Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels
Abstract
1. Introduction
2. Results
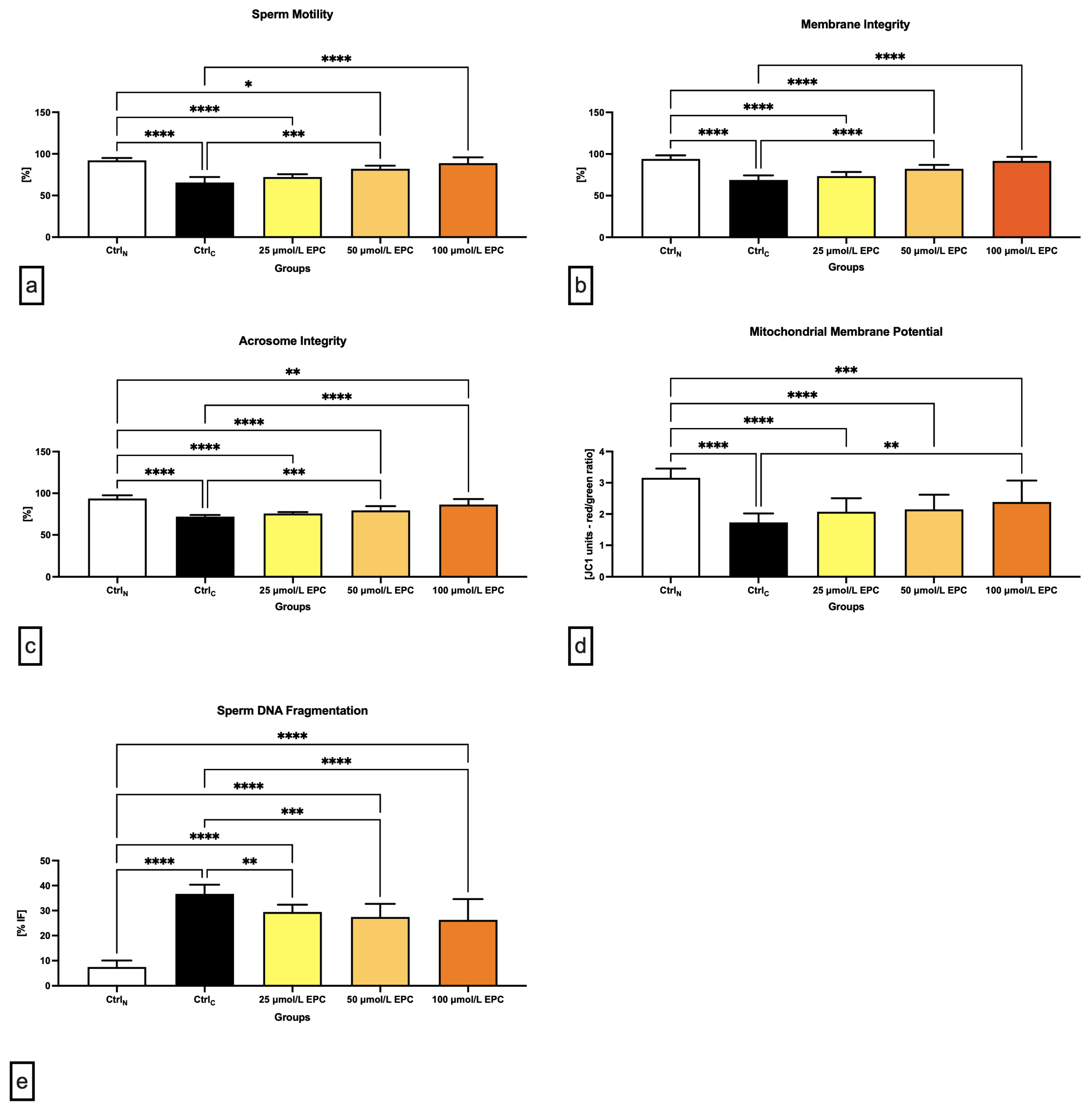
2.1. Conventional Sperm Quality Parameters
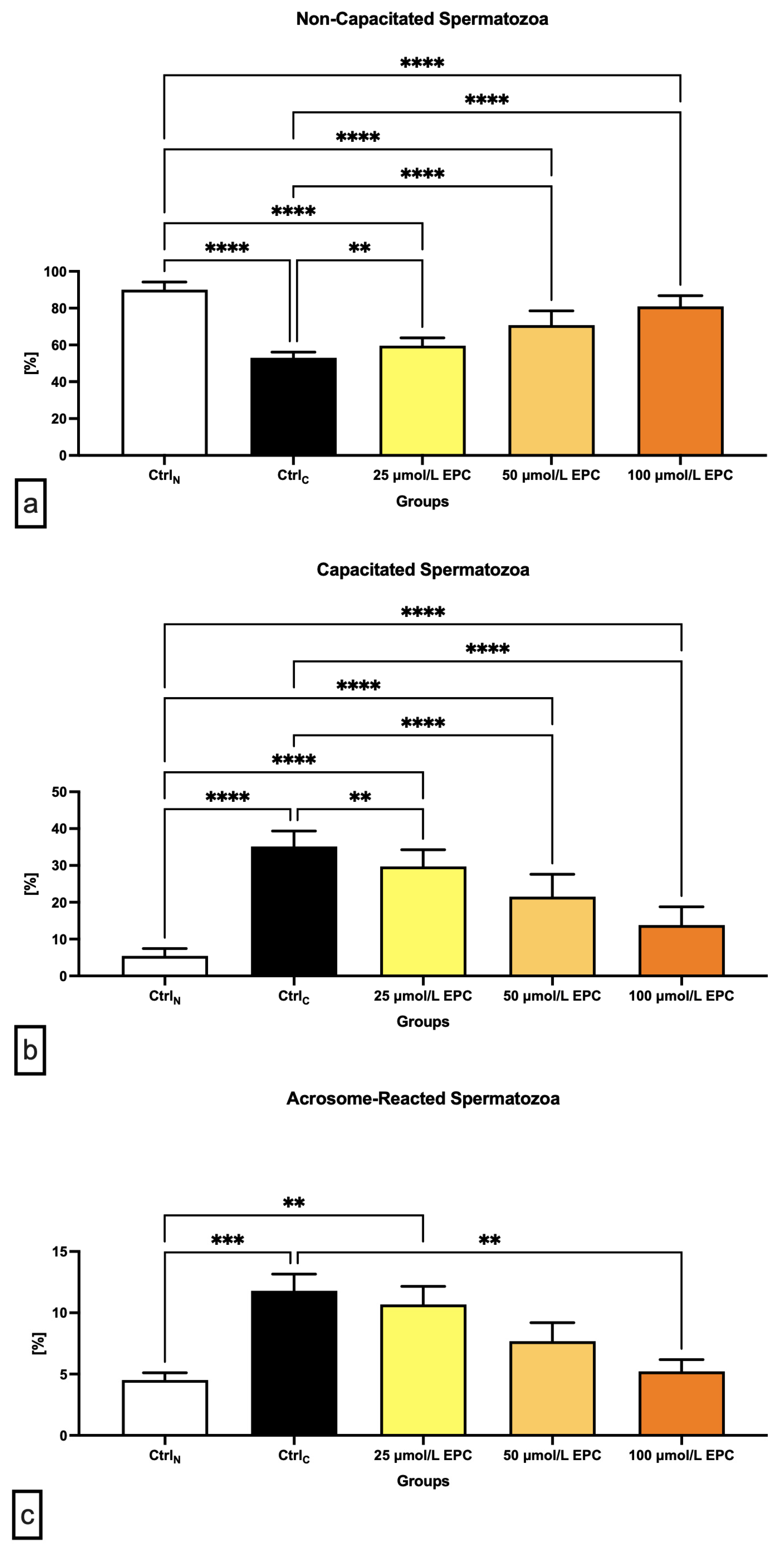
2.2. Capacitation Patterns
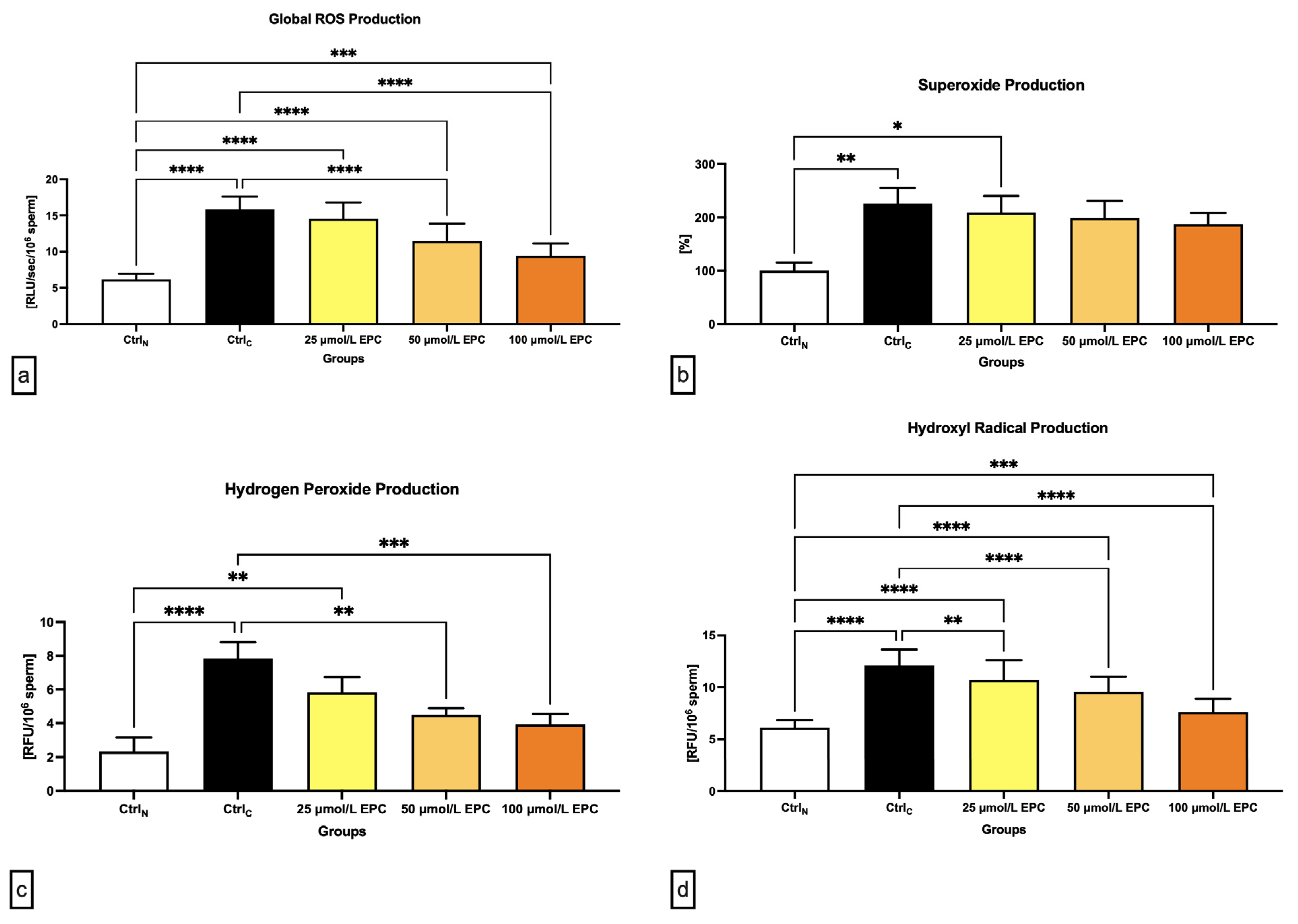
2.3. Oxidative Profile
2.4. Western Blots
3. Discussion
4. Materials and Methods
4.1. Semen Collection and Cryopreservation
4.2. Conventional Sperm Quality Parameters
4.3. Capacitation Patterns
4.4. Oxidative Profile
4.5. Western Blots
4.6. Statistics
- Native control (CtrlN) was compared to the cryopreserved control (CtrlC);
- Experimental groups were compared to both controls.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Curry, M.R. Cryopreservation of semen from domestic livestock. Rev. Reprod. 2000, 5, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, W.; Van Robays, J. Artificial insemination history: Hurdles and milestones. Facts Views Vis. Obgyn. 2015, 7, 137–143. [Google Scholar]
- John Morris, G.; Acton, E.; Murray, B.J.; Fonseca, F. Freezing injury: The special case of the sperm cell. Cryobiology 2012, 64, 71–80. [Google Scholar] [CrossRef]
- Ambar, R.F.; Gava, M.M.; Ghirelli-Filho, M.; Yoshida, I.H.; De Paula, T.S.; Glina, S. Tissue and sperm handling before assisted reproductive technology (ART): A systematic review. Arab J. Urol. 2021, 19, 238–246. [Google Scholar] [CrossRef]
- Vigolo, V.; Giaretta, E.; Da Dalt, L.; Damiani, J.; Gabai, G.; Bertuzzo, F.; Falomo, M.E. Relationships between Biomarkers of Oxidative Stress in Seminal Plasma and Sperm Motility in Bulls before and after Cryopreservation. Animals 2022, 12, 2534. [Google Scholar] [CrossRef]
- Aitken, R.J.; Bromfield, E.G.; Gibb, Z. Oxidative stress and reproductive function: The impact of oxidative stress on reproduction: A focus on gametogenesis and fertilization. Reproduction 2022, 164, F79–F94. [Google Scholar] [CrossRef]
- Arjun, V.; Kumar, P.; Dutt, R.; Kumar, A.; Bala, R.; Verma, N.; Jerome, A.; Virmani, M.; Patil, C.S.; Singh, S.; et al. Is addition or removal of seminal plasma able to compensate for the dilution effect of buffalo semen? Andrologia 2021, 53, e14123. [Google Scholar] [CrossRef]
- Castiglioni, V.C.; Siqueira, A.F.P.; Bicudo, L.C.; de Almeida, T.G.; Hamilton, T.R.D.S.; de Castro, L.S.; Mendes, C.M.; Nichi, M.; Losano, J.D.A.; Visitin, J.A.; et al. Lipid peroxidation in bull semen influences sperm traits and oxidative potential of Percoll®-selected sperm. Zygote 2021, 29, 476–483. [Google Scholar] [CrossRef]
- Tvrda, E.; Straka, P.; Galbavy, D.; Ivanic, P. Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress. Molecules 2019, 24, 3226. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Delgado-Bermúdez, A.; Mateo-Otero, Y.; Viñolas, E.; Hidalgo, C.O.; Ward, W.S.; Yeste, M. Determination of double- and single-stranded DNA breaks in bovine sperm is predictive of their fertilizing capacity. J. Anim. Sci. Biotechnol. 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.A.; Maulana, E.M.; Kaiin, E.M.; Purwantara, B.; Arifiantini, R.I. The quality of frozen semen of Limousin bull in various semen diluetnts. Trop. Anim. Sci. J. 2022, 45, 284–290. [Google Scholar] [CrossRef]
- Benko, F.; Fialková, V.; Žiarovská, J.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A. Int. J. Mol. Sci. 2022, 23, 14646. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Rajoriya, J.S.; Prasad, J.K.; Ramteke, S.S.; Perumal, P.; De, A.K.; Ghosh, S.K.; Bag, S.; Raje, A.; Singh, M.; Kumar, A.; et al. Exogenous cholesterol prevents cryocapacitation-like changes, membrane fluidity, and enhances in vitro fertility in bubaline spermatozoa. Reprod. Domest. Anim. 2020, 55, 726–736. [Google Scholar] [CrossRef]
- Benko, F.; Mohammadi-Sangcheshmeh, A.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In vitro versus cryo-induced capacitation of bovine spermatozoa, part 1: Structural, functional, and oxidative similarities and differences. PLoS ONE 2022, 17, e0276683. [Google Scholar] [CrossRef]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 2016, 169, 2–5. [Google Scholar] [CrossRef]
- Bahmyari, R.; Zare, M.; Sharma, R.; Agarwal, A.; Halvaei, I. The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: A systematic review and meta-analysis. Andrologia 2020, 52, e13514. [Google Scholar] [CrossRef]
- Robles, V.; Valcarce, D.G.; Riesco, M.F. The Use of Antifreeze Proteins in the Cryopreservation of Gametes and Embryos. Biomolecules 2019, 9, 181. [Google Scholar] [CrossRef]
- Mandal, R.; Badyakar, D.; Chakrabarty, J. Role of Membrane Lipid Fatty Acids in Sperm Cryopreservation. Adv. Androl. 2014, 2014, 190542. [Google Scholar] [CrossRef]
- Barakat, I.A.; Danfour, M.A.; Galewan, F.A.; Dkhil, M.A. Effect of various concentrations of caffeine, pentoxifylline, and kallikrein on hyperactivation of frozen bovine semen. BioMed Res. Int. 2015, 2015, 948575. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, S.N.; Malamiri, F.A.; Bossaghzadeh, F.; Esmaeili, A.; Moudi, E. The most important medicinal plants affecting sperm and testosterone production: A systematic review. JBRA Assist. Reprod. 2022, 26, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Roozbeh, N.; Amirian, A.; Abdi, F.; Haghdoost, S. A Systematic Review on Use of Medicinal Plants for Male Infertility Treatment. J. Family Reprod. Health 2021, 15, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The Role of Selected Natural Biomolecules in Sperm Production and Functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef]
- Dos Santos, A.N.; de Nascimento, T.R.L.; Gondim, B.L.C.; Velo, M.M.A.C.; de Rêgo, R.I.A.; do Neto, J.R.C.; Machado, J.R.; da Silva, M.V.; de Araújo, H.W.C.; Fonseca, M.G. Catechins as Model Bioactive Compounds for Biomedical Applications. Curr. Pharm. Des. 2020, 26, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Pirker, K.F.; Baratto, M.C.; Basosi, R.; Goodman, B.A. Influence of pH on the speciation of copper (II) in reactions with the green tea polyphenols, epigallocatechin gallate and gallic acid. J. Inorg. Biochem. 2012, 112, 10–16. [Google Scholar] [CrossRef]
- Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef]
- Rai, A.; Gill, M.; Kinra, M.; Dsouza, L.A.; Sumalatha, S.; Raj, S.; Shetty, R.; Nandakumar, K.; Chamallamudi, M.R.; Kumar, N. Assessment of preclinical effect of (+)-catechin hydrate on sexual function: An in silico and in vivo study. Andrologia 2020, 52, e13737. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, H.; Wu, Z.-B.; Zhao, J.; Zhang, S.; Li, W. Protection of murine spermatogenesis against ionizing radiation-induced testicular injury by a green tea polyphenol. Biol. Reprod. 2015, 114, 122333. [Google Scholar] [CrossRef]
- Abshenas, J.; Babaei, H.; Zare, M.H.; Allahbakhshi, A.; Sharififar, F. The effects of green tea (Camellia sinensis) extract on mouse semen quality after scrotal heat stress. Vet. Res. Forum 2012, 2, 242–247. [Google Scholar]
- Zanchi, M.M.; Manfredini, V.; Dos Santos Brum, D.; Vargas, L.M.; Spiazzi, C.C.; Soares, M.B.; Izaguirry, A.P.; Santos, F.W. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol. Rep. 2015, 2, 252–260. [Google Scholar] [CrossRef]
- Awoniyi, D.O.; Aboua, Y.G.; Marnewick, J.; Brooks, N. The effects of rooibos (Aspalathus linearis), green tea (Camellia sinensis) and commercial rooibos and green tea supplements on epididymal sperm in oxidative stress-induced rats. Phytother. Res. 2012, 26, 1231–1239. [Google Scholar] [CrossRef]
- Silva, E.C.B.; Arruda, L.C.P.; Vieira, J.I.T.; Soares, P.C.; Guerra, M.M.P. (+)-Catechin and (-)-epigallocatechin gallate: Are these promising antioxidant therapies for frozen goat semen? Arq. Bras. Med. Vet. Zootec. 2019, 71, 521–528. [Google Scholar] [CrossRef]
- Boonsorn, T.; Kongbuntad, W.; Narkkong, N.; Aengwanich, W. Effects of catechin addition to extender on sperm quality and lipid peroxidation in boar semen. Am. Eurasian J. Sustain. Agric. 2010, 7, 283–288. [Google Scholar]
- Wittayarat, M.; Ito, A.; Kimura, T.; Namula, Z.; Luu, V.V.; Do, L.T.; Sato, Y.; Taniguchi, M.; Otoi, T. Effects of green tea polyphenol on the quality of canine semen after long-term storage at 5 °C. Reprod. Biol. 2013, 13, 251–254. [Google Scholar] [CrossRef]
- Moretti, E.; Mazzi, L.; Terzuoli, G.; Bonechi, C.; Iacoponi, F.; Martini, S.; Rossi, C.; Collodel, G. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod. Toxicol. 2012, 34, 651–657. [Google Scholar] [CrossRef]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human Sperm Quality and Metal Toxicants: Protective Effects of some Flavonoids on Male Reproductive Function. Int. J. Fertil. Steril. 2016, 10, 215–523. [Google Scholar] [CrossRef]
- Baňas, Š.; Ďuračka, M.; Benko, F.; Žiarovská, J.; Lukáč, N.; Tvrdá, E. Epicatechin improves frozen sperm vitality by its antioxidant and cryoprotective actions. J. Microbiol. Biotech. Food Sci. 2022, 12, e9490. [Google Scholar] [CrossRef]
- De Amicis, F.; Santoro, M.; Guido, C.; Russo, A.; Aquila, S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol. Nutr. Food Res. 2012, 56, 1655–1664. [Google Scholar] [CrossRef]
- Ugur, M.R.; Saber Abdelrahman, A.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in Cryopreservation of Bull Sperm. Front. Vet. Sci. 2019, 6, 268. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, F.E.; Chen, H.C.; Colenbrander, B.; Verkleij, A.J. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology 1990, 27, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E.; Mohey-Elsaeed, O. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. Int. J. Vet. Sci. Med. 2017, 6, S49–S56. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ahmad, N.; Riaz, A.; Anzar, M. Sperm survival kinetics in different types of bull semen: Progressive motility, plasma membrane integrity, acrosomal status and reactive oxygen species generation. Reprod. Fertil. Dev. 2015, 27, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.B.; Egashira, J.; Katafuchi, N.; Endo, K.; Ogata, K.; Yamanaka, K.; Yamanouchi, T.; Matsuda, H.; Hashiyada, Y.; Yamashita, K. Bovine sperm selection procedure prior to cryopreservation for improvement of post-thawed semen quality and fertility. J. Anim. Sci. Biotechnol. 2019, 10, 91. [Google Scholar] [CrossRef]
- Madeja, Z.E.; Podralska, M.; Nadel, A.; Pszczola, M.; Pawlak, P.; Rozwadowska, N. Mitochondria Content and Activity Are Crucial Parameters for Bull Sperm Quality Evaluation. Antioxidants 2021, 10, 1204. [Google Scholar] [CrossRef]
- Trevizan, J.T.; Carreira, J.T.; Carvalho, I.R.; Kipper, B.H.; Nagata, W.B.; Perri, S.H.V.; Franco Oliveira, M.E.; Pierucci, J.C.; Koivisto, M.B. Does lipid peroxidation and oxidative DNA damage differ in cryopreserved semen samples from young, adult and aged Nellore bulls? Anim. Reprod. Sci. 2018, 195, 8–15. [Google Scholar] [CrossRef]
- Benko, F.; Duracka, M.; Lukac, N.; Tvrda, E. Cryocapacitation and its association with oxidative features in cryopreserved bovine spermatozoa. J. Microbiol. Biotech. Food Sci. 2022, 12, e9268. [Google Scholar] [CrossRef]
- Martin, G.; Sabido, O.; Durand, P.; Levy, R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 2004, 71, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Alshawa, E.; Laqqan, M.; Montenarh, M.; Hammadeh, M.E. Influence of cryopreservation on the CATSPER2 and TEKT2 expression levels and protein levels in human spermatozoa. Toxicol. Rep. 2019, 6, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Schmitt, B.M.; Berger, U.V.; Nsumu, N.N.; Boron, W.F.; Hediger, M.A.; Brown, D.; Breton, S. Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol. Reprod. 1999, 60, 573–579. [Google Scholar] [CrossRef]
- Flores, E.; Ramió-Lluch, L.; Bucci, D.; Fernández-Novell, J.M.; Peña, A.; Rodríguez-Gil, J.E. Freezing-thawing induces alterations in histone H1-DNA binding and the breaking of protein-DNA disulfide bonds in boar sperm. Theriogenology 2011, 76, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; White, I.G. Glycolytic enzymes in the spermatozoa and cytoplasmic droplets of bull, boar and ram, and their leakage after shock. J. Reprod. Fertil. 1972, 30, 105–115. [Google Scholar] [CrossRef]
- Lee-Estevez, M.; Herrera, L.; Díaz, R.; Beltrán, J.; Figueroa, E.; Dumorné, K.; Ulloa-Rodríguez, P.; Short, S.; Risopatrón, J.; Valdebenito, I.; et al. Effects of cryopreservation on cAMP-dependent protein kinase and AMP-activated protein kinase in Atlantic salmon (Salmo salar) spermatozoa: Relation with post-thaw motility. Anim. Reprod. Sci. 2019, 209, 106133. [Google Scholar] [CrossRef]
- Feliciello, A.; Gottesman, M.E.; Avvedimento, E.V. The biological functions of A-kinase anchor proteins. J. Mol. Biol. 2001, 308, 99–114. [Google Scholar] [CrossRef]
- Stival, C.; Ritagliati, C.; Xu, X.; Gervasi, M.G.; Luque, G.M.; Baró Graf, C.; De la Vega-Beltrán, J.L.; Torres, N.; Darszon, A.; Krapf, D.; et al. Disruption of protein kinase A localization induces acrosomal exocytosis in capacitated mouse sperm. J. Biol. Chem. 2018, 293, 9435–9447. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence-based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Silvestre, M.A.; Yániz, J.L.; Peña, F.J.; Santolaria, P.; Castelló-Ruiz, M. Role of Antioxidants in Cooled Liquid Storage of Mammal Spermatozoa. Antioxidants 2021, 10, 1096. [Google Scholar] [CrossRef]
- Al-Mutary, M.G. Use of antioxidants to augment semen efficiency during liquid storage and cryopreservation in livestock animals: A review. J. King Saud Univ. Sci. 2021, 33, 101226. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, H.; Liu, C.; Huang, J.; Liu, Z. A review for physiological activities of EGCG and the role in improving fertility in humans/mammals. Biomed. Pharmacother. 2020, 127, 110186. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox. Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef]
- Uekusa, Y.; Kamihira, M.; Nakayama, T. Dynamic behavior of tea catechins interacting with lipid membranes as determined by NMR spectroscopy. J. Agric. Food Chem. 2007, 55, 9986–9992. [Google Scholar] [CrossRef]
- Greifova, H.; Tvrda, E.; Jambor, T.; Lukac, N. Dose- and time-dependent effects of epicatechin on bovine spermatozoa in vitro. J. Microbiol. Biotech. Food Sci. 2018, 2, 235–239. [Google Scholar] [CrossRef]
- Purdy, P.H.; Ericsson, S.A.; Dodson, R.E.; Sternes, K.L.; Garner, D.L. Effects of flavonoids, silibinin and catechin, on the motility of extended cooled caprine sperm. Small Rumin. Res. 2004, 55, 239–243. [Google Scholar] [CrossRef]
- Heo, S.; Kim, S.; Kang, D. The Role of Hydrogen Peroxide and Peroxiredoxins throughout the Cell Cycle. Antioxidants 2020, 9, 280. [Google Scholar] [CrossRef]
- Tejero, I.; Gonzalez-Lafont, A.; Lluch, J.M.; Eriksson, L.A. Theoretical modeling of hydroxyl-radical-induced lipid peroxidation reactions. J. Phys. Chem. B. 2007, 111, 5684–5693. [Google Scholar] [CrossRef]
- Zarkovic, N. Antioxidants and Second Messengers of Free Radicals. Antioxidants 2018, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.; Cotter, T. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death. Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.A.; Amit, T.; Kalfon, L.; Reznichenko, L.; Weinreb, O.; Youdim, M.B. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: Special reference to epigallocatechin gallate (EGCG). J. Alzheimers Dis. 2008, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sapanidou, V.G.; Margaritis, I.; Siahos, N.; Arsenopoulos, K.; Dragatidou, E.; Taitzoglou, I.A.; Zervos, I.A.; Theodoridis, A.; Tsantarliotou, M.P. Antioxidant effect of a polyphenol-rich grape pomace extract on motility, viability and lipid peroxidation of thawed bovine spermatozoa. J. Biol. Res. 2014, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Gale, I.; Gil, L.; Malo, C.; González, N.; Martínez, F. Effect of Camellia sinensis supplementation and increasing holding time on quality of cryopreserved boar semen. Andrologia 2015, 47, 505–512. [Google Scholar] [CrossRef]
- Naaby-Hansen, S.; Herr, J.C. Heat shock proteins on the human sperm surface. J. Reprod. Immunol. 2010, 84, 32–40. [Google Scholar] [CrossRef]
- Degenhardt, K.; Sundararajan, R.; Lindsten, T.; Thompson, C.; White, E. Bax and Bak independently promote cytochrome C release from mitochondria. J. Biol. Chem. 2002, 277, 14127–14134. [Google Scholar] [CrossRef]
- Spinaci, M.; Volpe, S.; De Ambrogi, M.; Tamanini, C.; Galeati, G. Effects of epigallocatechin-3-gallate (EGCG) on in vitro maturation and fertilization of porcine oocytes. Theriogenology 2008, 69, 877–885. [Google Scholar] [CrossRef]
- Paál, D.; Strejček, F.; Tvrdá, E.; Formicki, G.; Klein, S.; Rath, D.; Massanyi, P. The in Vitro Effect of Taurine on Boar Spermatozoa Quality. Acta Univ. Agric. Silvic. Mendelianae Brun. 2018, 66, 131–137. [Google Scholar] [CrossRef]
- Ďuračka, M.; Husarčíková, K.; Jančov, M.; Galovičová, L.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Staphylococcus-Induced Bacteriospermia In Vitro: Consequences on the Bovine Spermatozoa Quality, Extracellular Calcium and Magnesium Content. Animals 2021, 11, 3309. [Google Scholar] [CrossRef]

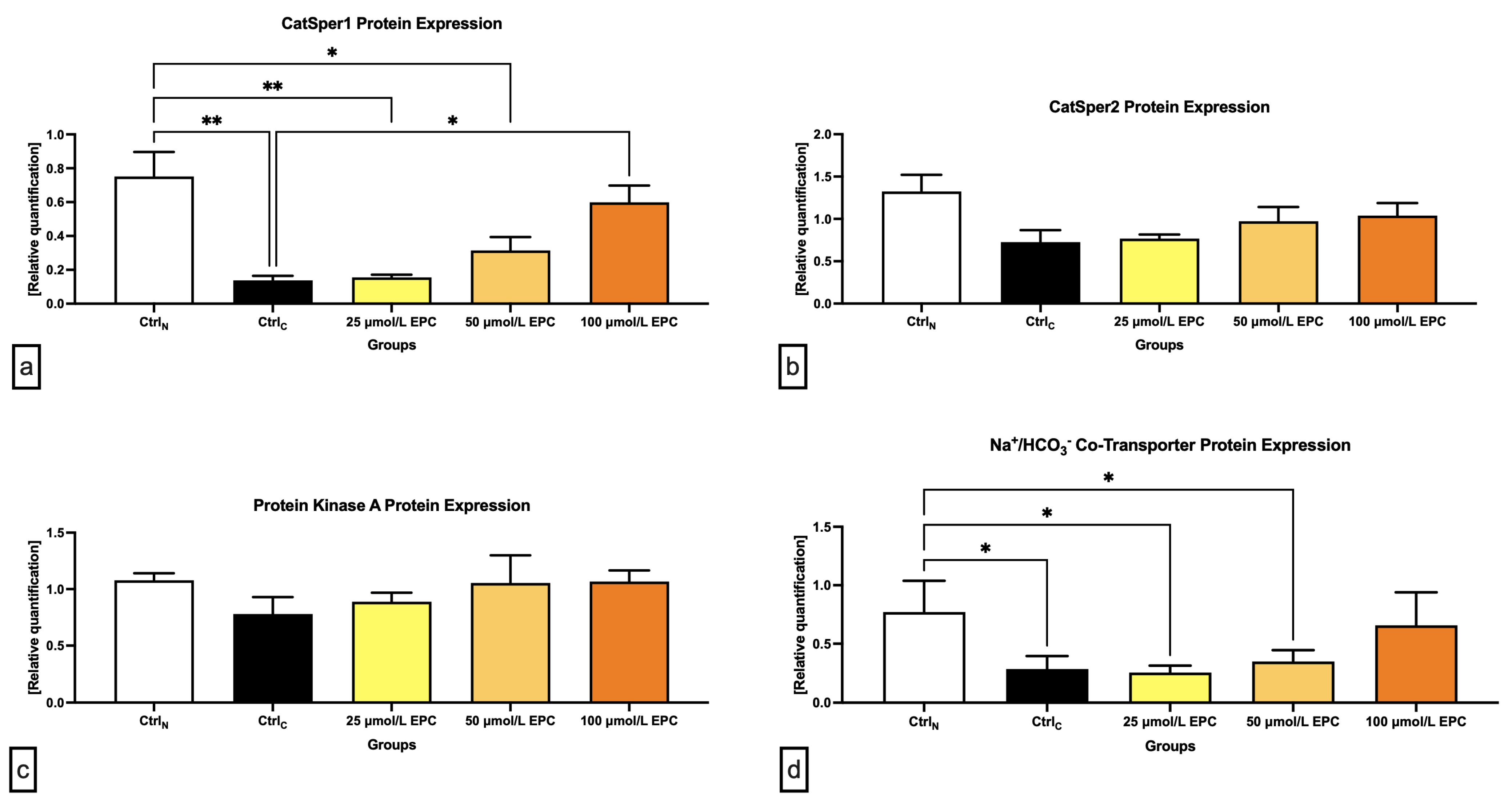
| Target Protein | Antibody | Source | Clonality/Isotype | Dilution | Blocking Solution | Cat. # | Manufacturer |
|---|---|---|---|---|---|---|---|
| CatSper1 | CATSPER1 Polyclonal Antibody | rabbit | Polyclonal/IgG | 1:1000 | 5% milk/TBS/0.1% Tween-20 | PA5-75788 | Invitrogen, Waltham, MA, USA |
| CatSper2 | CATSPER2 Polyclonal Antibody | rabbit | Polyclonal/IgG | 1:1000 | 5% milk/TBS/0.1% Tween-20 | PA5-41072 | Invitrogen, Waltham, MA, USA |
| NBC | Anti-Na+/HCO3− Contransporter Polyclonal Antibody | rabbit | Polyclonal/IgG | 1:500 | 5% milk/TBS/0.1% Tween-20 | AB3212-I | Merck Millipore, Temecula, CA, USA |
| PKA | PKA alpha Antibody | rabbit | Polyclonal/IgG | 1:1000 | 5% milk/TBS/0.1% Tween-20 | PA5-17626 | Invitrogen, Waltham, MA, USA |
| β-actin | beta Actin Polyclonal Antibody | rabbit | Polyclonal/IgG | 1:1000 | 5% milk/TBS/0.1% Tween-20 | PA1-46296 | Invitrogen, Waltham, MA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels. Int. J. Mol. Sci. 2023, 24, 2510. https://doi.org/10.3390/ijms24032510
Baňas Š, Benko F, Ďuračka M, Lukáč N, Tvrdá E. Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels. International Journal of Molecular Sciences. 2023; 24(3):2510. https://doi.org/10.3390/ijms24032510
Chicago/Turabian StyleBaňas, Štefan, Filip Benko, Michal Ďuračka, Norbert Lukáč, and Eva Tvrdá. 2023. "Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels" International Journal of Molecular Sciences 24, no. 3: 2510. https://doi.org/10.3390/ijms24032510
APA StyleBaňas, Š., Benko, F., Ďuračka, M., Lukáč, N., & Tvrdá, E. (2023). Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels. International Journal of Molecular Sciences, 24(3), 2510. https://doi.org/10.3390/ijms24032510








