Metabolic Signature of Energy Metabolism Alterations and Excess Nitric Oxide Production in Culture Media Correlate with Low Human Embryo Quality and Unsuccessful Pregnancy
Abstract
1. Introduction
2. Results
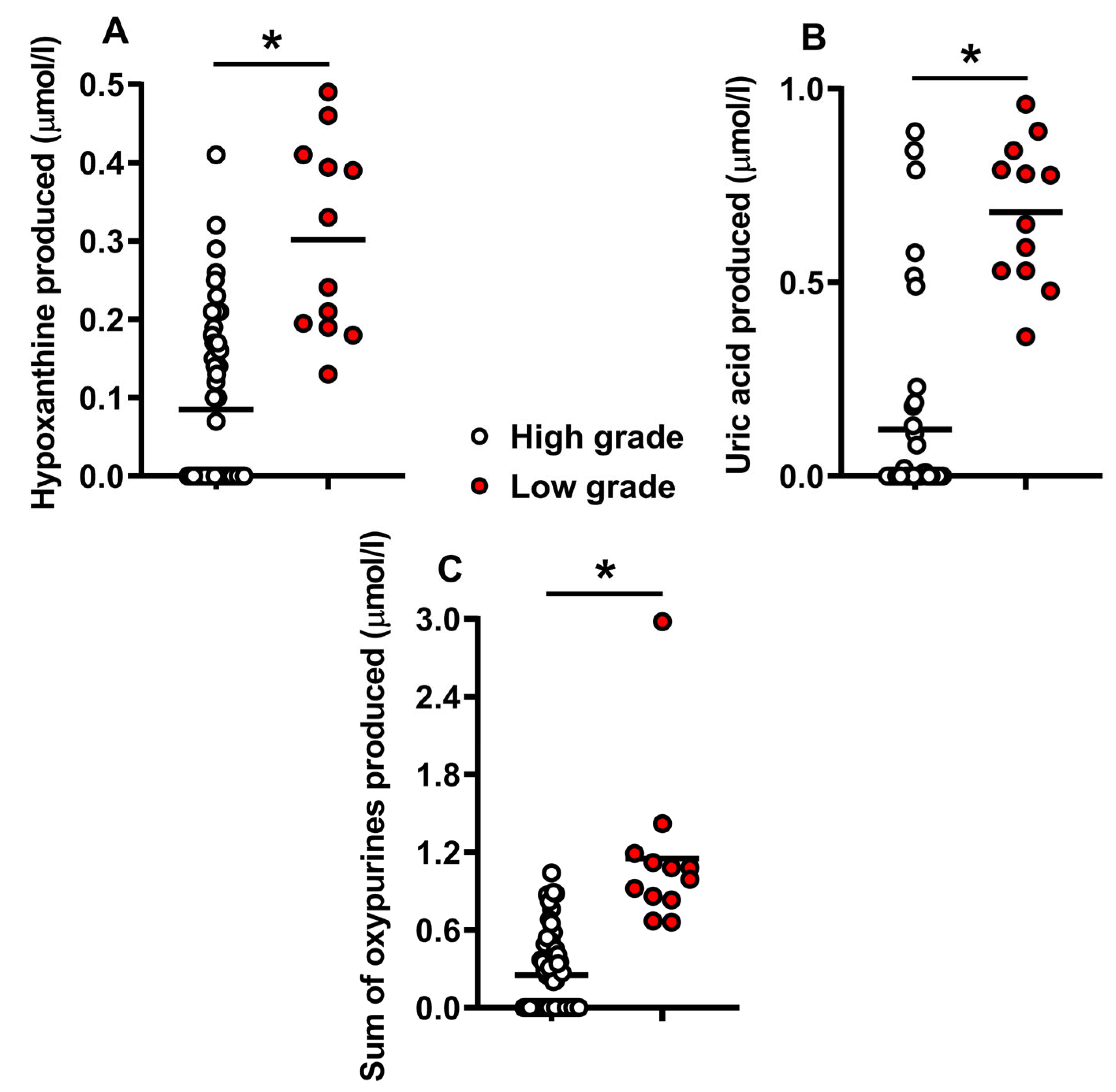
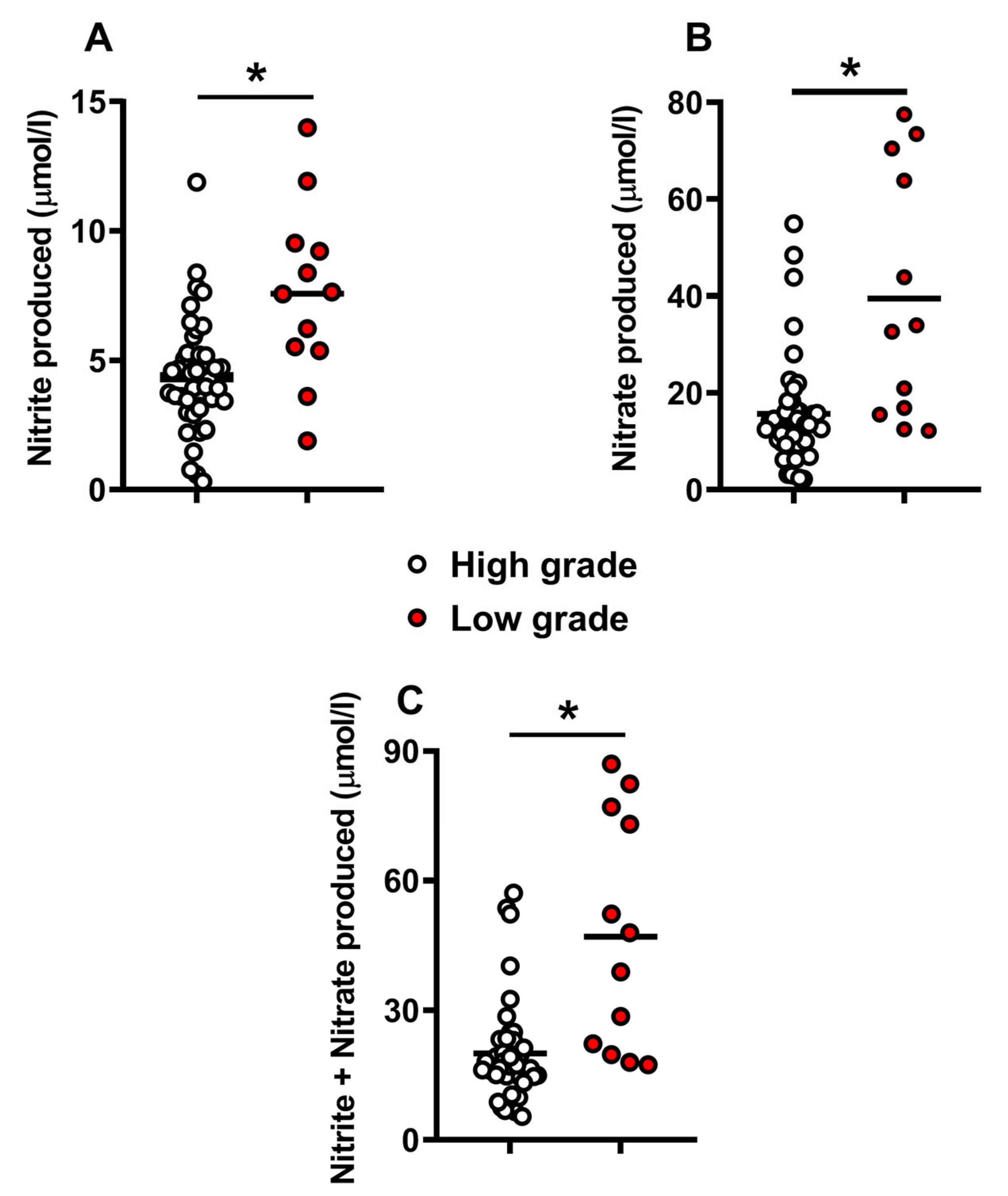
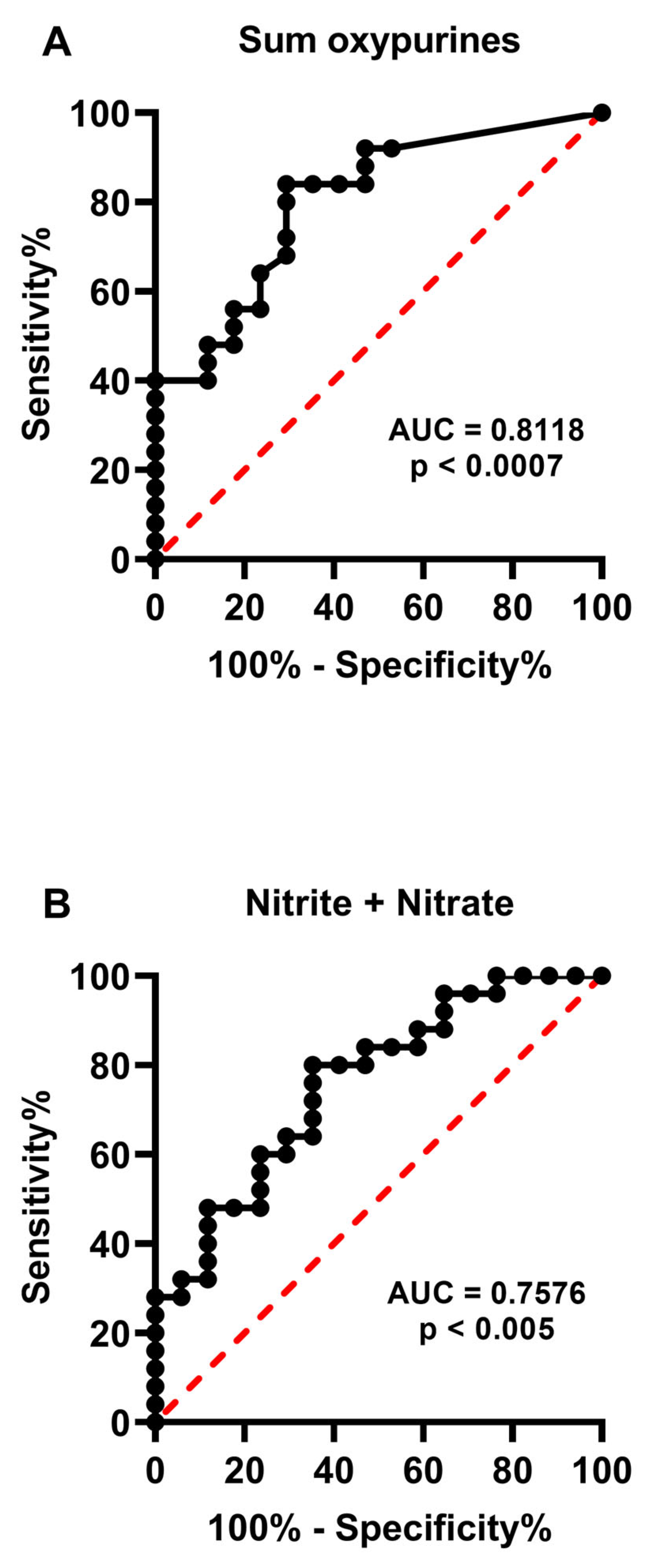
2.1. Metabolites in the Culture Media Correlate with Morphological Human Embryo Quality
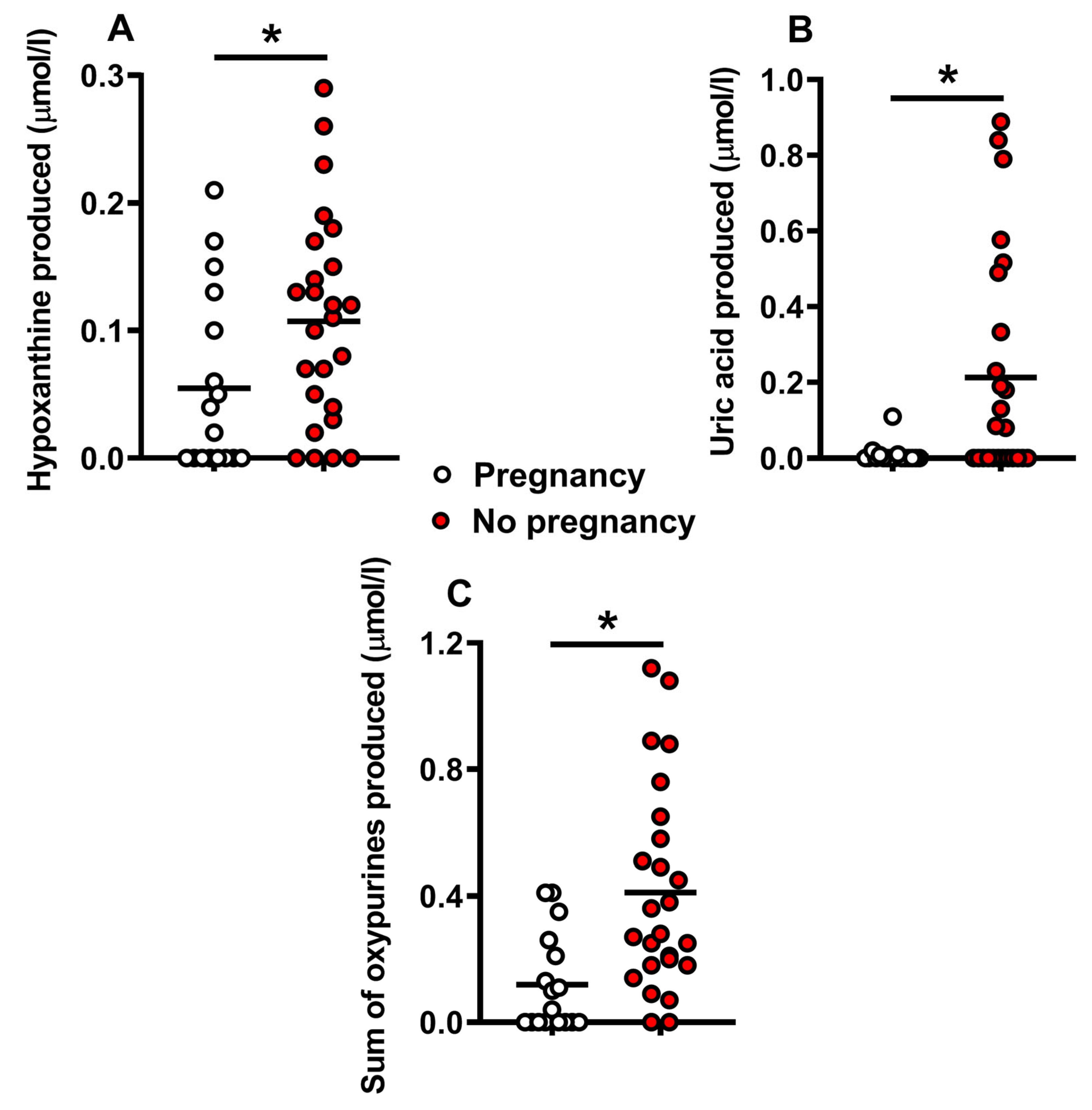
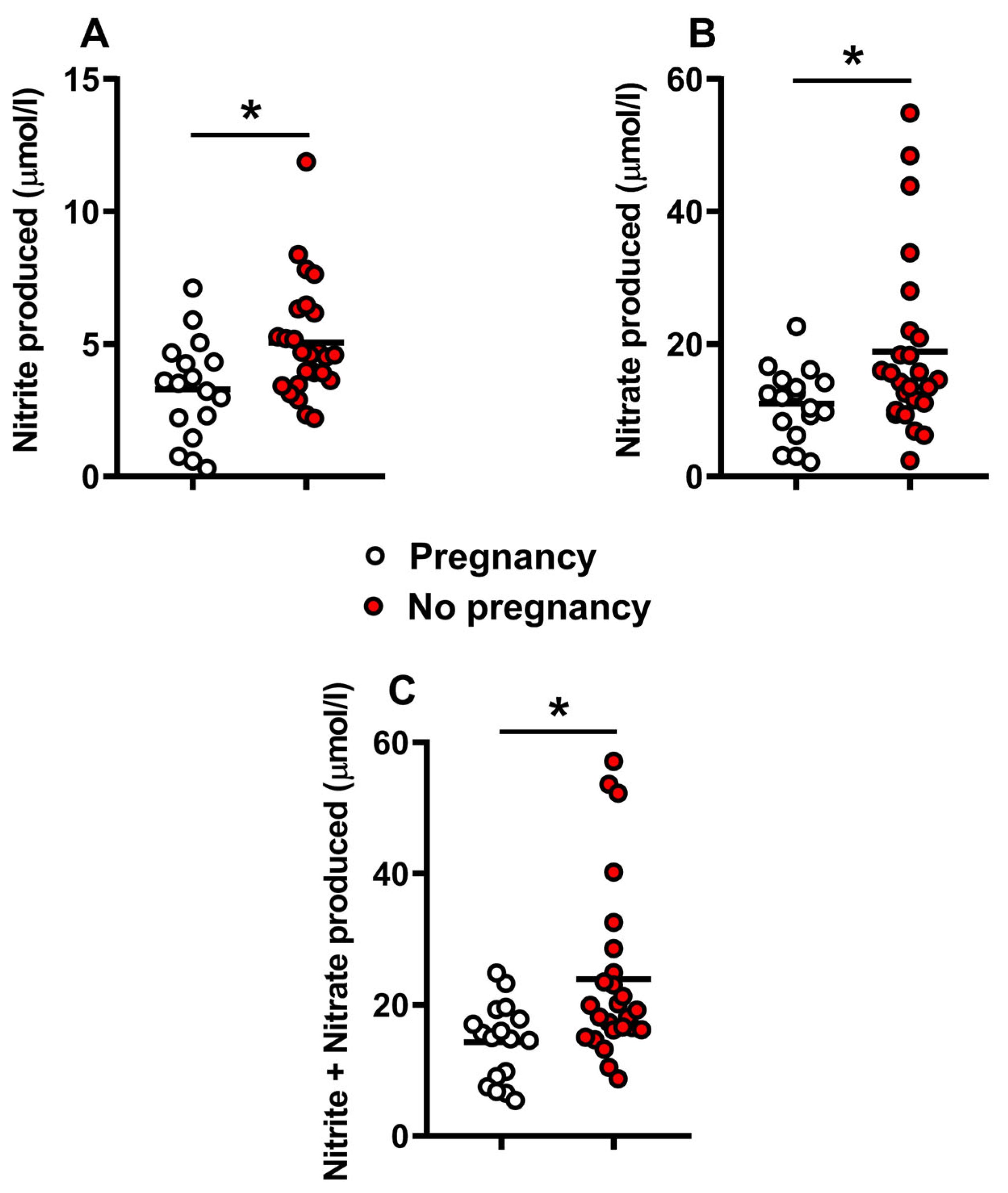
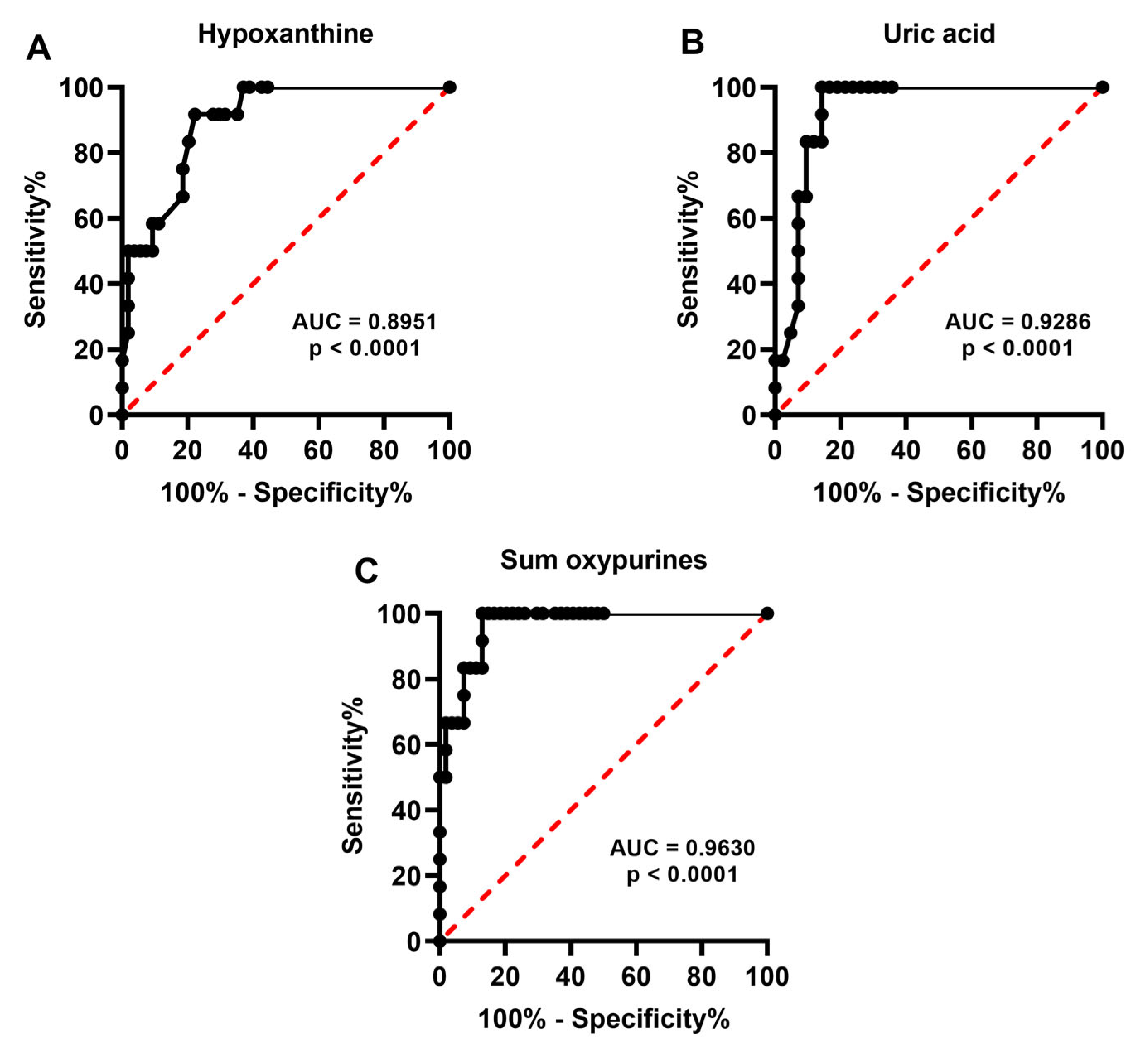
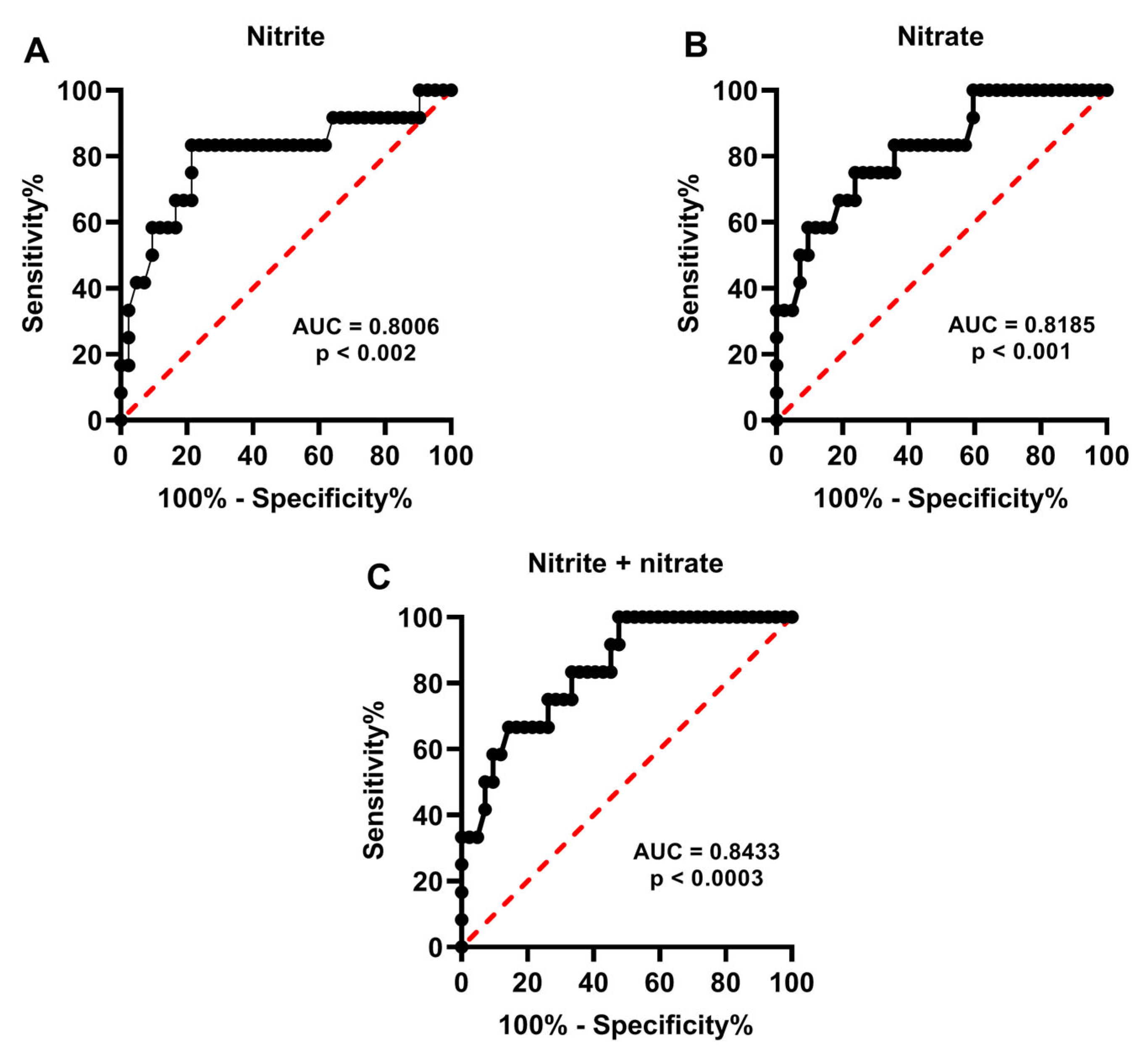
2.2. Metabolite Levels in the Culture Media Correlate with Successful Pregnancy and Live Births
2.3. Release of Catabolites in the Culture Media May Be Useful to Biochemically Grade Embryo Quality and to Select the Best Embryos for Implantation
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics and Protocols for Ovarian Stimulation
4.2. Fertilization Procedures, Embryo Culture, Evaluation of Biochemical Pregnancy, Clinical Pregnancy, and Birth Rate
4.3. Biochemical Analysis of Embryo Culture Media
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iss.it/documents/20126/0/14+Report_2020_dati+PMA_2018+%281%29.pdf/e956ec84-675b-6d73-8a92-ba73d8a95c28?t=1606929156366 (accessed on 29 September 2022).
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Rubio, I.; Gala´n, A.; Larreategui, Z.; Ayerdi, F.; Bellver, J.; Herrero, J.; Meseguer, M. Clinical validation of embryo culture and selection by morphokinetic analysis: A randomized, controlled trial of the EmbryoScope. Fertil. Steril. 2014, 102, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Basile, N.; Vime, P.; Florensa, M.; Aparicio Ruiz, B.; Garcia Velasco, J.A.; Remohi, J.; Meseguer, M. The use of morphokinetics as a predictor of implantation: A multicentric study to define and validate an algorithm for embryo selection. Hum. Reprod. 2015, 30, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Gott, A.; Hardy, K.; Winston, R.; Leese, H. Non-invasive measurement of pyruvate and glucose uptake and lactate production by single human preimplantation embryos. Hum. Reprod. 1990, 5, 104–108. [Google Scholar] [CrossRef]
- Guerif, F.; McKeegan, P.; Leese, H.J.; Sturmey, R.G. A simple approach for COnsumption and RElease (CORE) analysis of metabolic activity in single mammalian embryos. PLoS ONE 2013, 8, e67834. [Google Scholar] [CrossRef]
- Gardner, D.K.; Wale, P.L.; Collins, R.; Lane, M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum. Reprod. 2011, 26, 1981–1986. [Google Scholar] [CrossRef]
- Brison, D.R.; Houghton, F.D.; Falconer, D.; Roberts, S.A.; Hawkhead, J.; Humpherson, P.G.; Lieberman, B.A.; Leese, H.J. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum. Reprod. 2004, 19, 2319–2324. [Google Scholar] [CrossRef]
- Picton, H.M.; Elder, K.; Houghton, F.D.; Hawkhead, J.A.; Rutherford, A.J.; Hogg, J.E.; Leese, H.J.; Harris, S.E. Association between amino acid turnover and chromosome aneuploidy during human preimplantation embryo development in vitro. Mol. Hum. Reprod. 2010, 16, 557–569. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Bultman, S.J. Metaboloepigenetics: Interrelationships between energy metabolism and epigenetic control of gene expression. J. Cell Physiol. 2012, 227, 3169–3177. [Google Scholar] [CrossRef]
- Rambo, L.M.; Ribeiro, L.R.; Della-Pace, I.D.; Stamm, D.N.; da Rosa Gerbatin, R.; Prigol, M.; Pinton, S.; Nogueira, C.W.; Furian, A.F.; Oliveira, M.S.; et al. Acute creatine administration improves mitochondrial membrane potential and protects against pentylenetetrazol-induced seizures. Amino Acids 2013, 44, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Bracko, O.; Di Pietro, V.; Lazzarino, G.; Amorini, A.M.; Tavazzi, B.; Artmann, J.; Wong, E.C.; Buxton, R.B.; Weller, M.; Luft, A.R.; et al. 3-Nitropropionic acid-induced ischemia tolerance in the rat brain is mediated by reduced metabolic activity and cerebral blood flow. J. Cereb. Blood Flow Metab. 2014, 34, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Yakoub, K.M.; Lazzarino, G.; Amorini, A.M.; Caruso, G.; Scazzone, C.; Ciaccio, M.; Tavazzi, B.; Lazzarino, G.; Belli, A.; Di Pietro, V. Fructose-1,6-Bisphosphate Protects Hippocampal Rat Slices from NMDA Excitotoxicity. Int. J. Mol. Sci. 2019, 20, 2239. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Amorini, A.M.; Petzold, A.; Gasperini, C.; Ruggieri, S.; Quartuccio, M.E.; Lazzarino, G.; Di Stasio, E.; Tavazzi, B. Serum Compounds of Energy Metabolism Impairment Are Related to Disability, Disease Course and Neuroimaging in Multiple Sclerosis. Mol. Neurobiol. 2017, 54, 7520–7533. [Google Scholar] [CrossRef]
- Tavazzi, B.; Vagnozzi, R.; Signoretti, S.; Amorini, A.M.; Belli, A.; Cimatti, M.; Delfini, R.; Di Pietro, V.; Finocchiaro, A.; Lazzarino, G. Temporal window of metabolic brain vulnerability to concussions: Oxidative and nitrosative stresses--part II. Neurosurgery 2007, 61, 390–395. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef]
- Lazzarino, G.; Pallisco, R.; Bilotta, G.; Listorti, I.; Mangione, R.; Saab, M.W.; Caruso, G.; Amorini, A.M.; Brundo, M.V.; Lazzarino, G.; et al. Altered Follicular Fluid Metabolic Pattern Correlates with Female Infertility and Outcome Measures of In Vitro Fertilization. Int. J. Mol. Sci. 2021, 22, 8735. [Google Scholar] [CrossRef]
- Abd-Elfattah, A.S.; Jessen, M.E.; Lekven, J.; Wechsler, A.S. Differential cardioprotection with selective inhibitors of adenosine metabolism and transport: Role of purine release in ischemic and reperfusion injury. Mol. Cell Biochem. 1998, 180, 179–191. [Google Scholar] [CrossRef]
- Lee, W.H.; Gounarides, J.S.; Roos, E.S.; Wolin, M.S. Influence of peroxynitrite on energy metabolism and cardiac function in a rat ischemia-reperfusion model. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1385–H1395. [Google Scholar] [CrossRef]
- Lazzarino, G.; Raatikainen, P.; Nuutinen, M.; Nissinen, J.; Tavazzi, B.; Di Pierro, D.; Giardina, B.; Peuhkurinen, K. Myocardial release of malondialdehyde and purine compounds during coronary bypass surgery. Circulation 1994, 90, 291–297. [Google Scholar] [CrossRef]
- Igaki, Y.; Tanno, M.; Sato, T.; Kouzu, H.; Ogawa, T.; Osanami, A.; Yano, T.; Kuno, A.; Miki, T.; Nakamura, T.; et al. Xanthine oxidoreductase-mediated injury is amplified by upregulated AMP deaminase in type 2 diabetic rat hearts under the condition of pressure overload. J. Mol. Cell Cardiol. 2021, 154, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, E.; Diviney, T.; FitzGerald, U. Dysregulation of astrocytic mitochondrial function following exposure to a dopamine metabolite: Implications for Parkinson’s disease. Eur. J. Neurosci. 2021, 53, 2960–2972. [Google Scholar] [CrossRef] [PubMed]
- Strifler, G.; Tuboly, E.; Görbe, A.; Boros, M.; Pécz, D.; Hartmann, P. Targeting Mitochondrial Dysfunction with L-Alpha Glycerylphosphorylcholine. PLoS ONE 2016, 11, e0166682. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Chakraborty, A.; Wijesinghe, M.B.; Amorini, A.M.; Lazzarino, G.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Caraci, F.; Dhar, P.; et al. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Dis. 2018, 9, 245. [Google Scholar] [CrossRef]
- Zhang, G.; Han, H.; Zhuge, Z.; Dong, F.; Jiang, S.; Wang, W.; Guimarães, D.D.; Schiffer, T.A.; Lai, E.Y.; Ribeiro Antonino Carvalho, L.R.; et al. Renovascular effects of inorganic nitrate following ischemia-reperfusion of the kidney. Redox Biol. 2021, 39, 101836. [Google Scholar] [CrossRef]
- Fiorello, M.L.; Treweeke, A.T.; Macfarlane, D.P.; Megson, I.L. The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes. Sci. Rep. 2020, 10, 19547. [Google Scholar] [CrossRef]
- Laranjinha, J.; Nunes, C.; Ledo, A.; Lourenço, C.; Rocha, B.; Barbosa, R.M. The Peculiar Facets of Nitric Oxide as a Cellular Messenger: From Disease-Associated Signaling to the Regulation of Brain Bioenergetics and Neurovascular Coupling. Neurochem. Res. 2021, 46, 64–76. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schoolcraft, W.B. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil. Steril. 2001, 76, 1175–1180. [Google Scholar] [CrossRef]
- Leese, H.J.; Barton, A.M. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J. Reprod. Fertil. 1984, 72, 9–13. [Google Scholar] [CrossRef]
- Lee, Y.S.L.; Thouas, G.A.; Gardner, D.K. Developmental kinetics of cleavage stage mouse embryos are related to their subsequent carbohydrate and amino acid utilization at the blastocyst stage. Hum. Reprod. 2015, 30, 543–552. [Google Scholar] [CrossRef]
- Wale, P.L.; Gardner, D.K. Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol. Reprod. 2012, 87, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 1998, 49, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D.; Hawkhead, J.A.; Humpherson, P.G.; Hogg, J.E.; Balen, A.H.; Rutherford, A.J.; Leese, H.J. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum. Reprod. 2002, 17, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Ferrick, L.; Lee, Y.S.L.; Gardner, D.K. Metabolic activity of human blastocysts correlates with their morphokinetics, morphological grade, KIDScore and artificial intelligence ranking. Hum. Reprod. 2020, 35, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Racowsky, C.; Stern, J.E.; Gibbons, W.E.; Behr, B.; Pomeroy, K.O.; Biggers, J.D. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: Associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil. Steril. 2011, 95, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yang, S.; Gong, F.; Lu, C.; Zhang, S.; Lu, G.; Lin, G. The relationship between cell number, division behavior and developmental potential of cleavage stage human embryos: A time-lapse study. PLoS ONE 2016, 11, e0153697. [Google Scholar] [CrossRef]
- Nanni, S.; Aiello, A.; Salis, C.; Re, A.; Cencioni, C.; Bacci, L.; Pierconti, F.; Pinto, F.; Ripoli, C.; Ostano, P.; et al. Metabolic Reprogramming by Malat1 Depletion in Prostate Cancer. Cancers 2020, 13, 15. [Google Scholar] [CrossRef]
- Ciccarone, F.; Di Leo, L.; Lazzarino, G.; Maulucci, G.; Di Giacinto, F.; Tavazzi, B.; Ciriolo, M.R. Aconitase 2 inhibits the proliferation of MCF-7 cells promoting mitochondrial oxidative metabolism and ROS/FoxO1-mediated autophagic response. Br. J. Cancer 2020, 122, 182–193. [Google Scholar] [CrossRef]
- Giallongo, C.; Tibullo, D.; Camiolo, G.; Parrinello, N.L.; Romano, A.; Puglisi, F.; Barbato, A.; Conticello, C.; Lupo, G.; Anfuso, C.D.; et al. TLR4 signaling drives mesenchymal stromal cells commitment to promote tumor microenvironment transformation in multiple myeloma. Cell Death Dis. 2019, 10, 704. [Google Scholar] [CrossRef]
- Lazzarino, G.; Mangione, R.; Belli, A.; Di Pietro, V.; Nagy, Z.; Barnes, N.M.; Bruce, L.; Ropero, B.M.; Persson, L.I.; Manca, B.; et al. ILB® Attenuates Clinical Symptoms and Serum Biomarkers of Oxidative/Nitrosative Stress and Mitochondrial Dysfunction in Patients with Amyotrophic Lateral Sclerosis. J. Pers. Med. 2021, 11, 794. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Mangione, R.; Saab, M.W.; Di Stasio, E.; Di Rosa, M.; Tavazzi, B.; Lazzarino, G.; Onder, G.; Carfì, A. Biochemical Discrimination of the Down Syndrome-Related Metabolic and Oxidative/Nitrosative Stress Alterations from the Physiologic Age-Related Changes through the Targeted Metabolomic Analysis of Serum. Antioxidants 2022, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallisco, R.; Lazzarino, G.; Bilotta, G.; Marroni, F.; Mangione, R.; Saab, M.W.; Brundo, M.V.; Pittalà, A.; Caruso, G.; Capoccia, E.; et al. Metabolic Signature of Energy Metabolism Alterations and Excess Nitric Oxide Production in Culture Media Correlate with Low Human Embryo Quality and Unsuccessful Pregnancy. Int. J. Mol. Sci. 2023, 24, 890. https://doi.org/10.3390/ijms24010890
Pallisco R, Lazzarino G, Bilotta G, Marroni F, Mangione R, Saab MW, Brundo MV, Pittalà A, Caruso G, Capoccia E, et al. Metabolic Signature of Energy Metabolism Alterations and Excess Nitric Oxide Production in Culture Media Correlate with Low Human Embryo Quality and Unsuccessful Pregnancy. International Journal of Molecular Sciences. 2023; 24(1):890. https://doi.org/10.3390/ijms24010890
Chicago/Turabian StylePallisco, Romina, Giacomo Lazzarino, Gabriele Bilotta, Francesca Marroni, Renata Mangione, Miriam Wissam Saab, Maria Violetta Brundo, Alessandra Pittalà, Giuseppe Caruso, Elena Capoccia, and et al. 2023. "Metabolic Signature of Energy Metabolism Alterations and Excess Nitric Oxide Production in Culture Media Correlate with Low Human Embryo Quality and Unsuccessful Pregnancy" International Journal of Molecular Sciences 24, no. 1: 890. https://doi.org/10.3390/ijms24010890
APA StylePallisco, R., Lazzarino, G., Bilotta, G., Marroni, F., Mangione, R., Saab, M. W., Brundo, M. V., Pittalà, A., Caruso, G., Capoccia, E., Lazzarino, G., Tavazzi, B., Bilotta, P., & Amorini, A. M. (2023). Metabolic Signature of Energy Metabolism Alterations and Excess Nitric Oxide Production in Culture Media Correlate with Low Human Embryo Quality and Unsuccessful Pregnancy. International Journal of Molecular Sciences, 24(1), 890. https://doi.org/10.3390/ijms24010890











