Proanthocyanidins Delay Fruit Coloring and Softening by Repressing Related Gene Expression during Strawberry (Fragaria × ananassa Duch.) Ripening
Abstract
:1. Introduction
2. Results
2.1. Exogenous PA treatment Slows down Strawberry Softening and Coloring
2.2. Total Soluble Solids, Titratable Acidity, Total Phenolic Content, and Total Flavonoid Content Determination
2.3. In Vivo PA and Anthocyanin Content
2.4. PA treatment Altered ABA and Sugar Content
2.5. PAs Inhibited Anthocyanin-Related Gene Expression at the Key Point for Fruit Coloring
2.6. PAs Affected the Expression of Firmness-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Skin Color, Fruit Firmness, TSS, and TA Assays
4.3. TPC and TFC Evaluations
4.4. Measurement of ABA Content
4.5. Sugars Detection
4.6. PAs and Anthocyanins
4.7. Gene Expression
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannaujia, P.K.; Asrey, R. Effect of harvesting season and cultivars on storage behaviour, nutritional quality and consumer acceptability of strawberry (Fragaria × ananassa Duch.) fruits. Acta Physiol. Plant 2021, 43, 88. [Google Scholar] [CrossRef]
- Twitchen, C.; Else, M.; Hadley, P. The effect of temperature and light intensity on rate of strawberry fruit ripening. Acta Hortic. 2021, 1309, 643–648. [Google Scholar] [CrossRef]
- Han, Y.; Dang, R.; Li, J.; Jinzhu, J.; Zhang, N.; Jia, M.; Wei, L.; Li, Z.; Li, B.; Jia, W. SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE2.6, an ortholog of OPEN STOMATA1, is a negative regulator of strawberry fruit development and ripening. Plant Physiol. 2019, 167, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, Y.; Zhang, A.; You, C.X. Regulation of fleshy fruit ripening: From transcription factors to epigenetic modifications. Hortic. Res. 2022, 9, uhac013. [Google Scholar] [CrossRef]
- Li, B.-J.; Grierson, D.; Shi, Y.; Chen, K.-S. Roles of abscisic acid in regulating ripening and quality of strawberry, a model non-climacteric fruit. Hortic. Res. 2022, 9, uhac089. [Google Scholar] [CrossRef]
- Given, N.K.; Venis, M.A.; Gierson, D. Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta 1988, 174, 402–406. [Google Scholar] [CrossRef]
- Martínez, G.A.; Chaves, A.R.; Añón, M.C. Effect of gibberellic acid on ripening of strawberry fruits (Fragaria × ananassa Duch.). J. Plant Growth Reg. 1994, 13, 87–91. [Google Scholar] [CrossRef]
- Sánchez-Gómez, C.; Posé, D.; Martín-Pizarro, C. Insights into transcription factors controlling strawberry fruit development and ripening. Front. Plant Sci. 2022, 13, 1022369. [Google Scholar] [CrossRef]
- Cheng, J.; Niu, Q.; Zhang, B.; Chen, K.; Yang, R.; Zhu, J.-K.; Zhang, Y.; Lang, Z. Downregulation of RdDM during strawberry fruit ripening. Genome Biol. 2018, 19, 212. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, R.; Li, X.; Tian, S.; Li, B.; Qin, G. N6-methyladenosine RNA modification regulates strawberry fruit ripening in an ABA-dependent manner. Genome Biol. 2021, 22, 168. [Google Scholar] [CrossRef]
- Luo, Y.; Ge, C.; Ling, Y.; Mo, F.; Yang, M.; Jiang, L.; Chen, Q.; Lin, Y.; Sun, B.; Zhang, Y. ABA and sucrose co-regulate strawberry fruit ripening and show inhibition of glycolysis. Mol. Genet. Genom. 2020, 295, 421–438. [Google Scholar] [CrossRef]
- Mansouri, S.; Sarikhani, H.; Sayyari, M.; Soleimani Aghdam, M. Melatonin accelerates strawberry fruit ripening by triggering GAMYB gene expression and promoting ABA accumulation. Sci. Hortic. 2021, 281, 109919. [Google Scholar] [CrossRef]
- Guo, J.; Wang, S.; Yu, X.; Dong, R.; Li, Y.; Mei, X.; Shen, Y. Polyamines regulate strawberry fruit ripening by abscisic acid, auxin, and ethylene. Plant Physiol. 2018, 177, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Steelheart, C.; Alegre, M.L.; Baldet, P.; Rothan, C.; Bres, C.; Just, D.; Okabe, Y.; Ezura, H.; Ganganelli, I.; Gergoff Grozeff, G.E.; et al. The effect of low ascorbic acid content on tomato fruit ripening. Planta 2020, 252, 36. [Google Scholar] [CrossRef] [PubMed]
- Considine, M. Ascorbate and Glutathione in Fruit Ripening: Is Cycling Important for Fruit Health? Research Signpost: Ahmedabad, India, 2006; pp. 275–296. [Google Scholar]
- Zhou, Y.; Huang, X.; Li, R.; Lin, H.; Huang, Y.; Zhang, T.; Mo, Y.; Liu, K. Transcriptome and biochemical analyses of glutathione-dependent regulation of tomato fruit ripening. J. Plant Interact. 2022, 17, 537–547. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2022, 1–29. [Google Scholar] [CrossRef]
- Yu, D.; Huang, T.; Tian, B.; Zhan, J. Advances in biosynthesis and biological functions of proanthocyanidins in horticultural plants. Foods 2020, 9, 1774. [Google Scholar] [CrossRef]
- Hellstrom, J.K.; Torronen, A.R.; Mattila, P.H. Proanthocyanidins in common food products of plant origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef]
- Dasiman, R.; Nor, N.M.; Eshak, Z.; Mutalip, S.S.M.; Suwandi, N.R.; Bidin, H. A Review of Procyanidin: Updates on Current Bioactivities and Potential Health Benefits. Biointerface Res. Appl. Chem. 2021, 12, 5918–5940. [Google Scholar]
- Bagchi, D.; Garg, A.; Krohn, R.; Bagchi, M.; Tran, M.; Stohs, S. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar]
- Jia, L.; Wu, Q.; Ye, N.; Liu, R.; Shi, L.; Xu, W.; Zhi, H.; Rahman, A.N.; Xia, Y.; Zhang, J. Proanthocyanidins inhibit seed germination by maintaining a high level of abscisic acid in Arabidopsis thaliana. J. Integr. Plant Biol. 2012, 54, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Duan, M.; Xing, P.; Xie, H.; Tang, X. Foliar application of procyanidins enhanced the biosynthesis of 2-acetyl-1-pyrroline in aromatic rice (Oryza sativa L.). BMC Plant Biol. 2022, 22, 376. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Shen, Y.; Jin, S.; Huang, S.; Cheng, X.; Wang, Z.; Li, P.; Zhao, J.; Bao, M.; Ning, G. Overexpression of Rosa rugosa anthocyanidin reductase enhances tobacco tolerance to abiotic stress through increased ROS scavenging and modulation of ABA signaling. Plant Sci. 2016, 245, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Li, F.; Hong, K.; Yuan, D. Effects of procyanidin treatment on the ripening and softening of banana fruit during storage. Sci. Hortic. 2022, 292, 110644. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Li, Y.; Wang, Y.; Whitetm, W.; Yuan, D.-B.; Jiang, Y. Exogenous procyanidin treatment delays senescence of harvested banana fruit by enhancing antioxidant responses and in vivo procyanidin content. Postharvest Biol. Technol. 2019, 158, 110999. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.; Li, Y.; Li, F.; Chen, J.; Yuan, D.-b.; Hong, K. Transcriptome analysis reveals the procyanidin treatment-responsive genes involved in regulating procyanidin accumulation during banana ripening and senescence. J. Am. Soc. Hortic. Sci. 2021, 146, 1–9. [Google Scholar] [CrossRef]
- Moing, A.; Renaud, C.; Gaudillère, M.; Raymond, P.; Roudeillac, P.; Denoyes-Rothan, B.A. Biochemical changes during fruit development of four strawberry cultivars. J. Am. Soc. Hortic. Sci. 2001, 126, 394–403. [Google Scholar] [CrossRef]
- Montero, T.M.; Mollá, E.M.; Esteban, R.M.; López-Andréu, F.J. Quality attributes of strawberry during ripening. Sci. Hortic. 1996, 65, 239–250. [Google Scholar] [CrossRef]
- Balasooriya, H.; Dassanayake, K.; Ajlouni, S. High temperature effects on strawberry fruit quality and antioxidant contents. Acta Hotic. 2016, 1278, 225–234. [Google Scholar] [CrossRef]
- Martínez-Rivas, F.J.; Blanco-Portales, R.; Molina-Hidalgo, F.J.; Caballero, J.L.; Perez de Souza, L.; Alseekh, S.; Fernie, A.R.; Muñoz-Blanco, J.; Rodríguez-Franco, A. Azacytidine arrests ripening in cultivated strawberry (Fragaria × ananassa) by repressing key genes and altering hormone contents. BMC Plant Biol. 2022, 22, 278. [Google Scholar] [CrossRef]
- Li, G.; Qin, B.; Li, S.; Yin, Y.; Zhao, J.; An, W.; Cao, Y.; Mu, Z. LbNR-derived nitric oxide delays lycium fruit coloration by transcriptionally modifying flavonoid biosynthetic pathway. Front. Plant Sci. 2020, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed Pharm. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Sirijan, M.; Pipattanawong, N.; Saeng-on, B.; Chaiprasart, P. Anthocyanin content, bioactive compounds and physico-chemical characteristics of potential new strawberry cultivars rich in-anthocyanins. J. Berry Res. 2020, 10, 397–410. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-mediated regulation of anthocyanin biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, L.; Zhang, J.; Zhang, Y.; Wang, Y.; Chen, Q.; Luo, Y.; Zhang, Y.; Li, M.; Wang, X.; et al. Identification of anthocyanins-related glutathione S-transferase (GST) genes in the genome of cultivated strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2020, 21, 8708. [Google Scholar] [CrossRef]
- Lunkenbein, S.; Coiner, H.; de Vos, C.H.; Schaart, J.G.; Boone, M.J.; Krens, F.A.; Schwab, W.; Salentijn, E.M. Molecular characterization of a stable antisense chalcone synthase phenotype in strawberry (Fragaria x ananassa). J. Agric. Food Chem. 2006, 54, 2145–2153. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, H.; Huang, T.; Shen, X.; Xia, J.; Pang, F.; Wang, J.; Zhao, M. The complexity of the Fragaria x ananassa (octoploid) transcriptome by single-molecule long-read sequencing. Hortic. Res. 2019, 6, 46. [Google Scholar] [CrossRef]
- Salvatierra, A.; Pimentel, P.; Moya-León, M.A.; Herrera, R. Increased accumulation of anthocyanins in Fragaria chiloensis fruits by transient suppression of FcMYB1 gene. Phytochem 2013, 90, 25–36. [Google Scholar] [CrossRef]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Griesser, M.; Hoffmann, T.; Bellido, M.L.; Rosati, C.; Fink, B.; Kurtzer, R.; Aharoni, A.; Muñoz-Blanco, J.; Schwab, W. Redirection of flavonoid biosynthesis through the down-regulation of an anthocyanidin glucosyltransferase in ripening strawberry fruit. Plant Physiol. 2008, 146, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.C.; Mirbeth, B.; Rentsch, J.; Sutter, C.; Ring, L.; Flachowsky, H.; Habegger, R.; Hoffmann, T.; Hanke, M.V.; Schwab, W. Premature and ectopic anthocyanin formation by silencing of anthocyanidin reductase in strawberry (Fragaria × ananassa). New Phytol. 2014, 201, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Q.; Yu, D.; Zhou, S.; Tang, H. Expression analysis of the ANR and LAR gene in Fragaria × ananassa cv. Toyonaka. J. Agric. Sci. 2013, 5, 2. [Google Scholar] [CrossRef]
- Liao, L.; Vimolmangkang, S.; Wei, G.; Zhou, H.; Korban, S.S.; Han, Y. Molecular characterization of genes encoding leucoanthocyanidin reductase involved in proanthocyanidin biosynthesis in apple. Front. Plant Sci. 2015, 6, 243. [Google Scholar] [CrossRef]
- Xin, Y.; Meng, S.; Ma, B.; He, W.; He, N. Mulberry genes MnANR and MnLAR confer transgenic plants with resistance to Botrytis cinerea. Plant Sci. 2020, 296, 110473. [Google Scholar] [CrossRef]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Han, Y.; Vimolmangkang, S.; Soria-Guerra, R.E.; Korban, S.S. Introduction of apple ANR genes into tobacco inhibits expression of both CHI and DFR genes in flowers, leading to loss of anthocyanin. J. Exp. Bot. 2012, 63, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Fujino, N.; Tenma, N.; Waki, T.; Ito, K.; Komatsuzaki, Y.; Sugiyama, K.; Yamazaki, T.; Yoshida, S.; Hatayama, M.; Yamashita, S.; et al. Physical interactions among flavonoid enzymes in snapdragon and torenia reveal the diversity in the flavonoid metabolon organization of different plant species. Plant J. 2018, 94, 372–392. [Google Scholar] [CrossRef]
- Paniagua, C.; Santiago-Doménech, N.; Kirby, A.R.; Gunning, A.P.; Morris, V.J.; Quesada, M.A.; Matas, A.J.; Mercado, J.A. Structural changes in cell wall pectins during strawberry fruit development. Plant Physiol. Biochem. 2017, 118, 55–63. [Google Scholar] [CrossRef]
- Sanchez, N.; Gutiérrez-López, G.F.; Cáez-Ramírez, G. Correlation among PME activity, viscoelastic, and structural parameters for Carica papaya edible tissue along ripening. J. Food Sci. 2020, 85, 1805–1814. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Pimentel, P.; Dotto, M.C.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-León, M.A. Expression of five expansin genes during softening of Fragaria chiloensis fruit: Effect of auxin treatment. Postharvest Biol. Technol. 2009, 53, 51–57. [Google Scholar] [CrossRef]
- García-Gago, J.A.; Posé, S.; Muñoz-Blanco, J.; Quesada, M.A.; Mercado, J.A. The polygalacturonase FaPG1 gene plays a key role in strawberry fruit softening. Plant Signal Behav. 2009, 4, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Guan, S.-C.; Chen, J.Q.; Wen, C.J.; Cai, J.F.; Chen, X. Genome wide identification and functional characterization of strawberry pectin methylesterases related to fruit softening. BMC Plant Biol. 2020, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pizarro, C.; Vallarino, J.G.; Osorio, S.; Meco, V.; Urrutia, M.; Pillet, J.; Casañal, A.; Merchante, C.; Amaya, I.; Willmitzer, L.; et al. The NAC transcription factor FaRIF controls fruit ripening in strawberry. Plant Cell 2021, 33, 1574–1593. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Mo, X.; Wen, C.; Gao, Z.; Chen, X.; Xue, C. FvMYB79 Positively regulates strawberry fruit softening via transcriptional activation of FvPME38. Int. J. Mol. Sci. 2021, 23, 101. [Google Scholar] [CrossRef]
- Salentijn, E.M.; Aharoni, A.; Schaart, J.G.; Boone, M.J.; Krens, F.A. Differential gene expression analysis of strawberry cultivars that differ in fruit-firmness. Physiol. Plant 2003, 118, 571–578. [Google Scholar] [CrossRef]
- Dzhanfezova, T.; Barba-Espín, G.; Müller, R.; Joernsgaard, B.; Hegelund, J.N.; Madsen, B.; Larsen, D.H.; Martínez Vega, M.; Toldam-Andersen, T.B. Anthocyanin profile, antioxidant activity and total phenolic content of a strawberry (Fragaria × ananassa Duch) genetic resource collection. Food Biosci. 2020, 36, 100620. [Google Scholar] [CrossRef]
- Mihai, C.M.; Al, L.; Mrghita, B.O.; Tma, M. Estimation of flavonoid content in propolis by two different colorimetric methods. Sci. Pap. Anim. Sci. Biotechnol. Timis. 2009, 3, 407–410. [Google Scholar]
- Pérez, A.G.; Olías, R.; Espada, J.; Olías, J.M.; Sanz, C. Rapid determination of sugars, nonvolatile acids, and ascorbic acid in strawberry and other fruits. J. Agric. Food Chem. 1997, 45, 3545–3549. [Google Scholar] [CrossRef]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef]
- Donno, D.; Cavanna, M.; Beccaro, G.L.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.K.; Bounous, G. Currants and strawberries as bioactive compound sources: Determination of antioxidant profi les with HPLC-DAD/MS. J. Appl. Bot. Food Qual. 2013, 86, 1–10. [Google Scholar]
- Chen, Q.; Yu, H.W.; Wang, X.R.; Xie, X.L.; Yue, X.Y.; Tang, H.R. An alternative cetyltrimethylammonium bromide-based protocol for RNA isolation from blackberry (Rubus L.). Genet. Mol. Res. 2012, 11, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yue, M.; Liu, Y.; Ye, Y.; Zhang, Y.; Lin, Y.; Wang, X.; Chen, Q.; Tang, H. Alterations of phenylpropanoid biosynthesis lead to the natural formation of pinkish-skinned and white-fleshed strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2022, 23, 7375. [Google Scholar] [CrossRef] [PubMed]
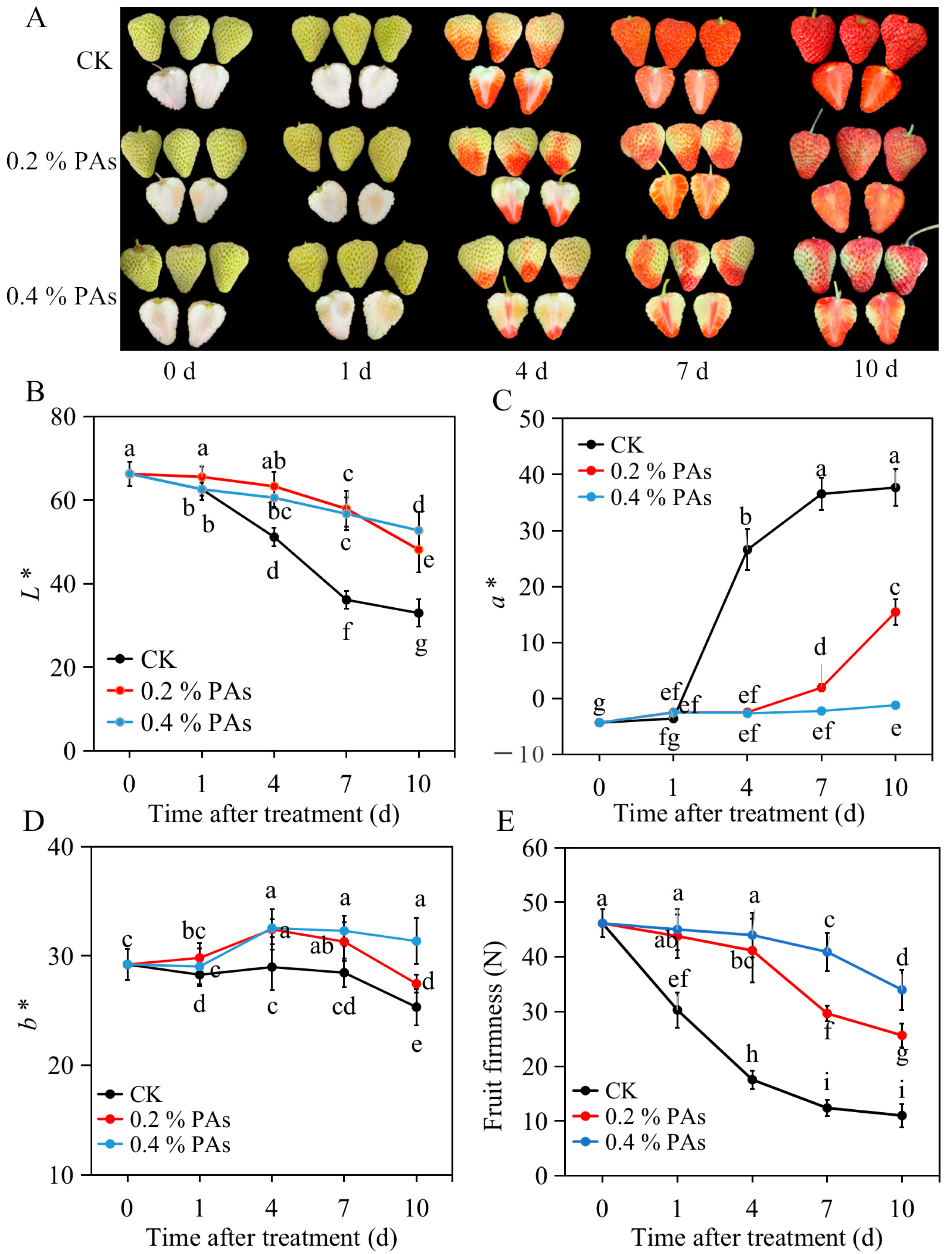



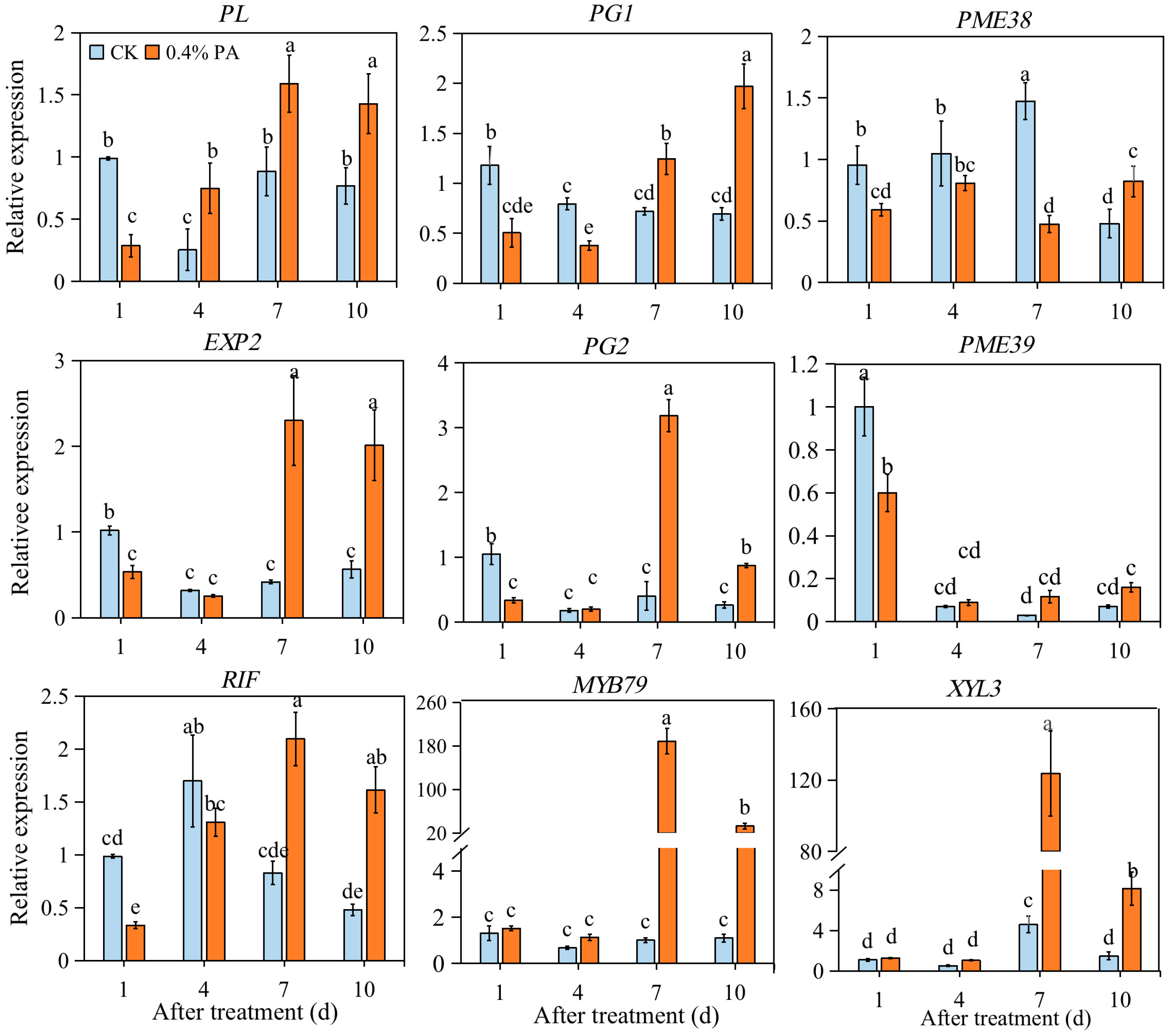
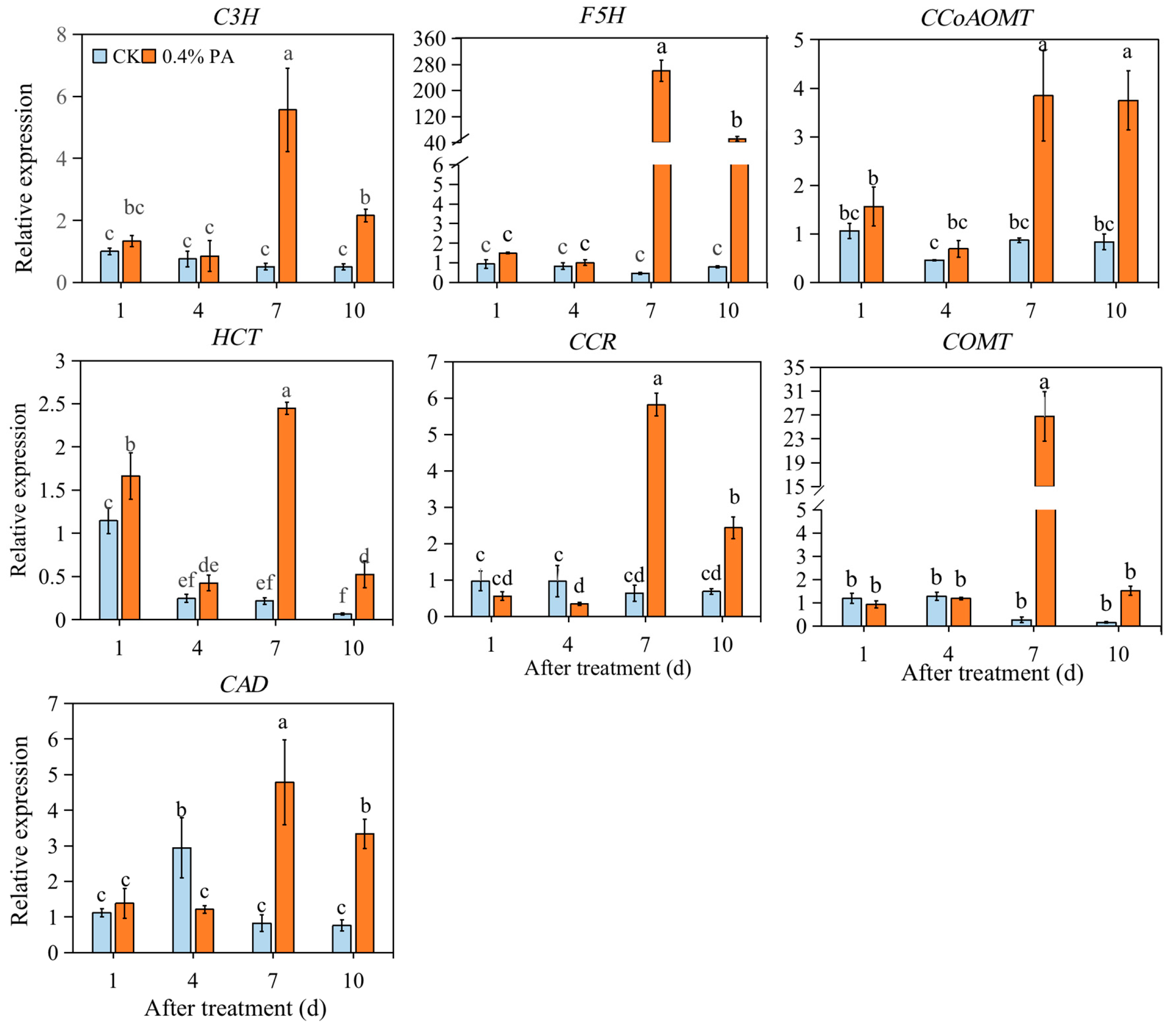
| Treatment | 0 d | 1 d | 4 d | 7 d | 10 d | |
|---|---|---|---|---|---|---|
| TSS (%) | CK | 7.69 ± 0.60 a | 7.29 ± 0.89 ab | 6.63 ± 0.74 cd | 5.74 ± 0.55 e | 5.22 ± 0.34 f |
| 0.2% | 7.69 ± 0.60 a | 6.43 ± 0.57 cd | 6.79 ± 0.48 bc | 6.20 ± 0.41 de | 5.20 ± 0.36 f | |
| 0.4% | 7.69 ± 0.60 a | 6.69 ± 0.50 cd | 6.31 ± 0.72 cd | 5.7 ± 0.59 de | 5.99 ± 0.66 de | |
| TA (%) | CK | 0.45 ± 0.04 h | 0.67 ± 0.07 fg | 0.91 ± 0.10 a | 0.82 ± 0.09 bc | 0.84 ± 0.04 b |
| 0.2% | 0.45 ± 0.04 h | 0.61 ± 0.07 g | 0.73 ± 0.03 de | 0.71 ± 0.03 def | 0.69 ± 0.04 ef | |
| 0.4% | 0.45 ± 0.04 h | 0.71 ± 0.08 def | 0.75 ± 0.06 de | 0.70 ± 0.02 ef | 0.77 ± 0.03 cd | |
| TPC (g·kg−1) | CK | 99.06 ± 8.54 bcd | 58.60 ± 21.84 e | 83.05 ± 31.52 bcde | 98.22 ± 22.28 b | 127.44 ± 29.10 a |
| 0.2% | 99.06 ± 8.54 bcd | 82.41 ± 15.83 bcde | 64.90 ± 8.99 de | 90.34 ± 15.41 bc | 127.96 ± 46.88 a | |
| 0.4% | 99.06 ± 8.54 bcd | 61.39 ± 8.44 e | 68.50 ± 5.02 cde | 79.08 ± 12.53 bcde | 129.96 ± 44.30 a | |
| TFC (g·kg−1) | CK | 426.54 ± 16.30 a | 322.53 ± 23.47 d | 386.24 ± 37.25 ab | 391.15 ± 24.32 a | 412.54 ± 13.13 a |
| 0.2% | 426.54 ± 16.30 a | 344.82 ± 13.15 cd | 333.15 ± 24.30 cd | 350.92 ± 84.88 bcd | 402.01 ± 37.91 a | |
| 0.4% | 426.54 ± 16.30 a | 329.13 ± 13.17 cd | 379.16 ± 15.56 abc | 379.56 ± 21.97 abc | 396.44 ± 84.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Wang, C.; Cao, S.; Sun, Z.; Zhang, Y.; Li, M.; He, W.; Wang, Y.; Chen, Q.; Zhang, Y.; et al. Proanthocyanidins Delay Fruit Coloring and Softening by Repressing Related Gene Expression during Strawberry (Fragaria × ananassa Duch.) Ripening. Int. J. Mol. Sci. 2023, 24, 3139. https://doi.org/10.3390/ijms24043139
Lin Y, Wang C, Cao S, Sun Z, Zhang Y, Li M, He W, Wang Y, Chen Q, Zhang Y, et al. Proanthocyanidins Delay Fruit Coloring and Softening by Repressing Related Gene Expression during Strawberry (Fragaria × ananassa Duch.) Ripening. International Journal of Molecular Sciences. 2023; 24(4):3139. https://doi.org/10.3390/ijms24043139
Chicago/Turabian StyleLin, Yuanxiu, Chunyan Wang, Shuaipeng Cao, Ziqing Sun, Yunting Zhang, Mengyao Li, Wen He, Yan Wang, Qing Chen, Yong Zhang, and et al. 2023. "Proanthocyanidins Delay Fruit Coloring and Softening by Repressing Related Gene Expression during Strawberry (Fragaria × ananassa Duch.) Ripening" International Journal of Molecular Sciences 24, no. 4: 3139. https://doi.org/10.3390/ijms24043139
APA StyleLin, Y., Wang, C., Cao, S., Sun, Z., Zhang, Y., Li, M., He, W., Wang, Y., Chen, Q., Zhang, Y., Wang, X., Luo, Y., & Tang, H. (2023). Proanthocyanidins Delay Fruit Coloring and Softening by Repressing Related Gene Expression during Strawberry (Fragaria × ananassa Duch.) Ripening. International Journal of Molecular Sciences, 24(4), 3139. https://doi.org/10.3390/ijms24043139







