Preparation, Characterization, and Biological Properties of Hydroxyapatite from Bigeye Snapper (Priancanthus tayenus) Bone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Bigeye Snapper Bone
2.2. Optimization of Hydroxyapatite Extraction Condition
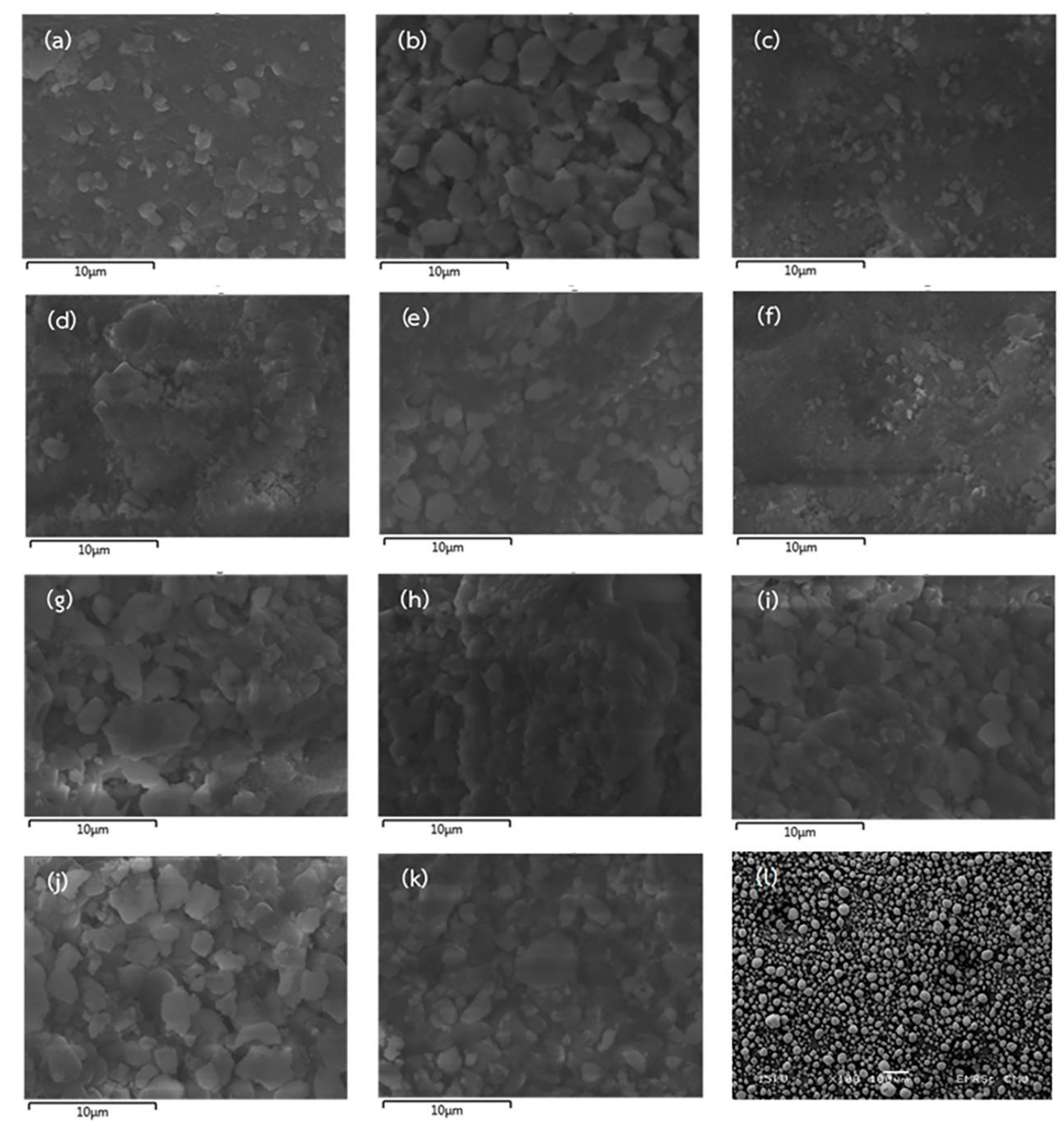
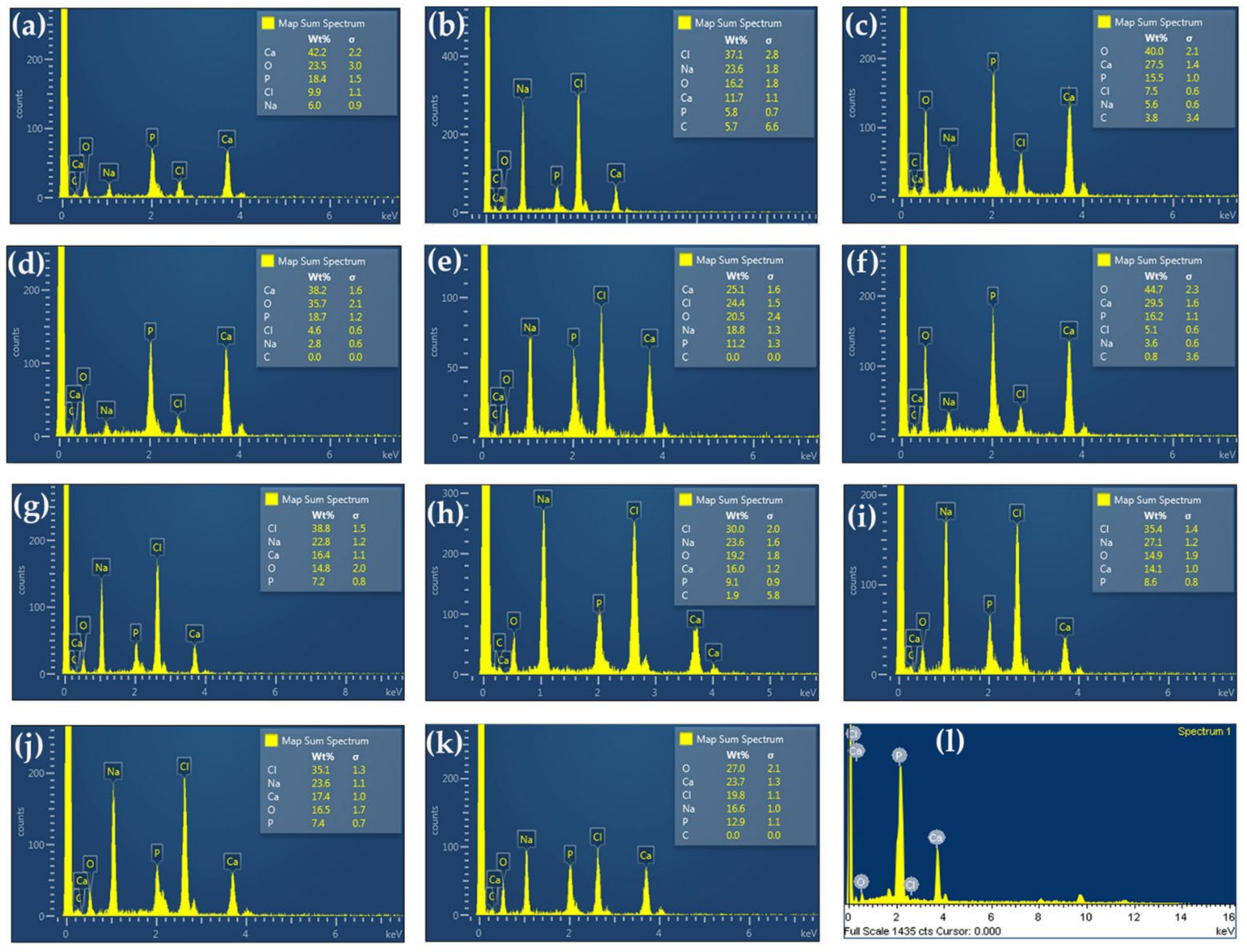
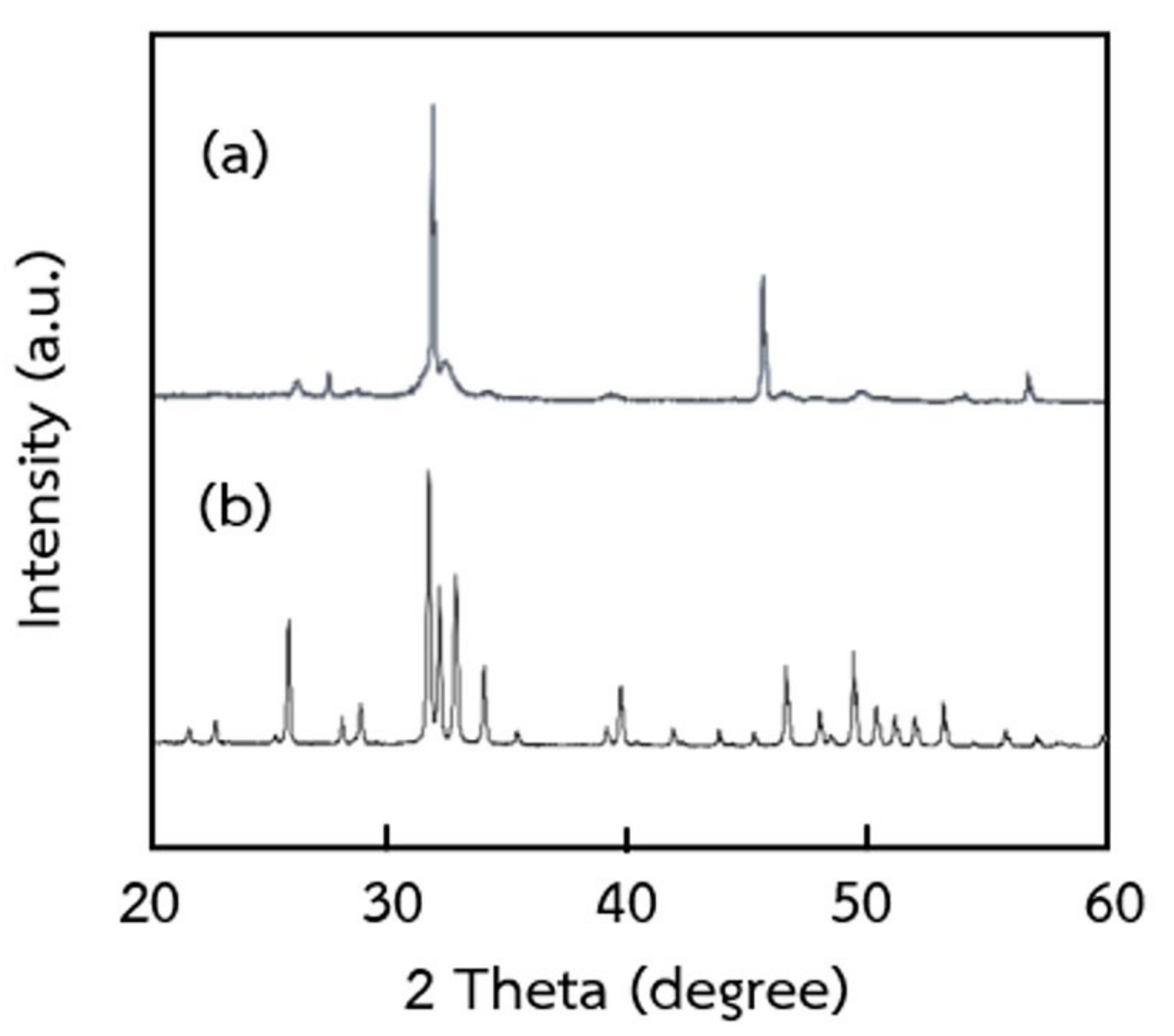
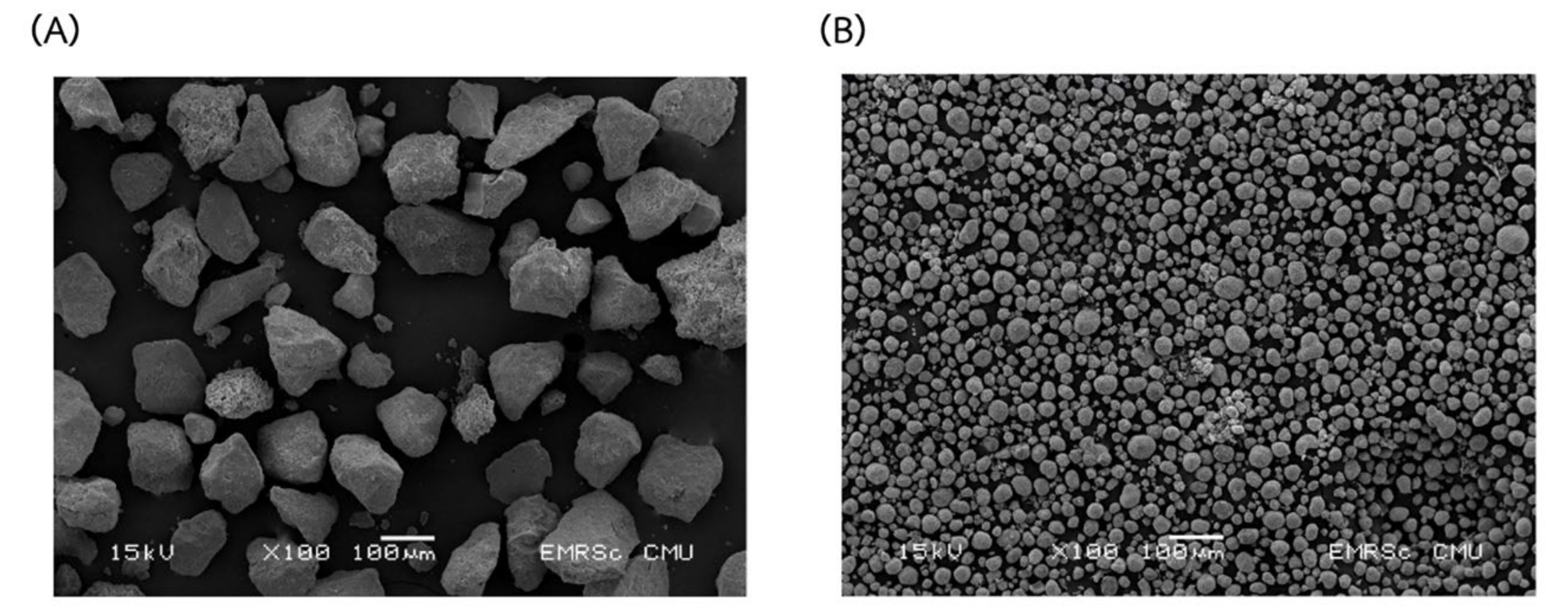
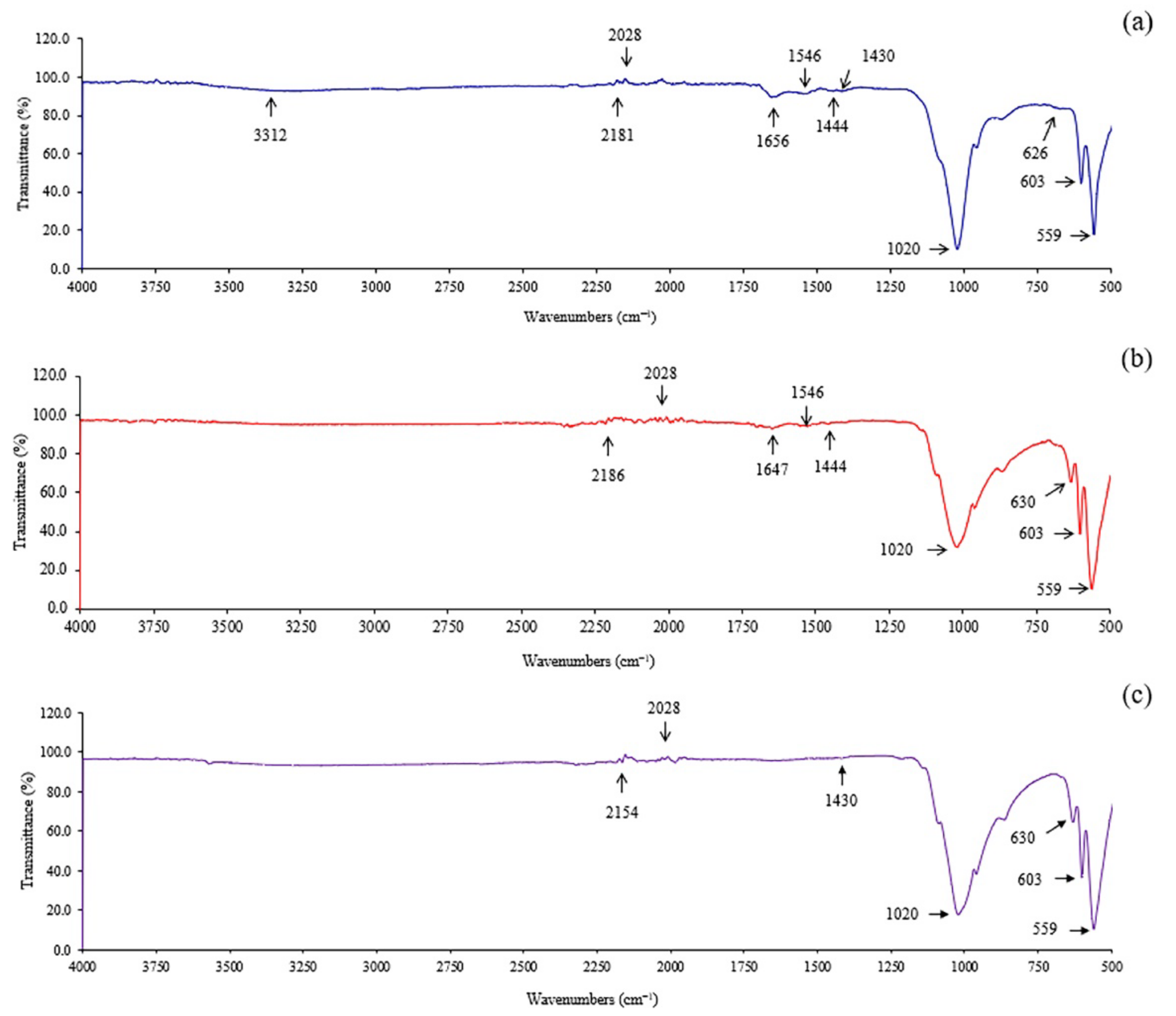
2.3. Characterization of Extracted Hydroxyapatite
2.4. Hydroxyapatite Precipitation from the Extracted Solution
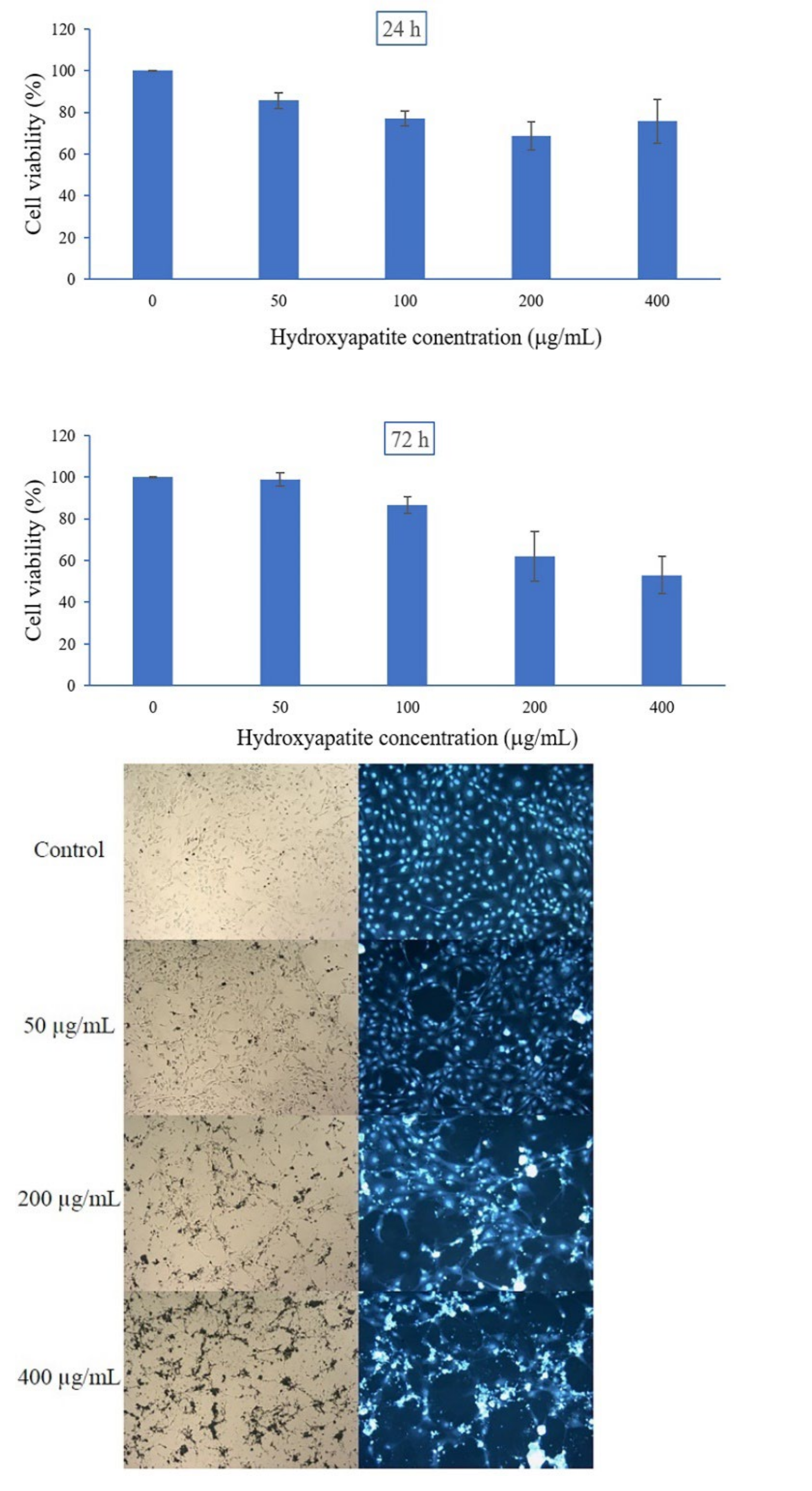
2.5. Cell Cytotoxicity
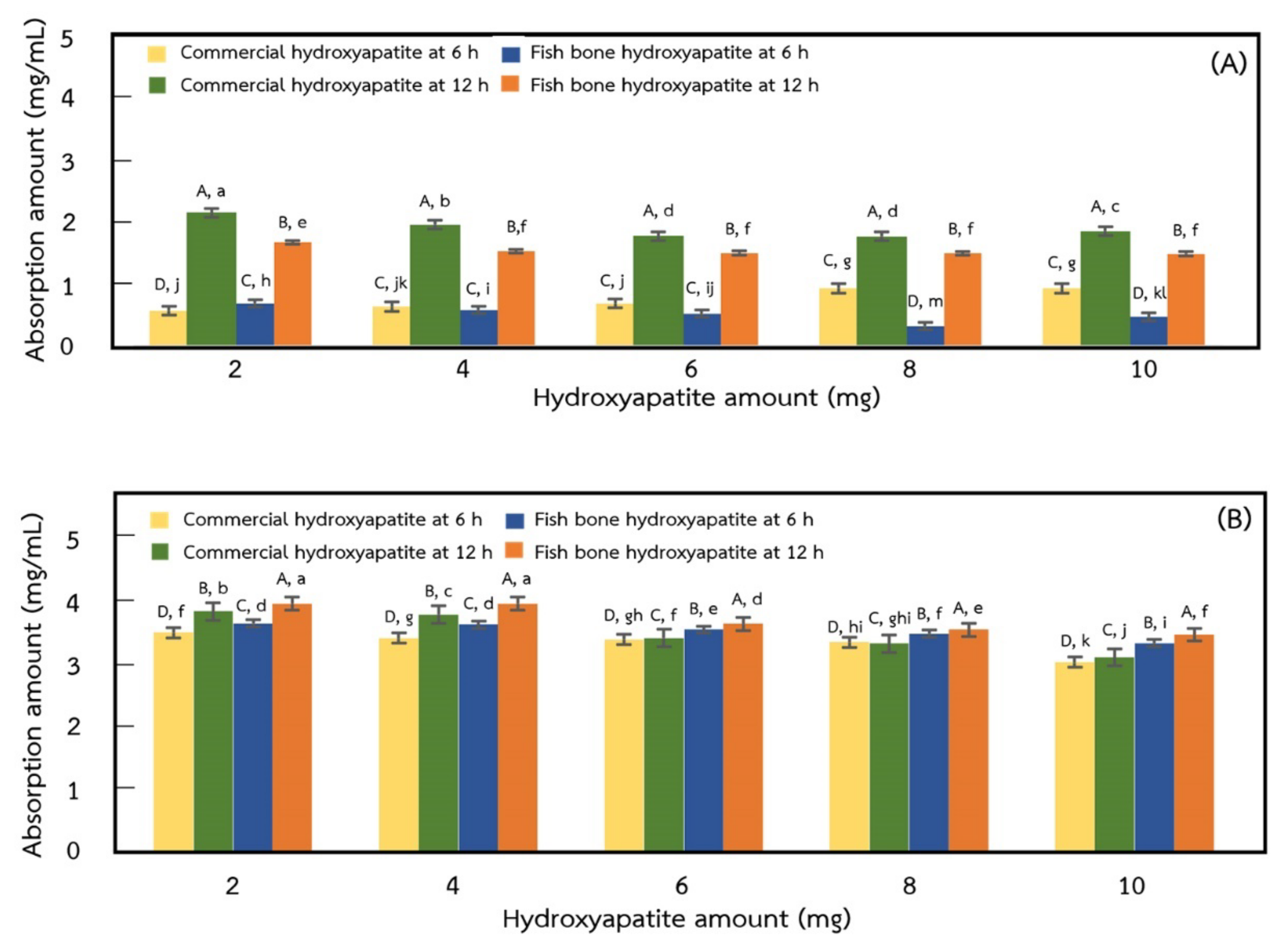
2.6. Adsorption of Protein on Hydroxyapatite
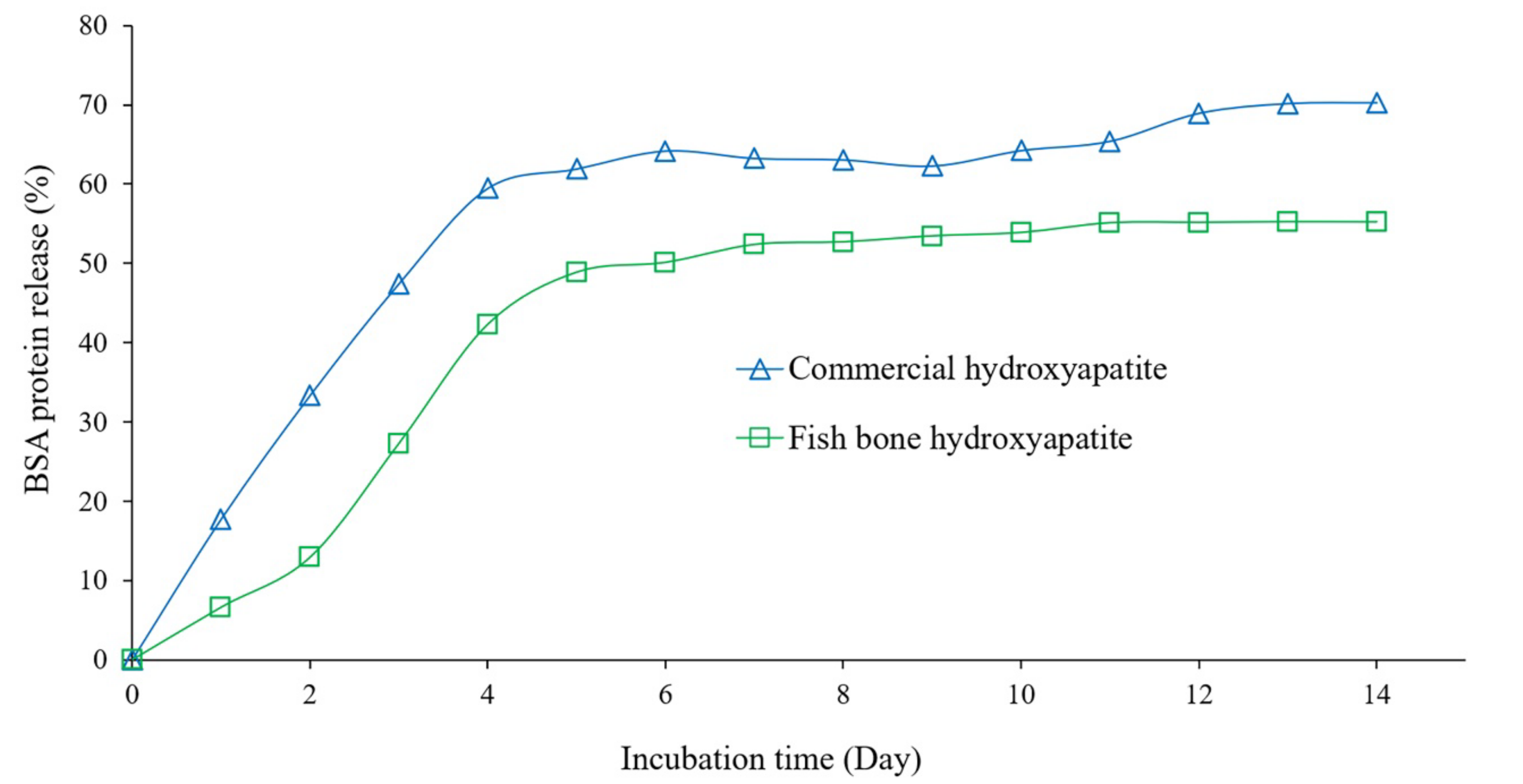
2.7. Desorption of Protein on Hydroxyapatite
3. Materials and Methods
3.1. Material and Preparation
3.2. Chemicals
3.3. Hydroxyapatite Extraction from Bigeye Snapper Bones
3.4. Optimization of Experimental Design
3.5. Analyzes
3.5.1. Proximate Compositions
3.5.2. Hydroxyapatite Extraction Yield
3.5.3. Color Determination
3.5.4. Calcium and Phosphorus Content
3.5.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.5.6. Scanning Electron Microscope (SEM) and Energy Dispersive X-ray (EDX)
3.5.7. X-ray Diffraction Analysis (XRD)
3.6. The Precipitation of Hydroxyapatite from the Extracted Solution
3.7. In Vitro Cell Viability
3.8. Adsorption of Protein on Hydroxyapatite
3.9. Desorption of Protein from Protein-Adsorbed Hydroxyapatite
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; pp. 73–81. [Google Scholar]
- Jaziri, A.A.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Chemical composition of lizardfish surimi by-product: Focus on macro and micro-minerals contents. Curr. Res. Nutr. Food Sci. 2021, 9, 52–61. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish waste: From problem to valuable resource. Mar. Drugs 2021, 19, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Bas, M.; Daglilar, S.; Kuskonmaz, N.; Kalkandelen, C.; Erdemir, G.; Kuruca, S.E.; Tulyaganov, D.; Yoshioka, T.; Gunduz, O.; Ficai, D.; et al. Mechanical and biocompatibility properties of calcium phosphate bioceramics derived from salmon fish bone wastes. Int. J. Mol. Sci. 2020, 21, 8082. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Janpet, C.; Manakit, P.; Klinmalai, P.; Kaewprachu, P.; Jaisan, C.; Surayot, U.; Chakrabandhu, Y.; Wangtueai, S. Characteristics and functional properties of gelatin and gelatin hydrolysate from bigeye snapper (Priacanthus tayenus) bone. Food Res. 2022, 6, 403–412. [Google Scholar] [CrossRef]
- Kojima, S.; Nakamura, H.; Lee, S.; Nagata, F.; Kato, K. Hydroxyapatite formation on self-assembling peptides with differing secondary structures and their selective adsorption for proteins. Int. J. Mol. Sci. 2019, 20, 4650. [Google Scholar] [CrossRef]
- Nam, V.P.; Hoa, N.V.; Trung, T.S. Properties of hydroxyapatites prepared from different fish bones: A comparative study. Ceram. Int. 2019, 45, 20141–20147. [Google Scholar] [CrossRef]
- Dabiri, S.M.H.; Rezaie, A.A.; Moghimi, M.; Rezaie, H. Extraction of hydroxyapatite from fish bones and its application in nickel adsorption. BioNanoScience 2018, 8, 823–834. [Google Scholar] [CrossRef]
- Khoo, W.; Nor, F.M.; Ardhyananta, H.; Kurniawan, D. Preparation of natural hydroxyapatite from bovine femur bones using calcination at various temperatures. Procedia Manuf. 2015, 2, 196–201. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Rajendran, A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O.; Pattanayak, D.K. Synthesis of organic derived hydroxyapatite scaffold from pig bone waste for tissue engineering applications. Adv. Powder Technol. 2018, 29, 1–8. [Google Scholar] [CrossRef]
- Ingole, V.H.; Hussein, K.H.; Kashale, A.A.; Ghule, K.; Vuherer, T.; Kokol, V.; Ling, Y.C.; Vinchurkar, A.; Dhakal, H.N.; Ghule, A.V. Ultrasound-assisted green economic synthesis of hydroxyapatite nanoparticles using eggshell biowaste and study of mechanical and biological properties for orthopedic applications. J. Biomed. Mater. Res. A 2017, 105, 2935–2947. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Manivasagan, P.; Kang, K.H.; Chalisserry, E.P.; Anil, S.; Kim, D.G.; Kim, S.K. Isolation and characterization of nano-hydroxyapatite from salmon fish bone. Materials 2015, 8, 5426–5439. [Google Scholar] [CrossRef]
- Shi, P.; Liu, M.; Fan, F.; Yu, C.; Lu, W.; Du, M. Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts. Mater. Sci. Eng. C 2018, 90, 706–712. [Google Scholar] [CrossRef]
- Yamamura, H.; da Silva, V.H.P.; Ruiz, P.L.M.; Ussui, V.; Lazar, D.R.R.L.; Renno, A.C.M.; Ribeiro, D.A. Physico-chemical characterization and biocompatibility of hydroxyapatite derived from fish waste. J. Mech. Behav. Biomed. Mater. 2018, 80, 137–142. [Google Scholar] [CrossRef]
- Tomoda, K.; Ariizumi, H.; Nakaji, T.; Makino, K. Hydroxyapatite particles as drug carriers for proteins. Colloids Surf. B Biointerfaces 2010, 76, 226–235. [Google Scholar] [PubMed]
- Wang, K.; Wang, M.H.; Wang, Q.W.; Lu, X.; Zhang, X.D. Computer simulation of proteins adsorption on hydroxyapatite surfaces with calcium phosphate ions. J. Eur. Ceram. Soc. 2017, 37, 2509–2520. [Google Scholar] [CrossRef]
- Lee, W.H.; Zavgorodniy, A.V.; Loo, C.Y.; Rohanizadeh, R. Synthesis and characterization of hydroxyapatite with different crystallinity: Effects on protein adsorption and release. J. Biomed. Mater. Res. A 2012, 100A, 1539–1549. [Google Scholar] [CrossRef]
- Kojima, C.; Watanabe, K. Adsorption and desorption of bioactive proteins on hydroxyapatite for protein delivery systems. J. Drug Deliv. 2012, 2012, 932461. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, S.; Deng, C. Effect of hydroxyapatite coating surface morphology on adsorption behavior of differently charged proteins. J. Bionic Eng. 2020, 17, 345–356. [Google Scholar] [CrossRef]
- Jaziri, A.A.; Hasanuddin, H.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Nutritional composition and mineral analysis of the by-products from tropical marine fish, purple-spotted bigeye (Priacanthus tayenus Richardson, 1846) and barracuda (Sphyraena obtusata Cuvier, 1829). IOP Conf. Ser. Earth Environ. Sci. 2022, 967, 012051. [Google Scholar] [CrossRef]
- Mongkonkamthorn, N.; Malila, Y.; Regenstein, J.M.; Wangtueai, S. Enzymatic hydrolysis optimization for preparation of tuna dark meat hydrolysate with antioxidant and angiotensin I-converting enzyme (ACE) inhibitory activities. J. Aquat. Food Prod. Technol. 2021, 30, 1090–1108. [Google Scholar] [CrossRef]
- Upata, M.; Siriwoharn, T.; Makkhun, S.; Yarnpakdee, S.; Regenstein, J.M.; Wangtueai, S. Tyrosinase inhibitory and antioxidant activity of enzymatic protein hydrolysate from jellyfish (Lobnema smithii). Foods 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Wang, D.; Yang, F.; Zhang, L.; Ji, S.; Wang, S. Enhanced extraction of hydroxyapatite from bighead carp (Aristichthys nobilis) scales using deep eutectic solvent. J. Food Sci. 2020, 85, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Gao, Y.; Iqbal, F.; Ahmad, P.; Ge, R.; Nishan, U.; Rahimb, A.; Gonfae, G.; Ullah, Z. Extraction of biocompatible hydroxyapatite from fish scales using novel approach of ionic liquid pretreatment. Sep. Purif. Technol. 2016, 161, 129–135. [Google Scholar] [CrossRef]
- Zhang, W.; Chai, Y.; Xu, X.; Wang, Y.; Cao, N. Rod-shaped hydroxyapatite with mesoporous structure as drug carriers for proteins. Appl. Surf. Sci. 2014, 322, 71–77. [Google Scholar] [CrossRef]
- Daupor, H. Extraction of hydroxyapatite by alkaline acid from budu waste and synthesis using calcination method. J. Phys. Conf. Ser. 2021, 2049, 012041. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; De Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Ramesh, S.; Loo, Z.Z.; Tan, C.Y.; Chew, W.J.K.; Ching, Y.C.; Tarlochan, F.; Chandran, H.; Krishnasamy, S.; Bang, L.T.; Ahmed, A.D.S. Characterization of biogenic hydroxyapatite derived from animal bones for biomedical applications. Ceram. Int. 2018, 44, 10525–10530. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Nasri, C.S.S.M.; Arshad, S.E.B. Hydrothermal synthesis of hydroxyapatite powders using Response Surface Methodology (RSM). PLoS ONE 2021, 16, e0251009. [Google Scholar] [CrossRef]
- Manoj, M.; Subbiah, R.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C.; Park, K. Influence of growth parameters on the formation of hydroxyapatite (HAp) nanostructures and their cell viability studies. Nanobiomedicine 2015, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Cursaru, L.M.; Iota, M.; Piticescu, R.M.; Tarnita, D.; Savu, S.V.; Savu, I.D.; Dumitrescu, G.; Popescu, D.; Hertzog, R.G.; Calin, M. Hydroxyapatite from natural sources for medical applications. Materials 2022, 15, 5091. [Google Scholar] [CrossRef] [PubMed]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Hydroxyapatite from fish scale for potential use as bone scaffold or regenerative material. Mater. Sci. Eng. C 2016, 62, 183–189. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, Determination of Nutritional Elements in Food Products, 20th ed.; The Association of Official Analytical Chemists: Rockville, MD, USA, 2016. [Google Scholar]
- Panda, N.N.; Pramanik, K.; Sukla, L.B. Extraction and characterization of biocompatible hydroxyapatite from fresh water fish scales for tissue engineering scaffold. Bioprocess Biosyst. Eng. 2014, 37, 433–440. [Google Scholar] [CrossRef]
- Behroozibakhsh, M.; Hajizamani, H.; Shekofteh, K.; Otadi, M.; Ghavami-Lahiji, M.; Nazari, N.S.F. Comparative assessment of the crystalline structures of powder and bulk human dental enamel by X-ray diffraction analysis. J. Oral Biosci. 2019, 61, 173–178. [Google Scholar] [CrossRef]
- Swain, S.K.; Sarkar, D. Study of BSA protein adsorption/release on hydroxyapatite nanoparticles. Appl. Surf. Sci. 2013, 286, 99–103. [Google Scholar] [CrossRef]
- Wassell, D.H.; Hall, R.C.; Embery, G. Adsorption of bovine serum albumin onto hydroxyapatite. Biomoterials 1995, 16, 697–702. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, T.; Wang, Y.; Wang, Z.; Zhang, P.; Liu, J. Environmental pH-controlled loading and release of protein on mesoporous hydroxyapatite nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2015, 46, 158–165. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, R.; Zhou, J.; Ye, Y.; Xu, Z.; Lin, C. A further insight into the adsorption mechanism of protein on hydroxyapatite by FTIR-ATR spectrometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 527–531. [Google Scholar] [CrossRef]
- Bharath, G.; Latha, B.S.; Alsharaeh, E.H.; Prakash, P.; Ponpandian, N. Enhanced hydroxyapatite nanorods formation on graphene oxide nanocomposite as a potential candidate for protein adsorption, pH controlled release and an effective drug delivery platform for cancer therapy. Anal. Methods 2017, 9, 240–252. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, Proximate Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]


| Treatment | X1 (min) | X2 (%) | Yield (%) | Ca ns (g/100 g) | P ns (g/100 g) | Ca/P Ratio ns | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 2.0 | 4.26 ± 0.20 e | 15.0 ± 4.87 | 7.38 ± 2.24 | 2.03 ± 0.03 | 79.8 ± 0.13 e | 4.16 ± 0.38 a | 19.3 ± 0.91 a | 31.1 ± 1.24 a |
| 2 | 10 | 5.0 | 10.6 ± 0.16 bc | 19.7 ± 1.58 | 9.43 ± 1.12 | 2.09 ± 0.06 | 90.3 ± 2.83 ab | 4.20 ± 1.63 a | 20.3 ± 2.18 a | 21.8 ± 1.89 c |
| 3 | 60 | 2.0 | 6.77 ± 0.43 d | 16.4 ± 2.44 | 8.08 ± 1.23 | 2.03 ± 0.00 | 75.2 ± 0.76 e | 3.62 ± 0.78 ab | 18.1 ± 1.33 ab | 29.0 ± 2.05 ab |
| 4 | 60 | 5.0 | 13.4 ± 1.30 a | 19.8 ± 3.90 | 9.55 ± 2.07 | 2.08 ± 0.03 | 91.3 ± 1.35 a | 2.84 ± 0.80 c | 16.5 ± 2.14 bc | 11.1 ± 2.39 f |
| 5 | 35 | 2.0 | 6.25 ± 0.11 d | 17.9 ± 0.03 | 8.65 ± 0.03 | 2.07 ± 0.00 | 76.9 ± 2.01 e | 3.11 ± 0.82 b | 18.6 ± 1.94 ab | 30.6 ± 0.67 e |
| 6 | 35 | 5.0 | 12.4 ± 1.03 a | 17.2 ± 2.58 | 8.28 ± 1.21 | 2.08 ± 0.00 | 89.7 ± 0.87 ab | 2.67 ± 0.29 c | 17.4 ± 1.42 b | 16.1 ± 0.43 b |
| 7 | 10 | 3.5 | 9.26 ± 0.15 c | 17.5 ± 1.16 | 8.30 ± 0.56 | 2.11 ± 0.00 | 87.1 ± 1.09 abc | 2.88 ± 0.25 c | 17.1 ± 1.57 b | 28.8 ± 1.57 d |
| 8 | 60 | 3.5 | 11.8 ± 0.71 ab | 20.1 ± 0.59 | 9.52 ± 0.39 | 2.11 ± 0.02 | 77.9 ± 2.55 de | 2.47 ± 0.23 c | 17.2 ± 0.73 b | 18.4 ± 1.31 c |
| 9 | 35 | 3.5 | 9.42 ± 0.12 c | 19.7 ± 0.80 | 9.27 ± 0.70 | 2.13 ± 0.05 | 85.6 ± 0.62 bc | 1.04 ± 0.35 d | 11.7 ± 2.55 d | 22.6 ± 0.28 c |
| 10 | 35 | 3.5 | 10.3 ± 0.15 bc | 16.2 ± 0.14 | 7.83 ± 0.30 | 2.06 ± 0.04 | 87.7 ± 0.62 abc | 1.05 ± 0.59 d | 10.8 ± 1.45 e | 22.9 ± 0.39 c |
| 11 | 35 | 3.5 | 10.4 ± 0.21 bc | 19.2 ± 2.24 | 9.23 ± 1.10 | 2.08 ± 0.00 | 84.4 ± 1.65 cd | 1.68 ± 0.44 d | 12.7 ± 1.49 d | 22.2 ± 2.00 c |
| Responses | Quadratic Polynomial Model | R2 | p-Value |
|---|---|---|---|
| Yield (%) | Y1 = −5.82 + 6.01X1 + 0.05X2 − 0.002X1X2 − 0.57X12 − 0.0001X22 | 0.9827 | 0.0002 |
| L* | Y2 = 81.3 + 1.59X1 + 0.05X2 − 0.012X1X2 − 0.03X12 + 0.00003X22 | 0.9000 | 0.0154 |
| a* | Y3 = 6.10 − 0.77X1 − 0.02X2 + 0.008X1X2 − 0.06X12 − 0.0002X22 | 0.9741 | 0.0006 |
| b* | Y4 = 17.5 + 2.44X1 + 0.004X2 + 0.01X1X2 − 0.78X12 − 0.001X22 | 0.9512 | 0.0027 |
| ΔE | Y5 = 45.2 − 14.8X1 − 0.43X2 − 0.02X1X2 + 2.165X12 + 0.01X22 | 0.8772 | 0.0250 |
| Ca content (g/100 g) | Y6 = 18.41 + 1.24X1 − 0.68X2 − 0.3X1X2 − 0.94X12 + 0.29X22 | 0.4770 | 0.5382 |
| P content (g/100 g) | Y7 = 8.77 − 0.53X1 + 0.34X2 − 0.15X1X2 − 0.31X12 + 0.14X22 | 0.4653 | 0.5587 |
| Ca/P ratio | Y8 = 2.10 + 0.02X1 − 0.002X2 − 0.003X1X2 − 0.035X12 + 0.0003X22 | 0.5782 | 0.3688 |
| Response & Factors | Parameter | Predicated Value | Experimental Value | Composite Desirability | ||||
|---|---|---|---|---|---|---|---|---|
| Goal | Lower | Upper | Weight | Importance | ||||
| X1 | Is in range | 10 | 60 | 1 | 3 | 60 | 60 | 0.908 |
| X2 | Is in range | 2 | 5 | 1 | 3 | 5 | 5 | |
| Yield (%) | maximize | 4.26 | 13.39 | 1 | 3 | 13.39 | 13.4 ± 1.30 | |
| L* | maximize | 84.96 | 88.65 | 1 | 3 | 88.0 | 84.5 ± 2.21 | |
| a* | Is in range | 1.03 | 4.20 | 1 | 3 | 1.47 | 2.84 ± 0.80 | |
| b* | Is in range | 10.83 | 20.32 | 1 | 3 | 12.25 | 16.5 ± 2.14 | |
| ΔE | Is in range | 8.25 | 20.39 | 1 | 3 | 16.31 | 15.6 ± 2.95 | |
| Ca content (g/100 g) | none | 15.03 | 20.07 | 1 | 3 | 19.31 | 19.8 ± 3.90 | |
| P content (g/100 g) | none | 7.38 | 9.55 | 1 | 3 | 9.29 | 9.55 ± 2.07 | |
| Ca/P ratio | none | 2.03 | 2.13 | 1 | 3 | 2.08 | 2.08 ± 0.03 | |
| Treatment | Yield ns (%) | L* | a* | b* | ∆E |
|---|---|---|---|---|---|
| 5% NaOH | 15.89 ± 1.22 | 85.84 ± 0.01 f | 2.81 ± 0.01 c | 15.10 ± 0.00 c | 16.76 ± 0.00 c |
| 10% NaOH | 15.93 ± 1.06 | 87.56 ± 0.01 d | 2.33 ± 0.01 e | 14.31 ± 0.02 e | 15.05 ± 0.01 e |
| 15% NaOH | 16.08 ± 0.30 | 86.59 ± 0.01 e | 2.56 ± 0.01 d | 14.40 ± 0.05 d | 15.78 ± 0.02 d |
| 20% NaOH | 16.09 ± 1.10 | 84.36 ± 0.03 h | 3.30 ± 0.00 a | 16.99 ± 0.05 b | 18.98 ± 0.04 a |
| 5% KOH | 15.11 ± 1.19 | 88.50 ± 0.02 b | 1.76 ± 0.00 h | 13.60 ± 0.03 g | 13.91 ± 0.03 g |
| 10% KOH | 16.08 ± 0.39 | 88.78 ± 0.03 a | 1.96 ± 0.01 g | 13.98 ± 0.03 f | 14.06 ± 0.02 f |
| 15% KOH | 16.87 ± 0.24 | 88.06 ± 0.01 c | 2.02 ± 0.01 f | 13.38 ± 0.02 h | 14.08 ± 0.02 f |
| 20% KOH | 17.05 ± 1.32 | 85.23 ± 0.01 g | 3.08 ± 0.01 b | 17.27 ± 0.02 a | 18.50 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jindapon, N.; Klinmalai, P.; Surayot, U.; Tanadchangsaeng, N.; Pichaiaukrit, W.; Phimolsiripol, Y.; Vichasilp, C.; Wangtueai, S. Preparation, Characterization, and Biological Properties of Hydroxyapatite from Bigeye Snapper (Priancanthus tayenus) Bone. Int. J. Mol. Sci. 2023, 24, 2776. https://doi.org/10.3390/ijms24032776
Jindapon N, Klinmalai P, Surayot U, Tanadchangsaeng N, Pichaiaukrit W, Phimolsiripol Y, Vichasilp C, Wangtueai S. Preparation, Characterization, and Biological Properties of Hydroxyapatite from Bigeye Snapper (Priancanthus tayenus) Bone. International Journal of Molecular Sciences. 2023; 24(3):2776. https://doi.org/10.3390/ijms24032776
Chicago/Turabian StyleJindapon, Nunnuth, Phatthranit Klinmalai, Utoomporn Surayot, Nuttapol Tanadchangsaeng, Woradej Pichaiaukrit, Yuthana Phimolsiripol, Chaluntorn Vichasilp, and Sutee Wangtueai. 2023. "Preparation, Characterization, and Biological Properties of Hydroxyapatite from Bigeye Snapper (Priancanthus tayenus) Bone" International Journal of Molecular Sciences 24, no. 3: 2776. https://doi.org/10.3390/ijms24032776
APA StyleJindapon, N., Klinmalai, P., Surayot, U., Tanadchangsaeng, N., Pichaiaukrit, W., Phimolsiripol, Y., Vichasilp, C., & Wangtueai, S. (2023). Preparation, Characterization, and Biological Properties of Hydroxyapatite from Bigeye Snapper (Priancanthus tayenus) Bone. International Journal of Molecular Sciences, 24(3), 2776. https://doi.org/10.3390/ijms24032776











