Abstract
Polymers based on renewable monomers are projected to have a significant role in the sustainable economy, even in the near future. Undoubtedly, the cationically polymerizable β-pinene, available in considerable quantities, is one of the most promising bio-based monomers for such purposes. In the course of our systematic investigations related to the catalytic activity of TiCl4 on the cationic polymerization of this natural olefin, it was found that the 2-chloro-2,4,4-trimethylpentane (TMPCl)/TiCl4/N,N,N′,N′-tetramethylethylenediamine (TMEDA) initiating system induced efficient polymerization in dichloromethane (DCM)/hexane (Hx) mixture at both −78 °C and room temperature. At −78 °C, 100% monomer conversion was observed within 40 min, resulting in poly(β-pinene) with relatively high Mn (5500 g/mol). The molecular weight distributions (MWD) were uniformly shifted towards higher molecular weights (MW) in these polymerizations as long as monomer was present in the reaction mixture. However, chain–chain coupling took place after reaching 100% conversion, i.e., under monomer-starved conditions, resulting in considerable molecular weight increase and MWD broadening at −78 °C. At room temperature, the polymerization rate was lower, but chain coupling did not occur. The addition of a second feed of monomer in the polymerization system led to increasing conversion and polymers with higher MWs at both temperatures. 1H NMR spectra of the formed polymers indicated high in-chain double-bond contents. To overcome the polarity decrease by raising the temperature, polymerizations were also carried out in pure DCM at room temperature and at −20 °C. In both cases, rapid polymerization occurred with nearly quantitative yields, leading to poly(β-pinene)s with Mns in the range of 2000 g/mol. Strikingly, polymerization by TiCl4 alone, i.e., without any additive, also occurred with near complete conversion at room temperature within a few minutes, attributed to initiation by adventitious protic impurities. These results convincingly prove that highly efficient carbocationic polymerization of the renewable β-pinene can be accomplished with TiCl4 as catalyst under both cryogenic conditions, applied widely for carbocationic polymerizations, and the environmentally benign, energy-saving room temperature, i.e., without any additive and cooling or heating. These findings enable TiCl4-catalyzed eco-friendly manufacturing of poly(β-pinene)s, which can be utilized in various applications, and in addition, subsequent derivatizations could result in a range of high-added-value products.
1. Introduction
As a direct consequence of population growth, the demand for petrochemicals, used mainly to produce polymers, is projected to increase significantly in the decades ahead of us [1]. Currently, these materials are largely produced from oil and gas, and only less than 5% of the chemical feedstock is made from biomass [2]. However, due mainly to considerations on the finite availability of fossil fuels and to environmental concerns as well, bio-based sources for monomers and polymers made therefrom have gained increasing interest in both academia and industry worldwide in recent years (see e.g., Refs. [3,4,5,6,7,8,9,10,11,12,13,14] and references therein). Among the natural sources of monomers, a variety of terpenes and their derivatives as renewable feedstock have recently been intensively investigated to produce various classes of polymers, such as polymyrcene, polylimonene, etc. (see e.g., Refs. [15,16,17,18,19,20,21,22,23,24,25,26] and references therein). Beyond doubt, β-pinene, which is the most abundant commercial terpene and is obtained from turpentine, belongs to a special class of natural olefins with a bicyclic substituent [25,26]. The reactive exo methylene group in β-pinene makes it able to undergo carbocationic, and to a certain extent radical and coordinative polymerizations. It has to be noted that in addition to poly(β-pinene), this compound is used not only in a range of products, but as a starting material [15,27,28,29,30,31,32] or therapeutic agent [33,34], and it even has potential in COVID-19 treatment [35].
The Lewis acid catalyzed carbocationic isomerization polymerization of β-pinene has been explored since the 1950s [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. A number of Lewis acids, such as AlCl3, AlBr3, EtAlCl2, Et2AlCl, BiCl3, SbCl3, ZrCl4, BF3, TiCl4, TiCl3(OiPr), SnCl4, and ZnCl2, and different reaction conditions were used in attempts to carry out the polymerization of β-pinene. However, usually, either low yields and/or polymers with number average molecular weight (Mn) less than 1000 g/mol were obtained, indicating the occurrence of severe chain-breaking reactions, i.e., termination and chain transfer, in these polymerizations. Keszler and Kennedy [43] reported that poly(β-pinene) with Mn up to 20,000 g/mol can be obtained with the “H2O”/EtAlCl2 initiating system (where “H2O” stands for adventitious moisture in the reactants and solvents) and up to 40,000 g/mol in the presence of a proton trap 2,6-di-tert-butylpyridine (DtBP) in methylchloride/methylcyclohexane (DCM/MeCHx) (50/50 v/v%) solvent mixture, but under energy-consuming cryogenic conditions, i.e., at −80 °C. It was concluded that chain transfer to monomer is the major chain-breaking reaction the extent of which determines the average molecular weights of the resulting polymer [43]. Similar results were found by Kamigaito et al. [44] with the “H2O”/EtAlCl2 initiating system in the polymerization of β-pinene under similar conditions in DCM/MeCHx solvent mixture at −78 °C. Other Lewis acids, such as Et2AlCl, TiCl4, and SnCl4, with less acidity than that of EtAlCl2, led to lower yields and lower molecular weights even after long reaction times [43,44]. Running the polymerization with EtAlCl2 in toluene at higher temperatures (10–50 °C) led to poly(β-pinene) oligomers with relatively low yields (44–65%) [45]. Higashimura and coworkers achieved quasiliving carbocationic polymerization of β-pinene by using the adduct of HCl with 2-chloroethylvinyl ether as initiator and TiCl3(OiPr) as co-initiator in the presence of nBu4NCl in dichloromethane (DCM) at −40 °C and −78 °C [46]. In subsequent research, this method was applied to synthesize various copolymers of β-pinene, i.e., random, block, and graft copolymers, with styrene, p-methylstyrene, and methyl methacrylate [47,48]. Kostjuk et al. [49] investigated the polymerization of β-pinene in DCM/n-hexane (40/60 v/v) mixture at 20 °C with the “H2O”/AlCl3/diphenyl ether (Ph2O)-initiating system, and found that poly(β-pinene) with relatively high Mn and exo-olefinic chain end could be obtained under the applied conditions. For promising optoelectronic purposes, Kamigaito and coworkers [50] synthesized poly(β-pinene)s by (di)cumyl chloride as initiator in conjunction with EtAlCl2 co-initiator in the presence of either diethyl ether (Et2O) or DtBP as additive in DCM/n-hexane mixtures (1/1 v/v) at various reaction temperatures from −78 °C to −15 °C. With the monofunctional cumyl chloride initiator, poly(β-pinene) with Mn of 6400 g/mol was obtained, while using the bifunctional dicumyl chloride initiator, the number average molecular weight of the polymers was up to 50,000 g/mol at low temperature [50]. Recently, Ballard et al. [51,64] reported on the carbocationic polymerization of β-pinene using either “H2O” or 1-(4-methoxyphenyl) ethanol initiator with tris(pentafluorophenyl)borane (B(C6F5)3) co-initiator as mild Lewis acid in DCM at room temperature, aiming at the preparation of tackifiers for pressure-sensitive adhesives. They found that long reaction times (8–10 h) were needed for high conversion, resulting in polymers with Mn of 700–1100 g/mol, indicating significant chain transfer in these polymerizations. Blending the resulting poly(β-pinene)s with commercial poly(styrene-b-isoprene-b-styrene) thermoplastic elastomer provided outstanding adhesive properties [51]. Efforts have also been made to prepare poly(β-pinene)s by various Lewis acids, such as SbCl3-AlCl3 mixtures [52], ZrCl4 [53], Nb- and Ta-halides [54], and GaCl3 [55]. All these polymerizations indicate that the reaction conditions, especially the presence or absence of an ion-generating initiator, the acidity of the co-initiator, solvent polarity, concentrations, temperature, and reaction time play critical roles in the outcome of the polymerization of β-pinene, mainly in terms of yield, while usually, polymers with multimodal molecular weight distributions and relatively low average molecular weights are formed.
Based in part on environmental considerations, attempts were also made to use various solid acid catalysts (co-initiators) in order to replace classical Lewis acids in the carbocationic polymerization of β-pinene [56,57,58,59,60,61,62]. For instance, the H3PW12O40 acid was reported to have considerably high catalytic activity, but the applied reaction time was rather long (22 h) and the presence of halogenic solvents and low reaction temperature (−10 °C) was necessary to obtain sufficient yields (10–60%) [56,57,58,59]. The Mn values of the resulting polymers were around 700–800 g/mol. When the phosphotungstic acid was supported by activated carbon [60] or silica [61], the reaction time was reduced to 2 h and the Mns were found to be in the ranges of 1200–1300 g/mol and 700–900 g/mol, respectively. Acidic montmorillonite clay was also applied as a “green” catalyst for the carbocationic polymerization of β-pinene [62]. In the absence of any solvent, the Mn of the polymers increased to some extent with reaction time and reached 3990 g/mol at 65% conversion after 8 h at 0 °C. In the presence of organic solvents, the Mn increased and reached 7400–7800 g/mol; however, the conversions decreased to low values of 20%–40%, even after 8 h reaction time at 18 °C reaction temperature [62].
Polymerizations of β-pinene by processes other than cationic polymerization were also attempted. Recently, Vieira et al. [67,68,69,70] reported on the atom transfer radical polymerization (ATRP) of this monomer, leading to low molecular weight oligomers. Coordination polymerization by Schiff-base nickel complex catalysts [71] was also accomplished. Copolymerization of β-pinene with ethylene by coordination polymerization with half titanocene/methylaluminumoxane (MAO) combinations has been also reported [72].
Copolymers of β-pinene with styrene [73] and isobutylene [74] were successfully prepared by Lewis-acid-catalyzed carbocationic copolymerization. Combination of cationic polymerization of β-pinene with other polymerization methods proved to be useful for obtaining various block copolymers [75,76,77,78,79]. For instance, cationic [76] and ATRP [77] of styrene from brominated poly(β-pinene) led to poly(β-pinene)-g-polystyrene graft copolymers, while ATRP of butyl acrylate and methyl methacrylate from the brominated poly(β-pinene) resulted in the corresponding graft copolymers [78]. Utilizing endfunctional poly(β-pinene) as a macroinitiator for the ring-opening polymerization of tetrahydrofuran resulted in poly(β-pinene)-b-poly(tetrahydrofuran) AB block copolymers [79]. Inverse vulcanization with β-pinene was also applied to obtain various copolymers for targeted applications [80,81].
Surprisingly, as the above in-depth literature overview on the carbocationic polymerization of β-pinene indicates, systematic polymerization investigations of this monomer with TiCl4, which is one of the most widely used mild and economic Lewis acids, and as such, is applied, for instance, as one of the components of Ziegler-Natta catalysts for olefin polymerizations in large quantities, have not been reported so far, to the best of our knowledge. TiCl4 has already been used as co-initiator in the quasiliving carbocationic polymerization of isobutylene and styrene, and also for producing commercial products from these monomers (see e.g., Refs. [82,83,84,85,86,87,88,89] and references therein). Only sporadic reports have been presented so far on the cationic polymerization of β-pinene by TiCl4 at low temperatures [42,43,44,46,55], without detailed investigations on the effect of the reaction conditions, especially polymerization temperature, on the conversion-time relationships and the major structural parameters of the resulting polymers in terms of molecular weight distribution, average molecular weights, and the structure of the formed poly(β-pinene). Herein, we report on the carbocationic polymerization of β-pinene by TiCl4 as catalyst (co-initiator) in the presence and absence of a cationic initiator at both low (cryogenic) temperature and energy-saving, environmentally advantageous room temperature (i.e., without any heating or cooling), and on the major kinetic observations together with the structural analysis of the poly(β-pinene)s formed in these reactions.
2. Results and Discussion
Among renewable resources for polymer production, β-pinene is one of the most abundant olefins that can undergo carbocationic polymerization. As shown in Scheme 1, the carbocationic polymerization of β-pinene occurs through the addition of the carbocationic species (initiator and propagating carbocation chain) to its reactive methylene group, followed by isomerization via β-scission of the tetracyclic ring, resulting in a reactive tertiary carbocationic species.
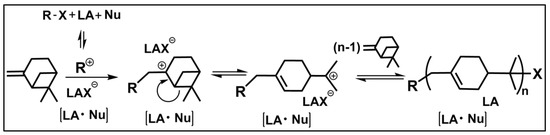
Scheme 1.
Carbocationic polymerization of β-pinene in the presence of an organic initiator (R-X), a Lewis acid (LA) catalyst (co-initiator), and a nucleophilic additive (Nu).
First, we carried out the polymerization of β-pinene with and without TMPCl initiator in conjunction with a TiCl4 catalyst in the presence of N,N,N′,N′-tetramethylethylenediamine (TMEDA) nucleophilic additive in dichloromethane/n-hexane (DCM/Hx) (45/55 v/v%) solvent mixture at −78 °C, the reaction temperature used also by others for β-pinene polymerization [42,43,44,55]. The applied DCM/Hx mixture is usual for carbocationic polymerizations, in which the solvent polarity plays a crucial role through the solvation of the carbocations and gegenions in such processes [82,90]. The TMEDA and other nucleophilic additives are used to suppress the chain transfer to the monomer, and thus to facilitate quasiliving carbocationic polymerization by complexing such additives with the Lewis acid, resulting in a decrease in the electrophilicity of the carbocationic chain end, as found with monomers such as isobutylene, styrene, and vinyl ethers [82,83,84,91]. As displayed in Figure 1, quantitative conversion of β-pinene takes place in the presence of TMPCl initiator at −78 °C polymerization temperature within 40 min. In contrast, the lack of the initiator results in a slow polymerization process, leading to only 40% monomer conversion in 60 min. This finding indicates the presence of low amounts of cationogenic (protic) impurities in this polymerization system, and thus it also signifies the necessity of the application of effective initiators in the carbocationic polymerization of β-pinene under the applied conditions for reaching high yields in short time. This result also explains the long reaction times used by others to obtain high yields in the absence of cationic initiator under similar conditions; e.g., 3 h [43] and 8 h with 66% conversion [44]. Plotting the observed data according to the first-order plot shows that the monomer is consumed in a pseudo first-order process (Figure 1, bottom), as expected on the basis of the polymerization mechanism in Scheme 1 in the absence of permanent termination.
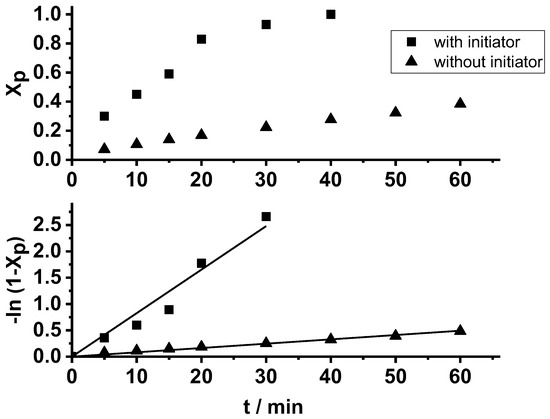
Figure 1.
The monomer conversion (Xp) as a function of time (top) and the first-order kinetic plot of monomer consumption (bottom) in the carbocationic polymerization of β-pinene (Pin) with the TMPCl/TiCl4/TMEDA initiating system and without TMPCl initiator in DCM/hexane (45/55 v/v%) solvent mixture at −78 °C ([TMPCl]/[Pin]/[TiCl4]/[TMEDA] = 1/73/10/1; [TMPCl] = 0.01 M with initiator).
As the GPC traces indicate in Figure 2, the molecular weight distributions (MWD) were monomodal and shifted towards higher molecular weights (MW) in the first 30 min of the polymerization in the presence of the TMPCl initiator, i.e., up to Mn of 3200 g/mol at 93% monomer conversion. This implies that propagating carbocationic species were permanently present in this polymerization. However, bimodal MWD of the resulting poly(β-pinene) with a shoulder in the higher MW range appeared at 100% conversion after 40 min reaction time, and the Mn increased to 5500 g/mol. This result indicates that in the absence of monomer, at least some fractions of the polymer chains still possessed the carbocationic chain ends, which can participate in chain–chain coupling reactions under monomer-starved conditions, similarly to that of isobutylene and styrene polymerizations [92,93,94,95]. It is interesting to note that the coupling was also supported by the determination of the molecular weight values at the maxima of the bimodal GPC traces. This evaluation resulted in 12,600 g/mol for the lower and 6400 g/mol for the higher elution volumes for the polymer obtained with 100% conversion at 40 min polymerization time. This clearly indicates that the molecular weight maximum of the polymer fraction with the higher molecular weight was nearly two times higher than that of the maximum belonging to the lower molecular weight. This finding allowed us to conclude that the majority of the higher molecular weight fraction contained two coupled chains. Plotting the Mn as a function of monomer conversion corroborated these findings (Figure 3). The Mn increased linearly, with conversion close to complete monomer consumption, but a significant increase in the molecular weight took place after 100% conversion.
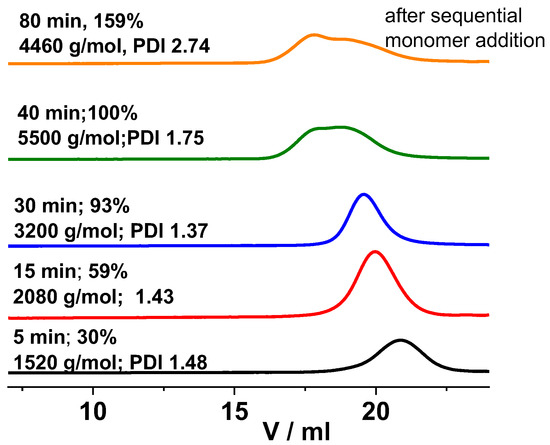
Figure 2.
GPC traces of poly(β-pinene)s obtained at different polymerization times by the TMPCl/TiCl4/TMEDA initiating system in DCM/hexane (45/55 v/v%) solvent mixture at −78 °C ([TMPCl]/[Pin]/[TiCl4]/[TMEDA] = 1/73/10/1; [TMPCl] = 0.01 M).
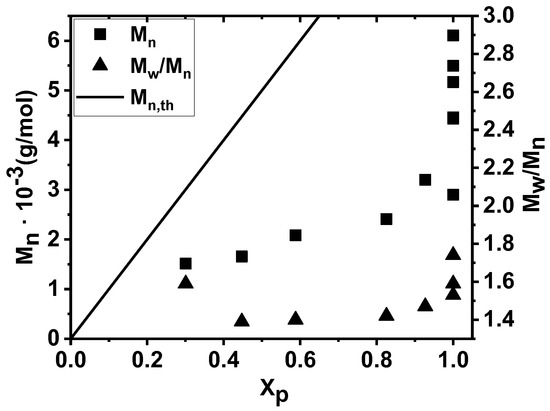
Figure 3.
Number average molecular weight (Mn) and polydispersity indices (Mw/Mn) of poly(β-pinene)s obtained by the TMPCl/TiCl4/TMEDA initiating system in DCM/hexane (45/55 v/v%) solvent mixture at −78 °C as a function of monomer conversion (Xp) ([TMPCl]/[Pin]/[TiCl4]/[TMEDA] = 1/73/10/1; [TMPCl] = 0.01 M).
As shown in Figure 2 and Figure 3, the Mw/Mn polydispersity indices (PDI) fell in the region of ~1.4; i.e., poly(β-pinene)s with relatively narrow MWDs were formed at lower than 100% conversion, while significant MWD broadening occurred at reaction times after reaching 100% conversion, which is another indication of chain–chain coupling. It has to be noted that the Mn data do not fall on the theoretical line (Mn,th) constructed by assuming 100% initiating efficiency and the absence of chain transfer. Instead, polymers with lower Mns were formed. However, as the data in Figure 2 and Figure 3 show, the GPC traces and thus the MWDs were uniformly shifted to higher molecular weights, indicating that all the formed chains were involved in the propagation step of this polymerization until monomer was present in the reaction mixture. This is in agreement with the mechanism shown in Scheme 1, according to which the cationic polymerization of β-pinene with TiCl4 catalyst proceeded via equilibrium between propagating (living) and nonpropagating (nonliving) polymer chains; that is, by quasiliving carbocationic polymerization [82,83,91,96] in the absence of permanent chain-breaking reactions, such as permanent termination or chain transfer.
As displayed in Figure 4, adding a second amount of β-pinene to the reaction mixture at 60 min polymerization time resulted in further polymer formation in the presence of the initiator at both −78 °C and at 25 °C (room temperature) (the results obtained at 25 °C are discussed later). However, this led to significant broadening of the MWD, as shown in Figure 2, when the polymerization was carried out at −78 °C. These results indicate that poly(β-pinene)s can be prepared with high yields and relatively high molecular weights, but with broad bimodal MWD, via sequential monomer addition by carrying out the polymerization with TMPCl/TiCl4/TMEDA initiating system in DCM/Hx (45/55 v/v%) solvent mixture under cryogenic conditions, i.e., at −78 °C.
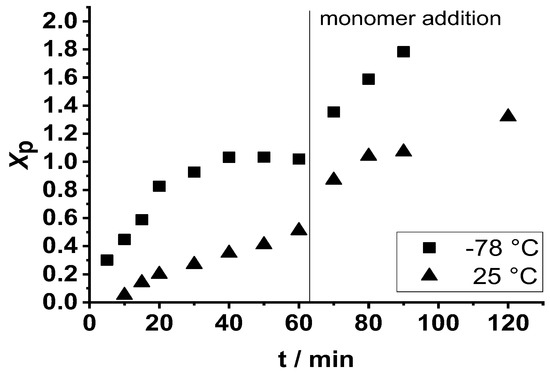
Figure 4.
Monomer conversion (Xp) as a function of time in the first 60 min and after addition of a second feed of monomer in the carbocationic polymerization of β-pinene (Pin) by the TMPCl/TiCl4/TMEDA initiating system in DCM/hexane (45/55 v/v%) solvent mixture at −78 °C and 25 °C ([TMPCl]/[Pin]/[TiCl4]/[TMEDA] = 1/73/10/1; [TMPCl] = 0.01 M).
When the polymerization of β-pinene was carried out in the absence of TMPCl initiator at −78 °C, the Mns of the resulting polymers obtained from the monomodal GPC curves (not shown) increased linearly with conversion from ~1000 g/mol to 1830 g/mol (40% monomer conversion), as displayed in Figure 5. However, these polymers possessed broad MWDs with Mw/Mn values in the range of ~3–3.4. These results, together with the observed slow polymerization in the absence of any cationic initiator in sufficiently high amounts, allow us to conclude that polymerization of β-pinene without added initiator is not an effective process for producing poly(β-pinene) with high yields and high molecular weights in DCM/Hx solvent mixture at −78 °C within the investigated reaction time range.
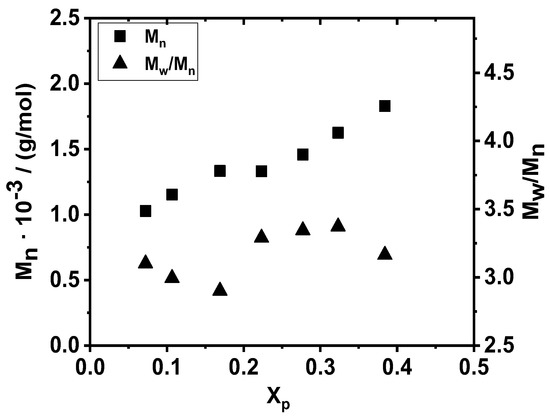
Figure 5.
Number average molecular weight (Mn) and polydispersity index (Mw/Mn) of poly(β-pinene) synthesized by the “H2O”/TiCl4/TMEDA initiating system in DCM/hexane (45/55 v/v%) solvent mixture at −78 °C as a function of monomer conversion (Xp) ([Pin]/[TiCl4]/[TMEDA] = 1/73/10).
Due to the interest in energy-efficient chemical processes, that is, especially chemical reactions without cooling or heating, the carbocationic polymerization of β-pinene was carried out with the TMPCl/TiCl4/TMEDA initiating system at room temperature as well. As shown in Figure 4, the polymerization proceeded at a slower rate at room temperature than at −78 °C. After 60 min polymerization, 50% monomer conversion was reached at room temperature, while 100% conversion was observed in 40 min at −78 °C. This was due to the decreased polarity of the reaction medium at higher temperatures. Adding a second feed of monomer to the reaction mixture after 60 min led to subsequent polymerization, resulting in an additional 80% monomer conversion in the next 60 min (Figure 4). As displayed in Figure 6, the monomer consumption showed pseudo first-order kinetics, indicating the absence of irreversible chain termination even at room temperature.
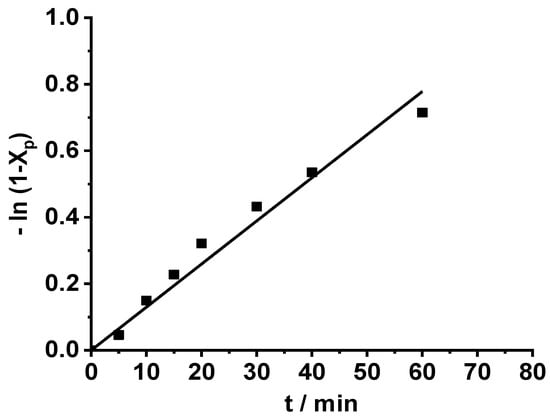
Figure 6.
First order kinetic plot for carbocationic polymerization of β-pinene (Pin) at 25 °C by the TMPCl/TiCl4/TMEDA initiating system in DCM/hexane (45/55 v/v%) solvent mixture ([TMPCl]/[Pin]/[TiCl4]/[TMEDA]= 1/73/10/1; [TMPCl]= 0.01 M).
As shown in Figure 7, the GPC traces of the polymers obtained with the TMPCl/TiCl4/TMEDA initiating system at room temperature were shifted to higher molecular weights with the increasing conversion together with some broadening of the MWD. However, in contrast to the result at −78 °C, chain–chain coupling leading to bimodal MWD was not detectable at 25 °C. The GPC chromatograms of the poly(β-pinene)s obtained at room temperature were monomodal, with Mw/Mn values in the range of 1.3–1.8, as presented in Figure 7.
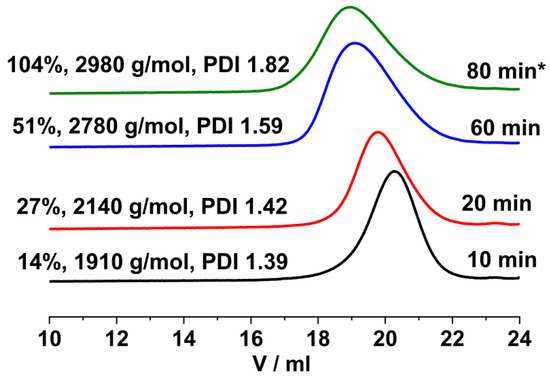
Figure 7.
GPC traces of poly(β-pinene)s obtained at different polymerization times by the TMPCl/TiCl4/TMEDA initiating system in DCM/hexane (45/55 v/v%) solvent mixture at room temperature ([TMPCl]/[Pin]/[TiCl4]/[TMEDA] = 1/73/10/1; [TMPCl] = 0.01 M; * after 60 min reaction time a second feed of monomer was added to the reaction mixture).
As depicted in Figure 8, the Mn of poly(β-pinene)s obtained by room temperature polymerization increased with conversion and reached 2780 g/mol after 60 min polymerization time. After the addition of the second feed of monomer, the Mn continued to increase, but at a lower rate, and it reached 3170 g/mol after 120 min polymerization. These findings indicate that carrying out the carbocationic polymerization of β-pinene with the TMPCl/TiCl4/TMEDA initiating system in DCM/Hx solvent mixture (45/55 v/v%) at room temperature is also suitable for producing poly(β-pinene)s with relatively high yields and molecular weights.
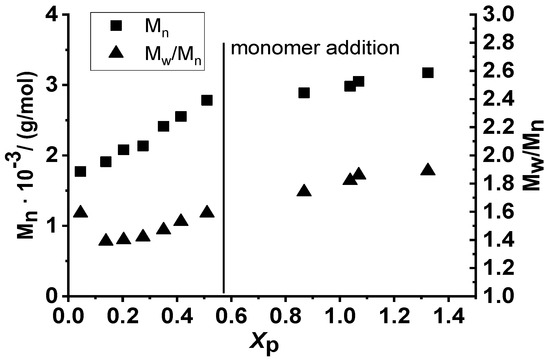
Figure 8.
Number average molecular weight (Mn) and polydispersity indices (Mw/Mn) of poly(β-pinene)s obtained by the TMPCl/TiCl4/TMEDA initiating system in DCM/hexane (45/55 v/v%) solvent mixture at room temperature as a function of monomer conversion (Xp) ([TMPCl]/[Pin]/[TiCl4]/[TMEDA] = 1/73/10/1; [TMPCl] = 0.01 M).
Considering that the carbocationic polymerization of β-pinene by the TMPCl/TiCl4/TMEDA initiating system in DCM/Hx proceeds with lower rates at room temperature than at −78 °C because of the lower polarity of the solvent at higher temperatures, polymerizations were also carried out in the polar dichloromethane. At room temperature, instantaneous polymerization occurred with quantitative monomer conversion, which led to boiling DCM as a consequence of the rapid formation of the full polymerization heat. The resulting poly(β-pinene) possessed monomodal MWD with Mn and Mw/Mn at 1800 g/mol and 1.26, respectively. In order to avoid the boiling of the DCM, the polymerization was also performed at −20 °C. This reaction led to polymer formation with complete monomer conversion in 5 min. The GPC analysis also found monomodal MWD with Mn at 2350 g/mol and Mw/Mn at 1.92 in this case. These findings show that the solvent polarity was indeed a crucial parameter in the carbocationic polymerization of β-pinene. On the other hand, these results also indicate that TiCl4 is a suitable co-initiator for this polymerization process under certain conditions, leading to high yields and poly(β-pinene)s with relatively high molecular weights.
In order to test whether polymerization occurred in the absence of both initiator and nucleophilic additive, the polymerization of β-pinene was also attempted by the addition of only TiCl4 in DCM/Hx 45/55 v/v% solvent mixture at room temperature. Strikingly, it was found that rapid polymerization took place, and nearly complete monomer conversion was observed with TiCl4 concentrations of 0.14 M and higher within 5 min reaction time. To reveal the effect of TiCl4 concentration on β-pinene polymerization under additive-free conditions at room temperature, a series of experiments were carried out by systematically varying the TiCl4 catalyst concentration. The monomer conversion as a function of polymerization time with various TiCl4 concentrations is displayed in Figure 9. As shown in this Figure, decreasing the TiCl4 concentration below 0.1 M decreased the polymerization rates, and reaction times of about 30, 60, and 90 min were required to reach complete monomer conversion with 99, 57, and 37 mM TiCl4 concentrations, respectively. Lower TiCl4 concentrations resulted in negligible amounts of polymer. This indicates that the concentration of adventitious protic impurities was in the range of about 30 mM in the investigated polymerization mixture. These results convincingly indicate that rapid protic initiation with TiCl4 occurs even at room temperature. In other words, TiCl4 is a highly efficient Lewis acid without any additive for the room temperature polymerization of β-pinene. As a representative GPC trace indicates in Figure 10, poly(β-pinene)s with monomodal and relatively narrow molecular weight distribution and relatively high average molecular weight with Mns in the range of 2000 g/mol were formed. This means that the addition of only TiCl4 into a mixture of the β-pinene monomer with suitable polymerization media (solvent) results in poly(β-pinene)s with high, practically quantitative yields and monomodal, relatively narrow MWDs. This finding may provide a unique opportunity to develop an improved production process for poly(β-pinene).
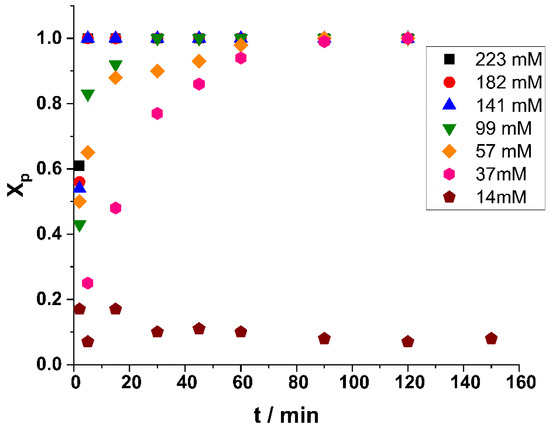
Figure 9.
Monomer conversion (Xp) as a function of time in the carbocationic polymerization of β-pinene (Pin) by the “H2O”/TiCl4 initiating system at different TiCl4 concentrations (14 mM–223 mM) in DCM/hexane (45/55 v/v%) solvent mixture at room temperature ([Pin] = 0.73 M).
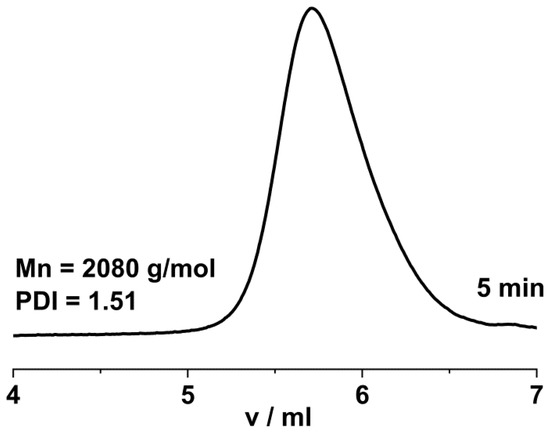
Figure 10.
GPC trace of poly(β-pinene) obtained by the “H2O”/TiCl4 initiating system in DCM/hexane (45/55 v/v%) solvent mixture at room temperature ([Pin]/[TiCl4] = 73/10; [TiCl4] = 99 mM, 5 min polymerization time).
The resulting polymers were also analyzed with 1H NMR spectroscopy. A representative 1H NMR spectrum of the obtained poly(β-pinene)s is shown in Figure 11. This spectrum is in good accordance with spectra reported in the literature [44,46,48,51,54]. Taking into account the integral values of the =CH– methine proton between 5.1–5.5 ppm and the rest of the protons of the monomer units between 0.6–2.5 ppm, the estimated average number of double bonds per monomer units was usually close to unity, falling in the range of 0.8–0.9 for most of the cases in the poly(β-pinene) samples obtained in the whole range of the polymerization conditions, and this was independent of the polymerization temperature. This indicates that ~10–20% of the in-chain double bonds may have participated in either the rearrangement reactions, such as the Wagner–Meerwein rearrangement, and/or the chain–chain coupling processes. However, it can be noted that the large majority of the intact double bonds in the formed poly(β-pinene)s may be utilized for subsequent polymer derivatizations.
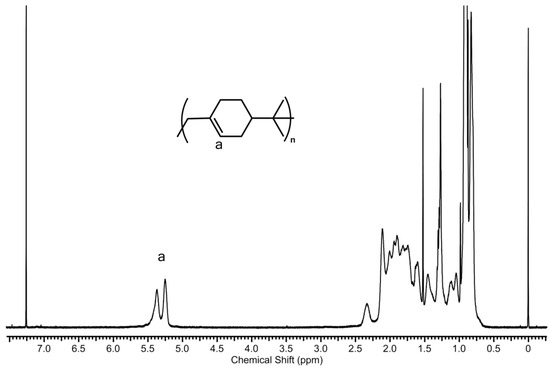
Figure 11.
1H NMR spectrum of poly(β-pinene) synthesized by the TMPCl/TiCl4/TMEDA initiating system in DCM/hexane (45/55 v/v%) solvent mixture at −78 °C ([TMPCl]/[Pin]/[TiCl4]/[TMEDA] = 1/73/10/1; [TMPCl] = 0.01 M; reaction time: 60 min).
Finally, we would like to note that all the polymers prepared in the course of our studies were solid white powders. Because the glass transition temperature (Tg) is one of the important properties of polymers, DSC measurements were carried out to determine the Tg of the poly(β-pinene)s obtained by TiCl4 co-initiator. Figure 12 shows a typical DSC curve for poly(β-pinene) prepared using TiCl4 as co-initiator for the polymerization of β-pinene. As displayed in this Figure, Tg of 90.2 °C was obtained by this measurement which corroborates well with the data reported in the literature [44,49]. It has to be noted here that usually, not only the Tg, but the so-called softening point, is used widely in industrial practice. As reported recently [97], the softening point of poly(β-pinene) increases with molecular weight and becomes constant at the value of 140 °C above Mn of ~1500 g/mol. Taking into account these characteristics of poly(β-pinene), it can be concluded that using TiCl4 as catalyst in the polymerization of β-pinene enables the production of polymers with Mn higher than 1500 g/mol even by polymerizations at room temperature. This indicates that such poly(β-pinene)s can be utilized in processes and applications that require polymers with such a softening point.
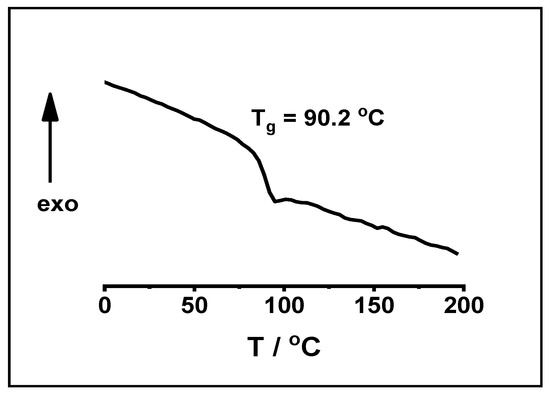
Figure 12.
A typical DSC curve of poly(β-pinene) obtained by the TiCl4 co-initiated carbocationic polymerization of β-pinene ([TMPCl]/[Pin]/[TiCl4]/[TMEDA] = 1/73/10/1; [TMPCl] = 0.01 M; DCM/hexane (45/55 v/v%) solvent mixture; −78 °C; reaction time: 90 min).
3. Materials and Methods
(-)β-pinene (99%, Sigma-Aldrich, St. Louis, MO, USA), TiCl4 (99.9%, Acros Organics, Geel, Belgium), N,N,N′,N′-tetramethylethylenediamine (TMEDA) (Sigma-Aldrich, St. Louis, MO, USA) were used as received. The olefinic impurities of hexane were removed by cc.H2SO4 treatment followed by filtering through Al2O3 and distillation from CaH2. Dichloromethane (DCM) was freshly distilled from CaH2 and stored under N2. The initiator 2-chloro-2,4,4-trimethylpentane (TMPCl) was synthesized as reported previously [92].
The carbocationic polymerization of β-pinene was carried out using TMPCl initiator, TiCl4 co-initiator, and TMEDA nucleophilic additive. The polymerizations were started by adding the TiCl4 catalyst to the solution containing the monomer, initiator, and nucleophilic additive. The flask was purged with nitrogen and was cooled by dry ice/isopropanol mixture for polymerizations carried out at −78 °C. During the polymerizations, samples were withdrawn, quenched, and precipitated with methanol (prechilled or room temperature), filtered, vacuum dried, and analyzed. A typical polymerization reaction was carried out as follows: in a three-necked round-bottom flask, a 120 mL mixture of dichloromethane/n-hexane (45:55 v/v%) was added, and then 0.187 g (1.1 mmol) of TMPCl, 12 g (88 mmol) of β-pinene, and 0.18 mL (1.2 mmol) of TMEDA were charged and mixed with a magnetic stirrer. Subsequently, this reaction mixture was either cooled to the required temperature or left at room temperature under nitrogen atmosphere. Finally, the polymerization was started with the addition of 2.1 mL (19 mmol) of TiCl4. At predetermined time intervals, samples of 10 mL were withdrawn and quenched immediately with methanol. In certain cases, fresh feed of monomer was added to the reaction (sequential monomer addition) after 60 min polymerization time in order to study the activity, especially the living polymerization character of these reactions. The monomer conversion was determined by gravimetry.
Gel permeation chromatography was carried out using two Styragel HR columns (HR1 and HR4), Waters 515 HPLC pump, Waters 717 Autosampler, Jetstream Column Thermostat, and Agilent 1260 Infinity refractive index detector. The flowrate of the tetrahydrofuran eluent was 0.3 mL/min. The temperature of the columns was 35 °C. Molecular weight distribution and average molecular weights were obtained on the basis of calibration with polystyrene standards.
1H NMR spectra were obtained with a Varian 300 MHz spectrometer in CDCl3 at room temperature. Differential scanning calorimetry (DSC) measurements were carried out with Mettler-Toledo (Greifensee, Switzerland) TC15 equipment under nitrogen atmosphere with 10 °C/min heating and cooling rate. The second heating scan was evaluated for the determination of the glass transition temperature (Tg).
4. Conclusions
Systematic investigations on the carbocationic polymerization of β-pinene, a bio-based, renewable, and sustainable monomer, were carried out with the commercial, cheap, widely available TiCl4 as catalyst under various conditions. The TMPCl/TiCl4/TMEDA initiating system led to 100% monomer conversion after only 40 min, resulting in poly(β-pinene) with Mn of 5500 g/mol in DCM/hexane solvent mixture at −78 °C. Without TMPCl, protic impurities as initiators led to polymerization with much lower rates, yielding polymers with lower Mns and higher polydispersities. With the TMPCl/TiCl4/TMEDA initiating system at room temperature, successful polymerization took place with remarkably decreased rates, resulting in polymers with similar Mns than that at −78 °C. The first-order plots of the monomer conversions, the GPC traces of the formed polymers at different reaction times, and the Mn-versus-conversion relationships clearly indicate that the resulting polymer chains were able to propagate until monomer was present in these polymerizations. At −78 °C, chain–chain coupling took place after reaching complete monomer conversion, leading to higher molecular weights and broadening MWDs. This process was absent in polymerizations at room temperature. Rapid polymerizations of β-pinene with nearly complete monomer conversions were observed with TiCl4 as co-initiator in DCM at room temperature and at −20 °C, due to the higher polarity of the pure solvent than that of its mixture with hexane. Surprisingly, carrying out the polymerizations of β-pinene by adding only TiCl4 in the absence of any organic initiators or additives at room temperature led to polymers reproducibly with high yields, relatively narrow MWDs, and sufficiently high molecular weights. This implies that TiCl4 can be considered as an advantageous alternative to β-pinene polymerizations in comparison to other Lewis acid catalysts, such as polymerizations based on AlCl3 and its derivatives. This highly efficient room-temperature polymerization, that is, without any additives, such as initiator and nucleophile, and without energy-consuming cooling or heating, can be considered as a remarkable eco-friendly polymerization process. In every case, the obtained polymers had high double bond/monomer unit ratios, which makes this bio-based polymer a strong platform for further modifications. These results convincingly indicate that TiCl4 is an efficient catalyst in the carbocationic polymerization of β-pinene, and the new results presented this study can be conveniently used in the production of poly(β-pinene)s on an industrial scale, even under energy saving conditions, i.e., at room temperature. Furthermore, subsequent chemical derivatizations of the reactive double bonds in the chain will provide opportunities to obtain a large variety of high-value-added products; for instance, materials useful from energy production to the biomaterial fields.
Author Contributions
Conceptualization: K.V., Á.S., Z.R., G.S., Á.V. and B.I.; methodology: K.V., Á.S., Z.R., G.S., Á.V. and B.I.; analysis: K.V., Á.S., Z.R., G.S., Á.V. and B.I.; data evaluation: K.V., Á.S., Z.R., G.S., Á.V. and B.I.; writing—original draft preparation: K.V., Á.S., Z.R., G.S. and B.I.; writing—review and editing: K.V., Á.S., Z.R., G.S., Á.V. and B.I.; visualization: K.V., Á.S., Z.R., G.S., Á.V. and B.I.; supervision: K.V. and B.I.; funding acquisition, B.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research, Development and Innovation Office, Hungary (K135946) and the State of Hungary, co-financed by the European Regional Development Fund (VEKOP-2.3.2-16-2017-00013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors thank Beatrix Sóvári for the technical assistance in the GPC measurements. Support by the National Research, Development and Innovation Office, Hungary (K135946) is acknowledged. This research was supported in part by the State of Hungary, co-financed by the European Regional Development Fund (VEKOP-2.3.2-16-2017-00013).
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEA. Oil 2019: Analysis and Forecasts to 2024; IEA: Paris, France, 2019. [Google Scholar] [CrossRef]
- Roddy, D.J. Biomass in a petrochemical world. Interface Focus 2013, 3, 20120038. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022, 7, 83–102. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent advances in antioxidant polymers: From sustainable and natural monomers to synthesis and applications. Polymers 2021, 13, 2465. [Google Scholar] [CrossRef]
- Voet, V.S.; Guit, J.; Loos, K. Sustainable photopolymers in 3D printing: A review on biobased, biodegradable, and recyclable alternatives. Macromol. Rapid Commun. 2021, 42, 2000475. [Google Scholar] [CrossRef]
- Lacruz, A.; Salvador, M.; Blanco, M.; Vidal, K.; Goitandia, A.M.; Martinková, L.; Kyselka, M.; de Ilarduya, A.M. Biobased waterborne polyurethane-ureas modified with POSS-OH for fluorine-free hydrophobic textile coatings. Polymers 2021, 13, 3526. [Google Scholar] [CrossRef]
- Hulnik, M.I.; Kuharenko, O.V.; Vasilenko, I.V.; Timashev, P.; Kostjuk, S.V. Quasiliving cationic polymerization of anethole: Accessing high-performance plastic from the biomass-derived monomer. ACS Sust. Chem. Eng. 2021, 9, 6841–6854. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Niedner, L.; Kali, G. Green engineered polymers: Solvent free, room-temperature polymerization of monomer from a renewable resource, without utilizing initiator. Chem. Select 2019, 4, 3495–3499. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Vivekanandhan, S.; Pin, J.M.; Misra, M. Composites from renewable and sustainable resources: Challenges and innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; M. Lacerda, T. Monomers and Macromolecular Materials from Renewable Resources: State of the Art and Perspectives. Molecules 2022, 27, 159. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Andicsová, A.E.; Mosnácek, J. Irreversible and Self-Healing Electrically Conductive Hydrogels Made of Bio-Based Polymers. Int. J. Mol. Sci. 2022, 23, 842. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, C.; Kordován, M.Á.; Czifrák, K.; Nagy, L.; Vadkerti, B.; Daróczi, L.; Zsuga, M.; Kéki, S. Synthesis of Sucrose-HDI Cooligomers: New Polyols for Novel Polyurethane Networks. Int. J. Mol. Sci. 2022, 23, 1444. [Google Scholar] [CrossRef] [PubMed]
- Mahamat Ahmat, Y.; Madadi, S.; Charbonneau, L.; Kaliaguine, S. Epoxidation of terpenes. Catalysts 2021, 11, 847. [Google Scholar] [CrossRef]
- Nishida, T.; Satoh, K.; Tamura, M.; Li, Y.; Tomishige, K.; Caillol, S.; Ladmiral, V.; Vayer, M.; Mahut, F.; Sinturel, C.; et al. Terpenoid-derived conjugated dienes with exo-methylene and a 6-membered ring: High cationic reactivity, regioselective living cationic polymerization, and random and block copolymerization with vinyl ethers. Polym. Chem. 2021, 12, 1186–1198. [Google Scholar] [CrossRef]
- Palenzuela, M.; Sánchez-Roa, D.; Damián, J.; Sessini, V.; Mosquera, M.E. Polymerization of terpenes and terpenoids using metal catalysts. Adv. Organomet. Chem. 2021, 75, 55–93. [Google Scholar] [CrossRef]
- Lamparelli, D.H.; Kleybolte, M.M.; Winnacker, M.; Capacchione, C. Sustainable myrcene-based elastomers via a convenient anionic polymerization. Polymers 2021, 13, 838. [Google Scholar] [CrossRef]
- Mosquera, M.E.G.; Jiménez, G.; Tabernero, V.; Vinueza-Vaca, J.; García-Estrada, C.; Kosalková, K.; Sola-Landa, A.; Monje, B.; Acosta, C.; Alonso, R.; et al. Terpenes and terpenoids: Building blocks to produce biopolymers. Sustain. Chem. 2021, 2, 467–492. [Google Scholar] [CrossRef]
- Wahlen, C.; Frey, H. Anionic polymerization of terpene monomers: New options for bio-based thermoplastic elastomers. Macromolecules 2021, 54, 7323–7336. [Google Scholar] [CrossRef]
- Derdar, H.; Mitchell, G.R.; Mahendra, V.S.; Benachour, M.; Haoue, S.; Cherifi, Z.; Bachari, K.; Harrane, A.; Meghabar, R. Green nanocomposites from rosin-limonene copolymer and Algerian clay. Polymers 2020, 12, 1971. [Google Scholar] [CrossRef]
- Sahu, P.; Bhowmick, A.K.; Kali, G. Terpene based elastomers: Synthesis, properties, and applications. Processes 2020, 8, 553. [Google Scholar] [CrossRef]
- Della Monica, F.; Kleij, A.W. From terpenes to sustainable and functional polymers. Polym. Chem. 2020, 11, 5109–5127. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef] [PubMed]
- Winnacker, M. Pinenes: Abundant and renewable building blocks for a variety of sustainable polymers. Angew. Chem. Int. Ed. 2018, 57, 14362–14371. [Google Scholar] [CrossRef] [PubMed]
- Nyamwihura, R.J.; Ogungbe, I.V. The pinene scaffold: Its occurrence, chemistry, synthetic utility, and pharmacological importance. RSC Adv. 2022, 12, 11346–11375. [Google Scholar] [CrossRef]
- Kleybolte, M.M.; Winnacker, M. β-pinene-derived polyesteramides and their blends: Advances in their upscaling, processing, and characterization. Macromol. Rapid Commun. 2021, 42, 2100065. [Google Scholar] [CrossRef]
- Stamm, A.; Öhlin, J.; Mosbech, C.; Olsén, P.; Guo, B.; Söderberg, E.; Biundo, A.; Fogelström, L.; Bhattacharyya, S.; Bornscheuer, U.T.; et al. Pinene-based oxidative synthetic toolbox for scalable polyester synthesis. JACS Au 2021, 1, 1949–1960. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Monaghan, O.R.; Elsmore, M.T.; Topham, P.D.; Toolan, D.T.; Derry, M.J.; Taresco, V.; Stockman, R.A.; De Focatiis, D.S.A.; Irvine, D.J.; et al. RAFT polymerisation of renewable terpene (meth)acrylates and the convergent synthesis of methacrylate–acrylate–methacrylate triblock copolymers. Polym. Chem. 2021, 12, 3177–3189. [Google Scholar] [CrossRef]
- Fried, A.D.; Brantley, J.N. Controlled polymerization of β-pinadiene: Accessing unusual polymer architectures with biomass-derived monomers. ACS Macro Lett. 2020, 9, 595–599. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, Z.; Yang, Y.; Chen, S.; Liao, S.; Luo, H.; Lu, H.; Wang, Z.; Fan, G. β-pinene derived products with enhanced in vitro antimicrobial activity. Nat. Prod. Commun. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, P.; Liu, X.; Hou, Y.; Yang, X.; Shan, S.; Ma, Y.; Pan, D.; Dong, B.; Guo, Z. Preparation of high-density fuel through dimerization of β-pinene. Chem. Eng. Technol. 2020, 43, 2259–2265. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LD Jayaweera, S.; ADias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, X.Z.; Xiao, Z.Q.; Fan, G.R.; Chen, S.X.; Liao, S.L.; Luo, H.; Wang, Z.D. Design, synthesis, antibacterial, antifungal and anticancer evaluations of novel β-pinene quaternary ammonium salts. Int. J. Mol. Sci. 2021, 22, 11299. [Google Scholar] [CrossRef]
- Adamski, A.; Adamska, J. The use of essential oils–alpha and β-pinene in the treatment of COVID-19. Ann. Clin. Med. Case Rep. 2021, 7, 1–4. [Google Scholar]
- Roberts, W.J.; Day, A.R. A study of the polymerization of α- and β-pinene with Friedel—Crafts type catalysts. J. Am. Chem. Soc. 1950, 72, 1226–1230. [Google Scholar] [CrossRef]
- Pietila, H.; Sivola, A.; Sheffer, H. Cationic polymerization of β-pinene, styrene and α-methylstyrene. J. Polym. Sci. Part A Polym. Chem. 1970, 8, 727–737. [Google Scholar] [CrossRef]
- Huet, J.M.; Marechal, E. Study of cationic polymerization of beta-pinene. C. R. Acad. Sci. Paris 1970, 271, 1058–1061. [Google Scholar]
- Snyder, C.; McIver, W.; Sheffer, H. Cationic polymerization of β-pinene and styrene. J. Appl. Polym. Sci. 1977, 21, 131–139. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Liao, T.P.; Guhaniyogi, S.; Chang, V.S. Poly (β-pinenes) carrying one, two, or three functional end groups. I. The effect of reaction conditions on the polymerization of β-pinene. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 3219–3227. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Liao, T.P.; Guhaniyogi, S.; Chang, V.S. Poly (β-pinenes) carrying one, two, and three functional end groups. II. Syntheses and characterization. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 3229–3240. [Google Scholar] [CrossRef]
- Martinez, F. Cationic polymerization of β-pinene. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 673–677. [Google Scholar] [CrossRef]
- Keszler, B.; Kennedy, J.P. Synthesis of high molecular weight poly(β-pinene). Adv. Polym. Sci. 1992, 100, 1–9. [Google Scholar] [CrossRef]
- Satoh, K.; Sugiyama, H.; Kamigaito, M. Biomass-derived heat-resistant alicyclic hydrocarbonpolymers: Poly(terpenes) and their hydrogenated derivatives. Green Chem. 2006, 8, 878–882. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Castro, J.A.A.M. Polymerization of β-pinene with ethylaluminum dichloride (C2H5AlCl2). J. Appl. Polym. Sci. 2001, 82, 2558–2565. [Google Scholar] [CrossRef]
- Lu, J.; Kamigaito, M.; Sawamoto, M.; Higashimura, T.; Deng, Y.X. Living cationic isomerization polymerization of β-pinene. 1. Initiation with HCl−2-chloroethyl vinyl ether adduct/TiCl3(OiPr) in conjunction with nBu4NCl. Macromolecules 1997, 30, 22–26. [Google Scholar] [CrossRef]
- Lu, J.; Kamigaito, M.; Sawamoto, M.; Higashimura, T.; Deng, Y.X. Living cationic isomerization polymerization of β-pinene. 2. Synthesis of block and random copolymers with styrene or p-methylstyrene. Macromolecules 1997, 30, 27–31. [Google Scholar] [CrossRef]
- Lu, J.; Kamigaito, M.; Sawamoto, M.; Higashimura, T.; Deng, Y.X. Living cationic isomerization polymerization of β-pinene. III. Synthesis of end-functionalized polymers and graft copolymers. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1423–1430. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Vasilenko, I.V.; Kostjuk, S.V. Room temperature cationic polymerization of β-pinene using modified AlCl3 catalyst: Toward sustainable plastics from renewable biomass resources. Green Chem. 2011, 13, 2362–2364. [Google Scholar] [CrossRef]
- Satoh, K.; Nakahara, A.; Mukunoki, K.; Sugiyama, H.; Saito, H.; Kamigaito, M. Sustainable cycloolefin polymer from pine tree oil for optoelectronics material: Living cationic polymerization of β-pinene and catalytic hydrogenation of high-molecular-weight hydrogenated poly(β-pinene). Polym. Chem. 2014, 5, 3222–3230. [Google Scholar] [CrossRef]
- Destephen, A.; González de San Román, E.; Martínez-Tong, D.E.; Ballard, N. Cationic polymerization of β-pinene using B(C6F5)3 as a Lewis acid for the synthesis of tackifiers in pressure sensitive adhesives. Macromol. Mater. Eng. 2021, 306, 2100194. [Google Scholar] [CrossRef]
- Lu, J.; Kamigaito, M.; Sawamoto, M.; Higashimura, T.; Deng, Y.-X. Cationic polymerization of β-pinene with the AlCl3/SbCl3 binary catalyst: Comparison with α-pinene polymerization. J. Appl. Polym. Sci. 1996, 61, 1011–1016. [Google Scholar] [CrossRef]
- Cataldo, F.; Gobbino, M.; Ursini, O.; Angelini, G. A study on the optically active polymer poly-β-pinene. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 1225–1234. [Google Scholar] [CrossRef]
- Hayatifar, M.; Marchetti, F.; Pampaloni, G.; Patil, Y.; Galletti, A.M.R. Room-temperature polymerization of β-pinene by niobium and tantalum halides. Catal. Today 2012, 192, 177–182. [Google Scholar] [CrossRef]
- Karasawa, Y.; Kimura, M.; Kanazawa, A.; Kanaoka, S.; Aoshima, S. New initiating systems for cationic polymerization of plant-derived monomers: GaCl3/alkylbenzene-induced controlled cationic polymerization of β-pinene. Polym. J. 2015, 47, 152–157. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Z.; Zhang, T.; Zeng, W.; An, X.; Lei, F. β-Pinene cationic polymerization using Keggin heteropolyacid catalysts. React. Kinet. Mech. Catal. 2010, 99, 463–470. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Z.; An, X.; Lei, F. Keggin heteropolyacids as catalyst for the polymerization of β-pinene. React. Kinet. Mech. Catal. 2010, 100, 355–361. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhu, H.L.; Wang, S.; Zhao, L.L.; Lei, F.H. Synthesis of substituted phosphotungstic acids and their catalytic performance on cationic polymerization of β-pinene. Adv. Mater. Res. 2013, 634, 616–619. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zeng, W.; Zhang, T.S.; Wang, S.; Zhu, H.L.; Lei, F.H. Cationic polymerization of β-pinene using Keggin phosphotungstates as catalysts. Adv. Mater. Res. 2012, 550, 296–300. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, S.; Wang, S.; Zeng, W.; Zhang, T.; Li, P.; Lei, F. Activated-carbon-supported phosphotungstic acid as novel heterogeneous catalysts for cationic polymerization of β-pinene. J. Chem. Eng. Jpn. 2015, 48, 29–34. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, S.; Wang, S.; Zeng, W.; Zhang, T. Silica-supported phosphotungstic acid as a novel heterogeneous catalyst for β-pinene polymerization. React. Kinet. Mech. Catal. 2014, 111, 577–590. [Google Scholar] [CrossRef]
- Akeb, M.; Harrane, A.; Belbachir, M. Polymerization of β-pinene by using natural montmorillonite clay as a green catalyst. Green Mater. 2018, 6, 58–64. [Google Scholar] [CrossRef]
- Poco, J.G.R.; Danese, M.; Giudici, R. Investigation of Cationic Polymerization of β-Pinene Using Calorimetric Measurements. Macromol. React. Eng. 2010, 4, 145–154. [Google Scholar] [CrossRef]
- Destephen, A.; Ballard, N. On the limitations of cationic polymerization of vinyl monomers in aqueous dispersed media. Polym. Chem. 2021, 12, 6444–6455. [Google Scholar] [CrossRef]
- Gaspar, A.S.; Cordeiro, J.B.; Simoes, P.A.; Gameiro, D.; Rocha, F.A.; Serra, A.C.; Coelho, J.F.J.; Fonseca, A.C. Poly(β-pinene) as an efficient biobased tackifier for metallocene poly(ethylene) based hotmelt adhesives. Int. J. Adh. Adh. 2022, 114, 103111. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Han, S.; Wei, Z.; Dai, B. Terpene resin prepared from renewable turpentine oil as a new type of cold flow improver for soybean biodiesel-diesel blends. Fuel 2022, 320, 123844. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Goncalves, S.A.; Vieira, R.P. Organocatalyzed β-pinene polymerization in UV light: Assessment of reaction conditions and material characterization. Eur. Polym. J. 2021, 147, 110303. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Junior, L.M.; de Souza, W.F.C.; Sato, H.H.; Alves, R.M.V.; Vieira, R.P. O-ATRP synthesized poly(β-pinene) blended with chitosan for antimicrobial and antioxidant bio-based films production. Int. J. Biol. Macromol. 2021, 193, 425–432. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Nascimento, L.E.S.; Godoy, H.T.; Vieira, R.P. Improving chitosan performance in the simultaneous adsorption of multiple polycyclic aromatic hydrocarbons by oligo(β-pinene) incorporation. Carbohydrate Polym. 2023, 302, 120379. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Wang, X.; Li, Z.; Lyu, J.; Wang, W.; Vieira, R.P. A new nano hyperbranched β-pinene polymer: Controlled synthesis and nonviral gene delivery. Colloids Surf. B Biointerfaces 2023, 222, 113032. [Google Scholar] [CrossRef]
- Yu, P.; Li, A.L.; Liang, H.; Lu, J. Polymerization of β-pinene with Schiff-base nickel complexes catalyst: Synthesis of relatively high molecular weight poly (β-pinene) at high temperature with high productivity. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3739–3746. [Google Scholar] [CrossRef]
- Kawamura, K.; Nomura, K. Ethylene copolymerization with limonene and β-pinene: New bio-based polyolefins prepared by coordination polymerization. Macromolecules 2021, 54, 4693–4703. [Google Scholar] [CrossRef]
- Sheffer, H.; Greco, G.; Paik, G. The characterization of styrene–β-pinene polymers. J. Appl. Polym. Sci. 1983, 28, 1701–1705. [Google Scholar] [CrossRef]
- Li, A.; Zhang, W.; Liang, H.; Lu, J. Living cationic random copolymerization of β-pinene and isobutylene with 1-phenylethyl chloride/TiCl4/Ti(OiPr)4/nBu4NCl. Polymer 2004, 45, 6533–6537. [Google Scholar] [CrossRef]
- Dey, A.; Haldar, U.; De, P. Block copolymer synthesis by the combination of living cationic polymerization and other polymerization methods. Front. Chem. 2021, 9, 644547. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Lu, J. Synthesis of poly (β-pinene)-g-polystyrene from allylic brominated poly (β-pinene). J. Appl. Polym. Sci. 2000, 75, 599–603. [Google Scholar] [CrossRef]
- Lu, J.; Liang, H.; Li, A.; Cheng, Q. Synthesis of block and graft copolymers of β-pinene and styrene by transformation of living cationic polymerization to atom transfer radical polymerization. Eur. Polym. J. 2004, 40, 397–402. [Google Scholar] [CrossRef]
- Lu, J.; Liang, H.; Zhang, W.; Cheng, Q. Synthesis of poly(β-pinene)-g-poly(meth)acrylate by the combination of living cationic polymerization and atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1237–1242. [Google Scholar] [CrossRef]
- Lu, J.; Liang, H.; Zhang, R.; Li, B. Synthesis of poly(β-pinene)-b-polytetrahydrofuran from β-pinene-based macroinitiator. Polymer 2004, 42, 4549–4553. [Google Scholar] [CrossRef]
- Derakhshan, A.A.; Pirsaheb, M.; Zinadini, S. Synthesis of sustainable poly(S-Abietic-co-pinene) through inverse vulcanization of Kurdica gum and used to fabricate durable and recyclable super-hydrophobic cotton wool filter: Oil-water separation application. Prog. Org. Coat. 2022, 168, 106862. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Wang, Y.; Dong, F.; Liu, H.; Xu, X. A natural polymer with desirable self-healing and recyclable, antibacterial, and adhesive properties based on turpentine monomer. Mater. Chem. Front. 2023, 7, 333–344. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Iván, B. Designed Polymers by Carbocationic Macromolecular Engineering: Theory and Practice; Hanser Publishers: Munich, NY, USA, 1992. [Google Scholar] [CrossRef]
- Iván, B.; Szanka, I.; Szabó, Á.; Pásztor, S.; Pásztói, B.; Stumphauser, T.; Kasza, G.; Szarka, G.; Kalocsai, D.; Bajcsi, Á.; et al. Endfunctional polyisobutylenes by quasiliving carbocationic polymerization and bi-and multicomponent macromolecular architectures therefrom. In Macromolecular Engineering: Design, Synthesis and Applications of Polymers; Lubnin, A., Erdodi, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 23–49. [Google Scholar] [CrossRef]
- Everland, H.; Kops, J.; Nielsen, A.; Iván, B. Living carbocationic polymerization of isobutylene and synthesis of ABA block copolymers by conventional laboratory techniques. Polym. Bull. 1993, 31, 159–166. [Google Scholar] [CrossRef]
- Tawada, M.; Faust, R. Living cationic polymerization of isobutylene with mixtures of titanium tetrachloride/titanium tetrabromide. Macromolecules 2005, 38, 4989–4995. [Google Scholar] [CrossRef]
- Fodor, Z.; Faust, R. Polyisobutylene-based thermoplastic elastomers. IV. Synthesis of poly (styrene-block-isobutylene-block-styrene) triblock copolymers using n-butyl chloride as solvent. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 33, 305–324. [Google Scholar] [CrossRef]
- Storey, R.F.; Thomas, Q.A. Quasi-living cationic polymerization of styrene and isobutylene: Measurement of run number and calculation of apparent rate constant of ionization by TiCl4. Macromolecules 2003, 36, 5065–5071. [Google Scholar] [CrossRef]
- Kaszas, G.; Puskas, J.E.; Kennedy, J.P.; Hager, W.G. Polyisobutylene-containing block polymers by sequential monomer addition. II. Polystyrene–polyisobutylene–polystyrene triblock polymers: Synthesis, characterization, and physical properties. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 427–435. [Google Scholar] [CrossRef]
- Makarevich, M.I.; Nikishau, P.A.; Berezianko, I.A.; Glushkova, T.V.; Rezvova, M.A.; Ovcharenko, E.A.; Bekmukhamedov, G.E.; Yakhvarov, D.G.; Kostjuk, S.V. Aspects of the Synthesis of Poly(styrene-block-isobutylene-block-styrene) by TiCl4-Co-initiated Cationic Polymerization in Open Conditions. Macromol 2021, 1, 243–255. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Maréchal, E. Carbocationic Polymerization; John Wiley & Sons Ltd.: New York, NY, USA, 1982. [Google Scholar] [CrossRef]
- Iván, B. Open mechanistic problems of quasiliving carbocationic polymerization of olefins mediated by nucleophilic additives. Macromol. Symp. 1988, 132, 65–74. [Google Scholar] [CrossRef]
- Held, D.; Iván, B.; Müller, A.H.; de Jong, F.; Graafland, T. Stability of propagating species in living cationic polymerization of isobutylene. ACS Symp. Ser. 1997, 665, 63–74. [Google Scholar]
- Storey, R.F.; Chisholm, B.J.; Masse, M.A. Morphology and physical properties of poly (styrene-b-isobutylene-b-styrene) block copolymers. Polymer 1996, 37, 2925–2938. [Google Scholar] [CrossRef]
- Nugay, N.; Nugay, T.; Deodhar, T.; Keszler, B.L.; Kennedy, J.P. Low cost bifunctional initiators for bidirectional living cationic polymerization of olefins. II. Hyperbranched styrene–isobutylene–styrene triblocks with superior combination of properties. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 705–713. [Google Scholar] [CrossRef]
- Kali, G.; Szesztay, M.; Bodor, A.; Iván, B. A New Synthetic Method for the Preparation of Star-Shaped Polyisobutylene with Hyperbranched Polystyrene Core. Macromol. Chem. Phys. 2007, 208, 1388–1393. [Google Scholar] [CrossRef]
- Iván, B. Terminology and classification of quasiliving polymerizations and ideal living polymerizations on the basis of the logic of elementary polymerization reactions, and comments on using the term “controlled”. Macromol. Chem. Phys. 2000, 201, 2621–2628. [Google Scholar]
- Soares, A.; Pestana, M. Polyterpene Resins: Part I—A Brief Historical Review. Silva Lusit. 2020, 28, 181–195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).