Abstract
LSUs (RESPONSE TO LOW SULFUR) are plant-specific proteins of unknown function that were initially identified during transcriptomic studies of the sulfur deficiency response in Arabidopsis. Recent functional studies have shown that LSUs are important hubs of protein interaction networks with potential roles in plant stress responses. In particular, LSU proteins have been reported to interact with members of the brassinosteroid, jasmonate signaling, and ethylene biosynthetic pathways, suggesting that LSUs may be involved in response to plant stress through modulation of phytohormones. Furthermore, in silico analysis of the promoter regions of LSU genes in Arabidopsis has revealed the presence of cis-regulatory elements that are potentially responsive to phytohormones such as ABA, auxin, and jasmonic acid, suggesting crosstalk between LSU proteins and phytohormones. In this review, we summarize current knowledge about the LSU gene family in plants and its potential role in phytohormone responses.
1. Introduction
Sulfur (S) is an essential macronutrient required for plant growth because it is a constituent of relevant biomolecules such as the amino acids methionine and cysteine, the antioxidant glutathione, coenzymes and prosthetic groups [1]. Therefore, plants cannot adequately complete their life cycle when subjected to an S-deficiency condition [2]. The symptoms mainly appear in the young parts of the plant and are characterized by reduced height, chlorosis of the leaves, and accumulation of anthocyanins [1,3]. Unlike other nutritional deficiencies, S deficiency typically results in reduced shoot growth compared to root growth [3].
At the molecular level, the response to S-deficiency can be divided into two main stages based on the duration and severity of the deficiency [3]. In the initial stage, plants alter the expression of primary genes involved in S-assimilation and uptake from the soil, and mobilize stored inorganic S from the vacuole [3,4]. However, if S remains a limiting factor, plants intensify organic S fluxes and activate stress defense responses, followed by the downregulation of genes responsible for nitrogen uptake and assimilation [5].
The advent of transcriptomics studies has allowed considerable progress in the identification of S-responsive genes, mainly in the model plant Arabidopsis thaliana [6]. An integrative metaanalysis of transcriptomic data from five different S experiments in public databases uncovered a robust set of genes whose expression depends only on the availability of S in Arabidopsis [7]. Interestingly, the biological function of approximately 45% of these robust S-responsive genes is currently unknown. A small gene family, “RESPONSE TO LOW SULFUR” (LSU), belongs to this group of consistently S-responsive genes, suggesting that these genes could be an essential component of this nutritional response [7].
Several studies have shown that LSUs are important hubs of protein interaction networks with potential function in the plant stress response [8,9,10]. Phytohormones play a critical role in helping plants adapt to adverse environmental conditions, including abiotic and biotic stresses [11]. Interestingly, it has been reported that LSU proteins interact with members of brassinosteroid signaling [12], jasmonate signaling [8], and the ethylene biosynthetic pathway [13], suggesting that LSUs could be involved in the response to plant stress by modulating phytohormones. Furthermore, in silico analysis of the promoter regions of LSU genes in Arabidopsis showed that they have cis regulatory elements which are potentially responsive to phytohormones such as ABA, auxin, and jasmonic acid [14]. This evidence suggests a possible crosstalk between LSU proteins and phytohormones.
The molecular functions of LSU proteins remain incompletely understood, but in recent years, several studies have shed light on their putative functions and evolution. In this review, we summarize the current knowledge about the LSU gene family in plants and their potential role in phytohormone responses.
2. General Features and Evolutionary History of the LSU Gene Family
2.1. The Discovery of the LSU Gene Family
LSU genes were first described in the context of the S-deficiency response in Arabidopsis thaliana by Maruyama–Nakashita [15]. This study identified LSU1 and LSU2 as two of 15 S-responsive genes that were significantly upregulated at multiple time points after plants were transferred to S-free medium [15]. Specifically, LSU1 was significantly induced 4, 8, 12, and 24 h after S-deficiency, whereas LSU2 was upregulated at 8, 12, and 24 h, indicating that the response of LSU genes to this nutritional deficiency is maintained during the first 24 hours in Arabidopsis roots [15]. In the same year, members of this gene family were also identified in tobacco plants in the context of S-deficiency by using the suppression subtractive hybridization approach [16], suggesting that the response of LSU genes to this nutritional deficiency may be conserved in plants.
More recently, S-deficiency has been reported to also induces the expression of the LSU3 and LSU4 genes in Arabidopsis roots and leaves [17]. However, the degree of induction was not the same among members of this family: the induction of LSU4 by S-deficiency is lower than LSU1/2/3 in both organs [17]. Furthermore, this study also showed that the mRNA levels of all tomato LSU genes and three wheat LSU genes increased by S-deficiency [17], supporting the idea that this gene family is associated with S-deficiency and the response to this nutritional deficiency could be conserved in angiosperm plants.
Analysis of the Arabidopsis genome revealed four members of the LSU family (LSU1–4), distributed in two chromosomes [14]: LSU1 and LSU3 are located on chromosome 3, and LSU2 and LSU4 are on chromosome 5. These two pairs of LSU genes are separated by a small distance of about 2 Kb [14]. In addition, LSU genes are characterized by their small size (approximately 300 bp of coding sequence) and the absence of introns [14].
2.2. Evolution of LSU Gene Family
The evolutionary history of the LSU gene family has recently been analyzed by using genomic information from 134 plant species that include representatives of the major phylogenetic groups of the Viridiplantae clade [17]. The first notable finding of this study was that the LSU family probably originated from the common ancestor of seed plants [17]. As shown in Figure 1, no homologous LSU sequences were found in the genomes of ancient vascular plants such as Selaginella moellendorffii, nonvascular plants, or microalgae. This result contrasts with the evolutionary history of other genes involved in the S-deficiency response, such as sulfate transporters, or genes encoding enzymes of S assimilation, such as ATP sulfurylase (APS) or APS reductase (APR), which are present in all Viridiplantae from microalgae to angiosperms (Figure 1) [17]. Furthermore, the family of the central transcriptional regulator of plant S-response, ETHYLENE-INSENSITIVE3-LIKE3 (EIL3), is present in all analyzed land plant genomes (Figure 1), indicating that the evolutionary appearance of LSU family is recent compared to other genes involved in the S-deficiency response [17]. In addition, several experimental-verified interactors of LSU genes in Arabidopsis, such as APS1, GAPC1, RAF2, FSD2, and RAP1, are also present in all analyzed Viridiplantae genomes [17].
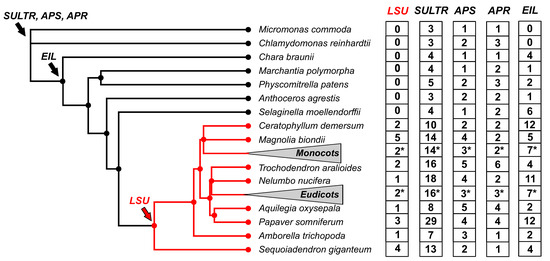
Figure 1.
The LSU gene family appeared recently in plant evolution compared to other S-responsive genes. The phylogenetic tree was constructed according to [17]. To improve visualization, 73 eudicotyledon and 31 monocotyledon species collapsed in the phylogenetic tree (triangle), and the average number of LSU genes are indicated with an asterisk. The copy number of LSUs, sulfate transporters (SULTR), ATP sulfurylases (APS), adenosine 5’-phosphosulfate reductase (APR), and ethylene-insensitive3-like transcription factors (EIL) was obtained from the PLAZA 5.0 database [18].
The number of LSU genes varies between angiosperm plants, ranging from 1 to 9 members [17]. This variation is mainly due to genome size, as a significant positive correlation has been found between the number of LSU genes and the size of the genome [17]. Furthermore, the analysis of the distribution of normalized LSU gene numbers in monocotyledon and eudicotyledons revealed no significant differences between these clades [14], suggesting that the LSU family does not expand during the evolution of angiosperm plants. Unlike the LSU copy number, the evolutionary distance between LSU genes of the same species in monocotyledons is more significant than in eudicotyledons, indicating a potential functional divergence of LSU genes within monocotyledon species such as wheat [17].
Phylogenetic analysis revealed that LSU genes could be divided into three main phylogenetic groups: Group A, which includes most of the monocotyledon species; Group B, including most of the malvid species; Group C, including most of the rosid species [17]. Protein sequence analysis based on 270 LSU sequences showed that the central region of LSU proteins has two highly conserved domains and also revealed the presence of three additional motifs that further support the classification by phylogenetic analyses [17]. The significance of conserved and group-specific motifs in LSU proteins is currently unknown, and further research should be undertaken to reveal the molecular function of these domains [17].
3. Functional Analyses of the LSU Family
3.1. Subcellular Localization of LSU Proteins
Biochemical fractionation has shown that LSU1 and LSU2 proteins localize in multiple cell compartments, including nuclear, cytosolic, and microsomal fractions [19], while LSU dimers are most probably located in the cytosol [12]. Data from the SUBA4 database [20] support a mainly nuclear and cytoplasmic localization for LSU1, and nuclear, cytoplasmic, chloroplastic, and mitochondrial localization for LSU2 and LSU3 (data for LSU4 is not available) (from Cell eFP viewer, ePlant, [21]). In tobacco, UP9C has a reported nuclear and cytosolic localization, and, generally, a putative nuclear localization signal has been found in this protein [14]. Although no nuclear localization signal has been found in Arabidopsis LSUs, their small size probably allows them to readily cross the nuclear pore [14].
3.2. Different Members of the LSU Gene Family Showed Tissue-Specific Expression
Analysis of LSU tissue expression has been limited to LSU1 and LSU2, showing that these proteins present specific tissue expressions consistent with a specialized role. For example, LSU1 is diffusely expressed in roots and strongly expressed in guard cells, indicating a role in stomata function, whereas LSU2 is ubiquitously expressed in leaves and roots [19]. Additionally, we performed a correlation analysis of LSU expression data across 69 samples of the Arabidopsis developmental atlas included in the eFP browser [21,22]. LSU1, LSU2, and LSU3 showed a high and significant positive correlation (p-value < 0.01, Figure 2A), indicating that they have similar expression patterns in the developmental atlas. In contrast, no significant correlation was found between LSU4 and LSU1/LSU2/LSU3, indicating that Arabidopsis LSU genes are grouped into two clusters according to developmental and tissue-specific expression (Figure 2A).

Figure 2.
Analysis of the LSU developmental expression atlas in Arabidopsis and wheat suggests the existence of two LSU subgroups with contrasting expression profiles. (A) Coexpression analyses of Arabidopsis (left panel) and wheat LSU family (right panel) performed with the r package “corrplot” [23] using all samples included in the developmental atlas of ePlant [21]. Only Pearson correlation values with a p-value < 0.01 are shown in the correlation heatmaps. (B) Expression profiles of Arabidopsis LSU4 and LSU2 in the three samples with the higher expression of each selected gene. (C) Expression profiles of wheat TraesCS1D03G0456700 and TraesCS6B03G0289700 in the three samples with the higher expression of each selected gene.
In Figure 2B, we compared the expression patterns of LSU2 (as a representative gene with a higher average expression of the LSU1/LSU2/LSU3 cluster) and LSU4 to illustrate the two different groups of Arabidopsis LSUs. In the case of LSU2, this gene is mainly expressed in leaf petiole, leaf vein, and pod of the senescent silique 1 (Figure 2B). In contrast, the maximum expression of LSU4 is detected in floral tissues (Figure 2C), supporting the contrasting correlation values between LSU4 and other LSUs obtained in Figure 2A. We then asked whether the existence of two contrasting groups of LSU expression also occurs in other plant species. To this end, we performed the same analysis with wheat LSU genes [17] as an example of a monocotyledon plant. We also found two contrasting groups of LSU genes in wheat according to their expression patterns throughout development (71 samples; Figure 2A,C), suggesting a possible functional divergence between members of this family in plants.
3.3. Functional Analyses of LSUs in Arabidopsis
Insights into the role of individual LSU proteins in Arabidopsis have been obtained by characterization of available T-DNA insertional lines, mainly for LSU2 and LSU4. Currently, no T-DNA lines for LSU1 are available, and although insertional lines for LSU3 exist, no reports have been published to date. The involvement of LSUs with biotic stress responses was first suggested in analyses of the Arabidopsis protein-protein interactome, showing that LSUs represented hubs in immune response-related networks [9]. Analysis of lsu2 mutants showed that this protein was necessary for normal immune plant response to the bacterium Pseudomonas syringae DC3000 (avrRpt2) and the fungi Hyaloperonospora arabidopsidis. LSU2 was identified as a target of pathogen effector proteins and was proposed to act as part of a growth-suppression mechanism mediated by the P. syringae 2 (RPS2) NB-LRR protein [9]. Later work using lsu2 mutants showed that stomatal closure in response to P. syringae DC3000 and the human pathogen Salmonella enterica subsp. enterica serovar Typhimurium strain 14028s was significantly reduced.
These results implicate LSU2 as part of an important plant defense mechanism that occurs in guard cells to prevent the entry of bacterial pathogens [24].
In addition to its role in the immune response to pathogens, LSU2 works as an integrator of light and chloroplast signaling. LSU2 is induced by light and lincomycin, a chloroplast biogenesis inhibitor [24]. lsu2 mutants have more than twofold chlorophyll contents compared to wild-type plants when deetiolation is performed in a wide range of light fluences [25]. As such, LSU2 (together with six other genes) was classified as an enhanced deetiolation (end) gene [25]. Consistent with the putative role of LSU2 in integrating light and plastid signaling, lsu2 mutants have a decreased expression of the photosynthesis-related genes Lhcb1.4, RbcS1A, PsbS, and CHS [25]. LSU2 has also been shown to act as part of a common response module of genes involved in plastid performance and retrograde signaling [26]. These functions of LSU2 are consistent with its subcellular localization in the chloroplast.
Regarding the LSU4 function, lsu4 mutants show a late flowering phenotype under short-day conditions, whereas flowers formed in the first flowering phase present aberrant developmental phenotypes and do not produce siliques [27]. This is accompanied by a decrease in the expression of critical flowering genes such as LFY, AP1, AP3, PI, and SEP3 transcripts and an increase in the expression of AP2, AG, and SEP2 [27]. Consistent with these phenotypes in the lsu4 mutant, LSU4 shows an induced expression during flowering and fruit formation [27]. The induction of LSU4 is also evident during deficiencies in different nutrients (phosphorous, nitrogen, potassium, iron), indicating a possible role of LSU4 as a coordinator of nutrient demand and flowering [27].
Given that no individual T-DNA lines exist for all LSU genes and to uncover phenotypes that can be masked by potential functional redundancy, Arabidopsis knockdown lines have been generated by using artificial microRNAs (amiRNAs) targeting all LSU members (>80% reduction in LSU1, LSU2 and LSU3 and 50% for LSU4) [19]. These lines present no obvious phenotypes when grown in standard soil or in vitro conditions [19]. However, closing of abaxial stomata in response to S-deficiency was impaired in the knockdown lines, leading to increased water loss and indicating a role for LSUs in this response [19]. This phenotype is consistent with the expression of LSU1 in guard cells [19] and the reported role of LSU2 in stomata closure [24].
Furthermore, H2O2 production in guard cell chloroplasts of knockdown lines was reduced compared to wild-type plants in response to S deficiency and other stresses such as high salt and Cu [19]. Consistent with this observation, the iron-dependent superoxide dismutase 2 (FSD2) was shown to physically interact with LSU1 and LSU2 in vitro and in vivo, and this interaction was shown to increase the enzymatic activity of FSD2; thus, the production of H2O2 from O2− [19]. Interestingly, the LSU1-FSD2 interaction is targeted and interfered by different virulence effectors, revealing a mechanism used by bacteria to abrogate pathogen-associated molecular pattern-triggered immunity [19]. As expected, the amiRNA lines are more susceptible to pathogen attack, and conversely, LSU1 overexpressor lines present an enhanced disease resistance phenotype under standard conditions, as well as under conditions of abiotic stress [19]. Interestingly, LSUs have also been linked to the function of beneficial bacteria such as Enterobacter sp. SA187, an endophytic bacterium that protects plants from abiotic stresses. Plant colonization with SA187 can completely suppress the increased ROS levels and alleviate growth suppression in LSU knockdown plants subjected to high salt stress [28].
3.4. Functional Analyses of LSUs in Other Plants
Similar to Arabidopsis, UP9 proteins, the LSU homologs in tobacco, play an important role in S-deficiency responses [14]. Knockdown of UP9 proteins by using a UP9C antisense line alters glutathione levels in roots and mature tobacco leaves, especially under S-deficiency conditions [29]. The effect of UP9 downregulation is organ-dependent, with mature leaves of UP9 transgenic plants having higher levels of total S and glutathione (GSH) than wild-type plants in S-deficiency, similar to plants grown in S sufficiency [29]. In the case of roots, total S and glutathione are more affected, presenting significantly decreased levels in the UP9 transgenics in both S conditions [29]. This resulted in knockdown plants presenting shorter roots, whereas shoot growth was unaffected. At the transcript expression level, UP9 knockdown resulted in altered levels of S-related enzymes and transporters, as well as genes related to ethylene, jasmonic acid, and polyamines [29]. UP9 knockdown plants also present altered metabolite profiles under S-deficiency, suggesting UP9s are key to adaptation to S-deficiency conditions [29]. By using these knockdown lines, UP9 was also shown to be required for the increased ethylene production that occurs during S-deficiency [13]. This is partly due to its interaction with the ACC oxidase protein [13]. UP9 downregulation affected the S-deficiency response of several genes, mainly involved in S metabolic processes, but also transcription regulation, defense response, and hormonal pathways such as ethylene, ABA, and CK [13].
The response of LSU genes to S deficiency has been demonstrated in several crops: tomato [17,30], rice [31], and wheat [17] (Table 1). In the case of wheat and tomato plants, the response of LSUs was verified by qPCR after two weeks of S deficiency in roots and leaves [17]. Furthermore, it has recently been reported that the three LSU genes of rice were upregulated in response to S deficiency in roots and shoots [31]. Interestingly, one of the LSU genes, Os10g0509600, was strongly induced by S deficiency but significantly downregulated in both the split-root half with S resupply and the split-root half that remained under S deficiency, suggesting a local and systemic response of this LSU gene to sulfate resupply [31]. In addition to these species, it has been proposed through mRNA-protein network analysis that LSU genes are also involved in the response to S deficiency in pea seeds [32] (Table 1).

Table 1.
List of crop species with experimental evidence for LSU genes.
LSU genes have also been described in the context of response to fungus infection [33] (Table 1). Recent research uncovered the dynamics of the transcriptome in sugarcane infected with S. scitamineum and identified an LSU homolog gene (S.off_newGene_71819) as a highly connected gene from a coexpression module linked to the metabolism associated with the defense response, suggesting an important role for this LSU in the plant–pathogen interaction [33]. Interestingly, the expression levels of this LSU homolog gene were higher in a sugarcane genotype resistant to S. scitamineum infection compared to a susceptible genotype. Together, these results suggest that LSU genes might play an important role in the defense response of sugarcane to fungus.
Glucosinolates are sulfur- and nitrogen-containing secondary metabolites of Brassicaceae plants that play an important role in plant defense by acting as a deterrent to herbivores and pathogens [35]. Interestingly, a recent genome-wide association study in Brassica juncea discovered that BjuA033112, a homolog gene of the Arabidopsis LSU2, is significantly associated with the gluconapin content, one of the main glucosinolates [34]. These findings suggest that LSU genes may be involved in the biosynthesis of glucosinolates in Brassica juncea.
4. LSU Protein Interactions and Phytohormone Signaling
4.1. LSU Protein Interactions Suggest Some Degree of Specialization within this Family in Arabidopsis
Computer modeling of different LSU proteins identified coiled-coil motifs in their structure [14]. Additional circular dichroism studies using a recombinant UP9C protein suggest that this protein is mostly alpha-helical, which further supports a coiled-coil structure [29]. Three-dimensional structure prediction by using AlphaFold is also consistent with an alpha-helical structure for Arabidopsis LSUs (https://alphafold.ebi.ac.uk/ (accessed on 21 November 2022); Q9SCK1, Q9FIR9, Q9SCK2, and Q8L8S2 for the LSU1-4, respectively). Although the 3D structure of LSUs has not been experimentally determined, the presence of a coiled-coil motif, which facilitates oligomerization, indicates that these small proteins can form multimers and interact with multiple kinds of proteins. Consistently, BiFC analyses have shown in planta formation for Arabidopsis LSU2, LSU3, and LSU4 homodimers [12]. However, Y2H analyses have confirmed interactions only for LSU1-LSU1 and LSU2-LSU2 homodimers, and for LSU1-LSU2, LSU1-LSU3, and LSU1-LSU4 heterodimers [12]. This suggests that the efficiency of interaction may vary between different LSU pairs or that LSUs associate forming multimeric complexes.
Additional structural modeling and spatial distribution of the electrostatic potential of LSU-LSU dimers reveal significant differences in homo- and heterodimer formation, suggesting that the dimer formation by LSU might have a regulatory function [12]. These analyses also suggested that dimers might bind to different molecular partners than monomeric forms [12]. Additionally, combined mutagenesis and Y2H analyses have identified that the conserved cysteine residues (C54) are not involved in the dimer stabilization and do not form S–S bridges between the monomers of LSUs. Because these cysteine residues are located on the surface of the protein and exposed to solvent, it has been proposed that they play a role in the interaction with the coiled-coil structure while they could also be involved in the recognition of protein interactors [12]. Consistently, LSU proteins were identified as protein hubs in high-throughput Y2H analyses that interrogated the Arabidopsis interactome [9]. Interestingly, despite their high similarity the LSU interactomes partially overlap [9,10], suggesting some degree of specialization. For example, from 100 protein interactors identified for LSU1 or LSU2, only 17 are shared by both LSU proteins [14].
LSU 1, 2, and 3 are able to interact with multiple partners, including pathogen effectors from Pseudomonas syringae and Hyaloperonospora arabidopsidis and proteins involved in different biological processes, including some related to plant immune processes [9]. From these interactors, the chloroplastic iron-dependent superoxide dismutase FSD2 has been independently validated as a partner for LSU1 and LSU2, and this interaction was shown to be relevant for stimulation of FSD2 activity and production of H2O2 [19]. Another study described MYB51, a transcription factor involved in glucosinolate biosynthesis as partner for LSU3 [36]. Further interactors for LSU1-4 were identified in vivo by using tandem affinity purification-mass spectrometry (TAP-MS) under sulfate sufficiency and deficiency conditions, and further confirmed by BiFC and Y2H [12]. Among confirmed proteins, ATP sulfurylase 1 (APS1), first enzyme in sulfate assimilation, the GRF8 transcription factor involved in plant growth, chlorophyll biosynthesis and seedling greening [37], the RAF2/SDIRIP1 protein involved in Rubisco assembly and ABA stress responses [38,39] and the GAPC1 C subunit of cytosolic GADPH enzyme (described as a redox switch with roles in plant responses to stress [40,41]) were validated by BiFC, whereas Y2H assays confirmed the interaction of LSUs and RAF2/SDIRIP1, the peroxisomal catalase 2 (CAT2) and the autophagy cargo receptor NBR1, involved in the crosstalk between autophagy and ABA signaling [42]. Interestingly, Y2H analysis in Nicotiana plumbaginifolia had identified a homolog of Arabidopsis NPR1, Joka2, as an interacting partner for UP9C [43], indicating a conserved function of LSUs and selective protein degradation by autophagy. Importantly, the strength of the interaction and protein partner varied among LSUs, and further Y2H analysis using LSUs with mutations in the conserved C54 cysteine residue further supported the idea that the shape of the dimer coiled coil is relevant for recognition of LSU targets [12].
These results are consistent with reported roles of LSUs in different aspects of abiotic and biotic stress responses in plants and with a specialization of LSU function in plants.
4.2. Crosstalk between LSUs and Phytohormones
Mapping of the Arabidopsis LSU interaction network [12] has revealed that the interacting partners of LSUs include diverse proteins, some of which have roles in hormonal signaling pathways. Y2H data support interactions of LSUs with JAZ1 and JAZ9, members of the JAZ (jasmonate ZIM-domain) family of repressors, the cytokinin B-type response regulator ARR14, and the ERF12 ethylene transcription factor [9,12]. In addition to these main signaling components of hormonal pathways, other proteins linked to hormonal responses have been described as LSU interactors, such as the GRF8 transcription factor, which is induced by brassinosteroids through BZR1 [44], the ZFP7 zinc finger, whose overexpression confers ABA insensitivity to seed germination [45], the H3K27 tri-methyltransferase SWN involved in the control of ABA-induced senescence-associated genes [46] or the EDS1 lipase, which promotes SA accumulation [47].
Furthermore, an interaction of UP9C and 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase (ACO2A), the enzyme that catalyzes ethylene synthesis from ACC, has been reported in tobacco [13]. Short-period sulfur deficiency triggers an accumulation of ethylene, which is absent in antisense UP9C plants, indicating the interaction between UP9C and ACO2A is relevant for its function [13].
4.3. Differential Expression of Arabidopsis LSU Genes in Response to Phytohormone Treatment
Besides LSU interaction with proteins involved in hormonal pathways, evidence suggest that hormones can have a direct impact on LSU expression. The central regulator of the ethylene signaling pathway, EIN3, can bind the LSU1 promoter in vivo and regulate its expression [48]. Specifically, a ChIP-qPCR assay showed that EIN3 protein bound strongly to fragments of LSU1 promoter, and this result was confirmed by EMSA and yeast one-hybrid analyses [48]. Furthermore, a transient dual-luciferase assay in Arabidopsis protoplast indicated that EIN3 transcriptionally represses LSU1, which agrees with higher LSU1 mRNA levels in ein3-1 mutants [48]. These results demonstrate that phytohormone signaling pathways can regulate LSUs in Arabidopsis.
To get new insights into the response of LSU genes to phytohormones, we reviewed the transcriptomic data of Arabidopsis LSUs in Plant Regulomics database [49], which integrates 11,090 Arabidopsis transcriptomic datasets, including phytohormone treatments. As shown in Figure 3A, LSU genes significantly respond to at least one of the following phytohormones: ABA, ethylene, auxin, and jasmonate. LSU1 is the member of this gene family with the highest number of experiments as a differentially expressed gene (adjusted p-value < 0.05) (eight experiments, Figure 3A). Specifically, LSU1 is down-regulated by ethylene in three experiments, which is consistent with the previously reported repression of this gene by EIN3 [48]. In addition, LSU1 is also downregulated by auxin (except in the experiment GSE1491) and jasmonate (Figure 3A). In contrast, LSU2 and LSU4 showed a positive response to auxin and the LSU3 gene to jasmonate, suggesting that the response to phytohormones differs among members of the LSU family in Arabidopsis (Figure 3A).
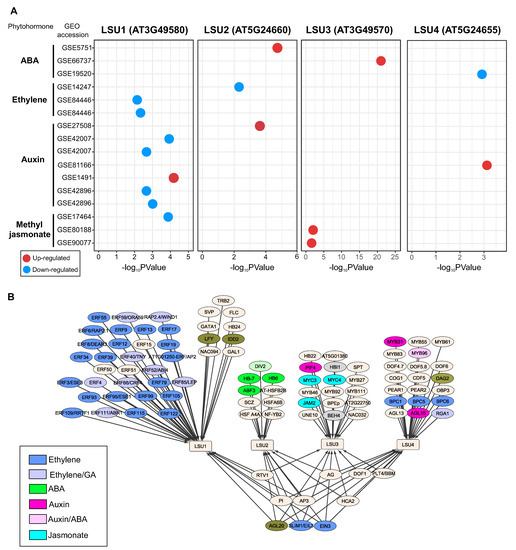
Figure 3.
The Arabidopsis LSU genes significantly respond to different phytohormone treatments (A), and the analysis of gene regulatory network (B) predicted that contrasting groups of phytohormone-related transcription factors regulate each LSU gene. Identification of the significant response of LSU genes to phytohormone treatments was performed by using transcriptome datasets available in the Plant Regulomics database [49]. The regulatory interactions between LSU genes and transcription factors were obtained PlantRegMap [50].
These differences between members of the LSU family in response to phytohormones are also reflected in the regulatory network predicted for these genes (Figure 3B). The predicted TF-target interaction obtained from PlantRegMap [50] suggests that LSU1 is mainly regulated by TFs from the ERF family, which are important regulatory components of ethylene signaling and are involved in plant development and stress responses by regulating the expression of ethylene-responsive genes [51]. On the contrary, LSU2 is predicted to be regulated by ABA-associated TFs, such as ABF3, and LSU3 by jasmonate-related TFs such as JAM2 (Figure 3B).
5. Conclusions
Although some information has been gathered about the LSUs, there are still many open questions about their functions. In this review, we have provided evidence that this group of proteins appears to display more multifaceted roles than previously expected.
Nevertheless, only a few plant LSU proteins have been functionally characterized. Members of the LSU family are likely to participate in fine-tuning responses to the different plant stresses, especially S limitation, and in various aspects of plant development, such as flowering and fruit formation. By modulating a variety of LSU in their protein–protein interactions, LSU might act in the crosstalk of various signaling pathways directly or indirectly linked to S metabolism.
It would be interesting to analyze the molecular mechanisms by which LSUs orchestrate metabolic homeostasis, plant stress responses, and plant growth and development. In this regard, we expect significant advances in connecting the structure and functions in this family of plant proteins in the following years.
The results accumulated in recent years in crops suggest that the LSU gene family could have significant implications for crop improvement in the future, particularly in regard to the S deficiency response and pathogen attack. The study of the LSUs in crops has the potential to lead to new strategies for crop improvement and sustainable agriculture.
Author Contributions
Conceptualization, J.C.; writing—original draft preparation, J.C., A.A.-M., J.M. and E.A.V.; writing—review and editing, J.C., A.A.-M., J.M. and E.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
J.C., A.A.-M. and E.A.V. were supported by the National Agency for Research and Development (ANID) Chile with Program FONDECYT Regular 1190812, FONDECYT Iniciación 11220937 and FONDECYT Regular 1211130. J.C., A.A.-M. and E.A.V. were also supported by ANID—Millennium Science Initiative Program—ICN17-022. J.M. was supported by the Ministerio de Ciencia e Innovación (MCIN) and Agencia Estatal de Investigación (AEI)/10.13039/501100011033/(PID2020-114165RR-381 C21). We also want to acknowledge the Severo Ochoa Program for Centres of Excellence in R&D (CEX2020-000999-S) supported by MCIN/AEI/10.13039/50110001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pramanik, K.; Panda, D.; Dutta, D.; Karmakar, S.; Bose, B. Sulfur in Seeds: An Overview. Plants 2022, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, F.; Naake, T.; Fernie, A.R.; Hoefgen, R. Coordinating Sulfur Pools under Sulfate Deprivation. Trends Plant Sci. 2020, 25, 1227–1239. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A. Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant Biol. 2017, 39, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Juhasz, A.; Islam, S.; Diepeveen, D.; Zhang, J.; Wang, P.; Ma, W. Impact of mid-season sulphur deficiency on wheat nitrogen metabolism and biosynthesis of grain protein. Sci. Rep. 2018, 8, 2499. [Google Scholar] [CrossRef]
- Watanabe, M.; Hoefgen, R. Sulphur systems biology—Making sense of omics data. J. Exp. Bot. 2019, 70, 4155–4170. [Google Scholar] [CrossRef]
- Henríquez-Valencia, C.; Arenas-M, A.; Medina, J.; Canales, J. Integrative Transcriptomic Analysis Uncovers Novel Gene Modules That Underlie the Sulfate Response in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 470. [Google Scholar] [CrossRef]
- Arabidopsis Interactome Mapping Consortium; Dreze, M.; Carvunis, A.R.; Charloteaux, B.; Galli, M.; Pevzner, S.J.; Tasan, M.; Ahn, Y.Y.; Balumuri, P.; Barabási, A.L.; et al. Evidence for network evolution in an Arabidopsis interactome map. Science 2011, 333, 601–607. [Google Scholar] [CrossRef]
- Mukhtar, M.S.; Carvunis, A.R.; Dreze, M.; Epple, P.; Steinbrenner, J.; Moore, J.; Tasan, M.; Galli, M.; Hao, T.; Nishimura, M.T.; et al. Independently Evolved Virulence Effectors Converge onto Hubs in a Plant Immune System Network. Science 2011, 333, 596–601. [Google Scholar] [CrossRef]
- Vandereyken, K.; Leene, J.V.; Coninck, B.D.; Cammue, B.P.A. Hub Protein Controversy: Taking a Closer Look at Plant Stress Response Hubs. Front. Plant Sci. 2018, 9, 694. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Niemiro, A.; Cysewski, D.; Brzywczy, J.; Wawrzyńska, A.; Sieńko, M.; Poznański, J.; Sirko, A. Similar but Not Identical—Binding Properties of LSU (Response to Low Sulfur) Proteins From Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, G.; Skoneczny, M.; Zientara-Rytter, K.; Wawrzyńska, A.; Głów, D.; Cristescu, S.M.; Harren, F.J.M.; Sirko, A. Tobacco LSU-like protein couples sulphur-deficiency response with ethylene signalling pathway. J. Exp. Bot. 2013, 64, 5173–5182. [Google Scholar] [CrossRef] [PubMed]
- Sirko, A.; Wawrzynska, A.; Rodriguez, M.C.; Sęktas, P. The family of LSU-like proteins. Front. Plant Sci. 2015, 5, 774. [Google Scholar] [CrossRef] [PubMed]
- Maruyama-Nakashita, A.; Nakamura, Y.; Watanabe-Takahashi, A.; Inoue, E.; Yamaya, T.; Takahashi, H. Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant J. 2005, 42, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyńska, A.; Lewandowska, M.; Hawkesford, M.J.; Sirko, A. Using a suppression subtractive library-based approach to identify tobacco genes regulated in response to short-term sulphur deficit. J. Exp. Bot. 2005, 56, 1575–1590. [Google Scholar] [CrossRef]
- Uribe, F.; Henríquez-Valencia, C.; Arenas-M, A.; Medina, J.; Vidal, E.A.; Canales, J. Evolutionary and Gene Expression Analyses Reveal New Insights into the Role of LSU Gene-Family in Plant Responses to Sulfate-Deficiency. Plants 2022, 11, 1526. [Google Scholar] [CrossRef]
- Bel, M.V.; Silvestri, F.; Weitz, E.M.; Kreft, L.; Botzki, A.; Coppens, F.; Vandepoele, K. PLAZA 5.0: Extending the scope and power of comparative and functional genomics in plants. Nucleic Acids Res. 2021, 50, D1468–D1474. [Google Scholar] [CrossRef]
- Garcia-Molina, A.; Altmann, M.; Alkofer, A.; Epple, P.M.; Dangl, J.L.; Falter-Braun, P. LSU network hubs integrate abiotic and biotic stress responses via interaction with the superoxide dismutase FSD2. J. Exp. Bot. 2017, 68, 1185–1197. [Google Scholar] [CrossRef]
- Hooper, C.M.; Castleden, I.R.; Tanz, S.K.; Aryamanesh, N.; Millar, A.H. SUBA4: The interactive data analysis centre for Arabidopsis subcellular protein locations. Nucleic Acids Res. 2016, 45, D1064–D1074. [Google Scholar] [CrossRef]
- Waese, J.; Fan, J.; Pasha, A.; Yu, H.; Fucile, G.; Shi, R.; Cumming, M.; Kelley, L.A.; Sternberg, M.J.; Krishnakumar, V.; et al. ePlant: Visualizing and Exploring Multiple Levels of Data for Hypothesis Generation in Plant Biology. Plant Cell 2017, 29, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Klepikova, A.V.; Kasianov, A.S.; Gerasimov, E.S.; Logacheva, M.D.; Penin, A.A. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. 2016, 88, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix. (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 15 December 2022).
- Oblessuc, P.R.; Bisneta, M.V.; Melotto, M. Common and unique Arabidopsis proteins involved in stomatal susceptibility to Salmonella enterica Pseudomonas syringae. FEMS Microbiol. Lett. 2019, 366, fnz197. [Google Scholar] [CrossRef]
- Ruckle, M.E.; Burgoon, L.D.; Lawrence, L.A.; Sinkler, C.A.; Larkin, R.M. Plastids Are Major Regulators of Light Signaling in Arabidopsis. Plant Physiol. 2012, 159, 366–390. [Google Scholar] [CrossRef] [PubMed]
- Gläßer, C.; Haberer, G.; Finkemeier, I.; Pfannschmidt, T.; Kleine, T.; Leister, D.; Dietz, K.J.; Häusler, R.E.; Grimm, B.; Mayer, K.F.X. Meta-Analysis of Retrograde Signaling in Arabidopsis thaliana Reveals a Core Module of Genes Embedded in Complex Cellular Signaling Networks. Mol. Plant 2014, 7, 1167–1190. [Google Scholar] [CrossRef] [PubMed]
- Myakushina, Y.A.; Milyaeva, E.L.; Romanov, G.A.; Nikiforova, V.Y. Mutation in LSU4 gene affects flower development in Arabidopsis thaliana. Dokl. Biochem. Biophys. 2009, 428, 257–260. [Google Scholar] [CrossRef]
- Andrés-Barrao, C.; Alzubaidy, H.; Jalal, R.; Mariappan, K.G.; de Zélicourt, A.; Bokhari, A.; Artyukh, O.; Alwutayd, K.; Rawat, A.; Shekhawat, K.; et al. Coordinated bacterial and plant sulfur metabolism in Enterobacter sp. SA187–induced plant salt stress tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107417118. [Google Scholar] [CrossRef]
- Lewandowska, M.; Wawrzyńska, A.; Moniuszko, G.; Łukomska, J.; Zientara, K.; Piecho, M.; Hodurek, P.; Zhukov, I.; Liszewska, F.; Nikiforova, V.; et al. A Contribution to Identification of Novel Regulators of Plant Response to Sulfur Deficiency: Characteristics of a Tobacco Gene UP9C Its Protein Product and the Effects of UP9C Silencing. Mol. Plant 2010, 3, 347–360. [Google Scholar] [CrossRef]
- Canales, J.; Uribe, F.; Henríquez-Valencia, C.; Lovazzano, C.; Medina, J.; Vidal, E.A. Transcriptomic analysis at organ and time scale reveals gene regulatory networks controlling the sulfate starvation response of Solanum lycopersicum. BMC Plant Biol. 2020, 20, 1–17. [Google Scholar] [CrossRef]
- Wang, R.Y.; Liu, L.H.; Zhao, F.J.; Huang, X.Y. Local and Systemic Response to Heterogeneous Sulfate Resupply after Sulfur Deficiency in Rice. Int. J. Mol. Sci. 2022, 23, 6203. [Google Scholar] [CrossRef]
- Henriet, C.; Balliau, T.; Aimé, D.; Signor, C.L.; Kreplak, J.; Zivy, M.; Gallardo, K.; Vernoud, V. Proteomics of developing pea seeds reveals a complex antioxidant network underlying the response to sulfur deficiency and water stress. J. Exp. Bot. 2021, 72, 2611–2626. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Pan, Y.B.; Su, Y.; Zou, W.; Xu, F.; Sun, T.; Grisham, M.P.; Yang, S.; Xu, L.; Que, Y. WGCNA Identifies a Comprehensive and Dynamic Gene Co-Expression Network That Associates with Smut Resistance in Sugarcane. Int. J. Mol. Sci. 2022, 23, 10770. [Google Scholar] [CrossRef]
- Tandayu, E.; Borpatragohain, P.; Mauleon, R.; Kretzschmar, T. Genome-Wide Association Reveals Trait Loci for Seed Glucosinolate Accumulation in Indian Mustard (Brassica juncea L.). Plants 2022, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Martínez-Ballesta, M.; Moreno, D.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Berger, B.; Gigolashvili, T. bHLH05 Is an Interaction Partner of MYB51 and a Novel Regulator of Glucosinolate Biosynthesis in Arabidopsis. Plant Physiol. 2014, 166, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, Y.; Shi, W.; Yu, P.; Hu, Y.; Lv, J.; Fu, C.; Fan, M.; Bai, M.Y. The miR396-GRFs Module Mediates the Prevention of Photo-oxidative Damage by Brassinosteroids during Seedling De-Etiolation in Arabidopsis. Plant Cell 2020, 32, 2525–2542. [Google Scholar] [CrossRef] [PubMed]
- Fristedt, R.; Hu, C.; Wheatley, N.; Roy, L.; Wachter, R.; Savage, L.; Harbinson, J.; Kramer, D.; Merchant, S.; Yeates, T.; et al. RAF2 is a RuBisCO assembly factor in Arabidopsis thaliana. Plant J. 2018, 94, 146–156. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, F.; Wu, Y.; Lou, L.; Liu, L.; Tian, M.; Ning, Y.; Shu, K.; Tang, S.; Xie, Q. The RING finger ubiquitin E3 ligase SDIR1 targets SDIR1-INTERACTING PROTEIN1 for degradation to modulate the salt stress response and ABA signaling in Arabidopsis. Plant Cell 2015, 27, 214–227. [Google Scholar] [CrossRef]
- Kim, S.; Guo, L.; Wang, X. Nuclear moonlighting of cytosolic glyceraldehyde-3-phosphate dehydrogenase regulates Arabidopsis response to heat stress. Nat. Commun. 2020, 11, 3439. [Google Scholar] [CrossRef]
- Schneider, M.; Knuesting, J.; Birkholz, O.; Heinisch, J.; Scheibe, R. Cytosolic GAPDH as a redox-dependent regulator of energy metabolism. BMC Plant Biol. 2018, 18, 184. [Google Scholar] [CrossRef]
- Tarnowski, L.; Rodriguez, M.; Brzywczy, J.; Piecho-Kabacik, M.; Krčkova, Z.; Martinec, J.; Wawrzynska, A.; Sirko, A. A selective autophagy cargo receptor NBR1 modulates abscisic acid signalling in Arabidopsis thaliana. Sci. Rep. 2020, 10, 7778. [Google Scholar] [CrossRef] [PubMed]
- Zientara-Rytter, K.; Łukomska, J.; Moniuszko, G.; Gwozdecki, R.; Surowiecki, P.; Lewandowska, M.; Liszewska, F.; Wawrzyńska, A.; Sirko, A. Identification and functional analysis of Joka2 a tobacco member of the family of selective autophagy cargo receptors. Autophagy 2011, 7, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shang, J.X.; Chen, Q.X.; Oses-Prieto, J.A.; Bai, M.Y.; Yang, Y.; Yuan, M.; Zhang, Y.L.; Mu, C.C.; Deng, Z.; et al. Identification of BZR1-interacting Proteins as Potential Components of the Brassinosteroid Signaling Pathway in Arabidopsis through Tandem Affinity Purification. Mol. Cell. Proteom. 2013, 12, 3653–3665. [Google Scholar] [CrossRef]
- Joseph, M.; Papdi, C.; Kozma-Bognár, L.; Nagy, I.; López-Carbonell, M.; Rigó, G.; Koncz, C.; Szabados, L. The Arabidopsis ZINC FINGER PROTEIN3 Interferes with Abscisic Acid and Light Signaling in Seed Germination and Plant Development. Plant Physiol. 2014, 165, 1203–1220. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, J.; Zhuang, Y.; Ye, L.; Li, Z.; Wang, Y.; Qi, M.; Xu, L.; Zhang, Y. Polycomb repressive complex 2 attenuates ABA-induced senescence in Arabidopsis. Plant J. 2019, 97, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J. A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef]
- Kong, X.; Li, C.; Zhang, F.; Yu, Q.; Gao, S.; Zhang, M.; Tian, H.; Zhang, J.; Yuan, X.; Ding, Z. Ethylene promotes cadmium-induced root growth inhibition through EIN3 controlled XTH33 LSU1 expression in Arabidopsis. Plant Cell Environ. 2018, 41, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Zhao, F.; Wang, Y.; Liu, J.; Zhuang, Y.; Ye, L.; Qi, M.; Cheng, J.; Zhang, Y. Plant Regulomics: A data-driven interface for retrieving upstream regulators from plant multi-omics data. Plant J. 2019, 101, 237–248. [Google Scholar] [CrossRef]
- Tian, F.; Yang, D.C.; Meng, Y.Q.; Jin, J.; Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2019, 48, D1104–D1113. [Google Scholar] [CrossRef]
- Yang, R.; Liu, J.; Lin, Z.; Sun, W.; Wu, Z.; Hu, H.; Zhang, Y. ERF transcription factors involved in salt response in tomato. Plant Growth Regul. 2018, 84, 573–582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).