The Comprehensive Steroidome in Complete TSPO/PBR Knockout Mice under Basal Conditions
Abstract
1. Introduction
- (i)
- TSPO is indispensable for the transfer of cholesterol into mitochondria and, thus, is a prerequisite for the generation of pregnenolone (PREG);
- (ii)
- (iii)
- Reduced plasma PREG concentration following tspo mutations in rats [6], reduced intratesticular and circulating testosterone levels in Tspo Amhr2-Cre-mediated global TSPO-KO mice [12] and unchanged levels of adrenal corticosterone following adrenocorticotropic hormone treatment in conditional TSPO-KO mice [13];
- (iv)
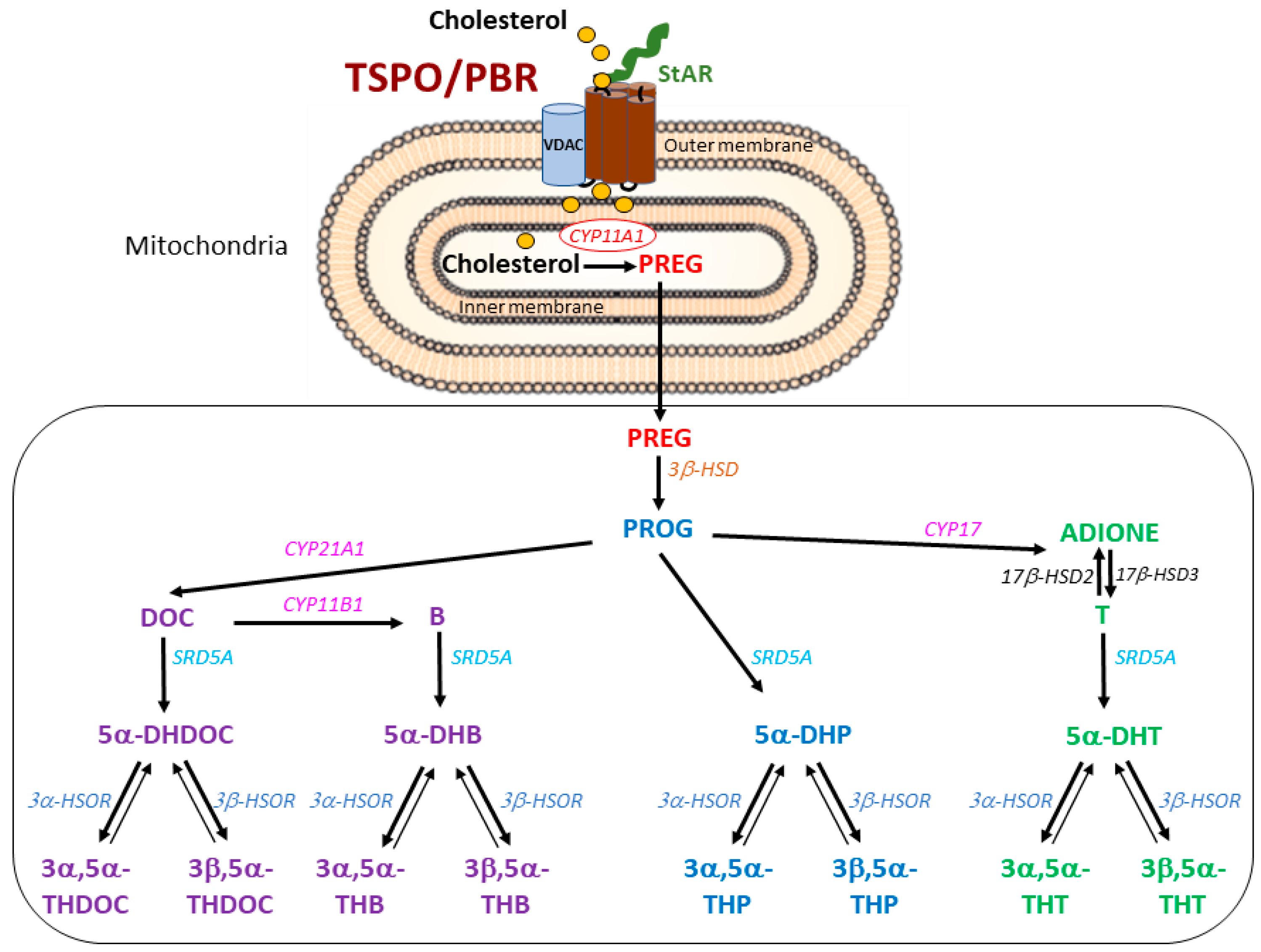
2. Results
2.1. Steroid Measurements in Wild-Type and TSPO-KO Mice under Basal Conditions
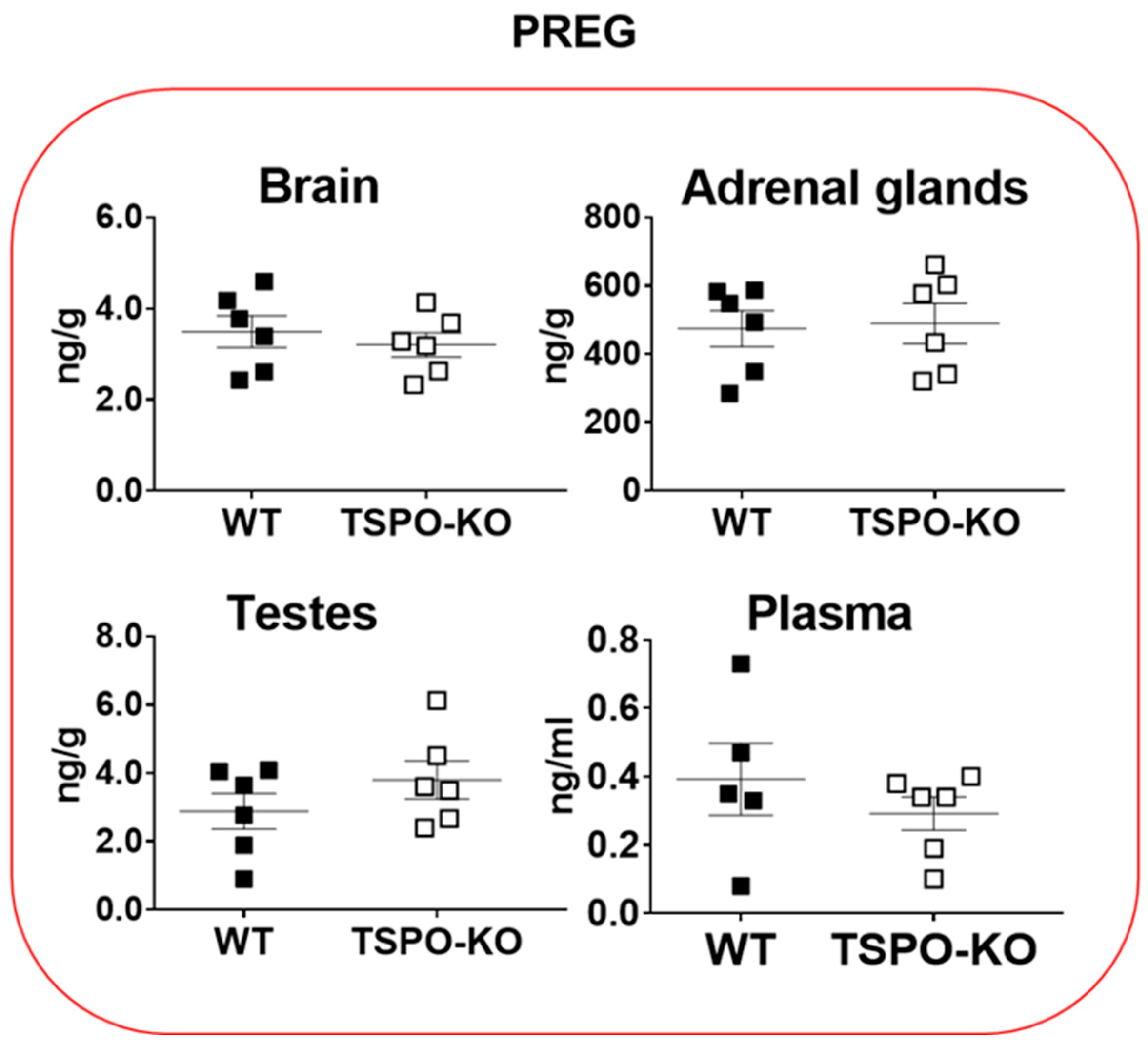
2.1.1. Basal Concentrations of PREG
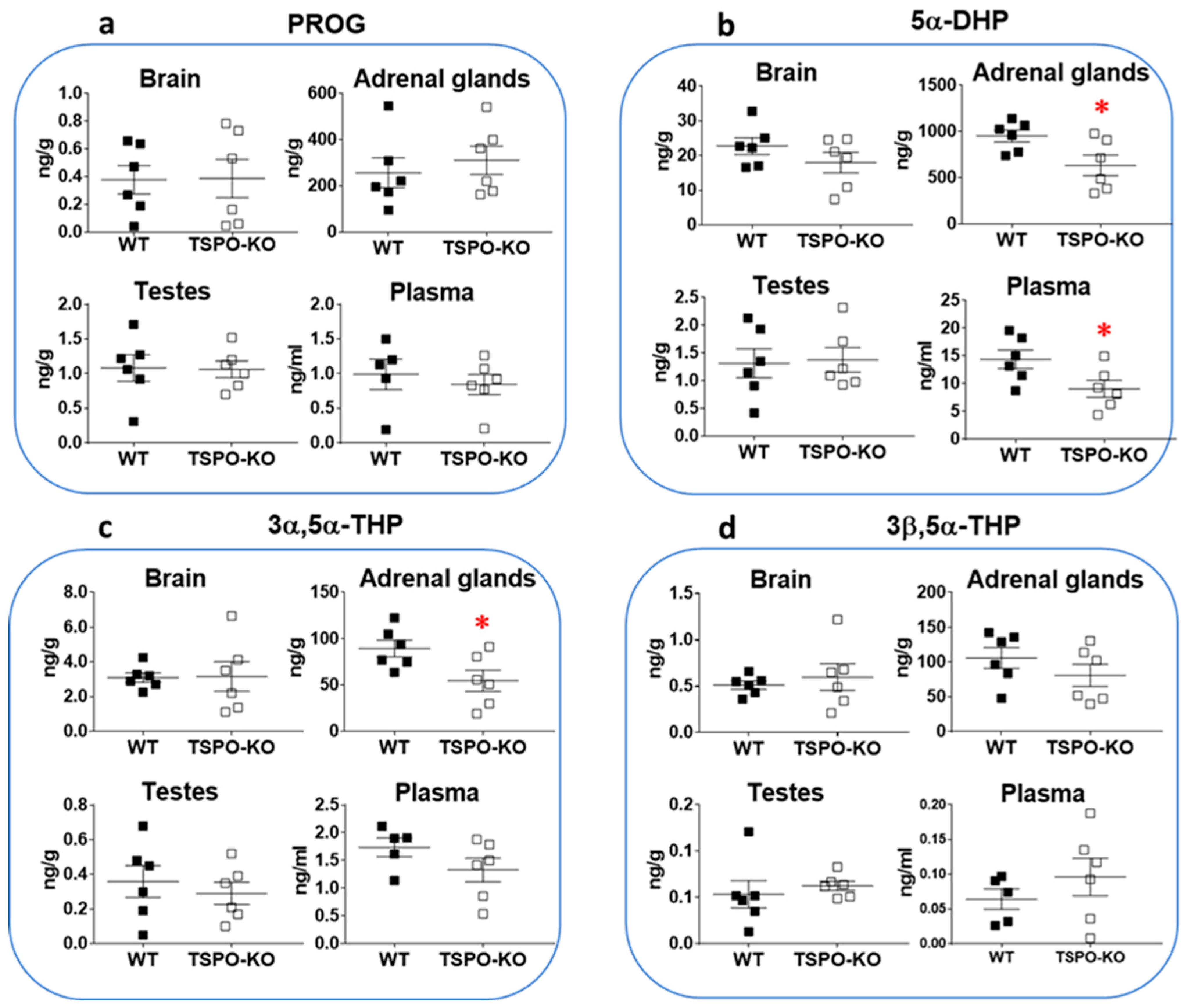
2.1.2. Basal Concentrations of Progestogens
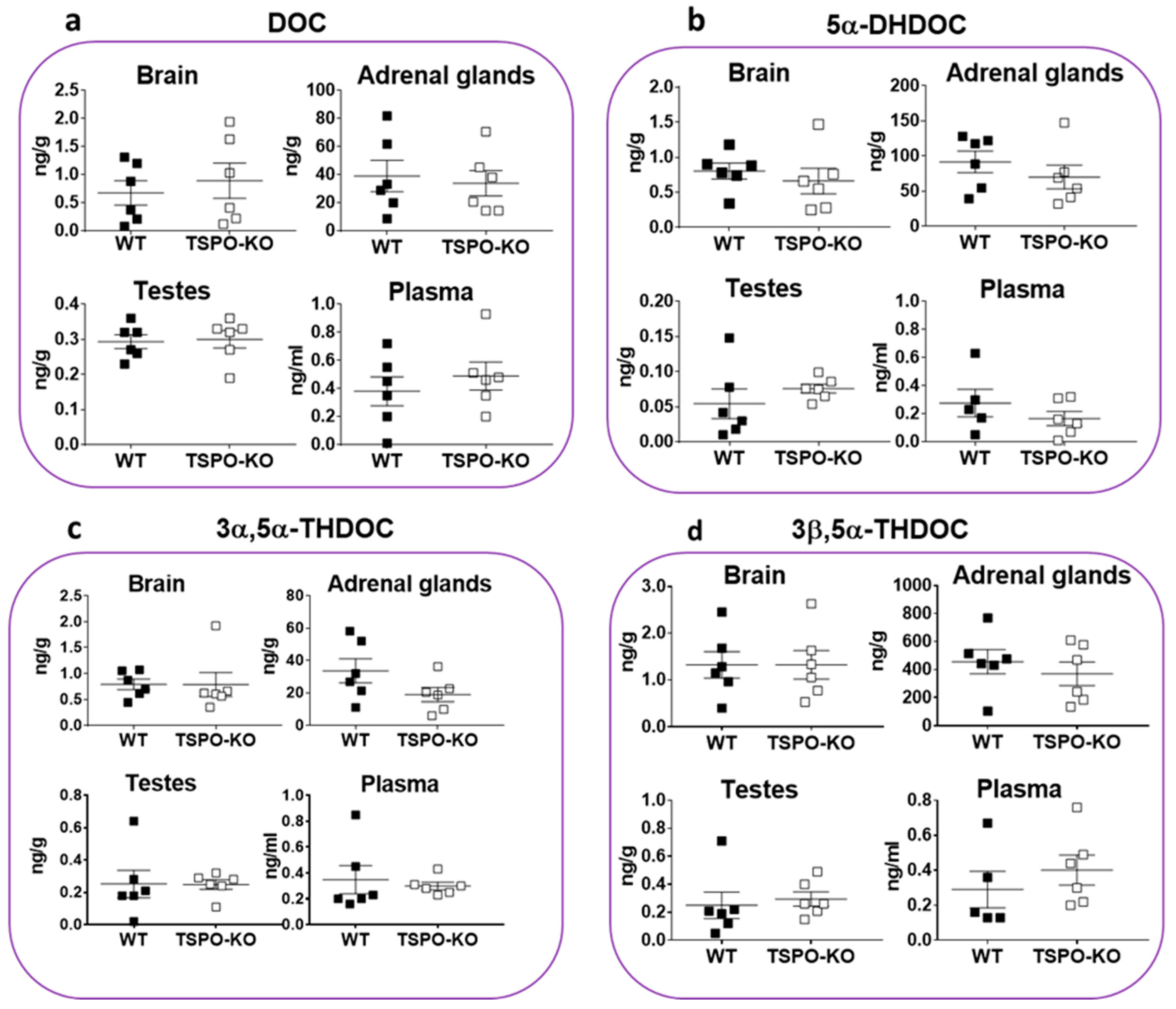
2.1.3. Basal Concentrations of Corticosteroids
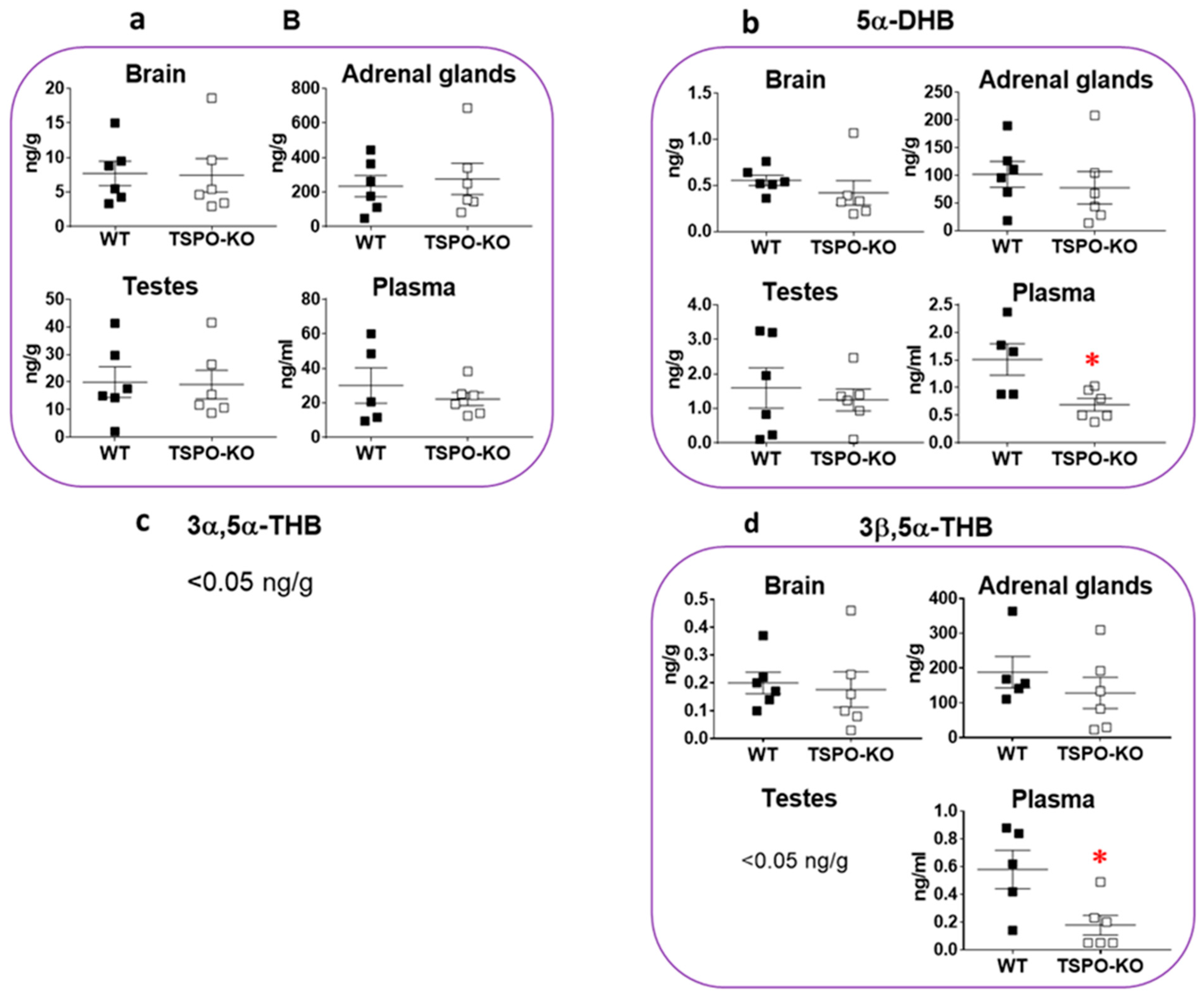
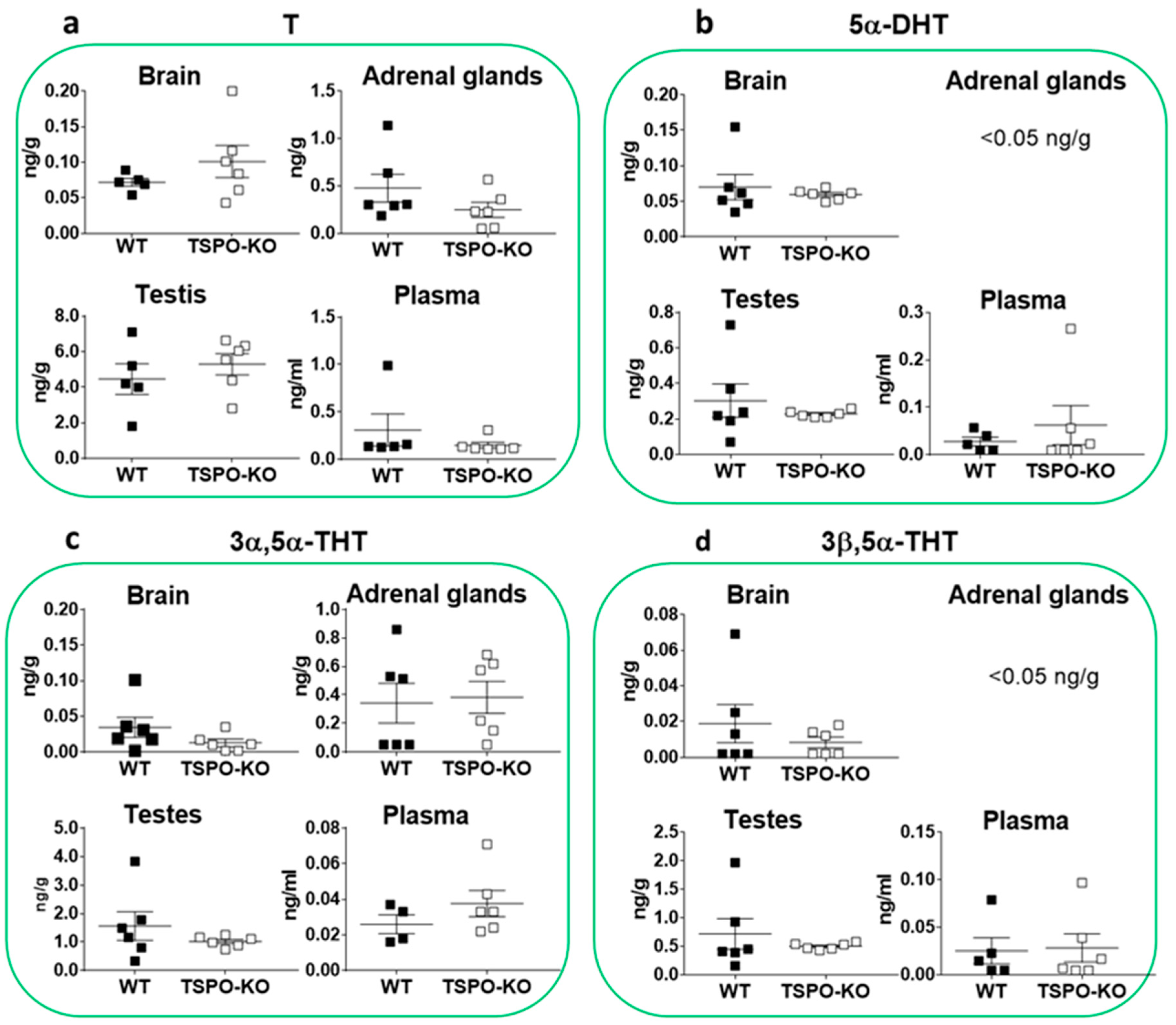
2.1.4. Basal Concentrations of Androgens
2.2. Basal Protein Levels of TSPO/PBR, VDAC-1, CYP11A1 and 5α-Reductase (SRD5A)
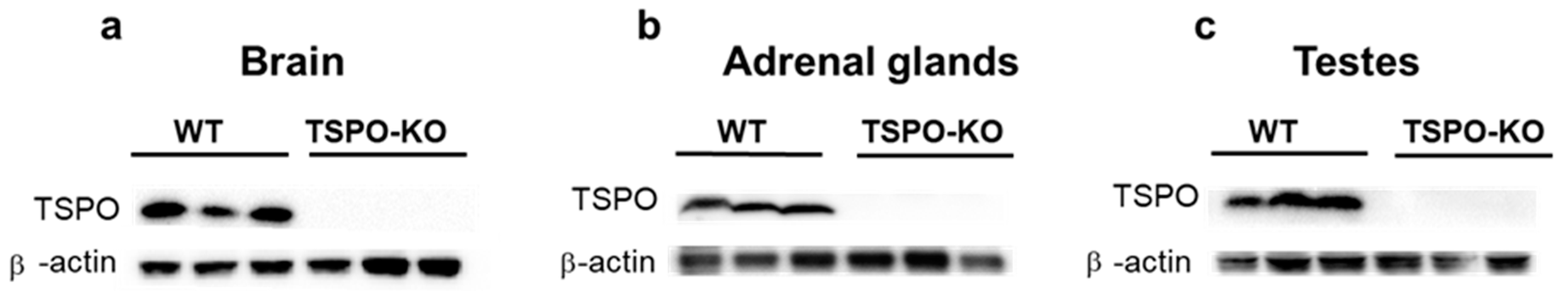
2.2.1. TSPO/PBR Protein Levels
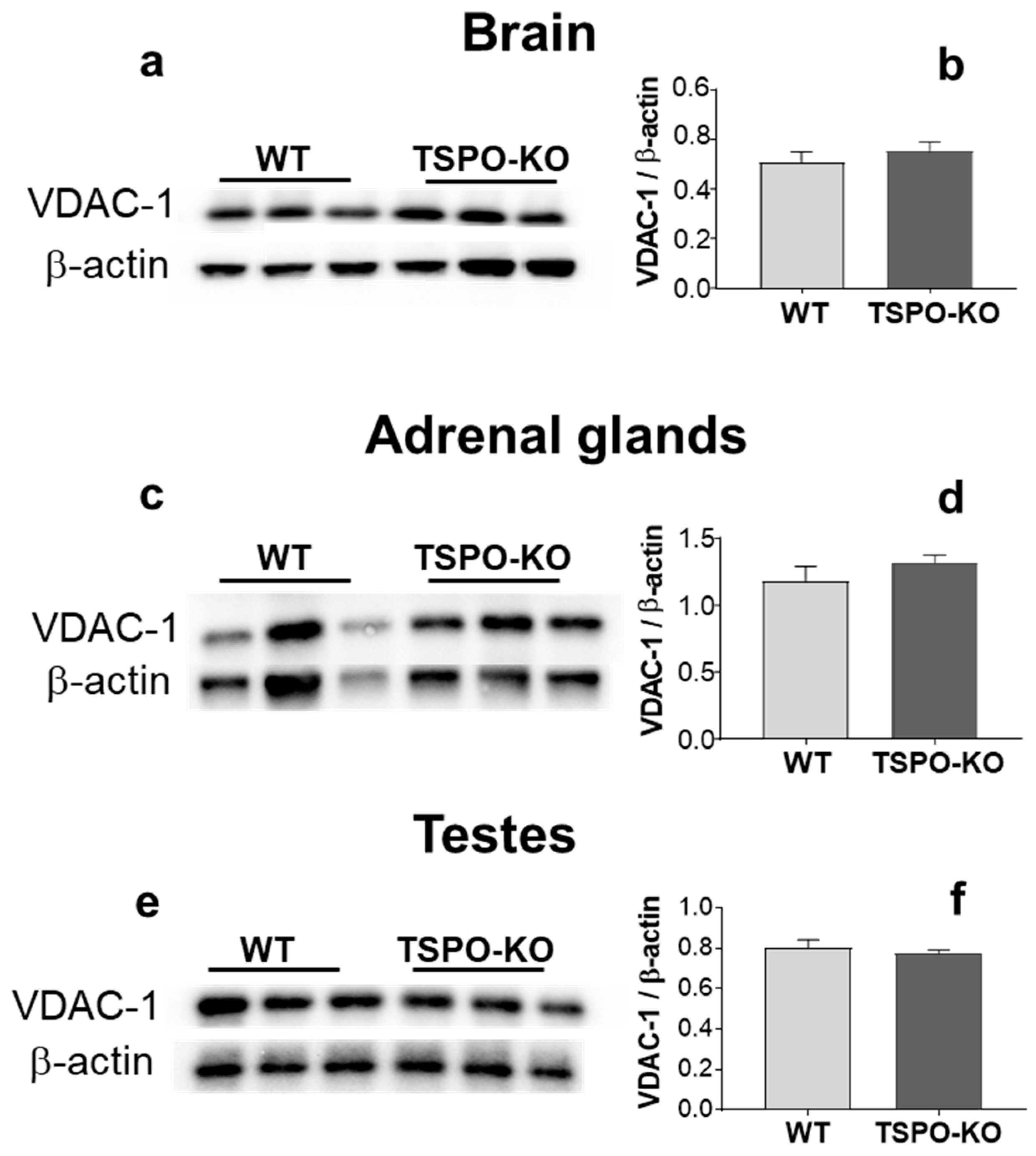
2.2.2. VDAC-1 Protein Levels
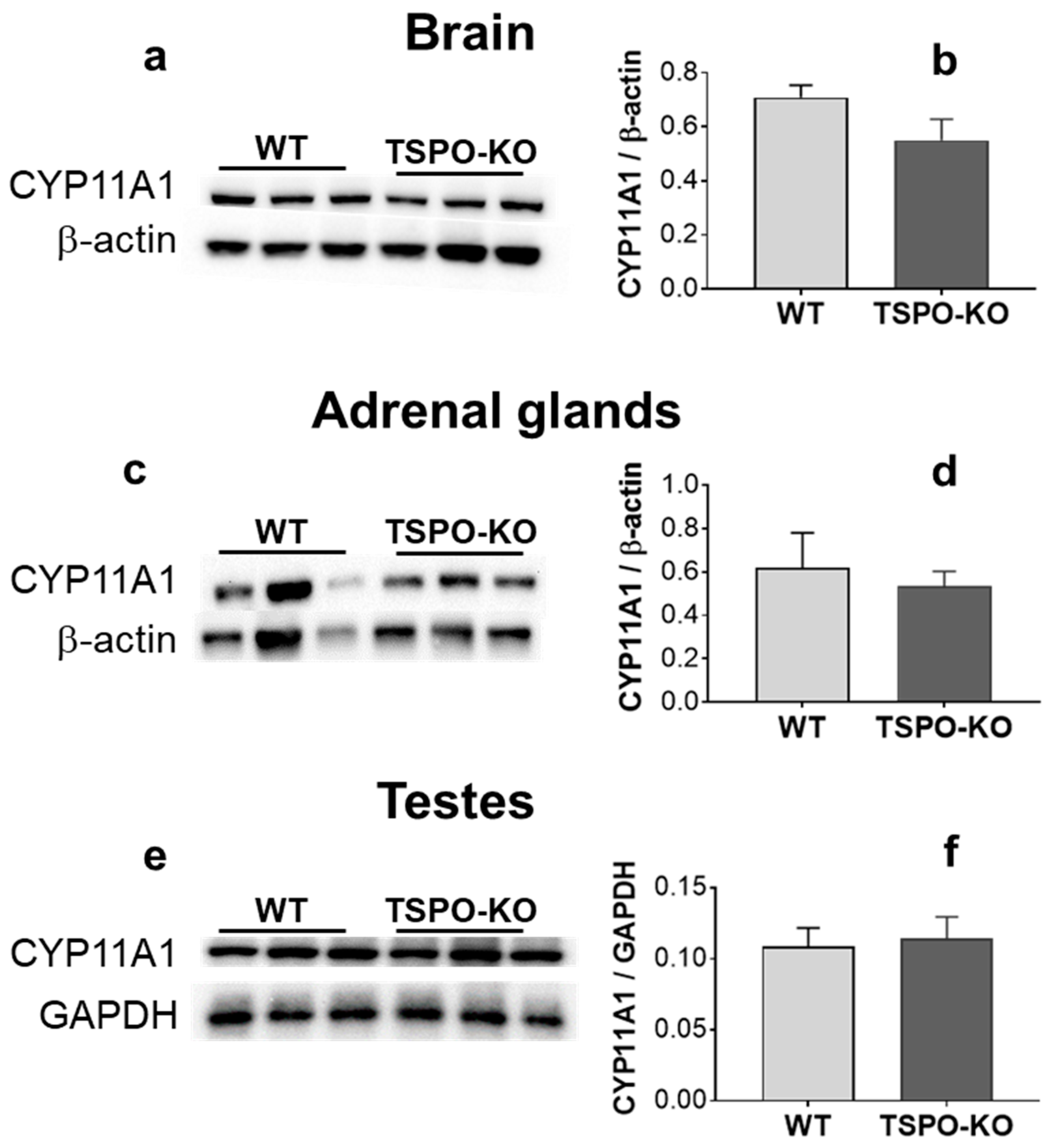
2.2.3. CYP11A1 Protein Levels
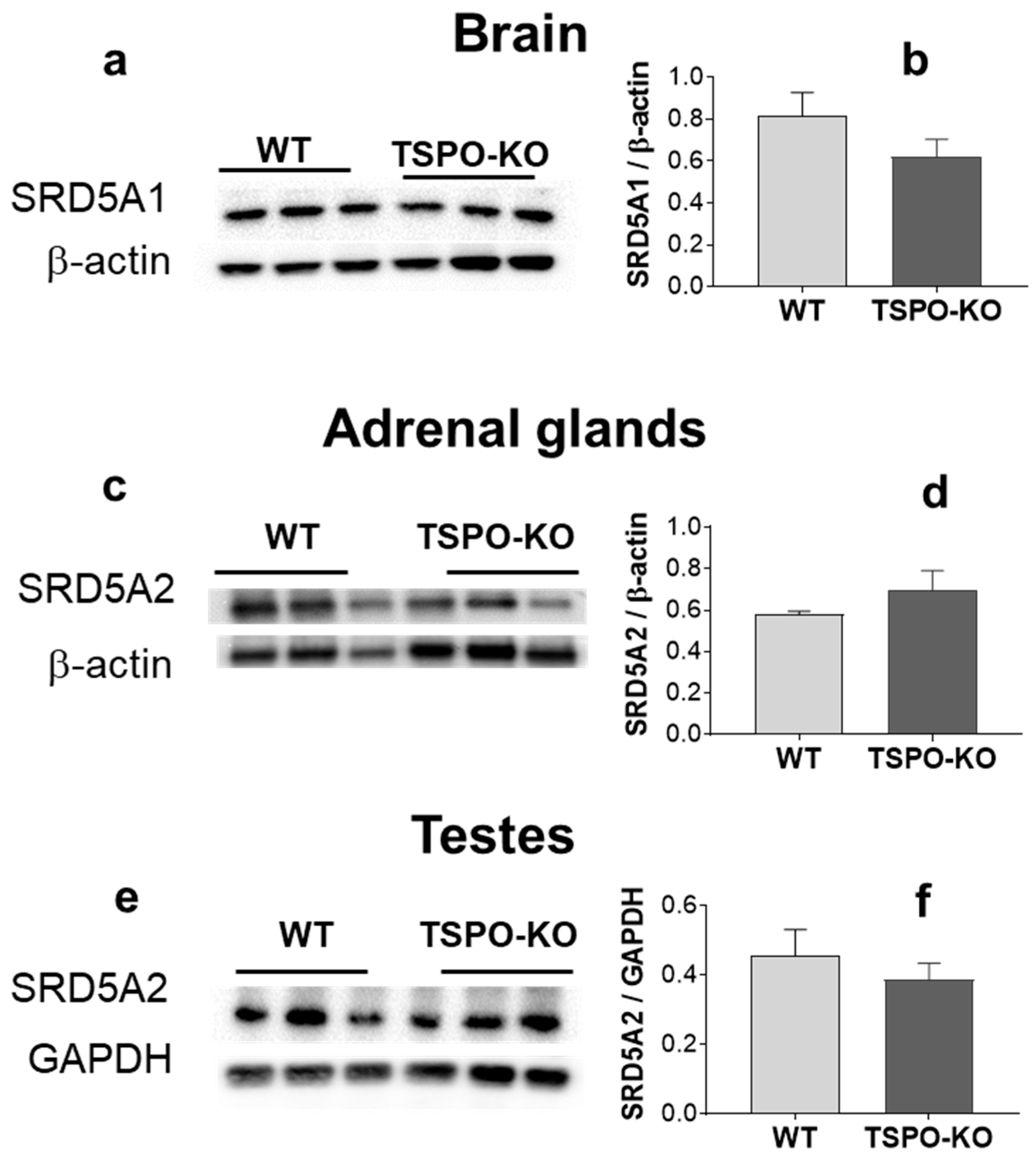
2.2.4. 5α-Reductase (SRD5A) Protein Levels
3. Discussion
4. Conclusions/Open Questions
- (1)
- Is there sufficient evidence to identify cholesterol translocation as the main function of the TSPO/PBR?
- (2)
- Does PREG synthesis involve enzymes other than mitochondrial CYP11A1, as recently suggested in human glial cells [49], or autooxidation pathways that are independent from mitochondrial cholesterol?
- (3)
- (4)
- While steroid concentrations in the adrenal glands, testes, brain and plasma appeared to be largely unchanged after a complete deletion of the tspo gene, there was a significant decrease in some 5α-reduced steroids in adrenal glands, which requires further investigation. Is the change in 5α-reductase enzymatic activity biologically significant?
- (5)
- Finally, in light of the above issues, should a conceptually more neutral, new nomenclature be used instead of TSPO—a naming according to a purported, no longer adequately substantiated main function—or PBR—an historical definition based on the affinity seen for certain benzodiazepines? In the meantime, one could consider the combined term “TSPO/PBR” in order to retain the unity of knowledge about PBR achieved before the name change in 2006 and the multiple perspectives on the putative function of the protein.
5. Materials and Methods
5.1. Ethics
5.2. Animals
5.3. Experimental Designs
5.4. Steroid Profiling by Gas Chromatography Coupled to Tandem Mass Spectrometry (GC-MS/MS)
5.5. Western Blot
5.6. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schumacher, M.; Weill-Engerer, S.; Liere, P.; Robert, F.; Franklin, R.J.; Garcia-Segura, L.M.; Lambert, J.J.; Mayo, W.; Melcangi, R.C.; Parducz, A.; et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog. Neurobiol. 2003, 71, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Roostaee, A.; Barbar, E.; Lehoux, J.G.; Lavigne, P. Cholesterol binding is a prerequisite for the activity of the steroidogenic acute regulatory protein (StAR). Biochem. J. 2008, 412, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Tugaeva, K.V.; Sluchanko, N.N. Steroidogenic Acute Regulatory Protein: Structure, Functioning, and Regulation. Biochemistry 2019, 84, S233–S253. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.; Degenhardt, B.; Teper, G.; Papadopoulos, V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 1267–1272. [Google Scholar] [CrossRef]
- Georges, E.; Sottas, C.; Li, Y.; Papadopoulos, V. Direct and specific binding of cholesterol to the mitochondrial translocator protein (TSPO) using PhotoClick cholesterol analogue. J. Biochem. 2021, 170, 239–243. [Google Scholar] [CrossRef]
- Owen, D.R.; Fan, J.; Campioli, E.; Venugopal, S.; Midzak, A.; Daly, E.; Harlay, A.; Issop, L.; Libri, V.; Kalogiannopoulou, D.; et al. TSPO mutations in rats and a human polymorphism impair the rate of steroid synthesis. Biochem. J. 2017, 474, 3985–3999. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Miller, W.L. Role of mitochondria in steroidogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 771–790. [Google Scholar] [CrossRef]
- Liu, G.J.; Middleton, R.J.; Banati, R.B. Subcellular distribution of the 18kDa translocator protein and transcript variant PBR-S in human cells. Gene 2017, 613, 45–56. [Google Scholar] [CrossRef]
- Rone, M.B.; Midzak, A.S.; Issop, L.; Rammouz, G.; Jagannathan, S.; Fan, J.; Ye, X.; Blonder, J.; Veenstra, T.; Papadopoulos, V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol. Endocrinol. 2012, 26, 1868–1882. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef]
- Antkiewicz-Michaluk, L.; Guidotti, A.; Krueger, K.E. Molecular characterization and mitochondrial density of a recognition site for peripheral-type benzodiazepine ligands. Mol. Pharmacol. 1988, 34, 272–278. [Google Scholar] [PubMed]
- Fan, J.; Campioli, E.; Sottas, C.; Zirkin, B.; Papadopoulos, V. Amhr2-Cre-Mediated Global Tspo Knockout. J. Endocr. Soc. 2020, 4, bvaa001. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Campioli, E.; Midzak, A.; Culty, M.; Papadopoulos, V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc. Natl. Acad. Sci. USA 2015, 112, 7261–7266. [Google Scholar] [CrossRef] [PubMed]
- Verleye, M.; Akwa, Y.; Liere, P.; Ladurelle, N.; Pianos, A.; Eychenne, B.; Schumacher, M.; Gillardin, J.M. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol. Biochem. Behav. 2005, 82, 712–720. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Aghazadeh, Y.; Fan, J.; Campioli, E.; Zirkin, B.; Midzak, A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol. Cell. Endocrinol. 2015, 408, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Liere, P.; Pianos, A.; Oudinet, J.-P.; Schumacher, M.; Akwa, Y. Differential effects of the 18-kDa translocator protein (TSPO) ligand etifoxine on steroidogenesis in rat brain, plasma and steroidogenic glands: Pharmacodynamic studies. Psychoneuroendocrinology 2017, 83, 122–134. [Google Scholar] [CrossRef]
- Lejri, I.; Grimm, A.; Halle, F.; Abarghaz, M.; Klein, C.; Maitre, M.; Schmitt, M.; Bourguignon, J.J.; Mensah-Nyagan, A.G.; Bihel, F.; et al. TSPO Ligands Boost Mitochondrial Function and Pregnenolone Synthesis. J. Alzheimers Dis. 2019, 72, 1045–1058. [Google Scholar] [CrossRef]
- Gut, P.; Zweckstetter, M.; Banati, R. Lost in translocation: The functions of the 18-kD translocator protein. Trends Endocrinol. Metab. 2015, 26, 349–356. [Google Scholar] [CrossRef]
- Banati, R.; Middleton, R.; Chan, R.; Hatty, C.; Wai-Ying Kam, W.; Quin, C.; Graeber, M.; Parmar, A.; Zahra, D.; Callaghan, P.; et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 2014, 5, 5452. [Google Scholar] [CrossRef]
- Middleton, R.J.; Liu, G.J.; Banati, R.B. Guwiyang Wurra—’Fire Mouse’: A global gene knockout model for TSPO/PBR drug development, loss-of-function and mechanisms of compensation studies. Biochem. Soc. Trans. 2015, 43, 553–558. [Google Scholar] [CrossRef]
- Morohaku, K.; Pelton, S.H.; Daugherty, D.J.; Butler, W.R.; Deng, W.; Selvaraj, V. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 2014, 155, 89–97. [Google Scholar] [CrossRef]
- Tu, L.N.; Morohaku, K.; Manna, P.R.; Pelton, S.H.; Butler, W.R.; Stocco, D.M.; Selvaraj, V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 2014, 289, 27444–27454. [Google Scholar] [CrossRef]
- Tu, L.N.; Zhao, A.H.; Stocco, D.M.; Selvaraj, V. PK11195 effect on steroidogenesis is not mediated through the translocator protein (TSPO). Endocrinology 2015, 156, 1033–1039. [Google Scholar] [CrossRef]
- Selvaraj, V.; Morohaku, K.; Koganti, P.P.; Zhang, J.; He, W.; Quirk, S.M.; Stocco, D.M. Commentary: Amhr2-Cre-Mediated Global Tspo Knockout. Front. Endocrinol. 2020, 11, 472. [Google Scholar] [CrossRef]
- Barron, A.M.; Ji, B.; Kito, S.; Suhara, T.; Higuchi, M. Steroidogenic abnormalities in translocator protein knockout mice and significance in the aging male. Biochem. J. 2018, 475, 75–85. [Google Scholar] [CrossRef]
- Zhu, X.; Frechou, M.; Liere, P.; Zhang, S.; Pianos, A.; Fernandez, N.; Denier, C.; Mattern, C.; Schumacher, M.; Guennoun, R. A Role of Endogenous Progesterone in Stroke Cerebroprotection Revealed by the Neural-Specific Deletion of Its Intracellular Receptors. J. Neurosci. 2017, 37, 10998–11020. [Google Scholar] [CrossRef]
- Hauet, T.; Liu, J.; Li, H.; Gazouli, M.; Culty, M.; Papadopoulos, V. PBR, StAR, and PKA: Partners in Cholesterol Transport in Steroidogenic Cells. Endocr. Res. 2002, 28, 395–401. [Google Scholar] [CrossRef]
- Luu-The, V.; Pelletier, G.; Labrie, F. Quantitative appreciation of steroidogenic gene expression in mouse tissues: New roles for type 2 5α-reductase, 20α-hydroxysteroid dehydrogenase and estrogen sulfotransferase. J. Steroid Biochem. Mol. Biol. 2005, 93, 269–276. [Google Scholar] [CrossRef]
- Hershkovitz, L.; Beuschlein, F.; Klammer, S.; Krup, M.; Weinstein, Y. Adrenal 20α-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology 2007, 148, 976–988. [Google Scholar] [CrossRef]
- Andersson, S.; Berman, D.M.; Jenkins, E.P.; Russell, D.W. Deletion of steroid 5α-reductase 2 gene in male pseudohermaphroditism. Nature 1991, 354, 159–161. [Google Scholar] [CrossRef]
- Steckelbroeck, S.; Watzka, M.; Reichelt, R.; Hans, V.H.; Stoffel-Wagner, B.; Heidrich, D.D.; Schramm, J.; Bidlingmaier, F.; Klingmuller, D. Characterization of the 5α-reductase-3α-hydroxysteroid dehydrogenase complex in the human brain. J. Clin. Endocrinol. Metab. 2001, 86, 1324–1331. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Poletti, A.; Cavarretta, I.; Celotti, F.; Colciago, A.; Magnaghi, V.; Motta, M.; Negri-Cesi, P.; Martini, L. The 5α-reductase in the central nervous system: Expression and modes of control. J. Steroid Biochem. Mol. Biol. 1998, 65, 295–299. [Google Scholar] [CrossRef]
- Thigpen, A.E.; Silver, R.I.; Guileyardo, J.M.; Casey, M.L.; McConnell, J.D.; Russell, D.W. Tissue distribution and ontogeny of steroid 5α-reductase isozyme expression. J. Clin. Investig. 1993, 92, 903–910. [Google Scholar] [CrossRef]
- Rheaume, E.; Lachance, Y.; Zhao, H.F.; Breton, N.; Dumont, M.; de Launoit, Y.; Trudel, C.; Luu-The, V.; Simard, J.; Labrie, F. Structure and expression of a new complementary DNA encoding the almost exclusive 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase in human adrenals and gonads. Mol. Endocrinol. 1991, 5, 1147–1157. [Google Scholar] [CrossRef]
- Yeliseev, A.A.; Kaplan, S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 1995, 270, 21167–21175. [Google Scholar] [CrossRef]
- Hiser, C.; Montgomery, B.L.; Ferguson-Miller, S. TSPO protein binding partners in bacteria, animals, and plants. J. Bioenerg. Biomembr. 2021, 53, 463–487. [Google Scholar] [CrossRef]
- Liu, G.J.; Middleton, R.J.; Kam, W.W.; Chin, D.Y.; Hatty, C.R.; Chan, R.H.; Banati, R.B. Functional gains in energy and cell metabolism after TSPO gene insertion. Cell Cycle 2017, 16, 436–447. [Google Scholar] [CrossRef]
- Gavish, M.; Veenman, L. Regulation of Mitochondrial, Cellular, and Organismal Functions by TSPO. Adv. Pharmacol. 2018, 82, 103–136. [Google Scholar] [CrossRef]
- Gatliff, J.; Campanella, M. TSPO is a REDOX regulator of cell mitophagy. Biochem. Soc. Trans. 2015, 43, 543–552. [Google Scholar] [CrossRef]
- El Chemali, L.; Akwa, Y.; Massaad-Massade, L. The mitochondrial translocator protein (TSPO): A key multifunctional molecule in the nervous system. Biochem. J. 2022, 479, 1455–1466. [Google Scholar] [CrossRef]
- Anholt, R.R.; De Souza, E.B.; Oster-Granite, M.L.; Snyder, S.H. Peripheral-type benzodiazepine receptors: Autoradiographic localization in whole-body sections of neonatal rats. J. Pharmacol. Exp. Ther. 1985, 233, 517–526. [Google Scholar]
- Hanukoglu, I.; Rapoport, R. Routes and regulation of NADPH production in steroidogenic mitochondria. Endocr. Res. 1995, 21, 231–241. [Google Scholar] [CrossRef]
- Miller, W.L. Mitochondrial specificity of the early steps in steroidogenesis. J. Steroid Biochem. Mol. Biol. 1995, 55, 607–616. [Google Scholar] [CrossRef]
- Bose, H.S.; Marshall, B.; Debnath, D.K.; Perry, E.W.; Whittal, R.M. Electron Transport Chain Complex II Regulates Steroid Metabolism. iScience 2020, 23, 101295. [Google Scholar] [CrossRef]
- Le Goff, J.M.; Martin, P.M.; Raynaud, J.P. (De)phosphorylation agents influence 5α-reduction of testosterone in human prostate. Endocrinology 1988, 123, 1693–1695. [Google Scholar] [CrossRef]
- Barron, A.M.; Higuchi, M.; Hattori, S.; Kito, S.; Suhara, T.; Ji, B. Regulation of Anxiety and Depression by Mitochondrial Translocator Protein-Mediated Steroidogenesis: The Role of Neurons. Mol. Neurobiol. 2021, 58, 550–563. [Google Scholar] [CrossRef]
- Falchi, A.M.; Battetta, B.; Sanna, F.; Piludu, M.; Sogos, V.; Serra, M.; Melis, M.; Putzolu, M.; Diaz, G. Intracellular cholesterol changes induced by translocator protein (18 kDa) TSPO/PBR ligands. Neuropharmacology 2007, 53, 318–329. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cheung, G.; Porter, E.; Papadopoulos, V. The neurosteroid pregnenolone is synthesized by a mitochondrial P450 enzyme other than CYP11A1 in human glial cells. J. Biol. Chem. 2022, 298, 102110. [Google Scholar] [CrossRef]
- Hatty, C.R.; Le Brun, A.P.; Lake, V.; Clifton, L.A.; Liu, G.J.; James, M.; Banati, R.B. Investigating the interactions of the 18kDa translocator protein and its ligand PK11195 in planar lipid bilayers. Biochim. Biophys. Acta 2014, 1838, 1019–1030. [Google Scholar] [CrossRef]
- Hatty, C.R.; Banati, R.B. Protein-ligand and membrane-ligand interactions in pharmacology: The case of the translocator protein (TSPO). Pharmacol. Res. 2015, 100, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Middleton, R.J.; Hatty, C.R.; Kam, W.W.; Chan, R.; Pham, T.; Harrison-Brown, M.; Dodson, E.; Veale, K.; Banati, R.B. The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. 2014, 24, 631–653. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Harrison-Brown, M.; Middleton, R.J.; Banati, R.; Liu, G.J. Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain. Int. J. Mol. Sci. 2018, 19, 2707. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Wetzel, C.H.; Dorostkar, M.; Herms, J.; Albert, N.L.; Schwarzbach, J.; Schumacher, M.; Neumann, I.D. Translocator protein (18kDa) TSPO: A new diagnostic or therapeutic target for stress-related disorders? Mol. Psychiatry 2022, 27, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Tremolanti, C.; Germelli, L.; Barresi, E.; Da Pozzo, E.; Simorini, F.; Castellano, S.; Taliani, S.; Da Settimo, F.; Martini, C.; Costa, B. Translocator Protein 18-kDa: A Promising Target to Treat Neuroinflammation-related Degenerative Diseases. Curr. Med. Chem. 2022, 29, 4831–4861. [Google Scholar] [CrossRef]
- Liere, P.; Pianos, A.; Eychenne, B.; Cambourg, A.; Liu, S.; Griffiths, W.; Schumacher, M.; Sjovall, J.; Baulieu, E.E. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J. Lipid Res. 2004, 45, 2287–2302. [Google Scholar] [CrossRef]
| Basal Steroid Concentrations | Brain (ng/g) | Adrenal Glands (ng/g) | Testes (ng/g) | Plasma (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | TSPO-KO | p | WT | TSPO-KO | p | WT | TSPO-KO | p | WT | TSPO-KO | p | |
| 20α-DHPREG | 0.41 ± 0.08 | 0.36 ± 0.04 | 0.590 | 0.32 ± 0.06 | 0.27 ± 0.08 | 0.627 | <0.05 | <0.05 | ------ | 0.021 ± 0.00 | 0.02 ± 0.00 | 0.948 |
| 20α-DHP | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.989 | 0.09 ± 0.02 | 0.10 ± 0.03 | 0.906 | 0.09 ± 0.02 | 0.08 ± 0.01 | 0.745 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.348 |
| 5α,20α-THP | 1.13 ± 0.15 | 1.11 ±0.22 | 0.959 | 0.72 ± 0.15 | 1.23 ± 0.36 | 0.218 | 0.05 ± 0.01 | 0.05 ± 0.01 | >0.99 | 0.12 ± 0.03 | 0.10 ± 0.02 | 0.500 |
| 3α,5α,20α-HHP | 0.55 ± 0.06 | 0.69 ± 0.11 | 0.600 | 1.63 ±0.04 | 1.63 ± 0.51 | >0.999 | 0.24 ± 0.06 | 0.32 ± 0.11 | 0.560 | 0.33 ± 0.04 | 0.27 ± 0.05 | 0.335 |
| 3β,5α,20α-HHP | 0.11 ± 0.01 | 0.15 ±0.05 | 0.457 | 2. 55 ± 0.35 | 2.61 ± 0.77 | 0.943 | 0.05 ± 0.01 | 0.05 ± 0.01 | >0.999 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.708 |
| ADIONE | 0.11 ± 0.05 | 0.09 ± 0.01 | 0.637 | 2.8 ± 0.7 | 3.0 ± 0.4 | 0.796 | 31 ± 7 | 31 ± 3 | 0.928 | 0.12 ± 0.02 | 0.11 ± 0.01 | 0.634 |
| Epiandrosterone | <0.05 | <0.05 | ------ | 1.31 ± 0.27 | 1.00 ± 0.36 | 0.506 | 0.11 ± 0.04 | 0.07 ± 0.01 | 0.354 | 0.01 ± 0.00 | 0.01 ± 0.00 | >0.99 |
| Androstenediol | 0.10 ± 0.01 | 0.11 ± 0.04 | 0.776 | <0.005 | <0.005 | ------ | 0.07 ± 0.03 | 0.06 ± 0.01 | 0.647 | <0.05 | <0.05 | ------ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liere, P.; Liu, G.-J.; Pianos, A.; Middleton, R.J.; Banati, R.B.; Akwa, Y. The Comprehensive Steroidome in Complete TSPO/PBR Knockout Mice under Basal Conditions. Int. J. Mol. Sci. 2023, 24, 2474. https://doi.org/10.3390/ijms24032474
Liere P, Liu G-J, Pianos A, Middleton RJ, Banati RB, Akwa Y. The Comprehensive Steroidome in Complete TSPO/PBR Knockout Mice under Basal Conditions. International Journal of Molecular Sciences. 2023; 24(3):2474. https://doi.org/10.3390/ijms24032474
Chicago/Turabian StyleLiere, Philippe, Guo-Jun Liu, Antoine Pianos, Ryan J. Middleton, Richard B. Banati, and Yvette Akwa. 2023. "The Comprehensive Steroidome in Complete TSPO/PBR Knockout Mice under Basal Conditions" International Journal of Molecular Sciences 24, no. 3: 2474. https://doi.org/10.3390/ijms24032474
APA StyleLiere, P., Liu, G.-J., Pianos, A., Middleton, R. J., Banati, R. B., & Akwa, Y. (2023). The Comprehensive Steroidome in Complete TSPO/PBR Knockout Mice under Basal Conditions. International Journal of Molecular Sciences, 24(3), 2474. https://doi.org/10.3390/ijms24032474






