Type IVb Hypersensitivity Reaction in the Novel Murine Model of Palladium–Induced Intraoral Allergic Contact Mucositis
Abstract
1. Introduction
2. Results
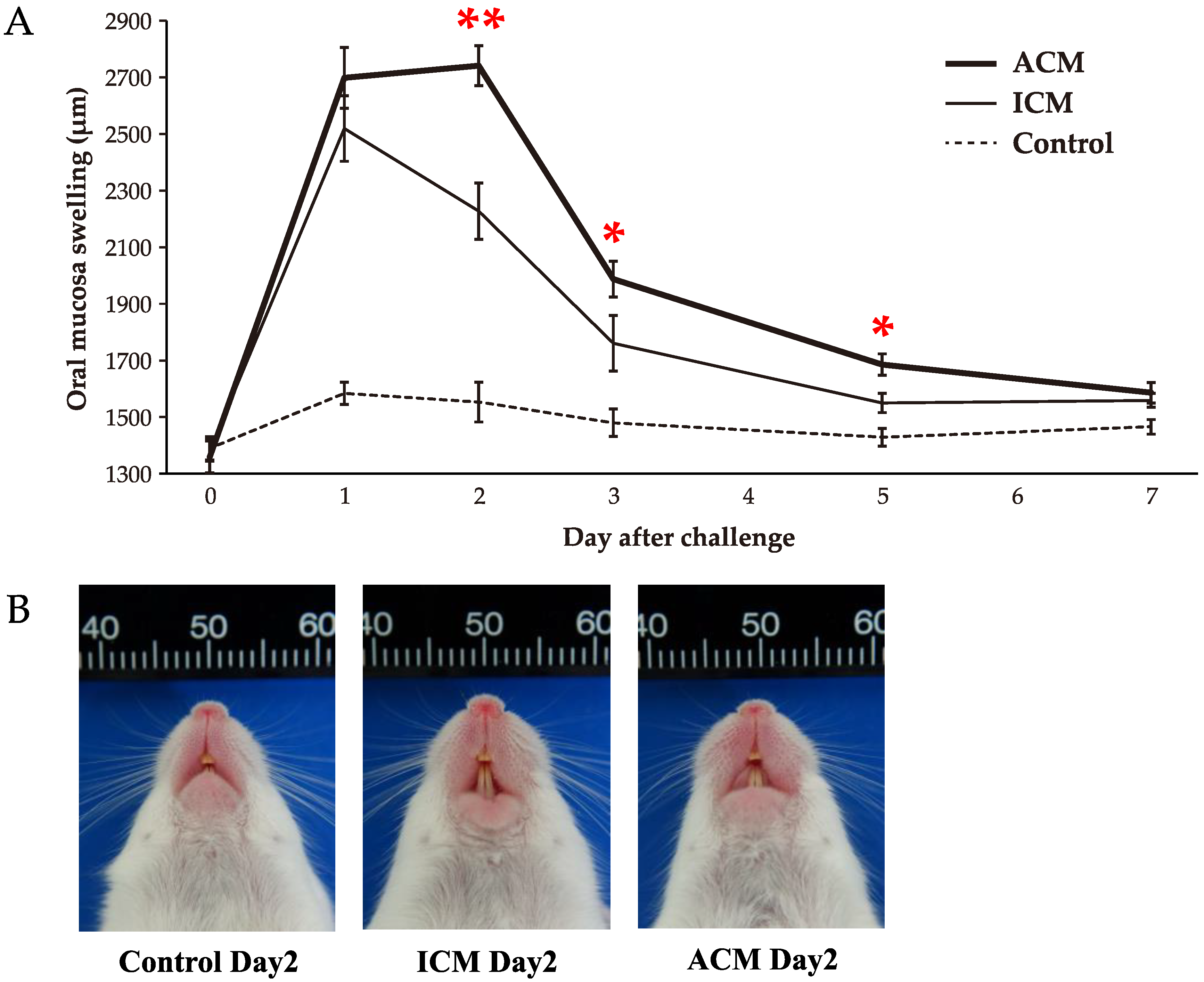
2.1. Pd–Induced Allergic BALB/cAJcl Mice Develop Swelling in the OM
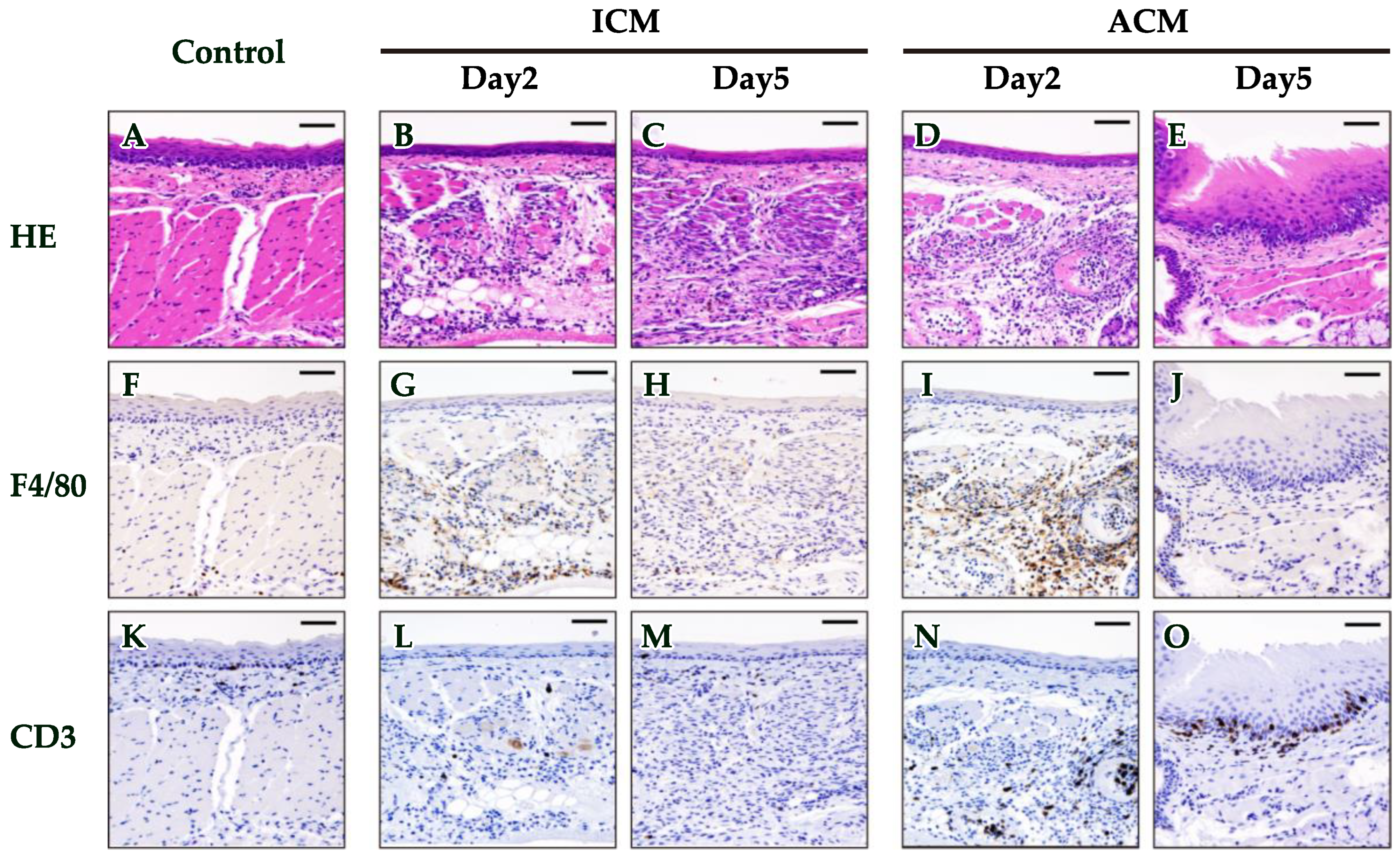
2.2. Histopathological and Immunohistochemical (IHC) Analyses of F4/80 and CD3 in the OM of Pd–Induced Allergic BALB/cAJcl Mice
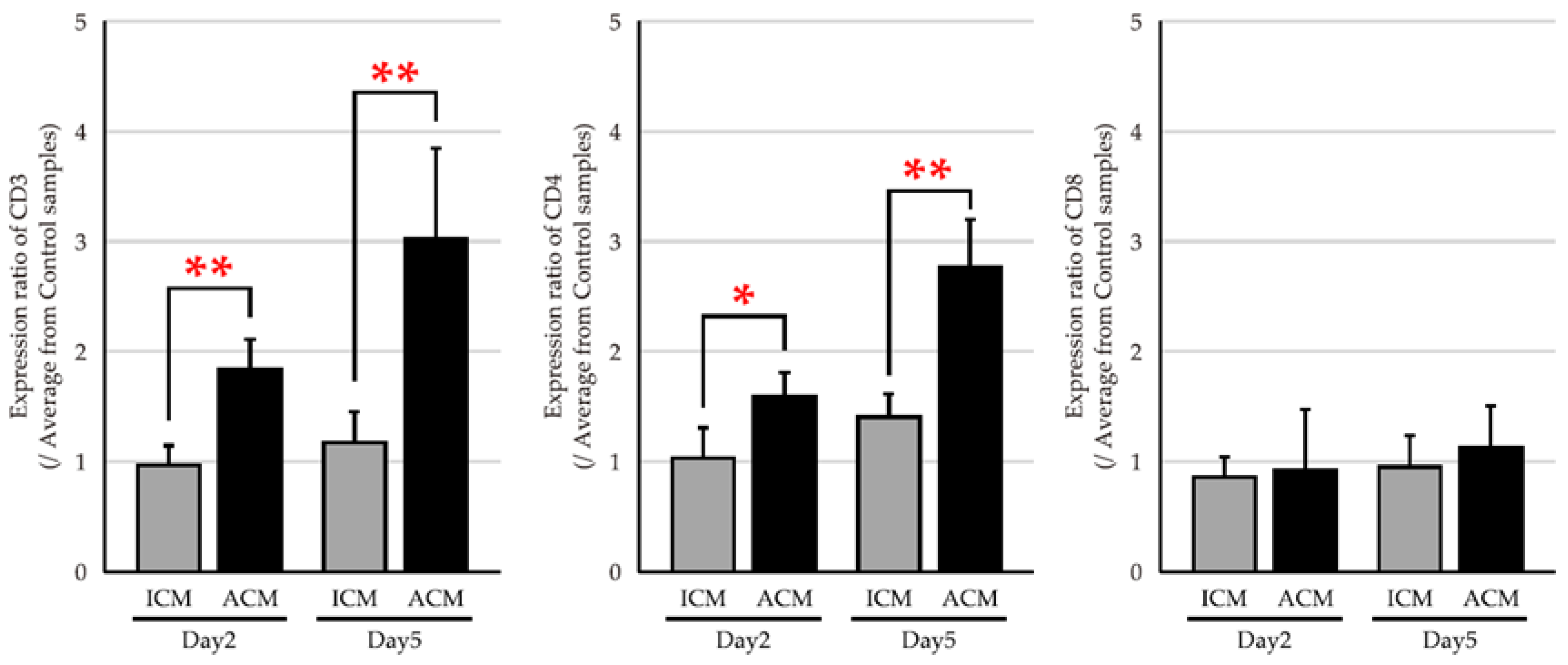
2.3. mRNA Expression Levels of T Cell Markers in the OM of Pd–Induced Allergic BALB/cAJcl Mice
2.4. mRNA Expression Levels of T Cell–Related Cytokines in the OM of Pd–Induced Allergic BALB/cAJcl Mice
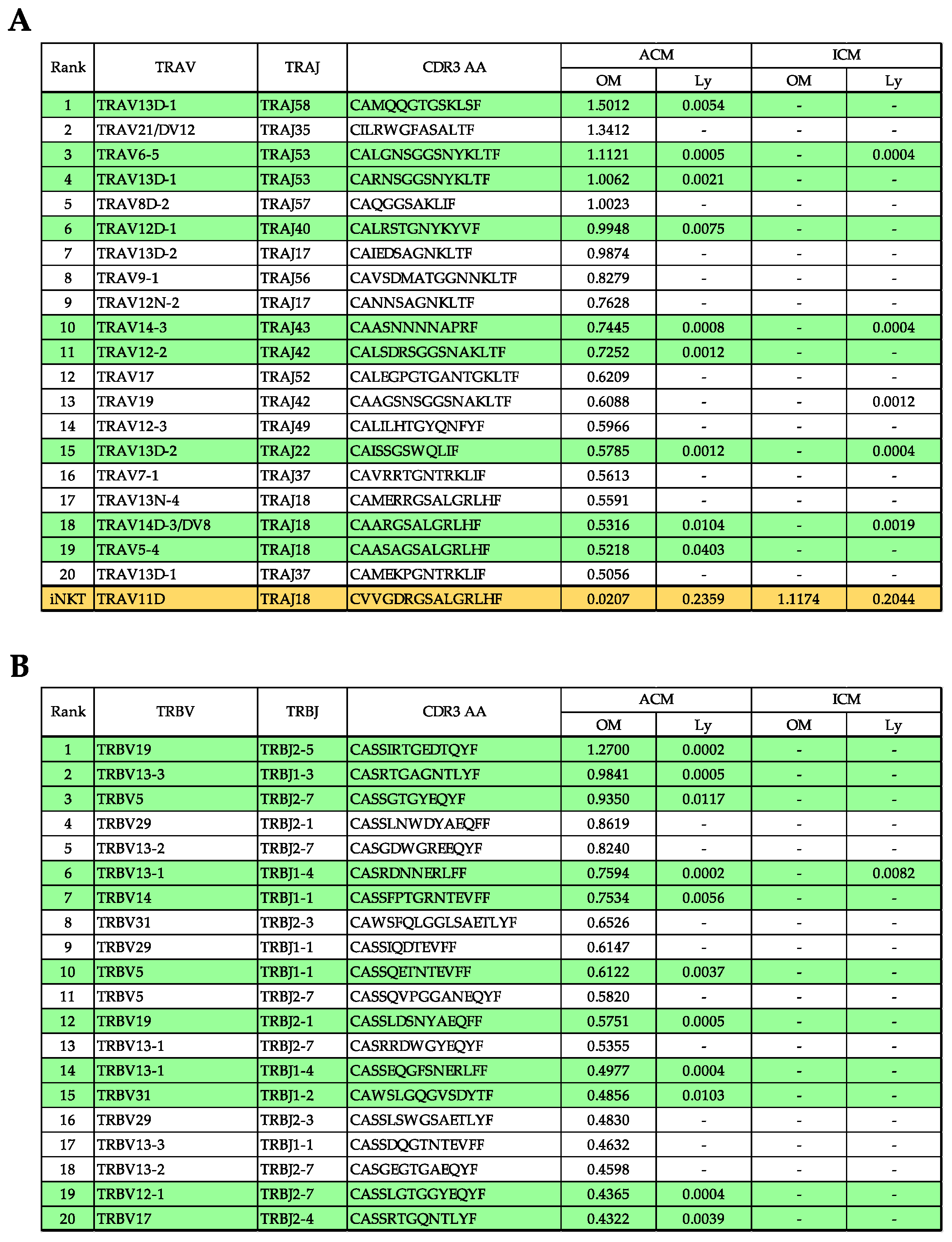
2.5. TRV–TRJ Combination and Diversity of TCR Repertoire in the OM and Cervical Lymph Nodes (Ly) of Pd–Induced ICM and ACM Mice on Day 5 after the Challenge
2.6. Commonality of TCR Repertoire in the OM and Ly of Pd–Induced ICM and ACM Mice on Day 5 after the Challenge
2.7. Evaluation of TRV and TRJ Gene Skew in Common Reads of ACM OM and ACM Ly Repertoire
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. Anesthetic Agents
4.4. Experimental Protocol of the Mouse Model of Pd–Induced Intraoral Metal Contact Allergy
4.5. Measurement of OM Swelling
4.6. Histological and IHC Analysis
4.7. RNA Extraction and cDNA Synthesis
4.8. qPCR
4.9. TCR Repertoire Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCR | T cell receptor |
| TRAV | TCRα–chain variable region |
| TRAJ | TCRα–chain joining region |
| TRBV | TCRβ–chain variable region |
| TRBJ | TCRβ–chain joining region |
| CDR3 | complementarity–determining region 3 |
| CD | cluster of differentiation |
References
- Raap, U.; Stiesch, M.; Reh, H.; Kapp, A.; Werfel, T. Investigation of contact allergy to dental metals in 206 patients. Contact Dermat. 2009, 60, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Murakami, S.; Akashi, Y.; Oka, H.; Shintani, T.; Ogawa, I.; Inoue, T.; Kurihara, H. Current status of dental metal allergy in Japan. J. Prosthodont. Res. 2019, 63, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Muris, J.; Goossens, A.; Gonçalo, M.; Bircher, A.J.; Giménez-Arnau, A.; Foti, C.; Bruze, M.; Andersen, K.E.; Rustemeyer, T.; Feilzer, A.J.; et al. Sensitization to palladium in Europe. Contact Dermat. 2015, 72, 11–19. [Google Scholar] [CrossRef]
- Faurschou, A.; Menné, T.; Johansen, J.D.; Thyssen, J.P. Metal allergen of the 21st century—A review on exposure, epidemiology and clinical manifestations of palladium allergy. Contact Dermat. 2011, 64, 185–195. [Google Scholar] [CrossRef]
- Kielhorn, J.; Melber, C.; Keller, D.; Mangelsdorf, I. Palladium—A review of exposure and effects to human health. Int. J. Hyg. Environ. Health 2002, 205, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Riedel, F.; Aparicio-Soto, M.; Curato, C.; Thierse, H.J.; Siewert, K.; Luch, A. Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. Int. J. Environ. Res. Public Health 2021, 18, 10867. [Google Scholar] [CrossRef]
- Ahlfors, E.; Czerkinsky, C. Contact sensitivity in the murine oral mucosa. I. An experimental model of delayed-type hypersensitivity reactions at mucosal surfaces. Clin. Exp. Immunol. 1991, 86, 449–456. [Google Scholar] [CrossRef]
- Larese Filon, F.; Uderzo, D.; Bagnato, E. Sensitization to palladium chloride: A 10-year evaluation. Am. J. Contact Dermat. 2003, 14, 78–81. [Google Scholar] [CrossRef]
- Ahlfors, E.; Jonsson, R.; Czerkinsky, C. Experimental T cell-mediated inflammatory reactions in the murine oral mucosa. II. Immunohistochemical characterization of resident and infiltrating cells. Clin. Exp. Immunol. 1996, 104, 297–305. [Google Scholar] [CrossRef]
- Nakasone, Y.; Kumagai, K.; Matsubara, R.; Shigematsu, H.; Kitaura, K.; Suzuki, S.; Satoh, M.; Hamada, Y.; Suzuki, R. Characterization of T cell receptors in a novel murine model of nickel-induced intraoral metal contact allergy. PLoS ONE 2018, 13, e0209248. [Google Scholar] [CrossRef]
- Kitaura, K.; Fujii, Y.; Hayasaka, D.; Matsutani, T.; Shirai, K.; Nagata, N.; Lim, C.K.; Suzuki, S.; Takasaki, T.; Suzuki, R.; et al. High clonality of virus-specific T lymphocytes defined by TCR usage in the brains of mice infected with West Nile virus. J. Immunol. 2011, 187, 3919–3930. [Google Scholar] [CrossRef]
- Kitaura, K.; Shini, T.; Matsutani, T.; Suzuki, R. A new high-throughput sequencing method for determining diversity and similarity of T cell receptor (TCR) α and β repertoires and identifying potential new invariant TCR α chains. BMC Immunol. 2016, 17, 38. [Google Scholar] [CrossRef]
- Kitaura, K.; Yamashita, H.; Ayabe, H.; Shini, T.; Matsutani, T.; Suzuki, R. Different Somatic Hypermutation Levels among Antibody Subclasses Disclosed by a New Next-Generation Sequencing-Based Antibody Repertoire Analysis. Front. Immunol. 2017, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Nakayama, M.; Aoshima, Y.; Nakamura, K.; Ono, M.; Nishiya, T.; Nakamura, S.; Takeda, Y.; Dobashi, A.; Takahashi, A.; et al. NKG2D+ IFN-γ+ CD8+ T cells are responsible for palladium allergy. PLoS ONE 2014, 9, e86810. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kumagai, K.; Eguchi, T.; Shigematsu, H.; Kitaura, K.; Kawano, M.; Horikawa, T.; Suzuki, S.; Matsutani, T.; Ogasawara, K.; et al. Characterization of T cell receptors of Th1 cells infiltrating inflamed skin of a novel murine model of palladium-induced metal allergy. PLoS ONE 2013, 8, e76385. [Google Scholar] [CrossRef]
- Ward, J.P.; Franks, S.J.; Tindall, M.J.; King, J.R.; Curtis, A.; Evans, G.S. Mathematical modelling of contact dermatitis from nickel and chromium. J. Math Biol. 2019, 79, 595–630. [Google Scholar] [CrossRef] [PubMed]
- Tanei, R.; Hasegawa, Y. Immunological Pathomechanisms of Spongiotic Dermatitis in Skin Lesions of Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 6682. [Google Scholar] [CrossRef]
- Yawalkar, N.; Hunger, R.E.; Buri, C.; Schmid, S.; Egli, F.; Brand, C.U.; Mueller, C.; Pichler, W.J.; Braathen, L.R. A comparative study of the expression of cytotoxic proteins in allergic contact dermatitis and psoriasis: Spongiotic skin lesions in allergic contact dermatitis are highly infiltrated by T cells expressing perforin and granzyme B. Am. J. Pathol. 2001, 158, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Uzzaman, A.; Cho, S.H. Chapter 28: Classification of hypersensitivity reactions. Allergy Asthma Proc. 2012, 33 (Suppl. 1), 96–99. [Google Scholar] [CrossRef]
- Saint-Mezard, P.; Berard, F.; Dubois, B.; Kaiserlian, D.; Nicolas, J.F. The role of CD4+ and CD8+ T cells in contact hypersensitivity and allergic contact dermatitis. Eur. J. Derm. 2004, 14, 131–138. [Google Scholar]
- Sumiwi, Y.A.; Soesatyo, M.H.; Sosroseno, W. The role of CD4+ T cells in the induction of contact hypersensitivity to mercury in a murine model. Cutan. Ocul. Toxicol. 2010, 29, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Unutmaz, D.; Gaspari, A.A. Natural killer T cells: An unconventional T-cell subset with diverse effector and regulatory functions. J. Investig. Derm. 2009, 129, 1628–1642. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Kronenberg, M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J. Clin. Investig. 2004, 114, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kumagai, K.; Kobayashi, H.; Shigematsu, H.; Kitaura, K.; Suzuki, S.; Horikawa, T.; Hamada, Y.; Ogasawara, K.; Suzuki, R. Accumulation of invariant NKT cells into inflamed skin in a novel murine model of nickel allergy. Cell Immunol. 2013, 284, 163–171. [Google Scholar] [CrossRef]
- Shigematsu, H.; Kumagai, K.; Kobayashi, H.; Eguchi, T.; Kitaura, K.; Suzuki, S.; Horikawa, T.; Matsutani, T.; Ogasawara, K.; Hamada, Y.; et al. Accumulation of metal-specific T cells in inflamed skin in a novel murine model of chromium-induced allergic contact dermatitis. PLoS ONE 2014, 9, e85983. [Google Scholar] [CrossRef]
- Kumagai, K.; Matsubara, R.; Nakasone, Y.; Shigematsu, H.; Kitaura, K.; Suzuki, S.; Haneji, K.; Hamada, Y.; Suzuki, R. Possible involvement of invariant natural killer T cells and mucosal-associated invariant T cells in a murine model of titanium allergy. J. Oral Maxillofac. Surg. Med. Pathol. 2018, 30, 1–9. [Google Scholar] [CrossRef]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef]
- Fujii, Y.; Kitaura, K.; Nakamichi, K.; Takasaki, T.; Suzuki, R.; Kurane, I. Accumulation of T-cells with selected T-cell receptors in the brains of Japanese encephalitis virus-infected mice. Jpn. J. Infect. Dis. 2008, 61, 40–48. [Google Scholar]
- Yoshida, R.; Yoshioka, T.; Yamane, S.; Matsutani, T.; Toyosaki-Maeda, T.; Tsuruta, Y.; Suzuki, R. A new method for quantitative analysis of the mouse T-cell receptor V region repertoires: Comparison of repertoires among strains. Immunogenetics 2000, 52, 35–45. [Google Scholar] [CrossRef]
- Lefranc, M.P.; Giudicelli, V.; Ginestoux, C.; Jabado-Michaloud, J.; Folch, G.; Bellahcene, F.; Wu, Y.; Gemrot, E.; Brochet, X.; Lane, J.; et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009, 37, D1006–D1012. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasu, K.; Kumagai, K.; Yoshizawa, T.; Kitaura, K.; Matsubara, R.; Suzuki, M.; Suzuki, R.; Hamada, Y. Type IVb Hypersensitivity Reaction in the Novel Murine Model of Palladium–Induced Intraoral Allergic Contact Mucositis. Int. J. Mol. Sci. 2023, 24, 3137. https://doi.org/10.3390/ijms24043137
Nasu K, Kumagai K, Yoshizawa T, Kitaura K, Matsubara R, Suzuki M, Suzuki R, Hamada Y. Type IVb Hypersensitivity Reaction in the Novel Murine Model of Palladium–Induced Intraoral Allergic Contact Mucositis. International Journal of Molecular Sciences. 2023; 24(4):3137. https://doi.org/10.3390/ijms24043137
Chicago/Turabian StyleNasu, Keisuke, Kenichi Kumagai, Takamasa Yoshizawa, Kazutaka Kitaura, Ryota Matsubara, Motoaki Suzuki, Ryuji Suzuki, and Yoshiki Hamada. 2023. "Type IVb Hypersensitivity Reaction in the Novel Murine Model of Palladium–Induced Intraoral Allergic Contact Mucositis" International Journal of Molecular Sciences 24, no. 4: 3137. https://doi.org/10.3390/ijms24043137
APA StyleNasu, K., Kumagai, K., Yoshizawa, T., Kitaura, K., Matsubara, R., Suzuki, M., Suzuki, R., & Hamada, Y. (2023). Type IVb Hypersensitivity Reaction in the Novel Murine Model of Palladium–Induced Intraoral Allergic Contact Mucositis. International Journal of Molecular Sciences, 24(4), 3137. https://doi.org/10.3390/ijms24043137




