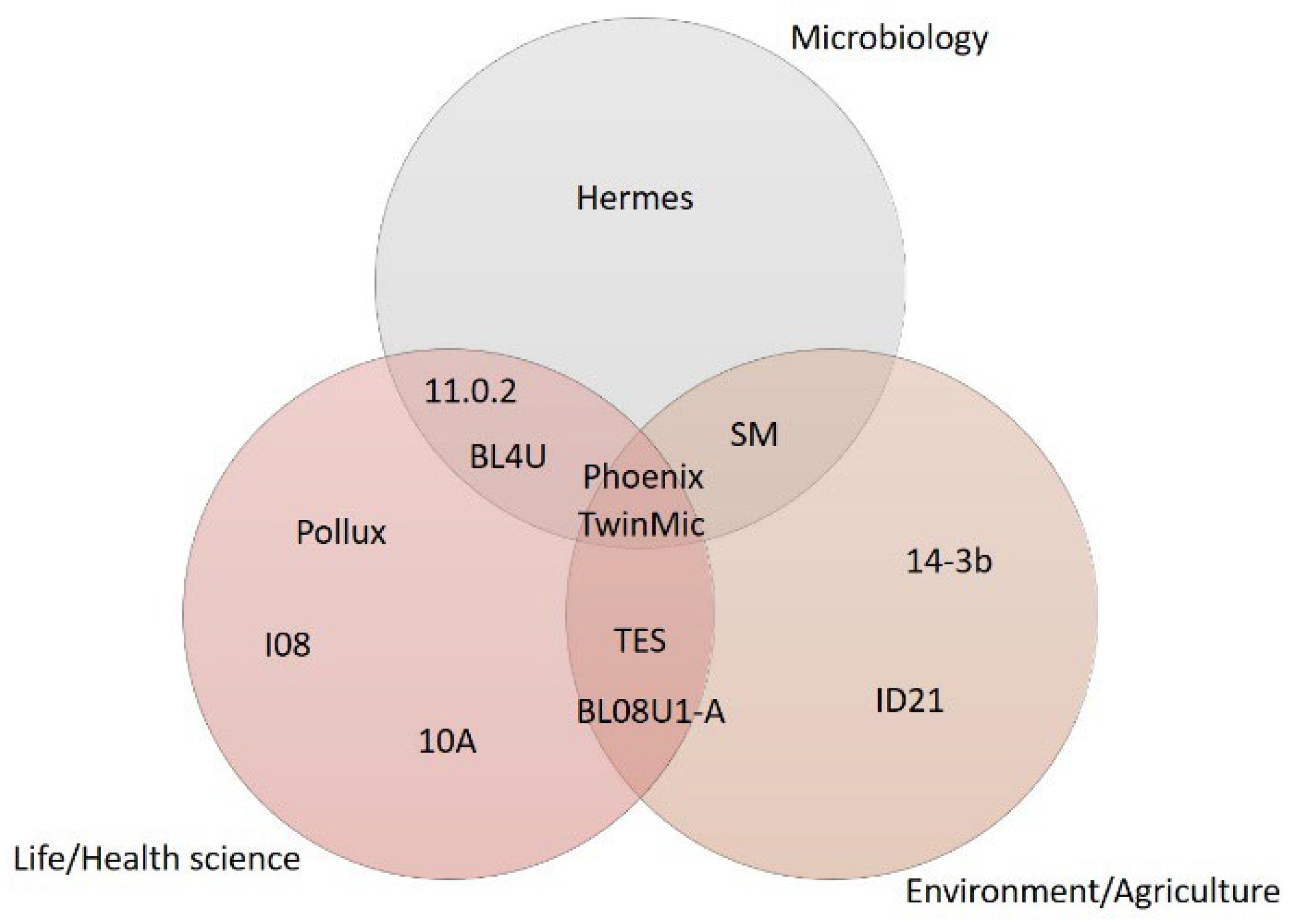
| Beamline | Facility | Country | Available Techniques | Spot Size/Spatial Resolution # [μm] | Energy Range [eV] | Application |
|---|---|---|---|---|---|---|
| 11.0.2 | Advanced Light Source (ALS) | USA | STXM, μ-XANES | 0.025 | 160–2000 | Life/Health science, Microbiology |
| MYSTIIC @EMIL * | Bessy II | Germany | STXM | 0.025# | 250–1500 | |
| SM | Canadian Light Source (CLS) | Canada | STXM, μ-XRF and μ-XANES | 0.03 | 130–2700 | Microbiology, Environment/Agriculture |
| I08 | Diamond | UK | STXM, μ-XRF and μ-XANES | 0.02# | 250–4200 | Life/Health science |
| TwinMic | Elettra | Italy | STXM, μ-XRF and μ-XANES | 0.1 | 400–2200 | Environment/Agriculture, Microbiology, Life/Health science |
| ID21 | European Synchrotron Radiation Facility (ESRF) | France | STXM, μ-XRF and μ-XANES | 0.07 × 0.03 | 2100–9200 | Environment/Agriculture |
| SoftiMAX * | MAX IV | Sweden | STXM, μ-XRF and μ-XANES | 0.06 | 275–2500 | |
| TES | National Synchrotron Light Source II (NSLS II) | USA | μ-XRF and μ-XANES | 2 | 2000–5500 | Environment/Agriculture, Life/Health science |
| Phoenix | Paul Scherrer Institute (PSI) | Switzerland | μ-XRF and μ-XANES | 2.5 | 400–2000 | Environment/Agriculture, Microbiology, Life/Health science |
| Pollux | Paul Scherrer Institute (PSI) | Switzerland | STXM | 0.02# | 250–1600 | Life/Health science |
| 10A | Pohang Light Source (PLS) | Korea | STXM, μ-XANES | 0.025# | 100–2000 | Life/Health science |
| BL08U1-A | Shanghai Synchrotron Radiation Facility (SSRF) | China | STXM, μ-XANES | 0.03# | 250–2000 | Life/Health science, Environment/Agriculture |
| Demeter * | Solaris | Poland | STXM | 10–2000 | ||
| Hermes | Soleil | France | STXM, μ-XANES | 0.025 | 70–2500 | Microbiology |
| 14-3b | Stanford Synchrotron Radiation Lightsource (SSRL) | USA | μ-XRF and μ-XANES | 5 | 2100–5000 | Environment/Agriculture |
| 27A * | Taiwan Photon Source (TPS) | Taiwan | 0.03# | 90–2500 | ||
| BL4U | UVSOR | Japan | STXM | 0.03 | 50–770 | Microbiology, Life/Health science |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanni, V.; Gianoncelli, A. Soft X-ray Fluorescence and Near-Edge Absorption Microscopy for Investigating Metabolic Features in Biological Systems: A Review. Int. J. Mol. Sci. 2023, 24, 3220. https://doi.org/10.3390/ijms24043220
Bonanni V, Gianoncelli A. Soft X-ray Fluorescence and Near-Edge Absorption Microscopy for Investigating Metabolic Features in Biological Systems: A Review. International Journal of Molecular Sciences. 2023; 24(4):3220. https://doi.org/10.3390/ijms24043220
Chicago/Turabian StyleBonanni, Valentina, and Alessandra Gianoncelli. 2023. "Soft X-ray Fluorescence and Near-Edge Absorption Microscopy for Investigating Metabolic Features in Biological Systems: A Review" International Journal of Molecular Sciences 24, no. 4: 3220. https://doi.org/10.3390/ijms24043220
APA StyleBonanni, V., & Gianoncelli, A. (2023). Soft X-ray Fluorescence and Near-Edge Absorption Microscopy for Investigating Metabolic Features in Biological Systems: A Review. International Journal of Molecular Sciences, 24(4), 3220. https://doi.org/10.3390/ijms24043220






