Brain Waste Removal System and Sleep: Photobiomodulation as an Innovative Strategy for Night Therapy of Brain Diseases
Abstract
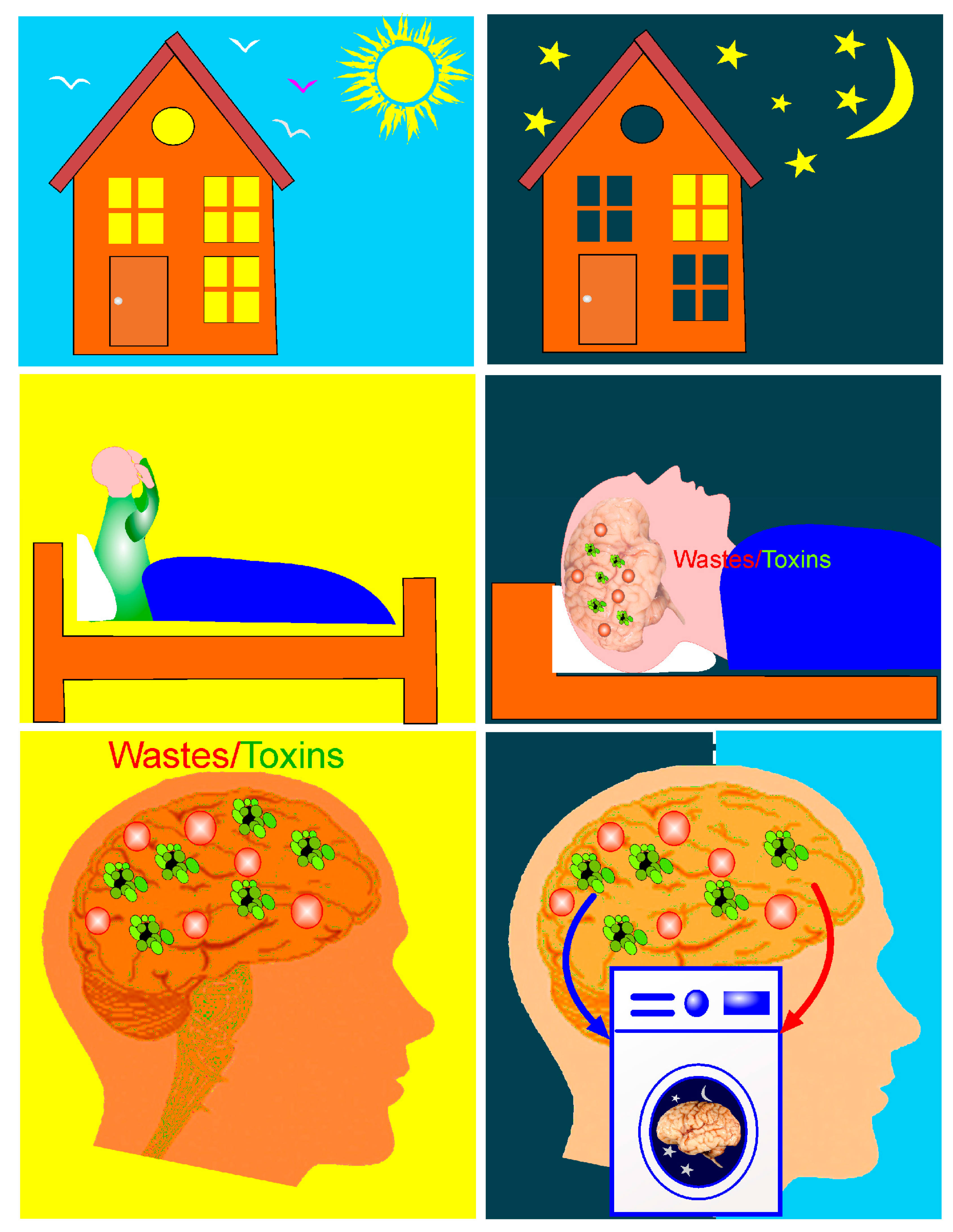
1. Therapeutic Properties of Activation of Brain Waste Removal System (BWRS) during Deep Sleep
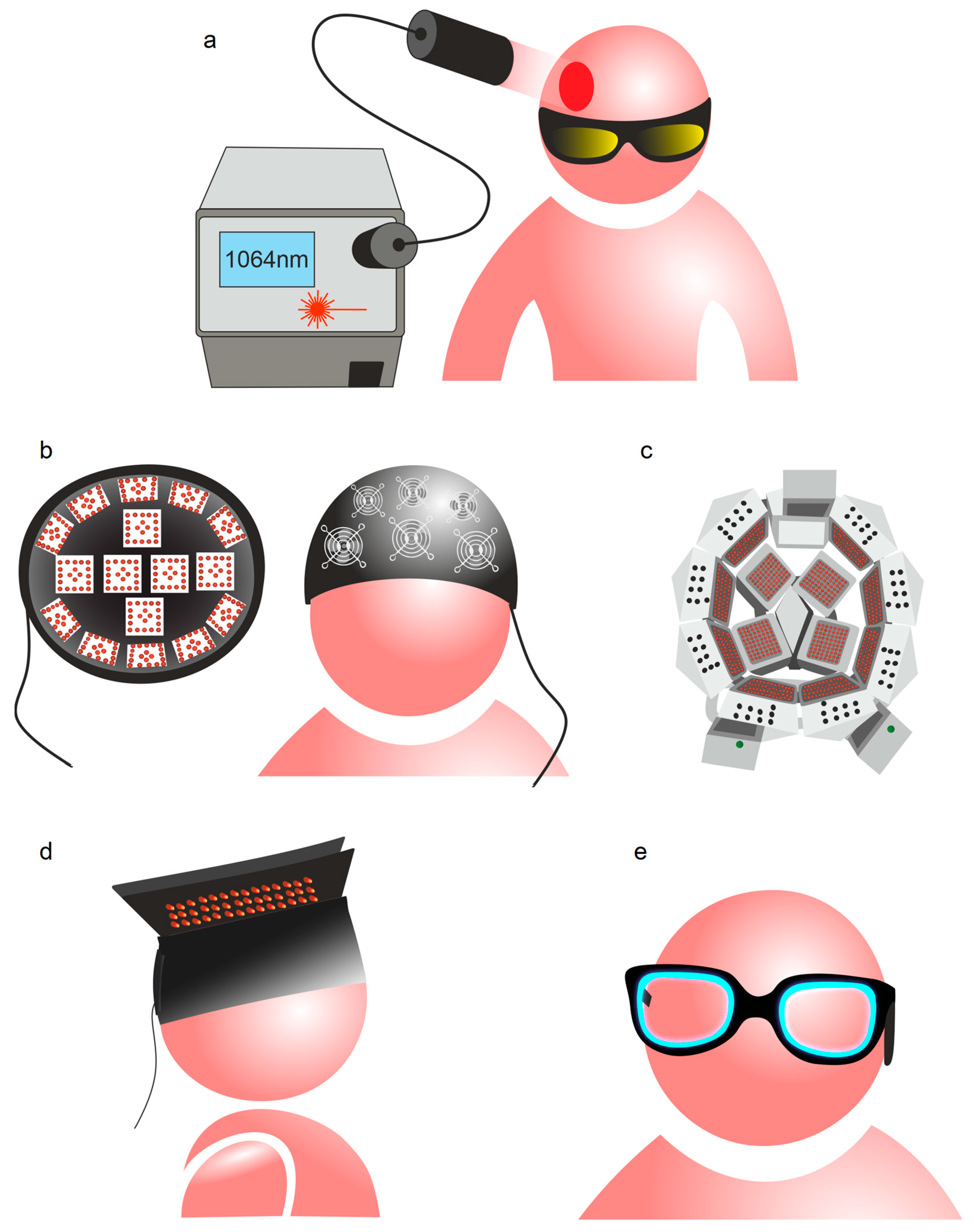
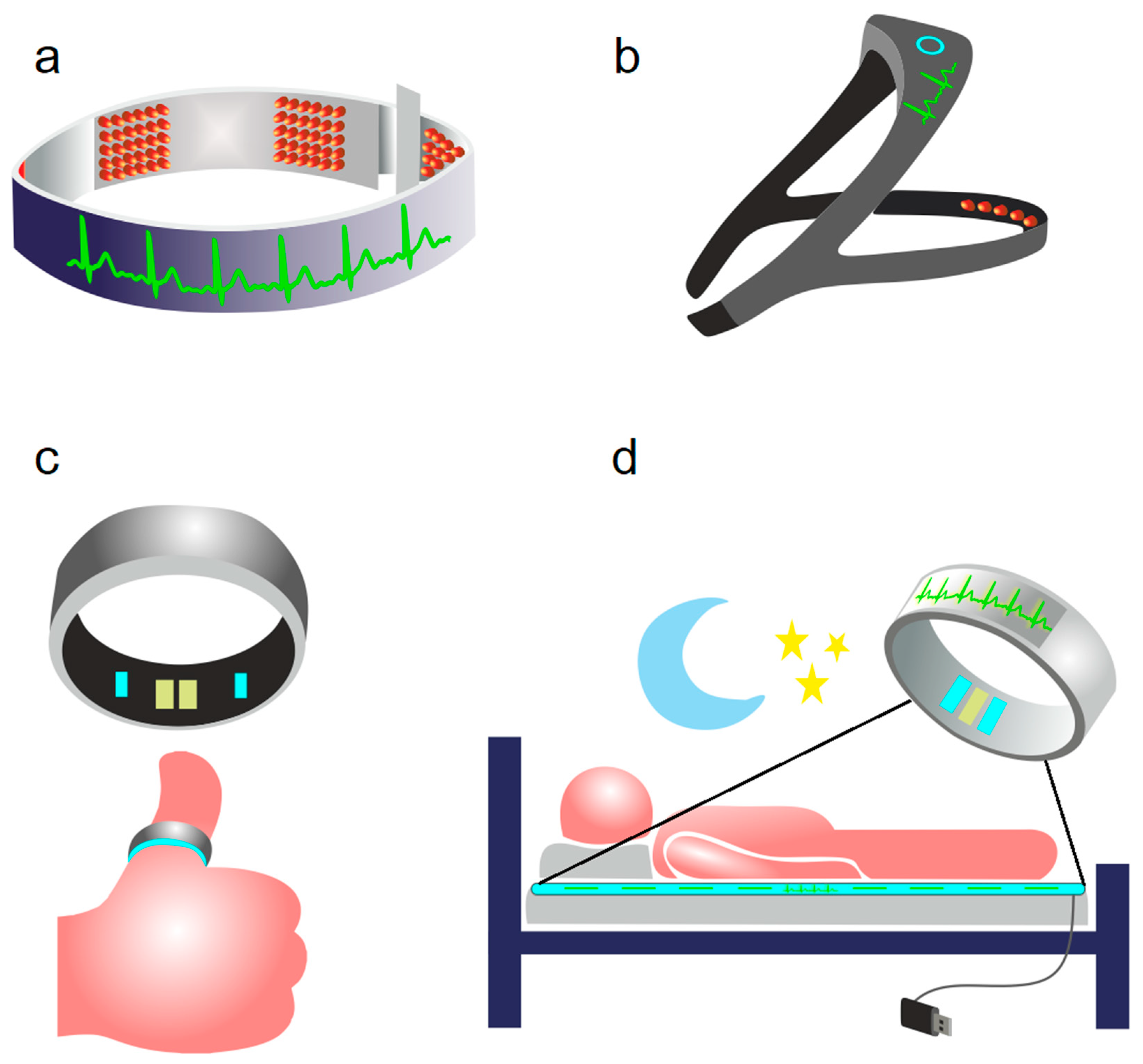
2. Photobiomodulation of BWRS: Innovative Strategies for Night Therapy of Brain Diseases
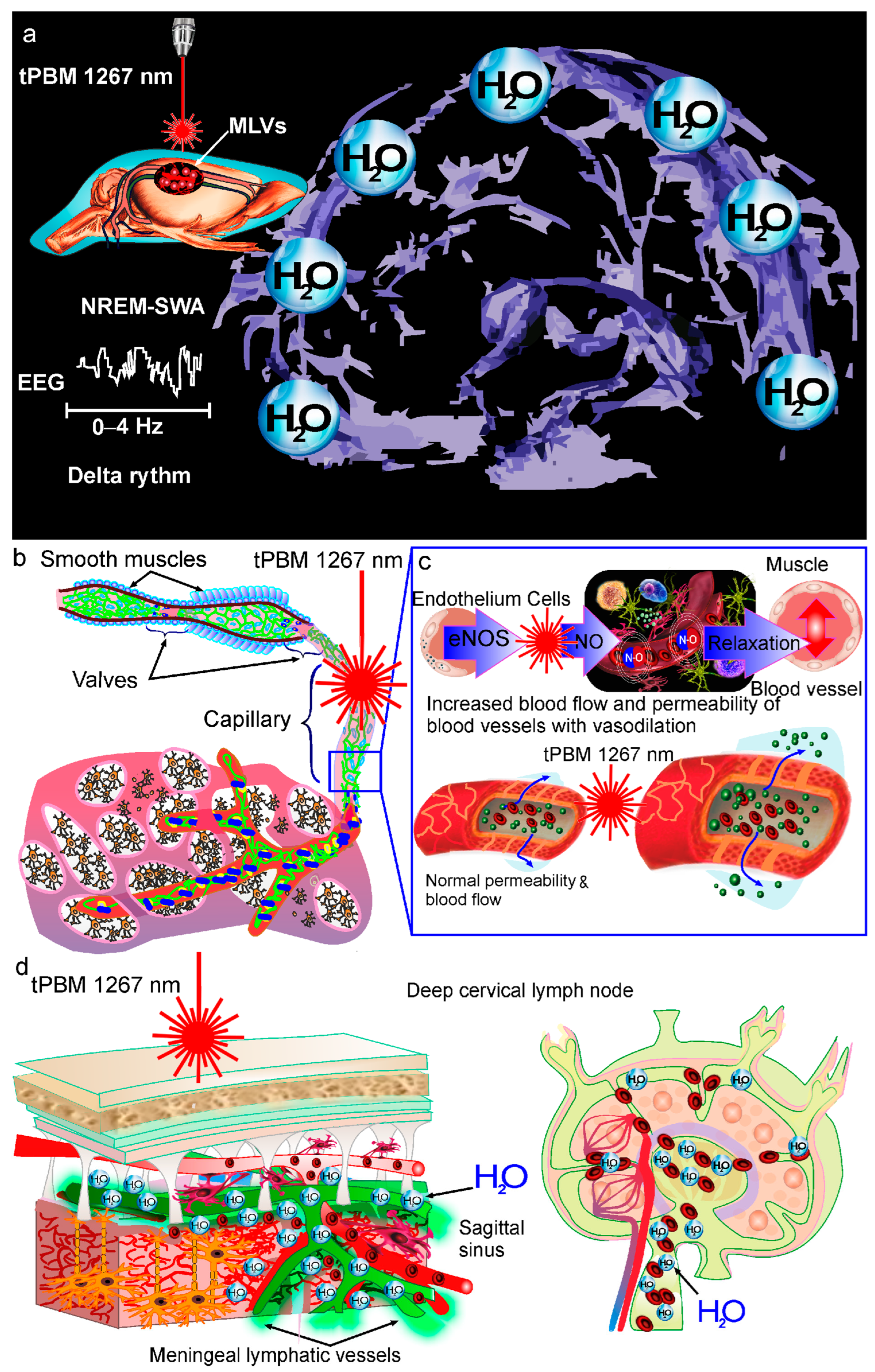
3. Mechanisms of tPBM of BWRS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franks, N.P.; Wisden, W. The inescapable drive to sleep: Overlapping mechanisms of sleep and sedation. Science 2021, 374, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Van Hees, V.T.; Paquet, C.; Sommerlad, A.; Kivimäki, M.; Dugravot, A.; Singh-Manoux, A. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 2021, 12, 2289. [Google Scholar] [CrossRef] [PubMed]
- Ooms, S.; Overeem, S.; Besse, K.; Rikkert, M.O.; Marcel Verbeek, M.; Claassen, J.A.H.R. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: A randomized clinical trial. JAMA Neurol. 2014, 71, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kojoria, E.; Wanga, G.-J.; Wiersa, C.E.; Demirala, S.B.; Guoa, M.; Kima, S.W.; Lindgrena, E.; Ramireza, V.; Zehraa, A.; Freemana, C.; et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Lucey, B.P.; McCullough, A.; Landsness, E.C.; Toedebusch, C.D.; McLeland, J.S.; Zaza, A.M.; Fagan, A.M.; McCue, L.; Xiong, C.; Morris, J.C.; et al. Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci. Transl. Med. 2019, 11, eaau6550. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Misialek, J.R.; Mosley, T.H.; Gottesman, R.F.; Punjabi, N.M.; Shahar, E.; MacLehose, R.; Ogilvie, R.P.; Knopman, D.; Alonso, A. Sleep characteristics and risk of dementia and Alzheimer’s disease: The atherosclerosis risk in communities study. Alzheimer Dement 2018, 14, 157–166. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. Sleep facilitates clearance of metabolites from the brain: Glymphatic function in aging and neurodegenerative diseases. Rejuven. Res. 2013, 16, 518–523. [Google Scholar] [CrossRef]
- Carvalho, D.Z.; St Louis, E.K.; Knopman, D.S.; Boeve, D.B.F.; Lowe, V.J.; Roberts, R.O.; Mielke, M.M.; Przybelski, S.A.; Machulda, M.M.; Petersen, R.C.; et al. Association of excessive daytime sleepiness with longitudinal beta-amyloid accumulation in elderly persons without dementia. JAMA Neurol. 2018, 75, 672–680. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Postnov, D.; Penzel, T.; Kurths, J. Sleep as a novel biomarker and a promising therapeutic target for cerebral small vessel disease: A review focusing on Alzheimer’s disease and the blood-brain barrier. Int. J. Mol. Sci. 2020, 21, 6293. [Google Scholar] [CrossRef]
- Izci-Balserak, B.; Zhu, B.; Wang, H.; Bronas, U.G.; Gooneratne, N.S. Independent associations between sleep duration, gamma gap, and cognitive function among older adults: Results from the NHANES 2013-2014. Geriatr. Nurs. 2022, 44, 1–7. [Google Scholar] [CrossRef]
- Spira, A.P.; Gamaldo, A.A.; An, Y.; Wu, M.N.; Simonsick, E.M.; Bilgel, M.; Zhou, Y.; Wong, D.F.; Ferrucci, L.; Resnick, S.M. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013, 70, 1537–1543. [Google Scholar]
- Minakawa, E.N.; Wada, K.; Nagai, Y. Sleep disturbance as a potential modifiable risk factor for Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 803. [Google Scholar] [CrossRef]
- Song, D.; Zhou, J.; Ma, J.; Chang, J.; Qiu, Y.; Zhuang, Z.; Xiao, H.; Zeng, L. Sleep disturbance mediates the relationship between depressive symptoms and cognitive function in older adults with mild cognitive impairment. Geriatr. Nurs. 2021, 42, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Heizhati, M.; Wang, L.; Li, M.; Yang, Z.; Lin, M.; Abudereyimu, R.; Hong, J.; Yang, W.; Yao, L.; et al. Poor sleep quality is negatively associated with low cognitive performance in general population independent of self-reported sleep disordered breathing. BMC Public Health 2022, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Mamedova, A.; Vinnik, V.; Klimova, M.; Saranceva, E.; Ageev, V.; Yu, T.; Zhu, D.; Penzel, T.; Kurths, J. Brain Mechanisms of COVID-19-Sleep Disorders. Int. J. Mol. Sci. 2021, 22, 6917. [Google Scholar] [CrossRef]
- Hurtado-Alvarado, G.; Becerril-Villanueva, E.; Contis-Montes, O.A.; Domínguez-Salazar, E.; Salinas-Jazmín, N.; Pérez-Tapia, S.M.; Pavon, L.; Velázquez-Moctezuma, J.; Gómez-González, B. The yin/yang of inflammatory status: Blood-brain barrier regulation during sleep. Brain Behav. Immun. 2018, 69, 154–166. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. The blood-brain barrier: Regulatory roles in wakefulness and sleep. Neuroscientist 2017, 23, 124–136. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Zhang, S.L.; Sehgal, A. Regulation of the blood-brain barrier by circadian rhythms and sleep. Trends Neurosci. 2019, 42, 500–510. [Google Scholar] [CrossRef]
- Medina-Flores, F.; Hurtado-Alvarado, G.; Contis-Montes de Oca, A.; López-Cervantes, S.P.; Konigsberg, M.; Deli, M.A.; Gómez-González, B. Sleep loss disrupts pericyte-brain endothelial cell interactions impairing blood-brain barrier function. Brain Behav. Immun. 2020, 89, 118–132. [Google Scholar] [CrossRef]
- Bellesi, M.; de Vivo, L.; Chini, M.; Gilli, F.; Tononi, G.; Chiara Cirelli, C. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J. Neurosci. 2017, 37, 5263–5273. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, C.S. Aristotle’s Theory of ‘Sleep and Dreams’ in the light of Modern and Contemporary Experimental Research. Electron. J. Philos. 2014, 17, 1–47. [Google Scholar] [CrossRef]
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.A.; Rosen, B.R.; Polimeni, J.R.; Lewis, L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.C.; Van der Werf, Y.D. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol Res Pract. 2021, 3, 5. [Google Scholar] [CrossRef]
- Yi, T.; Gao, P.; Zhu, T.; Yin, H.; Jin, S. Glymphatic System Dysfunction: A Novel Mediator of Sleep Disorders and Headaches. Front. Neurol. 2022, 13, 885020. [Google Scholar] [CrossRef]
- Yan, T.; Qiu, Y.; Yu, X.; Yang, L. Glymphatic Dysfunction: A Bridge Between Sleep Disturbance and Mood Disorders. Front. Psychiatry 2021, 12, 658340. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Blokhina, I.; Khorovodov, A.; Fedosov, I.; Yu, T.; Karandin, G.; Evsukova, A.; Elovenko, D.; Adushkina, V.; et al. Night photostimulation of clearance of beta-amyloid from mouse brain: New strategies in preventing Alzheimer’s disease. Cells 2021, 10, 3289. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef]
- Benveniste, H.; Heerdt, P.M.; Fontes, M.; Rothman, D.L.; Volkow, N.D. Glymphatic system function in relation to anesthesia and sleep states. Anesth. Analg. 2019, 128, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Xie, L.; Yu, M.; Kang, H.; Feng, T.; Deane, R.; Logan, J.; Nedergaard, M.; Benveniste, H. The effect of body posture on brain glymphatic transport. J. Neurosci. 2015, 35, 11034–11044. [Google Scholar] [CrossRef] [PubMed]
- Borb, A.A.; Achermann, P. Sleep homeostasis and models of sleep regulation. J. Biol. Rhythm 1999, 14, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Hauner, K.K.; Howard, J.D.; Zelano, C.; Gottfried, J.A. Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat. Neurosci. 2013, 16, 1553. [Google Scholar] [CrossRef]
- Lee, Y.F.; Gerashchenko, D.; Timofeev, I.; Bacskai, B.J.; Kastanenka, K.V. Slow wave sleep as a promising intervention target for Alzheimer’s disease. Front. Neurosci. 2020, 14, 705. [Google Scholar] [CrossRef]
- Niwa, Y.; Kanda, G.N.; Yamada, R.G.; Shi, S.; Sunagawa, G.A.; Ukai-Tadenuma, M.; Fujishima, H.; Matsumoto, N.; Masumoto, K.H.; Nagano, M.; et al. Muscarinic acetylcholine receptors Chrm1 and Chrm3 are essential for REM sleep. Cell Rep. 2018, 24, 2231–2247. [Google Scholar] [CrossRef]
- Funato, H.; Miyoshi, C.; Fujiyama, T.; Kanda, T.; Sato, M.; Wang, Z.; Ma, J.; Nakane, S.; Tomita, J.; Ikkyu, A.; et al. Forward-genetics analysis of sleep in randomly mutagenized mice. Nature 2016, 539, 378–383. [Google Scholar] [CrossRef]
- Cai, X.; Qiao, J.; Kulkarni, P.; Harding, I.C.; Ebong, E.; Ferris, C.F. Imaging the effect of the circadian light-dark cycle on the glymphatic system in awake rats. Proc. Natl. Acad. Sci. USA 2020, 117, 668–676. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F. Medicine in the fourth dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Ulv Larsen, S.M.; Landolt, H.P.; Berger, W.; Nedergaard, M.; Knudsen, G.M.; Holst, S.C. Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol. 2020, 18, 3000623. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Patton, A.P.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron 2017, 93, 1420–1435. [Google Scholar] [CrossRef]
- Cirelli, C.; Tononi, G. The why and how of sleep-dependent synaptic down-selection. Semin. Cell. Dev. Biol. 2022, 125, 91–100. [Google Scholar] [CrossRef]
- Rodriguez, A.V.; Funk, C.M.; Vyazovskiy, V.V.; Nir, Y.; Tononi, G.; Cirelli, C. Why does sleep slow-wave activity increase after extended wake? Assessing the effects of increased cortical firing during wake and sleep. J. Neurosci. 2016, 36, 12436–12447. [Google Scholar] [CrossRef]
- de Vivo, L.; Bellesi, M.; Marshall, W.; Bushong, E.A.; Ellisman, M.H.; Tononi, G.; Cirelli, C. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017, 355, 507–510. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef]
- Bedussi, B.; Naessens, D.M.P.; de Vos, J.; Olde Engberink, R.; Wilhelmus, M.M.M.; Richard, E.; ten Hove, M.; Van Bavel, E.; Bakker, E.N.P. Enhanced interstitial fluid drainage in the hippocampus of spontaneously hypertensive rats. Sci. Rep. 2017, 7, 744. [Google Scholar] [CrossRef]
- Hubbard, J.; Gent, T.C.; Hoekstra, M.M.B. Rapid fast-delta decay following prolonged wakefulness marks a phase of wake-inertia in NREM sleep. Nat. Commun. 2020, 11, 3130. [Google Scholar] [CrossRef]
- Hoekstra, M.; Emmenegger, Y.; Hubbard, J.; Franken, P. Cold-inducible RNA-binding protein (CIRBP) adjusts clock-gene expression and REM-sleep recovery following sleep deprivation. eLife 2019, 8, 43400. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.C.; Yu, X.; Miao, A.; Andrews, N.; Ma, Y.; Ye, Z.; Lignos, L.; Miracca, G.; Ba, W.; Yustos, R.; et al. A neuronal hub binding sleep initiation and body cooling in response to a warm external stimulus. Curr. Biol. 2018, 28, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.M.; Melanson, E.L.; Frydendall, E.J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J. Physiol. 2011, 589, 235–244. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.; Szymusiak, R. Keeping cool: A hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990, 13, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Burhan, H.; Cherian, I. Brain Cooling and Cleaning: A New Perspective in Cerebrospinal Fluid (CSF) Dynamics. In Cerebrovascular Diseases.; Bozzetto Ambrosi, P., Ahmad, R., Abdullahi, A., Agrawal, A., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Sundaram, S.; Hughes, R.L.; Peterson, E.; Müller-Oehring, E.M.; Brontë-Stewart, H.M.; Poston, K.L. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson’s disease. Neurosci. Biobehav. Rev. 2019, 103, 305–315. [Google Scholar] [CrossRef]
- Pu, T.; Zou, W.; Feng, W.; Zhang, Y.; Wang, L.; Wang, H. Persistent malfunction of glymphatic and meningeal lymphatic drainage in a mouse model of subarachnoid hemorrhage. Exp. Neurobiol. 2019, 28, 104–118. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Xu, H. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat. Commun. 2020, 11, 3159. [Google Scholar] [CrossRef]
- Liu, X.; Gao, C.; Yuan, J. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol. Commun. 2020, 8, 16. [Google Scholar] [CrossRef]
- Bolte, A.C.; Dutta, A.B.; Hurt, M.E. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 2020, 11, 4524. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Q.; Ma, L. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef]
- Ma, Q.; Schlegel, F.; Bachmann, S.B. Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci. Rep. 2019, 9, 14815. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Louveau, A.; Vaccari, A. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kastanenka, K.V.; Calvo-Rodriguez, M.; Hou, S.S.; Zhou, H.; Takeda, S.; Arbel-Ornath, M.; Lariviere, A.; Lee, Y.F.; Kim, A.; Hawkes, J.M.; et al. Frequency-dependent exacerbation of Alzheimer’s disease neuropathophysiology. Sci. Rep. 2019, 9, 8964. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.A.; Strittmatter, S.M.; Nygaard, H.B. Sleep and EEG power spectral analysis in three transgenic mouse models of Alzheimer’s disease: APP/PS1, 3xTgAD, and Tg2576. J. Alzheimer Dis. 2018, 64, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Castano-Prat, P.; Perez-Mendez, L.; Perez-Zabalza, M.; Sanfeliu, C.; Giménez-Llort, L.; Sanchez-Vives, M.V. Altered slow (<1 Hz) and fast (beta and gamma) neocortical oscillations in the 3xTg-AD mouse model of Alzheimer’s disease under anesthesia. Neurobiol. Ageing 2019, 79, 142–151. [Google Scholar]
- Mander, B.A.; Rao, V.; Lu, B.; Saletin, J.M.; Lindquist, J.R.; Ancoli-Israel, S.; Jagust, W.; Walker, M.P. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 2013, 16, 357. [Google Scholar] [CrossRef]
- Mander, B.A.; Marks, S.M.; Vogel, J.W.; Rao, V.; Lu, B.; Saletin, J.M.; Ancoli-Israel, S.; Jagust, W.J.; Walker, M.P. b-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat. Neurosci. 2015, 18, 1051–1057. [Google Scholar] [CrossRef]
- Westerberg, C.E.; Mander, B.A.; Florczak, S.M.; Weintraub, S.; Mesulam, M.M.; Zee, P.C.; Paller, K.A. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. JINS 2012, 18, 490. [Google Scholar] [CrossRef]
- Winer, J.R.; Mander, B.A.; Helfrich, R.F.; Maass, A.; Harrison, T.M.; Baker, S.L.; Knight, R.T.; Jagust, W.J.; Walker, M.P. Sleep as a potential biomarker of tau and b-amyloid burden in the human brain. J. Neurosci. 2019, 39, 6315–6324. [Google Scholar] [CrossRef]
- O’Donnell, J.; Zeppenfeld, D.; McConnell, E.; Pena, S.; Nedergaard, M. Norepinephrine: A neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res. 2012, 37, 2496–2512. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Li, R.; Vitullo, L. Perivascular innervation of penetrating brain parenchymal arterioles. J. Cardiovasc. Pharmacol. 2004, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kastanenka, K.V.; Hou, S.S.; Shakerdge, N.; Logan, R.; Feng, D.; Wegmann, S.; Chopra, V.; Hawkes, J.M.; Chen, X.; Bacskai, B.J. Optogenetic restoration of disrupted slow oscillations halts amyloid deposition and restores calcium homeostasis in an animal model of Alzheimer’s disease. PLoS ONE 2017, 12, e0170275. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Early network dysfunction in Alzheimer’s disease. Science 2019, 365, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Kang, J.E.; Lee, J.; Stewart, F.R.; Verges, D.K.; Silverio, L.M.; Bu, G.; Mennerick, S.; Holtzman, D.M. Endocytosis is required for synaptic activity-dependent release of amyloid-b in vivo. Neuron 2008, 58, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B.J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.R. Neuronal expression of glutamine synthetase in Alzheimer’s disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem. Int. 2000, 36, 471–482. [Google Scholar] [CrossRef]
- Poskanzer, K.E.; Yuste, R. Astrocytes regulate cortical state switching in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 2675–2684. [Google Scholar] [CrossRef]
- Iliff, J.; Wang, M.; Liao, Y.; Plogg, B.; Peng, W.; Gundersen, G.; Benveniste, H.; Vates, G.; Deane, R.; Goldman, S.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 147, ra111. [Google Scholar] [CrossRef]
- Jessen, N.; Munk, A.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 12, 2583–2599. [Google Scholar] [CrossRef]
- Hladky, S.; Barrand, M. The glymphatic hypothesis: The theory and the evidence. Fluids Barriers CNS 2022, 19, 9. [Google Scholar] [CrossRef]
- Salehpour, F.; Khademi, M.; Bragin, D.; DiDuro, J. Photobiomodulation therapy and the glymphatic system: Promising applications for augmenting the brain lymphatic drainage system. Int. J. Mol. Sci. 2022, 23, 2975. [Google Scholar] [CrossRef] [PubMed]
- Cecile, M.; Valverde, A.; Dole, M.; Hoh Kam, J. The effect of photobiomodulation on the brain during wakefulness and sleep. Front. Neurosci. 2022, 16, 1232. [Google Scholar]
- Semyachkina-Glushkovskaya, O.; Karavaev, A.; Prokhorov, M.; Runnova, A.; Borovkova, E.; Ishbulatov, Y.; Hramkov, A.; Kulminskiy, D.; Semenova, N.; Sergeev, K.; et al. EEG biomarkers of activation of the lymphatic drainage system of the brain during sleep and opening of the blood-brain barrier. Comput. Struct. Biotechnol. J. 2023, 21, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Spieth, L.; Berghoff, S.; Stumpf, S.; Winchenbach, J.; Michaelis, T.; Watanabe, T.; Gerndt, N.; Düking, T.; Hofer, S.; Ruhwedel, T.; et al. Anesthesia triggers drug delivery to experimental glioma in mice by hijacking caveolar transport. Neurooncol. Adv. 2021, 3, vdab140. [Google Scholar] [CrossRef] [PubMed]
- Tétrault, S.; Chever, O.; Sik, A.; Amzica, F. Opening of the blood-brain barrier during isoflurane anaesthesia. Eur. J. Neurosci. 2008, 28, 1330–1341. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X. The Crosstalk between the Blood–Brain Barrier Dysfunction and Neuroinflammation after General Anaesthesia. Curr. Issues Mol. Biol. 2022, 44, 5700–5717. [Google Scholar] [CrossRef]
- Sweeney, M.; Sagare, A.; Zlokovic, B. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship between Amyloid- Deposition and Blood–Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef]
- Al-Bachari, S.; Naish, J.; Parker, G.; Emsley, H.; Parkes, L. Blood–Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020, 11, 593026. [Google Scholar] [CrossRef]
- Pineda-Pardo, J.; Gasca-Salas, C.; Fernández-Rodríguez, B.; Rodríguez-Rojas, R.; Del Álamo, M.; Obeso, I.; Hernández-Fernández, F.; Trompeta, C.; Martínez-Fernández, R.; Matarazzo, M.; et al. Striatal Blood-Brain Barrier Opening in Parkinson’s Disease Dementia: A Pilot Exploratory Study. Mov. Disord. 2022, 37, 2057–2065. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Guo, X.; Pluimer, B.; Zhao, Z. Blood–Brain Barrier Dysfunction in Mild Traumatic Brain Injury: Evidence from Preclinical Murine Models. Front. Physiol. 2020, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Dion-Albert, L.; Cadoret, A.; Doney, E.; Kaufmann, F.N.; Dudek, K.A.; Daigle, B.; Parise, L.F.; Cathomas, F.; Samba, N.; Hudson, N.; et al. Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat. Commun. 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yin, Y.; Du, L. Blood-Brain Barrier Dysfunction in the Pathogenesis of Major Depressive Disorder. Cell. Mol. Neurobiol. 2022, 42, 2571–2591. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Sajja, R.; Naik, P.; Cucullo, L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J. Pharmacovigil. 2014, 2, 125. [Google Scholar] [PubMed]
- Banks, W.A. The Blood-Brain Barrier Interface in Diabetes Mellitus: Dysfunctions, Mechanisms and Approaches to Treatment. Curr. Pharm. Des. 2020, 26, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Krasemann, S.; Haferkamp, U.; Pfefferle, S.; Woo, M.; Heinrich, F.; Schweizer, M.; Appelt-Menzel, A.; Cubukova, A.; Barenberg, J.; Leu, J.; et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. 2022, 17, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Chen, F.; Cui, L. COVID-19 and cognitive impairment: Neuroinvasive and blood-brain barrier dysfunction. J. Neuroinflamm. 2022, 19, 222. [Google Scholar] [CrossRef]
- Louveau, A.; Plog, B.A.; Antila, S.; Alitalo, K.; Nedergaard, M.; Kipnis, J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Investig. 2017, 127, 3210–3219. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Fu, Z.; Kipnis, J. The meningeal lymphatic system: A new player in neurophysiology. Neuron 2018, 100, 375–388. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Postnov, D.; Kurths, J. Blood–brain barrier, lymphatic clearance, and recovery: Ariadne’s thread in labyrinths of hypotheses. Int. J. Mol. Sci. 2018, 19, 3818. [Google Scholar] [CrossRef]
- Tavares, G.A.; Louveau, A. Meningeal lymphatics: An immune gateway for the central nervous system. Cells 2021, 10, 3385. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.B.; Wang, X.X.; Xia, D.H.; Liu, H.; Tian, H.Y.; Fu, Y.; Chen, Y.K.; Qin, C.; Wang, J.Q.; Xiang, Z.; et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 2021, 27, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Olate-Briones, A.; Escalona, E.; Salazar, C.; Herrada, M.J.; Liu, C.; Herrada, A.A.; Escobedo, N. The meningeal lymphatic vasculature in neuroinflammation. FASEB J. 2022, 36, e22276. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.L.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef]
- Lan, Y.L.; Wang, H.; Chen, A.; Zhang, J. Update on the current knowledge of lymphatic drainage system and its emerging roles in glioma management. Immunology 2022, 10, 111. [Google Scholar] [CrossRef]
- Yanev, P.; Poinsatte, K.; Hominick, D.; Khurana, N.; Zuurbier, K.R.; Berndt, M.; Plautz, E.J.; Dellinger, M.T.; Stowe, A.M. Impaired meningeal lymphatic vessel development worsens stroke outcome. J. Cereb. Blood Flow Metab. 2020, 40, 263–275. [Google Scholar] [CrossRef]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain. 2020, 143, 2576–2593. [Google Scholar] [CrossRef]
- Lemprière, S. Meningeal lymphatic flow slows after mild traumatic brain injury. Nat. Rev. Neurol. 2020, 16, 600. [Google Scholar] [CrossRef]
- Zhinchenko, E.; Klimova, M.; Mamedova, A.; Agranovich, I.; Blokhina, I.; Antonova, T.; Terskov, A.; Shirokov, A.; Navolkin, N.; Morgun, A.; et al. Photostimulation of extravasation of beta-amyloid through the model of blood-brain barrier. Electronics 2020, 9, 1056. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Postnov, D.; Lavrova, A.; Fedosov, I.; Borisova, E.; Nikolenko, V.; Penzel, T.; Kurths, J.; Tuchin, V. Biophotonic strategies of measurement and stimulation of the cranial and the extracranial lymphatic drainage function. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 7400313. [Google Scholar] [CrossRef]
- Semyachkina-glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A. Photobiomodulation of lymphatic drainage and clearance: Perspective strategy for augmentation of meningeal lymphatic functions. BOE 2020, 11, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Klimova, M. Photostimulation of cerebral and peripheral lymphatic functions. Transl. Biophoton. 2020, 2, e201900036. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Shirokov, A. Photomodulation of lymphatic delivery of liposomes to the brain bypassing the blood-brain barrier: New perspectives for glioma therapy. Nanophotonics 2021, 10, 3215–3227. [Google Scholar] [CrossRef]
- Abad, V.; Guilleminault, C. Diagnosis and treatment of sleep disorders: A brief review for clinicians. Dialog Clin. Neurosci. 2022, 5, 371–388. [Google Scholar] [CrossRef]
- Wu, C.; Yang, L.; Feng, S.; Zhu, L. Therapeutic non-invasive brain treatments in Alzheimer’s disease: Recent advances and challenges. Inflamm. Regen. 2022, 42, 31. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation for Alzheimer’s disease: Has the light dawned? Photonics 2019, 6, 77. [Google Scholar] [CrossRef]
- Cardoso, F.; Gonzalez-Lima, F.; Gomes da Silva, S. Photobiomodulation for the aging brain. Ageing Res. Rev. 2021, 70, 101415. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Gong, S.Y.; Shu-Ying, X.; Wang, Y.; Peng, H.; Shen, Y.; Liu, C.-F. Light therapy: A new option for neurodegenerative diseases. Chin. Med. J. 2021, 134, 634–645. [Google Scholar] [CrossRef]
- Gutiérrez-Menéndez, A.; Marcos-Nistal, M.; Mendez, M.; Arias, J. Photobiomodulation as a promising new tool in the management of psychological disorders: A systematic review. Neurosci. Biobehav. Rev. 2020, 119, 242–254. [Google Scholar] [CrossRef]
- Srisurapanont, K.; Samakarn, Y.; Kamklong, B.; Siratrairat, P. Blue-wavelength light therapy for post-traumatic brain injury sleepiness, sleep disturbance, depression, and fatigue: A systematic review and network meta-analysis. PLoS ONE 2021, 16, e0246172. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Huang, Y.Y. Photobiomodulation in the Brain: Low-Level Laser (Light) Therapy in Neurology and Neuroscience; Academic Press: London, UK, 2019; 26p. [Google Scholar]
- Mosilhy, E.A.; Alshial, E.; Eltaras, M.M.; Rahman, M.M.A.; Helmy, H.I.; Elazoul, A.H.; Hamdy, O.; Haitham, S.M. Non-invasive transcranial brain modulation for neurological disorders treatment: A narrative review. Life Sci. 2022, 307, 120869. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Tedford, C.E.; DeLapp, S.; Jacques, S.; Anders, J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg. Med. 2015, 47, 312–322. [Google Scholar] [CrossRef]
- Salehpour, F.; Cassano, P.; Rouhi, N.; Hamblin, M.R.; De Taboada, L.; Farajdokht, F.; Mahmoudi, J. Penetration profiles of visible and near-infrared lasers and light-emitting diode light through the head tissues in animal and human species: A review of literature. Photobiomodul. Photomed. Laser Surg. 2019, 37, 581–595. [Google Scholar] [CrossRef]
- Wang, T.; Ouzounov, D.; Wu, C.; Horton, N.G.; Zhang, B.; Wu, C.-H. Three-photon imaging of mouse brain structure and function through the intact skull. Nat. Methods 2018, 15, 789–792. [Google Scholar] [CrossRef]
- Zinchenko, E.; Navolokin, N.; Shirokov, A.; Khlebtsov, B.; Dubrovsky, A.; Saranceva, E.; Abdurashitov, A.; Khorovodov, A.; Terskov, A.; Mamedova, A. Pilot study of transcranial photobiomodulation of lymphatic clearance of beta-amyloid from the mouse brain: Breakthrough strategies for non-pharmacologic therapy of Alzheimer’s disease. BOE 2019, 10, 4003–4017. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Yu, T.; Liu, Z.; Sun, S.; Bragin, D.; Navolokin, N.; Kurths, J.; Glushkovskaya-Semyachkina, O.; Zhu, D. Photostimulation of lymphatic clearance of red blood cells from the mouse brain after intraventricular hemorrhage. bioRxiv 2020, 16, 384149. [Google Scholar]
- Vargas, E.; Barratt, D.W.; Saucedo, C.L.; Huang, L.-D.; Abraham, J.A.; Tanaka, H.; Haley, A.P.; Gonzales-Lima, F. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med. Sci. 2017, 32, 1153–1162. [Google Scholar] [CrossRef]
- Wang, X.; Wanniarachchi, H.; Wu, A.; Gonzalez-Lima, F. Transcranial photobiomodulation and thermal stimulation induce distinct topographies of EEG alpha and beta power changes in healthy humans. Sci. Rep. 2021, 11, 18917. [Google Scholar] [CrossRef]
- Shahdadian, S.; Wang, X.; Wanniarachchi, H.; Chaudhari, A.; Truong, N.; Liu, H. Neuromodulation of brain topography and network topology by prefrontal transcranial photobiomodulation. J. Neural Eng. 2022, 19, 066013. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.G.F.; Can Ozan Tan, C.O.; Chan, S.-T.; Welt, J. Effect of transcranial low-level light therapy vs sham therapy among patients with moderate traumatic brain injury: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2017337. [Google Scholar] [CrossRef]
- Hipskind, S.G.; Grover, F.L.; Hipskind, S.G.; Fort, T.R. Pulsed transcranial red/near-infrared light therapy using light-emitting diodes improves cerebral blood flow and cognitive function in veterans with chronic traumatic brain injury: A case series. Photomed. Laser Surg. 2018, 37, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P.; Mischoulon, D.; Cusin, C.; Petrie, S. Transcranial photobiomodulation for the treatment of major depressive disorder. The ELATED-2 pilot trial. Photomed. Laser Surg. 2018, 36, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.H.; Halper, J.; Trent, N.; Jarrett, H. Photobiomodulation with near infrared light helmet in a pilot, placebo controlled clinical trial in dementia patients testing memory and cognition. J. Neurol. Neurosci. 2017, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Fang, P.; Liu, H.; Chen, L.; Fu, Y.; Xie, B.; Liu, Y.; Ye, H.; Gu, P. The clinical effect of blue light therapy on patients with delayed sleep-wake phase disorder. Nat. Sci. Sleep 2022, 14, 75. [Google Scholar]
- Valverde, A.; Mitrofanis, J.; Moro, C.; Hamilton, C. Lights at night: Does photobiomodulation improve sleep? Neural Regen. Res. 2022, 18, 474–477. [Google Scholar]
- Devine, J.K.; Schwartz, L.P.; Choynowski, J.; Hursh, S.R. Expert Demand for Consumer Sleep Technology Features and Wearable Devices. IoT 2021, 2, 18. [Google Scholar] [CrossRef]
- McCall, I.C.; Lua, C.; Minielly, N.; Illis, J. Owning ethical innovation: Claims about commercial wearable brain technologies. Neuron 2019, 102, 728–731. [Google Scholar] [CrossRef]
- Zambelli, Z.; Jakobsson, C.E.; Threadgokd, L.; Fidalgo, A.R.; Halstead, E.J.; Dimitriou, D. Exploring the feasibility and acceptability of a sleep wearable headband among a community sample of chronic pain individuals: An at-home observational study. Digit. Health 2022, 8, 20552076221097504. [Google Scholar] [CrossRef]
- De Fazio, R.; Mattei, V.; Al-Naami, B.; De Vittorio, M. Methodologies and Wearable Devices to Monitor Biophysical Parameters Related to Sleep Dysfunctions: An Overview. Micromachines 2022, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Cay, G.; Ravichandran, V.; Sadhu, S.; Zisk, A.H. Recent advancement in sleep technologies: A literature review on clinical standards, sensors, apps, and AI methods. IEEE Access 2022, 99, 104737–104756. [Google Scholar] [CrossRef]
- Wei, Z.; Li, W.Y.; Wang, X.; Qin, Z.; Zhou, J.; Wang, W. Evaluation of a non-contact ultra-wideband bio-radar sleep monitoring device for screening of sleep breathing disease. Sleep Breath. 2022, 26, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zarkada, G.; Yi, S.; Eichmann, A. Lymphatic endothelial cell junctions: Molecular regulation in physiology and diseases. Front. Physiol. 2020, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Scallan, J.P.; Huxley, V.H. In vivo determination of collecting lymphatic vessel permeability to albumin: A role for lymphatics in exchange. J. Physiol. 2010, 588, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the endothelial barrier: Identifying and reconciling controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Kuan, E.L.; Ivanov, S.; Bridenbaugh, E.A. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node–homing adipose tissue dendritic cells. J. Immunol. 2015, 194, 5200–5210. [Google Scholar] [CrossRef]
- Banerji, S.; Ni, J.; Wang, S.-X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. Lyve-1, a new homologue of the cd44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Afanasyeva, N.I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005, 36, 307–314. [Google Scholar] [CrossRef]
- Murad, F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci. Rep. 2004, 24, 452–474. [Google Scholar] [CrossRef]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and clinical applications of red and near-infrared photobiomodulation on endothelial dysfunction: A review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, Y.; Nawashiro, H.; Sato, S.; Kawauchi, S.; Shima, K.; Kikuchi, M. Targeted increase in cerebral blood flow by tran-scranial near-infrared laser irradiation. Lasers Surg. Med. 2010, 42, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Litscher, G.; Min, L.; Passegger, C.A.; Litscher, D.; Li, M.; Wang, M.; Ghaffari-Tabrizi-Wizsy, N.; Stelzer, I.; Feigl, G.; Gaischek, I.; et al. Transcranial Yellow, Red, and Infrared Laser and LED Stimulation: Changes of Vascular Parameters in a Chick Embryo Model. Integr. Med. Int. 2015, 2, 80–89. [Google Scholar] [CrossRef]
- Weihrauch, D.; Keszler, A.; Lindemer, B.; Krolikowski, J.; Lohr, N.L. Red light stimulates vasodilation through extracellular vesicle trafficking. J. Photochem. Photobiol. B Biol. 2021, 220, 112212. [Google Scholar] [CrossRef] [PubMed]
- Scallan, J.P.; Zawieja, S.D.; Castorena-Gonzalez, J.; Davis, M.J. Lymphatic pumping: Mechanics, mechanisms and malfunction. J. Physiol. 2016, 594, 5749–5768. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Maghzal, G.J.; Ayer, A.; Talib, J.; Giltrap, A.M.; Shengule, S.; Wolhuter, K.; Wang, Y.; Chadha, P.; Suarna, C.; et al. Singlet molecular oxygen regulates vascular tone and blood pressure in inflammation. Nature 2019, 566, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, S.D.; Ivanov, A.V. Light-oxygen effect in cells and its potential applications in tumour therapy (review). Quantum Electron. 1999, 29, 1031–1053. [Google Scholar] [CrossRef]
- Sokolovski, S.G.; Zolotovskaya, S.A.; Goltsov, A.; Pourreyron, C.; South, A.P.; Rafailov, E.U. Infrared laser pulse triggers increased singlet oxygen production in tumour cells. Sci. Rep. 2013, 3, 3484. [Google Scholar] [CrossRef]
- Khokhlova, A.; Zolotovskii, I.; Sokolovski, S.; Saenko, Y.; Rafailov, E.; Stoliarov, D.; Pogodina, E.; Svetukhin, V.; Sibirny, V.; Fotiadi, A. The light-oxygen effect in biological cells enhanced by highly localized surface plasmon-polaritons. Sci. Rep. 2019, 9, 18435. [Google Scholar] [CrossRef]
- Khokhlova, A.; Zolotovskii, I.; Stoliarov, D.; Vorsina, S.; Liamina, D.; Pogodina, E.; Fotiadi, A.A.; Sokolovski, S.G.; Saenko, Y.; Rafailov, E.U. The photobiomodulation of vital parameters of the cancer cell culture by low dose of near-IR laser Iirradiation. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Ronsein, G.E.; Oliveira, M.C.; Miyamoto, S.; Medeiros, M.H.G.; Di Mascio, P. Tryptophan oxidation by singlet molecular oxygen [O2(1∆g)]: Mechanistic studies using 18O-labeled hydroperoxides, mass spectrometry, and light emission measurements. Chem. Res. Toxicol. 2008, 21, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.-P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Lane, H. Cell biology: Power games. Nature 2006, 443, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Waypa, G.B.; Smith, K.A.; Schumacker, P.T. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol. Aspects Med. 2016, 47, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P.; Petrie, S.P.; Hamblin, M.R.; Henderson, T.A.; Iosifescu, D.V. Review of transcranial photobiomodulation for major depressive disorder: Targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophot 2016, 3, 031404. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Q.; Wu, X.; Zhang, D.; Xing, D. Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces: A levels in Alzheimer’s disease models. Aging Cell 2020, 19, e13054. [Google Scholar] [CrossRef]
- Duggett, N.A.; Chazot, P.L. Low-Intensity Light Therapy (1068 nm) Protects CAD Neuroblastoma Cells from Amyloid-Mediated Cell Death. Biol. Med. 2014, 1, 2. [Google Scholar]
- Tao, L.; Liu, Q.; Zhang, F.; Fu, Y.; Zhu, X.; Weng, X.; Wei, X. Microglia modulation with 1070-nm light attenuates: A burden and cognitive impairment in Alzheimer’s disease mouse model. Light Sci. Appl. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Yue, X.; Mei, Y.; Zhang, Y.; Tong, Z.; Cui, D.; Yang, J.; Wang, A.; Wang, R.; Fei, X.; Ai, L. New insight into Alzheimer’s disease: Light reverses: An obstructed interstitial fluid flow and ameliorates memory decline in APP/PS1 mice. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 671–684. [Google Scholar] [CrossRef]
- Lee, G.-H.; Kim, H.; Lee, G.H.; Kwon, W.; Too, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 5, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Jinno, H.; Yokota, Y.; Koizumi, M.; Yukita, W. Self-powered ultraflexible photonic skin for continuous bio-signal detection via air-operation-stable polymer light-emitting diodes. Nat. Commun. 2021, 12, 2234. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.; Wall, M.; Jiang, H.; Kodira, C.; de Lima, K.; Herz, J.; et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef]
- Lustenberger, C.; Ferster, M.L.; Huwiler, S.; Brogli, L.; Werth, E.; Huber, R.; Karlen, W. Auditory deep sleep stimulation in older adults at home: A randomized crossover trial. Commun. Med. 2022, 2, 30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semyachkina-Glushkovskaya, O.; Fedosov, I.; Penzel, T.; Li, D.; Yu, T.; Telnova, V.; Kaybeleva, E.; Saranceva, E.; Terskov, A.; Khorovodov, A.; et al. Brain Waste Removal System and Sleep: Photobiomodulation as an Innovative Strategy for Night Therapy of Brain Diseases. Int. J. Mol. Sci. 2023, 24, 3221. https://doi.org/10.3390/ijms24043221
Semyachkina-Glushkovskaya O, Fedosov I, Penzel T, Li D, Yu T, Telnova V, Kaybeleva E, Saranceva E, Terskov A, Khorovodov A, et al. Brain Waste Removal System and Sleep: Photobiomodulation as an Innovative Strategy for Night Therapy of Brain Diseases. International Journal of Molecular Sciences. 2023; 24(4):3221. https://doi.org/10.3390/ijms24043221
Chicago/Turabian StyleSemyachkina-Glushkovskaya, Oxana, Ivan Fedosov, Thomas Penzel, Dongyu Li, Tingting Yu, Valeria Telnova, Elmira Kaybeleva, Elena Saranceva, Andrey Terskov, Alexander Khorovodov, and et al. 2023. "Brain Waste Removal System and Sleep: Photobiomodulation as an Innovative Strategy for Night Therapy of Brain Diseases" International Journal of Molecular Sciences 24, no. 4: 3221. https://doi.org/10.3390/ijms24043221
APA StyleSemyachkina-Glushkovskaya, O., Fedosov, I., Penzel, T., Li, D., Yu, T., Telnova, V., Kaybeleva, E., Saranceva, E., Terskov, A., Khorovodov, A., Blokhina, I., Kurths, J., & Zhu, D. (2023). Brain Waste Removal System and Sleep: Photobiomodulation as an Innovative Strategy for Night Therapy of Brain Diseases. International Journal of Molecular Sciences, 24(4), 3221. https://doi.org/10.3390/ijms24043221








