Molecular Characterization of Non-Neurogenic and Neurogenic Lower Urinary Tract Dysfunction (LUTD) in SCI-Induced and Partial Bladder Outlet Obstruction Mouse Models
Abstract
1. Introduction
2. Results
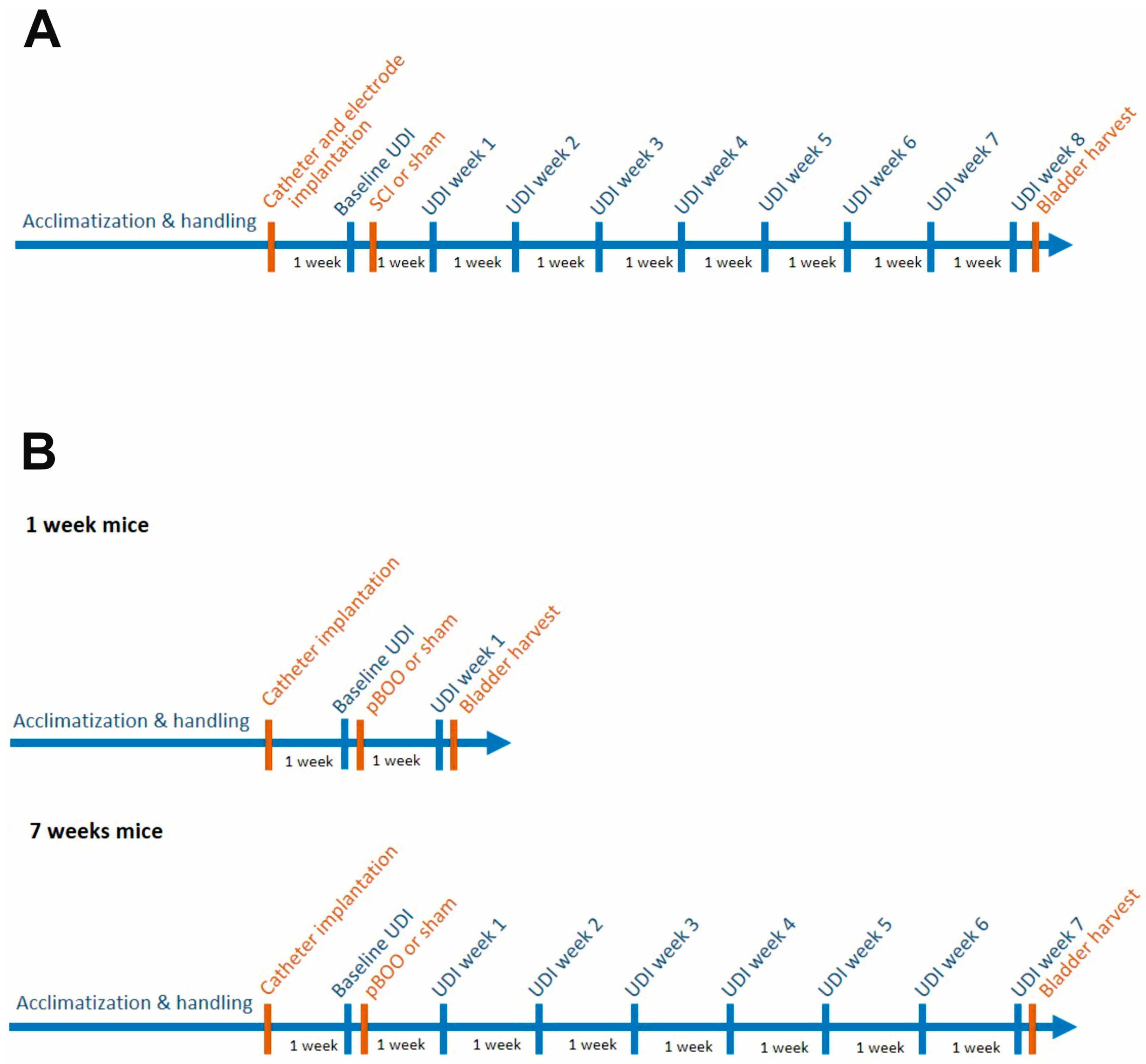
2.1. Study Design and Animal Grouping
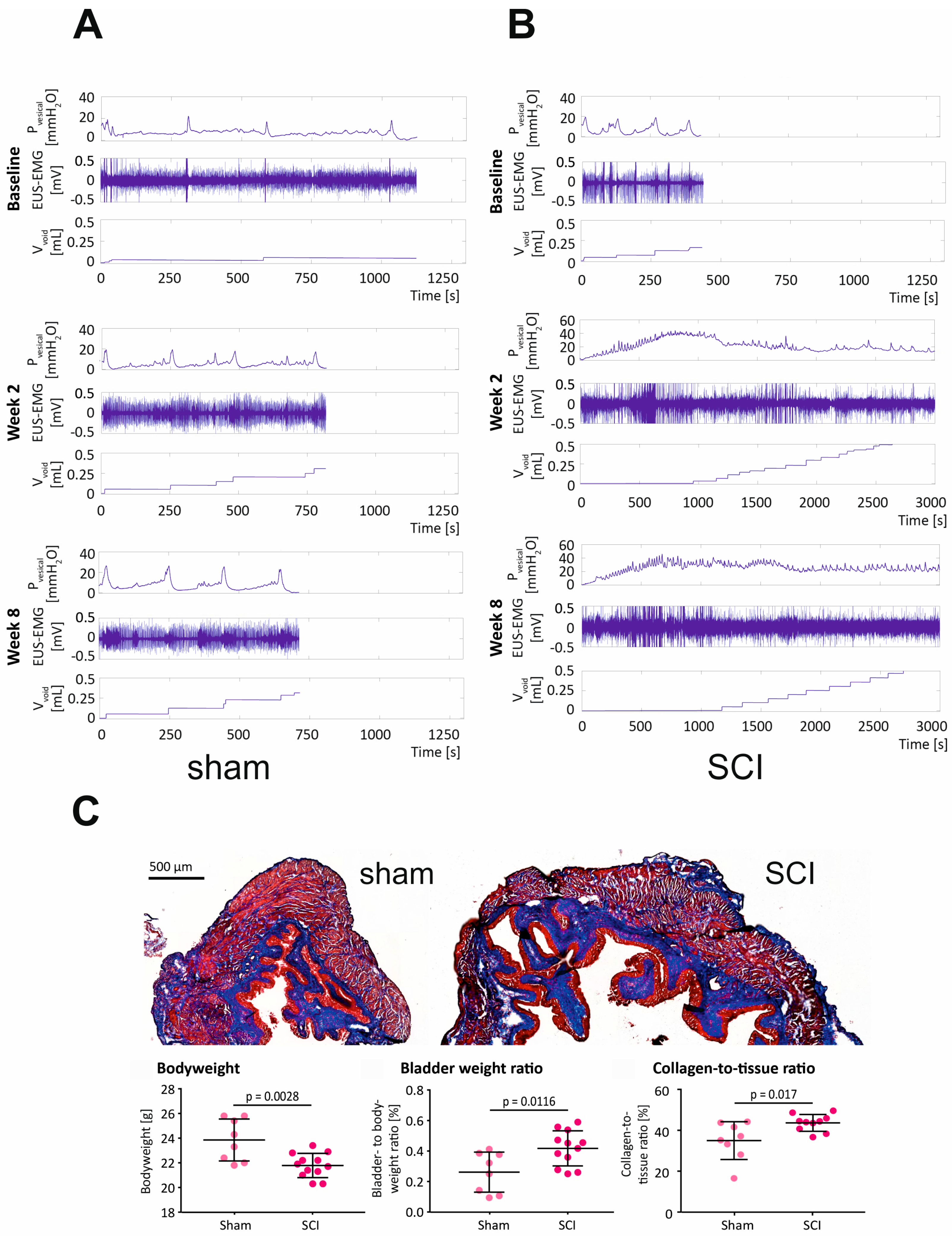
2.2. SCI in Mice Causes Severe LUTD, Leading to Bladder Fibrosis
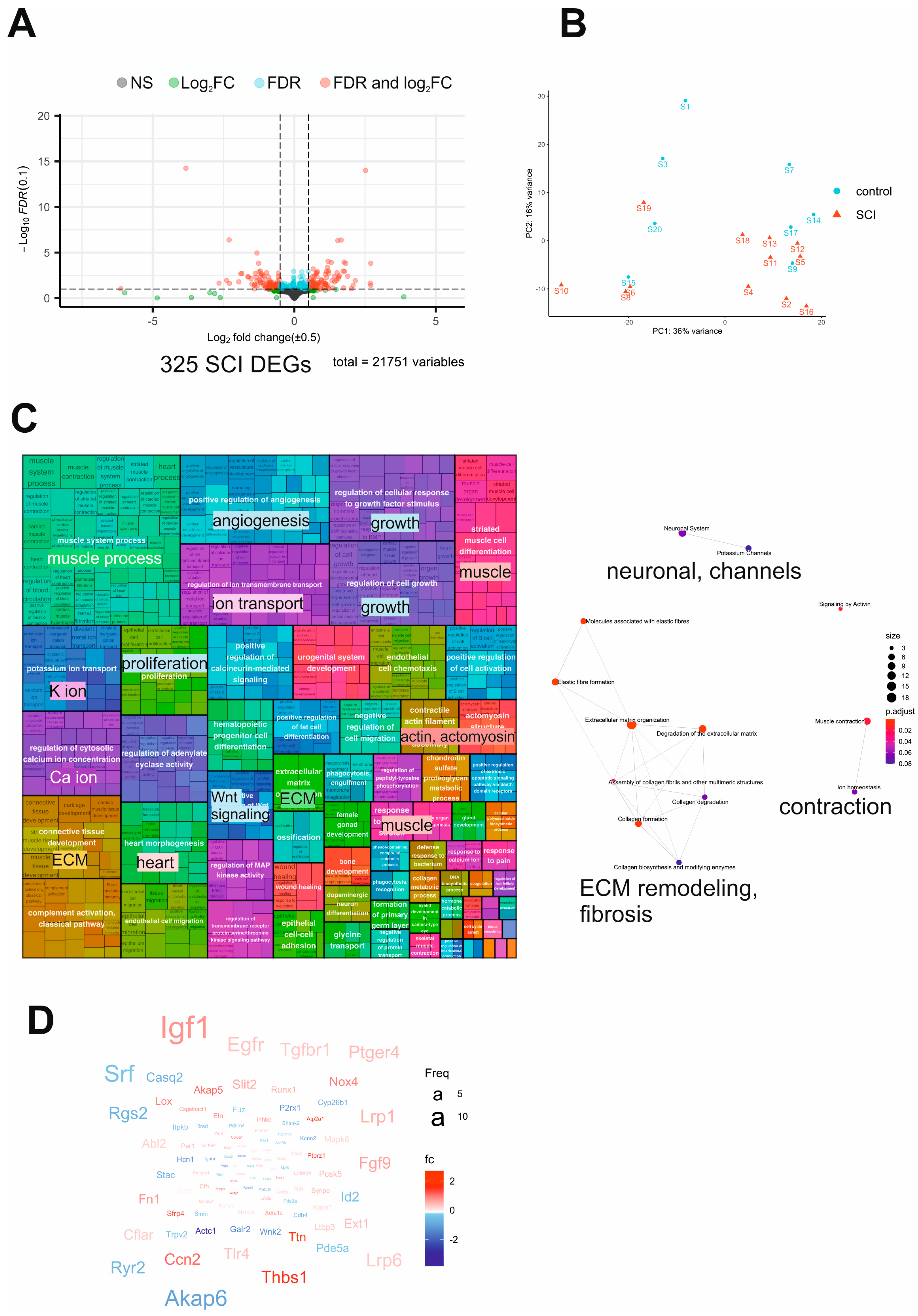
2.3. Transcriptome Analysis of SCI Mouse Bladders Reveals Dysregulation of Neuronal and Contractile Processes Accompanied by Fibrosis
2.4. Changes in Bladder Function and Morphology after pBOO and SCI Are Different
2.5. Transcriptome of the Mouse Bladders Progressively Deteriorates from One to 7 Weeks of pBOO
2.6. Comparative Transcriptome Analysis after SCI and pBOO Reveals Differences between Neurogenic and Obstructive LUTD
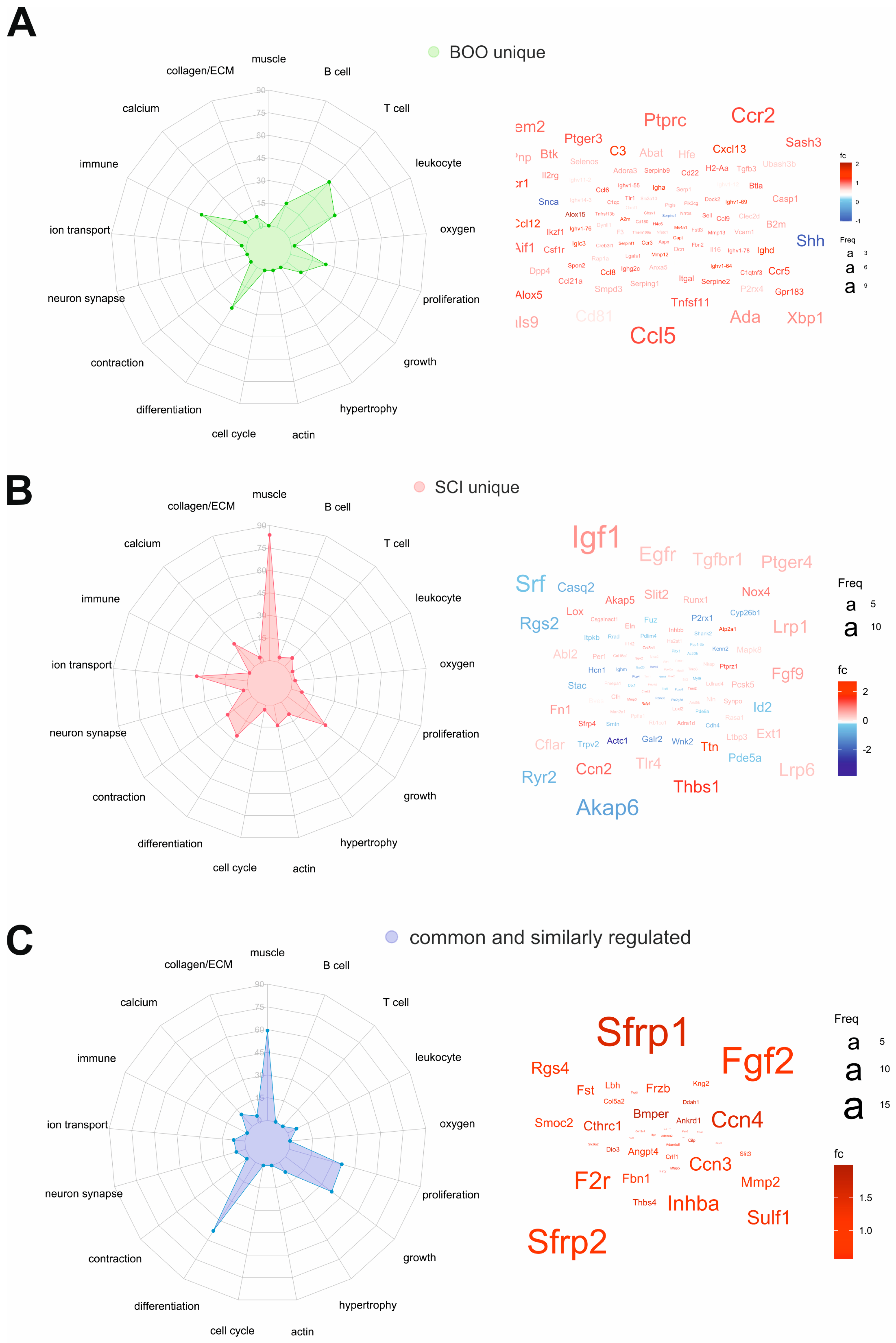
2.7. Common and Unique DEGs Define the Molecular Differences between SCI and pBOO Bladders
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Approval
4.3. Catheter and Electrode Implantation
4.4. Partial Bladder Outlet Obstruction (pBOO) Surgery
4.5. Spinal Cord Injury (SCI) Surgery
4.6. Urodynamic Investigation
4.7. UDI Analysis
4.8. Euthanasia and Bladder Harvest
4.9. Bladder Histology
4.10. Bladder Transcriptome Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.; Wullt, B.; Ballarini, S.; Zingg, D.; Naber, K.G. Social and economic burden of recurrent urinary tract infections and quality of life: A patient web-based study (GESPRIT). Expert Rev. Pharm. Outcomes Res. 2017, 18, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Durden, E.; Walker, D.; Gray, S.; Fowler, R.; Juneau, P.; Gooch, K. The economic burden of overactive bladder (OAB) and its effects on the costs associated with other chronic, age-related comorbidities in the United States. Neurourol. Urodyn. 2018, 37, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, P.D.; Wang, J.; Jiao, H.; Huang, Y.; Hori, K.; Moore, R.B.; Tredget, E.E. Bladder outlet obstruction: Progression from inflammation to fibrosis. BJU Int. 2010, 106, 1686–1694. [Google Scholar] [CrossRef]
- Gheinani, A.H.; Kiss, B.; Moltzahn, F.; Keller, I.; Bruggmann, R.; Rehrauer, H.; Fournier, C.A.; Burkhard, F.C.; Monastyrskaya, K. Characterization of miRNA-regulated networks, hubs of signaling, and biomarkers in obstruction-induced bladder dysfunction. J. Clin. Investig. 2017, 2, e89560. [Google Scholar] [CrossRef]
- Gheinani, A.H.; Bigger-Allen, A.; Wacker, A.; Adam, R.M. Systems analysis of benign bladder disorders: Insights from omics analysis. Am. J. Physiol. Physiol. 2020, 318, F901–F910. [Google Scholar] [CrossRef]
- Drzewiecki, B.A.; Anumanthan, G.; Penn, H.A.; Tanaka, S.T.; Thomas, J.C.; Adams, M.C.; Brock, J.W.; Pope, J.C.; Matusik, R.J.; Hayward, S.; et al. Modulation of the Hypoxic Response Following Partial Bladder Outlet Obstruction. J. Urol. 2012, 188, 1549–1554. [Google Scholar] [CrossRef]
- Iguchi, N.; Hou, A.; Koul, H.K.; Wilcox, D.T. Partial Bladder Outlet Obstruction in Mice May Cause E-Cadherin Repression through Hypoxia Induced Pathway. J. Urol. 2014, 192, 964–972. [Google Scholar] [CrossRef]
- Lemack, G.E.; Burkhard, F.; Zimmern, P.E.; McConnell, J.D.; Lin, V.K. Physiologic sequelae of partial infravesical obstruction in the mouse: Role of inducible nitric oxide synthase. J. Urol. 1999, 161, 1015–1022. [Google Scholar] [CrossRef]
- Felsen, D.; Dardashti, K.; Ostad, M.; Lemer, M.L.; Gross, S.S.; Chen, J.; Vaughan, E.; Poppas, D.P. Inducible Nitric Oxide Synthase Promotes Pathophysiological Consequences of Experimental Bladder Outlet Obstruction. J. Urol. 2003, 169, 1569–1572. [Google Scholar] [CrossRef]
- Boopathi, E.; Gomes, C.; Zderic, S.A.; Malkowicz, B.; Chakrabarti, R.; Patel, D.P.; Wein, A.J.; Chacko, S. Mechanical stretch upregulates proteins involved in Ca2+sensitization in urinary bladder smooth muscle hypertrophy. Am. J. Physiol. Physiol. 2014, 307, C542–C553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, M.; Lin, W.; Xiang, Z.; Huang, J.; Xu, P.; Shi, Y.; Wang, H. Responses of bladder smooth muscle to the stretch go through extracellular signal-regulated kinase (ERK)/p90 ribosomal S6 protein kinase (p90RSK)/Nuclear factor-κB (NF-κB) Pathway. Neurourol. Urodyn. 2019, 38, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.; Lin, C.; Jin, Y.; Ping-Lin, H.; Xun, L.; Bastian, A.; Arnulf, S.; Sha-Sha, X.; Xu, L.; Shu, C. Urethral meatus stricture BOO stimulates bladder smooth muscle cell proliferation and pyroptosis via IL-1β and the SGK1-NFAT2 signaling pathway. Mol. Med. Rep. 2020, 22, 219–226. [Google Scholar] [CrossRef]
- Thangavel, C.; Gomes, C.M.; Zderic, S.A.; Javed, E.; Addya, S.; Singh, J.; Das, S.; Birbe, R.; Den, R.B.; Rattan, S.; et al. NF-κB and GATA-Binding Factor 6 Repress Transcription of Caveolins in Bladder Smooth Muscle Hypertrophy. Am. J. Pathol. 2019, 189, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, P.; He, F.; Yang, X.; Wu, R.; Chen, W.; Li, L.; Yang, Z. Fibroblast Growth Factor 2 Promotes Bladder Hypertrophy Caused by Partial Bladder Outlet Obstruction. Front. Cell Dev. Biol. 2021, 9, 630228. [Google Scholar] [CrossRef]
- Hristov, K.L.; Afeli, S.A.Y.; Parajuli, S.P.; Cheng, Q.; Rovner, E.S.; Petkov, G.V. Neurogenic Detrusor Overactivity Is Associated with Decreased Expression and Function of the Large Conductance Voltage- and Ca2+-Activated K+ Channels. PLoS ONE 2013, 8, e68052. [Google Scholar] [CrossRef]
- Wilson, T.S.; Aziz, K.A.; Vazques, D.; Wuermser, L.-A.; Lin, V.K.; Lemack, G.E. Changes in detrusor smooth muscle myosin heavy chain mRNA expression following spinal cord injury in the mouse. Neurourol. Urodyn. 2004, 24, 89–95. [Google Scholar] [CrossRef]
- Lee, K.; O Park, S.; Choi, P.-C.; Ryoo, S.-B.; Lee, H.; Peri, L.E.; Zhou, T.; Corrigan, R.D.; Yanez, A.C.; Moon, S.B.; et al. Molecular and functional characterization of detrusor PDGFRα positive cells in spinal cord injury-induced detrusor overactivity. Sci. Rep. 2021, 11, 16268. [Google Scholar] [CrossRef]
- Shang, Z.; Ou, T.; Xu, J.; Yan, H.; Cui, B.; Wang, Q.; Wu, J.; Jia, C.; Cui, X.; Li, J. MicroRNA expression profile in the spinal cord injured rat neurogenic bladder by next-generation sequencing. Transl. Androl. Urol. 2020, 9, 1585–1602. [Google Scholar] [CrossRef]
- von Siebenthal, M.; Schneider, M.P.; Zheng, S.; Wuethrich, P.Y.; Burkhard, F.C.; Monastyrskaya, K. Effects of opioids and benzodiazepines on bladder function of awake restrained mice. Am. J. Clin. Exp. Urol. 2021, 9, 456–468. [Google Scholar]
- Gu, X.-J.; Ma, L.; Tang, J.-Y.; Zhou, J.-Y.; Zhu, C.; Zhang, X.; Zhou, P.; Yu, Q.; Wang, Y. Fluoxetine, a selective serotonin reuptake inhibitor used clinically, improves bladder function in a mouse model of moderate spinal cord injury. Neural Regen. Res. 2021, 16, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- DePaul, M.; Lin, C.-Y.; Silver, J.; Lee, Y.-S. Peripheral Nerve Transplantation Combined with Acidic Fibroblast Growth Factor and Chondroitinase Induces Regeneration and Improves Urinary Function in Complete Spinal Cord Transected Adult Mice. PLoS ONE 2015, 10, e0139335. [Google Scholar] [CrossRef] [PubMed]
- Kadekawa, K.; Yoshimura, N.; Majima, T.; Wada, N.; Shimizu, T.; Birder, L.A.; Kanai, A.J.; de Groat, W.C.; Sugaya, K.; Yoshiyama, M. Characterization of bladder and external urethral activity in mice with or without spinal cord injury—A comparison study with rats. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R752–R758. [Google Scholar] [CrossRef] [PubMed]
- Samson, G.; Cardenas, D.D. Neurogenic Bladder in Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Sidler, M.; Aitken, K.J.; Jiang, J.X.; Bägli, D.J. Nerve-sparing Mid-urethral Obstruction (NeMO) in Female Small Rodents. J. Vis. Exp. 2017, 122, e55288. [Google Scholar] [CrossRef]
- Bschleipfer, T.; Nandigama, R.; Moeller, S.; Illig, C.; Weidner, W.; Kummer, W. Bladder outlet obstruction influences mRNA expression of cholinergic receptors on sensory neurons in mice. Life Sci. 2012, 91, 1077–1081. [Google Scholar] [CrossRef]
- Pandita, R.K.; Fujiwara, M.; Alm, P.; Andersson, K.E. Cystometric evaluation of bladder function in non-anesthetized mice with and without bladder outlet obstruction. J. Urol. 2000, 164, 1385–1389. [Google Scholar] [CrossRef]
- Beamon, C.R.; Mazar, C.; Salkini, M.W.; Phull, H.S.; Comiter, C.V. The effect of sildenafil citrate on bladder outlet obstruction: A mouse model. BJU Int. 2009, 104, 252–256. [Google Scholar] [CrossRef]
- Pereira, M.; D’Ancona, C.A.L.; Rojas-Moscoso, J.A.; Filho, A.C.S.R.; Mónica, F.Z.; Antunes, E.; Ramos, A.C.S. Effects of nitric oxide inhibitors in mice with bladder outlet obstruction. Int. Braz. J. Urol. 2017, 43, 356–366. [Google Scholar] [CrossRef]
- Ekman, M.; Albinsson, S.; Uvelius, B.; Swärd, K. MicroRNAs in Bladder Outlet Obstruction: Relationship to Growth and Matrix Remodelling. Basic Clin. Pharmacol. Toxicol. 2016, 119, 5–17. [Google Scholar] [CrossRef]
- Gheinani, A.H.; Kock, I.; Vasquez, E.; Baumgartner, U.; Bigger-Allen, A.; Sack, B.S.; Burkhard, F.C.; Adam, R.M.; Monastyrskaya, K. Concordant miRNA and mRNA expression profiles in humans and mice with bladder outlet obstruction. Am. J. Clin. Exp. Urol. 2018, 6, 219–233. [Google Scholar] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Qin, L.; Li, G.; Tellides, G.; Simons, M. Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFβ)-dependent smooth muscle cell phenotype modulation. Sci. Rep. 2016, 6, 33407. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Xin, S. FGF signaling contributes to atherosclerosis by enhancing the inflammatory response in vascular smooth muscle cells. Mol. Med. Rep. 2019, 20, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Oparil, S.; Kelpke, S.S.; Chen, Y.-F.; Thompson, J.A. Fibroblast growth factor receptor-1 signaling induces osteopontin expression and vascular smooth muscle cell-dependent adventitial fibroblast migration in vitro. Circulation 2002, 106, 854–859. [Google Scholar] [CrossRef]
- MacKenzie, B.; Korfei, M.; Henneke, I.; Sibinska, Z.; Tian, X.; Hezel, S.; Dilai, S.; Wasnick, R.; Schneider, B.; Wilhelm, J.; et al. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir. Res. 2015, 16, 83. [Google Scholar] [CrossRef]
- Seitz, T.; Hellerbrand, C. Role of fibroblast growth factor signalling in hepatic fibrosis. Liver Int. 2021, 41, 1201–1215. [Google Scholar] [CrossRef]
- Gheinani, A.H.; Burkhard, F.C.; Rehrauer, H.; Fournier, C.A.; Monastyrskaya, K. MicroRNA MiR-199a-5p Regulates Smooth Muscle Cell Proliferation and Morphology by Targeting WNT2 Signaling Pathway. J. Biol. Chem. 2015, 290, 7067–7086. [Google Scholar] [CrossRef]
- Weerackoon, N.; Gunawardhana, K.L.; Mani, A. Wnt Signaling Cascades and Their Role in Coronary Artery Health and Disease. J. Cell. Signal. 2021, 2, 52–62. [Google Scholar] [CrossRef]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Guo, X. Transforming growth factor-β and smooth muscle differentiation. World J. Biol. Chem. 2012, 3, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Urs, S.; Boucher, J.; Bernaiche, T.; Venkatesh, D.; Spicer, D.B.; Vary, C.P.; Liaw, L. Notch and Transforming Growth Factor-β (TGFβ) Signaling Pathways Cooperatively Regulate Vascular Smooth Muscle Cell Differentiation. J. Biol. Chem. 2010, 285, 17556–17563. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Hollenbeck, S.T.; Ryer, E.J.; Edlin, R.; Yamanouchi, D.; Kundi, R.; Wang, C.; Liu, B.; Kent, K.C. TGF-β through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am. J. Physiol. Circ. Physiol. 2009, 297, H540–H549. [Google Scholar] [CrossRef] [PubMed]
- DiRenzo, D.M.; Chaudhary, M.A.; Shi, X.; Franco, S.R.; Zent, J.; Wang, K.; Guo, L.-W.; Kent, K.C. A crosstalk between TGF-β/Smad3 and Wnt/β-catenin pathways promotes vascular smooth muscle cell proliferation. Cell. Signal. 2016, 28, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Gheinani, A.H.; Akshay, A.; Besic, M.; Kuhn, A.; Keller, I.; Bruggmann, R.; Rehrauer, H.; Adam, R.M.; Burkhard, F.C.; Monastyrskaya, K. Integrated mRNA-miRNA transcriptome analysis of bladder biopsies from patients with bladder pain syndrome identifies signaling alterations contributing to the disease pathogenesis. BMC Urol. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.I.; Weng, S.; Gollub, J.; Jin, H.; Botstein, D.; Cherry, J.M.; Sherlock, G. GO::TermFinder--open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 2004, 20, 3710–3715. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]



| Pmax | Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 |
|---|---|---|---|---|---|---|---|---|---|
| sham | 18.82 ± 2.4 | 20.37 ± 2.35 | 21.78 ± 7.44 | 20.21 ± 3.07 | 21.07 ± 4.09 | 22.14 ± 4.51 | 24.11 ± 4.06 | 25.26 ± 6.77 | 29.21 ± 12.04 |
| SCI | 18.43 ± 4.13 | 41.49 ± 10.77 ** | 36.18 ± 7.09 ** | 39.77 ± 7.85 *** | 35.82 ± 8.02 ** | 35.14 ± 7.47 ** | 40.68 ± 9.99 ** | 41.29 ± 8.6 ** | 39.13 ± 6.71 ** |
| Pmax | Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 |
|---|---|---|---|---|---|---|---|---|
| Sham 1 wk | 18.7 ± 3.14 | 19.86 ± 2.6 | ||||||
| pBOO 1 wk | 16.9 ± 2.7 | 29.4 ± 4.6 ** | ||||||
| Sham 7 wks | 17.9 ± 5 | 21.09 ± 3 | 25.2 ± 5.3 | 23.6 ± 5.3 | 26.26 ± 7.5 | 22.3 ± 8.5 | 21.7 ± 6.6 | 22.12 ± 2.5 |
| pBOO 7 wks | 16.02 ± 4.34 | 37.5 ±12.7 * | 30.4 ± 6.7 | 29 ± 14.9 | 21 ± 7.2 | 21.45 ± 4.5 | 21.43 ± 5.1 | 20.5 ± 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Siebenthal, M.; Akshay, A.; Besic, M.; Schneider, M.P.; Hashemi Gheinani, A.; Burkhard, F.C.; Monastyrskaya, K. Molecular Characterization of Non-Neurogenic and Neurogenic Lower Urinary Tract Dysfunction (LUTD) in SCI-Induced and Partial Bladder Outlet Obstruction Mouse Models. Int. J. Mol. Sci. 2023, 24, 2451. https://doi.org/10.3390/ijms24032451
von Siebenthal M, Akshay A, Besic M, Schneider MP, Hashemi Gheinani A, Burkhard FC, Monastyrskaya K. Molecular Characterization of Non-Neurogenic and Neurogenic Lower Urinary Tract Dysfunction (LUTD) in SCI-Induced and Partial Bladder Outlet Obstruction Mouse Models. International Journal of Molecular Sciences. 2023; 24(3):2451. https://doi.org/10.3390/ijms24032451
Chicago/Turabian Stylevon Siebenthal, Michelle, Akshay Akshay, Mustafa Besic, Marc P. Schneider, Ali Hashemi Gheinani, Fiona C. Burkhard, and Katia Monastyrskaya. 2023. "Molecular Characterization of Non-Neurogenic and Neurogenic Lower Urinary Tract Dysfunction (LUTD) in SCI-Induced and Partial Bladder Outlet Obstruction Mouse Models" International Journal of Molecular Sciences 24, no. 3: 2451. https://doi.org/10.3390/ijms24032451
APA Stylevon Siebenthal, M., Akshay, A., Besic, M., Schneider, M. P., Hashemi Gheinani, A., Burkhard, F. C., & Monastyrskaya, K. (2023). Molecular Characterization of Non-Neurogenic and Neurogenic Lower Urinary Tract Dysfunction (LUTD) in SCI-Induced and Partial Bladder Outlet Obstruction Mouse Models. International Journal of Molecular Sciences, 24(3), 2451. https://doi.org/10.3390/ijms24032451






